Abstract
AF is a progressive disease of the atria, involving complex mechanisms related to its initiation, maintenance and progression. Computational modelling provides a framework for integration of experimental and clinical findings, and has emerged as an essential part of mechanistic research in AF. The authors summarise recent advancements in development of multi-scale AF models and focus on the mechanistic links between alternations in atrial structure and electrophysiology with AF. Key AF mechanisms that have been explored using atrial modelling are pulmonary vein ectopy; atrial fibrosis and fibrosis distribution; atrial wall thickness heterogeneity; atrial adipose tissue infiltration; development of repolarisation alternans; cardiac ion channel mutations; and atrial stretch with mechano-electrical feedback. They review modelling approaches that capture variability at the cohort level and provide cohort-specific mechanistic insights. The authors conclude with a summary of future perspectives, as envisioned for the contributions of atrial modelling in the mechanistic understanding of AF.
Keywords: AF mechanisms, computational modelling, arrhythmia simulations, precision medicine, personalised electrophysiology
The pathophysiology of AF is complex and incompletely understood to date.[1,2] AF is a progressive disease of the atria involving a multitude of mechanisms related to its initiation, maintenance and progression. Experimental evidence suggest that AF is characterised by alternations in atrial size, shape electrophysiology, autonomic innervation, and cardiomyocyte metabolism, as well as development of atrial fibrosis.[1] However, there are several challenges in translating these experimental findings into actionable treatment strategies applicable in clinical practice.[3]
The interplay between experimentally observed mechanisms on a cohort-specific or patient-specific basis, and their contribution in development and progression of AF is yet to be elucidated. Our incomplete understanding of AF mechanisms is reflected in the modest efficacy of current therapeutic approaches, particularly in patients with persistent AF, with recurrence rates of up to ~50%, despite advances in mapping and ablation technology.[4–8]
Computational modelling and simulations are essential tools in physical sciences and engineering, and over the last decades have been increasingly utilised in cardiac electrophysiology in the study of complex arrhythmias, such as AF.[9] Multi-scale models of cardiac electrophysiology provide a framework for integrating experimental and clinical findings, and linking micro-scale phenomena to whole-organ emergent behaviours. Computational modelling is now an essential part of mechanistic research in AF, because it can complement experimental observations and suggest novel mechanisms. Furthermore, whole-atria simulations are currently used in designing novel, individualised therapeutic strategies, contributing to the ongoing efforts towards precision medicine in cardiology.[10,11]
In this article, we focus on recent advancements in applications of atrial modelling in elucidating AF mechanisms. We summarise studies that use atrial modelling to investigate AF mechanisms that have taken place since our last review on the subject in 2014.[12] Recent advances in the use of atrial modelling in AF therapeutics and ablation planning are summarised in a separate contemporary review by our group.[10] Specifically, we summarise advancements in development of multi-scale AF models and then focus on the mechanistic links between alternations in atrial structure and electrophysiology with AF through the lens of computational modelling. We highlight how AF modelling complements experimental data, in ways that would not be possible outside the framework of simulations, as well as how AF models have revealed novel AF mechanisms. We also review modelling approaches that capture cohort-level variability and provide cohort-specific mechanistic insights. We conclude the review with a summary of the future perspectives for the contributions of atrial modelling in the mechanistic understanding of AF, towards the goal of understanding patient-specific AF mechanisms that would allow for personalised treatment.
Recent Advances in Atrial Modelling
Multi-scale atrial models are mathematical models that link electrophysiological phenomena at the cell, tissue and whole atria scale (Figure 1). The cell scale includes the equations that describe the kinetics of different ionic channels and regulatory proteins, that are coupled to produce the transmembrane potential of an atrial myocyte. The tissue scale includes cell-to-cell coupling and fibre orientation that govern electrical propagation. Electrical propagation is most commonly modelled using the mono-domain formulation coupled with cell models, while the bi-domain formulation is less frequently used due to its high computational cost. Last, the whole atria scale includes the entire complexity of atrial 3D anatomy and distribution of fibrosis.
Figure 1: Multi-scale Model Generation.
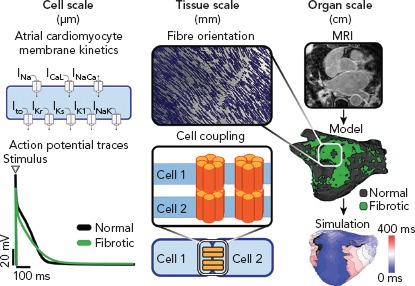
Atrial models are constructed in three different spatial scales: cell-level scale, tissue-level scale and organ-level scale. Cell-level atrial electrophysiology: schematic of the membrane channels and an example of the atrial action potentials obtained in fibrotic (green) and normal cells (black). Tissue scale: myocyte coupling and atrial tissue architecture create a preferential direction for wave propagation along the atrial fibres. Organ scale electrophysiology: late-gadolinium enhanced MRI can be used to segment normal tissue from fibrotic region and for the 3D reconstruction of the atrial geometry to simulate atrial activation.
In this section, first we focus on the current state and recent advances in the cell-scale representation of atrial electrophysiology in patients with AF. Next, we describe advances in tissue-scale and whole-atrial scale representation of atrial myocardium (atrial geometry, ultrastructure and fibrosis); the description is brief, as this topic has been extensively covered in a separate review by our group.[10] Last, we summarise advances in development of atrial models that incorporate mechano-electrical feedback.
Cell-scale Representation of Atrial Electrophysiology in AF
Biophysically Detailed Models of Atrial Cellular Electrophysiology
Biophysically detailed models of cellular electrophysiology typically follow the Hodgkin–Huxley model and represent current flow through ion channels, pumps, and exchangers, as well as sub-cellular calcium cycling. Markov models of ion channels are increasingly used to describe channel gating and modulation; and are important in modelling of the electrophysiological effect of medications and ion channel mutations.[13–16] The most commonly used human atrial cell models are those developed by Courtemanche-Ramirez-Nattel, Nygren et al., Koivumaki et al., Maleckar et al., Grandi et al. and Coleman et al.[17–22] The details of these and other atrial cell models have been recently reviewed.[12,23] These cell models have been used in studies that investigate AF mechanisms using tissue and whole-atria simulations.[24–38] Whole atria simulations using biophysically detailed cell models are typically computationally expensive, requiring execution on high-performance computer clusters.
Models Including the Ultra-rapid Outward K+ Current
The ultra-rapid outward K+ current (IKur) is a major repolarising current in human atria and accounts for the relatively short action potential duration (APD) of the atria.[39] Until recently, available atrial cellular electrophysiology models did not account for experimentally-observed IKur inactivation dynamics. Aguilar et al.incorporated an experimentally derived formulation of IKur in the biophysically detailed ionic model of Courtemanche et al.[40] This formulation accurately reproduces time-, voltage- and frequency-dependent inactivation of the channel.[40] This ionic model has been used in tissue-level simulations to gain mechanistic insights in the role of IKur in the presence or absence of AF-induced ionic remodelling. The model of Aguilar et al. has not been used in organ-level simulations yet. The significance of accurate modelling of IKur is that inhibition of IKur has been considered as an ideal anti-arrhythmic drug development strategy, due to the selective atrial localisation of IKur. Computational modelling has been used to evaluate the effect of different IKur inhibitors in terminating AF,understand the effect of AF-induced ionic remodelling on the efficacy of IKur inhibitionand define optimal IKur inhibitors properties, such as kinetics and state-dependent binding, that maximise AF selectivity in human atrial cardiomyocytes.[14,40,41] The efficacy of IKur inhibition in patients with paroxysmal AF has recently been evaluated in two clinical trials. DIAGRAF-IKUR demonstrated that IKur inhibitor S66913 failed to demonstrate efficacy in patients with paroxysmal AF.[42] The second trial evaluates BMS-919373 and has recently been completed, but the results are not available yet (NCT02156076).
Phenomenological Atrial Models
Phenomenological cell models do not provide a biophysically accurate description of cellular scale electrophysiology, but constitute simplified formulations that reproduce an accurate action potential shape and important electrophysiological properties, such as APD restitution, conduction velocity restitution and wave curvature. The two phenomenological models that have been used in tissue- or organ-level atrial simulations are the atrial Bueno-Orovio–Cherry–Fenton model and the Mitchel-Schaeffer model.
The Bueno-Orovio–Cherry–Fenton model is a phenomenological cell model that describes ventricular electrophysiology, using four state variables.[43] The atrial Bueno-Orovio–Cherry–Fenton model has been adapted to capture atrial electrophysiology and in 2D tissue-scale simulations it accurately replicates the characteristic features of re-entrant excitation patterns as they are observed in similar simulations using biophysically detailed models.[44] The modified Mitchel-Schaeffer model has two state variables and four parameters, and has been adapted to be robust to any pacemaker behaviour.[45] The simplicity of the Mitchel-Schaeffer model allows for ultra-fast computation. Phenomenological models, since they are considerably less complex than biophysically detailed models, require significantly reduced run time and are computationally more efficient.
Given their reduced complexity, phenomenological models are suitable for calibration to clinical measurements, such as activation sequences (as described in the following section), less prone to overfitting and easier to validate compared to biophysically detailed models.[46–49] However, since phenomenological models do not incorporate specific ionic channels, they may be less suitable for studies evaluating the complex effect of antiarrhythmic medications.
Development of Patient-specific Action Potential Models of Atrial Myocardium
Development of patient-specific atrial action potential models is an active area of research, since differences in cellular scale electrophysiology can dramatically affect the emergent atrial fibrillation dynamics in tissue- or organ-scale simulations.[47–49] This was highlighted in a recent study by Lombardo et al.[47] In this study, action potential morphology, APD restitution and conduction velocity restitution were assessed in five patients with AF during invasive electrophysiological studies, using monophasic action potential catheters and standard multi-electrode electrophysiology catheters. These parameters were used to calibrate a phenomenological (Fenton-Karma) and a biophysically detailed (Koivumäki) atrial ionic model with a stochastic optimisation approach. Parameters of the calibrated models were significantly different from published sets and between patients. Both biophysically detailed and phenomenological models produced spiral waves on 2D simulations that had similar dynamics for each patient, but largely varied between patients.[47] These results underscore the need for development of patient-specific atrial action potential models.
The Mitchel-Schaeffer model has been used for development of patient-specific atrial action potential models in tissue-and organ-scale studies, as it has a very small number of parameters, allowing for computationally tractable calibration.[46,48,49] Calibration of a tissue-scale formulation of the Mitchel-Schaeffer model using synthetic data and patient-derived atrial effective refractory periods and conduction velocity restitution was done in five patients with AF.[48,49] Calibrated models to different patients yielded different spiral wave dynamics in simple 2D simulations.[48,49] Atrial effective refractory period, local activation time and conduction velocity measurements from multiple locations in the left atrium, have been used to personalise the action potential parameters and tissue conductivity of a 2D implementation of the Mitchel-Schaeffer model on a realistic 3D atrial surface in seven patients with paroxysmal AF.[46] These models were derived during pacing from the coronary sinus and then validated, by accurately predicting the activation sequence of the left atrium during pacing from the high right atrium.[46]
Future studies should focus on developing optimisation approaches that are suitable for the unique nature of cardiac electrophysiology models. Cardiac electrophysiology models involve highly non-linear partial differential equations, with high-dimensional, frequently discontinuous parameter spaces and complex geometry. Recent research has suggested that hybrid optimisation approaches that combine stochastic and deterministic processes at each iteration are superior to purely stochastic or deterministic approaches.[50] AF models with personalised electrophysiology should be validated by predicting the location of re-entrant drivers that sustain AF and comparing it with clinical observations. It remains unclear how close we can come to electrophysiological personalisation of atrial models, and what level of personalisation is relevant for accurate mechanistical assessment and clinical predictions in patients with AF.
Modelling of Atrial Geometry, Ultrastructure and Fibrosis
A detailed review of the state-of-the-art methods for modelling atrial geometry, ultrastructure (fibre orientation) and fibrosis has recently been published by our team.[10] In brief, atrial models can have idealised or realistic geometry. Models with idealised atrial geometry abstract the left atrium (LA) as an ellipsoid surface (or volume) with orifices that correspond to the four pulmonary veins and the mitral valve (i.e. topologically equivalent to the LA).[51,52] Models with realistic atrial geometry are reconstructed from medical imaging and specifically from cardiac MRI, cardiac CT scans or invasively acquired electroanatomic maps.[31,35–37,46,53–70] The reconstructed models can be 3D surface models (manifolds), 3D bilayer models or full-thickness volumetric 3D models.[31,35–37,46,53–60,62–70] Myofibre orientation is incorporated in 3D atrial models using fibre orientation atlases derived from histology, rule-based methods or methods that use morphological data of the endo- and epicardial surfaces and the local solutions of Laplace’s equations.[65,71,72] Fibre orientation is critical for accurate organ-scale simulations.[73] There are ongoing efforts to develop fibre orientation atlases derived from diffusion-tensor imaging cardiac MRI and incorporate them in atrial models.[74] Novel approaches that derive fibre orientation from electroanatomic mapping or local electrograms are at different stages of development.[75,76]
Atrial fibrosis can be detected on late gadolinium enhancement MRI (LGE-MRI) as areas of increased gadolinium uptake using different thresholding techniques.[31,35,77,78] However, the precision in imaging of AF with currently available LGE-MRI technologies remains controversial. Areas of fibrosis can be then represented in atrial models as electrical conduction disturbances (lower conductivity, edge splitting, or percolation), transforming growth factor-beta1 ionic channel effects, myocyte-fibroblast coupling, discrete microstructural alternations in gap junction connectivity, and combinations of the preceding.[25,33,35,79–81] Selection of fibrosis modelling methodology is critical as the specific representation of fibrosis has a significant effect on rotor dynamics.[62,81] There is an urgent need for quantification of the uncertainty related to imaging, fibrosis detection and fibrosis representation, and incorporation of this uncertainty in model predictions.[82,83]
Modelling of Mechano-electrical Feedback
Atrial mechano-electric feedback refers to alterations in cell- or tissue-scale electrophysiological properties as a result of changes in the loading conditions of the atria. Atrial stretch has been associated with alternations in myocardial electrophysiology in experimental studies,but its role in AF pathophysiology remains unknown.[84,85] Incorporation of mechano-electric feedback in atrial models, although methodologically challenging, is necessary to understand the contribution of atrial mechanics and atrial stretch in the pathophysiology of AF. There are limited studies incorporating mechano-electrical feedback in atrial models, since simulations using strong electromechanical coupling are extremely computationally demanding. Strong electromechanical coupling refers to the modelling approach where changes in electrophysiological state variables result in atrial tissue deformation, and atrial tissue deformation alters the electrophysiological parameters that determine different ionic currents and the action potential. The first 2D atrial model that incorporated mechano-electrical feedback was developed by Brocklehurst et al.by strongly coupling the electrophysiological model of Colman et al.to the mechanical myofilament model of Rice et al., with parameters modified based on experimental data.[86–88] A stretch-activated channel was incorporated into the model to simulate the mechano-electrical feedback. Satriano et al.developed a 3D implementation of a strongly coupled electromechanical atrial model using reconstructed images from a porcine heart and ex vivo experimental validation.[89]
Mechanistic Insights into AF Initiation and Maintenance Using Computational Modelling
Atrial computational modelling allows integration of experimental and clinical findings, and provides insights in the fundamental mechanisms involved in initiation and perpetuation of AF. Increased pulmonary vein ectopy is the primary mechanism of paroxysmal AF initiation.[90] Maintenance of persistent AF occurs due to electroanatomical remodelling of the atria. Re-entrant drivers within regions of structural or functional inhomogeneities have a significant role in maintenance of persistent AF.[91] Structural and electrical remodelling have been incorporated in atrial models to investigate potential links between the altered electroanatomical substrate in AF, and the dynamics of AF re-entrant drivers. Key structural and functional alternations that are mechanistically linked to AF and have been studied using atrial modelling are pulmonary vein (PV) ectopy, presence of atrial fibrosis and its distribution, atrial wall thickness heterogeneity, atrial adipose tissue infiltration, development of repolarisation alternans, cardiac ion channel mutations, and atrial stretch with mechano-electrical feedback (Figure 2). Although atrial autonomic innervation and remodelling have a significant role in AF maintenance,current atrial modelling studies have not incorporated the distribution or remodelling of autonomic nerve fibres, primarily due to the limited ability to visualise these structures with clinically available imaging technologies.[92,93]
Figure 2: Mechanistic Insights from AF Modelling.
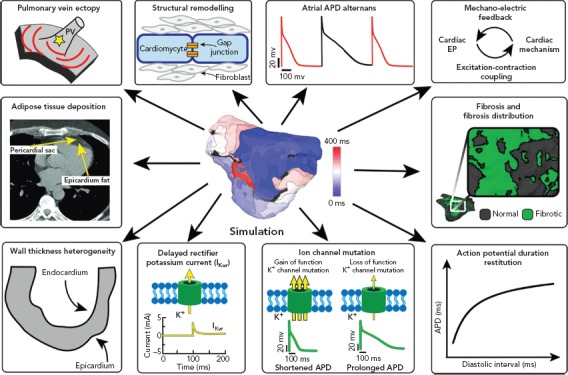
Clockwise: Pulmonary vein ectopy and AF inducibility, gap junctional uncoupling, development of repolarisation alternans, atrial stretch with mechano-electrical feedback, characterisation of the fibrotic atrial substrate, tissue restitution properties, ion channel mutations, atrial wall thickness heterogeneity and adipose tissue deposition (Source: Mahabadi et al. 2017.120 Reproduced with permission from the Public Library of Science). APD = action potential duration; PV = pulmonary vein.
Pulmonary Vein Ectopy as an AF Trigger and the Role of Pulmonary Veins in AF Maintenance
Increased PV ectopy is the primary mechanism of arrhythmia initiation in paroxysmal AF.[90] Cellular mechanisms that have been proposed for the generation of PV ectopy include increased automaticity and afterdepolarisations of the cardiomyocytes in the PV sleeves.[94] There are limited modelling studies evaluating PV ectopy, as previously reviewed.[12] Briefly, PV ectopy has been modelled as increased automaticity of PV atrial cells, through the incorporation of a hyperpolarisation-activated inward current to human atrial cell models, and micro-reentry within the PV sleeves. Recently, Roney et al. demonstrated that the electrophysiological properties and the extent of fibrosis of the PVs are associated with patient-specific susceptibility to AF initiation and maintenance.[31] Short PV APD and slow conduction velocity at the LA/PV junction was associated with increased arrhythmia susceptibility, while longer PV APD was protective. The presence of PV fibrosis was associated with increased incidence of re-entrant drivers in the PVs region.[31] Future studies should evaluate how a biophysically and structurally detailed model of ectopy in the PV sleeves would drive the atria into paroxysmal AF.
Role of Fibrosis in AF Dynamics
There is conflicting clinical evidence on the role of fibrosis in re-entrant drivers dynamics in patients with AF. In two studies (n=12–41), re-entrant drivers observed during AF, localised in the boundary zones between fibrotic and non-fibrotic atrial myocardium.[58,95] In three studies, there was no association between re-entrant driver localisation and LGE-MRI fibrosis.[96–98] Both invasive and non-invasive methods for rotor detection were used in these studies.[58,95–98] The discrepancy between the results of these studies could be due to differences in patient cohorts, LGE-MRI acquisition, image processing, and fibrosis definition, and electrical signal processing and rotor detection strategies. In the setting of this clinical equipoise, atrial modelling provides unique insights in the role of fibrosis in AF dynamics.
Atrial modelling studies strongly support that the extent and distribution of atrial fibrosis are critical determinants of AF initiation, maintenance, and re-entrant driver dynamics during AF. In a sensitivity analysis of simulations using realistic atrial geometry, the extent and distribution of fibrosis had a greater impact on re-entrant driver localisation over alternations in tissue wavelength.[31] Although diffuse fibrosis is sufficient for initiation of AF in simulations,patient-specific fibrosis distribution determines re-entrant driver dynamics.[25,32–35] In two separate studies using patient-specific atrial geometry and fibrosis distribution derived from LGE-MRI, the re-entrant drivers that occurred during AF localised in the boundary zones between fibrotic and non-fibrotic atrial myocardium.[33,34] These zones had a highly specific fibrosis spatial pattern, characterised by high fibrosis density and entropy, and corresponded to atrial areas with high degree of intermingling between fibrotic and non-fibrotic atrial myocardium.[33]
The role of fibrosis in AF dynamics has also been studied using a probabilistic approach with cellular automata models.[99] Cellular automata are simple models where a set of rules dictates the transition of each cell between a resting, excited or refractory state. Fibrotic atrial tissue has been incorporated in cellular automata models as decreased probability of a cell to be connected with its transverse neighbours, reflecting the lateralisation of connexin-43 that is present in fibrotic atrial tissue.[99] Progressive fibrosis significantly changed the frequency, duration, burden and dynamics of AF episodes. Similar to the simulations using biophysically detailed atrial models described above, micro-reentrant wavefronts in cellular automata models anchored at regions with critical fibrotic architectural patterns. The number of local critical patterns of fibrosis rather than the extent of global fibrosis determined AF dynamics.
The association between patient-specific fibrosis distribution and re-entrant driver dynamics has been experimentally validated using a single ex vivo atrial preparation from a patient with longstanding persistent AF.[55] In this study, a detailed 3D atrial model was reconstructed from both LGE-MRI and histology sections. Simulations using this model demonstrated that AF re-entrant drivers localise in areas with distinct structural features, specifically intermediate wall thickness and fibrosis as well as twisted myofibre orientation. Future studies, however, are needed to ascertain the association between re-entrant driver dynamics and fibrosis, as well as the contribution of re-entrant drivers to AF pathophysiology as it remains controversial.[100,101]
Role of Wall Thickness Heterogeneity in AF Re-entrant Driver Dynamics
Atrial wall thickness heterogeneity is a structural property of the atria that has a significant impact on AF re-entrant drivers’ trajectory and localisation.[36,37] In simulations using models with both idealised and realistic atrial geometry, re-entrant drivers drift from thicker to thinner regions along ridge-line structures.[36,37] In simulations using bi-atrial models reconstructed from MRIs of healthy volunteers (n=4) and patients with AF (n=2) the effect of wall thickness heterogeneity on re-entrant drivers trajectories was more prominent in the right atrium (RA), while in the LA, re-entrant driver trajectory was primarily determined by fibrosis distribution. In the RA, re-entrant drivers drifted toward and anchored to the large wall thickness gradient between the crista terminalis and the surrounding atrial wall. In the absence of such a gradient, re-entrant drivers drifted toward the superior vena cava or the tricuspid valve. In the presence of fibrosis, re-entrant drivers anchored to either the fibrotic region or between the crista terminalis and the fibrotic region, depending on the location in the RA from where they were elicited. The more uniform wall thickness of LA resulted in LA re-entrant drivers drifting towards the PVs in the absence of fibrosis, or anchoring in the fibrotic region in the presence of fibrosis.[37] A limitation of these studies is that fibre orientation was not included in the reconstructed atrial models. These findings highlight the complex interplay between atrial geometry, wall thickness gradients and fibrosis distribution that ultimately determine the dynamics of AF re-entrant drivers.
Adipose Tissue and its Effect on AF Dynamics
There is emerging evidence that AF-induced remodelling is characterised by increased deposition of epicardial adipose tissue and adipose tissue infiltration in the atrial myocardium. The presence of adipose tissue in or around the atrial myocardium has a paracrine pro-fibrotic effect.[102] Only one study to date uses atrial modelling to gain insight in the potential effects of fibro-fatty infiltration on AF dynamics.[26] In 2D simulations, the Courtemanche cell model was modified to represent atrial electrophysiology similar to what is experimentally observed when myocytes are co-cultured with adipocytes (69–87% increase in APD and 2.5–5.5A mV increase in resting membrane potential). The presence of adipose tissue-induced remodelling substantially affected spiral wave dynamics resulting in complex arrhythmias and wave breakup. Future studies are needed to elucidate the electrophysiological properties of adipocytes, and the electrophysiological alternations of atrial myocytes induced by the presence of adipose tissue. Incorporation of these findings in atrial models will allow us to understand how the presence of adipose tissue in or around the myocardium affect the propensity for and dynamics of AF.
Evaluating the Role of Atrial Stretch in AF Dynamics by Modelling Mechano-electrical Feedback
The role of atrial stretch in the onset and maintenance of AF is incompletely understood to date. Acute atrial stress is associated with conduction slowing and complex signal formation in the PV-LA junction in humans and prolongation of atrial refractory period in animal studies.[84,85] There is accumulating evidence that most voltage-sensitive ion channels that give rise to the cardiac action potential can be mechanically modulated,and that medications can affect the mechanosensitivity of ion channels (i.e. ranolazine inhibits mechanosensitivity of NaV1.5).[103,104] Computational modelling has been used to integrate the cell-level electrophysiological alternations induced by atrial stretching with the distribution of stretch on the atria, and its time evolution over the cardiac cycle, incorporating mechano-electric feedback in atrial models as described in above. In 2D simulations using the electromechanical model of Brocklehurst et al., the presence of mechano-electrical feedback significantly affected spiral wave tip trajectories, stability, excitation frequencies and meandering range (Figure 3).[86] Contrary to this, in whole organ 3D simulations using the Satriano et al. model, the role of stretch-activated channels was very small during a single-beat of sinus rhythm.[87] Simulations using the model of Satriano et al., however, demonstrated that higher strain occurs in areas adjacent to the mitral valve annulus, rim of the appendage, pulmonary vein trunks and Bachmann’s bundle.[89] These are regions where atrial arrhythmias are most likely to occur. Further studies are needed to assess the potential impact of mechano-electrical feedback on AF dynamics in whole heart simulations.
Figure 3: Electro-mechanical Dynamics of Spiral Waves.
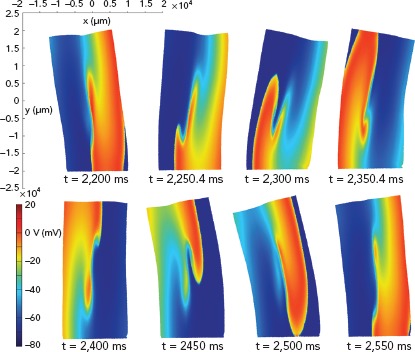
Snapshots of tissue deformation and membrane action potential conduction in the full electro-mechanical model during a spiral wave excitation in a 2D electro-mechanical coupling model of human atrial tissue (x, y: μm). Source: Brocklehurst et al. 2017.86 Reproduced from the Public Library of Science.
Mechanisms of Atrial Repolarisation Alternans and their Role in AF Dynamics
Atrial repolarisation alternans is the beat-to-beat alternation in APD that is observed in atrial myocytes when they are excited at fast rates. The onset of atrial repolarisation alternans has been associated with increased risk for development of AF in animal models and limited human data.[105–107] Simulations using biophysically detailed atrial models have provided a unique insight in the sub-cellular mechanisms of atrial repolarisation alternans.[27] Furthermore, multiscale models with realistic atrial geometry comprehensively describe how the cell-scale phenomenon of repolarisation alternans results in the organ-scale behaviour of AF.[38] In simulations, decreased ryanodine receptor inactivation has a central role in development of repolarisation alternans. Specifically, decreased ryanodine receptor inactivation results in augmentation of Ca++ alternans, ultimately manifesting as repolarisation alternans.[27] These results are consistent with experimental findings demonstrating that for the same sarcoplasmic reticulum C++ load, repolarisation alternans can occur due to changes of ryanodine receptor refractoriness.[108,109] In 3D simulations with realistic atrial geometry, elevated propensity to calcium-driven repolarisation alternans due to chronic AF electrical remodelling was associated with increased vulnerability to ectopy-induced arrhythmia. The presence of Ca++-induced electrical instabilities promoted disorganisation of AF through increased repolarisation heterogeneities, resulting in unstable scroll waves meandering and breaking in multiple wavelets.[38]
Mechanisms of AF in the Presence of Ion Channel Mutations
Rare cases of AF are associated with mutations in genes that encode critical cardiac ionic channels.[110–115] The sparsity of clinical data on these cases renders computational modelling a critical methodology for studying the pathophysiology of AF in the presence of genetic mutations. Mutations related to AF studied in a computational modelling framework are those in genes encoding for K+ channels and specifically hERG gene encodes for IKr channels and is associated with short-QT syndrome 1 (representative mutations L532P and N588K), KCNQ1 gene encodes for IKs channels and is associated with short-QT syndrome and familial/juvenile AF syndrome (representative mutations G229D, V307L and V141M), and KCNJ2 gene encodes for IKr1 which is an inward-rectifier K+ current, associated with short-QT syndrome 3 (representative mutations D172N and E299V). The effect of these mutations on cardiac dynamics has been evaluated in tissue-scale simulations,[24] and 3D atrial modelling studies incorporating realistic atrial geometry.[28–30]
Gain-of-function mutations in hERG, KCNQ1 and KCNJ2 genes result in shorter action potential duration. Atrial models incorporating gain-of-function mutations in these genes had shorter APD and refractory period compared to wild-type models.[24,28–30] These models exhibited greater inducibility of spiral wave re-entry in tissue level (Figure 4)and organ-level simulations, reduced tissue excitation wavelength, which caused greater inducibility of spiral wave re-entry in organ-level simulations,and increased lifespan of re-entrant drivers.[24,28–30] The presence of the KCNQ1 gain of function mutation G229D was associated with increased propensity for spiral wave breakup.[28]
Figure 4: Trajectories of the Rotor Centre.
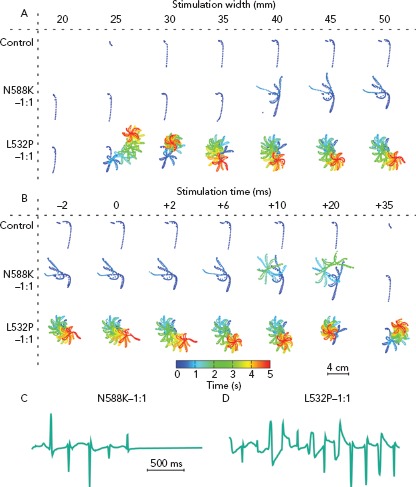
Trajectories of the rotor centre in 2D tissue patch for different stimulation widths (A) and times (B) in the modified Courtemanche–Ramirez–Nattel (control) model and the same model adapted to capture the electrophysiological effects of two representative hERG mutations that are associated with AF (L532P and N588K). The control model failed to initiate a spiral wave by using the S1–S2 cross-field protocol. The models adapted to L532P initiated a rotor for all investigated setups except a stimulus width of 20 mm (A). In contrast, the model adapted to N588K failed to initiate a rotor for stimuli <40 mm or later than 2 ms (B). Source: Loewe et al. 2014.24 Reproduced with permission from Oxford University Press.
Population of Models to Capture Cohort-specific Variability
Population of cell models calibrated to cohort-specific distributions of action potential properties are a powerful tool to capture the inter-subject, cohort-specific variability of atrial electrophysiology. In a study applying this approach, an initial population of >2,000 biophysically detailed atrial cell models were generated, in a way that each model had a unique set of ionic conductance combinations, stochastically selected over a wide range around their values in the original model.[116] Distributions of different properties of action potential morphology were estimated from experimental recordings in patients with AF. A subset of the initial population of models was then selected, such as the selected models had an action potential morphology that lies within the experimentally-derived action potential distribution (Figure 5).[116,117] Analysis of populations of models calibrated to recordings from atrial preparations of patients with AF (n=149) and sinus rhythm (n=214) demonstrated that models calibrated to AF have variations in IK1, IKur, and Ito conductances consistent with AF-induced remodelling.[116] Populations of models calibrated to action potential recordings from atria in sinus rhythm and AF identify potential ionic determinants of inter-subject variability in human APD and action potential morphology.[116] Populations of models calibrated to patients with AF have smaller APD variability and more stable dynamic restitution compared to populations of models calibrated patients in sinus rhythm.[117]
Figure 5: Simulated Normal Sinus Rhythm and Chronic AF.
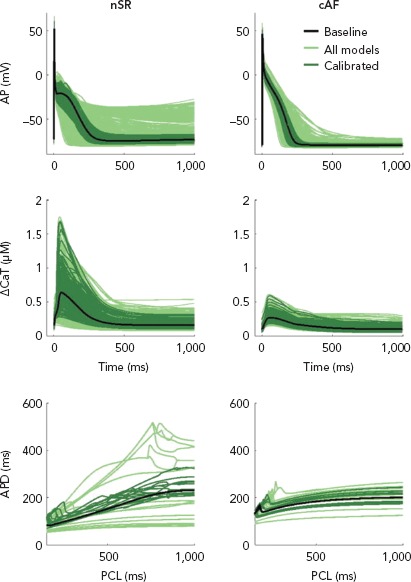
Simulated normal sinus rhythm (nSR) and chronic AF (cAF) populations: steady-state action potential (AP, upper) and calcium transient amplitude (CaT, middle) traces and a few representative dynamic restitution curves (lower). AP and CaT traces and restitution curves of the nSR baseline are also shown for comparison. The x-axis in restitution curves represents pacing cycle length in ms. The populations (1,000 models each) were functionally calibrated, resulting in 213 models in the nSR population and 357 in the cAF population. AP = action potential; APD = action potential duration; cAF = chronic AF; CaT = calcium transient amplitude; PCL = pacing cycle length. Source: Vagos et al. 2017.117 Reproduced with permission from AIP Publishing.
Populations of ionic models calibrated to recordings from patients with AF have been used in 3D simulations of idealised[51] and realistic[118] atrial geometry models to provide novel insights in the mechanisms involved in AF maintenance. Higher expression of INa and ICaL was associated with perpetuation of AF.[51] ICaL inhibition resulted in increased re-entrant drivers meandering and ultimately re-entrant driver collision and termination of AF, particularly in models with decreased INa.[51] Prolongation of APD in all phases of repolarisation caused slowing and regularisation of fibrillatory dynamics. Specifically, inhibition of IK1, INaK and INa resulted in organisation of AF.[118] The same inhibition of ionic currents was able to produce different effects on AF dynamics in atrial simulations using cells modelled to have the same variability as human experimental data.[51,118] The next frontiers in development of cohort-specific model populations are to develop model populations calibrated to different AF sub-types according to AF burden (i.e. paroxysmal, persistent and long-standing persistent AF) and use them in simulations to gain subtype-specific mechanistic insights, and to incorporate biomarkers that further refine model selection by describing more specific disease phenotypes.
Future Perspectives
Computational modelling of AF has emerged as a critical part of the scientific effort to better understand the complexity and variability in AF pathophysiology. Atrial models are becoming more sophisticated and capture fine details of atrial anatomy, ultrastructure, and fibrosis distribution. Personalisation of atrial models is slowly extending beyond geometrical, image-based model personalisation to functional and electrophysiological personalisation that can be cohort-specific or patient-specific. Simulations using atrial models have provided important insight in the mechanisms underlying AF, highlighting the importance of the atrial geometry, fibrotic substrate and altered atrial electrophysiology in initiation and maintenance of AF.
There are some limitations in the mechanistic insights that one can gain using currently available atrial models. There is a need for more accurate PV ectopy models. Future studies should focus on development of accurate models of PV electrophysiology, structure and fibrosis distribution, that can be used to investigate how patient-specific predisposition to PV ectopy, in conjunction with patient-specific substrate, result in the onset and maintenance of AF.
Computationally efficient models that incorporate mechano-electrical feedback need to be developed, to understand the impact of different loading conditions on the electrophysiological heterogeneities of the atrial and how these heterogeneities affect AF initiation and dynamics. Models with mechano-electrical feedback can also be used to evaluate the haemodynamic effect of AF and the potential benefit of rhythm control. Current atrial models capture patient-specific atrial anatomy and fibrosis distribution, but there are only limited studies incorporating patient-specific atrial electrophysiology.
Future modelling approaches should focus on developing models that capture patient-specific atrial electrophysiology. This could be accomplished by calibration of currently available models using patient-specific electrophysiological measurements, or utilisation of genomic and/or transcriptomic data. Addition of genomic and/or transcriptomic data to atrial models could allow for electrical personalisation by incorporating the predicted impact that different polymorphisms have on ionic channels and currents.
There is still incomplete understanding of the electrophysiological substrate present in different stages of AF progression. Characterisation of atrial electrophysiological properties, such as APD, APD restitution, conduction velocity, conduction velocity anisotropy and restitution at different stages of AF progression could allow computational models to more accurately describe AF dynamics, and provide insights in the mechanisms involved in progression of AF. Currently available models do not include a patient-specific representation of fibre orientation. Technological progress that will allow for derivation of patient-specific fibre orientation from imaging or electrophysiological measurements is an area of current investigation, with the potential to facilitate incorporation of patient-specific fibre orientation in atrial models. Currently available models do not include information about autonomic innervation of the heart. Future models should incorporate both the global effects of autonomic tone on atrial electrophysiology, as well as the local effects of atrial ganglionic innervation and remodelling.
The considerable uncertainty in atrial models arises both from natural variation in experimental and clinical data (aleatory uncertainty), and lack of knowledge (epistemic uncertainty). The impact of uncertainty on the outputs of atrial models is not well understood.[83] There are limited studies on verification, validation and uncertainty quantification of atrial models.[52,53] Future studies should prioritise uncertainty quantification and incorporation of it in atrial models and specifically determine how uncertainties in the cell-scale atrial models contribute to uncertainties in emergent behaviours of organ-level models, and how these uncertainties can be visualised and interpreted by experimentalists or clinicians. Statistical approaches such as Monte Carlo techniques, polynomial chaos expansions and Gaussian process emulation can be used to incorporate uncertainty in atrial models. A more detailed discussion of these techniques is provided in the review by Mirams et al.[83]
The mechanistic insights in AF provided by computational modelling and simulations will continue to grow in a virtuous cycle with experimental and clinical cardiac electrophysiology findings. The next frontier for atrial multi-scale modelling is to expand beyond the “whole-organ” scale to the “whole-patient” scale and incorporate mechanistic links for all clinical factors related to onset and progression of AF. Machine learning approaches have the potential to be combined with computational modelling to raise computational modelling to the ‘whole-patient’ scale.[119] Development of a patient-specific understanding of the mechanisms for AF initiation and progression is the most essential step towards precision medicine and development of personalised AF prevention or therapeutic strategies.
Clinical Perspective
Atrial models can be used to gain mechanistic insights into AF.
A complex interplay between atrial geometry, fibrosis distribution and wall thickness heterogeneity determines re-entrant driver localisation during AF.
While there is conflicting clinical evidence on re-entrant driver localisation with respect to atrial fibrosis, in simulations, re-entrant drivers coalesced in areas at the border between fibrotic and healthy myocardium.
Adipose tissue deposition in the left atrium promotes wave breakup and complex arrhythmia formation.
Development of atrial repolarisation alternans promotes repolarisation heterogeneity that results in arrhythmia instabilities and wave breakup.
Atrial modelling can be used to predict the effect of different ion channel mutations on AF initiation and maintenance.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–68. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 2.Mann I, Sandler B, Linton N, Kanagaratnam P. Drivers of atrial fibrillation: Theoretical considerations and practical concerns. Arrhythm Electrophysiol Rev. 2018;7:49–54. doi: 10.15420/aer.2017.40.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heijman J, Guichard JB, Dobrev D, Nattel S. Translational challenges in atrial fibrillation. Circ Res. 2018;122:752–73. doi: 10.1161/CIRCRESAHA.117.311081. [DOI] [PubMed] [Google Scholar]
- 4.Pallisgaard JL, Gislason GH, Hansen J et al. Temporal trends in atrial fibrillation recurrence rates after ablation between 2005 and 2014: a nationwide Danish cohort study. Eur Heart J. 2018;39:442–49. doi: 10.1093/eurheartj/ehx466. [DOI] [PubMed] [Google Scholar]
- 5.Kircher S, Arya A, Altmann D Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace. 2017. [Epub ahead of print] [DOI] [PubMed]
- 6.Conti S, Weerasooriya R, Novak P et al. Contact force sensing for ablation of persistent atrial fibrillation: A randomized, multicenter trial. Heart Rhythm. 2018;15:201–8. doi: 10.1016/j.hrthm.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Verma A, Jiang CY, Betts TR et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 8.Latchamsetty R, Morady F. Source determination in atrial fibrillation. Arrhythm Electrophysiol Rev. 2018;7:165–8. doi: 10.15420/aer:2018:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niederer SA, Lumens J, Trayanova NA. Computational models in cardiology. Nat Rev Cardiol. 2019;16:100–111. doi: 10.1038/s41569-018-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronis KN, Ali R, Trayanova NA. The role of personalized atrial modeling in understanding atrial fibrillation mechanisms and improving treatment. Int J Cardiol. 2019;287:139–47. doi: 10.1016/j.ijcard.2019.01.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trayanova N. From genetics to smart watches: developments in precision cardiology. Nat Rev Cardiol. 2019;16:72–3. doi: 10.1038/s41569-018-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trayanova NA. Mathematical approaches to understanding and imaging atrial fibrillation: significance for mechanisms and management. Circ Res. 2014;114:1516–31. doi: 10.1161/CIRCRESAHA.114.302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellinwood N, Dobrev D, Morotti S, Grandi E. Revealing kinetics and state-dependent binding properties of IKur-targeting drugs that maximize atrial fibrillation selectivity. Chaos. 2017;27:093918. doi: 10.1063/1.5000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellinwood N, Dobrev D, Morotti S, Grandi E. In silico assessment of efficacy and safety of IKur inhibitors in chronic atrial Fibrillation: Role of kinetics and state-dependence of drug binding. Front Pharmacol. 2017;8:799. doi: 10.1063/1.5000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morotti S, McCulloch AD, Bers DM et al. Atrial-selective targeting of arrhythmogenic phase-3 early afterdepolarizations in human myocytes. J Mol Cell Cardiol. 2016;96:63–71. doi: 10.1016/j.yjmcc.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittaker DG, Hancox JC, Zhang H. In silico assessment of pharmacotherapy for human atrial patho-electrophysiology associated with hERG-linked short QT syndrome. Front Physiol. 2018;9:1888. doi: 10.3389/fphys.2018.01888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998;275:H301–21. doi: 10.1152/ajpheart.1998.275.1.H301. [DOI] [PubMed] [Google Scholar]
- 18.Nygren A, Fiset C, Firek L et al. Mathematical model of an adult human atrial cell: The role of K+ currents in repolarization. Circ Res. 1998;82:63–81. doi: 10.1161/01.res.82.1.63. [DOI] [PubMed] [Google Scholar]
- 19.Koivumaki JT, Korhonen T, Tavi P. Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study. PLoS Comput Biol. 2011;7:e1001067. doi: 10.1371/journal.pcbi.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maleckar MM, Greenstein JL, Giles WR, Trayanova NA. K+ current changes account for the rate dependence of the action potential in the human atrial myocyte. Am J Physiol Heart Circ Physiol. 2009;297:H1398–410. doi: 10.1152/ajpheart.00411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grandi E, Pandit SV, Voigt N, Workman AJ, Dobrev D, Jalife J, Bers DM. Human atrial action potential and Ca2+ model: sinus rhythm and chronic atrial fibrillation. Circ Res. 2011;109:1055–66. doi: 10.1161/CIRCRESAHA.111.253955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colman MA, Aslanidi OV, Kharche S et al. Pro-arrhythmogenic effects of atrial fibrillation-induced electrical remodelling: insights from the three-dimensional virtual human atria. J Physiol. 2013;591:4249–72. doi: 10.1113/jphysiol.2013.254987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dossel O, Krueger MW, Weber FM et al. Computational modeling of the human atrial anatomy and electrophysiology. Med Biol Eng Comput. 2012;50:773–99. doi: 10.3389/fphys.2018.01888. [DOI] [PubMed] [Google Scholar]
- 24.Loewe A, Wilhelms M, Fischer F et al. Arrhythmic potency of human ether-a-go-go-related gene mutations L532P and N588K in a computational model of human atrial myocytes. Europace. 2014;16:435–43. doi: 10.1093/europace/eut375. [DOI] [PubMed] [Google Scholar]
- 25.Vigmond E, Pashaei A, Amraoui S et al. Percolation as a mechanism to explain atrial fractionated electrograms and reentry in a fibrosis model based on imaging data. Heart Rhythm. 2016;13:1536–43. doi: 10.1016/j.hrthm.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 26.De Coster T, Claus P, Seemann G et al. Myocyte remodeling due to fibro-fatty infiltrations influences arrhythmogenicity. Front Physiol. 2018;9:1381. doi: 10.1016/j.hrthm.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang KC, Bayer JD, Trayanova NA. Disrupted calcium release as a mechanism for atrial alternans associated with human atrial fibrillation. PLoS Comput Biol. 2014;10:e1004011. doi: 10.1371/journal.pcbi.1004011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zulfa I, Shim EB, Song KS, Lim KM. Computational simulations of the effects of the G229D KCNQ1 mutation on human atrial fibrillation. J Physiol Sci. 2016;66:407–15. doi: 10.1007/s12576-016-0438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whittaker DG, Colman MA, Ni H et al. Human atrial arrhythmogenesis and sinus bradycardia in KCNQ1-linked short QT syndrome: Insights from computational modelling. Front Physiol. 2018;9:1402. doi: 10.1007/s12576-016-0438-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whittaker DG, Ni H, El Harchi A et al. Atrial arrhythmogenicity of KCNJ2 mutations in short QT syndrome: Insights from virtual human atria. PLoS Comput Biol. 2017;13:e1005593. doi: 10.1371/journal.pcbi.1005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha M, Roney CH, Bayer JD et al. Wavelength and fibrosis affect phase singularity locations during atrial fibrillation. Front Physiol. 2018;9:1207. doi: 10.3389/fphys.2018.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y, Gong Y, Xia L. Simulation of atrial fibrosis using coupled myocyte-fibroblast cellular and human atrial models. Comput Math Methods Med 2017. 2017. p. 9463010. [DOI] [PMC free article] [PubMed]
- 33.Zahid S, Cochet H, Boyle PM et al. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res. 2016;110:443–54. doi: 10.1093/cvr/cvw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan R, Colman MA, Chubb H et al. Slow conduction in the border zones of patchy fibrosis stabilizes the drivers for atrial fibrillation: insights from multi-scale human atrial modeling. Front Physiol. 2016;7:474. doi: 10.3389/fphys.2016.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDowell KS, Zahid S, Vadakkumpadan F et al. Virtual electrophysiological study of atrial fibrillation in fibrotic remodeling. PLoS One. 2015;10:e0117110. doi: 10.1371/journal.pone.0117110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kharche SR, Biktasheva IV, Seemann G A computer simulation study of anatomy induced drift of spiral waves in the human atrium. Biomed Res Int 2015. 2015. p. 731386. [DOI] [PMC free article] [PubMed]
- 37.Roy A, Varela M, Aslanidi O. Image-based computational evaluation of the effects of atrial wall thickness and fibrosis on re-entrant drivers for atrial fibrillation. Front Physiol. 2018;9:1352. doi: 10.3389/fphys.2018.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang KC, Trayanova NA. Mechanisms of arrhythmogenesis related to calcium-driven alternans in a model of human atrial fibrillation. Sci Rep. 2016;6:36395. doi: 10.1038/srep36395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wettwer E, Hala O, Christ T et al. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 40.Aguilar M, Feng J, Vigmond E et al. Rate-dependent role of IKur in human atrial repolarization and atrial fibrillation maintenance. Biophys J. 2017;112:1997–2010. doi: 10.1016/j.bpj.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scholz EP, Carrillo-Bustamante P, Fischer F et al. Rotor termination is critically dependent on kinetic properties of Ikur inhibitors in an in silico model of chronic atrial fibrillation. PLoS One. 2013;8:e83179. doi: 10.1371/journal.pone.0083179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camm AJ, Dorian P, Hohnloser SH et al. A randomized, double-blind, placebo-controlled trial assessing the efficacy of S66913 in patients with paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Pharmacother. 2019;5:21–28. doi: 10.1093/ehjcvp/pvy022. [DOI] [PubMed] [Google Scholar]
- 43.Bueno-Orovio A, Cherry EM, Fenton FH. Minimal model for human ventricular action potentials in tissue. J Theor Biol. 2008;253:544–60. doi: 10.1016/j.jtbi.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Richter Y, Lind PG, Seemann G, Maass P. Anatomical and spiral wave reentry in a simplified model for atrial electrophysiology. J Theor Biol. 2017;419:100–7. doi: 10.1016/j.jtbi.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 45.Corrado C, Niederer SA. A two-variable model robust to pacemaker behaviour for the dynamics of the cardiac action potential. Math Biosci. 2016;281:46–54. doi: 10.1016/j.mbs.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corrado C, Williams S, Karim R et al. A work flow to build and validate patient specific left atrium electrophysiology models from catheter measurements. Med Image Anal. 2018;47:153–63. doi: 10.1016/j.media.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lombardo DM, Fenton FH et al. Comparison of detailed and simplified models of human atrial myocytes to recapitulate patient specific properties. PLoS Comput Biol. 2016;12:e1005060. doi: 10.1371/journal.pcbi.1005060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corrado C, Whitaker J, Chubb H et al. Personalized models of human atrial electrophysiology derived from endocardial electrograms. IEEE Trans Biomed Eng. 2017;64:735–42. doi: 10.1109/TBME.2016.2574619. [DOI] [PubMed] [Google Scholar]
- 49.Corrado C, Whitaker J, Chubb H Predicting spiral wave stability by personalized electrophysiology models Paper presented at: 2016 Computing in Cardiology Conference (CinC); 2016
- 50.Loewe A, Wilhelms M, Schmid J et al. Parameter estimation of ion current formulations requires hybrid optimization approach to be both accurate and reliable. Front Bioeng Biotechnol. 2015;3:209. doi: 10.3389/fbioe.2015.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liberos A, Bueno-Orovio A, Rodrigo M et al. Balance between sodium and calcium currents underlying chronic atrial fibrillation termination: An in silico intersubject variability study. Heart Rhythm. 2016;13:2358–65. doi: 10.1016/j.hrthm.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin YT, Chang ET, Eatock J et al. Mechanisms of stochastic onset and termination of atrial fibrillation studied with a cellular automaton model. J R Soc Interface. 2017;14:20160968. doi: 10.1098/rsif.2016.0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deng D, Murphy MJ, Hakim JB et al. Sensitivity of reentrant driver localization to electrophysiological parameter variability in image-based computational models of persistent atrial fibrillation sustained by a fibrotic substrate. Chaos. 2017;27:093932. doi: 10.1063/1.5003340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krueger MW, Rhode KS. O’Neill MD, et al. Patient-specific modeling of atrial fibrosis increases the accuracy of sinus rhythm simulations and may explain maintenance of atrial fibrillation. J Electrocardiol. 2014;47:324–8. doi: 10.1016/j.jelectrocard.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 55.Zhao J, Hansen BJ, Wang Y et al. Three-dimensional integrated functional, structural, and computational mapping to define the structural “fingerprints” of heart-specific atrial fibrillation drivers in human heart ex vivo. J Am Heart Assoc. 2017;6:e005922. doi: 10.1161/JAHA.117.005922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alessandrini M, Valinoti M, Unger L et al. A computational framework to benchmark basket catheter guided ablation in atrial fibrillation. Front Physiol. 2018;9:1251. doi: 10.3389/fphys.2018.01251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boyle PM, Hakim JB, Zahid S et al. The fibrotic substrate in persistent atrial fibrillation patients: comparison between predictions from computational modeling and measurements from focal impulse and rotor mapping. Front Physiol. 2018;9:1151. doi: 10.3389/fphys.2018.01151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyle PM, Hakim JB, Zahid S et al. Comparing reentrant drivers predicted by image-based computational modeling and mapped by electrocardiographic imaging in persistent atrial fibrillation. Front Physiol. 2018;9:414. doi: 10.3389/fphys.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phung TN, Moyer CB, Norton PT et al. Effect of ablation pattern on mechanical function in the atrium. Pacing Clin Electrophysiol. 2017;40:648–54. doi: 10.1111/pace.13086. [DOI] [PubMed] [Google Scholar]
- 60.Zahid S, Whyte KN, Schwarz EL et al. Feasibility of using patient-specific models and the “minimum cut” algorithm to predict optimal ablation targets for left atrial flutter. Heart Rhythm. 2016;13:1687–98. doi: 10.1016/j.hrthm.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roney CH, Bayer JD, Cochet H et al. Variability in pulmonary vein electrophysiology and fibrosis determines arrhythmia susceptibility and dynamics. PLoS Comput Biol. 2018;14:e1006166. doi: 10.1371/journal.pcbi.1006166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roney CH, Bayer JD, Zahid S et al. Modelling methodology of atrial fibrosis affects rotor dynamics and electrograms. Europace. 2016;18 iv:146–5. doi: 10.1093/europace/euw365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roney CH, Cantwell CD, Bayer JD et al. Spatial resolution requirements for accurate identification of drivers of atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e004899. doi: 10.1161/CIRCEP.116.004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shim J, Hwang M, Song JS et al. Virtual in-silico modeling guided catheter ablation predicts effective linear ablation lesion set for longstanding persistent atrial fibrillation: multicenter prospective randomized study. Front Physiol. 2017;8:792. doi: 10.3389/fphys.2017.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fastl TE, Tobon-Gomez C, Crozier A et al. Personalized computational modeling of left atrial geometry and transmural myofiber architecture. Med Image Anal. 2018;47:180–90. doi: 10.1016/j.media.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hwang M, Kwon SS, Wi J et al. Virtual ablation for atrial fibrillation in personalized in-silico three-dimensional left atrial modeling: comparison with clinical catheter ablation. Prog Biophys Mol Biol. 2014;116:40–7. doi: 10.1016/j.pbiomolbio.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Labarthe S, Bayer J, Coudiere Y et al. A bilayer model of human atria: mathematical background, construction, and assessment. Europace. 2014;16 Suppl 4 iv:21–iv29. doi: 10.1093/europace/euu256. [DOI] [PubMed] [Google Scholar]
- 68.Bayer JD, Roney CH, Pashaei A et al. Novel radiofrequency ablation strategies for terminating atrial fibrillation in the left atrium: a simulation study. Front Physiol. 2016;7:108. doi: 10.3389/fphys.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hwang M, Lim B, Song JS et al. Ganglionated plexi stimulation induces pulmonary vein triggers and promotes atrial arrhythmogenecity: In silico modeling study. PLoS One. 2017;12:e0172931. doi: 10.1371/journal.pone.0172931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lim B, Hwang M, Song JS et al. Effectiveness of atrial fibrillation rotor ablation is dependent on conduction velocity: An in-silico 3-dimensional modeling study. PLoS One. 2017;12:e0190398. doi: 10.1371/journal.pone.0190398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krueger MW, Schmidt V, Tobón C Modeling atrial fiber orientation in patient-specific geometries: a semi-automatic rule-based approach. 2011. pp. 223–32. [DOI] [PMC free article] [PubMed]
- 72.Labarthe S, Coudiere Y, Henry J, Cochet H. A semi-automatic method to construct atrial fibre structures: A tool for atrial simulations. 2012 Computing in Cardiology. 2012. pp. 881–4.
- 73.Krueger MW, Schmidt V, Tobón C . Berlin, Heidelberg: Springer: 2011. Modeling atrial fiber orientation in patient-specific geometries: a semi-automatic rule-based approach Functional Imaging and Modeling of the Heart; pp. 223–32. [Google Scholar]
- 74.Pashakhanloo F, Herzka DA, Ashikaga H et al. Myofiber architecture of the human atria as revealed by submillimeter diffusion tensor imaging. Circ Arrhythm Electrophysiol. 2016;9:e004133. doi: 10.1161/CIRCEP.116.004133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roney CH, Whitaker J, Sim I et al. A technique for measuring anisotropy in atrial conduction to estimate conduction velocity and atrial fibre direction. Comput Biol Med. 2019;104:278–90. doi: 10.1016/j.compbiomed.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muzikant AL, Hsu EW, Wolf PD, Henriquez CS. Region specific modeling of cardiac muscle: comparison of simulated and experimental potentials. Ann Biomed Eng. 2002;30:867–83. doi: 10.1114/1.1509453. [DOI] [PubMed] [Google Scholar]
- 77.Oakes RS, Badger TJ, Kholmovski EG et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khurram IM, Beinart R, Zipunnikov V et al. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm. 2014;11:85–92. doi: 10.1016/j.hrthm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boyle PM, Zahid S, Trayanova NA. Towards personalized computational modelling of the fibrotic substrate for atrial arrhythmia. Europace. 2016;18 iv:136–145. doi: 10.1093/europace/euw358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.MacCannell KA, Bazzazi H, Chilton L et al. A mathematical model of electrotonic interactions between ventricular myocytes and fibroblasts. Biophys J. 2007;92:4121–32. doi: 10.1529/biophysj.106.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gokhale TA, Medvescek E, Henriquez CS. Modeling dynamics in diseased cardiac tissue: Impact of model choice. Chaos. 2017;27:093909. doi: 10.1063/1.4999605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gray RA, Pathmanathan P. Patient-specific cardiovascular computational modeling: diversity of personalization and challenges. J Cardiovasc Transl Res. 2018;11:80–8. doi: 10.1007/s12265-018-9792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mirams GR, Pathmanathan P, Gray RA et al. Uncertainty and variability in computational and mathematical models of cardiac physiology. J Physiol. 2016;594:6833–47. doi: 10.1113/JP271671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Walters TE, Lee G, Spence S et al. Acute atrial stretch results in conduction slowing and complex signals at the pulmonary vein to left atrial junction: insights into the mechanism of pulmonary vein arrhythmogenesis. Circ Arrhythm Electrophysiol. 2014;7:1189–97. doi: 10.1161/CIRCEP.114.001894. [DOI] [PubMed] [Google Scholar]
- 85.Satoh T, Zipes DP. Unequal atrial stretch in dogs increases dispersion of refractoriness conducive to developing atrial fibrillation. J Cardiovasc Electrophysiol. 1996;7:833–42. doi: 10.1111/j.1540-8167.1996.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 86.Brocklehurst P, Ni H, Zhang H, Ye J. Electro-mechanical dynamics of spiral waves in a discrete 2D model of human atrial tissue. PLoS One. 2017;12:e0176607. doi: 10.1371/journal.pone.0176607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colman MA, Aslanidi OV, Kharche S et al. Pro-arrhythmogenic effects of atrial fibrillation-induced electrical remodelling: insights from the three-dimensional virtual human atria. J Physiol. 2013;591:4249–72. doi: 10.1113/jphysiol.2013.254987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rice JJ, Wang F, Bers DM, de Tombe PP. Approximate model of cooperative activation and crossbridge cycling in cardiac muscle using ordinary differential equations. Biophys J. 2008;95:2368–90. doi: 10.1529/biophysj.107.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Satriano A, Vigmond EJ, Schwartzman DS, Di Martino ES. Mechano-electric finite element model of the left atrium. Comput Biol Med. 2018;96:24–31. doi: 10.1016/j.compbiomed.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 90.Haissaguerre M, Jais P, Shah DC et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 91.Cochet H, Dubois R, Yamashita S et al. Relationship between fibrosis detected on late gadolinium-enhanced cardiac magnetic resonance and re-entrant activity assessed with electrocardiographic imaging in human persistent atrial fibrillation. JACC Clin Electrophysiol. 2018;4:17–29. doi: 10.1016/j.jacep.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Linz D, Elliott AD, Hohl M Role of autonomic nervous system in atrial fibrillation. Int J Cardiol. 2018. [DOI] [PubMed]
- 93.Kim MY, Sikkel MB, Hunter RJ et al. A novel approach to mapping the atrial ganglionated plexus network by generating a distribution probability atlas. J Cardiovasc Electrophysiol. 2018;29:1624–34. doi: 10.1111/jce.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Bakker JM, Ho SY, Hocini M. Basic and clinical electrophysiology of pulmonary vein ectopy. Cardiovasc Res. 2002;54:287–94. doi: 10.1016/s0008-6363(01)00532-6. [DOI] [PubMed] [Google Scholar]
- 95.Haissaguerre M, Shah AJ, Cochet H et al. Intermittent drivers anchoring to structural heterogeneities as a major pathophysiological mechanism of human persistent atrial fibrillation. J Physiol. 2016;594:2387–98. doi: 10.1113/JP270617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chrispin J, Gucuk Ipek E, Zahid S et al. Lack of regional association between atrial late gadolinium enhancement on cardiac magnetic resonance and atrial fibrillation rotors. Heart Rhythm. 2016;13:654–60. doi: 10.1016/j.hrthm.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 97.Schade A, Nentwich K, Costello-Boerrigter LC et al. Spatial relationship of focal impulses, rotors and low voltage zones in patients with persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27:507–14. doi: 10.1111/jce.12913. [DOI] [PubMed] [Google Scholar]
- 98.Sohns C, Lemes C, Metzner A et al. First-in-man analysis of the relationship between electrical rotors from noninvasive panoramic mapping and atrial fibrosis from magnetic resonance imaging in patients with persistent atrial fibrillation. Circ Arrhythm Electrophysiol. 2017;10:e004419. doi: 10.1161/CIRCEP.116.004419. [DOI] [PubMed] [Google Scholar]
- 99.Manani KA, Christensen K, Peters NS. Myocardial architecture and patient variability in clinical patterns of atrial fibrillation. Phys Rev E. 2016;94:042401. doi: 10.1103/PhysRevE.94.042401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Child N, Clayton RH, Roney CR et al. Unraveling the underlying arrhythmia mechanism in persistent atrial fibrillation: results from the STARLIGHT study. Circ Arrhythm Electrophysiol. 2018;11:e005897. doi: 10.1161/CIRCEP.117.005897. [DOI] [PubMed] [Google Scholar]
- 101.de Groot NM, Houben RP, Smeets JL et al. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: epicardial breakthrough. Circulation. 2010;122:1674–82. doi: 10.1161/CIRCULATIONAHA.109.910901. [DOI] [PubMed] [Google Scholar]
- 102.Abe I, Teshima Y, Kondo H et al. Association of fibrotic remodeling and cytokines/chemokines content in epicardial adipose tissue with atrial myocardial fibrosis in patients with atrial fibrillation. Heart Rhythm. 2018;15:1717–27. doi: 10.1016/j.hrthm.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 103.Peyronnet R, Nerbonne JM, Kohl P. Cardiac mechano-gated ion channels and arrhythmias. Circ Res. 2016;118:311–29. doi: 10.1161/CIRCRESAHA.115.305043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beyder A, Strege PR, Reyes S et al. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel Na(v)1.5: a novel mechanism of drug action. Circulation. 2012;125:2698–706. doi: 10.1161/CIRCULATIONAHA.112.094714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fuller H, Justo F, Nearing BD et al. Eleclazine, a new selective cardiac late sodium current inhibitor, confers concurrent protection against autonomically induced atrial premature beats, repolarization alternans and heterogeneity, and atrial fibrillation in an intact porcine model. Heart Rhythm. 2016;13:1679–86. doi: 10.1016/j.hrthm.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 106.Verrier RL, Fuller H, Justo F et al. Unmasking atrial repolarization to assess alternans, spatiotemporal heterogeneity, and susceptibility to atrial fibrillation. Heart Rhythm. 2016;13:953–61. doi: 10.1016/j.hrthm.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Narayan SM, Franz MR, Clopton P et al. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–30. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shkryl VM, Maxwell JT, Domeier TL, Blatter LA. Refractoriness of sarcoplasmic reticulum Ca2+ release determines Ca2+ alternans in atrial myocytes. Am J Physiol Heart Circ Physiol. 2012;302:H2310–20. doi: 10.1152/ajpheart.00079.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang L, Myles RC, De Jesus NM et al. Optical mapping of sarcoplasmic reticulum Ca2+ in the intact heart: ryanodine receptor refractoriness during alternans and fibrillation. Circ Res. 2014;114:1410–21. doi: 10.1161/CIRCRESAHA.114.302505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Andreasen L, Nielsen JB, Christophersen IE et al. Genetic modifier of the QTc interval associated with early-onset atrial fibrillation. Can J Cardiol. 2013;29:1234–40. doi: 10.1016/j.cjca.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 111.Sinner MF, Pfeufer A, Akyol M et al. The non-synonymous coding IKr-channel variant KCNH2-K897T is associated with atrial fibrillation: results from a systematic candidate gene-based analysis of KCNH2 (HERG) Eur Heart J. 2008;29:907–14. doi: 10.1093/eurheartj/ehm619. [DOI] [PubMed] [Google Scholar]
- 112.Hayashi K, Konno T, Tada H et al. Functional characterization of rare variants implicated in susceptibility to lone atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8:1095–104. doi: 10.1161/CIRCEP.114.002519. [DOI] [PubMed] [Google Scholar]
- 113.Sarquella-Brugada G, Campuzano O, Iglesias A et al. Short QT and atrial fibrillation: A KCNQ1 mutation-specific disease. Late follow-up in three unrelated children. HeartRhythm Case Rep. 2015;1:193–7. doi: 10.1016/j.hrcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Steffensen AB, Refsgaard L, Andersen MN et al. IKs Gain- and loss-of-function in early-onset lone atrial fibrillation. J Cardiovasc Electrophysiol. 2015;26:715–23. doi: 10.1111/jce.12666. [DOI] [PubMed] [Google Scholar]
- 115.Deo M, Ruan Y, Pandit SV et al. KCNJ2 mutation in short QT syndrome 3 results in atrial fibrillation and ventricular proarrhythmia. Proc Natl Acad Sci USA. 2013;110:4291–6. doi: 10.1073/pnas.1218154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanchez C, Bueno-Orovio A, Wettwer E et al. Inter-subject variability in human atrial action potential in sinus rhythm versus chronic atrial fibrillation. PLoS One. 2014;9:e105897. doi: 10.1371/journal.pone.0105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vagos MR, Arevalo H, de Oliveira BL et al. A computational framework for testing arrhythmia marker sensitivities to model parameters in functionally calibrated populations of atrial cells. Chaos. 2017;27:093941. doi: 10.1063/1.4999476. [DOI] [PubMed] [Google Scholar]
- 118.Sanchez C, Bueno-Orovio A, Pueyo E, Rodriguez B. Atrial fibrillation dynamics and ionic block effects in six heterogeneous human 3D virtual atria with distinct repolarization dynamics. Front Bioeng Biotechnol. 2017;5:29. doi: 10.3389/fbioe.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cantwell CD, Mohamied Y, Tzortzis KN et al. Rethinking multiscale cardiac electrophysiology with machine learning and predictive modelling. Comput Biol Med. 2019;104:339–51. doi: 10.1016/j.compbiomed.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mahabadi AA, Balcer B, Dykun I et al. Cardiac computed tomography-derived epicardial fat volume and attenuation independently distinguish patients with and without myocardial infarction. PLoS One. 2017;12:e0183514. doi: 10.1371/journal.pone.0183514. [DOI] [PMC free article] [PubMed] [Google Scholar]


