Abstract
Brown and its related beige adipose tissue (BAT) play a definitive role in maintaining body temperature by producing heat through uncoupling protein 1 (UCP1), which acts by dissociating oxidative phosphorylation from ATP production, resulting in the release of heat. Therefore, in order to maintain high thermogenic capacity, BAT must act as a metabolic sink by taking up vast amounts of circulating glucose and lipids for oxidation. This, along with the rediscovery of BAT in adult humans, has fueled the study of BAT as a putative therapeutic approach to manage the growing rates of obesity and metabolic syndromes. Notably, many of the beneficial consequences of BAT activity overlap with metabolic biomarkers of extended lifespan and healthspan. In this review, we provide background about BAT including the thermogenic program, BAT’s role as a secretory organ, and differences between BAT in mice and humans. We also provide details on BAT during aging, and perspectives on the potential of targeting BAT to promote lifespan and healthspan.
Keywords: Aging, Brown adipose tissue, Metabolism, Thermogenesis
Introduction
An unfortunate reality of modern medicine is that lifespan has increased more rapidly than healthspan (i.e., the period of life an individual is free of frailty and disease). This discrepancy has important geopolitical and economic ramifications since age-related diseases including type 2 diabetes, dementias, and cancers are responsible for the majority of healthcare costs in developed nations (Goldman et al. 2013; Kirkland 2016). Recently, there has been a shift towards focusing research efforts towards extending healthspan because of (i) the high maintenance cost of age-related diseases, (ii) the cost of finding a cure for one specific disease which in many cases only marginally would extend lifespan, and (iii) the fact that people do not want to live a third of their lives frail with diseases. Because of this, the “Geroscience Hypothesis” has been proposed (Austad 2016). The basis of this hypothesis is that it is cheaper and more efficient to target the fundamental processes of aging, thereby affecting many age-related diseases simultaneously, than to narrowly focus on one disease at a time.
Interestingly, one could propose a similar hypothesis for obesity and poor glycemic control since both are predictors of age-related diseases such as type 2 diabetes, cancers, dementias, cardiovascular diseases, and neuromuscular diseases, as well as others. Indeed, it is well-recognized that obesity and poor glycemic control over a period of time mimics an aged phenotype. Unfortunately, both obesity and metabolic syndromes are increased worldwide. Therefore, any therapeutic option that can target aging, obesity, and metabolic dysfunction is in high demand.
Since the rediscovery of BAT in adult humans (Cypess et al. 2009; Nedergaard et al. 2007; van Marken Lichtenbelt et al. 2009; Virtanen et al. 2009), many laboratories have attempted to utilize BAT to combat obesity and improve glycemic control. Although BAT is a small tissue, its metabolic effects on the body cannot be understated. For instance, in a cold-acclimated mouse, over 50% of consumed lipids and glucose are oxidized in BAT (Cannon and Nedergaard 2011; Golozoubova et al. 2004). Impressively, BAT has also been reported to have such a high rate of insulin-independent glucose uptake that cold acclimation can normalize glycemia in streptozotocin-treated rats (Takano et al. 1987). Interestingly, several studies have described improved BAT activity in long-lived animals (Darcy et al. 2016; Li et al. 2003; Ma et al. 2014; Ortega-Molina et al. 2012; Shabalina et al. 2017; Vatner et al. 2018), and diminished BAT activity in short-lived animals (Li et al. 2003; Wang et al. 2018), raising the possibility that BAT not only is integral to metabolism, but the aging process as well. This review is aimed to describe the function and physiology of BAT, and its role in aging.
Brown adipose tissue
Brown adipose tissue overview
In the classical view, adipose tissue is divided into two main categories, energy-storing white adipose tissue (WAT), and energy-expending BAT. However, this view is oversimplified as we now know there exists at least a third type of adipose tissue (termed beige/brite adipose tissue), and that there is tremendous heterogeneity not only within an adipose depot, but between different depots (e.g., subcutaneous and visceral WAT). Since WAT’s role in aging and adipose tissue heterogeneity are not the focus of this review, interested readers are directed to several relevant reviews that cover these topics in more detail (Lynes and Tseng 2018; Martyniak and Masternak 2017; Schosserer et al. 2018; Stout et al. 2017).
Developmentally, in mice, BAT comes from a mesoderm lineage that is paired box 7 (PAX7) (Lepper and Fan 2010) and myogenic factor 5 (MYF5) (Seale et al. 2008) positive. This lineage gives rise to both brown adipocyte precursors and myocyte precursors, and is separate from the MYF5-negative lineage that white adipocyte precursors arise from (Seale et al. 2008). Adipose tissue precursors are referred to as preadipocytes. It is worth noting that preadipocytes are replicative, whereas adipocytes are postmitotic. As with white preadipocytes, brown preadipocytes differentiate into mature adipocytes through the coordinated regulation of peroxisome proliferator-activated receptor gamma (PPARγ), and various isoforms of CCAAT/enhancer-binding proteins (C/EBPs) (Farmer 2006). Specific to brown adipocytes is the transcriptional control of the thermogenic program which is regulated by peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) (Puigserver et al. 1998) and PR domain containing 16 (PRDM16) (Seale et al. 2007). The control of the thermogenic gene program extends beyond PGC-1α and PRDM16 and includes other transcription factors, as well as input from nuclear receptors such as the thyroid hormone receptor (Cassard-Doulcier et al. 1994) and retinoic acid receptor (Alvarez et al. 1995). Although brown preadipocytes and adipocytes constitute the majority of BAT, other cell types such as immune cells, endothelial cells, and hematopoietic cells are also present in the tissue microenvironment (as reviewed in Lynes and Tseng 2018).
BAT is characterized by dense mitochondria along with a high degree of innervation and vascularization. Moreover, BAT contains multilocular lipid droplets, compared with the single, large unilocular lipid droplet present in WAT. Teleologically, these traits are straightforward given the need for high vascularization to bring nutrients to BAT and to dissipate heat throughout the body, innervation to trigger the thermogenic program (detailed below), and smaller lipid droplets that have an increased surface area in order to be mobilized for fuel more readily. The last, and arguably most defining characteristic of BAT is the presence of uncoupling protein 1 (UCP1) which is described in greater detail below.
The thermogenic program
The production of ATP is necessary for all cells to survive. While some ATP is formed through the catabolism of macromolecules, the overwhelming majority of ATP is derived from the electron transport chain (ETC). Here, H+ is displaced in the inner mitochondrial membrane space against chemical (acidic pH) and electrical (positive charge) gradients. The protons are able to move back to the mitochondrial matrix through complex V of the ETC, which harnesses the released energy to form ATP through the fusion of ADP and Pi. UCP1 is the essential protein in non-shivering thermogenesis because it “uncouples” the ETC by providing a means for protons to move down the electrochemical gradient without producing ATP (hence “uncoupling” oxidative phosphorylation from ATP production). The flow of protons through UCP1 still releases chemical energy, although the end product is heat rather than ATP. Because UCP1 disrupts the production of ATP, its expression is tightly regulated in brown and beige adipocytes, and thus, UCP1 serves as the defining marker for the thermogenic adipocytes. The expression of UCP1 is tightly regulated, combining epigenetic factors (JMJD1a and HDAC3) (Abe et al. 2015; Emmett et al. 2017), coordinated transcriptional machinery (PPARγ and PGC-1α) (Puigserver et al. 1998; Sears et al. 1996), and hormonal queues (thyroid hormone) (Cassard-Doulcier et al. 1994). The detailed mechanism by which UCP1 is transcriptionally regulated has been reviewed thoroughly in other reviews (Cannon and Nedergaard 2004; Lynes and Tseng 2015; Sambeat et al. 2017; Villarroya et al. 2017b).
Seminal studies have been published in recent years that highlight UCP1-independent forms of thermogenesis. These include the creatine-based substrate cycle present in both brown and beige adipocytes (Bertholet et al. 2017; Kazak et al. 2015, 2017), and the ATP-dependent calcium cycling present in beige adipocytes (Ikeda et al. 2017). These studies begin to explain the discrepancies found between UCP1-null mice and BAT-deficient mice (Cohen et al. 2014; Enerback et al. 1997; Feldmann et al. 2009; Ohno et al. 2013), and underscore just how much potentially remains unknown about thermogenic adipose tissue.
As for the thermogenic circuit itself, signaling at the tissue level begins with the release of norepinephrine (NE) from the sympathetic nervous system which interacts with β3-adrenergic receptors on brown adipocytes. Because of this, adrenergic stimulants have been sought after to activate BAT in humans; however, β-adrenergic agonists have unwanted side effects such as tachycardia and hypertension. Therefore, drugs that stimulate BAT without these unwanted side effects are in demand. The β3-adrenergic receptors are associated with a G protein-coupled receptor (GPCR) of the Gs subtype (Granneman 1988; Marette and Bukowiecki 1991), which activates adenylate cyclase, in turn raising cytosolic cAMP levels and activating protein kinase A (PKA) (Thonberg et al. 2001). PKA has several functions in the thermogenic pathway including activating mitogen-activated protein kinase (MAPK) p38 (Cao et al. 2001), and raising the cellular level of free fatty acids (FFAs). The rise in FFAs comes about through PKA-mediated phosphorylation of perilipin (Chaudhry and Granneman 1999), which causes the release of comparative gene identification-58 (CGI-58) that can in turn activate adipose triglyceride lipase (ATGL) (Granneman et al. 2007, 2009). ATGL is the major lipase responsible for the breakdown of triglycerides into diglycerides in BAT (Zimmermann et al. 2004). The breakdown of triglycerides into free fatty acids (FFAs) serves two main functions: the first function of FFAs in the thermogenic program is to be shuttled into the mitochondrial matrix to undergo β-oxidation with the resulting acetyl-CoA being shuttled into the tricarboxylic acid (TCA) cycle. Both β-oxidation of fatty acids and the TCA cycle generate ATP, but also provide a continuous flow of reduced electron carriers for the ETC (Cannon and Nedergaard 2004). The second function of FFAs is to act as activators of UCP1 (Shabalina et al. 2004). Specifically, UCP1 acts as a H+/long-chain fatty acid symporter (Fedorenko et al. 2012), meaning fatty acids are needed for UCP1 activity. Conversely to FFAs, purinergic nucleotides bind and inhibit UCP1 action (Shabalina et al. 2004).
Secretory function of BAT
Much of the current literature on BAT focuses on its beneficial effects on energy dissipation and fuel utilization due to its ability to act as a metabolic sink. An emerging area of BAT research, however, is on BAT as an endocrine organ. These BAT-secreted mediators, or “batokines,” can be protein peptides, metabolites, or miRNAs (Villarroya et al. 2017a). Many of the BAT-secreted factors that act in an autocrine/paracrine fashion also aid in modeling the BAT microenvironment for optimal function. For example, vascular endothelial growth factor A (VEGFa) and nitric oxide (NO) promote vascularization and increased blood flow (Asano et al. 1999; Nisoli et al. 1997), while fibroblast growth factor 2 (FGF2) and nerve growth factor (NGF) increase the sympathetic tone and preadipocyte recruitment in BAT (Nechad et al. 1994; Nisoli et al. 1996; Yamashita et al. 1994), all of which are necessary during thermogenic activation of BAT. Recently, our laboratory has identified the lipid mediator 12,13-diHOME which is secreted from BAT, and has cold and exercise mimetic properties by activating BAT in an autocrine fashion. 12,13-diHOME acts on BAT and skeletal muscle by stimulating fatty acid uptake by increasing translocation of CD36 and FATP1 to the cell membrane (Lynes et al. 2017; Stanford et al. 2018).
Beyond autocrine/paracrine action, BAT has been demonstrated to elicit endocrine actions as well. A direct way to identify secretion of batokines is through BAT transplantation studies. For example, BAT transplantation has been shown to improve glycemia in models of type 1 diabetes (both streptozotocin (STZ)-induced and autoimmune-mediated) (Gunawardana and Piston 2012, 2015). In models of type 2 diabetes (both high-fat diet–induced and ob/ob), mice receiving BAT transplantation have increased energy expenditure and improved glucose tolerance (Liu et al. 2013, 2015; Stanford et al. 2013; Zhu et al. 2014). It appears that BAT can also impact the central nervous system as one study reported that BAT transplantation increased the sympathetic tone to the endogenous BAT, WAT, heart, and muscle of recipient mice (Zhu et al. 2014). Other endocrine factors secreted from BAT include insulin-like growth factor-binding protein 2 (IGFBP2) and WNT10b which stimulate bone formation (Rahman et al. 2013). It would be particularly interesting to know if increased BAT function in postmenopausal women is able to slow the progression of osteoporosis by secreting WNT10b. Moreover, it appears that miRNAs are secreted from BAT. For example, mice with cold-stimulated BAT showed a decrease in miR-92a compared to mice housed at room temperature (Chen et al. 2016). Interestingly, humans showed an inverse relationship between BAT activity and serum levels of miR-92a, suggesting that miR-92a may serve as a biomarker of BAT activity.
One particularly important endocrine target of BAT is the liver. While the liver is the main site of production of FGF21 (Markan et al. 2014), BAT is capable of contributing significantly to the circulating levels of FGF21 under adrenergic stimulation (Hondares et al. 2011). Moreover, cold exposure not only causes an increase in the secretion of Fgf21 from BAT, but it represses hepatic Fgf21 expression. More recently, the use of the adipose tissue-specific Dicer knockout mouse model (ADicer KO) has demonstrated that BAT secretes at least one miRNA that acts on the liver and regulates Fgf21 expression. For example, ADicer KO mice have reduced levels of miR-99b in circulating exosomes, which can be rescued by transplanting BAT from wild-type mice (Thomou et al. 2017). Moreover, treatment of ADicer KO mice with exosomes containing miR-99b dramatically lowered hepatic Fgf21 levels, suggesting that BAT-secreted miR-99b is a regulator of hepatic Fgf21.
Interestingly, there appears to be endocrine crosstalk between BAT and the female reproductive tract. The classical example of this is observed in ovariectomized mice that develop obesity, which appears to be at least partially mediated through loss of BAT activity (Bartness and Wade 1984; Pedersen et al. 2001). This effect may be related to the estrogen receptors present in BAT (Wade and Gray 1978). In another example, BAT transplantation in a rat model of polycystic ovary syndrome reversed polycystic ovaries and hyperandrogenism, as well as improved fertility by reversing anovulation (Yuan et al. 2016). Recently, researchers were investigating ways to lower the well-documented postmenopausal increase in circulating the follicle-stimulating hormone (FSH) when they discovered that inhibiting FSH action through the use of an antibody specific to the β-subunit of FSH caused an increase in BAT activity (Liu et al. 2017). This study demonstrated that the postmenopausal increase in circulating FSH not only plays a role in alterations in adiposity and bone density, but also plays a role in the observed decrease in metabolic rate and BAT function during this stage of life. For decades, the so-called trade-offs between reproduction and longevity have been studied (for review please see Bartke et al. 2013). Although BAT’s function in metabolism and as an endocrine organ may play an important role in longevity, it is possible that BAT plays a yet undetermined role in the trade-off between longevity and reproduction.
Brown adipose tissue: of mice and men
In attempting to harness the function of BAT as a therapy, it is important to briefly discuss the similarities and differences of BAT between mice and humans. In mice, the largest depot of BAT resides in the interscapular region; however, BAT is also present in the cervical, axillary, paraaortic, cardiac, and perirenal regions (Lynes and Tseng 2018). BAT in humans is largely present in the supraclavicular region, but is also present in the cervical, axillary, paraaortic, paravertebral, and perirenal regions (Lynes and Tseng 2018; Ravussin and Galgani 2011; Sacks and Symonds 2013). Interestingly, a supraclavicular BAT depot has recently been described in mice (Mo et al. 2017). Interscapular BAT is present in human babies; however, this depot disappears during adolescence. In humans, the gold standard for measuring BAT location and activity is the use of positron emission tomography coupled with computed tomography (PET/CT) during the infusion of radiolabeled 18-fluorodeoxyglucose (18FDG), which takes advantage of the high rate of glucose uptake in BAT following stimulation with a “cold vest” or a β3-agonist such as mirabegron (Carpentier et al. 2018; Cypess et al. 2015). However, glucose uptake does not necessarily reflect thermogenic activity of BAT, and the use of 18FDG to estimate total volume of BAT has led to vastly different estimates of BAT volume in humans ranging from grams to kilograms (reviewed in Carpentier et al. 2018). Therefore, more accurate methodologies for detecting BAT activity in humans are highly sought after. In addition to additional imaging methods, such as magnetic resonance imaging (MRI), single photon emission-computed tomography (SPECT), ultrasound, and infrared imaging (Cypess et al. 2014), finding circulating biomarkers that reflect the activity level BAT provides cost-effective and easily accessible ways for both clinical and research purposes. Despite technological shortcomings, we do know that BAT is present in humans, and can significantly contribute to their metabolism. For example, spending several hours a day over a 10-day period at 15 °C has been shown to dramatically increase the glucose infusion rate (GIR) during a euglycemic clamp study of patients with type 2 diabetes (Hanssen et al. 2015).
Brown adipose tissue and aging
Changes in brown adipose tissue during aging
The most easily observed alteration in BAT during aging in both humans and mice is a loss of both tissue mass and activity, and a decrease in white fat browning (Cypess et al. 2009; Goncalves et al. 2017). The loss of thermogenic adipose tissue can be attributed to several external and internal factors. For example, forkhead box protein A3 (FOXA3) expression is increased in visceral fat during aging, and is reported to reduce BAT mass and beiging capacity of WAT (Ma et al. 2014). FOXA3 KO mice are long-lived and have increased BAT activity late into life, and are protected from age-related insulin resistance and high-fat diet–induced increases in visceral adiposity (Ma et al. 2014). Because adipocytes are postmitotic, having a large pool of preadipocytes is necessary to maintain tissue mass. Although most work with replicative senescence has been performed in WAT, there is evidence that replicative capacity and UCP1 expression is also greatly reduced during aging (Florez-Duquet et al. 1998). Several long-lived mouse models have less senescence and greater preadipocyte differentiation capacity in their WAT (Stout et al. 2014). It is of interest to know if this phenotype extends to BAT as well.
As with all tissues in the body, resident immune cells in BAT vary based on life stage. This is important because immune cells have been shown to play a large role in the thermogenic activity of BAT. For example, sympathetic neuron-associated macrophages (SAMs) are able to chelate NE from brown adipocytes (Pirzgalska et al. 2017), thereby decreasing BAT activity. It is worth noting that the immune cell function of BAT and WAT appears to have some similarities (such as infiltration of classically activated macrophages during obesity and aging); however, there are notable differences. For example, during aging and obesity, WAT classically secretes IL-6 as a pro-inflammatory cytokine. BAT, however, secretes IL-6 as an insulin sensitizer (Stanford et al. 2013) similar to IL-6 secretion from skeletal muscle (Pal et al. 2014). Moreover, BAT has the potential to secrete chemokines such as CXCL14 to attract alternatively activated macrophages (Cereijo et al. 2018).
A final and important way that BAT function can be diminished is through mitochondrial dysfunction. As with other tissues, there is a clear decline in mitochondrial function in adipose tissue during aging and obesity (Hallgren et al. 1989; Mennes et al. 2014). Moreover, reactive oxygen species (ROS) that are produced in the ETC can easily cause damage to the ETC components, and can oxidize the mitochondrial membrane lipids (causing a decrease in membrane potential), both of which lead to decreased respiratory function. Regardless, it is interesting that BAT-specific mitochondrial adaptations closely mirror those that are believed to be a mechanism behind life extension following calorie restriction (CR) (reviewed in Guarente 2008). For example, the major sites of ROS production in the mitochondria are complexes I and III of the ETC due to electron “stalling” (Barja 2007; Barros et al. 2004; Kushnareva et al. 2002; Pamplona and Barja 2007). A way that CR inadvertently reduces ROS is by increasing the use of lipids as metabolic fuel. The increased oxidation of lipids is preferential in terms of ROS production because oxidizing lipids produces a higher ratio of reduced FADH to NADH, which bypasses complex I of the ETC, and instead allows electrons to enter the ETC through complex II (Guarente 2008). Moreover, increased metabolic rate and mitochondrial uncoupling in isolated mitochondria from myocytes have been positively associated with increased longevity (Speakman et al. 2004), which aligns with the “Uncoupling to Survive Hypothesis” (Brand 2000). Whether this specific phenomenon holds true in mitochondria isolated from BAT of long-lived mice or humans remains to be elucidated, though studies provided below suggest it plausible. For completeness, it is worth mentioning that the Uncoupling to Survive Hypothesis may only apply to mammals since the opposing Free Radical Theory of Aging (Harman 1956) still applies when examining lower organisms such as flies (Farmer and Sohal 1987). Moreover, the use of an artificial mitochondrial uncoupler (2,4-dinitrophenol, DNP) increased the metabolic rate in zebra finch, but did not extend longevity (Stier et al. 2014). Despite this, it seems that mammals do benefit from increased uncoupling, and evidence suggests this may also extend to humans, since genetic variation in the human UCP1 gene is associated with longevity (Rose et al. 2011a, b).
Brown adipose tissue in long-lived mice
There is a dramatic falloff in BAT activity during aging and obesity (Cypess et al. 2009; van Marken Lichtenbelt et al. 2009; Virtanen et al. 2009). The intertwining aspects of BAT, aging, and obesity is illustrated particularly well in a study which used mouse models of progeria, extended longevity, and diet-induced obesity to show an overall downregulation of BAT miRNA expression in progeria and in obese animals, which was opposite to the upregulation of BAT miRNA in a longevity model (Oliverio et al. 2016). A mouse model in which BAT aids in extending longevity is the overexpression of Pten. The authors of this study reported an increase in BAT activity which presumably aided in the observed increases in energy expenditure, decreased adiposity, and improved glucose homeostasis of these animals (Ortega-Molina et al. 2012). More recently, mice lacking the regulator of G protein signaling 14 (RGS14) were found to be long-lived, which was at least partially due to enhanced BAT function (Vatner et al. 2018).
Particularly useful mammalian models for dissecting mechanisms of extended lifespan and healthspan are those that have alterations in the somatotropic axis (growth hormone and IGF-1). This includes the long-lived Ames dwarfs (Brown-Borg et al. 1996) and growth hormone receptor knockout (GHRKO) mice (Coschigano et al. 2000), and the short-lived bovine growth hormone (bGH) transgenic mice that overexpress growth hormone (Bartke 2003; Kopchick et al. 2014). Although improved glucose homeostasis has been a major focus of these animals, recent years have suggested that alterations in energy metabolism (e.g., increased energy expenditure) may also play a pivotal role in their longevity (Bartke and Darcy 2017). In the early 2000s, it was reported that GHRKO mice have an enlarged interscapular BAT depot and increased expression of Ucp1, as well as increased expression of Fgf21 (Li et al. 2003). Interestingly, Ucp1 expression is lower in bGH mice, suggesting that there is a negative relationship between circulating growth hormone and Ucp1 expression. Interestingly, microarray data from GHRKO mice at 6 months of age showed an increase in genes related to the mitochondria and metabolism, and a decrease in genes expressed by dendritic cells and macrophages (Stout et al. 2015).
The Ames dwarf mouse is long-lived due to a recessively-inherited Prop1 loss-of-function mutation that results in severe hormonal deficiencies (Brown-Borg et al. 1996). These animals lack production of growth hormone, T4, and prolactin from the anterior pituitary; therefore, Ames dwarf mice are also deficient in circulating IGF-1 and T3. Despite severe hypothyroidism, Ames dwarf mice have highly active BAT compared to their littermates as observed through depleted lipid stores, increased BAT tissue weight, increased expression of genes related to thermogenesis and lipid metabolism, and increased oxygen consumption and energy expenditure (Darcy et al. 2016, 2018; Westbrook et al. 2009). Surgical removal of BAT in Ames dwarf mice normalizes their oxygen consumption to that of control mice, while housing dwarf mice at thermoneutrality normalizes their lipid stores, genes expression, and oxygen consumption to that of control mice (Darcy et al. 2016, 2018). Moreover, thermoneutral housing and surgically removing BAT cause a mild decrease in insulin sensitivity in Ames dwarf mice, suggesting their BAT plays a role in insulin sensitivity which is thought to be a major contributor to their extended longevity.
Conclusions and perspectives
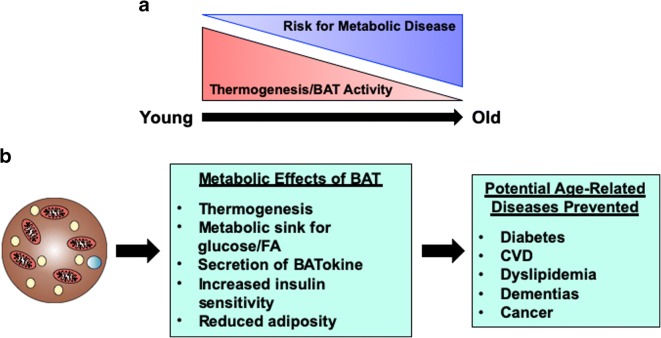
When maximally stimulated, BAT turns on the anabolism and catabolism of macromolecules. The reason for this is that BAT uniquely dissociates oxidative phosphorylation and ATP production in order to produce heat. Because of BAT’s unique metabolic phenotype to act as a metabolic sink, researchers have turned to BAT to combat the growing obesity and diabetes epidemics plaguing the developed world. Of course, the overlap with obesity and aging is not lost, and so there has also been interest in BAT’s role in delaying the underlying processes of aging (summarized in Fig. 1). Particularly, activating BAT appears to be a possible means to reverse the postmenopause and post-andropause decline in metabolic rate that is accompanied by increased visceral adiposity. While a direct link between increased BAT activity and longevity can be appreciated (and has been demonstrated in several studies), it is important to not forget the endocrine function of BAT. This review highlighted some of the known factors secreted from BAT; however, more have been described in the literature and many more unknown factors exist. Alterations in the secretory function of BAT during aging have been severely understudied, and are often unmentioned from studies that perform BAT removal surgeries which result in worsened metabolic phenotypes and shortened longevity. Given that BAT secretes multiple factors from miRNAs to peptide and lipid metabolites, it is plausible to hypothesize that secreted factors from activated BAT could influence both lifespan and healthspan. Regardless, when evaluating metabolic phenotypes of longevity, it is crucial to evaluate BAT activity as it is likely that BAT impacts longevity in a multifaceted manner.
Fig. 1.
Brown adipose tissue and aging. a Brown adipose tissue (BAT) mass and activity decreases throughout life. This decrease is associated with an increased risk of developing metabolic syndrome, especially in the postmenopause and post-andropause life stages. b BAT has major metabolic functions including thermogenesis, secreting batokines, and acting as a metabolic sink for glucose and lipids. BAT reduces obesity and increases insulin sensitivity, which help prevent age-related diseases such as diabetes, dementias, and CVD. Thus, BAT may potentially be a therapeutic option to treat aging, as well as treating obesity
Funding information
This work was supported in part by US National Institutes of Health (NIH) grants R01DK077097 and R01DK102898 (to Y.-H.T.), and P30DK036836 (to Joslin Diabetes Center’s Diabetes Research Center) from the National Institute of Diabetes and Digestive and Kidney Diseases, and by US Army Medical Research grant W81XWH-17-1-0428 (to Y.-H.T.). J.D was supported by institutional research training grant T32DK007260 from NIH. We apologize to those whose work we did not reference due to space limitations.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abe Y, Rozqie R, Matsumura Y, Kawamura T, Nakaki R, Tsurutani Y, Tanimura-Inagaki K, Shiono A, Magoori K, Nakamura K, Ogi S, Kajimura S, Kimura H, Tanaka T, Fukami K, Osborne TF, Kodama T, Aburatani H, Inagaki T, Sakai J. JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis. Nat Commun. 2015;6:7052. doi: 10.1038/ncomms8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez R, de Andrés J, Yubero P, Viñas O, Mampel T, Iglesias R, Giralt M, Villarroya F. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J Biol Chem. 1995;270:5666–5673. doi: 10.1074/jbc.270.10.5666. [DOI] [PubMed] [Google Scholar]
- Asano A, Kimura K, Saito M. Cold-induced mRNA expression of angiogenic factors in rat brown adipose tissue. J Vet Med Sci. 1999;61:403–409. doi: 10.1292/jvms.61.403. [DOI] [PubMed] [Google Scholar]
- Austad SN (2016) The geroscience hypothesis: is it possible to change the rate of aging? In: Sierra F (ed) Advances in Geroscience. Springer
- Barja G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: implications for aging studies. Rejuvenation Res. 2007;10:215–224. doi: 10.1089/rej.2006.0516. [DOI] [PubMed] [Google Scholar]
- Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78:210–216. doi: 10.1159/000073704. [DOI] [PubMed] [Google Scholar]
- Bartke A, Darcy J. GH and ageing: pitfalls and new insights. Best Pract Res Clin Endocrinol Metab. 2017;31:113–125. doi: 10.1016/j.beem.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93:571–598. doi: 10.1152/physrev.00006.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN. Effects of interscapular brown adipose tissue denervation on body weight and energy metabolism in ovariectomized and estradiol-treated rats. Behav Neurosci. 1984;98:674–685. doi: 10.1037//0735-7044.98.4.674. [DOI] [PubMed] [Google Scholar]
- Bertholet AM, Kazak L, Chouchani ET, Bogaczyńska MG, Paranjpe I, Wainwright GL, Bétourné A, Kajimura S, Spiegelman BM, Kirichok Y. Mitochondrial patch clamp of beige adipocytes reveals UCP1-positive and UCP1-negative cells both exhibiting futile creatine cycling. Cell Metab. 2017;25:811–822. doi: 10.1016/j.cmet.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD. Uncoupling to survive? The role of mitochondrial inefficiency in ageing. Exp Gerontol. 2000;35:811–820. doi: 10.1016/s0531-5565(00)00135-2. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- Cao W, Medvedev AV, Daniel KW, Collins S. Beta-adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem. 2001;276:27077–27082. doi: 10.1074/jbc.M101049200. [DOI] [PubMed] [Google Scholar]
- Carpentier AC, Blondin DP, Virtanen KA, Richard D, Haman F, Turcotte EE. Brown adipose tissue energy metabolism in humans. Front Endocrinol (Lausanne) 2018;9:447. doi: 10.3389/fendo.2018.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassard-Doulcier AM, Larose M, Matamala JC, Champigny O, Bouillaud F, Ricquier D. In vitro interactions between nuclear proteins and uncoupling protein gene promoter reveal several putative transactivating factors including Ets1, retinoid X receptor, thyroid hormone receptor, and a CACCC box-binding protein. J Biol Chem. 1994;269:24335–24342. [PubMed] [Google Scholar]
- Cereijo R, Gavaldà-Navarro A, Cairó M, Quesada-López T, Villarroya J, Morón-Ros S, Sánchez-Infantes D, Peyrou M, Iglesias R, Mampel T, Turatsinze JV, Eizirik DL, Giralt M, Villarroya F. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab. 2018;28:750–763.e6. doi: 10.1016/j.cmet.2018.07.015. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Granneman JG. Differential regulation of functional responses by beta-adrenergic receptor subtypes in brown adipocytes. Am J Phys. 1999;277:R147–R153. doi: 10.1152/ajpregu.1999.277.1.R147. [DOI] [PubMed] [Google Scholar]
- Chen Y, Buyel JJ, Hanssen MJW, Siegel F, Pan R, Naumann J, Schell M, van der Lans A, Schlein C, Froehlich H, Heeren J, Virtanen KA, van Marken Lichtenbelt W, Pfeifer A. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Commun. 2016;7:11420. doi: 10.1038/ncomms11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Levy JD, Zhang Y, Frontini A, Kolodin DP, Svensson KJ, Lo JC, Zeng X, Ye L, Khandekar MJ, Wu J, Gunawardana SC, Banks AS, Camporez JPG, Jurczak MJ, Kajimura S, Piston DW, Mathis D, Cinti S, Shulman GI, Seale P, Spiegelman BM. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. doi: 10.1016/j.cell.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: consensus points and experimental guidelines. Cell Metab. 2014;20:408–415. doi: 10.1016/j.cmet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Weiner LS, Roberts-Toler C, Elía EF, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy J, McFadden S, Fang Y, Huber JA, Zhang C, Sun LY, Bartke A. Brown adipose tissue function is enhanced in long-lived, male Ames dwarf mice. Endocrinology. 2016;157:4744–4753. doi: 10.1210/en.2016-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcy J, McFadden S, Fang Y, Berryman DE, List EO, Milcik N, Bartke A. Increased environmental temperature normalizes energy metabolism outputs between normal and Ames dwarf mice. Aging (Albany NY) 2018;10:2709–2722. doi: 10.18632/aging.101582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett MJ, Lim HW, Jager J, Richter HJ, Adlanmerini M, Peed LC, Briggs ER, Steger DJ, Ma T, Sims CA, Baur JA, Pei L, Won KJ, Seale P, Gerhart-Hines Z, Lazar MA. Histone deacetylase 3 prepares brown adipose tissue for acute thermogenic challenge. Nature. 2017;546:544–548. doi: 10.1038/nature22819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer KJ, Sohal RS. Effects of ambient temperature on free radical generation, antioxidant defenses and life span in the adult housefly, Musca domestica. Exp Gerontol. 1987;22:59–65. doi: 10.1016/0531-5565(87)90015-5. [DOI] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, Kirichok Y. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Florez-Duquet M, Horwitz BA, McDonald RB. Cellular proliferation and UCP content in brown adipose tissue of cold-exposed aging Fischer 344 rats. Am J Phys. 1998;274:R196–R203. doi: 10.1152/ajpregu.1998.274.1.R196. [DOI] [PubMed] [Google Scholar]
- Goldman DP, Cutler D, Rowe JW, Michaud PC, Sullivan J, Peneva D, Olshansky SJ. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Aff (Millwood) 2013;32:1698–1705. doi: 10.1377/hlthaff.2013.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golozoubova V, Gullberg H, Matthias A, Cannon B, Vennstrom B, Nedergaard J. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol Endocrinol. 2004;18:384–401. doi: 10.1210/me.2003-0267. [DOI] [PubMed] [Google Scholar]
- Goncalves LF, Machado TQ, Castro-Pinheiro C, de Souza NG, Oliveira KJ, Fernandes-Santos C. Ageing is associated with brown adipose tissue remodelling and loss of white fat browning in female C57BL/6 mice. Int J Exp Pathol. 2017;98:100–108. doi: 10.1111/iep.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granneman JG. Norepinephrine infusions increase adenylate cyclase responsiveness in brown adipose tissue. J Pharmacol Exp Ther. 1988;245:1075–1080. [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Mitochondria--a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132:171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardana SC, Piston DW. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes. 2012;61:674–682. doi: 10.2337/db11-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardana SC, Piston DW. Insulin-independent reversal of type 1 diabetes in nonobese diabetic mice with brown adipose tissue transplant. Am J Physiol Endocrinol Metab. 2015;308:E1043–E1055. doi: 10.1152/ajpendo.00570.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren P, Sjostrom L, Hedlund H, Lundell L, Olbe L. Influence of age, fat cell weight, and obesity on O2 consumption of human adipose tissue. Am J Phys. 1989;256:E467–E474. doi: 10.1152/ajpendo.1989.256.4.E467. [DOI] [PubMed] [Google Scholar]
- Hanssen MJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21:863–865. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hondares E, Iglesias R, Giralt A, Gonzalez FJ, Giralt M, Mampel T, Villarroya F. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–12990. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, Tajima K, Ajuwon KM, Soga T, Kajimura S. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23:1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, Kajimura S, Gygi SP, Spiegelman BM. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Lu GZ, Jedrychowski MP, Bare CJ, Mina AI, Kumari M, Zhang S, Vuckovic I, Laznik-Bogoslavski D, Dzeja P, Banks AS, Rosen ED, Spiegelman BM. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab. 2017;26:660–671. doi: 10.1016/j.cmet.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL. Translating the science of aging into therapeutic interventions. Cold Spring Harb Perspect Med. 2016;6:a025908. doi: 10.1101/cshperspect.a025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick JJ, List EO, Kelder B, Gosney ES, Berryman DE. Evaluation of growth hormone (GH) action in mice: discovery of GH receptor antagonists and clinical. Mol Cell Endocrinol. 2014;386:34–45. doi: 10.1016/j.mce.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnareva Y, Murphy AN, Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Knapp JR, Kopchick JJ. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med (Maywood) 2003;228:207–215. doi: 10.1177/153537020322800212. [DOI] [PubMed] [Google Scholar]
- Liu X, Zheng Z, Zhu X, Meng M, Li L, Shen Y, Chi Q, Wang D, Zhang Z, Li C, Li Y, Xue Y, Speakman JR, Jin W. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013;23:851–854. doi: 10.1038/cr.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, Lin J, Zhao Q, Zhang C, Yuan X, Hu T, Liu L, Huang Y, Zhang L, Wang D, Zhan J, Jong Lee H, Speakman JR, Jin W. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology. 2015;156:2461–2469. doi: 10.1210/en.2014-1598. [DOI] [PubMed] [Google Scholar]
- Liu P, Ji Y, Yuen T, Rendina-Ruedy E, DeMambro VE, Dhawan S, Abu-Amer W, Izadmehr S, Zhou B, Shin AC, Latif R, Thangeswaran P, Gupta A, Li J, Shnayder V, Robinson ST, Yu YE, Zhang X, Yang F, Lu P, Zhou Y, Zhu LL, Oberlin DJ, Davies TF, Reagan MR, Brown A, Kumar TR, Epstein S, Iqbal J, Avadhani NG, New MI, Molina H, van Klinken JB, Guo EX, Buettner C, Haider S, Bian Z, Sun L, Rosen CJ, Zaidi M. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546:107–112. doi: 10.1038/nature22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes MD, Tseng YH. The thermogenic circuit: regulators of thermogenic competency and differentiation. Genes Dis. 2015;2:164–172. doi: 10.1016/j.gendis.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes MD, Tseng YH. Deciphering adipose tissue heterogeneity. Ann N Y Acad Sci. 2018;1411:5–20. doi: 10.1111/nyas.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, Takahashi H, Hirshman MF, Schlein C, Lee A, Baer LA, May FJ, Gao F, Narain NR, Chen EY, Kiebish MA, Cypess AM, Blüher M, Goodyear LJ, Hotamisligil GS, Stanford KI, Tseng YH. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat Med. 2017;23:631–637. doi: 10.1038/nm.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Xu L, Gavrilova O, Mueller E. Role of forkhead box protein A3 in age-associated metabolic decline. Proc Natl Acad Sci U S A. 2014;111:14289–14294. doi: 10.1073/pnas.1407640111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marette A, Bukowiecki LJ. Noradrenaline stimulates glucose transport in rat brown adipocytes by activating thermogenesis. Evidence that fatty acid activation of mitochondrial respiration enhances glucose transport. Biochem J. 1991;277:119–124. doi: 10.1042/bj2770119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Martyniak K, Masternak MM. Changes in adipose tissue cellular composition during obesity and aging as a cause of metabolic dysregulation. Exp Gerontol. 2017;94:59–63. doi: 10.1016/j.exger.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes E, Dungan CM, Frendo-Cumbo S, Williamson DL, Wright DC. Aging-associated reductions in lipolytic and mitochondrial proteins in mouse adipose tissue are not rescued by metformin treatment. J Gerontol A Biol Sci Med Sci. 2014;69:1060–1068. doi: 10.1093/gerona/glt156. [DOI] [PubMed] [Google Scholar]
- Mo Q, Salley J, Roshan T, Baer LA, May FJ, Jaehnig EJ, Lehnig AC, Guo X, Tong Q, Nuotio-Antar AM, Shamsi F, Tseng YH, Stanford KI, Chen MH (2017) Identification and characterization of a supraclavicular brown adipose tissue in mice. JCI Insight 2. 10.1172/jci.insight.93166 [DOI] [PMC free article] [PubMed]
- Nechad M, Ruka E, Thibault J. Production of nerve growth factor by brown fat in culture: relation with the in vivo developmental stage of the tissue. Comp Biochem Physiol Comp Physiol. 1994;107:381–388. doi: 10.1016/0300-9629(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Benarese M, Liberini P, Carruba MO. Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology. 1996;137:495–503. doi: 10.1210/endo.137.2.8593794. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Briscini L, Carruba MO. Inducible nitric oxide synthase in rat brown adipocytes: implications for blood flow to brown adipose tissue. Endocrinology. 1997;138:676–682. doi: 10.1210/endo.138.2.4956. [DOI] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Ohyama K, Sharp LZ, Kajimura S. EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature. 2013;504:163–167. doi: 10.1038/nature12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliverio M, Schmidt E, Mauer J, Baitzel C, Hansmeier N, Khani S, Konieczka S, Pradas-Juni M, Brodesser S, van TM, Bartsch D, Brönneke HS, Heine M, Hilpert H, Tarcitano E, Garinis GA, Frommolt P, Heeren J, Mori MA, Brüning JC, Kornfeld JW. Dicer1-miR-328-Bace1 signalling controls brown adipose tissue differentiation and function. Nat Cell Biol. 2016;18:328–336. doi: 10.1038/ncb3316. [DOI] [PubMed] [Google Scholar]
- Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, Muñoz-Martin M, Gómez-López G, Cañamero M, Mulero F, Pastor J, Martinez S, Romanos E, Mar Gonzalez-Barroso M, Rial E, Valverde AM, Bischoff JR, Serrano M. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 2012;15:382–394. doi: 10.1016/j.cmet.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Pal M, Febbraio MA, Whitham M. From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol. 2014;92:331–339. doi: 10.1038/icb.2014.16. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G. Highly resistant macromolecular components and low rate of generation of endogenous damage: two key traits of longevity. Ageing Res Rev. 2007;6:189–210. doi: 10.1016/j.arr.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Bruun JM, Kristensen K, Richelsen B. Regulation of UCP1, UCP2, and UCP3 mRNA expression in brown adipose tissue, white adipose tissue, and skeletal muscle in rats by estrogen. Biochem Biophys Res Commun. 2001;288:191–197. doi: 10.1006/bbrc.2001.5763. [DOI] [PubMed] [Google Scholar]
- Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sánchez NM, Mahú I, Mendes R, Gres V, Kubasova N, Morris I, Arús BA, Larabee CM, Vasques M, Tortosa F, Sousa AL, Anandan S, Tranfield E, Hahn MK, Iannacone M, Spann NJ, Glass CK, Domingos AI. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med. 2017;23:1309–1318. doi: 10.1038/nm.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology. 2013;154:2687–2701. doi: 10.1210/en.2012-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin E, Galgani JE. The implication of brown adipose tissue for humans. Annu Rev Nutr. 2011;31:33–47. doi: 10.1146/annurev-nutr-072610-145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Crocco P, D'Aquila P, Montesanto A, Bellizzi D, Passarino G. Two variants located in the upstream enhancer region of human UCP1 gene affect gene expression and are correlated with human longevity. Exp Gerontol. 2011;46:897–904. doi: 10.1016/j.exger.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Rose G, Crocco P, De Rango F, Montesanto A, Passarino G. Further support to the uncoupling-to-survive theory: the genetic variation of human UCP genes is associated with longevity. PLoS One. 2011;6:e29650. doi: 10.1371/journal.pone.0029650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks H, Symonds ME. Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes. 2013;62:1783–1790. doi: 10.2337/db12-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambeat A, Gulyaeva O, Dempersmier J, Sul HS. Epigenetic regulation of the thermogenic adipose program. Trends Endocrinol Metab. 2017;28:19–31. doi: 10.1016/j.tem.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schosserer M, Grillari J, Wolfrum C, Scheideler M. Age-induced changes in white, brite, and brown adipose depots: a mini-review. Gerontology. 2018;64:229–236. doi: 10.1159/000485183. [DOI] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scimè A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears IB, MacGinnitie MA, Kovacs LG, Graves RA. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Mol Cell Biol. 1996;16:3410–3419. doi: 10.1128/mcb.16.7.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina IG, Jacobsson A, Cannon B, Nedergaard J. Native UCP1 displays simple competitive kinetics between the regulators purine nucleotides and fatty acids. J Biol Chem. 2004;279:38236–38248. doi: 10.1074/jbc.M402375200. [DOI] [PubMed] [Google Scholar]
- Shabalina IG, Vyssokikh MY, Gibanova N, Csikasz RI, Edgar D, Hallden-Waldemarson A, Rozhdestvenskaya Z, Bakeeva LE, Vays VB, Pustovidko AV, Skulachev MV, Cannon B, Skulachev VP, Nedergaard J. Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1. Aging (Albany NY) 2017;9:315–339. doi: 10.18632/aging.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, et al. 12, 13-diHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab. 2018;27:1111–1120. doi: 10.1016/j.cmet.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier A, Bize P, Roussel D, Schull Q, Massemin S, Criscuolo F. Mitochondrial uncoupling as a regulator of life-history trajectories in birds: an experimental study in the zebra finch. J Exp Biol. 2014;217:3579–3589. doi: 10.1242/jeb.103945. [DOI] [PubMed] [Google Scholar]
- Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, Oberg AL, LeBrasseur NK, Miller RA, Kopchick JJ, Bartke A, Kirkland JL. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY) 2014;6:575–586. doi: 10.18632/aging.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MB, Swindell WR, Zhi X, Rohde K, List EO, Berryman DE, Kopchick JJ, Gesing A, Fang Y, Masternak MM. Transcriptome profiling reveals divergent expression shifts in brown and white adipose tissue from long-lived GHRKO mice. Oncotarget. 2015;6:26702–26715. doi: 10.18632/oncotarget.5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology (Bethesda) 2017;32:9–19. doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Honma T, Motohashi Y, Kobayashi Y. Streptozotocin diabetes in rats after acclimation to cold environment. Prev Med. 1987;16:63–69. doi: 10.1016/0091-7435(87)90006-5. [DOI] [PubMed] [Google Scholar]
- Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonberg H, Lindgren EM, Nedergaard J, Cannon B. As the proliferation promoter noradrenaline induces expression of ICER (induced cAMP early repressor) in proliferative brown adipocytes, ICER may not be a universal tumour suppressor. Biochem J. 2001;354:169–177. doi: 10.1042/0264-6021:3540169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner DE, Zhang J, Oydanich M, Guers J, Katsyuba E, Yan L, Sinclair D, Auwerx J, Vatner SF. Enhanced longevity and metabolism by brown adipose tissue with disruption of the regulator of G protein signaling 14. Aging Cell. 2018;17:e12751. doi: 10.1111/acel.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F, Cereijo R, Villarroya J, Giralt M. Brown adipose tissue as a secretory organ. Nat Rev Endocrinol. 2017;13:26–35. doi: 10.1038/nrendo.2016.136. [DOI] [PubMed] [Google Scholar]
- Villarroya F, Peyrou M, Giralt M. Transcriptional regulation of the uncoupling protein-1 gene. Biochimie. 2017;134:86–92. doi: 10.1016/j.biochi.2016.09.017. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM. Cytoplasmic 17 beta-[3H]estradiol binding in rat adipose tissues. Endocrinology. 1978;103:1695–1701. doi: 10.1210/endo-103-5-1695. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xu X, Liu Y, Gao Y, Kang F, Liu B, Wang J. Assessment of the aging of the brown adipose tissue by (1)(8)F-FDG PET/CT imaging in the progeria mouse model Lmna. Contrast Media Mol Imaging. 2018;2018:8327089. doi: 10.1155/2018/8327089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in oxygen consumption, respiratory quotient, and heat production in long-lived GHRKO and Ames dwarf mice, and short-lived bGH transgenic mice. J Gerontol A Biol Sci Med Sci. 2009;64:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita H, Sato Y, Kizaki T, Oh S, Nagasawa J, Ohno H. Basic fibroblast growth factor (bFGF) contributes to the enlargement of brown adipose tissue during cold acclimation. Pflugers Arch. 1994;428:352–356. doi: 10.1007/BF00724518. [DOI] [PubMed] [Google Scholar]
- Yuan X, Hu T, Zhao H, Huang Y, Ye R, Lin J, Zhang C, Zhang H, Wei G, Zhou H, Dong M, Zhao J, Wang H, Liu Q, Lee HJ, Jin W, Chen ZJ. Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2016;113:2708–2713. doi: 10.1073/pnas.1523236113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Spicer EG, Gavini CK, Goudjo-Ako AJ, Novak CM, Shi H. Enhanced sympathetic activity in mice with brown adipose tissue transplantation (transBATation) Physiol Behav. 2014;125:21–29. doi: 10.1016/j.physbeh.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]