Abstract
Sarcopenia is a universal characteristic of the aging process and is often accompanied by increases in whole-body adiposity. These changes in body composition have important clinical implications, given that loss of muscle and gain of fat mass are both significantly and independently associated with declining physical performance as well as an increased risk for disability, hospitalizations, and mortality in older individuals. This increased fat mass is not exclusively stored in adipose depots but may become deposited in non-adipose tissues, such as skeletal muscle, when the oxidative capacity of the adipose tissue itself is exceeded. The redistributed adipose tissue is thought to exert detrimental local effects on the muscle environment given the close proximity. Thus, sarcopenia observed with aging may be better defined in the context of loss of muscle quality rather than loss of muscle quantity per se. In this perspective, we briefly review the age-related physiological changes in cellularity, secretory profiles, and inflammatory status of adipose tissue which drive lipotoxicity (spillover) of skeletal muscle and then provide evidence of how this may affect specific fiber type contractility. We focus on biological contributors (cellular machinery) to contractility for which there is some evidence of vulnerability to lipid stress distinguishing between fiber types.
Keywords: Lipotoxicity, Muscle fiber types, Aging, Muscle quality
Introduction
The age-related loss of muscle mass and function, referred to as sarcopenia, is a universal characteristic of the aging process (Buford et al. 2010; Fisher 2004; McKiernan et al. 2009; Morley et al. 2001; Roubenoff and Castaneda 2001) and is often accompanied by increases in whole-body adiposity (Visser et al. 1998). These changes in body composition have important clinical implications, given that loss of muscle and gain of fat mass are both significantly and independently associated with declining physical performance as well as an increased risk for disability, hospitalizations, and mortality in older individuals (Goodpaster et al. 2001; Newman et al. 2003).
This increased fat mass is not exclusively stored in adipose depots but may (B H Goodpaster et al. 2001, 2006; Newman 2015; Newman et al. 2003) become deposited in non-adipose tissues, such as skeletal muscle, when the oxidative capacity of the adipose tissue itself is exceeded. The redistributed adipose tissue is thought to exert detrimental local effects on the muscle environment given the close proximity. Importantly, inter- and intramuscular adipose tissue are not inert, but rather represent a dynamic system that plays a pivotal role in maintaining homeostatic equilibrium and modulating systemic metabolism and inflammation in skeletal muscle (Sepe et al. 2011; Zoico et al. 2010). Thus, sarcopenia observed with aging may be better defined in the context of loss of muscle quality rather than loss of muscle quantity per se.
In support of this concept, a recent review by Correa-de-Araujo et al. (2017) advocates for the need to develop standardized measurements and identify cellular mechanisms of muscle quality loss in the context of what they refer to as skeletal muscle function deficit (SMFD) as opposed to sarcopenia. This review was the result of a workshop sponsored by NIA at the International Conference on Frailty and Sarcopenia Research in Philadelphia, Pennsylvania, in 2016. This group identified physiological domains, including force production, as indicators of muscle quality and reflected on the ongoing research concerning the local and systemic effects of myosteatosis/lipotoxicity, and emerging methods to quantify changes in muscle tissue composition and function. Indeed, as reviewed above, recent evidence suggests that myosteatosis, the accumulation of lipid around and within myocytes, may play a role in the development SMFD. A central role for myosteotatic induction of skeletal muscle wasting and loss of quality is recognized for many disease conditions (e.g., cardiomyopathies, cancer cachexia, diabetes, obesity, Duchenne’s muscular dystrophy).
We extend this approach by providing a new layer of analyses that isolates fiber type quality vulnerability to lipotoxicity and the impact on contractility. Skeletal muscle groups are comprised of fibers with a generally specific or mixed metabolic profile: either more glycolytic (type II) using glucose, or more oxidative (type I) using lipids as a substrate (Janssen and Ross 2005; Zhang et al. 2006). These fiber types are also defined by their contractile properties such that type I fibers fire more slowly and work to maintain posture, whereas type II fibers fire more quickly and work to control more explosive movements. Furthermore, with aging, type II fibers are selectively lost whereas type I fibers are preserved (Zhang et al. 2006). Therefore, lipotoxicity may explain, in a novel way, why type II fibers are more vulnerable to aging.
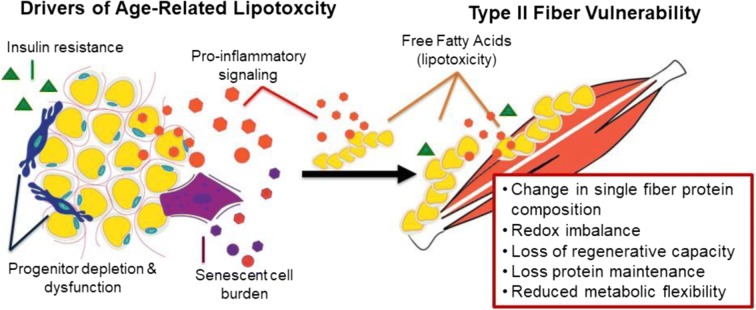
Thus, our central hypothesis states there is a primary role for lipotoxicity in the differential age-related loss and dysfunction/quality of type II fibers (Fig. 1). The consequences of age-related lipid over-accumulation are not homogenous across different fiber types; rather type II fibers are more vulnerable to this lipid stress. This concept is supported by data from high-fat feeding studies demonstrating that type I fibers have more “cellular machinery” to deal with the FA “spillover” than type II fibers, and muscle fiber types show differences in insulin sensitivity (Albers et al. 2015; Eshima et al. 2017; Shaw et al. 2008). For example, a fiber-type specific effect of high-fat feeding on intramyocellular lipid, triacylglycerol (TAG), and fatty acid (FA) content/intermediates accumulation is reported with high-fat feeding but only in type II fibers only, whereas type I fibers have greater adaptive response or resiliency (Janovská et al. 2010; Shortreed et al. 2009). We hypothesize there may be similar consequences during aging.
Fig. 1.
Several hypothesized mechanisms have emerged that may synergistically drive lipotoxicity in aging and its functional consequences on muscle and sarcopenia, including dedifferentiation of adipocyte-like progenitor cells, cellular senescence, and pro-inflammatory secretory factors. The consequences of age-related lipid over-accumulation are not homogenous across different fiber types; rather type II fibers are more vulnerable to this lipid stress
Several hallmarks of biological aging are implicated in lipotoxicity and impaired skeletal muscle contractility, and type II muscle fibers may be particularly vulnerable with advancing age (Table 1). These biological aging processes include reduced regenerative capacity and stem cell exhaustion, cellular senescence, pro-inflammatory intercellular signaling, insulin resistance and dysregulated nutrient sensing, mitochondrial dysfunction and reactive ROS/NOS production, and loss of single fiber proteostasis (López-Otín et al. 2013). Importantly, these processes do not occur independently, but involve interactions between multiple tissues, subcellular organelles, and coordinated signaling events across both adipose and skeletal muscle. In this regard, lipotoxicity may be a driver for both direct and indirect effects at the single fiber level. However, to date, the effects of lipid stress on single fiber type contractility concomitant with the single fiber protein profile are unexplored in the context of aging.
Table 1.
Several hallmarks of biological aging are implicated in lipotoxicity and impaired skeletal muscle contractility, and type II muscle fibers may be particularly vulnerable with advancing age. These biological aging processes include reduced regenerative capacity and stem cell exhaustion, cellular senescence, pro-inflammatory intercellular signaling, insulin resistance and dysregulated nutrient sensing, mitochondrial dysfunction and ROS/NOS production, and loss of single fiber proteostasis
In this perspective, we first briefly review the age-related physiological changes in cellularity, secretory profiles, and inflammatory status of adipose tissue which drive lipotoxicity (spillover) of skeletal muscle and then provide evidence of how this may affect specific fiber type contractility. It is not possible to discuss in detail every component in this brief perspective; however, here, we focus on biological contributors (cellular machinery) to contractility for which there is some evidence of vulnerability to lipid stress distinguishing between fiber types.
Cellular and physiological drivers of lipotoxicity (spillover)
Several hypothesized mechanisms have emerged that may synergistically drive lipotoxicity in aging and its functional consequences on muscle and sarcopenia, including dedifferentiation of adipocyte-like progenitor cells, cellular senescence, and pro-inflammatory secretory factors and are addressed below.
Adipose-like progenitor cells
Adipose tissue is an energy storage, endocrine, and immune organ (Sepe et al. 2011). A primary function is to store energy as neutral triglycerides. Lipases can then hydrolyze these intracellular triglycerides into glycerol and free fatty acids for transport to other tissues such as skeletal muscle to be oxidized in mitochondria. If the hydrolysis of triglycerides exceeds intracellular free fatty acid esterification, there is a net release of free fatty acids, which can lead to ectopic triglyceride storage and susceptibility to lipid stress. These fatty acids can exert cytotoxic effects that are the hallmark of lipotoxicity (Tchkonia et al. 2006). Adipose-like progenitor cells, or preadipocytes, express differentiation-dependent proteins that bind fatty acids and convert them into neutral triglycerides, thereby alleviating this lipotoxicity (Sepe et al. 2011). However, this process takes a toll even on the preadipocytes, and they must undergo rapid and lifelong turnover to help defend against lipotoxicity. Preadipocytes are estimated to account for up to 15–50% of cells in adipose tissue, but with aging, this renewing reservoir of progenitor cells becomes deplete at the same time that adipocytes become increasingly vulnerable to fatty acid’s lipotoxic effects (Guo et al. 2007). This lipotoxicity leads to cellular stress responses, and pro-inflammatory cytokine signaling. In turn, these processes result in depletion of the preadipocyte progenitor pool, and switch to a macrophage-like pro-inflammatory state (Cinti et al. 2005; Weisberg et al. 2003). Lipotoxicity can also contribute to dysdifferentiation of mesenchymal progenitors into partial differentiated adipocyte-like mesenchymal default cells (MAD cells), with consequences on preadipocytes and the progenitor pool of muscle, bone, and cartilage (Palmer and Kirkland 2016). Collectively, these processes lead to a feedback loop that furthers adipose redistribution, progenitor dysdifferentiation, inflammation, and lipotoxicity leading to cellular dysfunction and death in neighboring cells, including skeletal muscle fibers.
Cellular senescence
Cell stressors and molecular damage can initiate a deoxyribonucleic acid (DNA) damage response activating p53 and/or p16INK4a signaling cascades resulting in cellular senescence (Campisi and d Adda di Fagagna 2007; Kirkland 1992). Cellular senescence is a nonproliferative cell state that can entail a senescence-associated secretory phenotype (SASP) comprising cytokines, chemokines, pro-fibrotic factors, matrix metalloproteases (MMPs), factors causing stem/progenitor cell dysfunction, and growth factors that impose detrimental effects on the local and systemic environment (Zhu et al. 2014). Because senescent cells do not readily undergo apoptosis they can accumulate in tissues with age, with most notable burden of senescent cells in adipose tissues of aged mice, and animal models of obesity, metabolic dysfunction, diabetes, and age-related disease (Roos et al. 2016; Schafer et al. 2016; Tchkonia et al. 2010). For example, up to 30-fold greater senescent cell accumulation is observed in visceral adipose tissue from obese compared with non-obese adults, possibly owing to a combination of replicative, cytokine-induced, and metabolic stresses coupled with reduced apoptosis. Elevated cell senescence is accompanied by detrimental effects on life- and healthspan in animal models and human aging. In rodents, senescent cells within adipose directly contribute to age-dependent tissue alterations, characterized by decreased adipogenesis, adipocyte atrophy, and downregulation of key transcriptional regulators that can lead to multi-system physiologic decline and effects on physical function related to sarcopenia and frailty (LeBrasseur et al. 2015; Schafer et al. 2016; Zhu et al. 2015).
The physiologic relevance of cellular senescence is most convincingly demonstrated in vivo by (A) transplanting senescent cells or (B) targeted clearance of senescent cells through genetic manipulation or senolytic drugs. Xu et al. (2018) transplanted nonsenescent control or senescent preadipocytes (induced by radiation- or doxorubicin) into 6-month-old mice. Despite the limited number of cells transplanted in just 1 month post-intraperitoneal transplant, the previously healthy young mice had lower physical function as measured by slower walking speed, briefer hanging endurance, and weaker grip strength compared with controls. This functional loss can be prevented or alleviated by senescent cell clearance (genetic manipulation or “senolytic” drug combinations (Xu et al. 2018). In transgenic INK-ATTAC mice, activating a suicide protein that induces apoptosis in INK4a + senescent adipocyte progenitors alleviates multiple aging phenotypes including lipodystrophy and adipose tissue dysfunction, and it preserves muscle fiber diameter and prolongs time and distance achieved on a treadmill run to exhaustion (Baker et al. 2011; Palmer et al. 2019; Xu et al. 2015). Intriguingly, a single oral dose with the senescence-targeting drug combination in old mice reduced adipose p16INK4a expression and SASP within 5 days compared to vehicle-treated animals; in mice with mobility impaired by radiation exposure, a single senolytic course improved treadmill endurance within 4 days and this improvement persisted for over 7 months (Zhu et al. 2015). In a mouse model of pulmonary fibrosis, senescent cell clearance of ~ 30% reduces relative expression p16INK4a and SASP factors, improves lung compliance, and extends distance run to exhaustion (Schafer et al. 2017). Importantly, these findings extend to human aging, and a recent pilot study in patients with the age-related disease idiopathic pulmonary fibrosis found that 3 weeks of intermittent DQ administration was associated with clinically meaningful, statistically significant improvements in physical function: 6-min walk distance, 4-m gait speed, and 5-repeated chair-stand (Justice et al. 2019).
Interestingly, the functional consequences of senescent cell burden on muscle function are thought to be indirect. There is no conclusive evidence demonstrating age-related senescent cell accumulation in skeletal muscle fibers, likely because skeletal muscle fibers are fully differentiated post-mitotic cells. For example, up to 15% of dermal fibroblasts express senescence biomarkers telomere dysfunction induced foci (TIF) and 30% double strand DNA breaks (53BP1 foci) in very old baboons (26–30 years), whereas fewer than 3% of skeletal muscle myonuclei show DNA double strand breaks or other biomarkers of senescence (Jeyapalan et al. 2007). However, cell types that do senescence and express biomarkers of senescence, such as adipocytes and immune cells, can infiltrate skeletal muscle tissue and this senescent cell burden may exert detrimental effects on muscle fibers via paracrine signaling. For example, in subfascial/inter-muscular thigh adipose harvested from older humans, senescence measured by proportion of tumor suppressor protein p16INK4a expressing cells is associated with poorer mobility and physical function (Justice et al. 2018). Additionally, secreted factors such as fibroblast growth factors can induce senescence in mesenchymal stem cells isolated from human skeletal muscle in vitro (Sato et al. 2016). Collectively, this underscores the indirect contribution of senescent cell burden and senescence-associated secretory phenotype on skeletal muscle dysfunction.
Pro-inflammatory secretory factors
Adipose tissue expresses and secretes many different autocrine, paracrine, and endocrine factors, which include pro-inflammatory cytokines, chemokines, pro-thrombotic factors, and extracellular matrix proteins (Palmer and Kirkland 2016; Stout et al. 2017). Several reports indicate that preadipocytes, even independent of cell senescence, can develop expression and secretory phenotypes reminiscent of activated macrophages (Chung et al. 2006; Harkins et al. 2004). Moreover, macrophages within adipose tissue release pro-inflammatory factors such as TNF-α and MCP-1 that promote the activation of a variety of stress signaling cascades and NFκB activation (Hotamisligil et al. 1993; Schütze et al. 1995), resulting in adipose-resident macrophages with a pro-inflammatory secretory profile and upregulated CD11c surface expression (Trim et al. 2018). As a result, these secretory products exacerbate the pro-inflammatory microenvironment, potentiate stress signaling cascades, promote a positive feedback loop resulting in further free fatty acid release and lipotoxicity, and may contribute to unfavorable adipose-skeletal muscle cross-talk (Lafontan and Langin 2009; Suganami et al. 2007; Trim et al. 2018)). This process is exacerbated by aging, likely due to cellular stress responses brought about by lipotoxicity, hypoxia, and/or replicative arrest. The accumulation of senescent cells in adipose tissue with age and obesity add considerably to the pro-inflammatory milieu, including elevated expression of cytokines including Il-6, Il-1α, TNF-α, PAI-1, and MCP-1 (Lafontan and Langin 2009; Suganami et al. 2007; Trim et al. 2018). Collectively, these adipose tissues secretory factors—from preadipocytes, macrophages, or senescent cells—contribute to altered tissue architecture, foster local inflammation, lead to macrophage and lymphocyte infiltration, and enter the circulation leading to a pro-inflammatory systemic state. The combinatory effects of reduced capacity to store lipotoxic fatty acids, adipose, and skeletal muscle tissue damage by fatty acids, and inflammation creates a vicious feedback cycle leading to further cytokine release and pro-inflammatory signaling that has a profound effect on muscle fibers, motor function, and may contribute substantially to sarcopenia.
Specific fiber type lipotoxicity: impact on contractility (cellular machinery)
Single fiber proteostasis
Many of the effects of lipid stress on type II fiber function may be mediated by the composition of the fiber’s protein profile. Lipotoxicity may induce isoform transitions of the calcium cycling proteins (e.g., ryanodine receptor, dihydropyridine receptor, parvalbumin, SERCA, phospholamban) which impact single fiber contractility (fiber excitation, contraction, and relaxation), calcium signaling, and calcium homeostasis (Ciapaite et al. 2015; de Wilde et al. 2008; Eshima et al. 2017; Kaneko et al. 2011). In support of this, lipid excess resulted in phospholipid compositional changes linked to decreased activity of SERCA, suggesting alterations in the functional integrity of the sarcoplasmic reticulum (Funai et al. 2016). The structure of the sarcomeres may be compromised through changes in the expression of key skeletal muscle protein isoforms, such as myosin heavy chain, troponin, α-actinin, and desmin. Indeed, high-fat feeding induces not only a shift from type II/glycolytic toward type II/type I/oxidative fibers, but also a shift in Troponin T isoform expression from fast to slow (Ciapaite et al. 2015; Schilder et al. 2011). The transitions in protein isoforms within the sarcomere may contribute to impaired fiber contractility (calcium sensitivity/cooperativity, force and power production, contraction speed).
Cytoskeletal proteins are major determinants of sarcomeric architecture and are involved in force transmission along the muscle fiber (e.g., desmin, α-actinin). The potential for lipotoxic-enhanced fibrosis through extracellular matrix remodeling and collagen deposition impacts the structural integrity of the sarcomere, fiber, and neuromuscular junction resulting in enhanced stiffness (Bollinger 2017; Poudyal et al. 2013; Stearns-Reider et al. 2017), which increases injury susceptibility in type II fibers in addition to impaired force production. As a consequence of lipotoxicity, fiber inactivation, excitation-contraction uncoupling, altered calcium homeostasis, and impaired sarcomeric/fiber integrity would be observed in type II fibers. These actions contribute to type II fiber dysfunction and atrophy observed with aging.
Single fiber intercellular signaling (ROS and inflammation)
Lipid stress triggers increased ROS/NOS production (Silvestri et al. 2018). High levels of ROS accompanied by a decline in the antioxidant defense system alter the cellular redox balance and induce further oxidative stress. ROS accumulation can lead to many challenges: lipid perioxidation and disruption of the cellular membrane; ER stress, resulting in protein mis-folding and unfolding, and a decrease in protein synthesis, ultimately rendering muscle fibers incapable of clearing misfolded proteins; and activation of Caspase-3 and fiber apoptosis (Weiss et al. 2013). For instance, studies suggest a pivotal role for ER stress in palmitate-induced lipotoxicity leading to ROS production, protein palmitoylation, alteration in sphingolipid metabolism, depletion of ER Ca2+, and reduction of PPARα expression.
Additionally, studies demonstrate that type II fibers possess unique properties that potentiate mitochondrial ROS production. For example, mitochondrial free radical leak (H2O2 produced/O2 consumed) is two- to threefold higher in type IIB fibers during basal respiration supported by complex I or complex II substrates (Anderson and Neufer 2006; Pinho et al. 2017). In addition, there are striking differences in the topology and/or dismutation of superoxide in type IIB. In fact, type I fibers can rapidly dismutate O2−• relative to type IIB. Because this ROS potentiation in type II fibers is coupled with decreased ability to combat lipid stress in comparison to type I fibers, cellular homeostasis is disrupted predisposing the proteins and other cellular components to damage and dysfunction. In fact, compromised cellular redox homeostasis is suggested for impairments in NMJ integrity and function (Sakellariou et al. 2017) (Ahn et al. 2019) and likely contributes in the observed accelerated alterations in the NMJs of type II fibers with aging (Prakash and Sieck 1998).
Inflammation (an indirect consequence of lipotoxicity) also evokes a shift in cellular homeostasis, which further stresses the cellular environment. The results are redox imbalance, protein damage, and collateral effects of dysregulated homeostasis, which are likely important factors in the etiology of type II impaired activation at the neuromuscular junction, excitation-contraction uncoupling, and impaired cross-bridge cycling within the myofiber.
Collectively, the impact of the potentially deleterious effects of lipotoxicity on cellular ROS and inflammation stasis drives age-related type II fiber deterioration and further pathogenesis.
Single fiber regenerative capacity
Single fiber repair is through a series of complex, tightly controlled multistep processes involving degeneration, regeneration, and remodeling, ultimately restoring fiber structure and function. In single skeletal fibers, the satellite cells contribute significantly to this regenerative capacity through replenishing the satellite cell pool size, activation, proliferation and/or differentiation, and cellular programming. The investigations focused on aging and muscle satellite cells used numerous in vitro and in vivo models (D’Souza et al. 2015; Snijders et al. 2015). The preponderance of evidence indicates there is a decline in satellite cell content (satellite cell content expressed relative to the total number of myonuclei and per square millimeter of muscle fiber area) and this decline is associated with or precedes atrophy (Brack et al. 2007; Verdijk et al. 2007). Important to this perspective on lipotoxicity, the age-associated reduction in satellite cell content and atrophy is greater in type II fibers than in type I fibers (Verdijk et al. 2007). The underlying mechanisms for the fiber-type specific loss in satellite cell content are unknown; however, a stressed environment (e.g., pathological) limits a satellite cell’s ability to be activated, proliferate, and differentiate into a muscle fiber (D’Souza et al. 2015; Meng et al. 2015; Verdijk et al. 2014). Hence, type II fiber regenerative capacity is likely compromised in the presence of lipid overspill which leads to dysregulated homeostasis/oxidative stress and pro-inflammatory cytokine signaling. Taken together, lipid stress within the type II fiber drives fiber atrophy by compromising the regenerative capacity of the satellite cells and other key factors associated with the multistep processes.
Single fiber cellular growth and death
Dysregulation and/or perpetuation of intracellular protein turnover/autophagy may also contribute to differential age-related type II fiber atrophy, either by increasing proteolysis or initiating apoptosis or impairing synthesis. The cellular pathways responsible for protein maintenance consist of a well-orchestrated network that is responsive to stress (e.g., MAPKs, AMPK, HDAC). In fact, there is evidence that high-fat feeding influences skeletal muscle maintenance pathways via ERK1/2, p38 and CREB (Frier et al. 2012; Palacios et al. 2009; Putti et al. 2015). Potential contributing factors to a reduction in protein synthesis in type II fibers may be elevated lipid metabolites contributing to elevated TNF-α IL-1b, IL-6 levels, which activate transcription of MuRF-1 and MAFBx/atrogin-1, the key muscle atrophy pathway, through IGF/Akt-1 (Akhmedov and Berdeaux 2013; Brown et al. 2015). In this context, decreases in IGF-1 result in the inhibition of the muscle growth signaling pathway through IGF-1, P13K, Akt, and mTOR, effectively blunting protein synthesis (Brown et al. 2015). Thus, excess lipid stress in type II fibers leads to inflammation-mediated upregulation of atrophic pathways and a downregulation of growth pathways.
Single fiber insulin resistance and nutrient sensing
It is well-established that intramyocellular lipid accumulation is closely linked to insulin resistance in skeletal muscle through multiple mechanisms (Putti et al. 2015). Given that type II fibers have excess accumulation of lipids due to a lack of oxidative machinery, it also follows that metabolite intermediates such as diacylglycerol and ceramides are more abundant as well. These metabolites have varying consequences for cellular energy metabolism, resulting in the development of insulin resistance and impaired ATP production. In fact, these intermediates may serve as second messengers impairing signaling through PI3K and IRS-1 via activation of novel PKCs impairing muscle glucose uptake (Kitessa and Abeywardena 2016). On the other hand, ceramides and other sphingolipids feed into TNF-α and caspace-mediated apoptotic pathways (Bandet et al. 2019). In addition, other alterations in lipid metabolism are noted in the context of insulin resistance. For example, fatty acid oxidation and lipid storage as well as in the activity of lipid droplet coat proteins may activate signal transduction pathways which induce insulin resistance (Bosma 2016). Insulin sensitivity is also compromised through NF-kB activation by inflammation (Samuel and Shulman 2012; Silvestri et al. 2018). Interestingly, in humans, cytokine expression is fiber type specific such that pro-inflammatory cytokines TNF-α and IL18 are exclusively expressed in type II fibers, whereas expression of IL6 is largely observed in type 1 fibers (Plomgaard et al. 2005).
AMPK is a primary regulator of cellular energy homeostasis and is a positive regulator of glucose transport, mitochondrial function, and fatty acid oxidation, and a negative regulator of mTOR, ceramide/DAG production, inflammation, mTOR/SIRT signaling and ER and oxidative stress (Chavez et al. 2014; Rai and Demontis 2016). AMPK subunit expression is fiber type specific such that insulin-stimulated glucose uptake in the oxidative type I fiber–dominant muscles is higher than in muscles with a high degree of glycolytic type II fibers (Albers et al. 2015). In aged rats, insulin-induced glucose uptake is higher in type IIa (oxidative/glycolytic) compared with IIx and IIb (glycolytic) fibers (Mackrell et al. 2012). In human diabetes studies, a positive correlation exits between proportions of type I fibers and whole-body insulin sensitivity (Stuart et al. 2013). Thus, differential AMPK profiles in specific fiber types may contribute to insulin sensitivity in skeletal muscle.
There are certainly many other ways in which lipid stress may impact muscle metabolism and function, and have been reviewed in detail by others (Akhmedov and Berdeaux 2013; Janssen and Ross 2005; Kitessa and Abeywardena 2016; Miljkovic and Zmuda 2010; Palmer and Kirkland 2016; Samuel and Shulman 2012; Shoelson et al. 2006; Turpin et al. 2006; Zoico et al. 2010). However, taken together, the examples we describe provide evidence of how lipid stress leads to disruption of insulin signaling, increased apoptosis and inadequate nutrient sensing compensatory adaptations mediated within type II fibers. In turn, this may result in impaired myofiber contractility and reduced force production observed with aging.
Conclusion
Aging is associated with increased adipose deposition in non-adipose tissue, infiltrating tissues such as skeletal muscle (spillover), leading to loss of quality, contractility, and overall physical function (cellular machinery). This condition is referred to as lipotoxicity, or a deleterious accumulation of lipid in non-adipose tissues, which leads to cellular dysfunction, apoptosis, and eventual loss of function. Herein, we reviewed several hypothesized mechanisms that may synergistically drive lipotoxicity in aging and its functional consequences on muscle and sarcopenia, including dedifferentiation of adipocyte-like progenitor cells, cellular senescence, and pro-inflammatory secretory factors. More or less, each of these drivers impact skeletal muscle quality by placing deleterious fatty acid or inflammatory effectors in close proximity to or within the myocyte itself. Therefore, the myocyte must handle the spillover in order to maintain quality, contractility, and function.
Given that different skeletal muscle fiber types (I, II) are differentially defined by preferred substrate use and contractile properties, we have developed a novel hypothesis which espouses a primary role for lipotoxicity in the differential age-related loss and dysfunction of type II fibers. High-fat feeding studies in rodent suggests the rate at which the deleterious effects of lipid stress occurs and progresses is faster in type II fibers. Herein, we provide evidence to suggest how in healthy conditions type II fibers are highly adaptive through tightly coupled processes that regulate contractility including single fiber single fiber proteostasis, intercellular signaling (ROS and inflammation), regenerative capacity, growth/apoptosis, insulin resistance/nutrient sensing (cellular machinery). However, complications associated with lipid stress in type II fibers result in decoupling, resulting in dysregulated homeostasis and impaired contractility.
In summary, here, we provide a very cursory review of the distinct biochemical/metabolic and contractile properties of the type I and type II fibers that underlie the differential responses to lipid stress. In depth investigations are needed to unravel the precise contribution of these differences to muscle quality/contractility/sarcopenia and declining physical function. Furthermore, there is an imperative to measure and quantify lipid accumulation in muscle while simultaneously assessing integrity of cellular machinery governing contractility. Currently, there are very few methods for accomplishing this goal. Future studies must employ novel methodologies for providing these measurements in the context of contractility in specific fiber types and ultimately physical function in the context of aging.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahn B, Ranjit R, Premkumar P, Pharaoh G, Piekarz KM, Matsuzaki S, et al. Mitochondrial oxidative stress impairs contractile function but paradoxically increases muscle mass via fibre branching. J Cachexia Sarcopenia Muscle. 2019;10(2):411–428. doi: 10.1002/jcsm.12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmedov D, Berdeaux R. The effects of obesity on skeletal muscle regeneration. Front Physiol. 2013;4:371. doi: 10.3389/fphys.2013.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers PH, Pedersen AJT, Birk JB, Kristensen DE, Vind BF, Baba O, et al. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes. 2015;64(2):485–497. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol. 2006;290(3):C844–C851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandet CL, Tan-Chen S, Bourron O, Le Stunff H, Hajduch E (2019) Sphingolipid metabolism: new insight into ceramide-induced lipotoxicity in muscle cells. Int J Mol Sci 20(3). 10.3390/ijms20030479 [DOI] [PMC free article] [PubMed]
- Bollinger LM. Potential contributions of skeletal muscle contractile dysfunction to altered biomechanics in obesity. Gait Posture. 2017;56:100–107. doi: 10.1016/j.gaitpost.2017.05.003. [DOI] [PubMed] [Google Scholar]
- Bosma M. Lipid droplet dynamics in skeletal muscle. Exp Cell Res. 2016;340(2):180–186. doi: 10.1016/j.yexcr.2015.10.023. [DOI] [PubMed] [Google Scholar]
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317(5839):807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- Brown LA, Lee DE, Patton JF, Perry RA, Brown JL, Baum JI, Washington TA. Diet-induced obesity alters anabolic signalling in mice at the onset of skeletal muscle regeneration. Acta Physiol. 2015;215(1):46–57. doi: 10.1111/apha.12537. [DOI] [PubMed] [Google Scholar]
- Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9(4):369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Siddique MM, Wang ST, Ching J, Shayman JA, Summers SA. Ceramides and glucosylceramides are independent antagonists of insulin signaling. J Biol Chem. 2014;289(2):723–734. doi: 10.1074/jbc.M113.522847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147(11):5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- Ciapaite J, van den Berg SA, Houten SM, Nicolay K, van Dijk KW, Jeneson JA. Fiber-type-specific sensitivities and phenotypic adaptations to dietary fat overload differentially impact fast- versus slow-twitch muscle contractile function in C57BL/6J mice. J Nutr Biochem. 2015;26(2):155–164. doi: 10.1016/j.jnutbio.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: a symposium report. Front Physiol. 2017;8:87. doi: 10.3389/fphys.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DM, Trajcevski KE, Al-Sajee D, Wang DC, Thomas M, Anderson JE, Hawke TJ (2015) Diet-induced obesity impairs muscle satellite cell activation and muscle repair through alterations in hepatocyte growth factor signaling. Physiol Rep 3(8). 10.14814/phy2.12506 [DOI] [PMC free article] [PubMed]
- de Wilde J, Mohren R, van den Berg S, Boekschoten M, Dijk KW-V, de Groot P, et al. Short-term high fat-feeding results in morphological and metabolic adaptations in the skeletal muscle of C57BL/6J mice. Physiol Genomics. 2008;32(3):360–369. doi: 10.1152/physiolgenomics.00219.2007. [DOI] [PubMed] [Google Scholar]
- Eshima H, Tamura Y, Kakehi S, Kurebayashi N, Murayama T, Nakamura K et al (2017) Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol Rep (7):5. 10.14814/phy2.13250 [DOI] [PMC free article] [PubMed]
- Fisher AL. Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc. 2004;52(7):1185–1190. doi: 10.1111/j.1532-5415.2004.52320.x. [DOI] [PubMed] [Google Scholar]
- Frier BC, Wan Z, Williams DB, Stefanson AL, Wright DC. Epinephrine and AICAR-induced PGC-1α mRNA expression is intact in skeletal muscle from rats fed a high-fat diet. Am J Physiol Cell Physiol. 2012;302(12):C1772–C1779. doi: 10.1152/ajpcell.00410.2011. [DOI] [PubMed] [Google Scholar]
- Funai K, Lodhi IJ, Spears LD, Yin L, Song H, Klein S, Semenkovich CF. Skeletal muscle phospholipid metabolism regulates insulin sensitivity and contractile function. Diabetes. 2016;65(2):358–370. doi: 10.2337/db15-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Guo W, Pirtskhalava T, Tchkonia T, Xie W, Thomou T, Han J, et al. Aging results in paradoxical susceptibility of fat cell progenitors to lipotoxicity. Am J Physiol Endocrinol Metab. 2007;292(4):E1041–E1051. doi: 10.1152/ajpendo.00557.2006. [DOI] [PubMed] [Google Scholar]
- Harkins JM, Moustaid-Moussa N, Chung Y-J, Penner KM, Pestka JJ, North CM, Claycombe KJ. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134(10):2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Janovská A, Hatzinikolas G, Mano M, Wittert GA. The effect of dietary fat content on phospholipid fatty acid profile is muscle fiber type dependent. Am J Physiol Endocrinol Metab. 2010;298(4):E779–E786. doi: 10.1152/ajpendo.00356.2009. [DOI] [PubMed] [Google Scholar]
- Janssen I, Ross R. Linking age-related changes in skeletal muscle mass and composition with metabolism and disease. J Nutr Health Aging. 2005;9(6):408–419. [PubMed] [Google Scholar]
- Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Ageing Dev. 2007;128(1):36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, Nicklas BJ. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J Gerontol A Biol Sci Med Sci. 2018;73(7):939–945. doi: 10.1093/gerona/glx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S, Iida R-H, Suga T, Fukui T, Morito M, Yamane A. Changes in triacylglycerol-accumulated fiber type, fiber type composition, and biogenesis in the mitochondria of the soleus muscle in obese rats. Anat Rec. 2011;294(11):1904–1912. doi: 10.1002/ar.21472. [DOI] [PubMed] [Google Scholar]
- Kirkland JL. The biochemistry of mammalian senescence. Clin Biochem. 1992;25(2):61–75. doi: 10.1016/0009-9120(92)80047-k. [DOI] [PubMed] [Google Scholar]
- Kitessa SM, Abeywardena MY (2016) Lipid-induced insulin resistance in skeletal muscle: the chase for the culprit goes from total intramuscular fat to lipid intermediates, and finally to species of lipid intermediates. Nutrients 8(8). 10.3390/nu8080466 [DOI] [PMC free article] [PubMed]
- Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009;48(5):275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser. 2015;83:11–18. doi: 10.1159/000382054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackrell JG, Arias EB, Cartee GD. Fiber type-specific differences in glucose uptake by single fibers from skeletal muscles of 9- and 25-month-old rats. J Gerontol A Biol Sci Med Sci. 2012;67(12):1286–1294. doi: 10.1093/gerona/gls194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan SH, Colman R, Lopez M, Beasley TM, Weindruch R, Aiken JM. Longitudinal analysis of early stage sarcopenia in aging rhesus monkeys. Exp Gerontol. 2009;44(3):170–176. doi: 10.1016/j.exger.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Bencze M, Asfahani R, Muntoni F, Morgan JE. The effect of the muscle environment on the regenerative capacity of human skeletal muscle stem cells. Skelet Muscle. 2015;5:11. doi: 10.1186/s13395-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13(3):260–264. doi: 10.1097/MCO.0b013e328337d826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137(4):231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- Newman AB. Is the onset of obesity the same as aging? Proc Natl Acad Sci U S A. 2015;112(52):E7163. doi: 10.1073/pnas.1515367112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition study. J Am Geriatr Soc. 2003;51(3):323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging. 2009;1(9):771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Kirkland JL. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Exp Gerontol. 2016;86:97–105. doi: 10.1016/j.exger.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AK, Xu M, Zhu Y, Pirtskhalava T, Weivoda MM, Hachfeld CM, et al. Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell. 2019;18(3):e12950. doi: 10.1111/acel.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho RA, Sepa-Kishi DM, Bikopoulos G, Wu MV, Uthayakumar A, Mohasses A, et al. High-fat diet induces skeletal muscle oxidative stress in a fiber type-dependent manner in rats. Free Radic Biol Med. 2017;110:381–389. doi: 10.1016/j.freeradbiomed.2017.07.005. [DOI] [PubMed] [Google Scholar]
- Plomgaard P, Penkowa M, Pedersen BK. Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc Immunol Rev. 2005;11:53–63. [PubMed] [Google Scholar]
- Poudyal H, Panchal SK, Ward LC, Brown L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J Nutr Biochem. 2013;24(6):1041–1052. doi: 10.1016/j.jnutbio.2012.07.014. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21(7):887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Putti R, Migliaccio V, Sica R, Lionetti L. Skeletal muscle mitochondrial bioenergetics and morphology in high fat diet induced obesity and insulin resistance: focus on dietary fat source. Front Physiol. 2015;6:426. doi: 10.3389/fphys.2015.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai M, Demontis F. Systemic nutrient and stress signaling via myokines and myometabolites. Annu Rev Physiol. 2016;78:85–107. doi: 10.1146/annurev-physiol-021115-105305. [DOI] [PubMed] [Google Scholar]
- Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubenoff R, Castaneda C. Sarcopenia-understanding the dynamics of aging muscle. J Am Med Assoc. 2001;286(10):1230–1231. doi: 10.1001/jama.286.10.1230. [DOI] [PubMed] [Google Scholar]
- Sakellariou GK, Lightfoot AP, Earl KE, Stofanko M, McDonagh B. Redox homeostasis and age-related deficits in neuromuscular integrity and function. J Cachexia Sarcopenia Muscle. 2017;8(6):881–906. doi: 10.1002/jcsm.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Iso Y, Mizukami T, Otabe K, Sasai M, Kurata M, Suzuki H. Fibroblast growth factor-23 induces cellular senescence in human mesenchymal stem cells from skeletal muscle. Biochem Biophys Res Commun. 2016;470(3):657–662. doi: 10.1016/j.bbrc.2016.01.086. [DOI] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, et al. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016;65(6):1606–1615. doi: 10.2337/db15-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder RJ, Kimball SR, Marden JH, Jefferson LS. Body weight-dependent troponin T alternative splicing is evolutionarily conserved from insects to mammals and is partially impaired in skeletal muscle of obese rats. J Exp Biol. 2011;214(Pt 9):1523–1532. doi: 10.1242/jeb.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütze S, Wiegmann K, Machleidt T, Krönke M. TNF-induced activation of NF-kappa B. Immunobiology. 1995;193(2–4):193–203. doi: 10.1016/s0171-2985(11)80543-7. [DOI] [PubMed] [Google Scholar]
- Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57(1):66–75. doi: 10.1159/000279755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CS, Jones DA, Wagenmakers AJM. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol. 2008;129(1):65–72. doi: 10.1007/s00418-007-0349-8. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortreed KE, Krause MP, Huang JH, Dhanani D, Moradi J, Ceddia RB, Hawke TJ. Muscle-specific adaptations, impaired oxidative capacity and maintenance of contractile function characterize diet-induced obese mouse skeletal muscle. PLoS One. 2009;4(10):e7293. doi: 10.1371/journal.pone.0007293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri E, Cioffi F, De Matteis R, Senese R, de Lange P, Coppola M, et al. 3,5-Diiodo-L-thyronine affects structural and metabolic features of skeletal muscle mitochondria in high-fat-diet fed rats producing a co-adaptation to the glycolytic fiber phenotype. Front Physiol. 2018;9:194. doi: 10.3389/fphys.2018.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJC, Parise G. Satellite cells in human skeletal muscle plasticity. Front Physiol. 2015;6:283. doi: 10.3389/fphys.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns-Reider KM, D’Amore A, Beezhold K, Rothrauff B, Cavalli L, Wagner WR, et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell. 2017;16(3):518–528. doi: 10.1111/acel.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout MB, Justice JN, Nicklas BJ, Kirkland JL. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology. 2017;32(1):9–19. doi: 10.1152/physiol.00012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart CA, McCurry MP, Marino A, South MA, Howell MEA, Layne AS, et al. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J Clin Endocrinol Metab. 2013;98(5):2027–2036. doi: 10.1210/jc.2012-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, et al. Role of the toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27(1):84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Corkey BE, Kirkland JL. Current views of the fat cell as an endocrine cell: lipotoxicity. In: Bray GA, Ryan DH, editors. Overweight and the metabolic syndrome. Boston: Springer US; 2006. pp. 105–123. [Google Scholar]
- Tchkonia T, Morbeck DE, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, et al. Fat tissue, aging, and cellular senescence. Aging Cell. 2010;9(5):667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trim W, Turner JE, Thompson D. Parallels in immunometabolic adipose tissue dysfunction with ageing and obesity. Front Immunol. 2018;9:169. doi: 10.3389/fimmu.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin SM, Lancaster GI, Darby I, Febbraio MA, Watt MJ. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab. 2006;291(6):E1341–E1350. doi: 10.1152/ajpendo.00095.2006. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HHCM, van Loon LJC. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292(1):E151–E157. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- Verdijk LB, Snijders T, Drost M, Delhaas T, Kadi F, van Loon LJC. Satellite cells in human skeletal muscle; from birth to old age. Age. 2014;36(2):545–547. doi: 10.1007/s11357-013-9583-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68(3):584–590. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Sachet M, Zinngrebe J, Aschacher T, Krainer M, Hegedus B, et al. IL-24 sensitizes tumor cells to TLR3-mediated apoptosis. Cell Death Differ. 2013;20(6):823–833. doi: 10.1038/cdd.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. eLife. 2015;4:e12997. doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Morris KJ, Ng Y-C. Fiber type-specific immunostaining of the Na+,K+-ATPase subunit isoforms in skeletal muscle: age-associated differential changes. Biochim Biophys Acta. 2006;1762(9):783–793. doi: 10.1016/j.bbadis.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17(4):324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, et al. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 2010;65(3):295–299. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]