Abstract
Aging is a major risk factor for vascular cognitive impairment and dementia (VCID). Recent studies demonstrate that cerebromicrovascular dysfunction plays a causal role in the development of age-related cognitive impairment, in part via disruption of neurovascular coupling (NVC) responses. NVC (functional hyperemia) is responsible for adjusting cerebral blood flow to the increased energetic demands of activated neurons, and in preclinical animal models of aging, pharmacological restoration of NVC is associated with improved cognitive performance. To translate these findings, there is an increasing need to develop novel and sensitive tools to assess cerebromicrovascular function and NVC to assess risk for VCID and evaluate treatment efficacy. Due to shared developmental origins, anatomical features, and physiology, assessment of retinal vessel function may serve as an important surrogate outcome measure to study neurovascular dysfunction. The present study was designed to compare NVC responses in young (< 45 years of age; n = 18) and aged (> 65 years of age; n = 11) healthy human subjects by assessing flicker light-induced changes in the diameter of retinal arterioles using a dynamic vessel analyzer (DVA)-based approach. We found that NVC responses in retinal arterioles were significantly decreased in older adults as compared with younger subjects. We propose that the DVA-based approach can be used to assess NVC, as a surrogate cerebromicrovascular outcome measure, to evaluate the effects of therapeutic interventions in older individuals.
Keywords: Aging, Neurovascular coupling, Dynamic retinal vessel analysis, Endothelial dysfunction, Vascular cognitive impairment and dementia
Introduction
The central nervous system (CNS) is the most metabolically active organ system in the human body. It requires 20–25% of the body’s resting energy consumption and utilizes over 20% of the total oxygen consumption reflecting the high metabolic demand of neural processing. Constant and adequate provision of nutrients and oxygen to the CNS is essential to its health and normal function, since energy stores in the CNS are scarce. The CNS relies on the tightly regulated dense microcirculatory network for continuous supply of glucose and oxygen and for efficient washout of metabolic waste products. Oxygen and energy demand of the CNS show significant spatial and temporal variation with rapid changes in neuronal activity, which necessitates prompt adjustments of blood flow to maintain cellular homeostasis and ensure normal neural function (Enager et al. 2009; Mathiesen et al. 1998; Tarantini et al. 2017c). Moment-to-moment adjustment of blood flow to the metabolic demands of active neurons is accomplished through a process termed neurovascular coupling (or functional hyperemia) (Tarantini et al. 2017c). Active neurons in each regions of the CNS, including the brain (Attwell et al. 2010), the spinal cord (Piche et al. 2017), and the retina (Albanna et al. 2018; Lasta et al. 2013; Newman 2013; Noonan et al. 2015; Querques et al. 2019; Ravi Teja et al. 2017; Riva et al. 2005), depend on neurovascular coupling responses for the preservation of normal CNS function. Neurovascular coupling responses are orchestrated by an inter-cellular signaling network that is comprised of active neurons, astrocytes, and arteriolar vascular smooth muscle cells and endothelial cells (Chen et al. 2014; Petzold and Murthy 2011; Stobart et al. 2013; Wells et al. 2015).
There is strong evidence obtained both in older adults and laboratory rodent models of aging that advanced age results in significant impairment of neurovascular coupling responses (Balbi et al. 2015; Fabiani et al. 2013; Park et al. 2007; Sorond et al. 2013; Tong et al. 2012; Toth et al. 2014; Zaletel et al. 2005). Recent preclinical studies demonstrate that neurovascular dysfunction is an important factor contributing to the pathogenesis of cognitive decline in aging and pathological conditions associated with accelerated microvascular aging including hypertension, metabolic diseases, and Alzheimer’s disease (Girouard and Iadecola 2006; Tarantini et al. 2017b, 2018; Toth et al. 2015a, b; Tucsek et al. 2014). Selective pharmacological disruption of neurovascular coupling responses in young animals was shown to induce cognitive impairment mimicking the aging phenotype (Tarantini et al. 2015, 2017d). Importantly, rescue of functional hyperemia in aged animals by experimental anti-aging pharmacological interventions leads to improved cognitive performance (Tarantini et al. 2018; Toth et al. 2014). These preclinical observations show the feasibility of developing translationally relevant intervention strategies to improve neurovascular coupling responses and CNS blood flow in older individuals at risk for vascular cognitive impairment.
Translational studies to evaluate efficacy of novel interventions in older adults require standardized assessment of neurovascular outcome measures with high sensitivity and repeatability. Several methodologies have been adapted to study neurovascular coupling responses in humans including functional MRI (fMRI) (Duarte et al. 2015; Fabiani et al. 2013), functional near-infrared spectroscopy (fNIRS), and transcranial Doppler ultrasonography (Sorond et al. 2010, 2008). However, many of these methodologies have important disadvantages and technical constraints, which limit their use in follow-up studies in older individuals to assess treatment efficiency. These include limited sensitivity, artifacts (e.g., physiological and motion artifacts), the inherently highly complex relationship between neural activity and the blood oxygen level–dependent (BOLD) contrast, issues with patient compliance (e.g., claustrophobia) and contraindications to being in a magnetic field (e.g., metal in the body from a previous surgery in case of fMRI), high costs, and the frequent lack of an acoustic window in older adults. Due to these practical limitations, there is an urgent need to develop/adapt new sensitive, fast, and easy-to-use methodologies to aid in investigations of neurovascular coupling responses in older adults.
Due to shared developmental, anatomical, and physiological origins, retinal vessels may serve as an important surrogate vascular bed to study cerebromicrovascular structure and function in elderly human subjects. Clinical studies demonstrate that structural alterations of retinal vessels predict vascular pathologies affecting the brain. For example, abnormal retinal arteriovenous ratios (AVRs) predict the risk of stroke and vascular dementia (de Jong et al. 2011; Heitmar et al. 2015; McGrory et al. 2017). Similar to neurovascular coupling responses in the brain, which rely on the close interaction of neurons, glial cells, and cerebromicrovascular endothelial cells, a causal relationship between retinal neuronal activity, activation of glial cells, and endothelium-dependent vasodilation has been described in the retinal microcirculation (Metea and Newman 2006, 2007; Montero-Odasso et al. 2012). Recent developments also enable the assessment of neurovascular coupling responses in the retina (Kneser et al. 2009; Lim et al. 2013; Seshadri et al. 2016). The present study was designed to assess age-related changes in neurovascular coupling responses in healthy human subjects by assessing flicker light-induced changes in the diameter of retinal arterioles using a dynamic vessel analyzer (DVA)-based approach.
Materials and methods
Subjects
In this study, we have evaluated a subset of human subjects from an on-going clinical cohort study of vascular aging at the University of Oklahoma Health Sciences Center. Protocols were approved by the Institutional Review Board. Prior to participation in the study, participants were familiarized with study procedures and signed written consent forms which were collected. Eighteen healthy young subjects aged 21–45 (12 males and 6 females) and eleven clinically healthy elderly subjects above 65 years of age (6 male and 5 females) were selected. Exclusion criteria consisted of diabetes, active cancer, recent or active cardiovascular disease (angina, myocardial infarction, heart failure, stroke), presence of peripheral artery disease, active infection, and hormone replacement therapy. Subjects with blood pressure above 139/89 mmHg were excluded from this study. Patients did not consume caffeine nor use nicotine-containing products before the measurements.
Visual acuity, slit-lamp, and intraocular pressure examination
All subjects were evaluated for visual acuity using a Snellen chart in a well-lit room. After taking the extensive history, a slit-lamp examination was done to rule out any relevant ocular pathologies. Intraocular pressure was measured using a Goldmann applanation tonometer. Measurements were conducted in the right eye.
Dynamic retinal vessel analysis
The measurements were performed in a quiet, dimly lit, temperature-controlled room (22 °C). The pupil of the right eye was dilated using topical application of tropicamide (1% Tropicamide Ophtalmic Solution USP, AKORN, Lake Forest, IL), followed by a period of rest to achieve stable hemodynamic conditions. The patient’s fixation was adjusted using a fixation target so that the site of interest was located in the middle of the fundus picture. Evaluation of retinal arteriolar and venular diameters was performed using the dynamic vessel analyzer (DVA, IMEDOS Systems, Jena, Germany) according to published protocols (Garhofer et al. 2010; Gugleta et al. 2006). The DVA device allows for noninvasive and continuous assessment of retinal vessel diameters along a selected vessel segment before, during, and after flicker light stimulation. To allow these measurements, DVA is equipped with a mydriatic retinal camera (450 FF; Carl Zeiss, Jena, Germany), a charge-coupled device camera for electronic online imaging and a personal computer for system control, analysis, and recording. Each participant was evaluated for mean maximal arteriolar dilation and mean maximal venular dilation in response to flicker light stimulation (Kotliar et al. 2011). The flicker light was of the same wavelength as the illumination light. The flicker frequency was 12.5 Hz and its duration was 20 s. Before starting the flicker stimulation, a baseline recording for a minimum period of ~100 s was performed. After the flicker, 80 s of steady illumination was applied to allow the vessel diameter to return to baseline. The statistical mean of 3 consecutive examinations was calculated for each subject and each evaluated parameter. Vessel segments of approximately 1 mm in length, located in the upper temporal quadrant 1–3 optic disc diameters away from the optic disc edge, were assessed in the arteriolar and venular branches (Kotliar et al. 2011). Sites where two vessels were very close to each other were avoided.
Statistical analysis
All statistical tests were performed in Graphpad Prism 7.0e (Graphpad Software, La Jolla, CA, USA). Following the D’Agostino-Pearson normality test, measured parameters were compared using nonparametric Mann-Whitney U test or χ2 when appropriate. A p value of < 0.05 was considered significant.
Results
Participants
Table 1 shows the baseline characteristics of young (< 45 years old) and aged (> 65 years old) study participants. The average age for aged individuals was 74.5 ± 7.2 vs 32.7 ± 6.3 for young. There were no medications reported in the young subjects, while 7 out of 11 received prescribed anti-hypertensive medications, 3 received diuretics, and 3 received lipid-lowering medications. Mean arterial pressure for aged group was 91.1 ± 8.2 mmHg vs 91.3 ± 8.8 mmHg in young group (p = NS), average systolic pressure for aged group was 128.9 ± 8.2 vs 117.9 ± 10.6 in young (p = 0.006), average diastolic pressure for aged group was 75.3 ± 8.7 vs 78.1 ± 8.7 in young (p = NS). Although average systolic pressure was significantly higher in aged individuals as compared with young ones, individual levels were never above the 139 mmHg threshold.
Table 1.
Baseline characteristics of young (< 45 years old) and aged (> 65 years old) study participants
| Young (n = 18) | Aged (n = 11) | p value (t test or χ2 test, where applicable) | |
|---|---|---|---|
| Sex | |||
| Male | 12 | 6 | 0.67 |
| Female | 6 | 5 | |
| Age (years) | 32.7 ± 6.3 | 74.5 ± 7.2 | < 0.001* |
| Mean arterial pressure (mmHg) | 91.3 ± 8.8 | 93.1 ± 7.6 | 0.57 |
| Systolic blood pressure (mmHg) | 117.9 ± 10.6 | 128.9 ± 8.2 | 0.006* |
| Diastolic blood pressure (mmHg) | 78.1 ± 8.7 | 75.3 ± 8.7 | 0.41 |
| BMI (kg/m2) | 24.0 ± 3.2 | 25.6 ± 3.7 | 0.22 |
| Antihypertensive drugs | 0/18 | 7/11 | 0.99 |
| Diuretics | 0/18 | 3/11 | 0.99 |
| Lipid-lowering medications | 0/18 | 3/11 | 0.99 |
BMI, body mass index
*Significant difference by t test or χ2 test
Neurovascular coupling responses are impaired in retinal arterioles of aged individuals
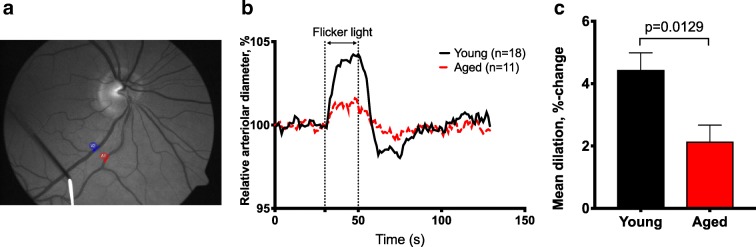
All subjects had a best-corrected distance visual acuity (VA) > 20/25 and an intraocular pressure below 21 mmHg. Exclusion criteria for both groups were photosensitive epilepsy, glaucoma, distinct cataract, and known intolerance of tetracaine and tropicamide. Aged individuals showed a 51.78% reduction in the mean maximal arteriolar dilation in response to flicker light stimulation (p = 0.0129, Fig. 1b and c). No statistical difference in the mean venular dilation between young and aged individuals was observed (p = 0.16).
Fig. 1.
Aging impairs neurovascular coupling in healthy individuals. (a) Representative fundus image showing a retinal arteriole (red arrow) and a retinal venule (blue arrow) in which flicker light stimulus-induced diameter changes were recorded using the dynamic vessel analysis (DVA) approach. (b) Time course of flicker light stimulation-induced changes in diameter of retinal arterioles in young (black, n = 18) and aged (red, n = 11) individuals. (c) Summary data showing that older individuals exhibit reduced mean maximal arteriolar dilation (% to baseline) in response to flicker light stimulation as compared to young controls. Data are mean ± SEM
Discussion
The major finding of our study is that advanced age is associated with impaired neurovascular coupling responses in retinal arterioles in otherwise healthy subjects. Our study extends the findings of previous investigations demonstrating age-related impairment of neurovascular coupling responses in the brain of older adults using other methodologies including fMRI (West et al. 2018).
Our study demonstrates the applicability of the DVA-based approach to assess microvascular reactivity in retinal vessels as a tool to evaluate age-related impairment of neurovascular coupling responses in older adults. Preclinical studies confirm the connection between neurovascular coupling and DVA microvascular reactivity by demonstrating selective increase in metabolic activity in inner retinal layers (Ames 3rd et al. 1992) and changes in oxygen tension in the retinal microcirculation in response to flicker light stimulation (Albanna et al. 2018; Shakoor et al. 2006). Human studies demonstrated the change in retinal blood flow, vessel diameter, and oxygen saturation coupled with retinal neural activity during flicker light stimulation (Falsini et al. 2002; Hammer et al. 2011) using DVA and laser Doppler velocimetry. The reproducibility and sensitivity of the DVA in healthy subjects is excellent: the coefficient of variation for the day-to-day variability of the instrument is ~5.2% for retinal arteries (Garhofer et al. 2010; Polak et al. 2000). Unlike fMRI, fNIRS, and transcranial Doppler (Huneau et al. 2015; Huppert et al. 2009; Sorond et al. 2011), which are based on indirect assessment of cerebral blood flow changes estimated from hemoglobin distribution in cortex regions or operator-dependent assessment of blood flow velocity, the DVA-based method provides direct assessment of microvascular reactivity during functional hyperemia. The non-invasive DVA-based method is also quick and its operational costs are significantly lower as compared with other methods (e.g., fMRI). Thus, the DVA-based approach (Kneser et al. 2009; Nagel et al. 2004; Seshadri et al. 2016) can be used for longitudinal studies to evaluate efficiency of anti-aging treatments on neurovascular coupling responses in older adults. As neurovascular coupling responses are also compromised in Alzheimer’s disease (Tarantini et al. 2017c), the DVA-based approach to asses retinal vascular reactivity may also be useful to monitor progression of the disease and/or to evaluate the effects of vasoprotective treatments in Alzheimer’s patients (Querques et al. 2019).
The cellular and molecular mechanisms by which aging impairs cerebromicrovascular function and compromise neurovascular coupling responses are multifaceted (Tarantini et al. 2017c). Neurovascular coupling responses in the brain involve, at least in part, release of NO from microvascular endothelial cells (Toth et al. 2015b). Similarly, microvascular responses measured by the DVA in the retina are also dependent on the activation of endothelial nitric oxide synthase (Lim et al. 2013). Aging is associated with generalized endothelial dysfunction due to an age-related increase in cellular oxidative stress and consequential decreases in bioavailability of nitric oxide (Ungvari et al. 2018). The mechanisms contributing to age-related increased oxidative stress and endothelial dysfunction include deficiency of vasoprotective IGF-1, heightened inflammatory status, and mitochondrial alterations (Pearson et al. 2008; Tarantini et al. 2018; Toth et al. 2015a, 2014; Ungvari et al. 2018). Novel experimental therapeutic interventions that reduce oxidative stress (Gioscia-Ryan et al. 2014; Pearson et al. 2008; Tarantini et al. 2018; Toth et al. 2014) were reported to significantly improve endothelial function in the cerebral circulation and rescue both neurovascular coupling responses and cognitive function in aged animals (Oomen et al. 2009; Tarantini et al. 2018). The DVA-based approach appears to be ideal for the design of studies translating the results of the aforementioned preclinical investigations.
This study has several limitations, including the fact that studied population had minor or no comorbidities. Therefore, further investigations are warranted to study the effect of age-related diseases and medication used on neurovascular coupling responses in the retina.
In conclusion, the DVA-based approach to asses neurovascular coupling in the retina represents an adjunct and clinically relevant cerebromicrovascular outcome measure that can be used to evaluate therapeutic interventions targeting cellular mechanisms of aging (Ashpole et al. 2017; Carlson et al. 2018; Csipo et al. 2018; Csiszar et al. 2017; Fulop et al. 2018; Lee et al. 2018; Nacarelli et al. 2018; Sarker and Franks 2018; Scerbak et al. 2018; Tarantini et al. 2017a; Ungvari et al. 2017) in older individuals. This method potentially also has the capacity to impact management strategies for the prevention of vascular cognitive impairment and dementia in older patients.
Funding information
This work was supported by grants from the American Heart Association (ST), the Oklahoma Center for the Advancement of Science and Technology (to AY, ZU), the National Institute on Aging (R01-AG055395, R01-AG047879; R01-AG038747), the National Institute of Neurological Disorders and Stroke (NINDS; R01-NS100782, R01-NS056218), the Oklahoma Shared Clinical and Translational Resources (OSCTR) program funded by the National Institute of General Medical Sciences (U54GM104938, to AY), and the Presbyterian Health Foundation (to ZU, AY, AC). The authors acknowledge the support from the NIA/NIH-funded Geroscience Training Program in Oklahoma (T32AG052363) and the Cellular and Molecular GeroScience CoBRE (1P20GM125528, sub#5337).
Compliance with ethical guidelines
Protocols were approved by the Institutional Review Board.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Agnes Lipecz and Tamas Csipo contributed equally to this work.
References
- Albanna W, Kotliar K, Lüke JN, Alpdogan S, Conzen C, Lindauer U, Clusmann H, Hescheler J, Vilser W, Schneider T, Schubert GA. Non-invasive evaluation of neurovascular coupling in the murine retina by dynamic retinal vessel analysis. PLoS One. 2018;13:e0204689. doi: 10.1371/journal.pone.0204689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames A, 3rd, Li YY, Heher EC, Kimble CR. Energy metabolism of rabbit retina as related to function: high cost of Na+ transport. J Neurosci. 1992;12:840–853. doi: 10.1523/JNEUROSCI.12-03-00840.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole NM, Logan S, Yabluchanskiy A, Mitschelen MC, Yan H, Farley JA, Hodges EL, Ungvari Z, Csiszar A, Chen S, Georgescu C, Hubbard GB, Ikeno Y, Sonntag WE. IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. GeroScience. 2017;39:129–145. doi: 10.1007/s11357-017-9971-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balbi M, Ghosh M, Longden TA, Vega MJ, Gesierich B, Hellal F, Lourbopoulos A, Nelson MT, Plesnila N. Dysfunction of mouse cerebral arteries during early aging. J Cereb Blood Flow Metab. 2015;35:1445–1453. doi: 10.1038/jcbfm.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BW, Craft MA, Carlson JR, Razaq W, Deardeuff KK, Benbrook DM. Accelerated vascular aging and persistent cognitive impairment in older female breast cancer survivors. Geroscience. 2018;40:325–336. doi: 10.1007/s11357-018-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csipo T, Fulop GA, Lipecz A, Tarantini S, Kiss T, Balasubramanian P, Csiszar A, Ungvari Z, Yabluchanskiy A. Short-term weight loss reverses obesity-induced microvascular endothelial dysfunction. GeroScience. 2018;40:337–346. doi: 10.1007/s11357-018-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiszar A, Tarantini S, Fülöp GA, Kiss T, Valcarcel-Ares MN, Galvan V, Ungvari Z, Yabluchanskiy A. Hypertension impairs neurovascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. GeroScience. 2017;39:359–372. doi: 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong FJ, Schrijvers EMC, Ikram MK, Koudstaal PJ, de Jong PTVM, Hofman A, Vingerling JR, Breteler MMB. Retinal vascular caliber and risk of dementia: the Rotterdam study. Neurology. 2011;76:816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte JV, Pereira JMS, Quendera B, Raimundo M, Moreno C, Gomes L, Carrilho F, Castelo-Branco M. Early disrupted neurovascular coupling and changed event level hemodynamic response function in type 2 diabetes: an fMRI study. J Cereb Blood Flow Metab. 2015;35:1671–1680. doi: 10.1038/jcbfm.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enager P, Piilgaard H, Offenhauser N, Kocharyan A, Fernandes P, Hamel E, Lauritzen M. Pathway-specific variations in neurovascular and neurometabolic coupling in rat primary somatosensory cortex. J Cereb Blood Flow Metab. 2009;29:976–986. doi: 10.1038/jcbfm.2009.23. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Gordon BA, Maclin EL, Pearson MA, Brumback-Peltz CR, Low KA, McAuley E, Sutton BP, Kramer AF, Gratton G. Neurovascular coupling in normal aging: a combined optical, ERP and fMRI study. Neuroimage. 2013;85:592–607. doi: 10.1016/j.neuroimage.2013.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsini B, Riva CE, Logean E. Flicker-evoked changes in human optic nerve blood flow: relationship with retinal neural activity. Invest Ophthalmol Vis Sci. 2002;43:2309–2316. [PubMed] [Google Scholar]
- Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. GeroScience. 2018;40:513–521. doi: 10.1007/s11357-018-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garhofer G, Bek T, Boehm AG, Gherghel D, Grunwald J, Jeppesen P, Kergoat H, Kotliar K, Lanzl I, Lovasik JV, Nagel E, Vilser W, Orgul S, Schmetterer L. Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 2010;88:717–722. doi: 10.1111/j.1755-3768.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- Gioscia-Ryan RA, LaRocca TJ, Sindler AL, Zigler MC, Murphy MP, Seals DR. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J Physiol. 2014;592:2549–2561. doi: 10.1113/jphysiol.2013.268680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol (1985) 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gugleta K, Zawinka C, Rickenbacher I, Kochkorov A, Katamay R, Flammer J, Orgul S. Analysis of retinal vasodilation after flicker light stimulation in relation to vasospastic propensity. Invest Ophthalmol Vis Sci. 2006;47:4034–4041. doi: 10.1167/iovs.06-0351. [DOI] [PubMed] [Google Scholar]
- Hammer M, Vilser W, Riemer T, Liemt F, Jentsch S, Dawczynski J, Schweitzer D. Retinal venous oxygen saturation increases by flicker light stimulation. Invest Ophthalmol Vis Sci. 2011;52:274–277. doi: 10.1167/iovs.10-5537. [DOI] [PubMed] [Google Scholar]
- Heitmar R, Kalitzeos AA, Patel SR, Prabhu-Das D, Cubbidge RP. Comparison of subjective and objective methods to determine the retinal arterio-venous ratio using fundus photography. J Optom. 2015;8:252–257. doi: 10.1016/j.optom.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneau C, Benali H, Chabriat H. Investigating human neurovascular coupling using functional neuroimaging: a critical review of dynamic models. Front Neurosci. 2015;9:467. doi: 10.3389/fnins.2015.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert TJ, Jones PB, Devor A, Dunn AK, Teng IC, Dale AM, Boas DA. Sensitivity of neural-hemodynamic coupling to alterations in cerebral blood flow during hypercapnia. J Biomed Opt. 2009;14:044038. doi: 10.1117/1.3210779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneser M, Kohlmann T, Pokorny J, Tost F. Age related decline of microvascular regulation measured in healthy individuals by retinal dynamic vessel analysis. Med Sci Monit. 2009;15:CR436–CR441. [PubMed] [Google Scholar]
- Kotliar KE, Lanzl IM, Schmidt-Trucksäss A, Sitnikova D, Ali M, Blume K, Halle M, Hanssen H. Dynamic retinal vessel response to flicker in obesity: a methodological approach. Microvasc Res. 2011;81:123–128. doi: 10.1016/j.mvr.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Lasta M, Pemp B, Schmidl D, Boltz A, Kaya S, Palkovits S, Werkmeister R, Howorka K, Popa-Cherecheanu A, Garhöfer G, Schmetterer L. Neurovascular dysfunction precedes neural dysfunction in the retina of patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2013;54:842–847. doi: 10.1167/iovs.12-10873. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Feliers D, Barnes JL, Oh S, Choudhury GG, Diaz V, Galvan V, Strong R, Nelson J, Salmon A, Kevil CG, Kasinath BS. Hydrogen sulfide ameliorates aging-associated changes in the kidney. Geroscience. 2018;40:163–176. doi: 10.1007/s11357-018-0018-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim M, Sasongko MB, Ikram MK, Lamoureux E, Wang JJ, Wong TY, Cheung CY. Systemic associations of dynamic retinal vessel analysis: a review of current literature. Microcirculation. 2013;20:257–268. doi: 10.1111/micc.12026. [DOI] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Akgoren N, Lauritzen M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol. 1998;512:555–566. doi: 10.1111/j.1469-7793.1998.555be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrory S, Cameron JR, Pellegrini E, Warren C, Doubal FN, Deary IJ, Dhillon B, Wardlaw JM, Trucco E, MacGillivray TJ. The application of retinal fundus camera imaging in dementia: a systematic review. Alzheimers Dement (Amst) 2017;6:91–107. doi: 10.1016/j.dadm.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Signalling within the neurovascular unit in the mammalian retina. Exp Physiol. 2007;92:635–640. doi: 10.1113/expphysiol.2006.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacarelli T, Azar A, Altinok O, Orynbayeva Z, Sell C. Rapamycin increases oxidative metabolism and enhances metabolic flexibility in human cardiac fibroblasts. Geroscience. 2018;40:243–256. doi: 10.1007/s11357-018-0030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel E, Vilser W, Lanzl I. Age, blood pressure, and vessel diameter as factors influencing the arterial retinal flicker response. Invest Ophthalmol Vis Sci. 2004;45:1486–1492. doi: 10.1167/iovs.03-0667. [DOI] [PubMed] [Google Scholar]
- Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab. 2013;33:1685–1695. doi: 10.1038/jcbfm.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan JE, Lamoureux EL, Sarossy M. Neuronal activity-dependent regulation of retinal blood flow. Clin Exp Ophthalmol. 2015;43:673–682. doi: 10.1111/ceo.12530. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Farkas E, Roman V, van der Beek EM, Luiten PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Front Aging Neurosci. 2009;1:4. doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cereb Blood Flow Metab. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Piche M, Paquette T, Leblond H. Tight neurovascular coupling in the spinal cord during nociceptive stimulation in intact and spinal rats. Neuroscience. 2017;355:1–8. doi: 10.1016/j.neuroscience.2017.04.038. [DOI] [PubMed] [Google Scholar]
- Polak K, Dorner G, Kiss B, Polska E, Findl O, Rainer G, Eichler HG, Schmetterer L. Evaluation of the Zeiss retinal vessel analyser. Br J Ophthalmol. 2000;84:1285–1290. doi: 10.1136/bjo.84.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querques G, Borrelli E, Sacconi R, de Vitis L, Leocani L, Santangelo R, Magnani G, Comi G, Bandello F. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci Rep. 2019;9:63. doi: 10.1038/s41598-018-37271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Teja KV, Tos Berendschot T, Steinbusch H, Carroll Webers AB, Praveen Murthy R, Mathuranath PS (2017) Cerebral and retinal neurovascular changes: a biomarker for Alzheimer’s disease. J Gerontol Geriatr Res 6. 10.4172/2167-7182.1000447 [DOI] [PMC free article] [PubMed]
- Riva CE, Logean E, Falsini B. Visually evoked hemodynamical response and assessment of neurovascular coupling in the optic nerve and retina. Prog Retin Eye Res. 2005;24:183–215. doi: 10.1016/j.preteyeres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Sarker MR, Franks SF. Efficacy of curcumin for age-associated cognitive decline: a narrative review of preclinical and clinical studies. Geroscience. 2018;40:73–95. doi: 10.1007/s11357-018-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbak C, Vayndorf E, Hernandez A, McGill C, Taylor B. Lowbush cranberry acts through DAF-16/FOXO signaling to promote increased lifespan and axon branching in aging posterior touch receptor neurons. GeroScience. 2018;40:151–162. doi: 10.1007/s11357-018-0016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri S, Ekart A, Gherghel D. Ageing effect on flicker-induced diameter changes in retinal microvessels of healthy individuals. Acta Ophthalmol. 2016;94:e35–e42. doi: 10.1111/aos.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor A, Blair NP, Mori M, Shahidi M. Chorioretinal vascular oxygen tension changes in response to light flicker. Invest Ophthalmol Vis Sci. 2006;47:4962–4965. doi: 10.1167/iovs.06-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: effects of healthy aging. Cortex. 2008;44:179–184. doi: 10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Galica A, Serrador JM, Kiely DK, Iloputaife I, Cupples LA, Lipsitz LA. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston study. Neurology. 2010;74:1627–1633. doi: 10.1212/WNL.0b013e3181df0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Kiely DK, Galica A, Moscufo N, Serrador JM, Iloputaife I, Egorova S, Dell'Oglio E, Meier DS, Newton E, Milberg WP, Guttmann CRG, Lipsitz LA. Neurovascular coupling is impaired in slow walkers: the MOBILIZE Boston study. Ann Neurol. 2011;70:213–220. doi: 10.1002/ana.22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorond FA, Hurwitz S, Salat DH, Greve DN, Fisher ND. Neurovascular coupling, cerebral white matter integrity, and response to cocoa in older people. Neurology. 2013;81:904–909. doi: 10.1212/WNL.0b013e3182a351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobart JL, Lu L, Anderson HD, Mori H, Anderson CM. Astrocyte-induced cortical vasodilation is mediated by D-serine and endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2013;110:3149–3154. doi: 10.1073/pnas.1215929110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Hertelendy P, Tucsek Z, Valcarcel-Ares MN, Smith N, Menyhart A, Farkas E, Hodges EL, Towner R, Deak F, Sonntag WE, Csiszar A, Ungvari Z, Toth P. Pharmacologically-induced neurovascular uncoupling is associated with cognitive impairment in mice. J Cereb Blood Flow Metab. 2015;35:1871–1881. doi: 10.1038/jcbfm.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Fulop GA, Kiss T, Farkas E, Zölei-Szénási D, Galvan V, Toth P, Csiszar A, Ungvari Z, Yabluchanskiy A. Demonstration of impaired neurovascular coupling responses in TG2576 mouse model of Alzheimer's disease using functional laser speckle contrast imaging. Geroscience. 2017;39:465–473. doi: 10.1007/s11357-017-9980-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer’s disease: contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol. 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Yabluchanksiy A, Fülöp GA, Hertelendy P, Valcarcel-Ares MN, Kiss T, Bagwell JM, O’Connor D, Farkas E, Sorond F, Csiszar A, Ungvari Z. Pharmacologically induced impairment of neurovascular coupling responses alters gait coordination in mice. GeroScience. 2017;39:601–614. doi: 10.1007/s11357-017-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Fulop GA, Hertelendy P, Gautam T, Farkas E, Perz A, Rabinovitch PS, Sonntag WE, Csiszar A, Ungvari Z. Treatment with the mitochondrial-targeted antioxidant peptide SS-31 rescues neurovascular coupling responses and cerebrovascular endothelial function and improves cognition in aged mice. Aging Cell. 2018;17:e12731. doi: 10.1111/acel.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong XK, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits. J Neurosci. 2012;32:4705–4715. doi: 10.1523/JNEUROSCI.0169-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice:role of improved cerebromicrovascular endothelial function and down-regulation of NADPH oxidas. Am J Physiol Heart Circ Physiol. 2014;306:H299–H308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14:1034–1044. doi: 10.1111/acel.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, Ballabh P, Sonntag WE, Baur JA, Csiszar A, Ungvari Z. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309:H1837–H1845. doi: 10.1152/ajpheart.00463.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Hertelendy P, Valcarcel-Ares MN, Fülöp GA, Logan S, Kiss T, Farkas E, Csiszar A, Yabluchanskiy A. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. GeroScience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JA, Christie IN, Hosford PS, Huckstepp RTR, Angelova PR, Vihko P, Cork SC, Abramov AY, Teschemacher AG, Kasparov S, Lythgoe MF, Gourine AV. A critical role for purinergic signalling in the mechanisms underlying generation of BOLD fMRI responses. J Neurosci. 2015;35:5284–5292. doi: 10.1523/JNEUROSCI.3787-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West KL, Zuppichini MD, Turner MP, Sivakolundu DK, Zhao Y, Abdelkarim D, Spence JS, Rypma B. BOLD hemodynamic response function changes significantly with healthy aging. Neuroimage. 2018;188:198–207. doi: 10.1016/j.neuroimage.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaletel M, Strucl M, Pretnar-Oblak J, Zvan B. Age-related changes in the relationship between visual evoked potentials and visually evoked cerebral blood flow velocity response. Funct Neurol. 2005;20:115–120. [PubMed] [Google Scholar]