Abstract
Cold temperatures are a major source of stress for plants and negatively impact crop yield. A possible way to protect plants is to treat them with antifreeze proteins (AFPs). Here, we investigated whether fish AFPs can shield the rare ornamental species Hosta capitata from low-temperature stress. We elucidated the expression patterns of the cold-inducible genes C-repeat binding factor 1 (CBF1) and dehydrin 1 (DHN1), as well as the antioxidant genes superoxide dismutase (SOD) and catalase (CAT). All were upregulated at low temperature (4 °C). With increasing exposure time, CBF1 and DHN1 expression generally rose (except CBF1 at 48 h). In contrast, SOD and CAT expression gradually declined from 6 to 48 h. Depending on exposure duration, AFP regulation of gene transcription varied with concentration. However, compared with other concentrations, 100 µg/L AFP reduced CBF1 and DHN1 expression and increased SOD and CAT expression in plants, regardless of exposure time. Both AFP I and III were likely to be most effective at protecting plants against cold stress at concentrations of 100 µg/L. Their involvement in H. capitata cold-stress treatment occurred through regulating the expression of important stress-response genes.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1859-5) contains supplementary material, which is available to authorized users.
Keywords: Antifreeze protein, Antioxidant, Gene expression, Ornamental plant, Reactive oxygen species
Introduction
Low-temperature (0–15 °C) stress is a serious threat to sustainable crop production and plant distribution, because it induces the production of excess reactive oxygen species (ROS), causing oxidative damage and disrupting the electron transport chain (Gill and Tuteja 2010). In response, plants rapidly upregulate multiple genes that increase cold tolerance (Gilmour et al. 1998; Doherty et al. 2009; Hsieh et al. 2004; Smallwood and Bowles 2002; Zhu et al. 2007). Two well-studied genes in this category are C-repeat binding factor 1 (CBF1) and dehydrin 1 (DHN1). The former is expressed within 15 min of cold exposure and begins to regulate other cold-induced genes after 2 h (Gilmour et al. 1998; Mantyla et al. 1995; Liu et al. 1998; Jaglo-Ottosen et al. 1998), an effect that has been confirmed through overexpression experiments in Arabidopsis (Liu et al. 1998; Jaglo-Ottosen et al. 1998; Maruyama et al. 2004). Effects of DHN1 have also been studied by overexpression experiments (Puhakainen et al. 2004; Shekhawat et al. 2011). DHN1 is upregulated in plants by low temperatures (Tommasini et al. 2008; Cramer et al. 2007; Xiao and Nassuth 2006) and has cryoprotective properties related to preventing cell-membrane destabilization (Bravo et al. 2003; Puhakainen et al. 2004). In addition to expressing cold-inducible genes, antioxidant defense systems are engaged (e.g., antioxidant genes SOD and CAT are upregulated) to scavenge the excess ROS induced by cold temperatures (Naing et al. 2018; Ciarmiello et al. 2011; Catala et al. 2012).
Another important plant-defense mechanism against low temperatures involves antifreeze proteins (AFPs). AFPs protect cells from injury in subzero temperatures (Cutler et al. 1989; Chapsky and Rubinsky 1997), with AFP I treatment improving cryoprotection in chrysanthemums (Jeon et al. 2015) and AFP II doing the same in potato (Seo et al. 2018). However, no studies are available regarding whether either AFP is involved in the expression patterns of cold-inducible genes and antioxidant genes. Indeed, we know relatively little about the regulatory mechanisms of AFPs in low-temperature tolerance. This study aimed to investigate the interactions of AFPs with genes linked to the cold-stress response, and hence, we exposed the ornamental plant Hosta capitata to low temperatures (4 °C) for different durations, and then characterized CBF1, DHN1, SOD, and CAT expression patterns. We then treated plants with AFP I and AFP III to understand the effects of these proteins on the four genes.
Materials and methods
Plant materials
Rhizomes of the ornamental plant H. capitata were planted in pots containing sandy soil and maintained in a greenhouse until new leaves emerged. For in vitro propagation, rhizomes with new leaves were thoroughly washed under tap water, surface-sterilized by dipping in 1% w/v sodium hypochlorite for 10 min, and then rinsed with sterile distilled water at least five times. Shoot tips were aseptically excised from the sterilized rhizomes and used as explants to evaluate in vitro shoot regeneration.
In vitro shoot regeneration
Sterilized explants were cultured on MS basal medium-containing 6-benzylaminopurine (BA) and naphthaleneacetic acid (NAA), 3% w/v sucrose, and 0.8% w/v agar (pH 5.8). The culture plates were incubated at 25 °C with cycles of 16 h light and 8 h dark (16/h light/8 h dark). After culturing for 8 weeks, each regenerated shoot was separated from the explants, transferred to MS basal medium, and maintained there for 4 weeks. Plants of uniform size were selected for cold-stress experiments.
Cold-stress treatments
Culture bottles containing five uniform plants were placed in a growth chamber under 16 h light/8 h dark conditions at 4 °C for 6 h, 12 h, 24 h, or 48 h (cold-stress treatments). Control plants were maintained at 25 °C. Each treatment consisted of three culture bottles (15 plants) with three replications. After low-temperature exposure, young leaves were collected from plants, placed in 2 mL tubes, and immersed in liquid nitrogen. These samples were stored at − 80 °C until further use.
Treatments with AFP I and AFP III
To clarify whether fish AFPs (types I and III) exert a cryoprotective effect, plants of uniform size were cultured on MS basal liquid medium-containing 0, 100, 300, 500, or 1000 µg/L of AFP I and AFP III. To simulate cold stress, culture bottles were placed in a 4 °C growth chamber under 16 h light/8 h dark conditions for 6 h, 12 h, 24 h, or 48 h. Plants cultured in AFP-free medium were used as control. Each treatment consisted of three culture bottles (15 plants) with three replications. After low-temperature exposure, leaves were collected, placed in 2 mL tubes, and immersed in liquid nitrogen. Samples were immediately stored at − 80 °C until further use.
RNA extraction and detection of cold-inducible and antioxidant genes
Total RNA was extracted from above stored leaves following published methods (Naing et al. 2018) with the RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). The cDNA was synthesized from 1 μg total RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Transcript levels of cold-inducible genes (CBF1 and DHN1) and antioxidant genes (SOD and CAT) were determined through quantitative real-time PCR (qRT-PCR; Applied Biosystems, Foster City, CA, USA). The α-tubulin gene was used as a reference standard. Primers and PCR conditions are listed in Supplementary Table 1. Three biological samples per treatment were used and assays were performed in triplicate.
Statistical analysis
Data were analyzed in SPSS v. 11.09 (IBM Corp., Armonk, NY, USA) and are presented as mean ± standard deviation (SD). Means were compared with Duncan’s multiple range test. Significance was set at P < 0.05.
Results
Analysis of CBF1 and DHN1 expression in response to cold stress
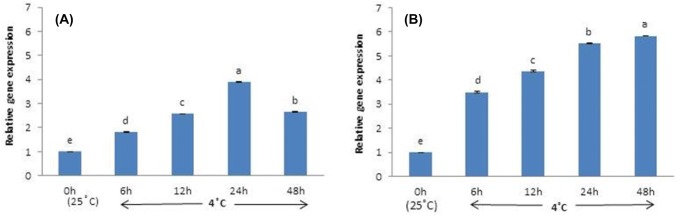
Plants subjected to control temperatures (25 °C) exhibit the lowest CBF1 expression. In contrast, CBF1 expression begins to increase after 6 h at 4 °C and continues to rise, peaking at 24 h, and then declines at 48 h (Fig. 1a). Similarly, DHN1 expression is low in control plants. However, after 6 h at 4 °C, DHN1 expression increases more sharply than CBF1, peaking at 24 h and 48 h (Fig. 1b).
Fig. 1.
Transcript levels of CBF1 (a) and DHN1 (b) in leaves of Hosta capitata treated with 4 °C for different time intervals. Plants at room temperature (25 °C) were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
Expression analysis of SOD and CAT in response to low-temperature stress
Both SOD and CAT are expressed at significantly higher levels in cold-treated plants than in control. Specifically, SOD expression is notably increased after 6 h at 4 °C, followed by a gradual decrease, reaching its lowest level (except in control plants) at 48 h (Fig. 2a). The expression pattern of CAT is similar, its expression increases after 6 h of cold stress and then slowly decreases over time (Fig. 2b).
Fig. 2.
Transcript levels of SOD (a) and CAT (b) in leaves of Hosta capitata treated with 4 °C for different time intervals. Plants at room temperature (25 °C) were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
Effects of AFPs on CBF1 and DHN1 expression at low temperature
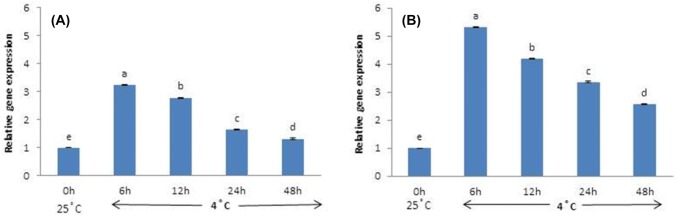
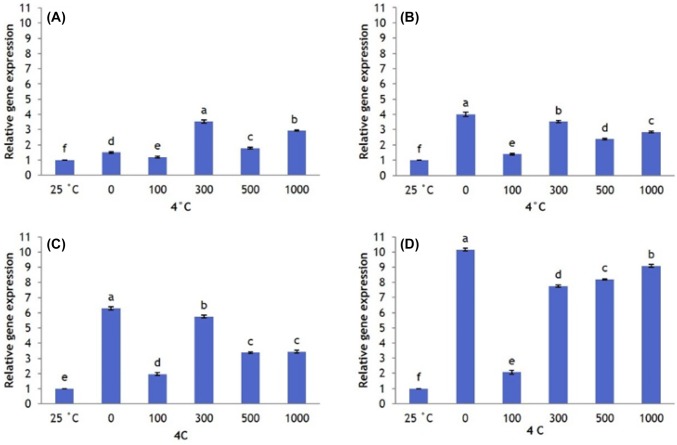
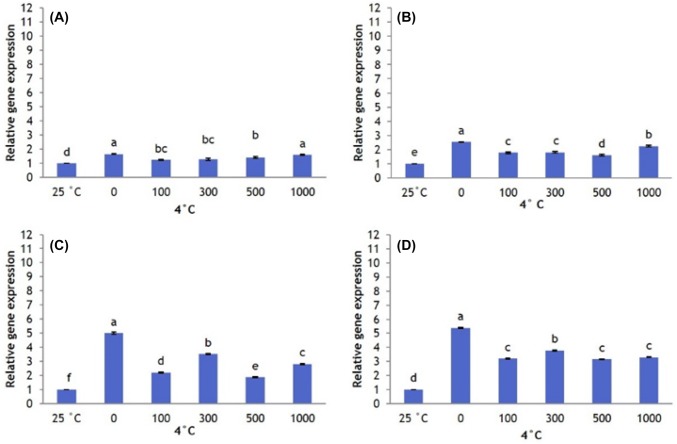
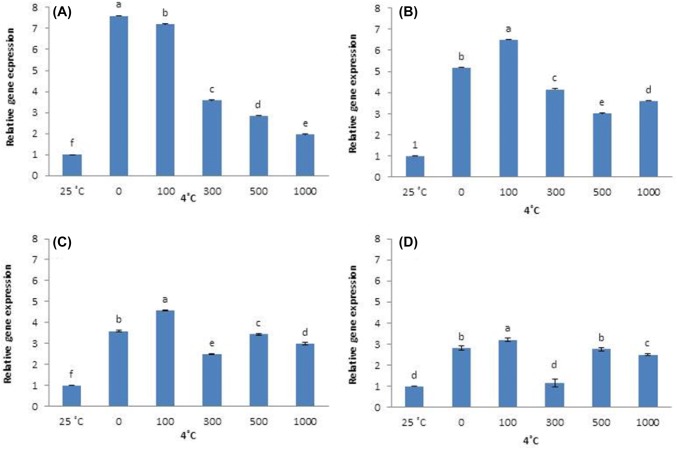
Overall, CBF1 expression is greater at 4 °C than at 25 °C (control). Plants that did not receive AFP I have significantly lower CBF1 expression than plants that received 300, 500, and 1000 µg/L AFP I. However, as the exposure to cold stress increased to 12 h, 24 h, and 48 h, CBF1 expression in plants that received no AFP I increases to significantly higher levels than in plants that received all other treatments (Fig. 3). Plants that received 100 µg/L AFP I have the lowest levels of CBF1 expression. In plants treated with AFP III, CBF1 expression patterns were similar. Transcript levels at 0 µg/L AFP III were significantly higher than those under other treatments, particularly after 24 h and 48 h exposure (Fig. 4).
Fig. 3.
Transcript levels of CBF1 in leaves of Hosta capitata treated with various AFP I concentrations at 4 °C for different time intervals (a 6 h; b 12 h; c 24 h; and d 48 h). Plants at room temperature (25 °C) were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
Fig. 4.
Transcript levels of CBF1 in Hosta capitata treated with various AFP III concentrations at 4 °C for different time intervals (a 6 h; b 12 h; c 24 h; and d 48 h). Plants at 25 °C were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
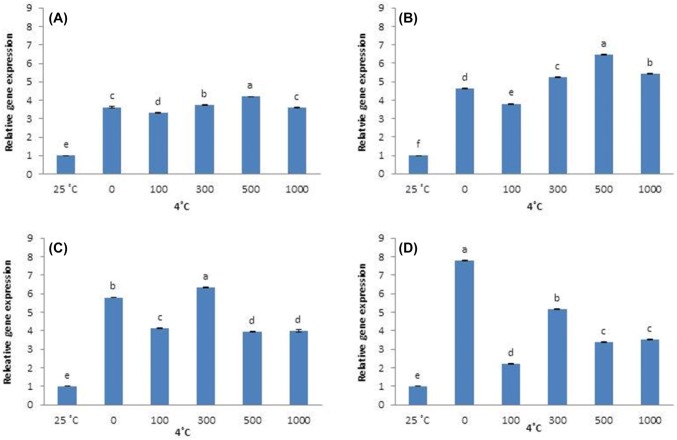
Likewise, DHN1 was significantly upregulated in all cold-stressed plants compared with control, regardless of AFP treatment or exposure time. After 6 h of cold stress, DHN1 expression is highest in plants treated with 0 and 1000 µg/L AFP I, and is lower in plants treated with 100, 300, and 500 μg/L AFP I. At 12 h, 24 h, and 48 h of cold stress, DHN1 expression in the absence of AFP I remains significantly higher than in AFP I-treated plants (Fig. 5). Plant treated with 100 µg/L AFP I have the lowest expression (especially after 24 h and 48 h). In contrast, DHN1 expression in plants treated with AFP III (300 and 500 µg/L) is significantly higher at 6 h and 12 h of cold stress than at other AFP III concentrations. However, DHN1 expression in plants treated with 500 µg/L AFP III is lower after 24 h of cold stress than in plants receiving no AFP III. Furthermore, DHN1 expression is significantly lower in plants receiving all concentrations of AFP III after 48 h of cold stress compared that in plants receiving no AFP III (Fig. 6). For all exposure times, 100 µg/L AFP III yielded the lowest DHN1 expression. In summary, 100 µg/L AFP I and AFP III minimally induced CBF1 and DHN1 expression regardless of exposure duration.
Fig. 5.
Transcript levels of DHN1 in leaves of Hosta capitata treated with various AFP I concentrations at 4 °C for different time intervals (a 6 h; b 12 h; c 24 h; and d 48 h). Plants at 25 °C were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
Fig. 6.
Transcript levels of DHN1 in leaves of Hosta capitata treated with various AFP III concentrations at 4 °C for different time intervals (a 6 h; b 12 h; c 24 h; and d 48 h). Plants at 25 °C were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
Effects of AFP I and AFP III on SOD and CAT expression in response to cold stress
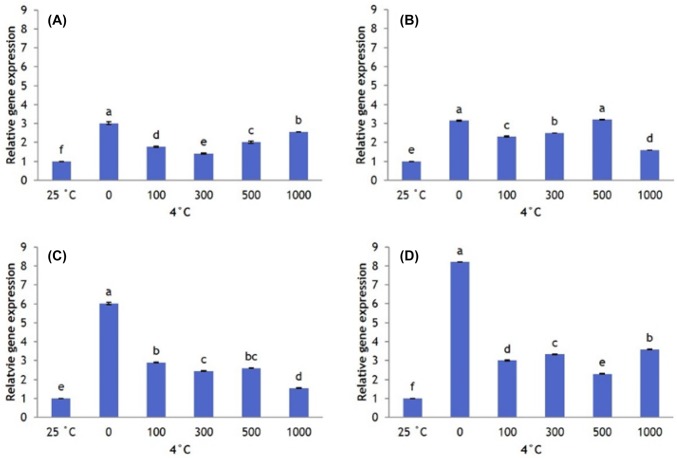
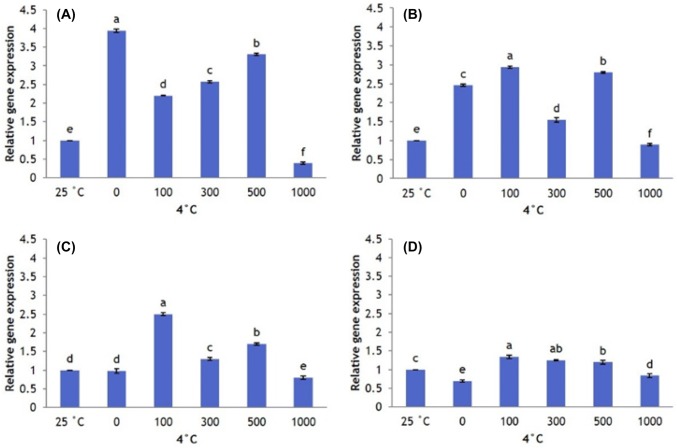
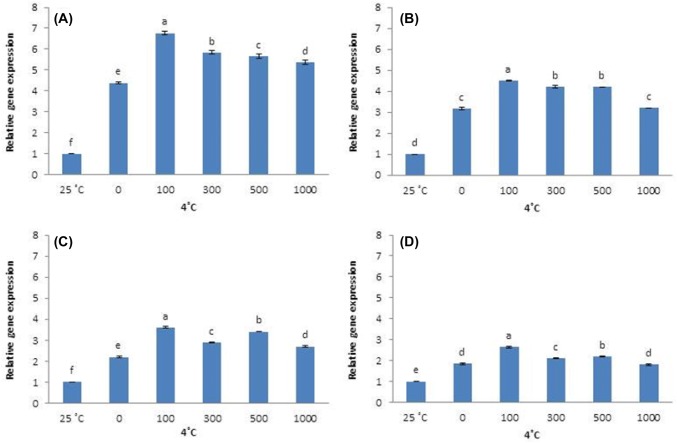
Cold-stressed plants significantly increase SOD expression. However, AFP type and concentration changes SOD gene expression under cold stress. SOD expression is higher in plants that received no AFP I than in plants that received any concentration of AFP I. However, SOD expression in plants that received no AFP I declines after 12 h, becoming significantly lower at 24 and 48 h. Plants that received 100 µg/L AFP I have higher levels of SOD expression than all other treatments from 12 to 48 h of cold stress (Fig. 7).
Fig. 7.
Transcript levels of SOD in leaves of Hosta capitata treated with various AFP I concentrations at 4 °C for different time intervals (a 6 h; b 12 h; c 24 h; and d 48 h). Plants at 25 °C were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
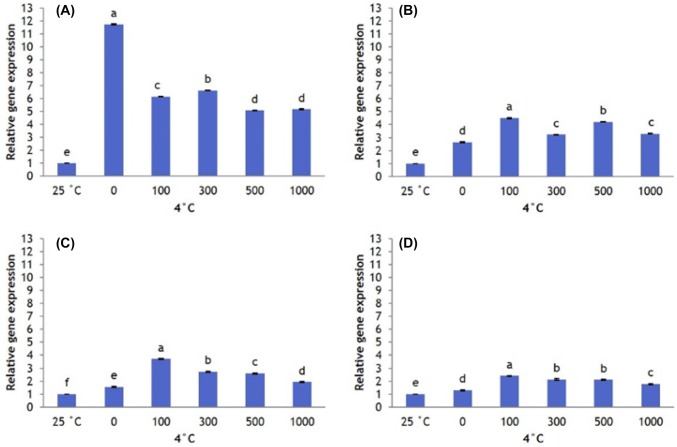
Similar SOD expression patterns were observed in AFP III-treated plants. Plants that received no AFP III have higher levels of SOD expression than plants that received AFP III at 6 h, followed by a steady decline to lower expression at 12–48 h; 100 µg/L AFP III induces the highest level of SOD expression during this period (Fig. 8). Cold stress also significantly upregulated CAT expression. All AFP I concentrations reduce CAT expression compared with plants that received no AFP I until 24 h and 48 h, when 100 µg/L AFP I upregulates CAT (Fig. 9). When plants were treated with AFP III, CAT expression at all concentrations tested is higher than that in the absence of AFP III for all exposure times, with 100 µg/L causing the greatest upregulation (Fig. 10).
Fig. 8.
Transcript levels of SOD in leaves of Hosta capitata treated with various AFP III concentrations at 4 °C for different time intervals (a 6 h; b 12 h; c 24 h; and d 48 h). Plants at 25 °C were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
Fig. 9.
Transcript levels of CAT in leaves of Hosta capitata treated with various AFP I concentrations at 4 °C for different time intervals (a 6 h; b 12 h; c 24 h; and d 48 h). Plants at 25 °C were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
Fig. 10.
Transcript levels of CAT in Hosta capitata leaves treated with various AFP III concentrations at 4 °C for different time intervals (a 6 h; b 12 h; c 24 h; and d 48 h). Plants at 25 °C were used as controls. Data are means of three replicates. Error bars indicate standard deviation of the means
Discussion
In this study, we characterized the expression patterns of cold-inducible genes CBF1 and DHN1 and antioxidant genes SOD and CAT in H. capitata under conditions of cold stress, while also clarifying the regulatory role of AFPs in cold responsiveness.
We observed relatively low CBF1 and DHN1 expression in control plants, but we found that these genes are significantly upregulated after exposure to cold at 4 °C. This molecular change appears important in plant adaption to cold stress. Indeed, multiple studies have demonstrated a link between CBF1 upregulation in plants and increased cold tolerance (Gilmour et al. 1998; Mantyla et al. 1995; Liu et al. 1998; Jaglo-Ottosen et al. 1998). Likewise, DHN1 upregulation protects cells from cold-induced membrane destabilization (Bravo et al. 2003; Puhakainen et al. 2004) and thus increases cold-stress tolerance (Tommasini et al. 2008; Cramer et al. 2007; Xiao and Nassuth 2006). We also observed a continuous increase in CBF1 and DHN1 expression as the duration of cold stress increased. This sustained upregulation may enhance the plant’s ability to withstand prolonged stress. However, subsequent decline in CBF1 expression at 48 h might reflect an inability of the plants to sustain further upregulation as energy stores become depleted. Overall, the association of CBF1 and DHN1 upregulation with cold-stress duration suggests that the two genes participate in H. capitata cold-stress tolerance.
Cold stress induces excess ROS and inhibits plant growth (Naing et al. 2018; Xi et al. 2013). Under these stressful conditions, plants appear to adaptively enhance antioxidant defense systems by upregulating SOD and CAT (Naing et al. 2017, 2018; Ciarmiello et al. 2011; Catala et al. 2012). In the present study, we also observed significant SOD and CAT upregulation in plants exposed to 4 °C compared with control plants, thus protecting plants from oxidative cell damage. However, SOD and CAT transcript levels steadily declined with increasing exposure to cold. This outcome may be attributable to a decrease in ROS concentration by the upregulation of antioxidant enzymes, which eventually results in the downregulation of enzyme expression.
AFP treatment may confer higher cold tolerance. In AFP-treated plants, the transcript levels of cold-inducible genes CBF1 and DHN1 were significantly lower than those in untreated plants, especially after 24 h and 48 h of cold exposure. Early in the cold-stress treatment, however, CBF1 and DHN1 expression either did not significantly differ between AFP-treated and untreated plants or were higher in the AFP-treated plants. These findings suggest that plants may strongly induce CBF1 and DHN1, so that they can adapt quickly to sudden cold stress, irrespective of AFP. Once plants adjust to the cold for several hours, the influence of AFP treatment becomes more noticeable. For both AFPs, 100 µg/L was the most effective concentration for lowering CBF1 and DHN1 transcript levels. Interestingly, this concentration also results in higher antioxidant expression for longer periods than is seen with other AFP concentrations. The observed discrepancy between cold-inducible and antioxidant gene expression may be due to different phytotoxicity with lower versus higher AFP concentrations. Specifically, if elevated AFP is more phytotoxic, then plants treated with lower AFP concentrations will be more capable of producing elevated antioxidant expression than plants treated with higher AFP concentrations. Corroborating this supposition, previous research showed that higher AFP concentrations exhibit comparatively lower or even negative antifreeze activity (Jeon et al. 2015; Seo et al. 2018 Lee et al. 2015; Hansen et al. 1993; Carpenter and Hansen 1992). We note the likelihood that the optimal AFP concentrations may differ among cell types. Thus, the practical application of AFPs as cryoprotectants in industries such as horticulture will require customizing AFP concentrations according to tissue origin.
In this study, both AFPs at all concentrations participated in H. capitata cold tolerance. The antifreeze activity of AFPs has been demonstrated in several other plant and animal cells. Specifically, AFP I blocks potassium and calcium ion channels and reduces lipid membrane ion leakage at 4 °C (Rubinsky et al. 1992; Baguisi et al. 1997), while AFP III stabilizes plasma membranes through lipid interactions (Wang and Huang 1996). However, until this study, there was little insight regarding the molecular mechanism of AFP involvement in cold-stress tolerance. Our results demonstrated that AFP I and III exert their cryoprotective effects through regulating key cold-inducible and antioxidant genes.
Conclusions
The present study demonstrates the involvement of cold-induced genes (CBF1 and DHN1) and antioxidant genes (SOD and CAT) in H. capitata cold tolerance. Moreover, we showed for the first time that AFP I and AFP III both participate in cold response by regulating these genes. We tested different AFP concentrations and found that 100 µg/L was the optimal concentration for effective resistance to cold stress in H. capitata. These findings suggest that both AFPs have potential application as cryoprotectants in biotechnology, metabolic genetic engineering, and cryopreservation research.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
AHN designed the study. PPWP conducted the experiments. AHN and PPWP wrote and revised the manuscript. CKK and KIP supervised experiments at all stages and performed critical revisions of the manuscript. MYC assisted in data analysis and molecular analysis. All authors read and approved the final manuscript.
Funding
This work was supported by a grant from the Next-Generation BioGreen 21 Program (Project no. PJ01368505), Rural Development Administration, Republic of Korea. This work was supported in part by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through Export Promotion Technology Development Program, funded Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 317020-5), and K. I. Park and P. P. W. Pe were recipients of the fund.
Compliance with ethical standards
Competing interest
There is no conflict of interest.
Contributor Information
Kyeung Il Park, Email: pki0217@yu.ac.kr.
Chang Kil Kim, Email: ckkim@knu.ac.kr.
References
- Baguisi A, Arav A, Crosby TF, Roche JF, Boland MP. Hypothermic storage of sheep embryos with antifreeze proteins: development in vitro and in vivo. Theriogenology. 1997;48:1017–1024. doi: 10.1016/S0093-691X(97)00328-2. [DOI] [PubMed] [Google Scholar]
- Bravo LA, Gallardo J, Navarrete A, Olave N, Martínez J, Alberdi M, Close TJ, Corcuera LJ. Cryoprotective activity of a cold induced dehydrin purified from barley. Physiol Plant. 2003;118:262–269. doi: 10.1034/j.1399-3054.2003.00060.x. [DOI] [Google Scholar]
- Carpenter JF, Hansen TN. Antifreeze protein modulates cell survival during cryopreservation: mediation through influence on ice crystal growth. Proc Natl Acad Sci USA. 1992;89:8953–8957. doi: 10.1073/pnas.89.19.8953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catala R, Diaz A, Salinas J. Molecular responses to extreme temperatures. Plant Biotechnol Agric. 2012;5:287–307. doi: 10.1016/B978-0-12-381466-1.00019-5. [DOI] [Google Scholar]
- Chapsky L, Rubinsky B. Kinetics of antifreeze protein-induced ice growth inhibition. FEBS Lett. 1997;412:241–324. doi: 10.1016/S0014-5793(97)00787-4. [DOI] [PubMed] [Google Scholar]
- Ciarmiello LF, Woodrow P, Fuggi A, Pontecorvo G, Carillo P. Plant genes for abiotic stress. In: Shanker A, Venkateswarlu B, editors. Abiotic stress in plants—mechanisms and adaptations. London: IntechOpen; 2011. pp. 283–308. [Google Scholar]
- Cramer GR, Ergul A, Grimplet J, Tillett RL, Tattersall EAR, Bohlman MC, Vincent D, Sonderegger J, Evans J, Osborne C. Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomics. 2007;7:111–134. doi: 10.1007/s10142-006-0039-y. [DOI] [PubMed] [Google Scholar]
- Cutler AJ, Saleem M, Kendall E, Gusta LV, Georges F, Fletcher GL. Winter flounder antifreeze protein improves the cold hardiness of plant tissues. J Plant Physiol. 1989;135:351–354. doi: 10.1016/S0176-1617(89)80131-2. [DOI] [Google Scholar]
- Doherty CJ, Van Buskirk HA, Myers SJ, Thomashow MF. Roles for Arabidopsis CAMTA transcription factors in cold regulated gene expression and freezing tolerance. Plant Cell. 2009;21:972–984. doi: 10.1105/tpc.108.063958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J. 1998;16:433–442. doi: 10.1046/j.1365-313x.1998.00310.x. [DOI] [PubMed] [Google Scholar]
- Hansen TN, Smith KM, Brockbank KG. Type I antifreeze protein attenuates cell recoveries following cryopreservation. Transpl Proc. 1993;25:3182–3184. [PubMed] [Google Scholar]
- Hsieh TH, Lee JT, Yang PT, Chiu LH, Chang YY, Wang YC, Chan MT. Heterology expression of the Arabidopsis C-repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. Plant Physiol. 2004;135:1145–1155. doi: 10.1104/pp.900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Jeon SM, Naing AH, Park KI, Kim CK. The effect of antifreeze protein on the cryopreservation of chrysanthemums. Plant Cell Tissue Organ Cult. 2015;123:665–671. doi: 10.1007/s11240-015-0852-x. [DOI] [Google Scholar]
- Lee HH, Lee HJ, Kim HJ, Lee JH, Ko Y, Kim SM, Lee JR, Suh CS, Kim SH. Effects of antifreeze proteins on the vitrification of mouse oocytes: comparison of three different antifreeze proteins. Hum Reprod. 2015;30:2110–2119. doi: 10.1093/humrep/dev170. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyla E, Lang V, Palva ET. Role of abscisic acid in drought induced freezing tolerance, cold acclimation, and accumulation of LT178 and RAB18 proteins in Arabidopsis thaliana. Plant Physiol. 1995;107:141–148. doi: 10.1104/pp.107.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Sakuma Y, Kasuga M, Ito Y, Seki M, Goda H, Shimada Y, Yoshida S, Shinozaki K, Yamaguchi-Shinozaki K. Identification of cold-inducible downstream genes of the Arabidopsis DREB1A/CBF3 transcriptional factor using two microarray systems. Plant J. 2004;38:982–993. doi: 10.1111/j.1365-313X.2004.02100.x. [DOI] [PubMed] [Google Scholar]
- Naing AH, Park KI, Ai TN, Chung MY, Han JS, Kang YW, Kim CK. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biol. 2017;17:65. doi: 10.1186/s12870-017-1015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naing AH, Ai TN, Lim KB, Lee IJ, Kim CK. Overexpression of Rosea1 from snapdragon enhances anthocyanin accumulation and abiotic stress tolerance in transgenic tobacco. Front Plant Sci. 2018;9:1070. doi: 10.3389/fpls.2018.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhakainen T, Hess MW, Makela P, Svensson J, Heino P, Palva ET. Overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis. Plant Mol Biol. 2004;54:743–753. doi: 10.1023/B:PLAN.0000040903.66496.a4. [DOI] [PubMed] [Google Scholar]
- Rubinsky B, Mattioli M, Arav A, Barboni B, Fletcher GL. Inhibition of Ca++ and K+ currents by “antifreeze” proteins. Am J Physiol. 1992;262:542–545. doi: 10.1152/ajpregu.1992.262.3.R542. [DOI] [PubMed] [Google Scholar]
- Seo JH, Naing AH, Jeon SM, Kim CK. Anti-freezing-protein type III strongly influences the expression of relevant genes in cryopreserved potato shoot tips. Plant Mol Biol. 2018;97:347–355. doi: 10.1007/s11103-018-0743-8. [DOI] [PubMed] [Google Scholar]
- Shekhawat UKS, Srinivas L, Ganapathi TR. MusaDHN-1, a novel multiple stress inducible SK3-type dehydrin gene, contributes affirmatively to drought- and salt- stress tolerance in banana. Planta. 2011;235:915–932. doi: 10.1007/s00425-011-1455-3. [DOI] [PubMed] [Google Scholar]
- Smallwood M, Bowles DJ. Plants in a cold climate. Philos Trans R Soc Lond B Biol Sci. 2002;357:831–846. doi: 10.1098/rstb.2002.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasini L, Svensson JT, Rodriguez EM, Wahid A, Malatrasi M, Kato K, Wanamaker S, Resnik J, Close TJ. Dehydrin gene expression provides an indicator of low temperature and drought stress: transcriptome-based analysis of barley (Hordeum vulgare L.) Funct Integr Genomics. 2008;8:387–405. doi: 10.1007/s10142-008-0081-z. [DOI] [PubMed] [Google Scholar]
- Wang JH, Huang CN. Antifreeze proteins: in hypothermic and cryogenic preservation. Chin J Cell Biol. 1996;18:107–111. [Google Scholar]
- Xi ZM, Wang ZZ, Fang YL, Hu ZY, Hu Y, Deng MM, Zhang ZW. Effects of 24-epibrassinolide on antioxidation defense and osmoregulation systems of young grapevines (V. vinifera L.) under chilling stress. Plant Growth Regul. 2013;71:57–65. doi: 10.1007/s10725-013-9809-4. [DOI] [Google Scholar]
- Xiao H, Nassuth A. Stress- and development-induced expression of spliced and unspliced transcripts from two highly similar dehydrin 1 genes in V. riparia and V. vinifera. Plant Cell Rep. 2006;25:968–977. doi: 10.1007/s00299-006-0151-4. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Dong CH, Zhu JK. Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr Opin Plant Biol. 2007;10:290–295. doi: 10.1016/j.pbi.2007.04.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.