Abstract
Introduction
To assess the efficacy and safety of the subcutaneous (s.c.) secukinumab 150 mg with loading (150 mg) or without loading (150 mg no-load) regimen through 104 weeks in patients with active psoriatic arthritis (PsA) in the FUTURE 4 (NCT02294227) study.
Methods
Patients with PsA (N = 341) were randomized to s.c. secukinumab 150 mg, 150 mg no-load or placebo at baseline, weeks 1, 2, 3 and every 4 weeks thereafter. All placebo patients were reassigned to secukinumab 150 mg no-load at either week 16 (non-responders) or week 24 (responders). The primary end point was ACR20 at week 16. Patients could have their dose escalated from 150 to 300 mg based on their physician’s decision starting at week 36. Pre- and post-escalation ACR and PASI responses were also assessed.
Results
A total of 95.6% (326/341), 84.5% (288/341) and 79.8% (272/341) patients completed 16, 52 and 104 weeks of treatment, respectively. The primary end point was met; ACR20 response rate at week 16 was 41.2% and 39.8% with the 150 mg and 150 mg no-load groups, respectively, versus placebo (18.4%; adjusted P value = 0.0003 for both treatment arms). Efficacy responses observed at week 16 in both treatment regimens were sustained up to week 52 and 104, with many patients continuing to show improvements up to week 104. After dose escalation to 300 mg, the proportion of patients with non-/low-level ACR/PASI response decreased with increasing proportions of patients having higher ACR/PASI responses. No new or unexpected safety signals were reported.
Conclusion
The secukinumab 150 mg or 150 mg no-load regimen demonstrated significant and sustained improvements in the signs and symptoms of psoriatic arthritis through 104 weeks; the loading regimen was associated with numerically higher and earlier responses for some high-hurdle end points. Improved efficacy was observed upon dose escalation from 150 to 300 mg. The safety profile was consistent with previous reports.
Trial Registration
ClinicalTrials.gov identifier, NCT02294227.
Funding
Novartis Pharma AG, Basel, Switzerland.
Electronic Supplementary Material
The online version of this article (10.1007/s40744-019-0163-5) contains supplementary material, which is available to authorized users.
Keywords: Biologics, Efficacy, Inflammation, Interleukins, Psoriatic arthritis, Safety
Introduction
Psoriatic arthritis, a chronic inflammatory disease that affects the peripheral and axial joints, entheses and skin, is often associated with impaired physical function and poor quality of life [1]. The prevalence of psoriatic arthritis ranges from 5% to 40% among patients with psoriasis [2]. Psoriatic arthritis can be effectively managed by targeting reduction of articular pain, enthesitis, structural damage, disability and dermatologic symptoms [3–6].
According to the American College of Rheumatology (ACR) 2018 treatment guidelines, the first-line therapy in treatment-naïve patients with psoriatic arthritis is anti-tumor necrosis factor (TNF) agents. However, an interleukin (IL)-17A inhibitor may be used in patients with active psoriatic arthritis having severe psoriasis and in patients for whom anti-TNF agents are contraindicated [7]. While anti-TNF agents are effective for treating patients with both psoriasis and psoriatic arthritis, a substantial number of patients report inadequate response and/or poor tolerability to these agents [8].
Secukinumab, a human monoclonal antibody that directly inhibits IL-17A, has demonstrated significant and sustained long-term improvement in the signs and symptoms of active psoriatic arthritis in several phase 3 trials [9–13]. In these studies, subcutaneous (s.c.) secukinumab 150 mg (approved dose), following either intravenous (i.v.) or s.c. loading, has demonstrated sustained efficacy and safety up to 5 years [14]. Results from the secukinumab phase 3 studies have shown that secukinumab 300 mg is associated with greater efficacy compared with the 150 mg dose for some patients with psoriatic arthritis, particularly for patients with more severe psoriasis and for patients with a previous inadequate response to anti-TNF therapy [9, 12, 13, 15]. Therefore, an escalation of dose from secukinumab 150 mg s.c. to 300 mg s.c. every 4 weeks, based on the physician’s judgment, was implemented in ongoing phase 3 trials.
FUTURE 4 (NCT02294227) is a phase 3 study that explores the efficacy and overall safety of subcutaneous (s.c.) secukinumab 150 mg administered with (hereafter referred as secukinumab 150 mg load) or without loading (hereafter referred as secukinumab 150 mg no-load) through 2 years. Patients in this study were allowed to have their dose escalated from secukinumab 150 to 300 mg based on physician’s judgment, and data for these patients are also presented.
Methods
FUTURE 4 is a multicenter, randomized, double-blind, parallel-group, placebo-controlled 2-year study (104 weeks), conducted at 58 centers in 13 countries (Australia, Belgium, Bulgaria, Canada, Czech Republic, France, Germany, Italy, Poland, Russian Federation, Sweden, UK, USA). The study was done in accordance with the principles delineated in Declaration of Helsinki as revised in Brazil in 2013 [16]. All centers received approval from an independent ethics committee or institutional review board (Supplementary Table S1), and all enrolled patients provided written informed consent before starting the study-related procedures.
Following a screening period of up to 10 weeks, interactive response technology was used to randomly assign 341 eligible patients in a 1:1:1 ratio to one of the three treatment groups:
Secukinumab 150 mg load at weeks 0, 1, 2 and 3 followed by dosing every 4 weeks starting at week 4;
Secukinumab 150 mg no-load received secukinumab treatment at baseline and placebo at weeks 1, 2 and 3 followed by secukinumab dosing every 4 weeks from week 4;
Placebo patients followed the same regime (placebo at weeks 0, 1, 2, 3, 4, 8, 12) and received secukinumab 150 mg s.c. without loading regimen from either week 16 (for placebo non-responders) or week 24 (for placebo responders), with patients being classified as responders when they had ≥ 20% improvement from baseline in tender and swollen joint counts (Supplementary Figure S1).
Randomization was stratified by anti-TNF status [anti-TNF-naïve and anti-TNF-inadequate response or intolerance to these agents (IR)] as pre-specified with no less than 65% of randomized patients planned to be anti-TNF naïve.
Following a protocol amendment patients were allowed to have their 150 mg dose escalated to the 300 mg dose based on physician’s judgment, starting at week 36. Post escalation, a dose of secukinumab 300 mg was administered as two single s.c. injections of secukinumab 150 mg; patients were not allowed to switch to lower doses once the dose had been escalated.
Patients
The key inclusion criteria were patients of either sex aged ≥ 18 years who met the ClASsification criteria for Psoriatic ARthritis (CASPAR) and had active disease (defined as ≥ 3 tender joints out of 78 and ≥ 3 swollen joints out of 76 at baseline), despite previous treatment with NSAIDs plus/minus conventional/biologic DMARDs were included. Patients who had previously used up to three anti-TNF agents at an approved dose for at least 3 months could enroll if they had an inadequate response or had stopped treatment because of safety or tolerability reasons (anti-TNF-IR) after an appropriate washout period prior to randomization. Patients on prescribed NSAIDs were required to be on a stable dose for at least 2 weeks before randomization and were required to remain on a stable dose up to week 24. Patients could continue to receive the following medications at a stable dose for at least 2 weeks: prednisone or equivalent (≤ 10 mg/day); methotrexate (≤ 25 mg/week).
Key exclusion criteria included patients with previous exposure to secukinumab or any other biologic drug directly targeting the IL-17 or IL-17 receptor. Also excluded were patients with active infection in the 2 weeks before randomization or those with a history of ongoing, chronic or recurrent infections, or evidence of tuberculosis infection or with active inflammatory diseases other than psoriatic arthritis. Patients with a history of malignant disease within the past 5 years (excluding basal cell carcinoma or actinic keratoses, in situ cervical cancer or non-invasive malignant colon polyps) and those having chest X-ray/magnetic resonance imaging (MRI) with evidence of an ongoing infectious or malignant process, obtained within 3 months prior to screening, were also excluded.
Outcomes
The primary efficacy end point was the proportion of patients achieving ACR20 response at week 16 with secukinumab versus placebo. Secondary end points were assessed as part of a predefined hierarchical hypothesis-testing strategy at week 16 with secukinumab versus placebo and included change from baseline in the 28-joint Disease Activity Score including levels of C-reactive protein (DAS28-CRP), Psoriasis Area Severity Index 75 (PASI 75) response in patients with psoriasis affecting ≥ 3% body surface area, change from baseline in the physical component summary score of the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36 PCS score), ACR50 response and ACR20 response at week 4. Overall safety and tolerability was also assessed.
Pre-specified exploratory end points included assessment of ACR70 responses, PASI 90 responses, change from baseline in Health Assessment Questionnaire-Disability Index (HAQ-DI) score and Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F), minimal disease activity (MDA) responses and resolution of dactylitis and enthesitis at week 16, 52 and 104, subgroup analyses of ACR20/50 responses by previous anti-TNF therapy status and comparison between the secukinumab regimens up to week 16 for all the primary and secondary end points. In addition, the primary and secondary end points were also assessed at week 52 and 104.
ACR and PASI responses were also evaluated pre- and post-escalation (12–16, 20–24 weeks after the escalation happened): patients were grouped into four ranges based on their response: no (< 20); low (≥ 20 to < 50); moderate (≥ 50 to < 70); high (≥ 70) ACR responses. Similarly, ranges for PASI responses were: no (< 50); low (≥ 50 and < 75); moderate (≥ 75 and < 90); high (≥ 90).
The overall safety and tolerability were assessed by monitoring frequency of adverse events (AEs), laboratory abnormalities, electrocardiogram findings and vital signs. Biochemical investigations were classified according to the Common Terminology Criteria for Adverse Events (version 4).
Statistical Analysis
A sequential hierarchical testing method was used to maintain the family-wise type 1 error rate at 5% across the primary and ranked secondary end points. If the primary efficacy analysis was significant, secondary analyses were completed in the following sequence: DAS-28-CRP, PASI 75, SF36-PCS and ACR50 at week 16.
The primary end point, ACR20 at week 16, was analyzed by logistic regression with treatment and anti-TNF status as a factor and weight as a covariate. Missing values were imputed as non-responders. Efficacy data for secukinumab 150 mg load and no-load regimens are reported for originally randomized patients after application of non-responder imputation to missing binary variables and a mixed-effect model repeated measures for continuous variables until week 52, and as observed through week 104.
After protocol amendment, starting as early as week 36, the secukinumab 150 mg load and no-load groups included all the patients who had their dose escalated from 150 to 300 mg. Post-escalation data are presented as Sankey plots.
Safety analysis included all patients who received ≥ 1 dose of secukinumab, and data are presented as exposure-adjusted incidence rates (EAIR) per 100 patient-years over the entire treatment period. Safety results are presented for any secukinumab 150 mg and 300 mg group. Any secukinumab 150 mg included all patients who were originally randomized to secukinumab 150 mg and placebo-switchers at week 16 or 24. Any secukinumab 300 mg group included patients who had their dose escalated from secukinumab 150 to 300 mg during the study. For those patients who had their dose escalated, safety data were summarized separately based on the actual treatment received before and after dose escalation.
Results
Patient Disposition
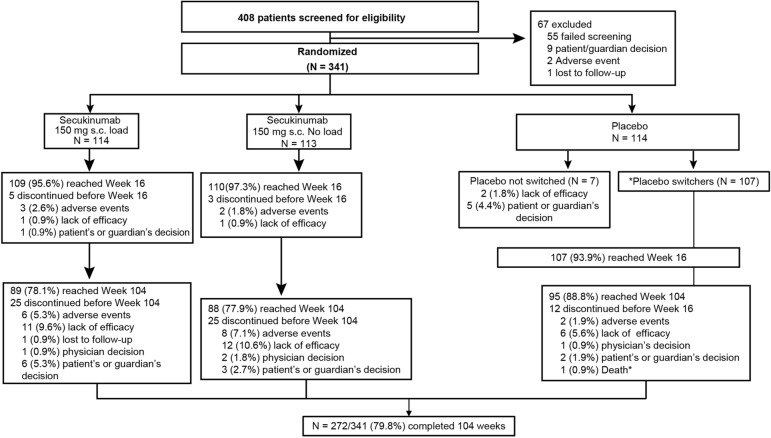
Of the 341 originally randomized patients, 288 (84.5%) and 272 (79.8%) completed 52 and 104 weeks of treatment, respectively. A total of 78.1% and 77.9% patients originally randomized to secukinumab 150 mg load and no-load, respectively, completed 2 years of treatment (Fig. 1). Seven patients in the placebo arm discontinued the study before week 16 and therefore did not switch to secukinumab treatment. In the 62 patients (18.6%) who discontinued the study before week 104, the most common reason for discontinuation was lack of efficacy (29 patients, 8.7%).
Fig. 1.
Patient disposition through week 104. The secukinumab groups received the 150 mg loading dose weekly at weeks 0, 1, 2 and 3, followed by a maintenance dose q4w starting at week 4 or s.c. secukinumab 150 mg without loading dose at baseline (with placebo doses at weeks 1, 2 and 3) followed by q4w dosing starting at week 4. Placebo was given on the same dosing schedule as the loading regimen, and all placebo patients were switched to s.c. secukinumab 150 mg q4w at week 16 or 24 depending on the patient responder status as evaluated at week 16. N is the number of randomized patients; *77 patients classed as placebo non-responders and 30 patients classed as placebo responders at week 16 received active treatment from week 16 and 24, respectively. Other than a death mentioned in the figure that occurred during the treatment period, one patient died during the follow-up period in the secukinumab 150 mg no-load group who was escalated to the secukinumab 300 mg dose. q4w every 4 weeks, s.c. subcutaneous
Demographic and baseline characteristics were comparable across the treatment groups; mean time since diagnosis of psoriatic arthritis ranged from 5.6 to 6.8 years (Table 1). Approximately one quarter of the patients had received prior anti-TNF therapy; approximately half of the patients were receiving concomitant methotrexate.
Table 1.
Demographic and baseline characteristics across groups
| Characteristic | Secukinumab 150 mg s.c. load (N = 114) | Secukinumab 150 mg s.c. no-load (N = 113) | Placebo (N = 114) |
|---|---|---|---|
| Age (years), mean (SD) | 48.3 (12.2) | 50.4 (11.8) | 48.5 (12.2) |
| Female, n (%) | 67 (58.8) | 62 (54.9) | 69 (60.5) |
| Weight (kg), mean (SD) | 85.7 (21.3) | 86.1 (20.3) | 83.6 (19.4) |
| Time (years) since first diagnosis of PsA, mean (SD) | 5.6 (7.3) | 5.7 (7.7) | 6.9 (7.6) |
| Disease history and baseline characteristics | |||
| TNFi-naïve, n (%) | 87 (76.3) | 86 (76.1) | 87 (76.3) |
| Methotrexate use at randomization, n (%) | 57 (50.0) | 53 (46.9) | 60 (52.6) |
| Systemic glucocorticoid use at randomization, n (%) | 19 (16.7) | 26 (23) | 20 (17.5) |
| TJC (78 joints), mean (SD) | 20.1 (15.5) | 19.0 (16.3) | 21.2 (15.7) |
| SJC (76 joints), mean (SD) | 9.6 (8.5) | 10.2 (9.1) | 9.4 (7.2) |
| Psoriasis (≥ 3% body surface area), n (%) | 55 (48.2) | 54 (47.8) | 62 (54.4) |
| Presence of dactylitis, n (%) | 40 (35.1) | 38 (33.6) | 44 (38.6) |
| Presence of enthesitis, n (%) | 74 (64.9) | 66 (58.4) | 76 (66.7) |
| Baseline DAS28-CRP Score, mean (SD) | 4.5 (1.0) | 4.5 (1.1) | 4.6 (1.0) |
DAS28-CRP Disease Activity Score 28 using C-reactive protein, PsA psoriatic arthritis, SD standard deviation, SJC swollen joint count, TJC tender joint count, TNFi tumor necrosis factor inhibitor, N number of randomized patients
Efficacy
Short-Term Efficacy
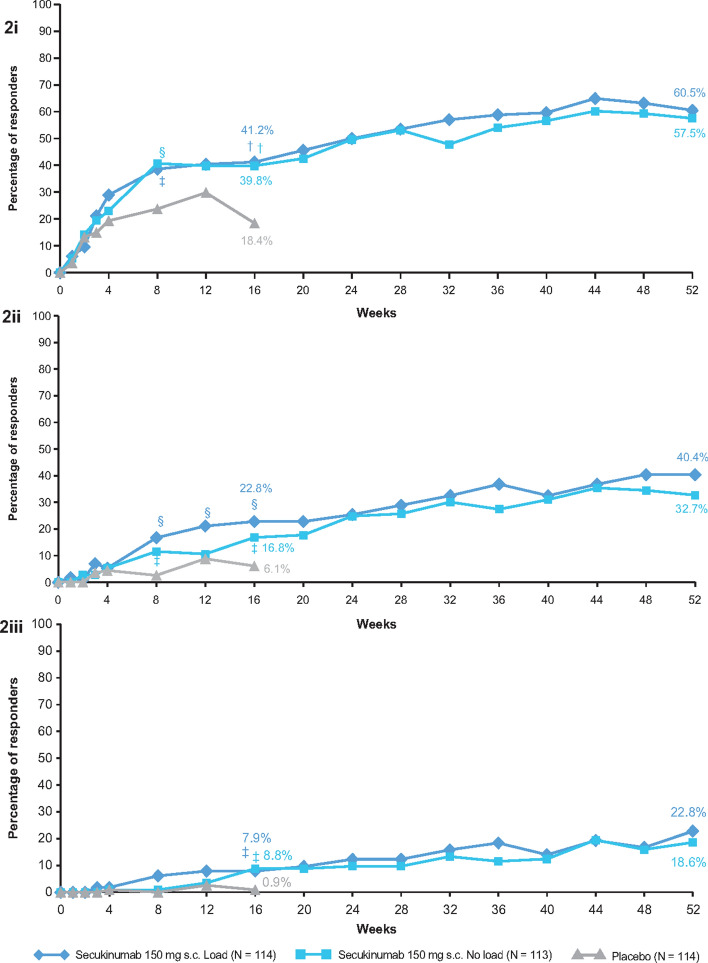
The primary end point of the study was met (Fig. 2i). At week 16, the ACR20 response rate using the conservative estimate of efficacy with missing values imputed as non-response was significantly higher with secukinumab 150 mg (41.2%) and secukinumab 150 mg no-load (39.8%) versus placebo (18.4%; adjusted P value = 0.0003 for both treatment arms). All secondary end points in the testing hierarchy were met for both 150 mg load and 150 mg no-load regimens compared with placebo, with the exception of ACR20 response at week 4 (Table 2). Earlier and numerically higher responses were observed for the load versus no-load secukinumab group at week 16 for some of the clinically relevant difficult-to-achieve (higher hurdle) end points (ACR50, PASI 90 and MDA) (Fig. 2ii, Table 2). Both secukinumab regimens improved ACR70, PASI 90 and MDA responses and change from baseline in FACIT-F score versus placebo; secukinumab no-load regimen improved HAQ-DI and resolution of enthesitis also. There was no statistical difference between two secukinumab regimens for all the primary and secondary end points at week 16.
Fig. 2.
i ACR20; ii ACR50; iii ACR70 response rates through week 52. Shown are the proportions of patients with an ACR20 response (panel i/ii/iii: improvement of ≥ 20/50/70% in both tender joint count and swollen joint count with no worsening by ≥ 20/50/70% in the remaining 3 of the 5 domains). †P < 0.001; §P < 0.01; ‡P < 0.05; P values versus placebo. Non-responder imputation through week 52. N number of patients in a cohort; ACR American College of Rheumatology response criteria
Table 2.
Summary of efficacy results at week 16 and 52
| Variables | Secukinumab | Placebo (N = 114) | |
|---|---|---|---|
| 150 mg s.c. load (N = 114) | 150 mg s.c. no-load (N = 113) | ||
| ACR20, % responders (n) | |||
| 16 | 41.2 (47)† | 39.8 (45)† | 18.4 (21) |
| 52 | 60.5 (69) | 57.5 (65) | – |
| DAS28-CRP, mean change from baseline (SE)b | |||
| 16 | − 0.98 (0.11)† | − 0.84 (0.11)† | − 0.21 (0.11) |
| 52 | − 1.73 (0.1) | − 1.67 (0.1) | – |
| PASI 75, % responders (n)a,c | |||
| 16 | 52.7 (55)† | 50.0 (54)† | 8.1 (62) |
| 52 | 60.0 (55) | 59.3 (54) | – |
| SF-36 PCS, mean change from baseline (SE)b | |||
| 16 | 3.42 (0.58)§ | 3.44 (0.58)§ | 0.63 (0.59) |
| 52 | 4.21 (0.74) | 4.48 (0.74) | – |
| ACR50, % responders (n) | |||
| 16 | 22.8 (26)§ | 16.8 (19)‡ | 6.1 (7) |
| 52 | 40.4 (46) | 32.7 (37) | – |
| ACR70, % responders (n) | |||
| 16 | 7.9 (9)‡ | 8.8 (10)‡ | 0.9 (1) |
| 52 | 22.8 (26) | 18.6 (21) | – |
| PASI 90, % responders (n) | |||
| 16 | 36.4 (55)† | 20.4 (54)§ | 1.6 (62) |
| 52 | 36.4 (55) | 38.9 (54) | – |
| MDA, % responders (n)a | |||
| 16 | 14.0 (114)§ | 10.6 (113)‡ | 2.6 (114) |
| 52 | 28.1 (114) | 24.8 (113) | – |
| FACIT-F score, mean change from baseline (SE)b | |||
| 16 | 2.81 (0.87)‡ | 4.23 (0.87)† | − 0.05 (0.89) |
| 52 | 4.56 (0.88) | 5.2 (0.87) | – |
| HAQ-DI, % response (n)b | |||
| 16 | 34.2 (114) | 42.5 (113)§ | 28.9 (114) |
| 52 | 38.6 (114) | 40.7 (113) | – |
| Resolution of enthesitis, % responders (n)a,d | |||
| 16 | 32.4 (74) | 39.4 (66)‡ | 21.1 (76) |
| 52 | 45.9 (74) | 50 (66) | – |
| Resolution of dactylitis, % responders (n)a,e | |||
| 16 | 32.5 (40) | 42.1 (38) | 31.8 (44) |
| 52 | 62.5 (40) | 76.3 (38) | – |
N number of randomized patients, n number of patients evaluated, SE standard error
†P < 0.001; §P < 0.01; ‡P < 0.05 versus placebo; P values at week 16 versus placebo are adjusted for multiplicity for all hierarchical end points except for ACR70, PASI 90, MDA, FACIT-F, HAQ-DI and resolution of enthesitis and dactylitis
Data are presented after application of anon-responder imputation through week 16 and 52/bMMRM through week 16 and 52
cN = 55 and N = 54 for secukinumab 150 mg and 150 mg no-load, respectively, for PASI responses; PASI reported only in patients with at least 3% body surface area affected with psoriasis at baseline
dN = 74 and N = 66 for secukinumab 150 mg and 150 mg no-load, respectively, for patients with enthesitis at baseline
eN = 40 and N = 38 for secukinumab 150 mg and 150 mg no-load, respectively, for patients with dactylitis at baseline
Long-Term Efficacy
Improvements in ACR20 response rates in the secukinumab 150 mg load (60.5%) and no-load (57.5%) groups were sustained through week 52 (Fig. 2i). Improvements in other efficacy end points were also sustained or further improved through 52 weeks (Fig. 2ii, iii and Table 2); improvements were also sustained or further improved in all end points with secukinumab at week 104 (Supplementary Table S2).
Results by Subgroup Analysis
Both secukinumab treatment regimens improved ACR20 and ACR50 responses at week 16 compared with placebo in anti-TNF-naïve patients (Supplementary Figure S2). ACR20 response rates at week 52 in anti-TNF-naïve patients were 65.5% and 62.8% with 150 mg and 150 mg no-load regimens, respectively; corresponding rates in anti-TNF-IR patients were 44.4% and 40.7%, respectively. ACR50 response rates at week 52 in anti-TNF-naïve patients were 44.8% and 39.5% with 150 mg and 150 mg no-load regimen, respectively.
Earlier and numerically higher ACR20/50 responses were observed for the secukinumab 150 mg versus 150 mg no-load in both anti-TNF-naïve and anti-TNF-IR patients with this trend generally persisting over 52 weeks (Supplementary Figure S2).
Dose Escalation Results
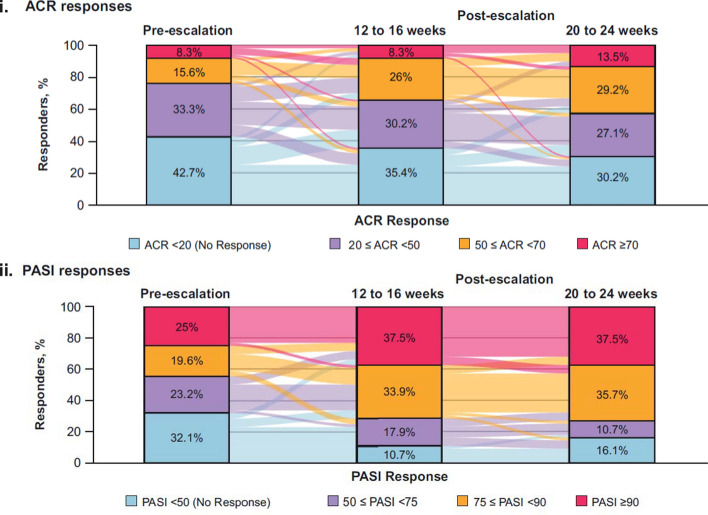
A total of 15 patients escalated from secukinumab 150 to 300 mg dose between week 36 to 52, and 136 patients escalated between week 52 to 104. After dose escalation, the proportion of patients with non-/low-level ACR response decreased with increasing proportions of patients having moderate or high ACR response (Fig. 3i and Supplementary Table S3). Lower proportions of patients had no ACR response (ACR < 20) at 12–16 weeks (34/96 patients, 35.4%) and 20–24 weeks (29/96 patients, 30.2%) after dose escalation compared with before dose escalation (41/96, 42.7%). Responses for higher hurdle ACR end points (ACR50 and ACR70) were achieved by greater proportions of patients at 12–16 weeks (33/96 patients, 34.4%) and 20–24 weeks (41/96 patients, 42.7%) post-dose escalation compared with before (23/96 patients, 24.0%), indicating an almost two-fold increase in response rate at 20–24 weeks following dose escalation from 150 to 300 mg.
Fig. 3.
i ACR and ii PASI responses before and after dose escalation. Pre-escalation is defined as the last assessment done on or before the patient was administered the 300 mg dose; patients with both pre- and all post-dose escalation assessment data available are included in the analysis; non-responders are considered in the dose escalation subset; PASI responses are reported only in patients with at least 3% body surface area affected with psoriasis at baseline. Colors flowing in the background indicate the proportion of patients changing the response over time. M is the number of patients evaluated (M = 96/56 for ACR/PASI). Overall, 136 patients were escalated to secukinumab 300 mg: 46 in secukinumab 150 mg load; 45 in secukinumab no-load; 45 in placebo-switchers
Most patients showed rapid improvements or sustainability in their PASI response after dose escalation from secukinumab 150 to 300 mg (Fig. 3ii and Supplementary Table S3). Lower proportions of patients had no PASI response (< 50) at 12–16 weeks (6/56 patients, 10.7%) and 20–24 weeks (9/56 patients, 16.1%) after dose escalation compared with before escalation (18/56 patients, 32.1%). Nearly three quarters of patients achieved PASI 75 or PASI 90 responses 12–16 weeks (40/56 patients, 71.4%) and 20–24 weeks (41/56 patients, 73.2%) after dose escalation compared with less than one half of patients before dose escalation (25/56 patients, 44.6%).
Safety
Over the entire treatment period, the mean [± SD] exposure to any secukinumab 300 mg (n = 136) and 150 mg (n = 334) was 278 ± 90.46 and 501.2 ± 193.05 days, respectively. The exposure-adjusted incidence rates (EAIR) (per 100 patient-years) of AEs by primary system organ class in any secukinumab 300 mg and 150 mg group were 226.4 (n = 103) and 211.9 (n = 285), respectively, over the entire treatment period. The AE with the highest EAIR was nasopharyngitis (n = 21/86, EAIR = 23.4/23.3 in any secukinumab group 300/150 mg group, respectively) (Table 3).
Table 3.
Summary safety data at week 104
| Any secukinumab s.c. 300 mg (N = 136) | Any secukinumab s.c. 150 mg (N = 334) | |
|---|---|---|
| Mean duration of exposure, days (SD) | 278.0 (90.46) | 501.2 (193.05) |
| Exposure, patient-years | 103.5 | 458.4 |
| No. of patients (no. of events/100 patient-years) | ||
| Patients reporting any AE, n (%) | 103 (75.7) | 285 (85.3) |
| SAEs, n (%) | 12 (8.8) | 47 (14.1) |
| Death, n (%) | 1 (0.7)a | 1 (0.3) |
| Discontinuation due to AEs, n (%) | 1 (0.7) | 16 (4.8) |
| Common AEse, EAIR (n) | ||
| Nasopharyngitis | 23.4 (21) | 23.3 (86) |
| URTI | 11.3 (11) | 11.6 (48) |
| Bronchitis | 8.0 (8) | 7.2 (31) |
| Sinusitis | 11.0 (11) | 6.7 (29) |
| Diarrhea | 4.0 (4) | 6.7 (29) |
| Selected AEs of interest, EAIR (n) | ||
| Candida infectionb | 0.0 (0) | 0.2 (1) |
| Serious infections and infestations | 1.9 (2) | 2 (9) |
| Major adverse cardiac events | 1.0 (1) | 0.7 (3) |
| Crohn’s disease | 0 | 0.2 (1) |
| Malignant or unspecified tumorsc | 1 (1) | 1.3 (6) |
| Inflammatory bowel diseased | 1 (1) | 0.2 (1) |
MedDRA version 20.1 was used for reporting
AE adverse event, EAIR exposure-adjusted incidence rate, SAE serious adverse event, SD standard deviation, URTI upper respiratory tract infection, IR incidence rate per 100 patient-years
aPatient died from pneumonia during follow-up period
bCandida infection (esophageal candidiasis) is reported as ‘Novartis MedDRA query’
cMalignant or unspecified tumor data are reported as ‘standard MedDRA query’ excluding basal cell carcinoma and squamous cell carcinoma
dData for inflammatory bowel disease is reported as Novartis MedDRA query
eThe most common AEs are reported as the preferred terms from the Medical Dictionary for Regulatory Activities and occurred at an incidence of at least 5 per 100 patient-years in the pooled secukinumab group during the entire treatment period
Inflammatory bowel disease was reported in one patients each in the any secukinumab 300/150 mg group. Malignant or unspecified tumors including basal cell carcinoma and squamous cell carcinoma were reported in two (one each with malignant melanoma and basal cell carcinoma) and seven (one each with chronic lymphocytic leukemia, malignant melanoma, breast cancer, papillary thyroid cancer, prostate cancer, squamous cell carcinoma and undifferentiated sarcoma) patients in the any secukinumab 300 mg and 150 mg group, respectively. Confirmed major adverse cardiac events (MACE) cases were reported in three patients in the any secukinumab 150 mg dose group (cardiovascular death and two events of myocardial infarction) and one patient in the any secukinumab 300 mg dose group (stroke). Candida infections (high level term) were reported in three and ten patients in the any secukinumab 300 mg and 150 mg group, respectively, during the entire treatment period. No cases of uveitis were reported.
The EAIRs for treatment-emergent non-fatal serious adverse events (SAEs) during the entire treatment period were similar in the any secukinumab 150 mg group (n = 47, IR 11.1) compared with the 300 mg group (n = 12, IR 12.0). According to primary system organ class, the most common SAEs in the any secukinumab dose group during the entire treatment period were infections and infestations (IR 1.9/2.0 for 300/150 mg group), neoplasms benign, malignant and unspecified (including cysts and polyps) (IR 2.9/1.8 for 300/150 mg group), injury, poisoning and procedural complications (IR 1.9/1.5), musculoskeletal and connective tissue disorders (IR 0/2.0 for 300/150 mg group) and vascular disorders (IR 1.0/0.9 for 300/150 mg group).
There were two deaths during the entire treatment period (one patient in each of the any 150 mg and any 300 mg secukinumab groups) (Table 3). One patient in the any secukinumab 300 mg group experienced worsening of pre-existing interstitial lung disease during the follow-up period, which was reported as the primary cause of death. Another patient in the 150 mg load arm experienced myocardial infarction as a severe SAE, which was considered the primary cause of death. None of the SAEs in either deaths was considered by the investigator to be related to the study drug.
Discussion
This randomized, double-blind, placebo-controlled phase 3 multicenter study of s.c. secukinumab 150 mg, with and without a loading regimen, assessed the efficacy, safety and tolerability in patients with active psoriatic arthritis over 104 weeks. The treatment regimens were well balanced with respect to demographics, disease history and baseline characteristics. The majority of patients enrolled at baseline remained in the study for 2 years, reflecting a high retention rate of 79.8%.
The primary end point was met by both secukinumab treatment regimens (150 mg s.c. and 150 mg s.c. no-load), demonstrating a significantly higher ACR20 response with secukinumab compared with placebo at week 16. Both secukinumab 150 mg and 150 mg no-load regimens improved other clinically important end points including DAS28-CRP, PASI 75, SF-36 PCS, ACR50, ACR70, PASI 90, MDA, FACIT-Fatigue and HAQ-DI response and resolution of enthesitis and dactylitis through 2 years. The results of this study are in agreement with those reported in four other phase 3 studies with secukinumab, which also showed rapid, significant and sustained improvements in the signs and symptoms of psoriatic arthritis with secukinumab 150 mg [9–13].
In general, the efficacy responses with secukinumab 150 mg observed in the present study were lower than the responses observed in previous studies with no obvious explanation other than inter-study variation. Earlier and higher responses were observed with the secukinumab 150 mg compared with the 150 mg no-load regimen for some higher hurdle end points including ACR50, PASI 90 and MDA.
Improvements in ACR20 and ACR50 responses with secukinumab through 52 weeks were demonstrated in both the anti-TNF-naïve and anti-TNF-IR subgroups. Responses were higher in anti-TNF-naïve patients compared with anti-TNF-IR patients, which is consistent with the results of previous secukinumab studies [9, 11–13, 15]. Earlier and higher ACR50/70 responses were observed with the secukinumab 150 mg compared with the 150 mg no-load regimen with this trend persisting over 52 weeks.
Patients who escalated from secukinumab 150 to 300 mg beginning at week 36 had improved ACR and PASI responses within 12–16 weeks. These responses were more pronounced in patients with low response levels at the time of dose escalation. Higher hurdle ACR end points (ACR50 or ACR70) were achieved by greater proportions of patients at 12–16 and 20–24 weeks after dose escalation compared with pre-escalation, with almost a twofold increase in each of these responses at 20–24 weeks post-escalation. Nearly three-quarters of patients in the analysis achieved PASI 75 and PASI 90 responses at 12–16 and 20–24 weeks after dose escalation compared with approximately half of the patients before dose escalation, suggesting a more pronounced high-hurdle PASI response following escalation from 150 to 300 mg. The dose escalation results are similar to those observed in FUTURE 1 and 2 studies [14, 17]. These results suggest that dose escalation to 300 mg may be an option for patients with a sub-optimal response to secukinumab 150 mg.
The safety profile of secukinumab was consistent with previous reports from phase 3 studies of secukinumab in patients with active psoriatic arthritis [9, 11–14, 17, 18]. The types, severity, nature and incidence of AEs with secukinumab at week 104 were similar to those already reported in other FUTURE studies, and with no apparent relationship with dose of secukinumab.
There are some limitations to the study. This trial was not designed to identify a difference between doses. The long-term efficacy and safety of secukinumab compared with placebo could not be determined, as patients on placebo had to be given rescue medication after a short period because of ethical considerations and study design. This was, however, consistent with clinical trials of other biologic agents.
Conclusion
In summary, secukinumab 150 mg s.c. administered as load or no-load regimen provided rapid and sustained improvements across multiple clinical domains of psoriatic arthritis with improvements sustained through 2 years. Patients receiving the loading regimen achieved an earlier and higher level of responses than those who received the no-load regimen for some higher hurdle clinical end points. Improved efficacy in patients whose dose was escalated from 150 to 300 mg was observed across end points assessing both skin and arthritic components of psoriatic arthritis. The safety profile of secukinumab showed no new or unexpected safety signals.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients who participated in this study, the study investigators and John Gallagher, medical consultant, Novartis Pharma AG, Switzerland.
Funding
The study was sponsored by Novartis Pharma AG, Basel, Switzerland, and designed by the scientific steering committee and Novartis personnel. Journal article processing charges are funded by Novartis. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
Medical writing support, under the guidance of the authors, was provided by Suchita Dubey, scientific writer, Novartis, India. Editorial support was provided by Neeta Pillai, scientific editor for Novartis, India. Medical writing support was funded by Novartis. The first draft of this manuscript was written by Suchita Dubey based on inputs from all the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Authorship Contributions
All authors were involved in the drafting and critical review of the manuscript and approved the final version for submission. A.J. Kivitz, P. Nash, H. Tahir, A. Everding, H. Mann and A. Kaszuba were involved in the acquisition of clinical data and participated as investigators in the clinical study from which data are reported in the manuscript. L. Pricop, K. Abrams, P. Pellet and A. Widmer were involved with the conception or design of the work and the development of the statistical analysis plan. A.J. Kivitz, L. Pricop, K. Abrams, A. Widmer and P. Pellet were involved with the interpretation of data in the manuscript. A. Widmer was involved with the analysis of the data in the manuscript. All authors agree to be accountable for all aspects of the work and attest to the accuracy and integrity of the work.
Disclosures
Alan J. Kivitz received consulting fees from AbbVie, UCB, Celgene, Janssen, Pfizer, Genentech, Novartis and Sanofi and on the speaker’s bureau of Celgene, Pfizer, Sanofi/Regeneron and Genentech. Peter Nash received research grants for clinical trials and honoraria for lectures and advice from Novartis, AbbVie, Roche, Pfizer, BMS, Janssen and Celgene. Hasan Tahir is on the speaker’s bureau of Novartis, Eli Lilly and AbbVie. Andrea Everding has received consulting fees from Novartis and Eli Lilly. Heřman Mann received consulting fees from AbbVie, Eli Lilly and Pfizer and on the speaker’s bureau of Novartis, AbbVie, Pfizer, Eli Lilly, Sanofi Genzyme, Merck and Roche. Andrzej Kaszuba received honoraria for lectures from Novartis, AbbVie and Janssen. Pascale Pellet is an employee of Novartis and owns Novartis stock. Albert Widmer is an employee of Novartis and owns Novartis stock. Luminita Pricop is an employee of Novartis and owns Novartis stock. Ken Abrams is an employee of Novartis and owns Novartis stock.
Compliance with Ethics Guidelines
The study was done in accordance with the principles delineated in Declaration of Helsinki as revised in Brazil in 2013 [16]. All centers received approval from an independent ethics committee or institutional review board (Supplementary Table S1), and all enrolled patients provided written informed consent before starting the study-related procedures.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available. Novartis is committed to sharing with qualified external researcher access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to: 10.6084/m9.figshare.8223971.
References
- 1.Kang EJ, Kavanaugh A. Psoriatic arthritis: latest treatments and their place in therapy. Ther Adv Chronic Dis. 2015;6(4):194–203. doi: 10.1177/2040622315582354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weger W. Current status and new developments in the treatment of psoriasis and psoriatic arthritis with biological agents. Br J Pharmacol. 2010;160(4):810–820. doi: 10.1111/j.1476-5381.2010.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, Boehncke W-H, et al. Treatment recommendations for psoriatic arthritis. Ann Rheum Dis. 2009;68(9):1387–1394. doi: 10.1136/ard.2008.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–441. doi: 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 6.Coates LC, On behalf of BSRCAC, Standards A, Guidelines Working G, the B. Tillett W, et al. The 2012 BSR and BHPR guideline for the treatment of psoriatic arthritis with biologics. Rheumatology. 2013;52(10):1754–1757. doi: 10.1093/rheumatology/ket187. [DOI] [PubMed] [Google Scholar]
- 7.Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken) 2019;71(1):2–29. doi: 10.1002/acr.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa L, Perricone C, Chimenti MS, Del Puente A, Caso P, Peluso R, et al. Switching between biological treatments in psoriatic arthritis: a review of the evidence. Drugs R&D. 2017;17(4):509–522. doi: 10.1007/s40268-017-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 10.Strand V, Mease P, Gossec L, Elkayam O, van den Bosch F, Zuazo J, et al. Secukinumab improves patient-reported outcomes in subjects with active psoriatic arthritis: results from a randomised phase III trial (FUTURE 1) Ann Rheum Dis. 2017;76(1):203–207. doi: 10.1136/annrheumdis-2015-209055. [DOI] [PubMed] [Google Scholar]
- 11.Mease PJ, Kavanaugh A, Reimold A, Tahir H, Rech J, Hall S, et al. Secukinumab in the treatment of psoriatic arthritis: efficacy and safety results through 3 years from the year 1 extension of the randomised phase III FUTURE 1 trial. RMD Open. 2018;4(2):e000723. doi: 10.1136/rmdopen-2018-000723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3) Arthritis Res Ther. 2018;20(1):47. doi: 10.1186/s13075-018-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mease P, van der Heijde D, Landewe R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77(6):890–897. doi: 10.1136/annrheumdis-2017-212687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mease PJ, KA, Reimold A, Tahir H, Rech J, Hall S, Geusens P, Pascale P, Delicha EM, Pricop L, Mpofu S. Secukinumab provides sustained improvements in the signs and symptoms in psoriatic arthritis: final 5 year efficacy and safety results from a phase 3 trial [abstract]. Arthritis Rheumatol. 2018;70(suppl 10). [DOI] [PMC free article] [PubMed]
- 15.Kavanaugh A, McInnes IB, Mease PJ, Hall S, Chinoy H, Kivitz AJ, et al. Efficacy of subcutaneous secukinumab in patients with active psoriatic arthritis stratified by prior tumor necrosis factor inhibitor use: results from the randomized placebo-controlled FUTURE 2 study. J Rheumatol. 2016;43(9):1713–1717. doi: 10.3899/jrheum.160275. [DOI] [PubMed] [Google Scholar]
- 16.World’s Medical Association; 2019. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/. Accessed 15 Apr 2019.
- 17.McInnes IB KA, Nash P, Rahman P, Rech J, Kirkham B, Navarra SV, Ding K, Ilsley E, Pricop L. Secukinumab provides sustained improvements in the signs and symptoms of active psoriatic arthritis: long-term (4-year) data from a phase 3 study [abstract]. Arthritis Rheumatol. 2018;70(suppl 10).
- 18.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available. Novartis is committed to sharing with qualified external researcher access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data may be requested from the corresponding author of the manuscript.