Abstract
Immunosuppression caused by avian leukemia virus J subgroup (ALV-J) infection includes atrophy or regeneration disorders of the lymphoid organs, decreased immune response, and termination of B lymphocyte maturation process and inhibition of T-lymphocyte development. The regulatory mechanism of the related resistance genes and protein expression is not clear. While searching for a molecular marker for the immune response to ALV-J infection, we detected differentially expressed proteins (DEPs) of spleens from chicken infected by ALV-J at 15th day and 30th day by the data-independent acquisition technique. Approximately 220 DEPs from the spleens of chickens infected by ALV-J were detected. To find a relatively stable biomarker molecule, we summarized the DEPs at two timepoints and selected activating signal cointegrator 1 complex subunit 3 (ASCC3), TBC1 domain family member 2 (TBC1D2), MHC class II beta chain 1 (BLB2), ensconsin (MAP7), complement component 1 Q subcomponent B chain (C1QB), and Follistatin-like 1 (FSTL1) from both comparisons for protein interaction network analysis. ASCC3, BLB2, C1QB, and FSTL1 were potential biomarkers for the complex infection mechanism of ALV-J and the dynamic immune mechanism of the body.
Keywords: ALV-J, Proteomic, ASCC3, BLB2, C1QB, FSTL1
Introduction
Avian leukosis viruses (ALV) belong to the retroviridae family. ALV infection not only induces a variety of neoplastic diseases, but also causes subclinical symptoms in most infected chickens (Rubin and Vogt 1962; Payne 1998), such as decreased egg production or growth retardation, making ALV an important disease endangering the development of China’s poultry industry (Li et al. 2019). The J subgroup of ALV (ALV-J) is the most infective subgroup to cause economic loss (Cui et al. 2009; Payne and Nair 2012). ALV-J infection can cause atrophy or regeneration disorders of the lymphoid organs, decrease the immune response level, terminate the maturation process of B lymphocyte, and inhibit the development of the T lymphocyte, leading to immunosuppression (Medzhitov and Janeway 1997). Because of the high level of genetic variation and the vertical and horizontal transmission of the ALV-J, currently, no effective vaccine exists against ALV-J (Fadly and Smith 1999). Therefore, improving resistance through genetic hybridization is the main method to control ALV-J. Selecting an effective biomarker can improve the cost-effectiveness of ALV-J purification in chickens. Proteomics techniques have greatly promoted the study of markers in animal husbandry and the interpretation of biological mechanisms (Liu et al. 2019). Two-dimensional gel electrophoresis–mass spectrometry (2-DE–MS) and isobaric tags for relative and absolute quantification–mass spectrometry (iTRAQ–MS) have been used to detect the possible mechanisms of virus infection, indicating that some proteins play important roles in apoptosis and oncogenicity (Fan et al. 2012; Li et al. 2015). However, the differentially expressed proteins in the DF-1 cells were not suitable for use as effective biomarkers for tissues of chicken infected by ALV-J.
In our study, to obtain the biomarkers of chicken injected by ALV-J, we characterized the expression patterns of proteins by data-independent acquisition (DIA) at the 15th and 30th days in spleens following infection with ALV-J. The results of this study will aid in developing potential biomarkers of ALV-J.
Materials and methods
Sample preparation and collection
Avian broilers were provided by the Zheng Da Company, Chengdu, China. Embryos were hatched separately in two incubators. After hatching, the chickens were injected with 100 μl ALV-J based on the TCID50 of the virus, whereas a control group was injected with 100 μl DMEM per embryos. After hatching, the female chicks were maintained separately in two pathogen-free negative pressure isolators (Strong Star Equipment Technology Co, Qingdao, China) and given ultraviolet sterilized food and boiled water. Chickens were euthanized on the 1st day, 7th day, 15th day, 30th day and 45th day, and the spleens were harvested. Based on an immune index (the weight of spleen divided by the body weight), the spleens of the chickens from the 15th day and 30th day were used for the DIA study. The samples were named as follows: the chickens euthanized on the 15th day from the control group were identified as 15DC, the chickens euthanized on the 15th day from the injected group were 15DI, the chickens euthanized on the 30th day from the control group were 30DC, and the chickens euthanized on the 30th day from the injected group were 30DI. The number of replications was three. The statistical analyses were conducted using the SAS 8.0 software for Windows, and the figures shown were developed in Graph Pad Prism 5.0. All data are expressed as the means ± SEM, and statistical analysis was performed using one-way ANOVA. P values < 0.05 were considered statistically significant.
DIA procedures
The DIA procedures were performed by the BGI company, Shenzhen, China. The spleen protein digestion procedure was performed according to the literature with minor modification (Yang et al. 2015, 2017). Lysis buffer 3 (8 M Urea, 40 mM Tris–HCl or tetraethyl-ammonium bromide (TEAB) with 1 mM Phenylmethanesulfonyl fluoride (PMSF), 2 mM Ethylene Diamine Tetraacetic Acid (EDTA) and 10 mM dithiothreitol (DTT), pH 8.5), and two magnetic beads (diameter 5 mm) were used to extract the proteins. The mixtures were placed into a Tissue Lyser for 2 min at 50 Hz to release proteins. After centrifugation at 25,000g at 4 °C for 20 min, the supernatant was transferred into a new tube, reduced with 10 mM DTT at 56 °C for 1 h and alkylated by 55 mM iodoacetamide (IAM) in the dark at room temperature for 45 min. Following centrifugation (25,000g, 4 °C, 20 min), the supernatant containing proteins was quantified by Bradford and sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE).
Trypsin Gold (Promega, Madison, WI, USA) was used to digest the 100 μg proteins for 4 h at a ratio of protein:trypsin of 40:1 at 37 °C. According to the above proportion, trypsin is added again and held at 37 °C until enzymolysis at 8 h. The peptides were hydrolyzed by the Strata X column and then desalinated and vacuum-dried.
The peptides were separated on a Shimadzu LC-20AB HPLC Pump system coupled with a high-pH RP column. The peptides were reconstituted with buffer A (5% acetonitrile, 95% H2O, with the pH adjusted to 9.8 with ammonia) to 2 ml and loaded onto a column containing 5-μm particles (Phenomenex). The peptides were separated at a flow rate of 1 ml/min with a gradient of 5% buffer B (5% H2O, 95% acetonitrile, adjust pH to 9.8 with ammonia) for 10 min, 5–35% buffer B for 40 min, and 35–95% buffer B for 1 min. The system was then maintained in buffer B for 3 min and equilibrated with 5% buffer B for 10 min. Elution was monitored by measuring absorbance at 214 nm, and fractions were collected every 1 min. The eluted peptides were pooled as 10 fractions and vacuum-dried.
The peptides separated in liquid phase were ionized by a NanoESI source and then placed on the Q-Exactive HF (Thermo Fisher Scientific, San Jose, CA) for DIA mode detection. The main parameters were as follows: the ion source voltage was 1.6 kV; the first-order mass spectrum scanning range was 350–1500 m/z; the resolution was 120,000; and 350–1500 Da was divided into 40 windows for fragmentation and signal collection. The fragment ions were detected in an Orbitrap. The dynamic exclusion time was 30 s.
This process relied mainly on the high-resolution mass spectrometer to produce sample data. For large-scale DIA data, the Spectronaut used constructed spectrum image database information to complete the deconvolution extraction of data, and the mProphet algorithm was used to complete the analysis and quality control of data. This process also completed the GO, COG, pathway, and other functional annotation analysis using the UniProt database. Based on the quantitative results, differential proteins between different comparison groups were searched, and functional analysis of the differentially enriched proteins was performed.
Results
Disease statistics
The spleen tissue indexes as observed for different times in the injected group and control group are shown in Fig. 1. On the 15th day and 30th day, the tissue indexes of the spleen of the injected group were separately significantly (P < 0.05) higher than control group.
Fig. 1.
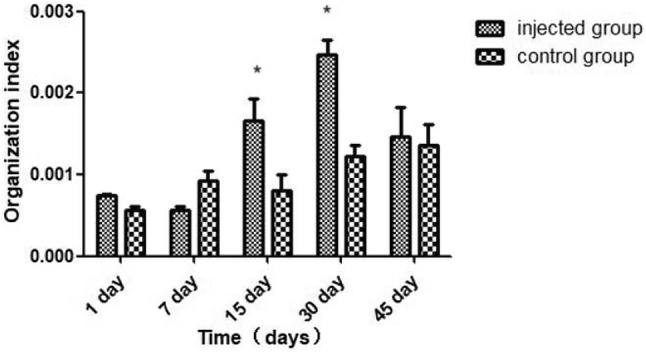
Spleen tissue index at different times in the injected group and control group. All values are presented as the means ± SEM (n = 3). Asterisk represents statistical significance (P < 0.05)
Identification of proteins
On average, approximately 29,565 peptides and 5898 proteins were identified. Table 1 briefly summarizes the information on the peptide number and protein number for each sample. Fold change ≥ 2 and P value < 0.05 were used as the screening criteria for the significantly differentially expressed proteins (DEPs), and the results are shown in Table 2. Sixty-five downregulated proteins and 38 upregulated proteins were detected between the 15DI group and 15DC group, 78 downregulated proteins and 44 upregulated proteins were detected between the 30 DI group and the 30 DC group.
Table 1.
Overview of the quantitative results of each sample
| Sample name | Peptide number | Protein number |
|---|---|---|
| 15DI-1 | 30210 | 5974 |
| 15DI-2 | 29323 | 5849 |
| 15DI -3 | 29737 | 5931 |
| 15DC-1 | 29197 | 5866 |
| 15DC-2 | 30960 | 6109 |
| 15DC-3 | 31248 | 6097 |
| 30DI-1 | 28717 | 5776 |
| 30DI-2 | 29282 | 5840 |
| 30DI-3 | 27923 | 5646 |
| 30DC-1 | 28371 | 5795 |
| 30DC-2 | 29539 | 5917 |
| 30DC-3 | 30274 | 5987 |
Table 2.
Statistical list of differential proteins
| Comparison group | Downregulated | Upregulated | Nonregulated |
|---|---|---|---|
| 15DI-vs-15DC | 65 | 38 | 6159 |
| 30DI-vs-30DC | 78 | 44 | 6004 |
GO analysis of DEPs
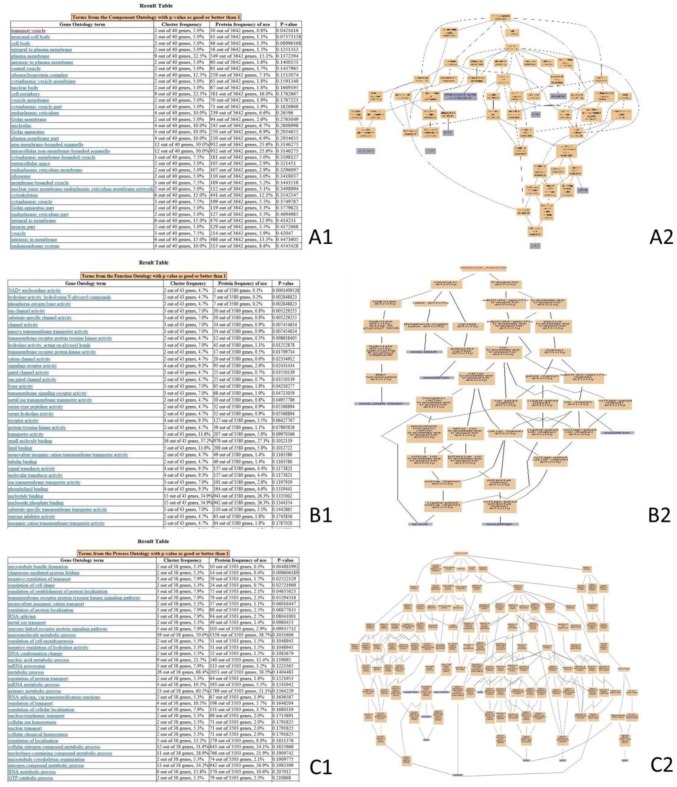
The DEPs of the 15DI group compared with 15DC group were enriched with the GO items (Fig. 2). The FLT4, ASIC1, BLB, CDC42SE2, ASPR, RHOQ, CHP1, XRN1, EGFR, and the VMA21 proteins (Fig. 2A2) were integral to the plasma membrane, MHC class II protein complex, phagocytic cup, plasma membrane, perkaryou, neuronal cell body, and transport vesicle (Fig. 2A1). CD38, BST1, GUCY1A3, ASIC1, ERG, and KCNA3 proteins (Fig. 2B2) were involved in molecular functions such as NAD + nucleosidase activity, phosphorus-oxygen lyase activity, guanylate cyclase activity, ligand-gated sodium channel activity, and voltage-gated potassium channel activity (Fig. 2B1). CHP1, EGFR, CDC42SE2, RHOQ, FLT4, GAPVD1, ASIC1, and HSPH1 proteins (Fig. 2C2) were involved in biological processes such as microtubule bundle formation, regulation of cell shape, vascular endothelial growth factor receptor signaling pathway, insulin receptor signaling pathway, and regulation of protein transport (Fig. 2C1).
Fig. 2.
GO-enrichment analysis of the DEPs of the 15DI group compared with the 15DC group. This pictures were the screenshot of GO-enrichment analysis. Cluster frequency indicates the ratio of annotation for the same GO term among all the DEPs and all of the proteins. (A) Gene-ontology analysis of the proteins in cellular component. A1 GO-enrichment results screenshot, P value < 0.05 is a significantly enriched GO entry; A2 genes annotated to the term. (B) Gene-ontology analysis of the proteins in molecular function. B1 GO-enrichment results screenshot, P value < 0.05 is a significantly enriched GO entry; B2 genes annotated to the term. (C) Gene-ontology analysis of the proteins in biological process. C1 GO-enrichment results screenshot, P value < 0.05 is a significantly enriched GO entry; C2 genes annotated to the term
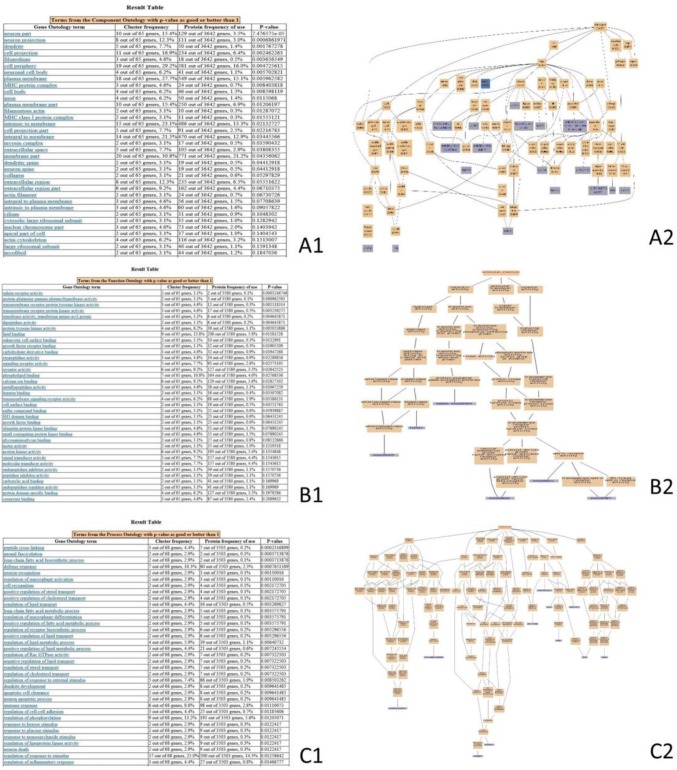
The DEPs of the 30DI group compared with the 30DC group enriched the GO items, as shown in Fig. 3. MYO5A, APP, BLB1, and MHC class I antigen proteins (Fig. 3A2) were involved in the photoreceptor outer segment, ciliary rootlet, MHC class II protein complex, and MHC I protein complex (Fig. 3A1). The CPQ, CNDP2, TGM4, PDGFRB, EPHB3, and EPHA4 proteins (Fig. 3B2) were involved in molecular function such as metallodipeptidase activity, dipeptidase activity, protein-glutamine gamma-glutamyltransferase activity, platelet-derived growth factor beta-receptor activity, and ephrin receptor activity (Fig. 3B1). CATHL1, LYG2, AvBD2, GAL6, LY86, AHSG, and THBS1 proteins (Fig. 3C2) were involved in biological processes such as the defense response to bacterium, defense response, inflammatory response, innate immune response, acute-phase response, and chronic inflammatory response (Fig. 3C1).
Fig. 3.
GO-enrichment analysis of the DEPs of the 30DI group compared with the 30DC group. This figure provides the screenshot of the GO-enrichment analysis. Cluster frequency indicates the ratio of the annotation of the same GO term between all of DEPs and all of proteins. (A) Gene-ontology analysis of the proteins in cellular component. A1 GO-enrichment results from the screenshot, P value < 0.05 is a significantly enriched GO entry; A2 genes annotated to the term. (B) Gene-ontology analysis of the proteins in molecular function. B1 GO-enrichment results screenshot, P value < 0.05 is a significantly enriched GO entry; B2 genes annotated to the term. (C) Gene-ontology analysis of the proteins in biological process. C1 GO-enrichment results’ screenshot, P value < 0.05 is a significantly enriched GO entry; C2 genes annotated to the term
Pathway analysis of DEPs
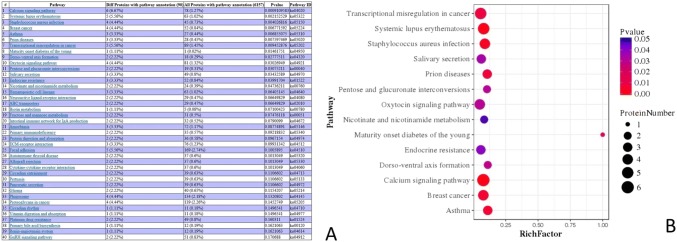
We perform pathway-enrichment analysis of the DEPs of the 15DI group and 15DC group based on the KEGG database. The DEPs were involved in pathways such as the calcium signaling pathway (6 proteins), systemic lupus erythematosus (5 proteins), Staphylococcus aureus infection (4 proteins), breast cancer (4 proteins), intestinal immune network for IgA production (3 proteins), and primary immunodeficiency (2 protein) (Fig. 4).
Fig. 4.
Pathway-enrichment analysis of the DEPs of the 15DI group and 15DC group. a This picture was the screenshot of the pathway enrichment. b This figure was the pathway that significantly enriched the DEPs. The enrichment factor is the number of DEPs annotated to the pathway divided by all the identified proteins annotated to the pathway. The higher the value is, the higher the proportion of the differentially expressed proteins annotated to this pathway. The dot size in the figure represents the number of DEPs annotated to this pathway
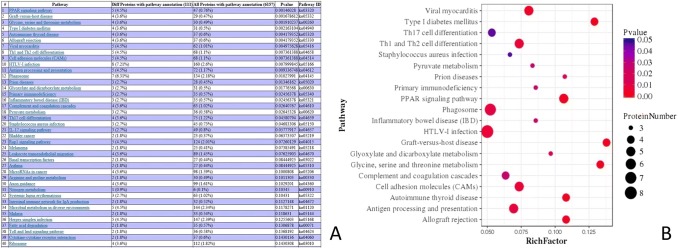
Pathway-enrichment analysis of the DEPs of the 30DI group and 30DC group was performed based on the KEGG database. The DEPs were involved in pathways such as the PPAR-signaling pathway (5 proteins), graft-versus-host disease (4 proteins), Th1 and Th2 cell differentiation (5 proteins), HTLV-I infection (8 proteins), phagosome (7 proteins), and primary immunodeficiency (3 protein) (Fig. 5).
Fig. 5.
Pathway-enrichment analysis of the DEPs of the 30DI group and 30DC group. a This picture was the screenshot of the Pathway enrichment. b This figure was the pathway that significantly enriched the DEPs. The enrichment factor is the number of DEPs annotated to the pathway divided by all the identified proteins annotated to the pathway. The higher the value is, the higher the proportion is of the differentially expressed proteins annotated to this pathway. The dot size in the figure represents the number of DEPs annotated to this pathway
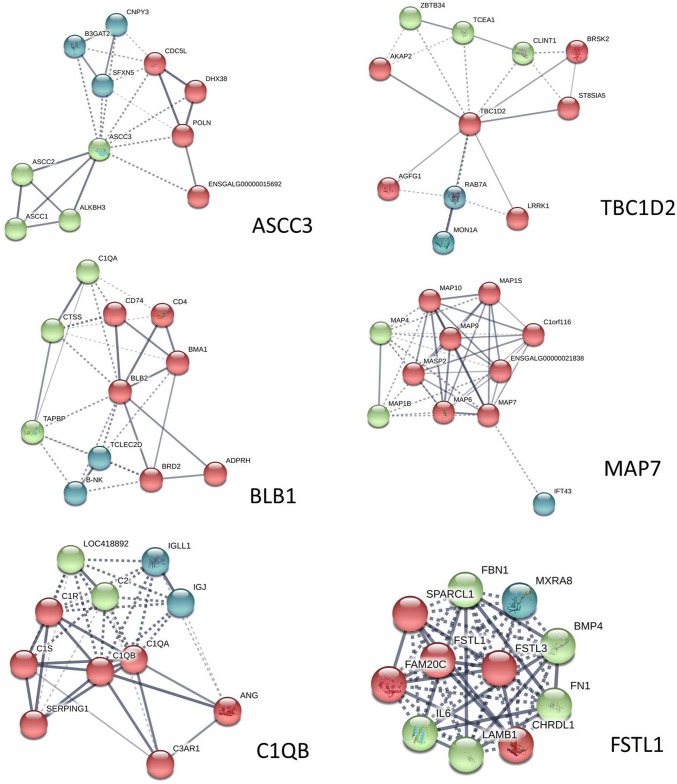
Interaction network of DEPs
Proteins usually do not work alone but rather interact with each other to perform various functions. To explore the protein interaction networks altered in chicken spleens after the chickens were infected with ALV-J, we identified the common DEPs in both comparisons, which we analyzed using the STRING software (Fig. 6). The DEPs were activating signal cointegrator 1 complex subunit 3 (ASCC3), TBC1 domain family member 2 (TBC1D2), MHC class II beta chain 1 (BLB2), ensconsin (MAP7), complement component 1 Q subcomponent B chain (C1QB), and Follistatin-like 1 (FSTL1).
Fig. 6.
Protein–protein interaction network of each of the common DEPs in both comparisons. In this network, the nodes represent proteins, and lines with different colors represent the predicted different associations
Discussion
Proteomic analysis is a powerful and relatively novel technique for investigating the biomarkers whose proteins respond to virus infection, and this analysis has been used for chickens (Desai et al. 2016; Li et al. 2015; Yang et al. 2015; Ouyang et al. 2017). Avian leukosis is caused by the avian leukosis virus. The complicated infection mechanism of the virus and the complex immune mechanism of chickens make investigation of the molecular markers of leukemia difficult. Only a few studies have used proteomics to study the molecular markers of leukemia such as ubiquitin carboxy-terminal hydrolase L1 (UCHL1), voltage-dependent anion channel 1 (VDAC1), high mobility group 1 (HMGB1), alpha-actinins (ACTNs), and peroxiredoxins (PRDXs) (Fan et al. 2012; Li et al. 2015). Chickens are the susceptible hosts of ALV-J, and the spleen is an important immune organ, which plays an anti-infection and immune response function in virus infection (Cesta 2006). In this study, we performed a DIA-based proteomic analysis of chicken spleens on the 15th day and 30th day, so we could focus on the immune response after the infection of ALV-J to find the potential biomarkers of chicken. We identified approximately 5400–6200 proteins of each group. Compared to the 15DI group and 15DC group, 65 proteins were downregulated and 38 proteins were upregulated. Compared with the 30DI group and 30DC group, 78 proteins were downregulated and 44 proteins were upregulated. Proteins related to the protein complex included the ATP-binding cassette, activating signal cointegrator complex subunit 3, ubiquitin-conjugating enzyme E2 variant, TBC1 domain family member 2A, MAP7 domain-containing protein 3, complement C1q subcomponent subunit B, Notch 1, Ankyrin, and major histocompatibility complex, which are involved in defense mechanisms, replication, recombination and repair, post-translational modification, protein turnover, cell wall/membrane/envelope biogenesis, intracellular trafficking, secretion, cell-cycle control, cell division, and chromosome partitioning. Considering the biomarker should be stable for detection at different times, we selected 6 DEPs (ASCC3, TBC1D2, BLB2, MAP7, C1QB, and FSTL1), which were expressed differentially in both comparisons, for the analysis of their protein–protein interaction network to investigate the potential biomarkers.
ASCC3 is 3′–5′ DNA helicase involved in repair of alkylated DNA, which is enriched in the mRNA splicing reactome pathways. The highest score predicted among the functional partner is the activating signal cointegrator 1 complex subunit 2 (ASCC2) and the alkylation repair homolog 3 (ALKBH3). The ALKBH3-ASCC alkylation-damage-signaling pathway is a critical regulator for the RNA-interacting domains in the alkylation-damage response (Soll et al. 2018; Brickner et al. 2017). ALV-J is a retrovirus and mainly integrates into the vicinity of tumor-related genes through its pre-viral DNA, so that the promoter of the virus can promote the expression of tumorigenic proteins or inhibit the expression of tumor suppressor proteins, thus leading to tumorigenesis (Hayward et al. 1981; Jing et al. 2014; Serva et al. 2012). The integrated progress might damage the DNA, and the ASCC3 may be related to the DNA reparation, but the mechanism needs more study to make such a determination. ASCC3 functioned as a negative regulator of the host defense response. Silencing of the ASCC3 resulted in the upregulation of multiple antiviral interferon-stimulated genes, which correlated with the inhibition of infection of several positive-strand RNA viruses (Li et al. 2013). According the proteomics, ASCC3 was downregulated in both comparisons, which means that, when the infected chicken developed a defense response, the ASCC3 was downregulated to promote the antiviral response.
TBC1D2 was involved in the TBC/RABGAPs and membrane trafficking. The top three predicted functional partners were the RAS oncogene family (RAB7A), ST8 alpha-N-acetyl-neuraminide alpha-2, 8-sialyltransferase 5 (ST8SIA5), and kinase (PRKA) anchor protein 2 (AKAP2). TBC1D2, which acted as a GTPase-activating protein of RAB7, targeted transcripts encoding the miR-17 to downregulate the RAB7 activity (Serva et al. 2012). The RAB7 would regulate the plasma cell survival and B-cell differentiation (Rowland et al. 2014). The occurrence of lymphocytic leukemia may be caused by the insertion of the ALV gene into the B-cell genome, which abnormally activates the myc gene and produces a large amount of myc phosphoprotein. Meanwhile, it is accompanied by the activation or inactivation of other cellular tumor genes, which eventually leads to the progressive growth and metastasis of the tumor and the generation of B lymphocytic tumor (Payne and Nair 2012). We speculated the reason of expressed protein of TBC1D2 being upregulated in the 15DI-vs-15DC group, but downregulated in the 30DI-vs-30DC group was the increased immune response over time, which leads to an increased demand for B cells, thus allowing the body to downregulate the genes that inhibit B-cell differentiation, such as TBC1D2, to strengthen the immune response. Considering the oppositely expressed protein of TBC1D2, we speculated that the TBC1D2 was related to the immune response caused by ALV-J, but was not suitable as a biomarker.
The MAP7 was involved in the microtubule cytoskeleton organization. The top three predicted functional partners were the microtubule-associated protein 6 homolog (MAP5), microtubule-associated protein 9 (MAP9), and microtubule-associated protein 10 (MAP10). The high expression of the MAP7 was related to tumor recurrence and poor prognosis in stage II colon cancer patients (Blum et al. 2008). MAP7 had the potential to accelerate proliferation, but not to influence apoptosis and cell-cycle progression in human lung adenocarcinoma cell line A549 (Yan et al. 2013). MAP7 may play an active role in the development of leukemia, and the high expression of MAP7 is a potentially predictive marker in cytogenetically normal acute myeloid leukemia (Fu et al. 2016). According to the proteomics, the protein expression of MAP7 was upregulated in the 15DI-vs-15DC group, but downregulated in the 30DI-vs-30DC group. This might be because of the complicated inflectional mechanism of ALV-J. We speculated that the MAP7 was related to the immune response caused by ALV-J, but was not suitable as a biomarker.
BLB2 was involved in herpes simplex infection, cell adhesion molecules, phagosome, intestinal immune network for IgA production, and influenza A through MHC class II antigen presentation and immune system reactome pathways. The top three predicted functional partners were invariant chain isoform p41 (CD74), CD4 molecule (CD4) and B locus M alpha chain 1 (BMA1). The genetic diversity of the BLB2 gene partially accounts for the high degree of MHC polymorphism and affects the immune system (Guo et al. 2012). MHC class II is crucial for downregulating the immune response, primarily through stimulating regulatory T cells (Weissler and Caton 2014). The immune response of chicken was caused by the infection of ALV-J. According to the proteomics, the expressed protein of BLB was downregulated in both comparisons. BLB2 showed consistently lower transcripts in the spleens of the MDV-infected than in the uninfected chickens, which agreed with our study results (Lian et al. 2010). We speculated that the ALV-J infection suppressant the MHC class II antigen presentation thus caused the immunosuppression of the chicken.
C1QB was involved in the regulation of the complement cascade, initially triggering the complement and T-classical antibody-mediated complement activation. The top three predicted functional partners were angiogenin (ANG); complement component 1, with q subcomponent in a chain (C1QA); and complement component 1, with s subcomponent (C1S). As one of the bone-marrow-derived macrophages expressed various complement-related genes, the relative expression of C1QB was highest (Luo et al. 2012). C1QB induce apoptosis of prostate cancer cells by targeting tumor suppressor factor to inhibit cell adhesion (Healy and Tipton 2007). The C1QB was upregulated, which was associated with the renal cell carcinoma tumor stage and grade (Zhang et al. 2016) and was consistent with our work. ALV-J C1QB was highly expressed after the infection of ALV-J, which meant C1QB may be related to the inhibition of tumor caused by ALV-J.
FSTL1 was involved in post-translational protein phosphorylation, regulation of insulin-like growth factor (IGF) transport and uptake by insulin-like growth factor binding proteins (IGFBPs), molecules associated with elastic fibers, integrin cell surface interactions, and degradation of the extracellular matrix reactome pathways. The top three predicted functional partners were follistatin-like-3 (FSTL3), interleukin-6 (IL6), and chordin-like protein 1 (CHRDL1). Some studies have found that FSTLl has elevated tolerance in the serum of patients with various autoimmune diseases (Tanaka et al. 2003; Clutter et al. 2009; Li et al. 2011). It activates immune cells, promotes gene expression, and releases pro-inflammatory cytokines (Le Luduec et al. 2008). Chronic inflammation is considered to be one of the definite factors leading to tumor development and progression. Damage of inflammatory factors to cells may lead to compensatory proliferation, accumulation of DNA damage/gene mutations and epigenetic changes. Given the specific role of FSTLl in inflammatory response, its influence on tumor progression has also aroused interest among researchers. FSTLl was closely related to primary glioblastoma (Reddy et al. 2008), prostate cancer cell lines (Trojan et al. 2005) and esophageal squamous cell carcinoma (Lau et al. 2017). In our data, FSTL1 was downregulated in both comparisons when an immune response occurred, which may be because of immunosuppression induced by ALV-J, but more work needs to be done to determine the mechanism.
Conclusion
Approximately 220 DEPs from the spleens of chicken infected with ALV-J were detected by DIA. To find a relatively stable biomarker molecule, we summarized the DEPs at two timepoints and selected six proteins (ASCC3, TBC1D2, BLB2, MAP7, C1QB, and FSTL1) from both comparisons for protein interaction network analysis. ASCC3, BLB2, C1QB, and FSTL1 were potential biomarkers for the complex infection mechanism of ALV-J and the dynamic immune mechanism of the body, but more research into the mechanisms involved is still needed.
Acknowledgements
The Sequencing work was financially supported by the China Agriculture Research System (CARS-41), the Engineering Centre of Chicken Breeding in Guangdong Province (2017-1649) and Guangdong Provincial Key Laboratory of Animal Molecular Design and Precise Breeding (2019B030301010). We would like to thank AMERICAN JOURNAL EXPERTS (https://secure.aje.com) for providing linguistic assistance during the preparation of this manuscript.
Author contributions
YW, HL, and QZ conceived and designed the experiments. FY and QJH performed the experiments. FY, CC, BL, FLY and HDY analyzed the data. HYX, DYL, and XLZ contributed the reagents/materials/analysis and tools. FY wrote the paper. YW, HL, and QZ revised the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Ethical statements
All animal care and experimental procedures were reviewed and approved by the Animal Care and Use Committee (#YYS130125) of the Animal Care Advisory at Sichuan Agricultural University. This study was carried out in strict accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals of the State Council of the People’s Republic of China.
Contributor Information
Hua Li, Email: okhuali@fosu.edu.cn.
Qing Zhu, Email: zhuqingsicau@163.com.
References
- Blum C, Graham A, Yousefzadeh M, Shrout J, Benjamin K, Krishna M, Hoda R, Hoda R, Cole DJ, Garrett-Mayer E. The expression ratio of Map7/B2M is prognostic for survival in patients with stage II colon cancer. Int J Oncol. 2008;33(3):579–584. [PMC free article] [PubMed] [Google Scholar]
- Brickner JR, Soll JM, Lombardi PM, Vågbø CB, Mudge MC, Oyeniran C, Rabe R, Jackson J, Sullender ME, Blazosky E. A ubiquitin-dependent signalling axis specific for ALKBH-mediated DNA dealkylation repair. Nature. 2017;551(7680):389. doi: 10.1038/nature24484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesta MF. Normal structure, function, and histology of the spleen. Toxicol Pathol. 2006;34(5):455–465. doi: 10.1080/01926230600867743. [DOI] [PubMed] [Google Scholar]
- Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-γ. J Immunol. 2009;182(1):234–239. doi: 10.4049/jimmunol.182.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Sun S, Zhang Z, Meng S. Simultaneous endemic infections with subgroup J avian leukosis virus and reticuloendotheliosis virus in commercial and local breeds of chickens. Avian Pathol. 2009;38(6):443–448. doi: 10.1080/03079450903349188. [DOI] [PubMed] [Google Scholar]
- Desai MA, Jackson V, Zhai W, Suman SP, Nair MN, Beach CM, Schilling MW. Proteome basis of pale, soft, and exudative-like (PSE-like) broiler breast (Pectoralis major) meat. Poult Sci. 2016;95(11):2696–2706. doi: 10.3382/ps/pew213. [DOI] [PubMed] [Google Scholar]
- Fadly AM, Smith EJ. Isolation and some characteristics of a subgroup J-like avian leukosis virus associated with myeloid leukosis in meat-type chickens in the United States. Avian Dis. 1999;43(3):391–400. doi: 10.2307/1592636. [DOI] [PubMed] [Google Scholar]
- Fan Z, Hu X, Zhang Y, Yu C, Qian K, Qin A. Proteomics of DF-1 cells infected with avian leukosis virus subgroup. J Virus Res. 2012;167(2):314–321. doi: 10.1016/j.virusres.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Fu L, Fu H, Zhou L, Xu K, Pang Y, Hu K, Wang J, Tian L, Liu Y, Wang J. High expression of MAP7 predicts adverse prognosis in young patients with cytogenetically normal acute myeloid leukemia. Sci Rep. 2016;6:34546. doi: 10.1038/srep34546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X-L, Zheng H-q, Li X-L, Li Y, Gu Z-L, Zheng C-S, Wei Z-H, Wang J-S, Zhou R-Y, Li L-H. Genetic variation of major histocompatibility complex BLB2 gene exon 2 in Hebei domestic chicken. Res Vet Sci. 2012;92(1):76–79. doi: 10.1016/j.rvsc.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Hayward WS, Neel BG, Astrin SM. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981;290(5806):475. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Healy J, Tipton K. Ceruloplasmin and what it might do. J Neural Trans. 2007;114(6):777. doi: 10.1007/s00702-007-0687-7. [DOI] [PubMed] [Google Scholar]
- Jing YY, Li YS, Xin JK, Chai JQ. Co-infection of ALV-J and Salmonella pullorum in laying hens. Pak Vet J. 2014;34(3):372–376. [Google Scholar]
- Lau MC-C, Ng KY, Wong TL, Tong M, Lee TK, Ming X-Y, Law S, Lee NP, Cheung AL, Qin Y-R. FSTL1 promotes metastasis and chemoresistance in esophageal squamous cell carcinoma through NFκB–BMP signaling cross-talk. Cancer Res. 2017;77(21):5886–5899. doi: 10.1158/0008-5472.CAN-17-1411. [DOI] [PubMed] [Google Scholar]
- Le Luduec J, Condamine T, Louvet C, Thebault P, Heslan JM, Heslan M, Chiffoleau E, Cuturi MC. An immunomodulatory role for follistatin-like 1 in heart allograft transplantation. Am J Trans. 2008;8(11):2297–2306. doi: 10.1111/j.1600-6143.2008.02398.x. [DOI] [PubMed] [Google Scholar]
- Li D, Wang Y, Xu N, Wei Q, Wu M, Li X, Zheng P, Sun S, Jin Y, Zhang G. Follistatin-like protein 1 is elevated in systemic autoimmune diseases and correlated with disease activity in patients with rheumatoid arthritis. Arthritis Res Ther. 2011;13(1):R17. doi: 10.1186/ar3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ding SC, Cho H, Chung BC, Gale M, Chanda SK, Diamond MS. A short hairpin RNA screen of interferon-stimulated genes identifies a novel negative regulator of the cellular antiviral response. MBio. 2013;4(3):e00313–e00385. doi: 10.1128/mBio.00385-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang Q, Gao Y, Qi X, Wang Y, Gao H, Gao Y, Wang X. Quantitative iTRAQ LC–MS/MS proteomics reveals the proteome profiles of DF-1 cells after infection with subgroup J Avian leukosis virus. Biomed Res Int. 2015 doi: 10.1155/2015/395307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wang P, Lin L, Shi M, Gu Z, Huang T, Ml Mo, Wei T, Zhang H, Wei P. The emergence of the infection of subgroup J avian leucosis virus escalated the tumour incidence in commercial Yellow chickens in Southern China in recent years. Transbound Emerg Dis. 2019;66(1):312–316. doi: 10.1111/tbed.13023. [DOI] [PubMed] [Google Scholar]
- Lian L, Qu L, Zheng J, Liu C, Zhang Y, Chen Y, Xu G, Yang N. Expression profiles of genes within a subregion of chicken major histocompatibility complex B in spleen after Marek’s disease virus infection. Poult Sci. 2010;89(10):2123–2129. doi: 10.3382/ps.2010-00919. [DOI] [PubMed] [Google Scholar]
- Liu L, Yi J, Ray WK, Vu LT, Helm RF, Siegel PB, Cline MA, Gilbert ER. Fasting differentially alters the hypothalamic proteome of chickens from lines with the propensity to be anorexic or obese. Nutr Diabetes. 2019;9(1):13. doi: 10.1038/s41387-019-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Chen M, Madden A, Xu H. Expression of complement components and regulators by different subtypes of bone marrow-derived macrophages. Inflammation. 2012;35(4):1448–1461. doi: 10.1007/s10753-012-9458-1. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91(3):295–298. doi: 10.1016/S0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Ouyang H, Wang Z, Chen X, Yu J, Li Z, Nie Q. Proteomic analysis of chicken skeletal muscle during embryonic development. Front Physiol. 2017;8:281. doi: 10.3389/fphys.2017.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. Retrovirus-induced disease in poultry. Poult Sci. 1998;77(8):1204–1212. doi: 10.1093/ps/77.8.1204. [DOI] [PubMed] [Google Scholar]
- Payne L, Nair V. The long view: 40 years of avian leukosis research. Avian Pathol. 2012;41(1):11–19. doi: 10.1080/03079457.2011.646237. [DOI] [PubMed] [Google Scholar]
- Reddy SP, Britto R, Vinnakota K, Aparna H, Sreepathi HK, Thota B, Kumari A, Shilpa B, Vrinda M, Umesh S. Novel glioblastoma markers with diagnostic and prognostic value identified through transcriptome analysis. Clin Cancer Res. 2008;14(10):2978–2987. doi: 10.1158/1078-0432.CCR-07-4821. [DOI] [PubMed] [Google Scholar]
- Rowland AA, Chitwood PJ, Phillips MJ, Voeltz GK. ER contact sites define the position and timing of endosome fission. Cell. 2014;159(5):1027–1041. doi: 10.1016/j.cell.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H, Vogt P. An avian leukosis virus associated with stocks of Rous sarcoma virus. Virology. 1962;17(1):184–194. doi: 10.1016/0042-6822(62)90096-X. [DOI] [PubMed] [Google Scholar]
- Serva A, Knapp B, Tsai Y-T, Claas C, Lisauskas T, Matula P, Harder N, Kaderali L, Rohr K, Erfle H. miR-17-5p regulates endocytic trafficking through targeting TBC1D2/Armus. PLoS One. 2012;7(12):e52555. doi: 10.1371/journal.pone.0052555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll JM, Brickner JR, Mudge MC, Mosammaparast N. RNA ligase-like domain in activating signal cointegrator 1 complex subunit 1 (ASCC1) regulates ASCC complex function during alkylation damage. J Biol Chem. 2018;293(35):13524–13533. doi: 10.1074/jbc.RA117.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Ozaki S, Kawabata D, Kishimura M, Osakada F, Okubo M, Murakami M, Nakao K, Mimori T. Potential preventive effects of follistatin-related protein/TSC-36 on joint destruction and antagonistic modulation of its autoantibodies in rheumatoid arthritis. Int Immunol. 2003;15(1):71–77. doi: 10.1093/intimm/dxg005. [DOI] [PubMed] [Google Scholar]
- Trojan L, Schaaf A, Steidler A, Haak M, Thalmann G, Knoll T, Gretz N, Alken P, Michel MS. Identification of metastasis-associated genes in prostate cancer by genetic profiling of human prostate cancer cell lines. Anticancer Res. 2005;25(1A):183–191. [PubMed] [Google Scholar]
- Weissler KA, Caton AJ. The role of T-cell receptor recognition of peptide: MHC complexes in the formation and activity of Foxp3+ regulatory T cells. Immunol Rev. 2014;259(1):11–22. doi: 10.1111/imr.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Liang H, Deng T, Zhu K, Zhang S, Wang N, Jiang X, Wang X, Liu R, Zen K. The identification of novel targets of miR-16 and characterization of their biological functions in cancer cells. Mol Cancer. 2013;12(1):92. doi: 10.1186/1476-4598-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zheng N, Zhao X, Zhang Y, Han R, Ma L, Zhao S, Li S, Guo T, Wang J. Proteomic characterization and comparison of mammalian milk fat globule proteomes by iTRAQ analysis. J Proteom. 2015;116:34–43. doi: 10.1016/j.jprot.2014.12.017. [DOI] [PubMed] [Google Scholar]
- Yang M, Song D, Cao X, Wu R, Liu B, Ye W, Wu J, Yue X. Comparative proteomic analysis of milk-derived exosomes in human and bovine colostrum and mature milk samples by iTRAQ-coupled LC–MS/MS. Food Res Int. 2017;92:17–25. doi: 10.1016/j.foodres.2016.11.041. [DOI] [PubMed] [Google Scholar]
- Zhang L, Jiang H, Xu G, Chu N, Xu N, Wen H, Gu B, Liu J, Mao S, Na R. iTRAQ-based quantitative proteomic analysis reveals potential early diagnostic markers of clear-cell renal cell carcinoma. Biosci Trends. 2016;10(3):210–219. doi: 10.5582/bst.2016.01055. [DOI] [PubMed] [Google Scholar]