Abstract
OBJECTIVE
Lifestyle interventions slow development of type 2 diabetes by half, but the impact of health payer reimbursement for delivery of intervention programs is not well known. We evaluated net commercial health payer expenditures when offering reimbursement for access to YMCA’s Diabetes Prevention Program (YDPP) in 42 states.
RESEARCH DESIGN AND METHODS
We used a nonequivalent comparison group design to evaluate net health care expenditures for adults with prediabetes who attended one or more YDPP visit between 1 July 2009 and 31 May 2013 (“YDPP users”). Rolling, 1:1 nearest neighbor propensity score (PS) matching was used to identify a comparison group of nonusers. Administrative data provided measures of YDPP attendance, body weight at YDPP visits, and health care expenditures. Random effects, difference-in-difference regression was used to estimate quarterly health care expenditures before and after participants’ first visit to YDPP.
RESULTS
Worksite screening identified 9.7% of the target population; 39.1% of those identified (19,933 participants through June 2015) became YDPP users. Mean weight loss for YDPP users enrolled before June 2013 (n = 1,725) was 7.5 lb (3.4%); 29% achieved ≥5% weight loss. Inclusive of added costs to offer YDPP, there were no statistically significant differences in mean per-person net health care expenditures between YDPP users and PS-matched nonusers over 2 years ($0.2 lower [95% CI $56 lower to $56 higher]). Mean reimbursement to the YMCA was $212 per YDPP user, with 92.8% of all expenditures made for those who attended at a high rate (≥9 completed YDPP visits).
CONCLUSIONS
Worksite screening was inefficient for identifying the population with prediabetes, but those identified achieved modest YDPP attendance and clinically meaningful weight loss. Over 2 years, added costs to offer the intervention were modest, with neutral effects on net health care costs.
Introduction
More than 30 million Americans have diabetes, imposing a substantial health and economic burden (1). Unfortunately, an additional 84 million Americans have prediabetes and are at high risk for developing type 2 diabetes over the next 5–10 years (1–3). This context warrants intensive efforts to prevent type 2 diabetes, particularly for those at high risk (4).
The Diabetes Prevention Program (DPP) clinical trial demonstrated that a resource-intensive lifestyle behavioral change intervention can reduce the rate of type 2 diabetes development in high-risk adults by 58% over ∼3 years (5). The DPP lifestyle intervention promotes healthful diet and moderate increases in physical activity to achieve modest weight loss (∼10–15 lb), which has been shown to convey benefits beyond diabetes prevention, such as improvement of other cardiovascular risk factors, reduced health care expenditures, and enhanced well-being (6–10). However, the high costs of implementation and frequent ongoing face-to-face visits impose a challenge for routine delivery of the DPP lifestyle intervention approach in real-world settings (11,12).
Studies involving lower-cost delivery of behaviorally based diabetes prevention interventions in community settings have demonstrated modest weight losses consistent with that seen in the DPP trial, often for <20% of the cost of the original DPP trial intervention (13–16). In 2009, UnitedHealth Group partnered with YMCA of the USA (YUSA) and the U.S. Centers for Disease Control and Prevention (CDC) to roll out a strategy involving health care sector payment for a low-cost, group-based adaptation of the DPP lifestyle intervention delivered by YMCAs (the YDPP) (17). Adopted in numerous geographic regions nationally, this approach involved performance-based payments made to YDPP providers to encourage high intervention attendance and achievement of at least a 5% weight loss goal for each participant.
At a population level, the reach and effectiveness of these efforts depend on the efficient identification of adults at high risk and the willingness of those adults to participate in the program (14). However, the optimal mix of strategies needed to maximize program participation is not yet known, and the financial sustainability of this approach from the health payer’s perspective will depend, in part, on how funding of the costs of YDPP impacts net health care expenditures.
Seizing this natural experimental opportunity, UnitedHealth Group, YUSA, and researchers at Northwestern and Indiana Universities partnered to evaluate the adoption and reach of these efforts (i.e., the extent to which adults at high risk participate in YDPP), as well as the effectiveness of the program in terms of 1) participant weight loss and 2) net health care expenditures.
Research Design and Methods
Overall Design and Study Setting
We used a nonequivalent, propensity score (PS)-matched comparison group design to evaluate the effects of YDPP on trends in health care expenditures for adult commercial health plan enrollees who were identified primarily during employer-based screening activities as being at high risk for developing type 2 diabetes. The study design is illustrated in Supplementary Fig. 1.
Study Population and Matching Approach
The study population included commercially insured adults ≥18 years old who were at increased risk for developing type 2 diabetes and were enrolled in a health plan that offered the YDPP free of charge to persons with prediabetes. For most participants, high risk for developing diabetes was determined by an A1C test result of 5.7–6.4% (39–47 mmol/mol). Our analysis excluded individuals who had prior evidence of a diabetes diagnosis, defined by at least one health system encounter with an ICD-9-CM 250.XX code or at least one past dispensing event for an oral antiglycemic class medication or insulin. Any enrollee with one or more visits to a YDPP class was classified as a “YDPP user.” The date of the first YDPP visit was defined as the “index date” of exposure (Supplementary Fig. 1). For the purposes of cost and outcome analyses, we excluded persons with an index date after 31 May 2013, as this ensured that all participants could have at least 2 years of follow-up data, so long as they remained enrolled in the health plan.
To identify a comparison group with similar baseline characteristics, we used a nearest-neighbor 1:1 PS matching approach (18,19). YDPP “nonusers” were eligible for the match if they met all eligibility criteria above and were enrolled in the health plan through an employer that offered YDPP but had zero visits to YDPP. For construction of baseline variables for the PS match, individuals in both groups were required to be enrolled in the health plan for at least 3 months before and 3 months after the index date. To enable balanced comparisons between exposure groups, we assigned each matched control subject the same index date as the YDPP user to which he/she was paired. Exposed and potential control patients were grouped by whether a health care provider had diagnosed prediabetes prior to employer screening (i.e., a prior medical visit with a prediabetes ICD code [binary 0/1]). By prediabetes diagnosis status, the patient-level propensity models used logistic regression to predict the odds of YDPP participation, including the following independent variables from the preindex date period: sex, age category, Charlson Comorbidity Index score (20), evidence for recent use of another “health seeking” preventive service (0/1), a prior fill for any antihypertensive medication (0/1), a prior fill for any antilipidemic medication (0/1), total health care expenditures prior to the index date, cardiometabolic drug costs prior to the index date, and employer-level variables for the number of total employees, average annual employee salary, and percent of employees in minority race categories. Supplementary Table 1 provides details for how these matching variables were constructed.
YDPP Lifestyle Intervention
The YDPP is a goal-based cognitive and behavioral training and support program that was derived from the intensive lifestyle intervention delivered during the U.S. DPP clinical trial (21). YDPP encourages and supports each participant to reach goals for moderate physical activity and dietary changes to achieve and maintain 5–7% weight loss. Face-to-face meetings with a lifestyle instructor are held approximately weekly over the first 4–5 months (16 total “core” lessons), followed by monthly maintenance meetings that combine education, skill building, and supportive accountability to help each individual reach their unique behavioral goals. Prior studies show that higher attendance in DPP-like interventions results in greater weight loss success (14) and that every 1 kg (∼1%) of weight loss achieved by a participant translates into an additional 16% reduction in the rate of developing type 2 diabetes (22). During YDPP implementation, the partnering health payer provided the YMCA with a scaled payment for each participant, based on three types of criteria: 1) the individual’s level of attendance in the 16 “core” lessons (i.e., completion of 1–3 lessons, 4–8 lessons, or 9–16 lessons), 2) their maximum weight loss recorded during the first 6 months (i.e., achievement of 5.0–8.9% weight loss or ≥9.0% weight loss), and 3) a modest payment for each maintenance lesson attended. This performance-based approach was intended to minimize intervention payments for participants who did not attend at a high level, while also encouraging YMCAs to deliver the program in a way that maximized attendance and weight loss by the greatest number of participants.
Measures and Outcomes
Study outcomes included temporal and geographic patterns of YDPP adoption, attendance levels for YDPP users, weight losses for YDPP users, and inpatient, outpatient, pharmacy, and total health care expenditures. Measures of YDPP participation included numbers of YDPP visits attended and body weight recorded at each attended visit. Total health care costs (including both health plan and patient cost share components) were assessed equally across all patients by applying a standardized cost to each claim (23). Total health care expenditures included payments by the health plan for delivery of the YDPP intervention. To minimize the effect of extreme outliers on mean cost estimates, we replaced costs that were >95th percentile with the value at the 95th percentile (24). The Charlson Comorbidity Index was constructed using all months of data available prior to the index date (20). A metabolic risk score was also constructed from the count of each of three risk factors (hypertension, dyslipidemia, and prediabetes) for which there was evidence in the claims record prior to the index date (see Supplementary Table 2 for details).
Data Sources
Data sources included national health plan member enrollment files, medical inpatient and ambulatory claims, and pharmacy claims made available by a large, U.S.-based commercial health insurer for the period of 1 July 2008 through 30 June 2015. This time range offered the potential for all individuals in the study population to have at least 1 year of data before and 2 years of data after their index date. By linking a YUSA tracking and billing system database with the health payer claims data, we constructed YDPP eligibility status and YDPP lesson attendance for each health plan enrollee, as well as weight loss at each visit among YDPP users. We calculated mean per-person total health payer reimbursement to the YMCA for intervention delivery by using information provided by both organizations regarding agreed-upon payments based on thresholds of individual patient attendance and weight loss achieved at YDPP visits. At the request of YUSA, we summarize these results in aggregate. The Northwestern University Institutional Review Board reviewed the parent study and determined that this work involved the use of coded, nonidentifiable data and was not classifiable as human subjects research.
Statistical Analysis
Temporal patterns in adoption of YDPP were summarized over time and by nine U.S. Census Bureau geographic divisions (25). Univariate and bivariate descriptive statistics for baseline characteristics were calculated for both YDPP users and the PS-matched nonuser comparison group. We assessed comparability of means and proportions for individual baseline variables before and after PS matching using standardized differences, with a value of <0.10 considered to represent an acceptable level of balance between groups (26). For inspection of the comparability of time trends between groups, we plotted means for each 90-day period (i.e., quarter), extending eight quarters before and eight quarters after the index date. Diminishing sample sizes resulting in greater cost volatility precluded cost comparisons beyond 2 years.
The unit of cost analysis was U.S. dollars ($) per person per quarter (or, in separate models, per person per year). We estimated mean between-group differences in quarterly cost outcomes over different time horizons (year 1; year 2) using enrollee-level difference-in-difference random effects regression models that included a dummy variable for exposure group (YDPP user; PS-matched nonuser), quarter (or, in separate models, year) indicators (i.e., relative to the index date), and group-by-quarter (or, separately, group-by-year) interaction terms. For estimating continuous outcomes, we used linear models because they provide estimates in natural units of the outcome variable and have been shown to produce reliable and unbiased estimates of CIs for mean cost differences when sample sizes are large (27). Analyses were conducted on the overall sample, as well as for prespecified patient subgroups, including 1) categories of age (<55 years vs. ≥55 years); 2) baseline Charlson Comorbidity Index (0 vs. ≥1); and 3) baseline metabolic risk score (≤1 vs. ≥2). We also conducted a sensitivity analysis restricting the study sample to only those YDPP users and PS-matched nonusers who had at least one encounter with a prediabetes diagnosis code prior to the index date.
Because our cost analysis adopted a health payer perspective, each patient who was no longer enrolled with the health plan was censored from future measurement periods. To avoid potential bias from differential dropout across the two comparison groups, we censored both the YDPP user and their PS-matched nonuser at any point when either subject was no longer enrolled in the health plan. This censoring approach reduced the size of the study population over time but ensured balanced comparisons throughout the evaluation period. Among YDPP users, 27% were no longer enrolled in the health plan at 24 months. There were no statistically significant differences in mean age, sex, or comorbidity scores between YDPP users who remained with their health plan and those who did not remain enrolled.
Results
Description of YDPP Adoption and Reach During Scale-up
Beginning in 2009, YUSA and the health payer worked collaboratively to identify U.S. regions where there was overlap among large populations of commercial health plan enrollees and YMCA networks that were selected by YUSA for early adoption of YDPP. YDPP delivery was offered in eight U.S. census divisions in 2010 and in all nine divisions every remaining year.
As of 30 June 2015, the YDPP had been delivered to 19,933 commercial health plan clients (regardless of our study eligibility criteria) in a total of 938 different delivery locations by 1,052 different instructors. YDPP participants were identified from 759 different employers over the 5 year scale-up period (54 in the last 6 months of 2010, 75 in 2011, 283 in 2012, 386 in 2013, 236 in 2014, and 91 in the first 6 months of 2015).
Identification of Employed, High-Risk Health Plan Enrollees
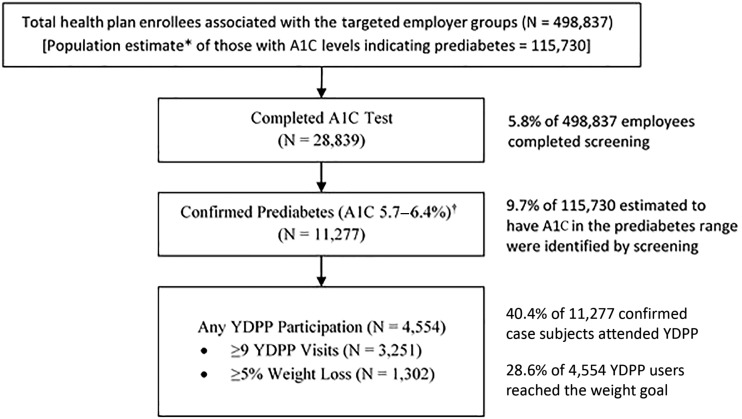
The reach of primarily employer-based strategies to identify and engage high-risk health plan enrollees in the YDPP is depicted in Fig. 1. With application of a U.S. population estimate of prediabetes prevalence of 23.2%, for using A1C testing alone (28), full participation in screening by all 498,837 employees who were enrolled in a partnering health plan would have identified ∼115,730 “high-risk” individuals with an A1C result of 5.7–6.4% (39–47 mmol/mol). However, only 28,839 (5.8%) of 498,837 employees completed testing, resulting in 11,277 (∼9.7% of the estimated 115,730-person target population) being identified with a high-risk A1C test result (Fig. 1).
Figure 1.
Population reach of YDPP following health payer–led screening and referral. *Multiplies the total employee population of 498,837 by the U.S. population prevalence estimate of 23.2% (28), who have A1C screening tests in the range of 5.7–6.4% (39–47 mmol/mol). †A1C of 5.7–6.4% (NGSP) is equivalent to 39–47 mmol/mol.
YDPP Participation and Weight Loss Achievement by High-Risk Health Plan Enrollees
Among the 11,277 individuals who were identified as having a qualifying A1C test, 4,554 (40.4%) attended at least one YDPP class. Levels of attendance and weight changes recorded by all of these YDPP users are detailed in Supplementary Table 3. Mean attendance among YDPP users overall was 12.4 (SD 6.3) classes; 71% completed 9 or more classes, and 1,801 (39.5% of YDPP users) completed all 16 of YDPP’s “core” intervention classes.
Among YDPP users, mean weight loss overall was 7.5 (SD 11.3) lb (3.4%), with 1,302 (28.6%) of those users reaching the weight loss goal of ≥5% of baseline body weight. There was a graded relationship between attendance level and weight loss achieved, with low attenders (1–3 classes completed) achieving a mean weight loss of only 0.5 (SD 2.8) lb (0.2%) and the highest attenders (≥16 classes completed) achieving a mean weight loss of 12.1 (SD 14.2) lb (5.4%). With application of the contracted payment amounts for each individual participant’s attendance and maximal weight loss, the mean total payout to the YMCA was estimated at $212 per YDPP user; 92.8% of the total payout was provided for YDPP users attending nine or more visits, for whom the mean weight loss was 9.8 lb (4.5%).
Characteristics of YDPP Users and Nonusers Examined in Health Care Cost Analysis
After exclusion of YDPP users with <2 years of potential follow-up after their index date (i.e., those with index dates on or after 1 June 2013) and those with <3 months of health plan enrollment before or after their index date, a total of 1,761 YDPP users remained eligible for the longitudinal economic evaluation, and 1,725 could be PS matched to YDPP nonusers for the difference-in-difference analysis. Prior to PS matching, YDPP users were more likely than nonusers to be women, to be ages 50–64 years, or to have a Charlson Comorbidity Index score >0 (Table 1). YDPP users also were more likely to have had a prior health care visit with a prediabetes diagnosis, recent use of another “health seeking” preventive service, and use of either blood pressure or lipid-lowering medication. Finally, YDPP users had higher total health care costs and higher costs for cardiometabolic class medications prior to exposure.
Table 1.
Baseline characteristics of YDPP users and nonusers before and after PS matching
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| Eligible YDPP users | All nonusers* | Stand. diff.† | Eligible YDPP users matched | PS-matched nonusers | Stand. diff.† | |
| N | 1,761 | 361,431 | 1,725 | 1,725 | ||
| Sex, % women | 72.4 | 49.5 | 0.46 | 72.1 | 71.9 | 0.01 |
| Age category, % | 0.66 | 0.05 | ||||
| 18–34 years | 2.5 | 15.3 | 2.6 | 2.1 | ||
| 35–49 years | 22.1 | 33.5 | 22.4 | 23.4 | ||
| 50–57 years | 30.8 | 19.9 | 31.0 | 32.5 | ||
| 58–64 years | 32.2 | 16.1 | 31.9 | 30.0 | ||
| ≥65 years | 12.4 | 15.3 | 12.2 | 11.9 | ||
| Age (years), mean (SD) | 55.0 (8.9) | 50.2 (14.0) | 0.34 | 53.0 (9.2) | 52.9 (9.2) | 0.01 |
| Charlson Comorbidity Index score, % by category‡ | 0.29 | 0.07 | ||||
| 0 | 57.1 | 71.1 | 57.2 | 55.5 | ||
| 1 | 20.7 | 14.2 | 20.6 | 20.0 | ||
| 2–3 | 15.1 | 9.9 | 15.1 | 17.8 | ||
| ≥4 | 7.1 | 4.8 | 7.1 | 6.7 | ||
| Mean metabolic risk factor score, mean (SD)§ | 1.2 (1.1) | 0.5 (0.8) | 0.87 | 1.2 (1.1) | 1.2 (1.1) | 0.00 |
| High blood pressure treatment, % | 52.7 | 33.0 | 0.41 | 52.5 | 53.3 | 0.02 |
| Abnormal blood cholesterol treatment, % | 27.2 | 15.5 | 0.29 | 27.0 | 27.8 | 0.02 |
| Prediabetes diagnosis, % | 40.8 | 6.5 | 0.88 | 39.7 | 39.7 | 0.00 |
| Recent use of health seeking services, %‖ | 23.3 | 15.3 | 0.2 | 23.0 | 23.6 | 0.02 |
| Percentile category of total health care costs, % by category¶ | 0.43 | 0.02 | ||||
| >90th percentile | 15.5 | 10.8 | 15.9 | 16.1 | ||
| 75th–90th percentile | 27.1 | 16.3 | 27.6 | 27.1 | ||
| 50th–74th percentile | 25.8 | 21.4 | 24.6 | 25.0 | ||
| <50th percentile | 31.6 | 51.5 | 31.8 | 31.8 | ||
| Percentile category of cardiovascular Rx costs, % by category¶ | 0.42 | 0.04 | ||||
| >95th percentile | 10.7 | 6.4 | 10.7 | 10.0 | ||
| 90th–95th percentile | 10.1 | 5.9 | 10.0 | 10.4 | ||
| 1st–89th percentile | 33.2 | 21.2 | 33.1 | 34.1 | ||
| 0 (no medication costs in these drug classes) | 46.0 | 66.6 | 46.2 | 45.5 | ||
| Year of first exposure# | 0.38 | 0.00 | ||||
| 2010 | 3.6 | 10.5 | 3.7 | 3.7 | ||
| 2011 | 15.4 | 24.3 | 15.6 | 15.6 | ||
| 2012 | 49.9 | 40.3 | 49.7 | 49.7 | ||
| 2013 | 31.1 | 24.9 | 31.0 | 31.0 | ||
| Employer-level variables, % | ||||||
| Employees in a high-deductible health plan | 27.6 | 22.3 | 0.15 | 27.8 | 26.9 | 0.02 |
| Employees reported as African American | 9.7 | 8.6 | 0.14 | 9.6 | 9.7 | 0.01 |
| Employees reported as Hispanic | 6.7 | 7.6 | 0.10 | 6.7 | 7.2 | 0.06 |
| Charlson Comorbidity Index, mean (SD) | 0.5 (0.2) | 0.6 (0.4) | 0.33 | 0.5 (0.2) | 0.5 (0.2) | 0.01 |
Rx, prescription.
*Eligible nonusers were health plan enrollees who were associated with an employer that had at least one eligible YDPP user (i.e., participating employers).
†The standardized difference (Stand. diff.) compares the difference in means in units of the pooled SD. Unlike t tests and other statistical tests of hypothesis, the standardized difference is not influenced by sample size. Covariates with a standardized difference <0.10 are generally considered to be well matched (26).
‡Higher scores represent increasing numbers of chronic disease diagnoses (i.e., comorbidity).
§Score range 0–3, based on the sum of metabolic risk traits that could be identified in claims data sources (Supplementary Table 2).
‖Indicator for any occurrence of a preventive service procedure or diagnosis code in the 6 months prior to the index date (Supplementary Table 1).
¶Percentile categories for the sum of costs accruing between 6 and 18 months before the index date.
#Year of index date for YDPP users and PS-matched nonusers; for nonmatched nonusers, represents year the first employee of the same employer was exposed to YDPP.
After PS matching, there were no remaining imbalances between the two comparison groups in any of these baseline characteristics (Table 1). Almost three-quarters of eligible YDPP users were women, and approximately three-quarters were ≥50 years of age. More than 40% had an encounter with a prediabetes diagnosis before their index date, suggesting some recognition of their high-risk status by a health care provider. Slightly more than half received a blood pressure medication and just more than one-quarter received an antilipidemic class medication during the 6–18 months before the index date. Despite these metabolic risk factors, less than one-quarter had evidence of recent use of another health promotion/disease prevention service in the 6 months before the index date.
Temporal Comparisons in Health Care Costs
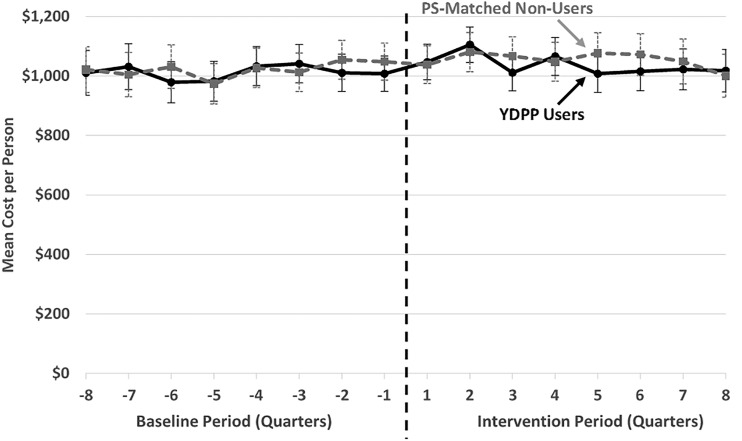
Figure 2 displays quarterly trends in mean per-person total health care expenditures of YDPP user and PS-matched nonuser groups before and after index dates. Visually, the trends between matched groups are generally comparable before the index date.
Figure 2.
Raw mean quarterly total health care expenditure trends, per commercially insured YDPP user and PS-matched nonuser. *To ensure at least 2 years of potential follow-up for all participants in the evaluation sample, we restricted the cost analysis to health plan enrollees in both groups with an index date on or before 1 June 2013; error bars reflect 95% CI for each quarterly mean.
Difference-in-difference estimates of mean quarterly health care expenditures across each of the first 2 years of participation are displayed in Table 2. During year 1, the estimated mean total health care expenditures for YDPP users, inclusive of payments for delivery of the YDPP, were not statistically significantly different (P = 0.731) from those for PS-matched nonusers (mean for YDPP users was $10 lower [95% CI $68 lower to $48 higher]). Over the first 2 years combined, mean total health care expenditures for YDPP users were estimated to be $0 lower (95% CI $56 lower to $56 higher) than for PS-matched nonusers. In exploratory analyses, there were no statistically significant differences during either follow-up year in any specific expenditure subcategory, including inpatient and emergency department, total pharmacy, or ambulatory expenditures (estimates not shown).
Table 2.
Difference-in-difference estimates of mean per-patient quarterly health care expenditures ($), comparing YDPP users and PS-matched nonusers
| Intervention period | Change in quarterly total costs for YDPP users* | Change in quarterly total costs for PS-matched nonusers* | Difference in net quarterly total costs between YDPP users and PS-matched nonusers† | P |
|---|---|---|---|---|
| Quarter 1 | 109.2 (58.8, 159.2) | 82.0 (26.7, 137.4) | 27.5 (−47.2, 102.3) | 0.47 |
| Quarter 2 | 7.5 (−45.2, 60.1) | 57.3 (0.5, 114.2) | −49.3 (−126.7, 28.2) | 0.21 |
| Quarter 3 | 45.2 (−10.2, 100.7) | 12.1 (−46.4, 70.7) | 33.4 (−47.3, 114.0) | 0.42 |
| Quarter 4 | −10.0 (−66.6, 46.6) | 45.8 (−21.0, 112.7) | −55.5 (−143.1, 32.1) | 0.21 |
| Quarter 5 | −14.7 (−72.9, 43.6) | 27.6 (−38.9, 94.1) | −42.0 (−130.4, 46.4) | 0.35 |
| Quarter 6 | −7.0 (−69.7, 55.7) | 15.7 (−56.0, 87.5) | −22.6 (−117.8, 72.6) | 0.64 |
| Quarter 7 | −32.1 (−99.3, 35.1) | −42.9 (−113.4, 27.6) | 11.4 (−85.9, 108.6) | 0.82 |
| Quarter 8 | 46.8 (−29.4, 122.8) | −85.5 (−158.6, −12.4) | 132.5 (27.1, 238.0) | 0.01 |
| Year 1 overall | 39.1 (0.4, 77.8) | 49.7 (6.3, 93.2) | −10.2 (−68.4, 47.9) | 0.73 |
| Year 2 overall | −4.0 (−52.0, 44.0) | −15.9 (−69.5, 37.6) | 12.3 (−59.4, 84.1) | 0.74 |
| Year 1 and 2 overall | 20.4 (−16.7, 57.6) | 21.0 (−21.0, 63.1) | −0.2 (−56.2, 55.9) | 0.99 |
Data are mean (95% CI).
*Adjusted estimate from difference-in-difference models of the difference between each quarterly mean and the quarterly mean prior to the index date. Yearly differences in means are estimated from separate models. Negative numbers indicate that mean per person expenditures are lower in the follow-up period than at baseline.
†Adjusted estimate of difference in difference between the YDPP and PS-matched comparison group; negative numbers indicate that mean per-person expenditures are lower for YDPP users than for the PS-matched nonusers.
Comparisons of Cost Trends and Differences Across Prespecified Patient Subgroups
Quarterly trends in total health care expenditures for the three prespecified subgroups of YDPP users, as well as for those with and without a prior ambulatory visit for a prediabetes diagnosis, are depicted graphically in Supplementary Fig. 2A–D, including analyses by age category (Supplementary Fig. 2A), count of metabolic risk factors (Supplementary Fig. 2B), numbers of comorbidities (Supplementary Fig. 2C), and prediabetes encounter status (Supplementary Fig. 2D). YDPP users with higher age or with higher comorbidity levels generated higher mean health care expenditures both before and after the index date than those who were younger or who had lower levels of metabolic risk factors or comorbidity. However, difference-in-difference estimates comparing mean costs between YDPP users and PS-matched nonusers did not achieve statistical significance within any of the higher risk subgroups.
Conclusions
Our evaluation demonstrates considerable growth in YDPP delivery from 2010 to 2015, reaching a scale of >1,000 instructors serving almost 20,000 individuals in ∼1,000 locations throughout the U.S. This growth also appears to be sustained; as of June 2018, YUSA reported having offered the YDPP to >61,000 total people nationally, using >1,100 locations in 40 states (29). Despite this growth in capacity, health payer efforts focused primarily on worksite prediabetes screening, and referral of high-risk employees to YDPP fell short of expectations as a strategy to enroll high numbers of participants. Though 40% of employees who were identified with prediabetes attended at least one YDPP class, and 28% overall attended nine or more YDPP classes, the requirement for completion of a blood test to identify prediabetes outside of health care settings proved to be a large barrier to YDPP program enrollment. Only ∼6% of all employees enrolled in the participating health plan completed prediabetes testing, which resulted in identification of only ∼10% of the expected target population.
Despite the relatively low reach of the engagement strategy, high-risk adult health plan enrollees who did participate in YDPP achieved modest but meaningful weight loss levels (7.5 lb), with ∼29% reaching the weight loss goal of 5% or more. YDPP users with higher attendance had higher levels of mean weight loss. Despite the incremental cost of health payer reimbursement for use of YDPP by eligible health plan enrollees, net health care expenditures for YDPP users were not statistically significantly different from PS-matched nonusers over the first 2 years of YDPP exposure.
Ongoing maintenance of the use of a performance-based payment arrangement between YUSA and commercial payers provides encouraging evidence for the sustainability of health plan coverage of YDPP (17). With federal policies now requiring full health plan coverage of intensive lifestyle interventions for enrollees with cardiovascular risk conditions such as prediabetes (30), the CDC reports that >60 health payers currently provide coverage for National DPP programs offered by YMCAs or other recognized program vendors (31). Similarly, in April 2018 the Centers for Medicare and Medicaid Services began providing payment to National DPP providers for access to analogous interventions for high-risk Medicare fee-for-service beneficiaries (32).
Although this study was not designed to provide a detailed analysis of YDPP implementation costs, we were able to estimate the mean per-person health payer reimbursement made to the YMCA using administrative records for YDPP attendance and weight loss levels. The estimated mean YMCA reimbursement of ∼$212 per person is lower than the previously reported costs of ∼$424 for community-based delivery of DPP-like interventions (16). In the context of the original DPP clinical trial intervention, which demonstrated 15 lb of mean weight loss in year 1 for ∼$1,399 in program delivery costs ($94/lb) (5,11), the YDPP achieved approximately half the mean weight loss for only ∼15% of the delivery expense ($28/lb). Importantly, this reimbursement amount does not include any additional costs that may have been incurred by the YMCA, health plans, or employers for other activities beyond program delivery. For example, operational costs for employer-based prediabetes screening or for administering YDPP referrals were not included in the analysis.
Our study has some limitations. First, we only had access to administrative data sources, which do not capture outcomes such as quality of life or changes in A1C, blood pressure, or other intermediate clinical outcomes including diabetes onset, which may signal the potential for cost savings over longer time horizons. In the DPP, every 1 kg of additional weight loss was associated with a 16% further reduction in risk of developing type 2 diabetes over ∼3 years (22), and modest weight loss improved health-related quality of life and reduced the need for medications used to address cardiometabolic risk factors (9,10). Second, we were only able to analyze direct medical costs. Patients may also incur or avoid nonmedical costs (e.g., pay for transportation to YDPP visits) or indirect costs (e.g., have lower lost wages from missed workdays) after being offered a behavioral change program (11). Third, as is the case in any propensity match, it is possible that there were remaining differences between YDPP users and PS-matched nonusers at baseline. For example, all YDPP users completed employer-based blood testing that confirmed the presence of prediabetes, but a similar data source for blood test results was not available for nonusers. The presence of a prior health care visit for prediabetes was included as a covariate in the PS match, but it is possible that some YDPP users without a prior health care visit for prediabetes could have been PS matched to nonusers with an unknown prediabetes status. Though sensitivity analyses did not show differences in cost outcomes for YDPP users with and without a prior health care visit for prediabetes, it is still possible that there were other remaining differences between YDPP users and PS-matched nonusers that could have impacted the cost comparison. Fourth, our study population was limited to individuals who enrolled in YDPP before June 2013, which focuses on a subset of earlier-adopting employers from a time when YDPP enrollment and delivery processes may not yet have been optimized; thus, these findings may not generalize to more recent YDPP users, to older or younger individuals, to those without commercial health insurance, or to those who are identified as having prediabetes through other channels such as routine health care–based screening and referral. Notably, another recent study found that Medicare enrollees participating in YDPP did have significantly lower total health care expenditures in the 1st year after enrolling in the program (33), which may suggest that older adults, who begin with higher baseline health care expenditures, may experience earlier and more pronounced health benefits after YDPP enrollment.
Conclusion
A strategy involving worksite A1C testing to identify prediabetes, followed by referral for free of charge access to the YDPP, yields relatively inefficient success identifying high-risk individuals, albeit modest YDPP enrollment by those who are identified, and relatively high attendance with meaningful weight loss by those who do enroll. Over a 2-year time horizon, this approach to intervention delivery has a relatively low marginal intervention delivery cost for health payers and is likely to be cost neutral with respect to net health care expenditures. One large remaining challenge is maximizing participant engagement in the program without dramatically increasing net costs. With U.S. Preventive Services Task Force recommendations that should encourage health care providers to identify prediabetes and offer DPP referrals more routinely for all high-risk patients (34,35), cost-effective approaches for maximizing participant engagement and attendance should be a priority for future research.
Supplementary Material
Article Information
Funding. This research was supported by CDC grant U58DP002718 as part of the Natural Experiments for Translation in Diabetes (NEXT-D) Study.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.T.A. contributed to the study design, performed data analysis, and drafted the manuscript. R.K. contributed to the study design, performed data analysis, and reviewed and edited the manuscript. A.J.C. contributed to the study design, performed data analysis, and reviewed/edited the manuscript. D.T.L. contributed to the study design and reviewed/edited the manuscript. A.M.H. contributed to the study design and reviewed/edited the manuscript. M.M. contributed to the study design and reviewed/edited the manuscript. C.S. contributed to the study design and reviewed/edited the manuscript. R.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-2071/-/DC1.
See accompanying article, p. 1612.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Statistical Report, 2017: Estimates of Diabetes and Its Burden in the United States [Internet], 2017. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 5 August 2017
- 2.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890 [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Steffes MW, Gregg E, Brancati FL, Coresh J. Performance of A1C for the classification and prediction of diabetes. Diabetes Care 2011;34:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayan KM, Williamson DF. Prevention of type 2 diabetes: risk status, clinic, and community. J Gen Intern Med 2010;25:154–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackermann RT, Edelstein SL, Narayan KM, et al.; Diabetes Prevention Program Research Group . Changes in health state utilities with changes in body mass in the Diabetes Prevention Program. Obesity (Silver Spring) 2009;17:2176–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackermann RT, Marrero DG, Hicks KA, et al. . An evaluation of cost sharing to finance a diet and physical activity intervention to prevent diabetes. Diabetes Care 2006;29:1237–1241 [DOI] [PubMed] [Google Scholar]
- 8.Diabetes Prevention Program Research Group The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012;35:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman WH, Hoerger TJ, Brandle M, et al.; Diabetes Prevention Program Research Group . The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratner R, Goldberg R, Haffner S, et al.; Diabetes Prevention Program Research Group . Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care 2005;28:888–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diabetes Prevention Program Research Group Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care 2003;26:2518–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garfield SA, Malozowski S, Chin MH, et al.; Diabetes Mellitus Interagency Coordinating Committee (DIMCC) Translation Conference Working Group . Considerations for diabetes translational research in real-world settings. Diabetes Care 2003;26:2670–2674 [DOI] [PubMed] [Google Scholar]
- 13.Ackermann RT, Finch EA, Brizendine E, Zhou H, Marrero DG. Translating the Diabetes Prevention Program into the community. The DEPLOY Pilot Study. Am J Prev Med 2008;35:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75 [DOI] [PubMed] [Google Scholar]
- 15.Balk EM, Earley A, Raman G, Avendano EA, Pittas AG, Remington PL. Combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med 2015;163:437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Qu S, Zhang P, et al. . Economic evaluation of combined diet and physical activity promotion programs to prevent type 2 diabetes among persons at increased risk: a systematic review for the Community Preventive Services Task Force. Ann Intern Med 2015;163:452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vojta D, Koehler TB, Longjohn M, Lever JA, Caputo NF. A coordinated national model for diabetes prevention: linking health systems to an evidence-based community program. Am J Prev Med 2013;44(Suppl. 4):S301–S306 [DOI] [PubMed] [Google Scholar]
- 18.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–2281 [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics 1996;52:249–264 [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 21.Ackermann RT, Marrero DG. Adapting the Diabetes Prevention Program lifestyle intervention for delivery in the community: the YMCA model. Diabetes Edu 2007;33:69, 74–75, 77–78. [DOI] [PubMed] [Google Scholar]
- 22.Hamman RF, Wing RR, Edelstein SL, et al. . Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diehr P, Yanez D, Ash A, Hornbrook M, Lin DY. Methods for analyzing health care utilization and costs. Annu Rev Public Health 1999;20:125–144 [DOI] [PubMed] [Google Scholar]
- 24.Thomas JW, Ward K. Economic profiling of physician specialists: use of outlier treatment and episode attribution rules. Inquiry 2006;43:271–282 [DOI] [PubMed] [Google Scholar]
- 25.United States Census Bureau. Census regions and divisions [Internet], 2016. Available from https://www.census.gov/programs-surveys/economic-census/geographies/levels/2017-levels.html. Accessed 22 May 2017
- 26.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health 2002;23:151–169 [DOI] [PubMed] [Google Scholar]
- 28.Aponte J. Prevalence of normoglycemic, prediabetic and diabetic A1c levels. World J Diabetes 2013;4:349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Y. YMCA’s Diabetes Prevention Program [Internet], 2018. Available from http://www.ymca.net/diabetes-prevention. Accessed 9 August 2018
- 30.Konchak JN, Moran MR, O’Brien MJ, Kandula NR, Ackermann RT. The state of diabetes prevention policy in the USA following the Affordable Care Act. Curr Diab Rep 2016;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. National Diabetes Prevention Program [Internet], 2016. Available from http://www.cdc.gov/diabetes/prevention/index.html. Accessed 3 June 2017
- 32.Centers for Medicare & Medicaid Services. Medicare Diabetes Prevention Program (MDPP) expanded model [Internet], 2016. Available from https://innovation.cms.gov/initiatives/medicare-diabetes-prevention-program/. Accessed 30 September 2017
- 33.Alva ML, Hoerger TJ, Jeyaraman R, Amico P, Rojas-Smith L. Impact of The YMCA of the USA Diabetes Prevention Program on Medicare spending and utilization. Health Aff (Millwood) 2017;36:417–424 [DOI] [PubMed] [Google Scholar]
- 34.LeFevre ML; U.S. Preventive Services Task Force . Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2014;161:587–593 [DOI] [PubMed] [Google Scholar]
- 35.Siu AL; U.S. Preventive Services Task Force . Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2015;163:861–868 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.