Abstract
OBJECTIVE
This study was conducted to update national estimates of the economic burden of undiagnosed diabetes, prediabetes, and gestational diabetes mellitus (GDM) in the United States for year 2017 and provide state-level estimates. Combined with published estimates for diagnosed diabetes, these updated statistics provide a detailed picture of the economic costs associated with elevated blood glucose levels.
RESEARCH DESIGN AND METHODS
This study estimated medical expenditures exceeding levels occurring in the absence of diabetes or prediabetes and the indirect economic burden associated with reduced labor force participation and productivity. Data sources analyzed included Optum medical claims for ∼5.8 million commercially insured patients continuously enrolled from 2013 to 2015, Medicare Standard Analytical Files containing medical claims for ∼2.8 million Medicare patients in 2014, and the 2014 Nationwide Inpatient Sample containing ∼7.1 million discharge records. Other data sources were the U.S. Census Bureau, Centers for Disease Control and Prevention, and Centers for Medicare & Medicaid Services.
RESULTS
The economic burden associated with diagnosed diabetes (all ages), undiagnosed diabetes and prediabetes (adults), and GDM (mothers and newborns) reached nearly $404 billion in 2017, consisting of $327.2 billion for diagnosed diabetes, $31.7 billion for undiagnosed diabetes, $43.4 billion for prediabetes, and nearly $1.6 billion for GDM. Combined, this amounted to an economic burden of $1,240 for each American in 2017. Annual burden per case averaged $13,240 for diagnosed diabetes, $5,800 for GDM, $4,250 for undiagnosed diabetes, and $500 for prediabetes.
CONCLUSIONS
Updated statistics underscore the importance of reducing the burden of prediabetes and diabetes through better detection, prevention, and treatment.
Introduction
Diabetes is a metabolic condition characterized by elevated blood glucose due to insufficient insulin production (type 2) or inability to produce insulin (type 1), and/or peripheral tissue resistance to the action of insulin. Diabetes is linked to higher medical costs, reduced labor force participation and productivity, and early mortality due to complications caused by insufficient blood glucose control (1–3). Individuals with prediabetes—higher-than-normal glucose levels that do not meet the criteria for diabetes—have higher rates of diabetes-associated complications compared with populations with normal glucose levels (3,4).
The estimated economic burden of diabetes and prediabetes exceeded $322 billion in 2012 (3). Changes have occurred since 2012 in the prevalence and characteristics of the population with diabetes and prediabetes, the health care system and medical costs, and labor force participation and earnings. The Centers for Disease Control and Prevention (CDC) reports that between 2012 and 2015, the number of people with diagnosed diabetes increased from 21.0 to 23.1 million, the number with undiagnosed diabetes fell from 8.1 to 7.2 million, and the number with prediabetes fell from 86.0 to 84.1 million (5,6). In 2012, an estimated 222,000 child births were to mothers with gestational diabetes mellitus (GDM) (3). GDM prevalence increases with mother’s age, and CDC reports that the mean age of new mothers is rising (7).
The American Diabetes Association (ADA) estimates the economic burden associated with diagnosed diabetes exceeded $327 billion in 2017, reflecting a 25% real increase from 2012 after adjusting for inflation (2). The main drivers of this increase were increased prevalence of diabetes among older Americans and rising cost per case. This study updates national and state economic burden estimates for undiagnosed diabetes, prediabetes, and GDM to provide a more comprehensive estimate of the total economic burden of diabetes and prediabetes in 2017. Such information informs national and state policies and resource allocation decisions to improve diabetes detection, prevention, and treatment.
Research Design and Methods
Data sources and methods replicate previous studies in 2007 and 2012 (3,4,8–10). Many data sources for this study were used to estimate diagnosed diabetes prevalence and associated costs for 2017 (2). All costs are in 2017 dollars, with the hospital services, physician services, and prescription drug components of the medical Consumer Price Index used to adjust medical costs and the total Consumer Price Index used to adjust indirect costs.
Estimating Disease Prevalence
Underlying the analysis is a constructed population database containing a representative sample of each state’s population in 2017, with each person categorized by disease status: diagnosed diabetes, undiagnosed diabetes, prediabetes, or normal glucose levels. The database starts with the 2016 American Community Survey (ACS) (n = 3,156,487), which includes age, sex, race/ethnicity, type of medical insurance, family income, and whether the person resides in a group setting. We projected the population to 2017 using states’ population growth projections by demographic.
We used random sampling with replacement to statistically match each person in ACS with other data sources containing information on diagnosed diabetes status and risk factors correlated with undiagnosed diabetes and prediabetes (i.e., overweight or obesity, current tobacco smoker, hypertension, and presence or history of other chronic diseases). We matched each community-based ACS individual to a person in the combined 2015–2016 files of the Behavioral Risk Factor Surveillance System (n = 986,552) from the same state, age-group, sex, race/ethnicity, family income level, and medical insurance type. For ACS individuals living in a group setting, we matched people residing in a residential care facility or nursing home to a person of similar state, age-group, sex, and race/ethnicity from the 2013 Medicare Beneficiary Survey (n = 13,924) or the 2015 Nursing Home Minimum Data Set (635,060 adults and 1,474 children), respectively. Diagnosed diabetes status in the Behavioral Risk Factor Surveillance System is based on the question “Have you EVER been told by a doctor or health professional that you have diabetes or sugar diabetes?” Diabetes status in the Medicare Beneficiary Survey and Nursing Home Minimum Data Set is based on clinical diagnosis.
For adults without diagnosed diabetes, we estimated their probability of undiagnosed diabetes or prediabetes using a prediction equation estimated from the National Health and Nutrition Examination Survey (NHANES). NHANES is nationally representative of the noninstitutionalized population, with the 2007–2014 files containing 22,655 adults with laboratory results for detecting diabetes or prediabetes status and excluding 165 pregnant women and 2,683 adults previously diagnosed with diabetes or using antidiabetic agents. We followed CDC’s approach to calculate national prevalence by identifying adults with undiagnosed diabetes if HbA1c was ≥6.5% or fasting plasma glucose was ≥126 mg/dL, and identified adults with prediabetes if A1C was ≥5.7 to 6.4% or fasting plasma glucose was ≥100 to 125 mg/dL (5).
The predictive model used multinomial logistic regression with the dependent variable having three values: normal glucose levels, prediabetes, and undiagnosed diabetes (11,12). Explanatory variables reflect risk factors for diabetes common to both NHANES and the constructed population database (13). Applying the predictive model to the constructed population database produced estimates of undiagnosed diabetes and prediabetes prevalence by state, insurance type, and demographic.
GDM estimates are based on the 2014 National Inpatient Sample (NIS) (n = 108,660 births with GDM [ICD-9 code 648.8X] of 1,659,115 total births), excluding women with diagnosed type 1 or type 2 diabetes. We estimated the percentage of births where the mother has GDM by mother’s age for each of the nine U.S. Census regions (state identifier is unavailable in NIS) and multiplied these percentages by 2016 published statistics on total live births by state and mother’s age. Birth estimates for 2016 were scaled to 2017 based on national trends by mother’s age-group.
Estimating Health Care Use and Costs
The approach estimates health care use above levels expected to occur in the absence of diabetes or prediabetes. Utilization patterns of commercially insured patients came from the Optum deidentified Normative Health Information (dNHI) database (n = 5,831,940), analyzing medical claims for patients continuously enrolled from January 2013 through December 2015 and including 81,460 adults with a diabetes diagnosis in 2015 but no history of diabetes in 2014 or 2013. Utilization patterns of Medicare beneficiaries came from the 2014 Medicare Standard Analytical Files (n = 2,836,269). We estimated total national health care use by demographic group and insurance type, which we projected to 2017 based on population growth between the data source year and 2017. National estimates came from the 2014 NIS (n = 7,071,762 discharges) for hospital inpatient care, the 2013–2015 National Ambulatory Medical Care Survey (n = 128,915) for office-based care, and the National Hospital Ambulatory Medical Care Survey for outpatient/clinic care (years 2009–2011, n = 100,502) and emergency care (years 2012–2014, n = 78,074). Estimates of expenditures for visits, prescriptions, medical supplies, and equipment came from the 2011–2015 Medical Expenditure Panel Survey (MEPS) (n = 181,529), with hospital inpatient costs estimated from NIS using hospital-specific cost-to-charge ratios. Cost data include a blend of commercially insured, Medicare, Medicaid, and self-pay patients.
Identifying Patients by Diabetes Category to Analyze Health Care Use
We identified patients with diagnosed diabetes in the dNHI and Medicare Standard Analytical Files using diagnosis codes for diabetes, pharmacy claims, and laboratory results. We compared their utilization to all other adults.
For the undiagnosed diabetes analysis, we identified a proxy population in the dNHI on the verge of diagnosis. This proxy had no indication of diabetes in 2013 and 2014, but had indications of diabetes in 2015. We compared their 2013 and 2014 utilization to patients without diabetes.
The prediabetes population consists of patients in dNHI with claims or laboratory results indicating prediabetes. The control group was patients with no laboratory results or a laboratory result indicating normal glucose levels. We used data from 1 year to categorize patients (2013 or 2014) and used their utilization in the following year (2014 or 2015) to analyze health care use.
Women in dNHI who gave birth in 2014 were identified with GDM using the diagnosis code within 9 months before delivery. The sample (n = 21,900) consists of mother-child pairs where the mother was continuously enrolled starting 271 days before delivery and extending 365 days after delivery. The control group was mothers with no indication of GDM. We compared health care utilization of mothers from 9 months preceding through 12 months after childbirth. We compared utilization for newborns for 12 months after birth. We excluded women with GDM from the undiagnosed diabetes and prediabetes analyses and excluded women with diagnosed diabetes and prediabetes from the GDM analysis.
Estimating Excess Use of Health Care Services and Medical Costs
We estimated rate ratios for undiagnosed diabetes, prediabetes, and GDM using Poisson regression with dNHI data where the dependent variable was annual health care use (ambulatory visits, emergency visits, and inpatient days by comorbidity category). We used the primary diagnosis code to categorize medical claims into comorbidity groupings: neurological symptoms, peripheral vascular disease, cardiovascular disease, renal complications, endocrine complications, ophthalmic complications, hypertension, and other complications. An “all other” category included primary care and medical conditions not considered complications of diabetes. For GDM, we included excess costs associated with 1) mothers’ cesarean delivery, polyhydramnios, urinary tract infection, amniotic cavity infection, preeclampsia and eclampsia, other hypertension issues complicating pregnancy, and other pregnancy-related events; and 2) infants’ intrauterine hypoxia and birth asphyxia, macrosomia, endocrine and metabolic disturbances specific to the fetus and newborn, birth trauma due to long gestation and high birth weight, fetus or newborn affected by other complications of labor and delivery, respiratory distress syndrome, jaundice, congenital anomalies, and all other neonatal events.
The rate ratios reflect annual service use for people with diabetes or prediabetes versus their control group. The regressions controlled for age, sex, medical insurance type, U.S. Census region, year of utilization, and costly health conditions (HIV/AIDS, organ transplantation, cancer, and pregnancy). Regressions for prediabetes also controlled for peripheral vascular disease, cardiovascular disease, hypertension, endocrine complications, and ophthalmic complications. Estimates of diabetes and prediabetes prevalence were combined with rate ratios to create etiological fractions indicating the proportion of health care use exceeding levels expected in the absence of diabetes or prediabetes (14). Etiological fraction equations by age-group, care delivery setting (hospital inpatient, emergency departments, ambulatory visits), and medical condition category are published elsewhere (8).
Rate ratios based on medical claims potentially are overstated by failing to control for confounders correlated both with diabetes and health care use. We estimated two multivariate Poisson regressions using 2011–2015 MEPS for the 10 medical conditions that are the largest contributors to the overall cost of diabetes, comprising general medical conditions, other chronic ischemic heart disease, myocardial infarction, heart failure, hypertension, conduction disorders and cardiac dysrhythmias, cellulitis, occlusion of cerebral arteries, end-stage renal disease, and renal failure and its sequelae. A naïve model controlled only for age and sex when estimating the link between diabetes status and annual use of health care services. A full model controlled for age, sex, education level, family income, marital status, medical insurance status, and race/ethnicity as covariates (2). We created scalars by taking the ratio of the diabetes effect from the full model divided by the diabetes effect from the naïve model to scale down the diagnosed and undiagnosed diabetes rate ratios.
Estimating Indirect Burden
Indirect burden estimates for diagnosed diabetes come from a recent ADA analysis (2). The approach for modeling undiagnosed diabetes burden mirrors previous studies (3,8). The modeled components of indirect costs are 1) absenteeism—missed work days due to poor health (15,16), 2) presenteeism—reduced productivity while at work (17–19), 3) inability to work—conservatively modeled as unemployed due to disability, and 4) reduced productivity for adults younger than age 65 not in the workforce. Premature mortality is included for diagnosed diabetes only.
For modeling diagnosed diabetes indirect cost, we analyzed the 2014–2016 National Health Interview Survey (NHIS) (n = 101,307) to estimate labor force participation rates (logistic regression) and missed work days (negative binomial regression) for adults with diabetes compared with adults without diabetes controlling for demographics (age, sex, race/ethnicity), body weight status (underweight or normal, overweight, obese, and unknown), and whether the person had diagnosed hypertension. We modeled presenteeism by using the same estimate from our previous study that diagnosed diabetes reduces productivity at work by 6.6%, which is an estimate at the lower end of the 1.8–38% range reported in the literature (15,17–19).
Undiagnosed diabetes status, by definition, is unavailable in NHIS and in employer surveys used to calculate reduced productivity. Analysis of medical claims data finds that our proxy population for undiagnosed diabetes uses fewer health care services, on average, compared with a similar population with diabetes. We therefore assume that adults with undiagnosed diabetes are healthier, on average, then adults with diagnosed diabetes and thus have smaller productivity loss. To estimate indirect costs associated with undiagnosed diabetes, we first calculated what lost productivity would be for the population with undiagnosed diabetes by applying average productivity loss from the population with diagnosed diabetes by demographic. We then scaled down these estimates by multiplying the ratio of hospital inpatient days for people with undiagnosed diabetes to people with diagnosed diabetes by demographic. This approach suggests that adults with undiagnosed diabetes have indirect costs that are ∼63%, 56%, and 65% lower, on average, compared with adults with diagnosed diabetes aged <45, 45–64, and ≥65, respectively. This approach produced more conservative estimates of indirect costs than using other proxies for the health of people with undiagnosed diabetes relative to people with diagnosed diabetes, such as the ratio of ambulatory visits.
The indirect burden estimates for diagnosed diabetes include lost productivity associated with the estimated 277,000 premature deaths annually associated with diabetes and attributed sequelae (2). Productivity loss was not calculated for prediabetes and GDM due to data limitations. Because prediabetes is largely asymptomatic, average productivity loss likely is small.
Results
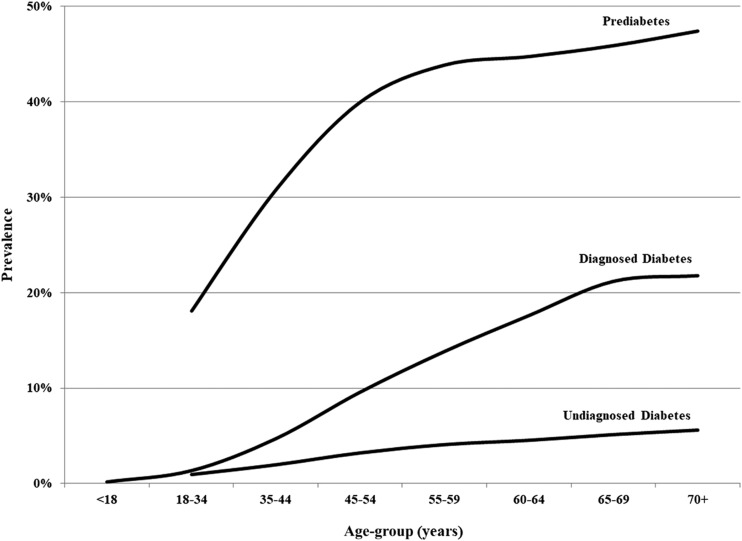
In 2017, an estimated 24.7 million people had diagnosed diabetes, 7.5 million had undiagnosed diabetes, and 85.9 million had prediabetes in the U.S. Among adults, 9.8% had diagnosed diabetes, 3% had undiagnosed diabetes, and 34% had prediabetes. Prevalence rates rise with age, and among the population aged ≥70, 21.8% had diagnosed diabetes, 5.6% had undiagnosed diabetes, and 47.4% had prediabetes (Fig. 1).
Figure 1.
Prevalence of diabetes and prediabetes in the U.S., 2017.
An estimated 268,900 infants were born to mothers with GDM in 2017. Nationally, 6.9% of births were to mothers with GDM, although incidence rises with mothers’ age, ranging from 1.9% for new mothers aged <20 to 14.3% for new mothers aged >39.
People with diagnosed diabetes had more ambulatory visits, emergency visits, and inpatient days compared with the population without diagnosed diabetes. Adults with undiagnosed diabetes had higher utilization for many of these condition categories compared with adults without diabetes, but the differences were much smaller. Adults with prediabetes had slightly higher rates of ambulatory visits compared with adults with no indication of abnormal glucose levels. For diagnosed diabetes, many of the rate ratios indicating higher utilization of services were in the 3–6 range (indicating 3- to 6-times the level of services used by people without diagnosed diabetes). In contrast, many of the rate ratios for undiagnosed diabetes were in the 1.5–3 range, and many of the rate ratios for prediabetes were in the 1.1–2.0 range.
Each case of GDM was associated with ∼$5,800 in higher medical expenditures. GDM only slightly raised medical costs for newborns (average $40/newborn) but substantially raised the mother’s costs related to the pregnancy and birth (average $5,760/mother). The breakdown for higher costs includes inpatient care ($3,140), prescription medicine ($1,200), ambulatory visits ($1,140), and emergency care ($280).
The national annual cost associated with elevated blood glucose in 2017 reached nearly $404 billion, including $327.2 billion for diagnosed diabetes, $31.7 billion for undiagnosed diabetes, $43.4 billion for prediabetes, and $1.56 billion for GDM (Table 1). On average, prediabetes costs $500 annually per person (medical costs only), undiagnosed diabetes costs $4,250, and diagnosed diabetes costs $13,240. Of $4 in total economic burden associated with diabetes and prediabetes, $3 was associated with medical costs and $1 was associated with nonmedical costs.
Table 1.
U.S. economic annual costs associated with diabetes and prediabetes, by age-group, 2017
| Cost by age-group | Diagnosed* | Undiagnosed | Prediabetes | GDM | Total |
|---|---|---|---|---|---|
| Total national cost (millions of dollars) | 327,198 | 31,726 | 43,391 | 1,558 | 403,874 |
| Medical costs | 237,269 | 19,841 | 43,391 | 1,558 | 302,059 |
| Nonmedical costs | 89,929 | 11,886 | 101,814 | ||
| Average cost per case ($) | |||||
| Total | 13,240 | 4,250 | 500 | 5,800 | |
| Medical costs (by age-group) | 9,600 | 2,660 | 500 | 5,800 | |
| <45 years (<26 for GDM)Ϯ | 5,980 | 890 | 330 | 8,840 | |
| 45–64 years (26–35 for GDM) | 6,870 | 2,030 | 470 | 5,610 | |
| ≥65 years (≥36 for GDM) | 13,240 | 4,320 | 820 | 5,050 | |
| Nonmedical costs (by age-group) | 3,640 | 1,590 | |||
| 18–44 yearsϮ | 5,580 | 2,060 | |||
| 45–64 years | 5,320 | 2,320 | |||
| ≥65 years | 1,480 | 520 |
*Source: American Diabetes Association (2).
ϮNonmedical costs not calculated for the age <18 population.
Diagnosed diabetes and GDM prevalence varies geographically (Table 2). Diagnosed diabetes rates can differ slightly from CDC-reported rates because these numbers cover the entire population and not just the community-based population. Diagnosed diabetes prevalence rates are highest in West Virginia and many Southern states (Alabama, Florida, Louisiana, Kentucky, and Mississippi) and are lowest in Mountain and Western states (Alaska, Colorado, and Utah).
Table 2.
State-level prevalence of diabetes and prediabetes, 2017, in thousands (prevalence rate %)
| State | Diagnosed* | Undiagnosed | Prediabetes | GDM |
|---|---|---|---|---|
| Alabama | 483 (12.7) | 119 (3.1) | 1,316 (34.6) | 3.5 (6.0) |
| Alaska | 34 (6.4) | 15 (2.8) | 182 (33.8) | 0.9 (8.0) |
| Arizona | 555 (10.1) | 164 (3.0) | 1,893 (34.5) | 4.6 (5.5) |
| Arkansas | 262 (11.4) | 70 (3.0) | 796 (34.6) | 2.1 (5.5) |
| California | 2,764 (9.0) | 884 (2.9) | 10,320 (33.4) | 44.2 (9.1) |
| Colorado | 270 (6.2) | 117 (2.7) | 1,444 (32.9) | 3.9 (5.9) |
| Connecticut | 236 (8.3) | 79 (2.8) | 944 (33.1) | 2.3 (6.4) |
| Delaware | 72 (9.5) | 24 (3.1) | 268 (35.4) | 0.7 (6.7) |
| District of Columbia | 42 (7.5) | 14 (2.5) | 164 (29.0) | 0.7 (7.3) |
| Florida | 1,944 (11.6) | 546 (3.3) | 5,973 (35.7) | 14.9 (6.7) |
| Georgia | 853 (10.7) | 234 (2.9) | 2,674 (33.7) | 8.3 (6.4) |
| Hawaii | 99 (9.0) | 39 (3.5) | 410 (37.1) | 1.6 (8.8) |
| Idaho | 104 (8.2) | 36 (2.9) | 427 (33.8) | 1.2 (5.4) |
| Illinois | 902 (9.0) | 296 (3.0) | 3,393 (34.0) | 11.0 (7.2) |
| Indiana | 512 (10.0) | 146 (2.9) | 1,707 (33.5) | 5.3 (6.5) |
| Iowa | 199 (8.3) | 70 (2.9) | 820 (34.1) | 2.6 (6.8) |
| Kansas | 194 (8.8) | 66 (3.0) | 782 (35.3) | 2.5 (6.6) |
| Kentucky | 411 (11.9) | 101 (2.9) | 1,168 (33.8) | 3.3 (6.0) |
| Louisiana | 441 (12.2) | 113 (3.1) | 1,243 (34.4) | 3.6 (5.7) |
| Maine | 89 (8.4) | 32 (3.0) | 373 (35.1) | 0.7 (5.8) |
| Maryland | 496 (10.5) | 139 (2.9) | 1,600 (33.7) | 5.2 (7.1) |
| Massachusetts | 449 (8.2) | 144 (2.6) | 1,743 (31.8) | 4.7 (6.6) |
| Michigan | 762 (9.8) | 239 (3.1) | 2,701 (34.7) | 7.6 (6.8) |
| Minnesota | 339 (7.9) | 118 (2.8) | 1,441 (33.7) | 5.1 (7.3) |
| Mississippi | 278 (12.2) | 75 (3.3) | 814 (35.6) | 2.2 (5.8) |
| Missouri | 535 (11.3) | 139 (2.9) | 1,594 (33.6) | 4.8 (6.5) |
| Montana | 64 (7.9) | 24 (3.0) | 282 (34.7) | 0.7 (5.3) |
| Nebraska | 112 (7.8) | 44 (3.0) | 522 (36.2) | 1.8 (6.9) |
| Nevada | 205 (8.8) | 70 (3.0) | 816 (35.1) | 2.0 (5.6) |
| New Hampshire | 86 (8.0) | 29 (2.7) | 351 (32.9) | 0.8 (6.2) |
| New Jersey | 616 (8.7) | 207 (2.9) | 2,395 (34.0) | 7.3 (7.1) |
| New Mexico | 174 (10.7) | 53 (3.2) | 587 (36.1) | 1.2 (5.1) |
| New York | 1,474 (9.5) | 456 (2.9) | 5,228 (33.5) | 16.3 (7.0) |
| North Carolina | 798 (10.0) | 244 (3.1) | 2,765 (34.6) | 7.7 (6.5) |
| North Dakota | 49 (8.6) | 15 (2.7) | 183 (32.2) | 0.7 (6.6) |
| Ohio | 970 (10.7) | 263 (2.9) | 3,039 (33.6) | 9.1 (6.7) |
| Oklahoma | 322 (10.8) | 93 (3.1) | 1,040 (34.9) | 2.9 (5.7) |
| Oregon | 285 (8.7) | 93 (2.8) | 1,097 (33.5) | 4.0 (8.7) |
| Pennsylvania | 1,006 (9.9) | 303 (3.0) | 3,484 (34.1) | 9.0 (6.5) |
| Rhode Island | 75 (8.8) | 23 (2.7) | 280 (33.1) | 0.7 (6.1) |
| South Carolina | 441 (11.3) | 123 (3.1) | 1,361 (34.9) | 3.6 (6.3) |
| South Dakota | 55 (8.6) | 19 (2.9) | 220 (34.0) | 0.8 (6.5) |
| Tennessee | 592 (11.3) | 158 (3.0) | 1,792 (34.3) | 4.9 (6.2) |
| Texas | 2,163 (10.3) | 621 (3.0) | 7,142 (34.0) | 24.0 (6.1) |
| Utah | 148 (6.8) | 51 (2.3) | 652 (30.1) | 2.8 (5.5) |
| Vermont | 35 (7.1) | 14 (2.8) | 165 (33.6) | 0.4 (6.1) |
| Virginia | 639 (9.6) | 189 (2.8) | 2,208 (33.3) | 6.9 (6.9) |
| Washington | 473 (8.2) | 164 (2.9) | 1,938 (33.7) | 8.0 (8.9) |
| West Virginia | 184 (12.7) | 45 (3.1) | 502 (34.8) | 1.1 (5.9) |
| Wisconsin | 389 (8.6) | 135 (3.0) | 1,560 (34.4) | 4.6 (7.0) |
| Wyoming | 34 (7.8) | 12 (2.8) | 148 (33.6) | 0.4 (5.3) |
| Total U.S. | 24,714 (9.8) | 7,464 (3.0) | 85,948 (34.0) | 268.9 (6.9) |
*Source: American Diabetes Association (2).
Geographic variation is less for the calculated prevalence rates for undiagnosed diabetes and prediabetes because the prediction equations only consider variation across states in demographics and modeled risk factors. Projected prediabetes prevalence rates in adults ranged from 37.1% in Hawaii to 29.0% in the District of Columbia.
State estimates of economic burden reflect population size and demographics and variation in disease prevalence, earnings, and medical costs (Table 3).
Table 3.
State annual economic burden by diabetes category, 2017 (millions of dollars)
| Medical costs |
Indirect costs |
||||||
|---|---|---|---|---|---|---|---|
| State | DDM* | UDM | PDM | GDM | DDM* | UDM | Total costs |
| Alabama | 4,188 | 293 | 603 | 18 | 1,715 | 173 | 6,990 |
| Alaska | 419 | 50 | 115 | 8 | 156 | 36 | 783 |
| Arizona | 5,108 | 420 | 935 | 27 | 1,655 | 217 | 8,362 |
| Arkansas | 2,210 | 168 | 364 | 11 | 885 | 96 | 3,733 |
| California | 27,011 | 2,323 | 5,192 | 273 | 12,454 | 1,773 | 49,026 |
| Colorado | 2,556 | 310 | 750 | 24 | 1,033 | 181 | 4,854 |
| Connecticut | 2,697 | 241 | 553 | 15 | 960 | 135 | 4,601 |
| Delaware | 703 | 66 | 137 | 4 | 279 | 36 | 1,224 |
| District of Columbia | 428 | 43 | 84 | 4 | 271 | 34 | 864 |
| Florida | 19,320 | 1,479 | 3,066 | 83 | 5,480 | 720 | 30,147 |
| Georgia | 7,781 | 610 | 1,295 | 46 | 3,139 | 341 | 13,212 |
| Hawaii | 1,015 | 110 | 209 | 11 | 465 | 99 | 1,910 |
| Idaho | 956 | 96 | 219 | 7 | 314 | 50 | 1,643 |
| Illinois | 8,726 | 784 | 1,697 | 62 | 3,169 | 431 | 14,868 |
| Indiana | 4,696 | 371 | 824 | 30 | 1,791 | 212 | 7,924 |
| Iowa | 1,900 | 185 | 416 | 15 | 646 | 100 | 3,262 |
| Kansas | 1,703 | 165 | 374 | 14 | 693 | 93 | 3,042 |
| Kentucky | 3,597 | 250 | 554 | 18 | 1,566 | 154 | 6,138 |
| Louisiana | 4,231 | 316 | 642 | 21 | 1,453 | 174 | 6,836 |
| Maine | 1,007 | 92 | 213 | 4 | 365 | 55 | 1,737 |
| Maryland | 4,922 | 358 | 757 | 27 | 2,085 | 272 | 8,420 |
| Massachusetts | 5,485 | 464 | 1,065 | 31 | 2,131 | 285 | 9,460 |
| Michigan | 7,033 | 640 | 1,355 | 41 | 2,685 | 336 | 12,090 |
| Minnesota | 3,529 | 353 | 823 | 32 | 1,173 | 188 | 6,098 |
| Mississippi | 2,419 | 199 | 395 | 12 | 995 | 102 | 4,122 |
| Missouri | 4,916 | 371 | 807 | 28 | 1,795 | 199 | 8,114 |
| Montana | 628 | 69 | 154 | 4 | 191 | 38 | 1,084 |
| Nebraska | 993 | 111 | 259 | 10 | 384 | 64 | 1,822 |
| Nevada | 2,043 | 185 | 412 | 12 | 704 | 109 | 3,464 |
| New Hampshire | 944 | 87 | 209 | 5 | 319 | 51 | 1,616 |
| New Jersey | 6,660 | 566 | 1,242 | 41 | 2,511 | 369 | 11,390 |
| New Mexico | 1,497 | 128 | 280 | 7 | 475 | 63 | 2,450 |
| New York | 15,135 | 1,223 | 2,637 | 92 | 6,092 | 880 | 26,058 |
| North Carolina | 7,711 | 728 | 1,534 | 47 | 2,896 | 362 | 13,278 |
| North Dakota | 471 | 45 | 102 | 5 | 190 | 24 | 837 |
| Ohio | 9,015 | 695 | 1,525 | 50 | 3,332 | 386 | 15,004 |
| Oklahoma | 2,762 | 225 | 480 | 17 | 1,071 | 133 | 4,687 |
| Oregon | 3,143 | 261 | 599 | 24 | 1,165 | 182 | 5,374 |
| Pennsylvania | 9,342 | 772 | 1,684 | 47 | 3,539 | 472 | 15,856 |
| Rhode Island | 778 | 67 | 156 | 4 | 283 | 42 | 1,330 |
| South Carolina | 4,248 | 358 | 737 | 22 | 1,642 | 186 | 7,192 |
| South Dakota | 510 | 51 | 112 | 5 | 183 | 30 | 890 |
| Tennessee | 5,157 | 387 | 829 | 26 | 2,107 | 225 | 8,732 |
| Texas | 18,903 | 1,387 | 3,102 | 132 | 6,699 | 801 | 31,025 |
| Utah | 1,249 | 120 | 304 | 16 | 498 | 79 | 2,266 |
| Vermont | 362 | 40 | 93 | 2 | 158 | 26 | 681 |
| Virginia | 6,048 | 518 | 1,133 | 39 | 2,335 | 304 | 10,377 |
| Washington | 5,044 | 499 | 1,124 | 53 | 1,693 | 273 | 8,687 |
| West Virginia | 1,661 | 112 | 240 | 6 | 638 | 68 | 2,725 |
| Wisconsin | 4,099 | 421 | 925 | 30 | 1,356 | 204 | 7,034 |
| Wyoming | 311 | 32 | 74 | 2 | 113 | 19 | 551 |
| Total U.S. | 237,269 | 19,841 | 43,391 | 1,558 | 89,929 | 11,886 | 403,874 |
State numbers might not sum to national totals due to rounding.
DDM, diagnosed diabetes mellitus; PDM, prediabetes mellitus; UDM, undiagnosed diabetes mellitus.
*Source: American Diabetes Association (2).
Conclusions
These findings help frame the diabetes burden within a broader economic picture of health care spending. In 2017, elevated glucose levels contributed to an additional $302 billion in medical expenditures and a nearly $102 billion loss from reduced labor force participation, early mortality, and lower productivity. This annual $404 billion burden represents a hidden “tax” averaging $1,240 per American in the form of higher medical costs and reduced national productivity. The average household size in 2017 was 2.54 members, and median income was $61,372, suggesting that this diabetes burden equates to $3,150 for the typical household or 5.1% of income (20,21).
Nationally, nearly half of adults have diabetes (12.8%) or prediabetes (34%). The higher cost to treat people with diabetes underscores the importance of efforts to help prevent diabetes onset, such as the National Diabetes Prevention Program. The 268,900 babies born to mothers with GDM in 2017 represent 6.9% of the nation’s 3.9 million births and underscores the importance of screening for and management of GDM.
This economic burden of nearly $404 billion in 2017 grew from $322 billion in 2012 ($344 billion in 2017 dollars). Adjusted for inflation, the burden increased by 3.5% per year ($11.9 billion annually). Between 2012 and 2017, the inflation-adjusted average cost for diagnosed diabetes grew by 13%, reflecting an aging population with diabetes. In contrast, the average cost per case for prediabetes and GDM declined 8% and 6%, respectively, reflecting narrowing differences in health care use patterns for people with prediabetes and GDM relative to comparison populations. The undiagnosed diabetes cost estimates in this report are not directly comparable to the published 2012 estimates because a calculation error overstated the 2012 estimate. The corrected 2012 medical cost estimate for undiagnosed diabetes is $16.9 billion (rather than the $23.4 billion estimate reported). The average cost per undiagnosed diabetes case in 2017 increased 23% after adjusting for inflation relative to the corrected 2012 estimate, increasing from $3,230 in 2012 ($3,450 in 2017 dollars) to $4,250 in 2017. This increase reflects an increase in the average age of people with undiagnosed diabetes (Table 1).
Not all cases of diabetes and prediabetes can be prevented, and for those that experience disease onset, not all complications can be prevented. Consequently, these burden estimates overstate preventable medical costs.
Study limitations include the following:
Undiagnosed diabetes, by definition, is not identified in medical claims; therefore, we used a proxy population of people within 2 years of diagnosis.
Undiagnosed diabetes status is unavailable in NHIS and in employer surveys used to calculate reduced productivity. We used estimates of productivity loss among people with diagnosed diabetes, by demographic, scaled down by the ratio of hospital inpatient days for people with undiagnosed diabetes to people with diagnosed diabetes to reflect that people with diagnosed diabetes tend to be less healthy than people with undiagnosed diabetes.
Prediabetes is largely asymptomatic, with a small expected average productivity loss. However, one study estimating average annual productivity loss from absenteeism and presenteeism for people with chronic conditions reports productivity loss for two asymptomatic conditions: hypertension ($260 in 2017 dollars) and hypercholesterolemia ($20) (22). If the 62 million adults aged 18–64 (employed and unemployed) experienced similar productivity loss, this equates nationally to an additional $1.2–$16.1 billion in productivity loss associated with prediabetes.
The regression analyses to calculate rate ratios using medical claims lack controls for potential lifestyle confounders. Consequently, we conducted additional analyses with MEPS data and reduced rate ratios to account for these confounders.
For the Medicare, Medicaid, and uninsured populations, we lacked data to directly estimate differences in health care use (rate ratios), by care delivery setting and medical condition category, for prediabetes and undiagnosed diabetes. We therefore extrapolated rate ratios estimated for a commercially insured population to these other populations. One study reports that Medicaid expenditures averaged 2.6-times higher for patients with diabetes compared with patients without diabetes, which is higher than our national estimate of 2.3 (adjusting for age and sex), so our estimate for the Medicaid population might be conservative (23). The Transformed Medicaid Statistical Information System may make comprehensive Medicaid claims data accessible for future research.
The study omits cost of services for podiatry, some vision care provided by optometrists, and dental care and omits indirect costs for mothers with GDM (e.g., increased time off from work). Thus, the burden estimates likely are conservative.
Estimates of the national prevalence of undiagnosed diabetes and prediabetes reported by CDC using NHANES data and the predictive equations used to generate state-level estimates in our analysis are based on results for one blood glucose or A1C test. Standard clinical practice is to use two blood samples to confirm the diagnosis (1). Similarly, patients categorized as having prediabetes in medical claims analysis (for which laboratory values are available for some patients) could be based on the presence of a single blood glucose or A1C reading. This assumption could produce conservative estimates of the impact of prediabetes on health care use because patients without prediabetes are inadvertently placed in the wrong category.
Insufficient data exist to directly estimate state-level prevalence of undiagnosed diabetes and prediabetes. Our use of prediction equations based on national NHANES data likely mitigates state-to-state variation in prediabetes and undiagnosed diabetes burden.
Burden estimates for prediabetes and undiagnosed diabetes were unavailable for children.
Patients with diabetes or prediabetes who have more contact with the health care system are more likely to have their condition diagnosed. This could overstate the health care use rate ratios relative to the comparison groups.
The GDM burden estimate could be understated due to 1) underdiagnosis of GDM based on ICD-9 codes in NIS, or 2) omission of the increased risk and cost of intrauterine fetal death.
This study estimates the association of elevated glycemic levels with higher medical costs and patient increased risk for diabetes sequelae. Obesity increases the risk for both diabetes and sequelae, so part of the economic burden of elevated blood glucose cannot be distinguished from the economic burden of obesity (24,25).
Health utilization data have a large percentage of zeros, so negative binomial regression or correction for overdispersion often are preferred to the standard Poisson regression used to compute rate ratios (26,27). Correction for overdispersion reduces the SEs but leaves rate ratios unaffected; however, ratios based on Poisson tend to be smaller than ratios based on negative binomial regression, thus making our burden estimates conservative. Furthermore, some regressions failed to converge when using negative binomial.
The above limitations are areas for future research, as are 1) quantifying productivity loss associated with prediabetes and GDM, and 2) including optometry, podiatry, and oral health as cost categories for diabetes burden.
Conclusion
The economic burden associated with diagnosed diabetes (all ages), undiagnosed diabetes and prediabetes (adults), and GDM (mother and infants) is estimated to be nearly $404 billion in 2017. This includes $302 billion in higher medical expenditures and $102 billion in reduced productivity. This annual burden exceeds $1,240 for each person in the U.S. These findings underscore the urgency to adopt more comprehensive screening approaches as well as better prevention and treatment strategies, including continued scaling of the National Diabetes Prevention Program and greater uptake of diabetes self-management education and support.
Article Information
Acknowledgments. The authors thank Theodore F. Kirby, of the Lewin Group, and Ritashree Chakrabarti of IHS Markit for assistance with data preparation.
Funding. Funding for this study was provided by Novo Nordisk.
Duality of Interest. K.G., M.M., and E.B. are employees and shareholders of Novo Nordisk, a global health care company with products to treat people with diabetes. W.Y., I.C., and P.F.H. are employees of the Lewin Group (a subsidiary of Optum Health, part of UnitedHealth Group) and own stock in UnitedHealth Group. T.M.D., W.Y., I.C., K.B., A.P.S., W.I., and P.F.H. provide paid consulting services to federal and state governments, nonprofit entities (including the ADA), and for-profit entities (including Novo Nordisk). No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.M.D. wrote the manuscript and researched data. W.Y. contributed to and edited the manuscript and directed research on medical expenditures. K.G. and P.F.H. contributed to discussion and reviewed and edited the manuscript. M.M. contributed to study conceptualization and reviewed and edited the manuscript. E.B. contributed to study conceptualization and discussion. I.C. and K.B. researched data and contributed to discussion. A.P.S. researched data on GDM and contributed to discussion. W.I. researched data and contributed to discussion on prediabetes and undiagnosed diabetes. T.M.D. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
See accompanying article, p. 1609.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.American Diabetes Association 3. Comprehensive medical evaluation and assessment of comorbidities: Standards of Medical Care in Diabetes–2018. Diabetes Care 2018;41(Suppl. 1):S28–S37 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dall TM, Yang W, Halder P, et al. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 2014;37:3172–3179 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Dall TM, Chen Y, et al. Medical cost associated with prediabetes. Popul Health Manag 2009;12:157–163 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report, 2017 [Internet], 2017. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 12 November 2018
- 6.Centers for Disease Control and Prevention; National Center for Chronic Disease Prevention and Health Promotion. National Diabetes Statistics Report, 2014 [Internet], 2014. Available from http://www.thefdha.org/pdf/diabetes.pdf. Accessed 12 November 2018
- 7.Matthews TJ, Hamilton BE. Mean Age of Mothers is on the Rise: United States, 2000–2014 [Internet]. NCHS Data Brief, No 232. Hyattsville, MD, National Center for Health Statistics, 2016. Available from https://www.cdc.gov/nchs/products/databriefs/db232.htm. Accessed 12 November 2018
- 8.Dall TM, Zhang Y, Chen YJ, Quick WW, Yang WG, Fogli J. The economic burden of diabetes. Health Aff (Millwood) 2010;29:297–303 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Dall TM, Mann SE, et al. The economic costs of undiagnosed diabetes. Popul Health Manag 2009;12:95–101 [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Quick WW, Yang W, et al. Cost of gestational diabetes mellitus in the United States in 2007. Popul Health Manag 2009;12:165–174 [DOI] [PubMed] [Google Scholar]
- 11.Biesheuvel CJ, Vergouwe Y, Steyerberg EW, Grobbee DE, Moons KGM. Polytomous logistic regression analysis could be applied more often in diagnostic research. J Clin Epidemiol 2008;61:125–134 [DOI] [PubMed] [Google Scholar]
- 12.Dall TM, Narayan KMV, Gillespie KB, et al. Detecting type 2 diabetes and prediabetes among asymptomatic adults in the United States: modeling American Diabetes Association versus US Preventive Services Task Force diabetes screening guidelines. Popul Health Metr 2014;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turi KN, Buchner DM, Grigsby-Toussaint DS. Predicting risk of type 2 diabetes by using data on easy-to-measure risk factors. Prev Chronic Dis 2017;14:E23 [DOI] [PMC free article] [PubMed]
- 14.Flegal KM, Panagiotou OA, Graubard BI. Estimating population attributable fractions to quantify the health burden of obesity. Ann Epidemiol 2015;25:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breton M-C, Guénette L, Amiche MA, Kayibanda J-F, Grégoire J-P, Moisan J. Burden of diabetes on the ability to work: a systematic review. Diabetes Care 2013;36:740–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asay GRB, Roy K, Lang JE, Payne RL, Howard DH. Absenteeism and employer costs associated with chronic diseases and health risk factors in the US workforce. Prev Chronic Dis 2016;13:E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiBonaventura M, Link C, Pollack MF, Wagner JS, Williams SA. The relationship between patient-reported tolerability issues with oral antidiabetic agents and work productivity among patients having type 2 diabetes. J Occup Environ Med 2011;53:204–210 [DOI] [PubMed] [Google Scholar]
- 18.Loeppke R, Taitel M, Haufle V, Parry T, Kessler RC, Jinnett K. Health and productivity as a business strategy: a multiemployer study. J Occup Environ Med 2009;51:411–428 [DOI] [PubMed] [Google Scholar]
- 19.Rodbard HW, Fox KM, Grandy S; Shield Study Group . Impact of obesity on work productivity and role disability in individuals with and at risk for diabetes mellitus. Am J Health Promot 2009;23:353–360 [DOI] [PubMed] [Google Scholar]
- 20.U.S. Census Bureau. Average Number of People Per Household, By Race And Hispanic Origin, Marital Status, Age, and Education of Householder: 2017 [Internet], 2017. Available from https://www.census.gov/data/tables/time-series/demo/families/households.html. Accessed 12 November 2018
- 21.US Census Bureau. Income and Poverty in the United States [Internet], 2017. Available from https://www.census.gov/library/publications/2018/demo/p60-263.html. Accessed 12 November 2018
- 22.Mitchell RJ, Bates P. Measuring health-related productivity loss. Popul Health Manag 2011;14:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaiser Family Foundation. The Role of Medicaid for People with Diabetes [Internet], 2012. Available from https://kaiserfamilyfoundation.files.wordpress.com/2013/01/8383_d.pdf. Accessed 2 January 2019
- 24.Li Q, Blume SW, Huang JC, Hammer M, Graf TR. The economic burden of obesity by glycemic stage in the United States. Pharmacoeconomics 2015;33:735–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung MYM, Carlsson NP, Colditz GA, Chang S-H. The burden of obesity on diabetes in the United States: medical expenditure panel survey, 2008 to 2012. Value Health 2017;20:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weaver CG, Ravani P, Oliver MJ, Austin PC, Quinn RR. Analyzing hospitalization data: potential limitations of Poisson regression. Nephrol Dial Transplant 2015;30:1244–1249 [DOI] [PubMed] [Google Scholar]
- 27.Payne EH, Hardin JW, Egede LE, Ramakrishnan V, Selassie A, Gebregziabher M. Approaches for dealing with various sources of overdispersion in modeling count data: scale adjustment versus modeling. Stat Methods Med Res 2017;26:1802–1823 [DOI] [PubMed] [Google Scholar]