Abstract
The production and application of nanomaterials have grown tremendously during last few decades. The widespread exposure of nanoparticles to the public is provoking great concerns regarding their toxicity to the human body. However, in comparison with the extensive studies carried out to examine nanoparticle toxicity to the human body/organs, one especially vulnerable organ, the eye, is always neglected. Although it is a small part of the body, 90% of outside information is obtained via the ocular system. In addition, eyes usually directly interact with the surrounding environment, which may get severer damage from toxic nanoparticles compared to inner organs. Therefore, the study of assessing the potential nanoparticle toxicity to the eyes is of great importance. Here, the recent advance of some representative manufactured nanomaterials on ocular toxicity is summarized. First, a brief introduction of ocular anatomy and disorders related to particulate matter exposure is presented. Following, the factors that may influence toxicity of nanoparticles to the eye are emphasized. Next, the studies of representative manufactured nanoparticles on eye toxicity are summarized and classified. Finally, the limitations that are associated with current nanoparticle‐eye toxicity research are proposed.
Keywords: eyes, nanomaterials, nanosafety, ocular damage, toxicity
1. Introduction
Nanoparticles have become omnipresent in workplaces and consumer products at this century.1, 2 The widespread exposure of nanoparticles to workers who make or use them in manufacturing plants is provoking great concerns regarding their toxicity to human body by scientists and the public.3 Over the years, many studies have examined the toxicity profile of nanoparticles on main organs such as respiratory tract, lung, brain, liver, kidney, skin, as well as immune system.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 However, in comparison with the flourishing studies carried out to testify nanoparticle toxicity to those organs mentioned above, one especially vulnerable organ, the eye, is always neglected. As a superficial organ, eyes are usually directly exposed to the surrounding environment. Direct contact of eyes with hazardous substance in the environment can lead to ocular damage. According to the statistics provided by Centers for Disease Control and Prevention, about 300 000 workplace eye injuries are sent to emergency room every year in the USA.16 The importance of the eye is needless to say as 90% of outside information is obtained via the ocular system. Severe injuries could lead to significant morbidity and even disability. Therefore, with the increasing amount of nanoparticles released from the widespread nanoproduction and nanoapplication, their potential toxicity to the eyes should be well‐studied. U.S. Food and Drug Administration (FDA) had established the guidance for industry: safety of nanomaterials in cosmetic products (Docket number: FDA‐2011‐D‐0489), which provokes the importance of eye safety in this area. The standard eye irritation test established by The Organisation for Economic Co‐operation and Development (OECD) for the testing of chemicals is used as the standard to measure nanomaterials eye toxicity. The safety use of nanomaterials to eyes surely has received certain attention internationally but the studies about the toxicity effect of nanomaterials on eyes are still at their early stage.
Due to its small size, the toxicity effects of nanomaterials on ocular surface have their own characteristics when compared with mechanical or chemical‐induced injuries.17, 18, 19, 20 As there are a series of anatomical barriers on the eyeballs, the particles with large size are excluded out of the ocular surface with blinking and washing away by tear film. However, the small size of nanoparticles ensures the close contact with ocular surfaces, anchoring to the cornea for longer residence time, penetrates the barriers of ocular surface, and reaches posterior segments of the eye.21 It demonstrated that the size of the nanoparticles determines the speed and amount to penetrate the ocular surface, which makes the small particles hard to be washed out.22 Therefore, nanosized particles are well tolerated and more easily to migrate across epithelial barrier to cause cytotoxicity and inflammatory response.21, 22, 23, 24, 25 Once entering eyes, nanoparticles may subsequently induce cellular toxicity as well as systematic immune response not only at ocular surface, but also to lens, retina, or even optic nerve and macula. For example, studies have shown that metal‐containing nanoparticles, after entering the eyes, could be conveyed into the nasal cavity through the nasolacrimal duct by drainage from the eye socket, which subsequently enter the central nervous system via the eye–nose–brain route.25 These reports clearly showed that the mechanism of nanomaterials toxicity effects on eyes is quite different from other materials and therefore more attention should be received to this important field.
Previous studies have reported many workplace related eye toxicity such as cosmetic/personal care products, pesticide exposure at agricultural sector, hazardous chemicals at industrial place, as well as environmental particles such as building products, air pollutions (PM 2.5), or volcano particles.17, 18, 26, 27, 28, 29, 30, 31, 32, 33, 34 However, to our best knowledge, a review focus on the summarization of toxicity effects of nanomaterials to eyes is still rare. Herein, instead of covering every single nanomaterial, we choose some representative manufactured nanomaterials from the list made by the Working Party on Manufactured Nanomaterials (WPMN) of the OECD35 and classify them into three categories: metal nanomaterials, metal oxide nanomaterials, and carbon nanomaterials. We hope this review could give a general introduction of nanoparticles toxicity to eyes, and at the same time provoke more incisive studies in this important field.
2. Nanoparticle Caused Ocular Syndromes
In this chapter, we give a brief description of ocular anatomy and typical ocular disorders with a focus on occupational or environmental particulate materials exposure. Although the ocular surface is the foremost layer that contacts with nanomaterials, once nanoparticles are absorbed into eyes, toxic effect could also be induced within the eye (such as iris and lens) or inner surface of retina, optic nerve, and macula (Scheme 1).36, 37 What is worth to be noted here is that some symptoms mentioned below may have not yet found in nanomaterial‐related workplace. However, considering the pathogenesis of nanoparticle to eyes, these symptoms might also be induced by nanoparticles and need to acquire equal attention for future study.
Scheme 1.
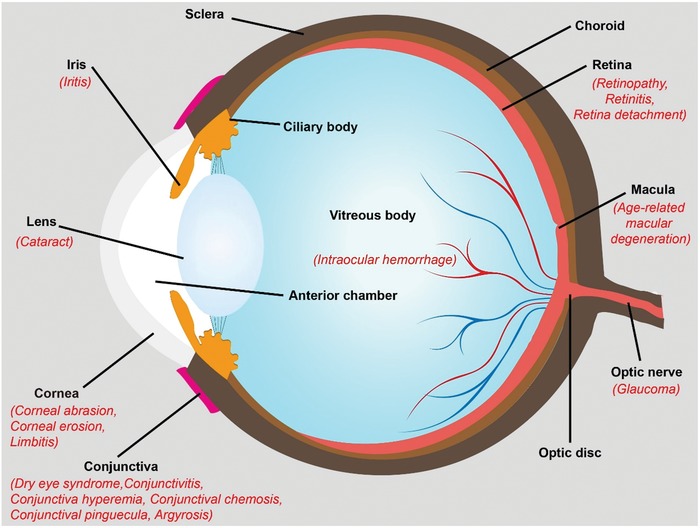
Typical eye disorders caused by nanoparticle exposure.
2.1. Nanoparticles Caused Damage on Eye Surface
Most of the researches regarding the direct influence of nanoparticles on eyes focus on their damage to eye's surfaces. The ocular surface includes two major parts: the cornea and the conjunctiva. Different from other parts of the body covered by skin, the ocular surface is covered with a thin layer of tear film that is supported by lacrimal and accessory lacrimal gland, conjunctival goblet cells, Meibomian gland, and glands of Zeis and Moll.38, 39 It is proposed that the potential mechanism of ocular symptoms caused by the dysfunction of any ocular surface component is inflammation. The introduction of foreign substances on the epithelial surface could induce a series of inflammatory events, which may subsequently cause tear film instability as well as cell damage.40, 41, 42 Common clinical features of ocular surface inflammation include dryness, burning, itching, gritty eyes, conjunctival injection, dilatation of conjunctival vasculature, conjunctival chemosis, limbitis, redness, and swelling of eyelids.43
2.1.1. Dry Eye Syndrome
Also known as keratoconjunctivitis sicca, it is an inflammatory disorder that affects ocular surface and lacrimal gland.44 It happens when either the eye produces deficient tear or when tear evaporates too quickly.45 The common symptoms usually include feeling of dryness, grittiness, and burning; further complications associated with dry eye syndrome are conjunctivitis and keratitis. Study has shown that particulate matters such as TiO2 are more harmful to dry eyes when compared with normal eyes.46
2.1.2. Conjunctivitis
Also known as pink eyes, it is an inflammation or swelling of the conjunctiva. People with conjunctivitis may experience pink discoloration, gritty, itching, burning feeling of one or both eyes, excessive tearing, and swollen eyelids. Conjunctival hyperemia and ocular discharge are common symptoms of conjunctivitis. It is reported that the particulate matters could increase the risk of allergic conjunctivitis.47
2.1.3. Conjunctival Hyperemia
Also known as conjunctival injection, it is one cause of eye redness, which results from dilation of conjunctival vessels and increased blood flow. Particulate matter triggered allergic cascade (by releasing histamine) could result in vasodilation and increased blood flow.48
2.1.4. Conjunctival Chemosis
Chemosis is the swelling of the conjunctiva, resulting from the extravasation of plasma.48
2.1.5. Conjunctival Pinguecula
It is a common type of conjunctival degeneration, which is usually associated with yellow‐white deposit on conjunctiva near to limbus. It is found that people with silicosis have a high risk for conjunctival pinguecula in silica exposure environment.49
2.1.6. Argyrosis
Also know as argyria, it is caused by the gradual accumulation of silver products in eyes. Argyrosis particularly indicates the argyria of the conjunctiva. Once developed argyrosis, conjunctiva may turn blue‐gray.
2.1.7. Corneal Abrasion
Scratched cornea is a common ocular injury that results from damage or loss of corneal epithelial cells. The usual symptoms of corneal abrasion are pain/gritty feeling, red eyes, and hypersensitivity to light. Several studies have shown that nanoparticle exposure could cause destruction to corneal epithelium cells through different mechanisms such as cell membrane damage, mitochondrial dysfunction, or cell death.50
2.1.8. Corneal Erosion
The common symptom is similar to the initial state of corneal abrasion with tearing on awakening. Its recurrence may occur years after a corneal abrasion.
2.1.9. Limbitis
The inflammation of limbus. Limbus, the special site where corneal epithelial stem cells reside, is the border of the conjunctiva and cornea.
2.1.10. Uveitis
Also called iritis, it is the inflammation of uvea, which includes iris, ciliary body, and choroid. The common symptoms are redness or burning of eyes, blurred vision, sensitivity to light, and so on. Bacteria, virus, or particulate matter‐induced over‐reactive immune system could be the causes of this disease.51
2.2. Nanoparticles Induced Damage on Lens
It has been well stated that cigarette smoking, solid fuel (such as wood) combustion, and metal iron could cause damage to lens. Smoking is found to be related with metal accumulation such as cadmium which can cause oxidative DNA damage or per oxidative damage to lens cell membrane for triggering cataractogenesis;52 the alteration in metal ions may be a contributor to cataract formation by inducing oxidative stress, inhibiting antioxidant pathways, or modifying the structure/formation of lens extracellular matrix;53 an iron intraocular foreign body sedimentation may lead to siderosis which may cause cataract and lens discoloration.54 It is proposed that metal irons could cause oxidative damage to lens through the metal catalyzed Fenton reaction.55
2.2.1. Cataract
Cataract is a clouding of the internal lens mainly caused by aging, radiation, genetics, complication of other disease, etc.56 People who have cataract often suffer from diminished ability in vision. The common symptoms include reduced color intensity, blurry vision, and tough seeing with bright light and at night.57
2.3. Nanoparticles Induced Damage on Retina
As mentioned above, particulate materials could induce inflammatory factors production, which further elicit systematic inflammatory response with enhanced cytokine production.58, 59, 60 Therefore, we could also speculate that the induction of chronic exacerbation of immune response could cause damage to layers of retinal vessels, degeneration of retinal cells, and neovascularization.61 For instance, studies showed that gold, silver nanoparticles, or multiwalled carbon nanotubes (MWCNT) could cause increased cell apoptosis and oxidative stress in animal retinal cells or tissue.62, 63 In addition, it is reported that the particulate materials could increase serum homocysteine level, and further reduce the ocular blood flow velocity.64 Besides, excess iron ions dissolved from iron nanoparticles in eyes are associated with retinal detachment, age‐related macular degeneration, and intraocular hemorrhage;54, 65, 66 ZnO nanoparticles could increase retinopathy.67
2.3.1. Retinopathy
Retinopathy is the damage to the retina, which may lead to vision impairment or even vision loss. It usually refers to retinal vascular damage or retina damage induced by abnormal blood flow.68 Retinopathy is usually the complication from diabetes, which is also called diabetic retinopathy.
2.3.2. Retinitis
It is the inflammation of the retina which can cause permanent damage to retina. People with retinitis may experience slow night/peripheral vision loss or eventually blindness. Iron is reported to cause retinitis.69
2.3.3. Retina Detachment
A disorder in which the neural retina separates from the underneath retinal pigment epithelium.70 People with retinal detachment may experience symptoms such as flashes of light, visual floaters, shadow at peripheral vision, or central visual loss.70
2.3.4. Age‐Related Macular Degeneration
It is one specific kind of retinopathy which affects the macula, the structure that is responsible for the high‐acuity vision. Although it may not cause total blindness, it gets worse as ageing.
2.3.5. Intraocular Hemorrhage
It is a disorder of bleeding into the eyeball. It may be caused by the abnormal vessels or damage of normal vessels. The common symptoms include floaters and visual loss. It is also a potential complication of proliferative diabetic retinopathy or retina vasculitis.71, 72
2.4. Nanoparticle Induced Damage on Optic Nerve
Upon nanoparticles exposure, ocular surface including corneal and conjunctival epithelium provides protection against their absorption into vitreous humour.36 Vitreous humour is located at the anterior and posterior chambers of the eye, which is in close contact with retina, optic nerve, and macula.37 Therefore, particulate materials from vitreous humour also injure optic nerves. In addition, it has shown that fine particles also result in increasing intraocular pressure under constant exposure,73 and cause damage to optic nerves.
2.4.1. Glaucoma
A disease that causes damage to the retinal ganglion cells and optic nerve, usually caused by the high intraocular pressure. Particulate matters might cause glaucoma by “clogging” the trabecular meshwork and interfere the outflow channels.74 It could cause diminished peripheral vision or even blindness.
3. Nanoparticle Toxicity Influencing Factors on Eyes
Current studies regarding nanoparticle eye toxicity factors mostly put their focus only on size or exposure time, however, there are much more distinguished parameters that may influence the eye toxicity of nanoparticle, due to their unique physiochemical properties. Besides, the hazard of one specific nanoparticle is often influenced by several kinds of properties. The resulted toxicity is usually due to a combination of the properties instead of one single factor. The important factors that influence toxicity of nanoparticles toward eye are summarized as follows. These factors could also be used as a reference for future nanoparticle eye toxicity studies.
3.1. Chemical Components and Nanoparticle Chemistry
The two notions of nanoparticle chemistry and chemical components are different. Chemical components refer to the chemical elements that nanoparticle contain; although two kinds of nanoparticle have the same components, they may possess different chemical or crystalline structure. These two profiles are both critical in determining nanoparticle toxicity. Nanomaterials usually contain more than one chemical. Some are of great toxicity due to their chemical components such as heavy metal or other kind of poisonous elements.75 For instance, the high toxicity of ZnO nanoparticles is probably due to the release of toxic Zn ion in the lysosomes after internalized into cells.76 Different metals of metal oxide nanoparticles can cause different levels of toxicity due to their ability to cause reactive oxygen species (ROS) generation.77
Besides, the chemistry of nanoparticle may influence toxicity profile in a few ways, such as affecting cellular uptake, subcellular localization, and oxidative stress.78 For example, there are two forms of TiO2, rutile and anatase. Although they contain same chemical composition, due to the different crystalline structure, rutile nanoparticles exhibited high oxidative damage to human bronchial epithelial cells in the absence of photoactivation, while anatase nanoparticle at the same size did not.79
3.2. Size and Shape
Size can influence toxicity in many ways. It is known that as nanoparticle size decreases, the surface area increases exponentially.80 Thus more chemical molecules may attach to the big surface, which further increase its surface reactivity as well as toxic effects.81 For example, SiO2 nanoparticles with diameters less than 40 nm show a higher toxicity to human corneal epithelial cells than those bigger than 50 nm.50 In addition, smaller sized nanoparticles are more easily to migrate across epithelial barrier to cause cytotoxicity and inflammatory response.82, 83
Apart from size, shape is another factor to influence nanoparticle toxicity. For instance, nanoparticles with higher aspect radio tend to be more toxic.84 It is indicated that fiber‐shaped nanoparticles are more toxic than spherical‐shaped nanoparticles of the same chemical composition.85 Studies also show that compared to amorphous carbon black (spherical particles), carbon nanotubes with long aspect ratio are more toxic.86, 87, 88 In addition, 2D nanomaterials such as graphene with sharp sheets can also exert toxicity by causing damage to cell membrane.89
3.3. Surface Area/Charge and Modification
The surface of nanoparticle also plays major role in regulating nanoparticle toxicity. The surface area, surface charge, as well as surface modification are all important factors. As mentioned above, as nanoparticles are smaller in size, their surface area becomes bigger correspondingly. When nanoparticles are of same mass, same chemical composition, and same crystalline structure, it is well established that the inflammatory response is proportional to their total surface area.90, 91, 92 Several studies have demonstrated that larger surface area can induce increased reactivity as well as oxidative and DNA damage.91, 93, 94 Additionally, changes in surface charge also result in differences in toxicity of nanoparticles, as it can influence biodistribution as well as cellular uptake efficiency of the nanoparticles.95, 96, 97, 98 It is reviewed previously that positively charged nanoparticles can be more easily taken up by cells than negatively or neutrally charged ones, which leads to more inflammatory effects and cell death.85 Recently, the oxidation state is also found to be important in some nanomaterials such as GO, where higher oxidation state induces more harmful effects to the eyes.99
As it is the surface of nanoparticle that directly interacts with cells and biological materials, surface modification/functionalization can significantly change the bio‐physicochemical properties of nanoparticles such as chemical reactivity, magnetic or optical properties, stability, and biocompatibility.100, 101, 102 These properties are closely associated with the safe or toxic profile of nanoparticles. For example, GO nanoparticles are found to cause damage to ocular surface both in vivo and in vitro, while this toxicity could be alleviated by using proper functionalization with polyethylene glycol (PEG) or antioxidant glutathione (GSH).99, 103
3.4. Dosage and Concentration
Some studies show that the toxicity of nanoparticles is not closely related to nanoparticle mass dose when compared to size differences.100, 104 For example, it is found that low dose (10 mg m−3) exposure to 20 nm TiO2 nanoparticles induced much higher long tumor incidence than high dose (250 mg m−3) exposure of 300 nm particles.105 Also higher concentrated GO exposure is reported to cause damage to human corneal/conjunctiva epithelium cells.103 However, one thing should be noted that high concentration of nanoparticles could induce the formation of particle aggregation, which may reduce its toxic effects compared to lower concentrations of the same nanoparticles.79, 106, 107 Besides, several studies also showed that high dose of nanoparticles could expose adverse effects to health.108, 109, 110 Therefore, it is believed that concentration of nanoparticle is another determinant of its toxicity profile.
3.5. Behavior in Solvent or Biological Media
Nanoparticles tend to have different behaviors regarding dispersion or agglomeration state in different solvents, which in turn influence their size as well as toxicity profile.111 It is indicated that TiO2 and ZnO nanoparticles show considerably bigger size in phosphate buffer saline (PBS) than in water.95, 112 Accordingly, their corresponding toxicity varies in different solvents. Besides, it is also well accepted that nanoparticles display different sizes or agglomeration state in biological media because of the formation of protein corona.113 Such protein corona also changes the size and surface properties of nanoparticles, so as to influence their absorption, transportation, as well as toxicity. For instance, the introduction of foetal bovine serum (FBS), which might provide sufficient protein for the formation of protein corona around nanoparticles, was found to alleviate the toxicity of ultrafine SiO2 nanoparticles to primary human corneal epithelial cells.50
4. Ocular Toxicity toward Workplace Nanomaterials
Numerous toxicity studies have demonstrated short‐term associations between different levels of nanoparticle exposure and increased acute morbidity. It is proposed that nanoparticles could cause cellular toxicity mainly by four mechanisms. a) Generation of oxidative stress. For instance, metal nanoparticles such as iron or Au/Ag nanoparticle could cause ROS production and further impact cellular functions (i.e., apoptosis) to cause damage to cells.62, 114 b) Disruption of cell membrane. Some nanoparticles such as graphene oxide (GO) are reported to cause damage to the membrane of cells due to their sharp sheets.89 c) Induction of inflammation response. Once absorbed by cells or entering blood circulation, nanoparticles tend to activate inflammatory responses. Several nanoparticles such as gold, TiO2, Fe, GO nanoparticles possess inflammatory nature, which could increase inflammatory cells and proinflammatory cytokine production.115 d) Genotoxicity. Due to the typical physicochemical characteristic and large surface area to volume ratio, nanoparticles may induce unpredictable genotoxicity.116 It is reported that the long‐term oxidative stress and inflammation caused by nanoparticles could eventually cause DNA damage.117, 118 Metal nanoparticles such as gold or silver with oxidative nature are found to cause genetic damage.119, 120 Understanding the mechanism of nanoparticle toxicity, we can better design a proper way to lessen or even avoid their toxic exposure.
Here we present a few commonly manufactured nanomaterials listed from WPMN of the OECD. These important nanomaterials are now widely used in many industrial and commercial products, which may cause the most direct toxicity to the public. Efforts have been put to study their ocular toxicity. Here, we generally classify them into three parts: metal, metal oxide, and carbon nanomaterials.
4.1. Metal Nanomaterials
Metallic nanomaterials are among the most widely used nanomaterials in workplace.4, 121 For instance, silver nanoparticles due to their antimicrobial ability are widely applied in household detergents, antibacterial sprays, apparel, socks, and shoes;122, 123 ionic Ag and Au can be reduced to their metallic nanoparticles by dissolved organic matter under natural sunlight;124 many metal nanoparticles can be spontaneously generated from metal objects, including wire and earrings.125 Although bulk metals have been considered to be safe, several research groups have found that their nanoscale particles may exert toxic effects on eyes. For instance, Soderstjerna et al. studied both silver and gold nanoparticles internalization, apoptosis, and oxidative stress using an in vitro cell/tissue culture model of the mouse retina.62 Both 20 and 80 nm Ag and Au nanoparticles were used in this study. After 72 h nanoparticle exposure, the researchers found that even low concentration (<0.0065 µg mL−1 for 20 nm nanoparticles and <0.4 µg mL−1 for 80 nm nanoparticles) of nanoparticles demonstrated undesired effects on the retina cells, such as significant oxidative stress and cell apoptosis (Figure 1). The results also revealed that these nanoparticles exerted neurotoxic effects especially on the photoreceptors, which may lead to visual impairment or even blindness. Biswas's group found that the ocular toxicity of Au nanoparticles is closely related to their size, shape, concentration, as well as surface area.126 Gold nanoparticles also disrupted Zebrafish ocular development and pigmentation.127 In addition, the work of Sriram et al. showed that silver nanoparticle induced cytotoxicity and apoptosis by producing increased ROS in bovine retinal endothelial cells in a size‐dependent manner.128 Besides, Kim et al. evaluated the acute eye irritation potential of Ag nanoparticle using New Zealand white rabbits according to OECD test guidelines.129 Conjunctival redness, edema, and discharge were observed 1 h after removing the Ag nanoparticles, while no signs of irritation to the cornea, iris, or conjunctiva were found 24, 48, and 72 h after removing the substance. However, Maneewattanapinyo et al. in a study using guinea pigs exposed to 5000 ppm Ag nanoparticles, a grade 1 conjunctivae irritation during first 24 h was observed.130 Despite these results, it is also known that long‐term exposure to silver or colloidal silver could cause argyrosis (pigmentation of the eye).131, 132, 133, 134 Therefore, the chronic occupational exposure of Ag nanoparticles to eye toxicity may need to be studied at this point.
Figure 1.
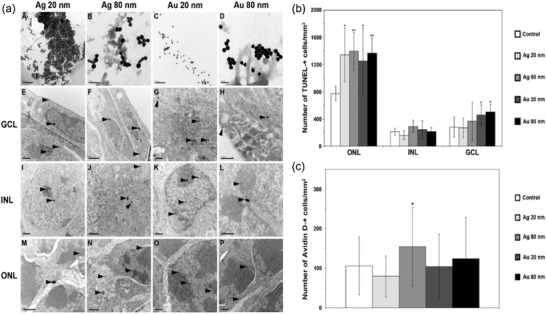
a) TEM‐images demonstrating uptake of Ag and Au NPs in the mouse retina. The top row shows the respective NP structure, i.e., AgNP 20 nm (A), AgNP 80 nm (B), AuNP 20 nm (C), and AuNP 80 nm (D). (E)–(P) All four types of NPs were taken up by the cultured retina and found in all three retinal nuclear neuronal layers, i.e., the GCL, INL, and ONL. Both 20 and 80 nm sized NPs were found either as single NPs (e.g., E, M (upper arrowheads) or as clusters of NPs (e.g., E, I, M (lower arrowheads). The 20 nm sized Ag and Au NPs, respectively, were found in the nucleus (E, G, I, K, M, O), the nucleolus (I, K, O), the mitochondria (G), the cytosol (E, G, M) and in the extracellular space (M). In the majority of cells the largest fraction of the 20 nm sized NPs were located in the nucleus (I, K, O) compared to the fraction of NPs found in the other cell compartments. Notably, all NPs were found both in the eu‐chromatin (e.g., I, K, N) and heterochromatin (e.g., I, K, L, P, M). Ag and Au NPs, sized 80 nm, were detected in the nucleus, but to a lesser extent compared to the 20 nm NPs (F, J, L, N, P) and in the extracellular space (H, L, P). NPs, sized 80 nm, were not detected in the nucleolus and within mitochondria. GCL = ganglion cell layer, INL = the inner nuclear layer, ONL = the outer nuclear layer. Arrowheads show NPs that have been taken up by the retinal cells. Scale bars equal 0.2 mm for images (A–D); 1 mm for (E,F), (I,J), and (M,N); and 0.5 mm for (G,H), (K,L), and (O,P). b) Increased number of apoptotic cells in the mouse retina after exposure to 20 and 80 nm Ag and Au NPs. Graph shows numbers of TUNEL‐positive cells and results are presented as mean ±SD (n = 5–8 explants per group). *p < 0.05 compared to control, **p < 0.01 compared to control. Scale bars equal 100 mm. c) Oxidative stress. Graph shows numbers of AvidinD‐positive cells and results are presented as mean ±SD (n = 4 explants per group). *p < 0.05 compared to control. Scale bars equal 100 mm. Reproduced with permission.62 Copyright 2014, Public Library of Science.
In contrast to the toxicity study, the eye safety researches of Au and Ag nanoparticles were also studied to some point. Kim et al. once examined whether intravenously administered Au nanoparticle could pass through the blood–retinal barrier (BRB) and cause toxicity in C57BL/6 mice.24 They found that 100 nm nanoparticles were not detected in the retina, whereas 20 nm nanoparticles could pass through BRB and affected little cellular viability of retinal endothelial cells and astrocytes. Another safety study also indicated that intravitreal nanogold at concentrations of 67 × 10−3 m/0.1 mL and 670 × 10−3 m/0.1 mL exhibited no retinal or optic nerve toxicity within 1 month histologic examination.135 Additionally, in a series of study made by Gurunathan group, Ag nanoparticles were found to not only inhibit cell survival via PI3K/Akt dependent pathway in bovine retinal endothelial cells,136, 137 but also inhibit vascular endothelial growth factor and interleukin‐1 (IL‐1) induced vascular permeability via Src dependent pathway in porcine retinal endothelial cells.138 These results showed that Ag nanoparticles could be used as therapeutic agents to inhibit the ocular diseases such as diabetic retinopathy.
In addition to heavy metals, other metals such as iron also commonly exist in workplace and can exert potential toxicity to eyes. Previously, it was well reviewed by Dunaief's group that iron could induce ocular siderosis and be associated with a broad range of ocular disease including iris heterochromia, glaucoma, cataract, lens discoloration, retinal detachment, age‐related macular degeneration, as well as intraocular hemorrhage.54, 65 Recently, Park et al. further examined the toxicity mechanisms of nanosized iron particles in human corneal epithelial cells.139 After 24 h incubation, they found that Fe nanoparticles could elevate levels of inflammatory mediator such as nitric oxide, cytokines, and chemokine, increase level of multiple cell death‐related pathway indicators, as well as generate mistranscripted RNA. However, their study was only at in vitro experiment level, and thus further in vivo research is required to investigate the ocular toxicity of iron nanoparticle in a more practical way.
4.2. Metal Oxide Nanomaterials
The manufacturing of metal oxide nanoparticles is reportedly done in large production quantities. According to the National Nanotechnology Initiative of America, silica and ceria in the form of ultrafine abrasive particles including nanoparticles are produced each year in thousands of tons scales for precisely polishing silicon wafers.4 Until 2012, nearly 1.5 million tons of silica nanoparticles had been placed in global market for agriculture, food, and consumer products such as cosmetics.140, 141 In recent years, cerium oxide nanoparticles and cerium oxide nanoparticle‐containing materials have intensely been used in polishing glass and jewelry, and in catalytic converters for many industrial and commercial applications.142, 143 In addition, titanium dioxide and zinc oxide nanoparticles are applied in sunscreens and other cosmetics in a large scale for decades.144 Such large‐scale production and use of metal oxide nanoparticles have increased the risk of human exposure to them, while their toxicity study on eyes is only at early stage.
Photoreceptor cells are characterized with super high rate of oxygen metabolism.145 Thus, photoreceptor cells are constantly exposed to the adverse effects of oxidative stress and light photons. In blindness causing diseases, including inherited retinal degeneration, diabetic retinopathy, macular degeneration, and retinal detachment, regardless of the initiating pathogeny, the intracellular reactive oxygen species are thought to be generated in either chronic or acute ways and are able to induce cell death.146, 147, 148, 149 Due to their redox capacity, cerium oxides are widely used as antioxidant which can reduce oxidative stress in eye.150, 151, 152, 153 Notably, McGinnis's group has utilized the antioxidative feature of nanoceria particles to treat different retinal diseases. For example, they found that cerium oxide nanoparticles could prevent ROS‐induced cell death in primary cell culture of rat retina and prevent vision loss caused by light‐induced degeneration of photoreceptor cells.149 They also reported that in a model of tubby mutant mice, which generally exhibit inherited retinal and cochlear degeneration, nanoceria could delay photoreceptor degeneration and protect retinal function by upregulating genes related to neuroprotection, survival signal pathways, oxidative stress, antioxidant defense, and key photoreceptor‐specific gene, as well as down‐regulating apoptosis signaling pathways and reducing mislocalization of photoreceptor‐specific proteins.154, 155 In addition, they also studied the antioxidant as well as neovascularization inhibiting function of nanoceria particles in wet age‐related macular degeneration mouse models (very low density lipoprotein receptor knockout (vldlr−/−) mouse).156, 157 Recently, they further explored the catalytic activity of cerium oxide nanoparticles in another photoreceptor degeneration P23H‐1 rat model.158 Upon antioxidant research, several studies also showed that nanoceria particles exert no genotoxic effect on human lens epithelial cells159 as well as no toxicity in rat retina using intravitreal injection for a long time.160, 161
In comparison with the huge production and exposure of silica nanoparticles in our society, the study of their eye toxicity is greatly limited. It is well known that the inhalation and retention of crystalline silica may cause severe silicosis, a fibrotic disease of the lungs. However, it was not until 2008 that researchers started studying the effects of silica exposure to the eyes. Yoruk et al. for the first time suggested that eyes can be also considerably affected in the patients with silicosis.49 They found that after 40 ± 26 months silica exposure, patients with silicosis exhibited significant conjunctival hyperaemia and pingueculae. Later on, researchers started putting their attention to the ocular toxicity of silica nanoparticles. For instance, Park's group reported several studies that silica nanoparticles of 50, 100, and 150 nm did not induce significant cytotoxicity in cultured human corneal epithelial cell162 and human corneal keratocytes.163 The in vivo studies using Sprague–Dawley rats also confirmed the safe application of silica nanoparticles to ocular topical administration.164 However, a recent study by our group found that ultrafine (30 and 40 nm) SiO2 nanoparticles caused toxicity such as cell membrane damage, cell death, and mitochondrial dysfunction to primary human corneal epithelial cells as well as observable corneal injury in Sprague–Dawley rats (Figure 2a–d).50 With regard to this, it is suggested that the size difference (≤40 nm in our study and ≥50 nm in Park's study) might exhibit different toxicity both in vitro and in vivo. Further study may be needed to find out the comprehensive influencing factors of silica nanoparticle toxicity on eyes, in order to determine the standard safe use of silica nanoparticle. Interestingly, we also found that the ultrafine SiO2 nanoparticles induced toxicity could be significantly reduced by the introduction of FBS (Figure 2e,f), likely due to the formation of a protective protein corona formation around the nanoparticles. Although other antioxidants such as GSH, resveratrol, and curcumin were also examined for their ability to prevent SiO2 nanoparticle cytotoxicity, none of them exhibited sufficient protective effects. This study provides an alternative clinical treatment (FBS or its derivatives) for reducing corneal toxicity caused by ultrafine particulates.
Figure 2.
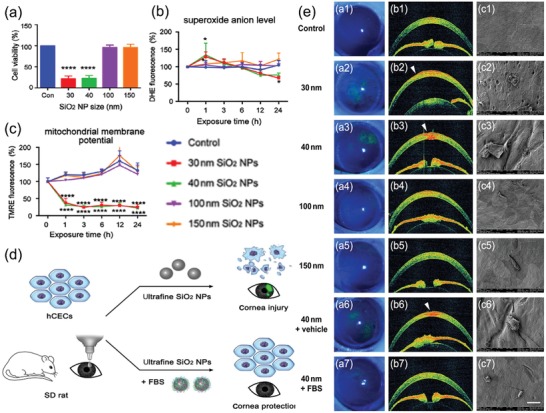
a) Effect of SiO2 NPs on cell viability for range of diameters and concentrations. Mean cell viability of hCECs, measured by CCK8 with a microplate reader following treatment with SiO2 NPs of diameters 30, 40, 100, and 150 nm, at 100 µg mL−1, for 24 h. n = 3 per bar; error bars, standard deviation (SD); ****P < 0.0001 by one‐way ANOVA and Tukey's post hoc test. b,c) Damage to hCECs exposed to SiO2 NPs with varying size. b) Mean intracellular O2 ●−, as measured by dihydroethidium (DHE) fluorescence of hCECs exposed to 100 µg mL−1 SiO2 NPs of different diameters between 0 and 24 h. c) Mean mitochondrial membrane potential, as measured by TMRE fluorescence of hCECs exposed to 100 µg mL−1 SiO2 NPs of different diameters for 0–24 h. n = 3 per bar; error bars show SD; *P < 0.05, ****P < 0.0001, compared with control. d) Scheme of toxicity of silicon dioxide nanoparticles on the cornea and protein corona as a strategy for therapy. e) Structural corneal damage caused by exposure to SiO2 NPs with varying size and therapeutic effect of FBS. a1–a5) Representative images of corneal fluorescein staining under cobalt blue light following exposure to distilled water, and 30, 40, 100 and 150 nm SiO2 NPs, respectively. Corneal damage is revealed by green fluorescence. b1–b5) Representative AS‐OCT images following exposure to the same agents in (a1–a5). White arrowheads indicate corneal defects. c1–c5) Representative SEM images following exposure to the same agents as in (a1–a5). Scale bar, 400 mm. Representative images of corneal fluorescein staining (a6, a7), AS‐OCT (b6, b7), and SEM (c6, c7) for cornea treated with vehicle or FBS after 40 nm SiO2 NPs, respectively. Reproduced with permission.50 Copyright 2018, Elsevier.
Another two typical metal oxide nanoparticles, titanium dioxide and zinc oxide, are also common in industrial and commercial products. Generally, primary TiO2 and ZnO nanoparticles are 10–20 nm in size, but they are typically used in cosmetics as 30 to 150 nm aggregates.165 As size plays an important role in nanoparticle toxicity, different groups measured ocular toxicity using different sizes of TiO2 nanoparticles. Previous study showed that whole‐body exposure of airborne TiO2 microparticles to rats could induce ocular surface immune system, where the Type 2 helper T‐cell pathway plays a key role.42 It resulted in the increasing of interferon‐c (IFN‐c), IL‐4, and IL‐17 levels in anterior segment of eyeball. Several studies showed that TiO2 nanoparticles of 20–50 nm did not induce noticeable toxicity to retinal constituent cells as well as retina of C57BL/5 mice and zebrafish.166, 167 Besides, in an acute toxicity study in rabbits, Warheit et al. found that 129.4 nm fine TiO2 nanoparticles could cause very low toxicity and reversible ocular conjunctival redness in rabbits.168 However, with repeated exposure, Kim's group found that TiO2 of less than 75 nm could induce ocular surface damage, where the area of conjunctival goblet cells decreased after TiO2 exposure.169 In addition, the ocular surface of dry eyes is also reported to be more vulnerable to TiO2 nanoparticles exposure than that of normal eyes (using normal and experimental dry eye rat models).46 Apart from TiO2 nanoparticles, the ocular toxicity of ZnO nanoparticles is also tested in many researches. For instance, Bi's group utilized rat retinal ganglion cells to study the toxicity effect as well as mechanisms of ZnO nanoparticles. As reported, ZnO nanoparticle could cause cell death via overproducing ROS and caspase‐12, decreasing the expression and activity of plasma membrane calcium ATPase and bcl‐2/caspase‐9, as well as disrupting the intracellular calcium homeostasis (Figure 3a,b).170, 171, 172 Moreover, Kim et al. also reported that treatment with 20 nm, negatively charged ZnO nanoparticles could increase retinopathy, which is associated with their local distribution in ocular lesions.67
Figure 3.

a) Principle of ZnO nanoparticle‐induced the apoptosis/necrosis of RGC‐5 cells. Reproduced with permission.170 Copyright 2013, Elsevier. b) Schematic illustration of putative calcium signaling pathway in RGC5 cell death induced by ZnO nanoparticles. The scheme illustrates the possible pathway of intracellular calcium ion elevation mediated by plasma membrane calcium ATPases. Because of exposure to ZnO nanoparticles, the Ca2+‐ATPase activity is inhibited, leading to influx of extracellular calcium and elevation of intracellular calcium ion level, then mitochondria produce excessive ROS. The excessive ROS can also decrease the production of PMCA2 at gene and protein levels, inhibit the Ca2+‐ATPase activity, and further aggravate the disrupted intracellular calcium homeostasis, finally initiate the cellular apoptosis/necrosis. ER = endoplasmic reticulum. Reproduced with permission.171 Copyright 2013, Elsevier.
4.3. Carbon Nanomaterials
The carbon nanoparticles considered here include fullerenes, carbon nanotubes (both single‐walled carbon nanotubes (SWCNTs) and MWCNTs), and graphene and its derivatives (such as GO or reduced graphene oxide (rGO)). Fullerenes represent a group of nanoparticles comprising the entire of carbon atoms (Cx), which have been widespreadly used in many skin care, cosmetics, and biomedical applications such as bioimaging and tumor treatment.173, 174 Pristine fullerene C60 is the most common and stable type among fullerene family (such as C70, C76, C90, C28, C36, etc.).174 On the other hand, carbon nanotubes are the cylindrical shape of fullerenes. With their unique 1D hollow nanostructure and special characteristics, they are also broadly used in energy storage, molecular electronics, artistic materials, medical and health devices, and many others.175 It was reported that the annual global production of carbon nanotubes was running over 100 tons in 2004 and the global revenues from carbon nanotubes were about $230 million with a growth rate of ≈170% in 2006.176, 177 In addition, graphene and its derivatives are the 2D form of carbon in nature. Since its discovery in 2004, graphene and its derivatives are considered to be the new revolutionary materials and are widely used for a variety of industrial, environmental, and biomedical applications such as electronics, next generation semiconductor, batteries, radient heat materials, and electrochemical biosensors.178, 179 This ever‐increasing demand for carbon‐based nanomaterials gives rise to the concerns of their potential toxicity to the environment and human health. Especially when suspended in a gaseous phase or liquids, these nanomaterials can lead to an enhanced exposure to workers and consumers, increasing the potential toxicity by entering body through skin pores or eyes.
Back to 1999, Huczko et al. conducted an eye irritation test to study the toxic effects of fullerene matter on Draize rabbit. Among the two eyes of each rabbit, one eye was instilled with 0.2 mL fullerene water suspension while the other eye was set as control. The author found no eye effects within 24, 48, and 72 h, respectively.180 Later, in order to evaluate the safety of highly purified fullerenes used in cosmetic industry on eyes, Aoshima et al. performed a toxicity study using laboratory animals, where they found that the highly purified fullerenes could cause conjunctival redness and corneal epithelial defects on rabbits, but these symptoms disappeared in two days after eye‐irritation test.181 However, although these researches claimed relatively safe use of fullerene matter, the same results may not be able to directly apply to fullerene nanoparticles, as size generally plays an important role in toxicity. More recently, Ema et al. reported one of their studies on acute ocular irritation of fullerene nanoparticles.182 The results showed that no corneal opacity, abnormality of the iris, or chemosis eye were found on experimental rabbits at all the time point after fullerene nanoparticle application. Although conjunctival redness and blood vessel hyperemia were caused by fullerenes at 1 h, 24 h experiment did not exhibit such outcomes. The previous study indeed offered some important information on acute irritation of fullerene nanoparticles, but the study of long term eye toxicity as well as toxicological effects of other fullerene derivatives are still limited. Further study may be needed to clarify the unsolved toxicity regarding fullerenes.
When it comes to carbon nanotubes, different groups tested their eye toxicity using different in vitro or in vivo model. For instance, as shown in a series studies from Liu's group, both SWCNTs and MWCNTs were toxic to human ocular cells. SWCNTs showed a high toxicity to ARPE‐19 cells with a decrease in cell viability, changes in superoxide dismutase (SOD) levels, membrane integrity, and cell apoptosis.183 On the other hand, MWCNTs were found to cause a decrease in cell survival rate, an increase in lactate dehydrogenase (LDH) release, and enhancement of ROS generation and apoptotic cells on human retinal pigment epithelium cells.63 However, compared to the severe toxicity on cell models, carbon nanotubes show much less adverse effects in experimental animal models. Huczko and Lange found that soot with a high content of SWCNTs had no eye damage in a modified rabbit eye test.184 Kishore et al. examined two different sizes of MWCNT ((MWCNT 1:5–8 µm in length with 3–8 nm inside diameter and outside diameter of 140 ± 30 nm; MWCNT 2:1–10 µm in length with 2–6 nm inside diameter and outside diameter of 10–15 nm) for their eye irritation potential both in vivo and in vitro.185 As the results showed, only reversible conjunctival redness and discharge were found in rabbits and zero irritation score was found in a Hen Egg Chorion Allantoic Membrane (HET‐CAM) test (a test to investigate eye irritancy in vitro). As no significant toxicity differences were found between two sizes of MWCNTs and the in vivo and in vitro results were in a good correlation, their study further indicated low toxicity of carbon nanotubes on eyes. More recently, Ema et al. investigated eye irritation of two products of SWCNTs and two products of MWCNTs in rabbits, where they found that only one of the MWCNTs (among four) showed a very weak, reversible acute irritant (conjunctival redness and vessel hyperemia) to the eyes 1 h after irritation application.186 The other three carbon nanotubes exhibited nonvisible toxic under test conditions.
Graphene with its unique functions is widely used in eye‐related biomedical applications nowadays. For instance, due to its outstanding electrical and mechanical properties as well as excellent biocompatibility, graphene is used to coat contact lenses for electromagnetic interference shielding and dehydration protection.187 Such direct eye‐contact requires higher safety of graphene application. Previously, Tan et al. used human corneal stromal fibroblasts to study graphene toxicity, they found that graphene showed excellent short‐term biocompatibility with corneal cells and tissues.188 However, in practical industrial application, not only graphene but its derivatives such as GO or rGO are also widely used. Therefore, many eye‐toxicity studies also put their focus on graphene derivatives. Yan et al. investigated the toxicity of GO both on human retinal pigment epithelium cells and rabbits.189 Their results indicated that GO did not induce any significant toxicity to cell growth and proliferation or damage to rabbit eyes. However, our previous study showed that GO nanoparticles were toxic to primary human corneal epithelium cells and human conjunctiva epithelium cells in a time‐ and dose‐dependent way (Figure 4a–d).103 In addition, although GO did not cause acute eye irritation on rabbit experiment, short‐term repeated GO exposure to Sprague–Dawley rats induced reversible damage to the eyes via oxidative stress. Our research used both in vitro and in vivo models to study the GO toxicity onto eyes (Figure 4e–g). In consistence to this result, more recently, An et al. studied the ocular toxicity of rGO and GO using both in vitro and in vivo methods.190 In their research, rGO exposure did not cause significant ocular toxicity in mice. On the contrary, short‐term repeated GO exposure could result in obvious intraocular inflammation, an incrassated corneal stromal layer, cell apoptosis in cornea, and iris neovascularization in Kunming mice, as well as significant cell death in corneal epidermal cells. Considering the toxic effect of GO, researchers started putting their focus on alleviating the toxicity of GO by using proper functionalization. For instance, we recently investigated the toxicity of PEGylated GO in ocular tissue, and found that the cytotoxicity of PEG‐GO is dependent on oxidation level instead of surface charge.99 The exposure of human ocular cells to higher oxidized PEG‐GO could lead to oxidative stress‐related cytotoxicity. Notably, instead of simply testing the “rough” cytotoxicity of PEG‐GO, we also explored the molecular mechanism of PEG‐GO toxicity with different surface charge and oxidation degree using whole‐cell gne expression profiling, it showed that highly oxidized PEG‐GO sample induced ROS‐dependent cytotoxicity through NDUFB9‐mediated pathway. This method may become a powerful tool for studying the toxicity effect of nanomaterials because it provides sufficient information of complex interactions between nanoparticles and biological systems, which could help to better design useful surface modifications to reduce toxicity of graphene‐based nanomaterials for biomedical application.
Figure 4.

a–d) The toxicity of GO to hCorECs. a,b) WST‐8 assay analysis of viability in hCorECs exposed to GO for 2 and 24 h, respectively. c,d) Flow cytometry analysis of apoptosis in hCorECs exposed to GO (50 mg mL−1) for 2 and 24 h, respectively. All the assays were conducted in three independent experiments. Data are presented as mean ± SEM. *p < 0.05. **p < 0.01. e–g) Schematic illustration of the experiments on evaluation of ocular irritation potential of GO exposure using e) in vitro and f,g) in vivo models. Reproduced with permission.103 Copyright 2016, Taylor & Francis.
5. Conclusion
In recent years, a dramatic increase in utilization of nanoparticles in food, industry, or cosmetic field has led to an increased concern of their adverse effects on eyes. In this review, we first summarized a few typical ocular disorders upon particulate materials exposure by categories: ocular surface, lens, retina, and optic nerve. As foreign body could induce systematic inflammatory response inside biological bodies, environmental nanoparticles may exert toxicity to all parts of eyes. In addition, we also give a brief introduction of the recent advances of environmental nanoparticle toxicity on eyes. A few number of commonly used nanomaterials in industrial or environmental places (recommended by OECD) are chose to delineate their eye toxicity studies in detail (Table 1).
Table 1.
The list of toxicity studies about commonly used nanomaterials in industrials or environmental places. NP: nanoparticle; SWCNT: single‐walled carbon nanotubes; MWCNT: multiwalled carbon nanotubes; GO: graphene oxide; ROS: reactive oxygen species; SOD: superoxide dismutase; LDH: lactate dehydrogenase
| Compound | Biological model | Mechanism | Outcome | Reference |
|---|---|---|---|---|
| Au NP | Zebrafish eye | Disrupt eye development and pigmentation | 127 | |
| Ag/Au NP | Cell and tissue culture of mouse retina | Oxidative stress | Apoptosis, Neurotoxic effect, and even visual impairment | 62 |
| Ag NP | Bovine retinal endothelial cells | Oxidative stress | Cytotoxicity and apoptosis | 128 |
| Ag NP | New Zealand white rabbits | Conjunctival redness, edema, and discharge | 129 | |
| Ag NP | Guinea pigs | Grade 1 conjunctivae irritation | 130 | |
| Fe NP | Human corneal epithelial cells | Elevated inflammatory response, cell death‐related pathway indicators and generated mistranscripted RNA | Cell death | 139 |
| CeO2 NP | Rat retina primary cells, tubby mutant mice, and very low density lipoprotein receptor knockout mouse | Antioxidative effect | 149, 154, 155, 156, 157 | |
| SiO2 NP | Human corneal epithelial cells and Sprague–Dawley rats | Cell membrane damage, cell death, and mitochondrial dysfunction | Corneal injury | 50 |
| TiO2 NP | Rabbits | Reversible ocular conjunctival redness | 168 | |
| TiO2 NP | New Zealand white rabbits | Ocular surface damage | 169 | |
| ZnO NP | Rat retinal ganglion cells | Overproducing ROS, caspase 12, decreasing plasma membrane calcium ATPase and bcl 2/caspase 9, disrupting intracellular calcium homeostasis | Cell death | 170, 171, 172 |
| ZnO NP | Sprague–Dawley rats | Retinopathy | 67 | |
| Fullerene | Rabbit | Conjunctival redness and corneal epithelial defects | 181 | |
| Fullerene | Rabbits | Conjunctiva redness and blood vessel hyperemia | 182 | |
| SWCNT | ARPE‐19 | Changes in SOD levels, membrane integrity and cell apoptosis | Cell death | 183 |
| MWCNT | Human retinal pigment epithelium cells | increase in LDH release, ROS generation and apoptosis | Decrease in cell survival rate | 63 |
| MWCNT | Rabbit | Conjunctival redness/discharge and vessel hyperemia | 185, 186 | |
| GO NP | Primary human corneal epithelium cells and human conjunctiva epithelium cells; Sprague–Dawley rats | Oxidative stress | Cell death | 103 |
| GO NP | Kunming mice and corneal epidermal cells | Inflammation and apoptosis | Incrassated corneal stromal layer and iris neovascularization | 190 |
Efforts may have been put into the eye toxicity examination of some nanoparticles, it is far from enough. There are still limitations regarding current studies.
In nanoparticle eye toxicity studies, different research groups utilized different biological models including cell lines, aquatic organisms (e.g., embryonic zebrafish), and whole animals (e.g., rabbits or rodents). Sometimes, even using the same biological model, taking cell lines for example, the type of cell lines, culturing conditions, and incubation time also vary from group to group.50, 162 Considering the nature of nanoparticles during toxicity examinations, however, it is irrational to compare results from different research groups and determine whether the obtained toxicity results are physiologically relevant. It is also difficult for us to get a systematic toxicity conclusion of certain nanoparticles from various different research results. Therefore, the standard strategies and interpretation of research outcomes are called for the correct understanding of nanomaterial toxic effects to eyes.191
Although some researches claim that nanoparticle could cause reversible toxicity to eyes, almost all of them fail to testify such conclusion in a long‐term repeated study. Most workers or residents in nanomaterial‐workplace expose their eyes to nanomaterials for years. Therefore, only weeks or months repeated study is not potent to claim the safety of nanomaterials to eyes. Consequently, long‐term toxicity studies are still needed to claim the safe use of nanomaterials.
As seen from the current outcome we have obtained, the toxicity effect of nanoparticle on eyes might be worse than we imagine. Starting with mild discomfort on eyes, conditions could progress to pretty severe nerve or visual impairment, or even loss of vision. Besides, it is also proposed that, in addition to causing direct damage to eyes, the exposure of nanoparticles to eye could also induce some inner damage. For instance, silver and TiO2 nanoparticles could translocate into central nervous system though eye‐to‐brain pathways, which could induce neuroinflammation as well as other problems, such as entering blood.25, 192 Such blood‐assisted distribution can lead to a severe problem. As nanomaterials generally cannot be metabolized and only ultrasmall nanoparticles have a potential to be excreted by renal pathway, most nanomaterials tend to accumulate in various tissues including spleen, liver, or lymph nodes.193 From this point of view, nanoparticle exposure could not only induce adverse effect to eyes, but also expose potential safe concern on other parts of body through ocular absorption. Further studies regarding their inner toxicity examination should also be carried out to realize a comprehensive safety study of eye‐absorbed nanomaterials.
Taken together, eye safety in nanomaterials industry is of equal importance as skin or pulmonary safety. The study of their toxicity can not only help us understand the haphazardness of nanoparticle use, but also give us the hint on how to better protect ourselves in such environment. It is reported that adequate eye protection could prevent 90% of work‐related eye injuries.194 In order to protect eyes from particulate dust and vapors, goggles with side shields, and proper cleaning solutions for contact lens users should be provided in the nanomaterials workplaces. With better understanding of the nanoparticle toxicity to eyes, we can make a safer environment for nanomaterial contacts.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
This work was supported by the National Basic Research Program of China (Grant Nos. 2016YFA0201600 and 2018YFA0107302), National Natural Science Foundation of China (Grant Nos. 551822207, 51772292, 31571015, 11621505, and 11435002), Key Research Program of Frontier Sciences (Grant No. QYZDJ‐SSW‐SLH022), Youth Innovation Promotion Association CAS (Grant No. 2013007), and the Foundation of Southwest Hospital (Grant Nos. SWH2017ZDCX2007 and SWH2016LHJC‐07).
Biographies
Shuang Zhu received her B.S. (2013) and M.S. (2016) degrees at Shandong University and Seoul National University, respectively. She currently works as a research assistant under the supervision of Prof. Zhanjun Gu at CAS Key Laboratory for Biomedical Effects of Nano‐materials & Nanosafety of the Institute of High Energy Physics, Chinese Academy of Science. Her research focuses on the synthesis of nanomaterials and the exploration of their biosafety and biomedical applications.

Zhanjun Gu received his B.E. degree (2002) from Huazhong University of Science and Technology and Ph.D. degree (2007) from Institute of Chemistry, Chinese Academy of Science, under the direction of Prof. Jiannian Yao. He then became a postdoctoral fellow at the University of Georgia. In 2009, he joined the faculty at the CAS Key Lab for Biomedical Effects of Nano‐materials & Nanosafety of the Institute of High Energy Physics, Chinese Academy of Science. His current research interests include nanomaterials synthesis, optical spectroscopy, and bioapplications of luminescent nanomaterials.

Yuliang Zhao is the founder of the CAS Key Lab for Biomedical Effects of Nano‐materials & Nanosafety, and Professor and Deputy Director‐General of the National Center for Nanoscience and Technology of China. He was elected as a member of the Chinese Academy of Science in 2017. His research interests mainly include analytical chemistry, nanotoxicology, and nanomedicine.

Zhu S., Gong L., Li Y., Xu H., Gu Z., Zhao Y., Safety Assessment of Nanomaterials to Eyes: An Important but Neglected Issue. Adv. Sci. 2019, 6, 1802289 10.1002/advs.201802289
Contributor Information
Haiwei Xu, Email: xuhaiwei@tmmu.edu.cn.
Zhanjun Gu, Email: zjgu@ihep.ac.cn.
References
- 1. Bowman D. M., van Calster G., Friedrichs S., Nat. Nanotechnol. 2010, 5, 92. [DOI] [PubMed] [Google Scholar]
- 2. Tsang M. P., Kikuchi‐Uehara E., Sonnemann G. W., Aymonier C., Hirao M., Nat. Nanotechnol. 2017, 12, 734. [DOI] [PubMed] [Google Scholar]
- 3. Auffan M., Rose J., Bottero J.‐Y., Lowry G. V., Jolivet J.‐P., Wiesner M. R., Nat. Nanotechnol. 2009, 4, 634. [DOI] [PubMed] [Google Scholar]
- 4. Ray P. C., Yu H., Fu P. P., J. Environ. Sci. Health, Part C: Environ. Carcinog. Ecotoxicol. Rev. 2009, 27, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hirai T., Yoshioka Y., Higashisaka K., Tsutsumi Y., in Biological Effects of Fibrous and Particulate Substances, Springer, Japan, Tokyo: 2016. [Google Scholar]
- 6. Pietroiusti A., Stockmann‐Juvala H., Lucaroni F., Savolainen K., Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2018, 10, e1513. [DOI] [PubMed] [Google Scholar]
- 7. Iavicoli I., Fontana L., Nordberg G., Crit. Rev. Toxicol. 2016, 46, 490. [DOI] [PubMed] [Google Scholar]
- 8. Reijnders L., J. Mater. Sci. 2012, 47, 5061. [Google Scholar]
- 9. Feng X., Chen A., Zhang Y., Wang J., Shao L., Wei L., Int. J. Nanomed. 2015, 10, 4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oberdörster G., Elder A., Rinderknecht A., J. Nanosci. Nanotechnol. 2009, 9, 4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zolnik B. S., Gonzalez‐Fernandez A., Sadrieh N., Dobrovolskaia M. A., Endocrinology 2010, 151, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sahu S. C., Hayes A. W., Toxicol. Res. Appl. 2017, 1, 2397847317726352. [Google Scholar]
- 13. De Matteis V., Toxics 2017, 5, 29. [Google Scholar]
- 14. Yang Y., Qin Z., Zeng W., Yang T., Cao Y., Mei C., Kuang Y., Nanotechnol. Rev. 2017, 6, 279. [Google Scholar]
- 15. Brohi R. D., Wang L., Talpur H. S., Wu D., Khan F. A., Bhattarai D., Rehman Z. U., Farmanullah F., Huo L. J., Front. Pharmacol. 2017, 8, 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Workplace Eye Injury Statistics—Don't Be One of Them, https://www.hexarmor.com/posts/workplace‐eye‐injury‐statistics (accessed: April 2017).
- 17. Jung S. J., Mehta J. S., Tong L., Ocul. Surf. 2018, 16, 198. [DOI] [PubMed] [Google Scholar]
- 18. Kamboj A., Spiller H. A., Casavant M. J., Chounthirath T., Smith G. A., Ophthalmic Epidemiol. 2019, 26, 84. [DOI] [PubMed] [Google Scholar]
- 19. Buzea C., Pacheco I. I., Robbie K., Biointerphases 2007, 2, MR17. [DOI] [PubMed] [Google Scholar]
- 20. Taghavi S. M., Momenpour M., Azarian M., Ahmadian M., Souri F., Taghavi S. A., Sadeghain M., Karchani M., Electron. Physician 2013, 5, 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Araújo J., Garcia M. L., Mallandrich M., Souto E. B., Calpena A. C., Nanomed.: Nanotechnol., Biol. Med. 2012, 8, 1034. [Google Scholar]
- 22. Uğurlu N., Aşık M. D. a., Çakmak H. B., Tuncer S., Turk M., Çağıl N., Denkbas E. B., J. Biomed. Nanotechnol. 2019, 15, 830. [DOI] [PubMed] [Google Scholar]
- 23.How Nanotechnology Will Revolutionize Eye Care, http://www.reviewofcontactlenses.com/article/how‐nanotechnology‐will‐revolutionize‐eye‐care (accessed: November 2011).
- 24. Kim J. H., Kim J. H., Kim K. W., Kim M. H., Yu Y. S., Nanotechnology 2009, 20, 505101. [DOI] [PubMed] [Google Scholar]
- 25. Boyes W. K., Chen R., Chen C., Yokel R. A., NeuroToxicology 2012, 33, 902. [DOI] [PubMed] [Google Scholar]
- 26. Jaga K., Dharmani C., Environ. Health Prev. Med. 2006, 11, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bhattacharya S. K., Hom G. G., Fernandez C., Hom L. G., Cutaneous Ocul. Toxicol. 2007, 26, 203. [DOI] [PubMed] [Google Scholar]
- 28. Klopfer J., J. Am. Optom. Assoc. 1989, 60, 773. [PubMed] [Google Scholar]
- 29. Wolkoff P., Indoor Air 2017, 27, 246. [DOI] [PubMed] [Google Scholar]
- 30. Rozanova E., Heilig P., Godnic‐Cvar J., Arch. Ind. Hyg. Toxicol. 2009, 60, 205. [DOI] [PubMed] [Google Scholar]
- 31. Li Z., Bian X., Yin J., Zhang X., Mu G., J. Ophthalmol. 2016, 2016, 3628762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang C. J., Yang H. H., Chang C. A., Tsai H. Y., Invest. Opthalmol. Visual Sci. 2012, 53, 429. [DOI] [PubMed] [Google Scholar]
- 33. Tesone A. I., Vitar R. M. L., Tau J., Maglione G. A., Llesuy S., Tasat D. R., Berra A., Environ. Res. 2018, 167, 87. [DOI] [PubMed] [Google Scholar]
- 34. Luo H., Shrestha S., Zhang X., Saaddine J., Zeng X., Reeder T., Ophthalmic Epidemiol. 2018, 25, 280. [DOI] [PubMed] [Google Scholar]
- 35. Organization for Economic Co‐operation and Development , Series on the safety of manufactured nanomaterials, number 6, List of manufactured nanomaterials and list of endpoints for phase one of the OECD testing programme, www.olis.oecd.org/olis/2008doc.nsf/LinkTo/NT00003282/$FILE/JT03246895.PDF (accessed: July 2008).
- 36. Lam H. R., Hougaard K. S., Larsen P. B., Christensen F., Vogel U., The Danish Environmental Protection Agency, 2015.
- 37. Zhou H. Y., Hao J. L., Wang S., Zheng Y., Zhang W. S., Int. J. Ophthalmol. 2013, 6, 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Takahashi Y., Watanabe A., Matsuda H., Nakamura Y., Nakano T., Asamoto K., Ikeda H., Kakizaki H., Ophthalmic Plast. Reconstr. Surg. 2013, 29, 215. [DOI] [PubMed] [Google Scholar]
- 39. Gipson I. K., Invest. Opthalmol. Visual Sci. 2007, 48, 4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Torricelli A. A., Novaes P., Matsuda M., Alves M. R., Monteiro M. L., Arquivos Brasileiros de Oftalmologia 2011, 74, 377. [DOI] [PubMed] [Google Scholar]
- 41. Matsuda M., Bonatti R., Marquezini M. V., Garcia M. L., Santos U. P., Braga A. L., Alves M. R., Saldiva P. H., Monteiro M. L., PLoS One 2015, 10, e0143131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eom Y., Song J. S., Lee H. K., Kang B., Kim H. C., Lee H. K., Kim H. M., Invest. Opthalmol. Visual Sci. 2016, 57, 6580. [DOI] [PubMed] [Google Scholar]
- 43. Ong H. S., Dart J. K., Community Eye Health 2016, 29, 44. [PMC free article] [PubMed] [Google Scholar]
- 44. Zoukhri D., Exp. Eye Res. 2006, 82, 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Javadi M.‐A., Feizi S., J. Ophthalmic Vision Res. 2011, 6, 192. [PMC free article] [PubMed] [Google Scholar]
- 46. Han J. Y., Kang B., Eom Y., Kim H. M., Song J. S., Cornea 2017, 36, 605. [DOI] [PubMed] [Google Scholar]
- 47. Mimura T., Ichinose T., Yamagami S., Fujishima H., Kamei Y., Goto M., Takada S., Matsubara M., Sci. Total Environ. 2014, 487, 493. [DOI] [PubMed] [Google Scholar]
- 48. Camara J. G., Lagunzad J. K. D., Hawaii Med. J. 2011, 70, 262. [PMC free article] [PubMed] [Google Scholar]
- 49. Yoruk O., Ates O., Araz O., Aktan B., Alper F., Sutbeyaz Y., Altas E., Erdogan F., Ucuncu H., Akgun M., Rhinology 2008, 46, 328. [PubMed] [Google Scholar]
- 50. Sun D., Gong L., Xie J., Gu X., Li Y., Cao Q., Li Q., A L., Gu Z., Xu H., Sci. Bull. 2018, 63, 907. [DOI] [PubMed] [Google Scholar]
- 51. Caspi R. R., J. Clin. Invest. 2010, 120, 3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ahmad A., Ahmad I., Khan M. A., Munshi A. B., Siddiqui I., Anzar O., Anzar A., J. Environ. Anal. Toxicol. 2014, 5, 1000252. [Google Scholar]
- 53. Langford‐Smith A., Tilakaratna V., Lythgoe P. R., Clark S. J., Bishop P. N., Day A. J., PLoS One 2016, 11, e0147576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. He X., Hahn P., Iacovelli J., Wong R., King C., Bhisitkul R., Massaro‐Giordano M., Dunaief J. L., Prog. Retinal Eye Res. 2007, 26, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Avunduk A. M., Yardimci S., Avunduk M. C., Kurnaz L., Kockar M. C., Exp. Eye Res. 1997, 65, 417. [DOI] [PubMed] [Google Scholar]
- 56. Sinha R., Kumar C., Titiyal J. S., Indian J. Ophthalmol. 2009, 57, 165. [Google Scholar]
- 57. Allen D., Vasavada A., BMJ 2006, 333, 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fullerton D. G., Bruce N., Gordon S. B., Trans. R. Soc. Trop. Med. Hy. 2008, 102, 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Banerjee A., Mondal N. K., Das D., Ray M. R., Inflammation 2012, 35, 671. [DOI] [PubMed] [Google Scholar]
- 60. Brito J. M., Belotti L., Toledo A. C., Antonangelo L., Silva F. S., Alvim D. S., Andre P. A., Saldiva P. H., Rivero D. H., Toxicol. Sci. 2010, 116, 67. [DOI] [PubMed] [Google Scholar]
- 61. West S. K., Bates M. N., Lee J. S., Schaumberg D. A., Lee D. J., Adair‐Rohani H., Chen D. F., Araj H., Int. J. Environ. Res. Public Health 2013, 10, 5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Soderstjerna E., Bauer P., Cedervall T., Abdshill H., Johansson F., Johansson U. E., PLoS One 2014, 9, e105359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yan L., Li G., Zhang S., Sun F., Huang X., Zhang Q., Dai L., Lu F., Liu Y., Chin. Sci. Bull. 2013, 58, 2347. [Google Scholar]
- 64. Memisogullari R., Yuksel H., Coskun A., Yuksel H. K., Yazgan O., Bilgin C., Tohoku J. Exp. Med. 2007, 212, 247. [DOI] [PubMed] [Google Scholar]
- 65. Raju H. B., Hu Y., Vedula A., Dubovy S. R., Goldberg J. L., PLoS One 2011, 6, e17452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Song D., Dunaief J. L., Front. Aging Neurosci. 2013, 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim Y. H., Kwak K. A., Kim T. S., Seok J. H., Roh H. S., Lee J. K., Jeong J., Meang E. H., Hong J. S., Lee Y. S., Kang J. S., Toxicol. Res. 2015, 31, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Duh E. J., Sun J. K., Stitt A. W., JCI Insight 2017, 2, e93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Is B., M K., Am E., Rh E. L. R., J. Environ. Anal. Toxicol. 2017, 07, 1000473. [Google Scholar]
- 70. Fraser S., Steel D., BMJ Clin. Evidence 2010, 2010, 0710. [PMC free article] [PubMed] [Google Scholar]
- 71. Kimura S. J., Carriker F. R., Hogan M. J., A.M.A. Arch. Ophthalmol. 1956, 56, 361. [DOI] [PubMed] [Google Scholar]
- 72. Shi L., Huang Y.‐F., J. Res. Med. Sci. 2012, 17, 865. [PMC free article] [PubMed] [Google Scholar]
- 73. Oliva G., Perri F., Di Clemente D., Avino P., Manigrasso M., Vernale C., Bailardi F., Trevisani B., Giannico C., Prev. Res. 2014, 3, 148. [Google Scholar]
- 74. Yanoff M., Duker J. S., Ophthalmology, Elsevier, London 2018. [Google Scholar]
- 75. Bahadar H., Maqbool F., Niaz K., Abdollahi M., Iran. Biomed. J. 2016, 20, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cho W. S., Duffin R., Howie S. E., Scotton C. J., Wallace W. A., Macnee W., Bradley M., Megson I. L., Donaldson K., Part. Fibre Toxicol. 2011, 8, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Horie M., Kato H., Fujita K., Endoh S., Iwahashi H., Chem. Res. Toxicol. 2012, 25, 605. [DOI] [PubMed] [Google Scholar]
- 78. Xia T., Kovochich M., Brant J., Hotze M., Sempf J., Oberley T., Sioutas C., Yeh J. I., Wiesner M. R., Nel A. E., Nano Lett. 2006, 6, 1794. [DOI] [PubMed] [Google Scholar]
- 79. Gurr J. R., Wang A. S., Chen C. H., Jan K. Y., Toxicology 2005, 213, 66. [DOI] [PubMed] [Google Scholar]
- 80. Shin S. W., Song I. H., Um S. H., Nanomaterials 2015, 5, 1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Suh W. H., Suslick K. S., Stucky G. D., Suh Y. H., Prog. Neurobiol. 2009, 87, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lefebvre D. E., Venema K., Gombau L., L. G. Valerio, Jr. , Raju J., Bondy G. S., Bouwmeester H., Singh R. P., Clippinger A. J., Collnot E. M., Mehta R., Stone V., Nanotoxicology 2015, 9, 523. [DOI] [PubMed] [Google Scholar]
- 83. Bellmann S., Carlander D., Fasano A., Momcilovic D., Scimeca J. A., Waldman W. J., Gombau L., Tsytsikova L., Canady R., Pereira D. I., Lefebvre D. E., Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2015, 7, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lippmann M., Environ. Health Perspect. 1990, 88, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Braakhuis H. M., Park M. V., Gosens I., De Jong W. H., Cassee F. R., Part. Fibre Toxicol. 2014, 11, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shvedova A. A., Kisin E. R., Mercer R., Murray A. R., Johnson V. J., Potapovich A. I., Tyurina Y. Y., Gorelik O., Arepalli S., Schwegler‐Berry D., Hubbs A. F., Antonini J., Evans D. E., Ku B. K., Ramsey D., Maynard A., Kagan V. E., Castranova V., Baron P., Am. J. Physiol.: Lung Cell. Mol. Physiol. 2005, 289, L698. [DOI] [PubMed] [Google Scholar]
- 87. Lam C. W., James J. T., McCluskey R., Hunter R. L., Toxicol. Sci. 2004, 77, 126. [DOI] [PubMed] [Google Scholar]
- 88. Bottini M., Bruckner S., Nika K., Bottini N., Bellucci S., Magrini A., Bergamaschi A., Mustelin T., Toxicol. Lett. 2006, 160, 121. [DOI] [PubMed] [Google Scholar]
- 89. Chang X. L., Yang S. T., Xing G., J. Biomed. Nanotechnol. 2014, 10, 2828. [DOI] [PubMed] [Google Scholar]
- 90. Saber A. T., Jensen K. A., Jacobsen N. R., Birkedal R., Mikkelsen L., Moller P., Loft S., Wallin H., Vogel U., Nanotoxicology 2012, 6, 453. [DOI] [PubMed] [Google Scholar]
- 91. Stoeger T., Reinhard C., Takenaka S., Schroeppel A., Karg E., Ritter B., Heyder J., Schulz H., Environ. Health Perspect. 2006, 114, 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jacobsen N. R., Moller P., Jensen K. A., Vogel U., Ladefoged O., Loft S., Wallin H., Part. Fibre Toxicol. 2009, 6, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sager T. M., Kommineni C., Castranova V., Part. Fibre Toxicol. 2008, 5, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sager T. M., Castranova V., Part. Fibre Toxicol. 2009, 6, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jiang J., Oberdörster G., Biswas P., J. Nanopart. Res. 2009, 11, 77. [Google Scholar]
- 96. Asati A., Santra S., Kaittanis C., Perez J. M., ACS Nano 2010, 4, 5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Frohlich E., Int. J. Nanomed. 2012, 7, 5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schaeublin N. M., Braydich‐Stolle L. K., Schrand A. M., Miller J. M., Hutchison J., Schlager J. J., Hussain S. M., Nanoscale 2011, 3, 410. [DOI] [PubMed] [Google Scholar]
- 99. Wu W., Yan L., Chen S., Li Q., Gu Z., Xu H., Yin Z. Q., Nanotoxicology 2018, 12, 819. [DOI] [PubMed] [Google Scholar]
- 100. Oberdorster G., Oberdorster E., Oberdorster J., Environ. Health Perspect. 2005, 113, 823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yin H., Too H. P., Chow G. M., Biomaterials 2005, 26, 5818. [DOI] [PubMed] [Google Scholar]
- 102. Gupta A. K., Gupta M., Biomaterials 2005, 26, 1565. [DOI] [PubMed] [Google Scholar]
- 103. Wu W., Yan L., Wu Q., Li Y., Li Q., Chen S., Yang Y., Gu Z., Xu H., Yin Z. Q., Nanotoxicology 2016, 10, 1329. [DOI] [PubMed] [Google Scholar]
- 104. Donaldson K., Stone V., Ann. Ist. Super Sanita 2003, 39, 405. [PubMed] [Google Scholar]
- 105. Hoet P. H., Bruske‐Hohlfeld I., Salata O. V., J. Nanobiotechnol. 2004, 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Takenaka S., Karg E., Roth C., Schulz H., Ziesenis A., Heinzmann U., Schramel P., Heyder J., Environ. Health Perspect. 2001, 109, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Churg A., Stevens B., Wright J. L., Am. J. Physiol. 1998, 274, L81. [DOI] [PubMed] [Google Scholar]
- 108. Oberdorster G., J. Intern. Med. 2010, 267, 89. [DOI] [PubMed] [Google Scholar]
- 109. Singh S., Shi T., Duffin R., Albrecht C., van Berlo D., Hohr D., Fubini B., Martra G., Fenoglio I., Borm P. J., Schins R. P., Toxicol. Appl. Pharmacol. 2007, 222, 141. [DOI] [PubMed] [Google Scholar]
- 110. Clift M. J., Rothen‐Rutishauser B., Brown D. M., Duffin R., Donaldson K., Proudfoot L., Guy K., Stone V., Toxicol. Appl. Pharmacol. 2008, 232, 418. [DOI] [PubMed] [Google Scholar]
- 111. Gatoo M. A., Naseem S., Arfat M. Y., Mahmood Dar A., Qasim K., Zubair S., Biomed. Res. Int. 2014, 2014, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sager T. M., Porter D. W., Robinson V. A., Lindsley W. G., Schwegler‐Berry D. E., Castranova V., Nanotoxicology 2007, 1, 118. [Google Scholar]
- 113. Walkey C. D., Chan W. C., Chem. Soc. Rev. 2012, 41, 2780. [DOI] [PubMed] [Google Scholar]
- 114. Prow T. W., Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol. 2010, 2, 317. [DOI] [PubMed] [Google Scholar]
- 115. Yah C. S., Simate G. S., Iyuke S. E., Pak. J. Pharm. Sci. 2012, 25, 477. [PubMed] [Google Scholar]
- 116. Scherzad A., Meyer T., Kleinsasser N., Hackenberg S., Materials 2017, 10, 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rim K. T., Song S. W., Kim H. Y., Saf. Health Work 2013, 4, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Khanna P., Ong C., Bay B. H., Baeg G. H., Nanomaterials 2015, 5, 1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dogan‐Topal B., Uslu B., Ozkan S. A., in Nanoscale Fabrication, Optimization, Scale‐Up and Biological Aspects of Pharmaceutical Nanotechnology, William Andrew Publishing, Oxford 2018. [Google Scholar]
- 120. Wan R., Mo Y., Feng L., Chien S., Tollerud D. J., Zhang Q., Chem. Res. Toxicol. 2012, 25, 1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Vance M. E., Kuiken T., Vejerano E. P., McGinnis S. P., Hochella M. F. Jr., Rejeski D., Hull M. S., Beilstein J. Nanotechnol. 2015, 6, 1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Marambio‐Jones C., Hoek E. M. V., J. Nanopart. Res. 2010, 12, 1531. [Google Scholar]
- 123. Quadros M. E., Marr L. C., Environ. Sci. Technol. 2011, 45, 10713. [DOI] [PubMed] [Google Scholar]
- 124. Yin Y., Liu J., Jiang G., ACS Nano 2012, 6, 7910. [DOI] [PubMed] [Google Scholar]
- 125. Glover R. D., Miller J. M., Hutchison J. E., ACS Nano 2011, 5, 8950. [DOI] [PubMed] [Google Scholar]
- 126. Karakocak B. B., Raliya R., Davis J. T., Chavalmane S., Wang W. N., Ravi N., Biswas P., Toxicol. In Vitro 2016, 37, 61. [DOI] [PubMed] [Google Scholar]
- 127. Kim K. T., Zaikova T., Hutchison J. E., Tanguay R. L., Toxicol. Sci. 2013, 133, 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sriram M. I., Kalishwaralal K., Barathmanikanth S., Gurunathani S., Nanosci. Methods 2012, 1, 56. [Google Scholar]
- 129. Kim J. S., Song K. S., Sung J. H., Ryu H. R., Choi B. G., Cho H. S., Lee J. K., Yu I. J., Nanotoxicology 2013, 7, 953. [DOI] [PubMed] [Google Scholar]
- 130. Maneewattanapinyo P., Banlunara W., Thammacharoen C., Ekgasit S., Kaewamatawong T., J. Vet. Med. Sci. 2011, 73, 1417. [DOI] [PubMed] [Google Scholar]
- 131. Rosenman K. D., Seixas N., Jacobs I., Br. J. Ind. Med. 1987, 44, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Moss A. P., Sugar A., Hargett N. A., Atkin A., Wolkstein M., Rosenman K. D., Arch. Ophthalmol. 1979, 97, 906. [DOI] [PubMed] [Google Scholar]
- 133. Lansdown A. B., Adv. Pharmacol. Sci. 2010, 2010, 910686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Drake P. L., Hazelwood K. J., Ann. Occup. Hyg. 2005, 49, 575. [DOI] [PubMed] [Google Scholar]
- 135. Bakri S. J., Pulido J. S., Mukherjee P., Marler R. J., Mukhopadhyay D., Retina 2008, 28, 147. [DOI] [PubMed] [Google Scholar]
- 136. Kalishwaralal K., Banumathi E., Ram Kumar Pandian S., Deepak V., Muniyandi J., Eom S. H., Gurunathan S., Colloids Surf., B 2009, 73, 51. [DOI] [PubMed] [Google Scholar]
- 137. Gurunathan S., Lee K. J., Kalishwaralal K., Sheikpranbabu S., Vaidyanathan R., Eom S. H., Biomaterials 2009, 30, 6341. [DOI] [PubMed] [Google Scholar]
- 138. Sheikpranbabu S., Kalishwaralal K., Venkataraman D., Eom S. H., Park J., Gurunathan S., J. Nanobiotechnol. 2009, 7, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Park E. J., Chae J. B., Kang S., Lyu J., Jeong U., Yeom C., Kim Y., Chang J., Toxicol. In Vitro 2017, 42, 348. [DOI] [PubMed] [Google Scholar]
- 140. Liljenström C., Lazarevic D., Finnveden G., Silicon‐Based Nanomaterials in a Life‐Cycle Perspective, Including a Case Study on Self‐Cleaning Coatings, KTH Royal Institute of Technology, Stockholm: 2013. [Google Scholar]
- 141. Murugadoss S., Lison D., Godderis L., Van Den Brule S., Mast J., Brassinne F., Sebaihi N., Hoet P. H., Arch. Toxicol. 2017, 91, 2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Trovarelli A., Catal. Rev. 1996, 38, 439. [Google Scholar]
- 143. Kašpar J., Fornasiero P., Graziani M., Catal. Today 1999, 50, 285. [Google Scholar]
- 144.Literature review on the safety of titanium dioxide and zinc oxide nanoparticles in sunscreens, https://www.tga.gov.au/literature‐review‐safety‐titanium‐dioxide‐and‐zinc‐oxide‐nanoparticles‐sunscreens (accessed: January 2017).
- 145. Punzo C., Xiong W., Cepko C. L., J. Biol. Chem. 2012, 287, 1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Barnham K. J., Masters C. L., Bush A. I., Nat. Rev. Drug Discovery 2004, 3, 205. [DOI] [PubMed] [Google Scholar]
- 147. Caldwell R. B., Bartoli M., Behzadian M. A., El‐Remessy A. E., Al‐Shabrawey M., Platt D. H., Caldwell R. W., Diabetes/Metab. Res. Rev. 2003, 19, 442. [DOI] [PubMed] [Google Scholar]
- 148. Lewis G. P., Erickson P. A., Anderson D. H., Fisher S. K., Exp. Eye Res. 1991, 53, 629. [DOI] [PubMed] [Google Scholar]
- 149. Chen J., Patil S., Seal S., McGinnis J. F., Nat. Nanotechnol. 2006, 1, 142. [DOI] [PubMed] [Google Scholar]
- 150. Cao H. L., Chen J., Zhou X., McGinnis J. F., Invest. Opthalmol. Visual Sci. 2007, 48, 3714. [Google Scholar]
- 151. Patil S., Reshetnikov S., Haldar M. K., Seal S., Mallik S., J. Phys. Chem. C 2007, 111, 8437. [Google Scholar]
- 152. Cai X., Seal S., McGinnis J. F., in Studies on Retinal and Choroidal Disorders, Humana Press, Totowa, NJ: 2012. [Google Scholar]
- 153. Kyosseva S. V., McGinnis J., World J. Ophthalmol. 2015, 5, 23. [Google Scholar]
- 154. Kong L., Cai X., Zhou X., Wong L. L., Karakoti A. S., Seal S., McGinnis J. F., Neurobiol. Dis. 2011, 42, 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Cai X., Sezate S. A., Seal S., McGinnis J. F., Biomaterials 2012, 33, 8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Zhou X., Wong L. L., Karakoti A. S., Seal S., McGinnis J. F., PLoS One 2011, 6, e16733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Cai X., Seal S., McGinnis J. F., Biomaterials 2014, 35, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Wong L. L., Pye Q. N., Chen L., Seal S., McGinnis J. F., PLoS One 2015, 10, e0121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Pierscionek B. K., Li Y., Yasseen A. A., Colhoun L. M., Schachar R. A., Chen W., Nanotechnology 2010, 21, 035102. [DOI] [PubMed] [Google Scholar]
- 160. Wong L. L., Hirst S. M., Pye Q. N., Reilly C. M., Seal S., McGinnis J. F., PLoS One 2013, 8, e58431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cai X., Seal S., McGinnis J. F., Mol. Vision 2016, 22, 1176. [PMC free article] [PubMed] [Google Scholar]
- 162. Park J. H., Jeong H., Hong J., Chang M., Kim M., Chuck R. S., Lee J. K., Park C. Y., Sci. Rep. 2016, 6, 37762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Yim B., Park J. H., Jeong H., Hong J., Shin Y. J., Chuck R. S., Park C. Y., Invest. Opthalmol. Visual Sci. 2017, 58, 362. [DOI] [PubMed] [Google Scholar]
- 164. Kim M., Park J. H., Jeong H., Hong J., Choi W. S., Lee B. H., Park C. Y., Sci. Rep. 2017, 7, 8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Schilling K., Bradford B., Castelli D., Dufour E., Nash J. F., Pape W., Schulte S., Tooley I., van den Bosch J., Schellauf F., Photochem. Photobiol. Sci. 2010, 9, 495. [DOI] [PubMed] [Google Scholar]
- 166. Jo D. H., Kim J. H., Son J. G., Song N. W., Kim Y. I., Yu Y. S., Lee T. G., Kim J. H., Nanomed.: Nanotechnol., Biol. Med. 2014, 10, e1109. [Google Scholar]
- 167. Wang Y. J., He Z. Z., Fang Y. W., Xu Y., Chen Y. N., Wang G. Q., Yang Y. Q., Yang Z., Li Y. H., Int. J. Ophthalmol. 2014, 7, 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Warheit D. B., Hoke R. A., Finlay C., Donner E. M., Reed K. L., Sayes C. M., Toxicol. Lett. 2007, 171, 99. [DOI] [PubMed] [Google Scholar]
- 169. Eom Y., Song J. S., Lee D. Y., Kim M. K., Kang B. R., Heo J. H., Lee H. K., Kim H. M., Ocul. Surf. 2016, 14, 224. [DOI] [PubMed] [Google Scholar]
- 170. Guo D., Bi H., Liu B., Wu Q., Wang D., Cui Y., Toxicol. In Vitro 2013, 27, 731. [DOI] [PubMed] [Google Scholar]
- 171. Guo D., Bi H., Wang D., Wu Q., Int. J. Biochem. Cell Biol. 2013, 45, 1849. [DOI] [PubMed] [Google Scholar]
- 172. Guo D., Bi H., Wu Q., Wang D., Cui Y., J. Nanosci. Nanotechnol. 2013, 13, 3769. [DOI] [PubMed] [Google Scholar]
- 173. Lalwani G., Sitharaman B., Nano LIFE 2013, 03, 1342003. [Google Scholar]
- 174. Mousavi S. Z., Nafisi S., Maibach H. I., Nanomed.: Nanotechnol., Biol. Med. 2017, 13, 1071. [Google Scholar]
- 175.Engineered nanoparticles: Review of health and environmental safety (ENRHES). Project final report, https://www.nanowerk.com/nanotechnology‐report.php?reportid=133 (accessed: September 2010).
- 176.London, New Nanotubes Research and Daily Nanotechnology Updates, http://www.nanotech‐now.com/Cientifica‐release‐02162004.htm (accessed: February 2004).
- 177. Donaldson K., Aitken R., Tran L., Stone V., Duffin R., Forrest G., Alexander A., Toxicol. Sci. 2006, 92, 5. [DOI] [PubMed] [Google Scholar]
- 178. Singh V., Joung D., Zhai L., Das S., Khondaker S. I., Seal S., Prog. Mater. Sci. 2011, 56, 1178. [Google Scholar]
- 179. Tung T. T., Nine M. J., Krebsz M., Pasinszki T., Coghlan C. J., Tran D. N. H., Losic D., Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar]
- 180. Huczko A., Lange H., Calko E., Fullerene Sci. Technol. 1999, 7, 935. [Google Scholar]
- 181. Aoshima H., Saitoh Y., Ito S., Yamana S., Miwa N., J. Toxicol. Sci. 2009, 34, 555. [DOI] [PubMed] [Google Scholar]
- 182. Ema M., Matsuda A., Kobayashi N., Naya M., Nakanishi J., Cutaneous Ocul. Toxicol. 2013, 32, 128. [DOI] [PubMed] [Google Scholar]
- 183. Yan L., Zhang S., Zeng C., Xue Y. H., Zhou Z. L., Lu F., Chen H., Qu J., Dai L. M., Liu Y., Adv. Mater. Res. 2011, 287–290, 32. [Google Scholar]
- 184. Huczko A., Lange H., Fullerene Sci. Technol. 2001, 9, 247. [Google Scholar]
- 185. Kishore A. S., Surekha P., Murthy P. B., Toxicol. Lett. 2009, 191, 268. [DOI] [PubMed] [Google Scholar]
- 186. Ema M., Matsuda A., Kobayashi N., Naya M., Nakanishi J., Regul. Toxicol. Pharmacol. 2011, 61, 276. [DOI] [PubMed] [Google Scholar]
- 187. Lee S., Jo I., Kang S., Jang B., Moon J., Park J. B., Lee S., Rho S., Kim Y., Hong B. H., ACS Nano 2017, 11, 5318. [DOI] [PubMed] [Google Scholar]
- 188. Tan X. W., Thompson B., Konstantopoulos A., Goh T. W., Setiawan M., Yam G. H., Tan D., Khor K. A., Mehta J. S., Invest. Opthalmol. Visual Sci. 2015, 56, 6605. [DOI] [PubMed] [Google Scholar]
- 189. Yan L., Wang Y., Xu X., Zeng C., Hou J., Lin M., Xu J., Sun F., Huang X., Dai L., Lu F., Liu Y., Chem. Res. Toxicol. 2012, 25, 1265. [DOI] [PubMed] [Google Scholar]
- 190. An W., Zhang Y., Zhang X., Li K., Kang Y., Akhtar S., Sha X., Gao L., Exp. Eye Res. 2018, 174, 59. [DOI] [PubMed] [Google Scholar]
- 191. Kolle S. N., Sauer U. G., Rey Moreno M. C., Teubner W., Wohlleben W., Landsiedel R., Part. Fibre Toxicol. 2015, 13, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Wu T., Tang M., Nanomedicine 2018, 13, 233. [DOI] [PubMed] [Google Scholar]
- 193. Ai J., Biazar E., Jafarpour M., Montazeri M., Majdi A., Aminifard S., Zafari M., Akbari H. R., Rad H. G., Int. J. Nanomed. 2011, 6, 1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Peate W. F., Am. Fam. Physician 2007, 75, 1017. [PubMed] [Google Scholar]


