Abstract
Exogenous ghrelin reduces glucose-stimulated insulin secretion and endogenous ghrelin protects against hypoglycemia during starvation. Islet ε-cells produce ghrelin and δ-cells express growth hormone secretagogue receptor (GHSR), suggesting the possibility of a paracrine mechanism for islet ghrelin to reach high local concentrations and affect insulin secretion. GHSR has high constitutive activity and may act independently of ghrelin. The objective in this study was to determine whether an intraislet ghrelin-GHSR axis modulates insulin secretion and glucose metabolism using mouse models lacking ghrelin (Ghrl−/−) or GHSR (Ghsr−/−). Ghsr−/− and Ghsr+/+ mice had comparable islet ghrelin concentrations. Exogenous ghrelin decreased insulin secretion in perifused isolated islets in a GHSR-dependent manner. Islets isolated from Ghrl−/− or Ghsr−/− mice did not differ from controls in glucose-, alanine-, or GLP-1–stimulated insulin secretion during perifusion. Consistent with this finding, Ghrl−/− and Ghsr−/− male mice studied after either 6 or 16 h of fasting had blood glucose concentrations comparable with those of controls following intraperitoneal glucose, or insulin tolerance tests, or after mixed nutrient meals. Collectively, our data provide strong evidence against a paracrine ghrelin-GHSR axis mediating insulin secretion or glucose tolerance in lean, chow-fed adult mice.
Introduction
Ghrelin is a 28-peptide hormone originally discovered as a ligand for the growth hormone secretagogue receptor (GHSR) (1). Beyond its reported orexigenic properties and role in feeding behavior (2), ghrelin has been proposed as a mechanism for regulating fuel substrate metabolism in health and during the challenges of high-fat diet (3–6), starvation (4,7), and exercise (8). Leptin-deficient ob/ob mice with a genetic deletion of ghrelin had improved glucose tolerance and insulin secretion compared with ob/ob mice (3). Exogenous ghrelin administration reduces insulin secretion in rodents (9–12) and humans (13–15), albeit at concentrations that exceed circulating values. While most circulating ghrelin originates from the stomach, pancreatic islet ε-cells also express ghrelin (16–20), and ghrelin concentrations in the rat pancreatic vein exceed those measured in the pancreatic artery (12). It is possible that local ghrelin levels within the islet may be substantially higher than circulating concentrations. Within the pancreatic islet, GHSR expression is localized to somatostatin-secreting δ-cells (20–22), supporting a mechanism whereby ghrelin binds to GHSR to increase somatostatin secretion, thereby reducing insulin secretion (21,22). Work from several groups indicates that the GHSR has high constitutive activity (23–28) and GHSR antagonism increased insulin secretion during static rat islet culture (29) and in the perfused rat pancreas (12), suggesting that either islet ghrelin-dependent or ghrelin-independent (constitutive) GHSR activity could regulate insulin secretion. Moreover, whether ghrelin acts on islet GHSRs through endocrine or paracrine mechanisms warrants further investigation.
The objective of the current study is to establish the role of the islet ghrelin-GHSR axis in the physiologic regulation of insulin secretion in rodents. We hypothesized that removal of ghrelin or GHSR would increase insulin secretion in response to stimuli, resulting in improved glucose tolerance in vivo. This article describes a series of experiments using genetic models of GHSR and ghrelin deletion to determine their contributions to islet-intrinsic regulation of insulin secretion.
Research Design and Methods
Animals
Animal studies were approved by the Duke University Institutional Animal Care and Use Committee. Male and female C57Bl/6J mice (8–12 weeks old) were used for pharmacologic experiments where described. Cohorts of transgenic animals lacking ghrelin (Ghrl−/−) or GHSR (Ghsr−/−) included littermate, age-matched, and cage-matched Ghrl+/+ and Ghsr+/+ controls. Transgenic lines were on a C57Bl/6J background and backcrossed every 10 generations. All mice were fed standard chow for the duration of study. In vivo experiments were performed at 8–10 weeks of age, and islets were isolated at 9–13 weeks of age. Briefly, Ghrl−/− mice were generated using high-throughput recombinant VelociGene technology (30) and obtained from Dr. Mark Sleeman (Regeron Pharmaceuticals, Inc.) (31). Ghsr−/− mice have a loxP-flanked transcriptional blocking cassette upstream of the GHSR gene and were obtained from Drs. Joel Elmquist and Jeffrey Zigman (University of Texas Southwestern Medical Center) (32). Ghrl−/− and Ghrl+/+ mice were genotyped used a common forward primer (5′-TCGTCCAGCAGTCCTTACTT-3′) and wild-type reverse (5′-TTAGGGAGACAGACTGACTGA) and knockout reverse (5′-AACAACCCGTCGGATTCTCC). Ghsr−/− and Ghsr+/+ mice were genotyped with primers (5′-GAT GCT TGG GGA AGA GAG AAG TGA-3′, 5′-CAG ATG TAG CTA AAA GGC CTA TCA CA-3′, and 5′-CGG TCT CCA CCC TTC ATT ACT TTA-3′).
In Vivo Experiments
Intraperitoneal glucose tolerance tests (IPGTTs) (1.5 g/kg body wt) were performed after a 6-h (8:00 a.m.–2:00 p.m.) or 16-h overnight (7:00 p.m.–11:00 a.m.) fast during which mice were kept on fresh Sani-Chips bedding with free access to water. Fasting blood samples were collected at baseline to measure total ghrelin before IPGTT. Mixed-meal tolerance tests (MMTTs) were performed after a 6-h fast and consisted of a 200-μL oral gavage of Ensure. Tail vein blood glucose was collected throughout experiments where indicated, and insulin was measured at baseline and 10 min after oral gavage where indicated (cat. no. 90080, Ultra-Sensitive Mouse Insulin ELISA; Crystal Chem). Intraperitoneal insulin tolerance tests (ITTs) (0.5 units/kg body wt) were performed after a 6-h fast. Body composition was measured by nuclear magnetic resonance (NMR) (Bruker minispec Whole Body Composition Analyzer).
Islet Isolation
Islets were isolated by inflating the pancreas with collagenase type V (0.8 mg/mL, in Hanks’ balanced salt solution) injected retrograde through the pancreatic duct. Digestion occurred at 37°C and was stopped with application of ice-cold RPMI (2 mmol/L L-glutamine, 0.25% BSA). Islets were separated from pancreatic tissue using a histopaque gradient and allowed to recover in RPMI (11.1 mmol/L glucose, 10% FBS, 1% penicillin/streptomycin) overnight before experiments were performed.
Islet Perifusion
For islet perifusion, 100 islets were handpicked and loaded into 0.275-mL chambers containing Krebs-Ringer phosphate-HEPES (KRPH) (140 mmol/L NaCl, 4.7 mmol/L KCl, 1.5 mmol/L CaCl2, 1 mmol/L NaH2PO4, 1 mmol/L MgSO4, 5 mmol/L HEPES, 2 mmol/L NaHCO3, 1% fatty acid–free BSA) in 2.8 mmol/L glucose. Prior to all experiments, KRPH with 2.8 mmol/L glucose was perifused at a rate of 200 μL/min for 48 min to equilibrate using the BioRep Perifusion system. Following equilibration, experimental conditions were applied and perifusate was collected each minute. Acyl ghrelin (Bachem) and GLP-1 (AS-22463; AnaSpec) were reconstituted according to the manufacturer’s instructions, and alanine was diluted in KRPH prior to experiment. Perifusate insulin concentrations were measured with AlphaLISA (PerkinElmer).
Measurement of Plasma and Tissue Ghrelin
For measurement of circulating ghrelin, tail blood was collected after 6- or 16-h fasting and 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride was added (1 mg/mL final concentration). After centrifugation, plasma was acidified by addition of 1 N HCl and samples were stored at −80°C until assay. For measurement of islet ghrelin, ∼150 islets per mouse were isolated as described above and lysed in 100 mmol/L glycine-HCl (0.1% BSA, pH 3) with 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (1 mg/mL), vortexed, and stored at −20°C until assay; ghrelin values were normalized to islet protein content. These measures used a Rat/Mouse Ghrelin ELISA (EZRGRT-91K; Millipore) that measured total ghrelin (acylated and deacylated forms). A subset of islet lysates were measured by Dr. Bruce Gaylinn at the University of Virginia for acyl and desacyl ghrelin content using specific, two-site sandwich assays (33).
Analysis/Statistics
Data are presented as means ± SEM, and glucose tolerance is expressed as integrated area under the curve (AUC). Analysis was done using GraphPad Prism. Comparisons between genotypes were done within sex. Differences between two groups were compared by unpaired t test or Welch test. Differences between more than two groups were compared by one-way ANOVA with Sidak post hoc test for selected comparisons. Tests are described in the legend of figures and tables for each comparison.
Results
Islet Ghrelin Content and Plasma Ghrelin Concentrations
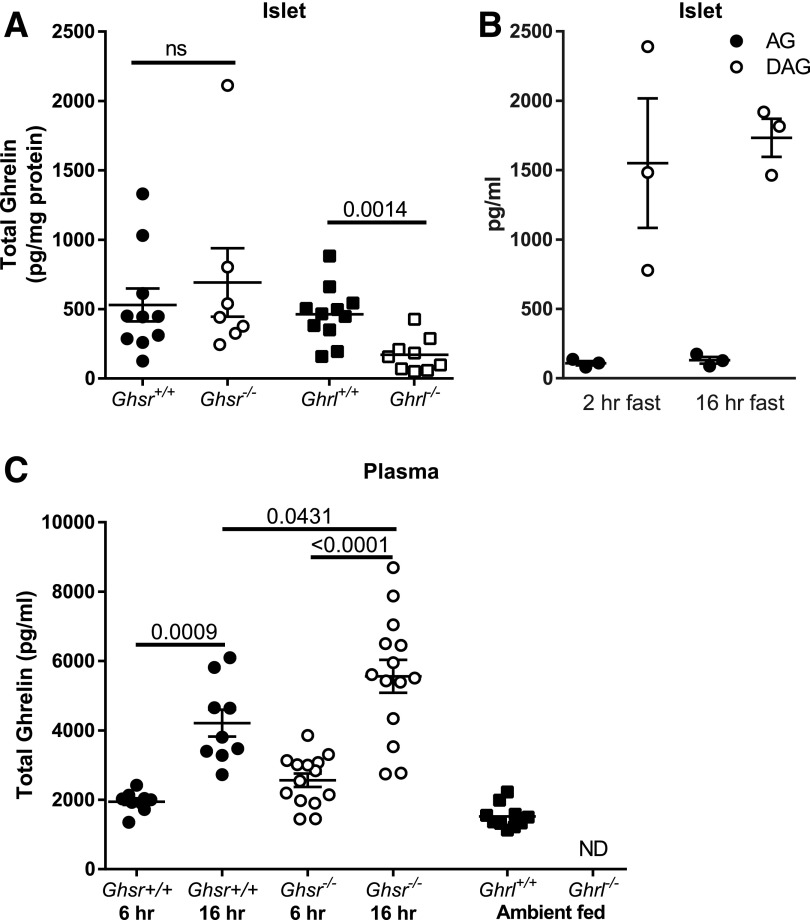
Ghrelin concentrations measured in islet lysates were easily detectable in both the Ghsr+/+ (530 ± 118 pg/mg) and Ghsr−/− (692 ± 247 pg/mg) mice (P = 0.52 for the between-group comparison), as well as the Ghrl+/+ mice (462 ± 61 pg/mg) (Fig. 1A). Ghrl−/− mice had a minimal amount of detectable ghrelin in islet extracts, though considerably less than in Ghrl+/+ and at the very low end of the standard curve of the assay (Fig. 1A). In Ghrl−/− islet lysate samples (n = 10), one sample was below the range of the assay (40 pg/mL, per the manufacturers specifications). When the remaining nine Ghrl−/− samples were diluted 1:1 with lysis buffer and the measurement was repeated, ghrelin was undetectable or below the range in six samples, suggesting that the peptide, if present at all, is very low in Ghrl−/− mice. A limited number of islet lysates from wild-type mice (n = 3 per group) were measured for acyl and desacyl ghrelin after 2- or 16-h fasts. Acyl ghrelin was detectable (2-h fast 108 ± 16.2 pg/mL and 16-h fast 130 ± 24.5 pg/mL); however, desacyl ghrelin was greater in concentration (2-h fast 1,551 ± 467 pg/mL and 16-h fast 1,733 ± 138 pg/mL) (Fig. 1B).
Figure 1.
Ghrelin concentrations in islets and plasma for Ghsr−/− and Ghrl−/− mice. A: Total islet ghrelin content did not differ between Ghsr+/+ and Ghsr−/− and was significantly greater in Ghrl+/+ mice compared with Ghrl−/−. Upon 1:1 dilution, most Ghrl−/− islet ghrelin levels were undetectable or below the range of detection. B: Islet desacyl ghrelin (DAG) was greater than acyl ghrelin (AG) after 2-h and 16-h fasts in a subset of mice (n = 3 per group). C: Total ghrelin in plasma increased between 6-h and 16-h fasts in Ghsr+/+ and Ghsr−/− male mice. Ghsr−/− had greater ghrelin after 16-h fast compared with Ghsr+/+, and no significant differences were detected between Ghsr−/− and Ghsr+/+ mice after 6-h fast. Circulating total ghrelin was present in Ghrl+/+ and undetectable in Ghrl−/− mice. All panels: mean ± SEM, with each symbol representing an individual animal. Unpaired t test (A); one-way ANOVA with Sidak post hoc test (C). P values are indicated between significant comparisons. hr, hour; ND, not detectable; ns, not significant.
To test the effect of fasting on circulating ghrelin concentrations, we measured plasma ghrelin in male (Fig. 1C) and female (Supplementary Fig. 1) Ghsr−/− and Ghsr+/+ mice after a 6-h (8:00 a.m.–2:00 p.m.) or 16-h (overnight [7:00 p.m.–11:00 a.m.]) fast. Ghrelin concentrations were greater after the overnight fast compared with the 6-h fast in all groups (Fig. 1C and Supplementary Fig. 1). Male Ghsr−/− mice had greater ghrelin concentration after a 16-h fast compared with Ghsr+/+ (Fig. 1C), and female Ghsr−/− mice had a trend for increased ghrelin compared with Ghsr+/+ (P = 0.072) (Supplementary Fig. 1). Ghrl−/− mice did not have detectable plasma ghrelin (Fig. 1C).
Glucose Tolerance and Insulin Sensitivity in Ghsr−/− Mice
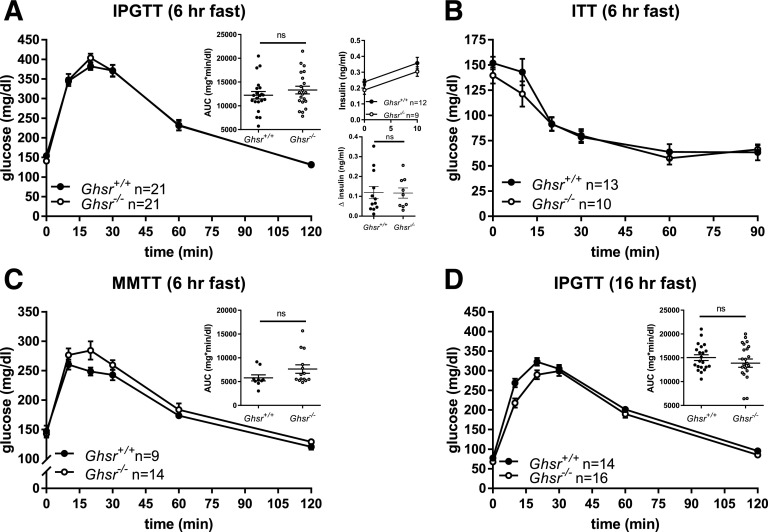
For determination of whether GHSR deletion alters glucose metabolism in adult mice, glucose and insulin tolerance was compared in Ghsr−/− and control Ghsr+/+ mice following 6 or 16 h of fasting. Ghsr−/− mice had modestly lower fasting glucose and body weight compared with controls (Table 1). However, after a 6-h fast, Ghsr−/− and Ghsr+/+ male mice had comparable glucose excursions and insulin secretion (Fig. 2A). Similar decreases in blood glucose were observed in Ghsr−/− and Ghsr+/+ mice during the ITT (Fig. 2B), suggesting similar insulin sensitivity between groups. Ghsr+/+ and Ghsr−/− mice also had comparable responses to a mixed-nutrient challenge (Ensure) (Fig. 2C). Given the important role of ghrelin to defend against hypoglycemia during starvation, the effect of GHSR removal on glucose tolerance was tested after an overnight fast, when circulating concentrations of ghrelin are elevated (Fig. 1C and Supplementary Fig. 1). Even in this setting, Ghsr−/− and Ghsr+/+ mice had similar glycemic responses to intraperitoneal glucose (Fig. 2D).
Table 1.
Characteristics of Ghsr+/+ and Ghsr−/− male and female mice
| Variable | Male |
Female |
||
|---|---|---|---|---|
|
Ghsr+/+ |
Ghsr−/− | Ghsr+/+ | Ghsr−/− | |
| Body composition | (n = 12) | (n = 9) | (n = 12) | (n = 8) |
| % lean mass | 75.8 ± 1.9 | 76.5 ± 2.0 | 72.5 ± 1.6 | 73.5 ± 1.4 |
| % fat mass | 11.8 ± 1.6 | 10.6 ± 2.1 | 13.9 ± 1.9 | 13.2 ± 1.7 |
| % fluid | 4.7 ± 0.3 | 4.8 ± 0.2 | 5.2 ± 0.3 | 5.2 ± 0.4 |
| Body weight before 6-h fast (g) | 25.2 ± 2.0 (n = 21) | 23.3 ± 1.9 (n = 23)* | 20.3 ± 1.4 (n = 23) | 20.4 ± 1.0 (n = 17) |
| Body weight before 16-h fast (g) | 24.9 ± 2.1 (n = 21) | 22.9 ± 2 (n = 23)* | 20.1 ± 1.4 (n = 22) | 19.7 ± 1.1 (n = 17) |
| Body weight after 16-h fast, before IPGTT (g) | 20.2 ± 1.5 (n = 12) | 19.7 ± 1.9 (n = 9) | 17.1 ± 1.1 (n = 12) | 17.0 ± 1.4 (n = 7) |
| Weight loss over 16-h fast (g) | 3.4 ± 0.4 (n = 12) | 3.0 ± 0.5 (n = 9)* | 2.3 ± 0.3 (n = 12) | 2.5 ± 0.3 (n = 7) |
| Fasting glucose, 6-h IPGTT (mg/dL) | 154.3 ± 21.1 (n = 21) | 141.9 ± 18.6 (n = 23)* | 135.9 ± 16.6 (n = 23) | 133.8 ± 15.1 (n = 17) |
| Fasting glucose, 16-h IPGTT (mg/dL) | 77.3 ± 14.2 (n = 21) | 68.4 ± 10.3 (n = 23)* | 68.7 ± 7.8 (n = 22) | 62.9 ± 11.43 (n = 17)† |
Data are means ± SD unless otherwise indicated. Body composition was gathered by NMR. Comparisons made with t test between genotypes (within sex).
†P < 0.1;
*P < 0.05 compared with Ghsr+/+.
Figure 2.
Glucose tolerance in Ghsr+/+ and Ghsr−/− male mice. A: IPGTT (1.5 g/kg body wt) following 6-h fast and glucose AUC, insulin concentrations at baseline (0 min) and 10 min, and change in insulin between 0 and 10 min. B: ITT (0.5 units/kg body wt). C: MMTT (200 μL; Ensure) and glucose AUC. D: IPGTT (1.5 g/kg body wt) following 16-h fast and glucose AUC. Glucose concentrations are shown as mean ± SEM, and glucose AUCs are shown as mean ± SEM with each symbol representing an individual animal. Unpaired t test for all comparisons. hr, hour; ns, not significant.
Ghsr−/− mice lost less weight over the course of the 16-h fast so that their body weights were comparable to those of controls before the IPGTT (Table 1). There were no differences in body composition (% lean mass, fat mass, fluid) between Ghsr−/− and Ghsr+/+ mice (Table 1). Glucose tolerance following an intraperitoneal glucose challenge was reanalyzed in a subset of Ghsr+/+ and Ghsr−/− mice (n = 4–11/group) of comparable weights (within 1 g), and when body weight was matched, there was no detectable difference in glucose tolerance (data not shown). Therefore, body weight differences are not masking differences in glucose tolerance.
In parallel experiments with female Ghsr−/− and Ghsr+/+ mice, results varied only slightly from males (Supplementary Fig. 2). After a 6-h fast, Ghsr−/− female mice had a modest impairment of intraperitoneal glucose tolerance, in concert with lower insulin secretion (Supplementary Fig. 2A). Similar to male mice, female Ghsr−/− mice did not differ from Ghsr+/+ controls in insulin sensitivity, glucose tolerance during MMTT, or IPGTT following a 16-h fast (Supplementary Fig. 2B–D). Ghsr−/− female mice did not differ in body weight, fasting glucose, or weight loss in response to 16-h fast (Table 1). These data raise the possibility of a subtle, sex-specific glucose phenotype in the absence of GHSR.
Glucose Tolerance and Insulin Sensitivity in Ghrl−/− Mice
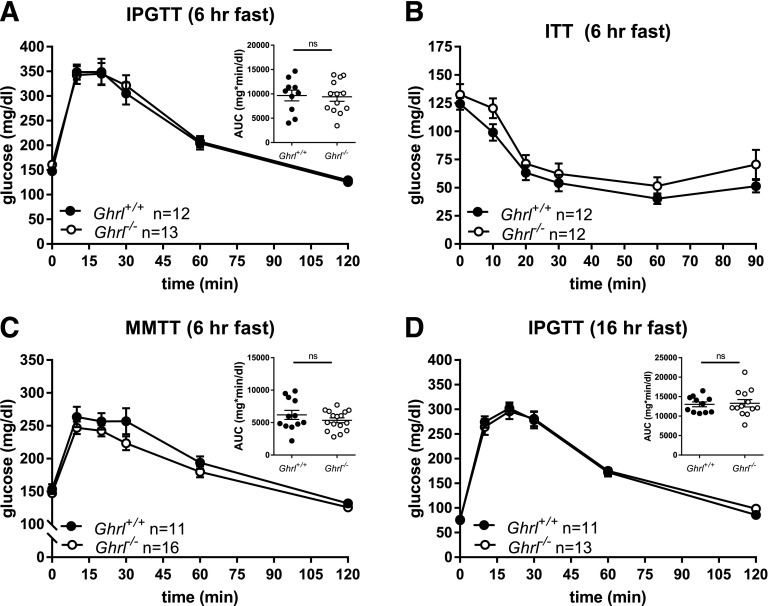
For testing of the importance of ghrelin in glucose and meal tolerance, Ghrl−/− and Ghrl+/+ mice had a similar series of experiments as described above. Following a 6-h fast, male Ghrl−/− mice had elevated fasting glucose compared with Ghrl+/+ (Table 2) but similar responses to intraperitoneal glucose (Fig. 3A), intraperitoneal insulin (Fig. 3B), and oral mixed-nutrient challenges (Fig. 3C). There were also no differences in fasting glucose concentrations or glucose excursion following a 16-h fast (Fig. 3D and Table 2). Parallel experiments performed in Ghrl−/− and Ghrl+/+ female mice (Supplementary Fig. 3 and Table 2) conformed to the results in males: comparable intraperitoneal glucose tolerance (after both 6- and 16-h fasts) and insulin sensitivity. However, female Ghrl−/− mice had a small but significant decrease in glucose concentrations following mixed-meal gavage (Supplementary Fig. 3C). There were no differences in body composition between Ghrl−/− and Ghrl+/+ male or female mice (Table 2).
Table 2.
Characteristics of Ghrl+/+ and Ghrl−/− male and female mice
| Variable | Male |
Female |
||
|---|---|---|---|---|
|
Ghrl+/+ |
Ghrl−/− | Ghrl+/+ | Ghrl−/− | |
| Body composition | (n = 12) | (n = 13) | (n = 15) | (n = 10) |
| % lean mass | 73.5 ± 3.1 | 72.8 ± 3.7 | 75 ± 3.6 | 75 ± 4.2 |
| % fat mass | 13.7 ± 2.6 | 15 ± 3.6 | 12.4 ± 3.4 | 12.9 ± 4.3 |
| % fluid | 4.9 ± 0.6 | 4.7 ± 0.2 | 4.7 ± 0.3 | 4.8 ± 0.2 |
| Body weight before 6-h fast (g) | 26.4 ± 1.9 (n = 12) | 26.3 ± 1.4 (n = 13) | 21 ± 1 (n = 15) | 22.7 ± 1.5 (n = 11)† |
| Body weight before 16-h fast (g) | 26.9 ± 1.8 (n = 12) | 26.3 ± 1.5 (n = 13) | 21.0 ± 0.8 (n = 16) | 22.2 ± 1.4 (n = 11)* |
| Fasting glucose, 6-h IPGTT (mg/dL) | 145.3 ± 17.5 (n = 12) | 160.8 ± 19.4 (n = 13)* | 162.3 ± 22.2 (n = 16) | 172 ± 10.3 (n = 11) |
| Fasting glucose, 16-h IPGTT (mg/dL) | 75.5 ± 10.6 (n = 12) | 76.2 ± 19.2 (n = 13) | 75.5 ± 15.1 (n = 16) | 74.5 ± 11.0 (n = 11) |
Data are means ± SD unless otherwise indicated. Body composition was gathered by NMR. Comparisons made with t test or by Welch test when SDs were different between genotypes (within sex).
†P < 0.1;
*P < 0.05 compared with Ghrl+/+.
Figure 3.
Glucose tolerance in Ghrl+/+ and Ghrl−/− male mice. A: IPGTT (1.5 g/kg body wt) following 6-h fast and glucose AUC. B: ITT (0.5 units/kg body wt). C: MMTT (200 μL; Ensure) and glucose AUC. D: IPGTT (1.5 g/kg body wt) following 16-h fast and glucose AUC. Glucose concentrations are shown as mean ± SEM, and glucose AUCs are shown as mean ± SEM with each symbol representing an individual animal. A and D: Unpaired t test for all comparisons. C: Welch test. hr, hour; ns, not significant.
Ghrelin Reduces Glucose-Stimulated Insulin Secretion via the GHSR
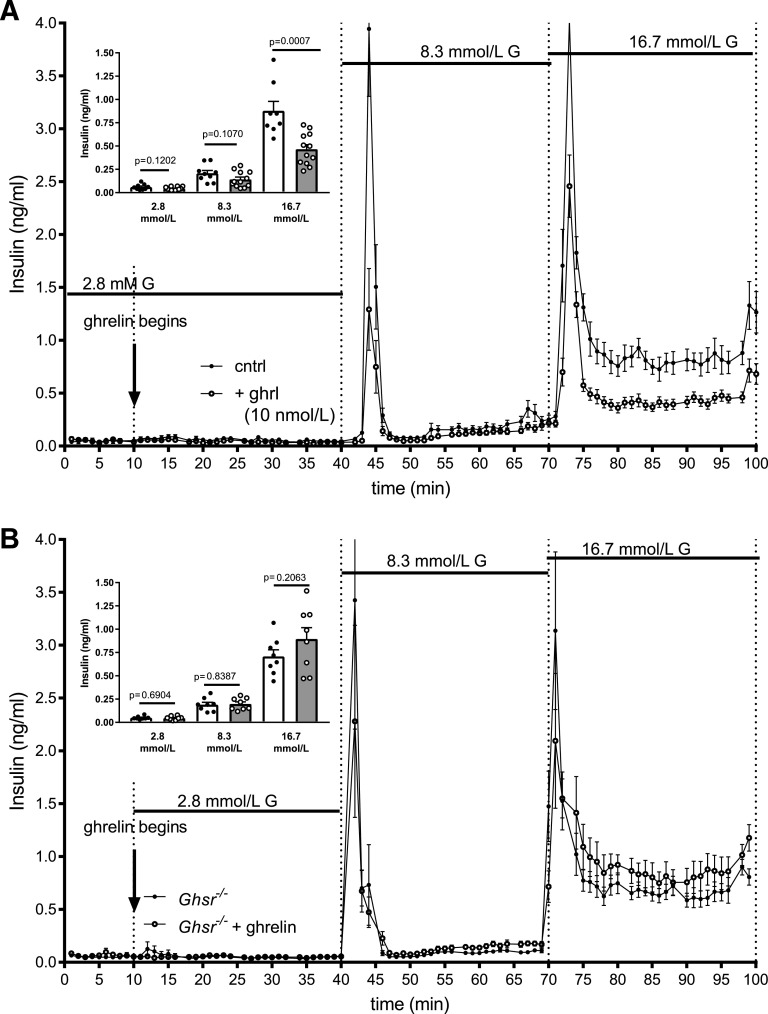
To determine whether ghrelin or GHSR action within the islet regulates insulin secretion, isolated islets were studied ex vivo in perifusion experiments. In the first experiments, wild type islets received a graded glucose infusion in the presence or absence of 10 nmol/L ghrelin (Fig. 4A). While ghrelin had no effect on insulin secretion at 2.8 mmol/L glucose, it blunted the first-phase insulin secretion at 8.3 mmol/L glucose and caused a ∼40% reduction in total insulin secretion during 16.7 mmol/L glucose. Thus, the inhibitory effect of 10 nmol/L ghrelin became more obvious at higher glucose concentrations. This paradigm was repeated in islets from Ghsr−/− mice to evaluate the necessity of the GHSR to mediate the suppressive effect of ghrelin on insulin secretion (Fig. 4B). In this setting, ghrelin did not decrease insulin secretion at any glucose concentration, demonstrating the necessity of the GHSR for this effect.
Figure 4.
Ghrelin reduces GSIS in a GHSR-dependent manner. Insulin secretion from islets perifused without ghrelin (closed circles, open bar) and with ghrelin (open circles, gray bar) at 2.8, 8.3, and 16.7 mmol/L glucose (G) in islets from wild-type (A) and Ghsr−/− (B) mice. Insulin concentrations are shown as mean ± SEM. Inserts for each graph represent mean ± SEM insulin concentrations at each glucose concentration. Unpaired t test between control and ghrelin-treated islets at each glucose concentration with P values are indicated. cntrl, perifused without ghrelin; + ghrl, perifused with ghrelin.
Islet Ghrelin and GHSR Are Dispensable for Insulin Secretion
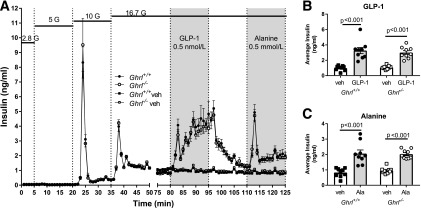
To directly test whether islet ghrelin from ε-cells regulates insulin secretion, insulin secretion was compared in Ghrl−/− and Ghrl+/+ islets during a glucose ramp from 2.8 to 16.7 mmol/L (Fig. 5A). Islets from both male and female mice were used and analyzed separately. However, there were no differences in the sexes and the data from males and females were combined for subsequent analysis. Insulin secretion did not differ between genotypes in response to increasing glucose concentrations, indicating that islet ghrelin has a minimal effect on glucose-stimulated insulin secretion (GSIS). We then compared additional β-cell stimuli, GLP-1 and alanine, in isolated islets from both mouse strains. GLP-1 and alanine substantially increased insulin secretion equally in Ghrl−/− and Ghrl+/+ islets (Fig. 5A–C). Thus, the presence of islet ghrelin does not affect insulin secretion in response to glucose, GLP-1, or alanine.
Figure 5.
Insulin secretion from perifused islets isolated from Ghrl+/+ and Ghrl−/−. A: Insulin was measured in perifusate from islets exposed to stepwise increases (2.8, 5, 10, and 16.7 mmol/L) in glucose (G). From 80 to 95 min, islets were exposed to 0.5 nmol/L GLP-1 or vehicle, and from 110 to 125 min, islets were exposed to 0.5 mmol/L alanine or vehicle. Insulin concentrations are shown as mean ± SEM. B and C: Average insulin concentrations during GLP-1 (B) and alanine (C) were greater than vehicle controls for Ghrl+/+ and Ghrl−/− islets. Mean ± SEM during specific treatments. One-way ANOVA with Sidak post hoc test; P values are indicated between significant comparisons. Ala, alanine; veh, vehicle.
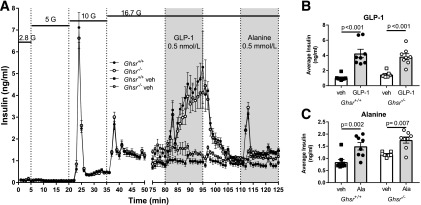
The reportedly high constitutive activity of GHSR may affect insulin secretion in the absence of ghrelin ligand (12,23), so we compared islets from Ghsr−/− and Ghsr+/+ mice in a paradigm identical to that described for Ghrl−/− and Ghrl+/+ islets. Similar GSIS was observed between Ghsr−/− and Ghsr+/+ mice in a glucose ramp from 2.8 to 16.7 mmol/L (Fig. 6A). Both Ghsr−/− and Ghsr+/+ islets responded similarly to GLP-1 and alanine (Fig. 6B and C), suggesting that insulin secretion in response to incretins and amino acids is not impacted by loss of GHSR. While Ghsr−/− mice appeared to have higher insulin secretion during vehicle treatment (Fig. 6 [time 75–125 min]), it was not statistically significant.
Figure 6.
Insulin secretion from perifused islets isolated from Ghsr+/+ and Ghsr−/−. A: Insulin was measured in perifusate from islets exposed to stepwise increases (2.8, 5, 10, and 16.7 mmol/L) in glucose (G). From 80 to 95 min, islets were exposed to 0.5 nmol/L GLP-1 or vehicle, and from 110 to 125 min, islets were exposed to 0.5 mmol/L alanine or vehicle. Insulin concentrations are shown as mean ± SEM. B and C: Average insulin concentrations during GLP-1 (B) and alanine (C) were greater than vehicle controls for Ghsr+/+ and Ghsr−/− islets. Mean ± SEM during specific treatments. One-way ANOVA with Sidak post hoc test; P values are indicated between significant comparisons. Ala, alanine; veh, vehicle.
Discussion
Following the discovery of ghrelin as an orexigenic peptide, there has been a steady accumulation of evidence supporting a role for the peptide in maintaining or modulating glycemia via specific actions on β-cells (9–12,21,22,29). Infusions of exogenous ghrelin to healthy humans decrease first-phase insulin secretion during intravenous glucose administration (14,15) and impair β-cell function after meal ingestion (34). In rodent models, pharmacologic doses of exogenous ghrelin can inhibit insulin secretion (12,21,22). These in vivo studies of the insulinostatic effects of ghrelin in rodents and in humans have used exogenous infusions of peptide that raised circulating ghrelin concentrations to supraphysiologic levels (10,21,22,29)—even higher than what is seen under severe caloric restriction. Thus, how these findings fit into physiologic regulation is unclear. Ghrelin is produced by ε-cells in the islet (17,29), and arterio-venous differences in ghrelin concentrations suggest net release from the pancreas (12). Ghrelin action on GHSR increases somatostatin and decreases insulin secretion (21,22), offering a potential ε-to-δ-cell modulation of insulin secretion by intraislet ghrelin or extrapancreatic ghrelin via endocrine fashion. However, the GHSR’s unique constitutive activity (23–25) offers a potential means to affect insulin secretion in the absence of ghrelin. Based on these findings, we hypothesized that an intraislet ghrelin system could regulate insulin secretion in a paracrine manner, assuming that local islet ghrelin concentrations approximate the high plasma levels achieved with exogenous infusions that suppress insulin secretion. Using complementary in vivo and ex vivo experiments with loss-of-function models for ghrelin and GHSR, we observed no significant effects on glucose tolerance or insulin secretion. Based on these results, we find no support for a mechanism in which ghrelin released from ε-cells, or constitutive GHSR activity on other islet cells, regulates insulin secretion in lean adult mice.
Numerous studies have pointed to a potential role for ghrelin and GHSR in glucose tolerance regulation (3,4,12,35), but the relative contributions of circulating or islet ghrelin have not been thoroughly investigated. To address the question of local regulation of insulin by ghrelin, we isolated islets from mice lacking ghrelin or its receptor, GHSR. Using islet perifusion we were able to compare a range of β-cell stimuli independent from endocrine and neural influences to isolate the paracrine effect. In this system, we report that the insulinostatic effect of exogenous ghrelin is comparable to what has been demonstrated in static islet culture (29) and perfused pancreata (12,21). This effect was absent in Ghsr−/− islets, demonstrating the necessity of this receptor for ghrelin’s action on insulin secretion. In our hands, Ghsr−/− and Ghrl−/− islets secreted insulin similarly to controls in response to glucose, GLP-1, and alanine, suggesting that islet ghrelin and GHSR are dispensable for insulin secretion. These ex vivo experiments were amply powered, and the lack of differences between ghrelin and GHSR knockouts and controls was unequivocal. We compared in vivo glucose and insulin tolerance in multiple cohorts of male and female Ghrl−/− and Ghsr−/− mice with their respective controls in response to various stimuli (intraperitoneal glucose, oral mixed meal). Moreover, given the apparent role of ghrelin to mitigate hypoglycemia during starvation (7,36,37), and its rise to maximal concentrations during fasting, we tested the roles of ghrelin or GHSR following short- and long-term fasts. This approach allowed us to interrogate persistent effects on separate days and comprehensively characterize the effect(s) of ghrelin and/or GHSR. We did not find evidence that ghrelin-dependent or ghrelin-independent GHSR signaling plays an important role in the shift from the fasted to the fed state or in mediating whole-body glucose and insulin tolerance in lean, adult mice.
We also examined the relevance of ghrelin or GHSR in systemic glucose regulation. In response to a variety of challenges, neither Ghsr−/− nor Ghrl−/− male mice had glucose or insulin tolerance that differed appreciably from controls. In male mice, Dezaki et al. (12) found nearly twofold higher GSIS and twofold lower glycemic response to IPGTT in overnight-fasted Ghrl−/− mice compared with controls (n = 9–10 per group). Despite our increased sample size and multiple paradigms of fasting and nutrient stimulation, none of our in vivo testing recapitulated this finding. While our results do not speak directly to the studies on the role of ghrelin in maintaining basal blood glucose during starvation (7,36,37), we did observe significantly lower fasting glucose concentration in GHSR−/− male mice. This finding is consistent with previous studies (32) and ghrelin’s inhibition of insulin secretion via locally released somatostatin (21,22), as well as its effects to stimulate GH secretion and lipolysis and enhance hepatic gluconeogenesis (37). Taken together, our data suggest that the ghrelin-GHSR axis is most relevant in the control of fasting, rather than postprandial, glucose. Previous studies have shown that ghrelin or GHSR deletion improves glucose tolerance and prevents weight gain or fat mass accumulation in mice with genetic or diet-induced obesity (3,4,12,32,38). It is therefore possible that the ghrelin system is more relevant and important in the setting of extreme energy abundance or deficit. The role of endogenous ghrelin and GHSR as it relates to glucose metabolism appears, if at all, to be very modest in the lean and nondiabetic rodent model during β-cell stimulation.
A functional intraislet ghrelin regulation system hinges upon the availability of sufficient amounts of ghrelin secreted by ε-cells to signal to other endocrine cells, especially δ-cells. Detection of ε-cells in adult mouse islets has reported low numbers, with some studies unable to detect ε-cells (reviewed in 39). We found that total ghrelin concentration measured from islet lysate was ∼500 pg/mg protein, despite only small numbers of ε-cells visible in the adult rat pancreas (40), human pancreas (19,20), and pancreatic islets (16). Most studies of pancreatic ghrelin have used gene expression (40), immunohistochemistry, or immunofluorescence to mark its presence (29,41). To our knowledge, no other measures of islet ghrelin content in adult mouse islets have been published. Although comparisons across species and assays have limitations, the islet ghrelin concentrations we detected are roughly comparable with immunoassay measures of total ghrelin in fetal rat pancreatic tissue (18). While it is unlikely that stomach-produced ghrelin is present in considerable amount after islet isolation and overnight culture, we cannot exclude its contribution to our islet-ghrelin measure. Notably, the majority of the detectable ghrelin in the mouse islet is desacyl ghrelin (Fig. 1B). This is consistent with the reported lack of ghrelin-O-acetyltransferase in mouse islets to date (42). The relevance of desacyl ghrelin on insulin secretion is unclear (22,43–45). In our experiments, absence of islet ghrelin did not suggest a role for ghrelin in modulating insulin secretion, as isolated Ghrl−/− islets did not differ from their respective controls in response to a glucose ramp, alanine, and GLP-1. Our ex vivo perifusions recapitulate our in vivo experiments and indicate that ghrelin produced in the islet is dispensable for insulin secretion in adult mice.
The role of constitutive activity of the GHSR in the regulation of insulin secretion was first investigated by Dezaki and colleagues in a series of foundational studies (10,12,29). Recent independent studies by DiGruccio et al. (22) and Adriaenssens et al. (21) refined this mechanism by demonstrating ghrelin action on δ-cell GHSRs (21,22), signals through Gαq (22), causing somatostatin secretion to ultimately decrease insulin secretion (21,22). It is important to note that GHSR has numerous downstream signaling cascades (Gαq, Gαi, β-arrestin) that are implicated for its different effects in pancreatic and nonpancreatic target tissues (27). Pharmacologic agents differentially affect the ligand-dependent and -independent signaling of GHSR (28), necessitating a reevaluation of previously published studies. For example, [D-Lys3]-GHRP-6, a purported GHSR antagonist used by Dezaki and colleagues, increased GSIS in rat islets and perfused pancreas (10,12,29); however, it can act as a biased antagonist for β-arrestin and promote GHSR internalization instead of antagonizing Gαq signaling (28). To avoid any bias or misinterpretation due to pharmacologic agents, we used Ghsr−/− mice. Our ex vivo islet perifusion data allow us to confidently conclude that Ghsr−/− islets do not have altered sensitivity to insulin secretagogues and that endogenous ghrelin signaling in the islet has a limited role in the local regulation of insulin secretion.
There are several important limitations to our study that must be considered in interpreting our results. First, our study leaned heavily on genetic mouse models with lack of either ghrelin or GHSR throughout their life span. Ghrelin-positive cells in the islet are greatest during gestation (∼10% of human islet cells) and less frequent in adults (16,20,46), suggesting that islet ghrelin may be more important during development (18,39). It can be argued that developmental knockouts adapt to elimination of important systems with compensatory mechanisms that could blur the impact of specific loss of function. However, these mouse lines have demonstrated phenotypes to prevent diabetes and obesity (3,4,12,32), so compensatory mechanisms for the gene deletions are not complete. Second, while we did detect some minor differences between genotypes in female mice, we did not adjust for estrus cycle, and it is possible that our data could be influenced by differences in dietary intake or metabolic changes that occur during estrus cycles (47,48). While others have reported that orexigenic actions are inhibited by estrogen (47), we caution overinterpretation of our reported sex differences, as such effects were not observed in the 16-h fast IPGTT or during the MMTT. Third, the genetic deletion of GHSR approach does not test the contribution of GHSR constitutive activity to insulin secretion directly. However, there is no “pure” GHSR inverse agonist available currently to address this question, and our study does not examine potential theoretical heterodimerization of GHSR with other G-coupled protein receptors (49). Finally, we did not study either of our animal lines following diet-induced obesity or when crossed onto genetic models of obesity—settings where other groups have demonstrated important effects of ghrelin signaling on glucose tolerance (4,12,32). While our focus was on the potential role of islet ghrelin signaling in lean mice, it may be that the GHSR has a role in glucose regulation during caloric excess.
In summary, the results presented here demonstrate that, in adult murine islets, ghrelin and GHSR activity are dispensable for insulin secretion under stimulated conditions (intraperitoneal glucose, mixed-meal gavage). Moreover, under stimulated conditions, this ligand-receptor pair does not affect glucose tolerance in healthy chow-fed male and female mice. Disruption of the ghrelin-GHSR axis produced strongest effects on glucose concentrations during fasting, suggesting a setting where local ghrelin-GHSR action may be most relevant. Specifically, how this disruption in fasting glucose could affect normal physiology or conditions of metabolic disturbance is still undetermined. Our findings favor a system whereby any ghrelin effects on nutrient-stimulated insulin secretion come from outside the pancreas, either through the circulation or nervous system, rather than from within the islet.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Bruce Gaylinn (University of Virginia) for measurement of acyl and desacyl ghrelin; Dr. Derek Nunez (Duke University) for critical feedback and discussion; Drs. Megan Capozzi, Ramamani Arumugam, Jonathan Douros, and Radha Krishna (all at Duke University) for technical assistance; and April Wittman (Duke University) for assistance with mouse colonies.
Funding. This study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (grants T32DK007012 to S.M.G. and R01DK097550 to J.T.) and by grants from the Carlsberg Foundation (CF16-0996) and Lundbeck Foundation (2016-2394) to B.S.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.M.G. designed, performed, and interpreted experiments and wrote the manuscript. J.N. and A.Z. performed experiments. B.S. contributed to discussion and edited the manuscript. J.E.C. and D.A.D. interpreted experiments and edited the manuscript. J.T. conceived, designed, and interpreted experiments and edited the manuscript. J.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data and Resource Availability. The data sets generated during and analyzed during the current study are available from the corresponding author upon reasonable request.
Prior Presentation. Parts of this study were presented in abstract form at The Ghrelin Symposium at the International Congress of Neuroendocrinology, Toronto, Canada, 13–14 July 2018, and at ENDO 2019, New Orleans, LA, 23–26 March 2019.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0079/-/DC1.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 2.Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 2010;151:4745–4755 [DOI] [PubMed] [Google Scholar]
- 3.Sun Y, Asnicar M, Saha PK, Chan L, Smith RG. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 2006;3:379–386 [DOI] [PubMed] [Google Scholar]
- 4.Sun Y, Butte NF, Garcia JM, Smith RG. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 2008;149:843–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briggs DI, Lockie SH, Wu Q, Lemus MB, Stark R, Andrews ZB. Calorie-restricted weight loss reverses high-fat diet-induced ghrelin resistance, which contributes to rebound weight gain in a ghrelin-dependent manner. Endocrinology 2013;154:709–717 [DOI] [PubMed] [Google Scholar]
- 6.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 2003;23:7973–7981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao T-J, Liang G, Li RL, et al. . Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci USA 2010;107:7467–7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani BK, Castorena CM, Osborne-Lawrence S, et al. . Ghrelin mediates exercise endurance and the feeding response post-exercise. Mol Metab 2018;9:114–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reimer MK, Pacini G, Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 2003;144:916–921 [DOI] [PubMed] [Google Scholar]
- 10.Dezaki K, Kakei M, Yada T. Ghrelin uses Gαi2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet β-cells: novel signal transduction of ghrelin. Diabetes 2007;56:2319–2327 [DOI] [PubMed] [Google Scholar]
- 11.Salehi A, Dornonville de la Cour C, Håkanson R, Lundquist I. Effects of ghrelin on insulin and glucagon secretion: a study of isolated pancreatic islets and intact mice. Regul Pept 2004;118:143–150 [DOI] [PubMed] [Google Scholar]
- 12.Dezaki K, Sone H, Koizumi M, et al. . Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006;55:3486–3493 [DOI] [PubMed] [Google Scholar]
- 13.Damjanovic SS, Lalic NM, Pesko PM, et al. . Acute effects of ghrelin on insulin secretion and glucose disposal rate in gastrectomized patients. J Clin Endocrinol Metab 2006;91:2574–2581 [DOI] [PubMed] [Google Scholar]
- 14.Tong J, Prigeon RL, Davis HW, et al. . Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010;59:2145–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tong J, Prigeon RL, Davis HW, Bidlingmaier M, Tschöp MH, D’Alessio D. Physiologic concentrations of exogenously infused ghrelin reduces insulin secretion without affecting insulin sensitivity in healthy humans. J Clin Endocrinol Metab 2013;98:2536–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wierup N, Svensson H, Mulder H, Sundler F. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept 2002;107:63–69 [DOI] [PubMed] [Google Scholar]
- 17.Prado CL, Pugh-Bernard AE, Elghazi L, Sosa-Pineda B, Sussel L. Ghrelin cells replace insulin-producing β cells in two mouse models of pancreas development. Proc Natl Acad Sci USA 2004;101:2924–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chanoine J-P, Wong AC. Ghrelin gene expression is markedly higher in fetal pancreas compared with fetal stomach: effect of maternal fasting. Endocrinology 2004;145:3813–3820 [DOI] [PubMed] [Google Scholar]
- 19.Dominguez Gutierrez G, Kim J, Lee A-H, et al. Gene signature of the human pancreatic ε cell. Endocrinology 2018;159:4023–4032 [DOI] [PMC free article] [PubMed]
- 20.Segerstolpe Å, Palasantza A, Eliasson P, et al. . Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab 2016;24:593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adriaenssens AE, Svendsen B, Lam BY, et al. . Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia 2016;59:2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiGruccio MR, Mawla AM, Donaldson CJ, et al. . Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol Metab 2016;5:449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holst B, Cygankiewicz A, Jensen TH, Ankersen M, Schwartz TW. High constitutive signaling of the ghrelin receptor--identification of a potent inverse agonist. Mol Endocrinol 2003;17:2201–2210 [DOI] [PubMed] [Google Scholar]
- 24.Holst B, Holliday ND, Bach A, Elling CE, Cox HM, Schwartz TW. Common structural basis for constitutive activity of the ghrelin receptor family. J Biol Chem 2004;279:53806–53817 [DOI] [PubMed]
- 25.Petersen PS, Woldbye DP, Madsen AN, et al. . In vivo characterization of high basal signaling from the ghrelin receptor. Endocrinology 2009;150:4920–4930 [DOI] [PubMed] [Google Scholar]
- 26.Fernandez G, Cabral A, Andreoli MF, et al. . Evidence supporting a role for constitutive ghrelin receptor signaling in fasting-induced hyperphagia in male mice. Endocrinology 2018;159:1021–1034 [DOI] [PubMed] [Google Scholar]
- 27.M'Kadmi C, Leyris J-P, Onfroy L, et al. Agonism, antagonism and inverse agonism bias at the ghrelin receptor signaling. J Biol Chem 2015;290:27021–27039 [DOI] [PMC free article] [PubMed]
- 28.Ramirez VT, van Oeffelen WEPA, Torres-Fuentes C, et al. Differential functional selectivity and downstream signaling bias of ghrelin receptor antagonists and inverse agonists. FASEB J 2019;33:518–531 [DOI] [PubMed]
- 29.Dezaki K, Hosoda H, Kakei M, et al. . Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in β-cells: implication in the glycemic control in rodents. Diabetes 2004;53:3142–3151 [DOI] [PubMed] [Google Scholar]
- 30.Valenzuela DM, Murphy AJ, Frendewey D, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol 2003;21:652–659 [DOI] [PubMed]
- 31.Wortley KE, Anderson KD, Garcia K, et al. . Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci U S A 2004;101:8227–8232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zigman JM, Nakano Y, Coppari R, et al. . Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 2005;115:3564–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Prudom CE, Nass R, et al. . Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab 2008;93:1980–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tong J, Davis HW, Gastaldelli A, D’Alessio D. Ghrelin impairs prandial glucose tolerance and insulin secretion in healthy humans despite increasing GLP-1. J Clin Endocrinol Metab 2016;101:2405–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma X, Lin Y, Lin L, et al. . Ablation of ghrelin receptor in leptin-deficient ob/ob mice has paradoxical effects on glucose homeostasis when compared with ablation of ghrelin in ob/ob mice. Am J Physiol Endocrinol Metab 2012;303:E422–E431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstein J, Zhao T-J, Li R, Sherbet D, Liang G, Brown M. Surviving starvation: essential role of the ghrelin-growth hormone axis. In Cold Spring Harbor Symposia on Quantitative Biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, p. 121–127 [DOI] [PubMed] [Google Scholar]
- 37.Li RL, Sherbet DP, Elsbernd BL, Goldstein JL, Brown MS, Zhao TJ. Profound hypoglycemia in starved, ghrelin-deficient mice is caused by decreased gluconeogenesis and reversed by lactate or fatty acids. J Biol Chem 2012;287:17942–17950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wortley KE, del Rincon J-P, Murray JD, et al. . Absence of ghrelin protects against early-onset obesity. J Clin Invest 2005;115:3573–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wierup N, Sundler F, Heller RS. The islet ghrelin cell. J Mol Endocrinol 2013;52:R35–R49 [DOI] [PubMed] [Google Scholar]
- 40.Wierup N, Yang S, McEvilly RJ, Mulder H, Sundler F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) cells. J Histochem Cytochem 2004;52:301–310 [DOI] [PubMed] [Google Scholar]
- 41.Date Y, Nakazato M, Hashiguchi S, et al. . Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes 2002;51:124–129 [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008;132:387–396 [DOI] [PubMed] [Google Scholar]
- 43.Tong J, Davis HW, Summer S, et al. Acute administration of unacylated ghrelin has no effect on basal or stimulated insulin secretion in healthy humans. Diabetes 2014:63:2309–2319 [DOI] [PMC free article] [PubMed]
- 44.Benso A, St-Pierre DH, Prodam F, et al. . Metabolic effects of overnight continuous infusion of unacylated ghrelin in humans. Eur J Endocrinol 2012;166:911–916 [DOI] [PubMed] [Google Scholar]
- 45.Gauna C, Uitterlinden P, Kramer P, et al. . Intravenous glucose administration in fasting rats has differential effects on acylated and unacylated ghrelin in the portal and systemic circulation: a comparison between portal and peripheral concentrations in anesthetized rats. Endocrinology 2007;148:5278–5287 [DOI] [PubMed] [Google Scholar]
- 46.Andralojc KM, Mercalli A, Nowak KW, et al. . Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 2009;52:486–493 [DOI] [PubMed] [Google Scholar]
- 47.Clegg DJ, Brown LM, Zigman JM, et al. . Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes 2007;56:1051–1058 [DOI] [PubMed] [Google Scholar]
- 48.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol 2015;402:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schellekens H, Dinan TG, Cryan JF. Taking two to tango: a role for ghrelin receptor heterodimerization in stress and reward. Front Neurosci 2013;7:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.