Abstract
Recent studies implicate a strong association between elevated plasma branched-chain amino acids (BCAAs) and insulin resistance (IR). However, a causal relationship and whether interrupted BCAA homeostasis can serve as a therapeutic target for diabetes remain to be established experimentally. In this study, unbiased integrative pathway analyses identified a unique genetic link between obesity-associated IR and BCAA catabolic gene expression at the pathway level in human and mouse populations. In genetically obese (ob/ob) mice, rate-limiting branched-chain α-keto acid (BCKA) dehydrogenase deficiency (i.e., BCAA and BCKA accumulation), a metabolic feature, accompanied the systemic suppression of BCAA catabolic genes. Restoring BCAA catabolic flux with a pharmacological inhibitor of BCKA dehydrogenase kinase (BCKDK) ( a suppressor of BCKA dehydrogenase) reduced the abundance of BCAA and BCKA and markedly attenuated IR in ob/ob mice. Similar outcomes were achieved by reducing protein (and thus BCAA) intake, whereas increasing BCAA intake did the opposite; this corroborates the pathogenic roles of BCAAs and BCKAs in IR in ob/ob mice. Like BCAAs, BCKAs also suppressed insulin signaling via activation of mammalian target of rapamycin complex 1. Finally, the small-molecule BCKDK inhibitor significantly attenuated IR in high-fat diet–induced obese mice. Collectively, these data demonstrate a pivotal causal role of a BCAA catabolic defect and elevated abundance of BCAAs and BCKAs in obesity-associated IR and provide proof-of-concept evidence for the therapeutic validity of manipulating BCAA metabolism for treating diabetes.
Introduction
The three branched-chain amino acids (BCAAs) are leucine, isoleucine, and valine. A strong association between elevated plasma BCAAs and insulin resistance (IR) has been repeatedly observed in human and rodent models (1–9). Moreover, longitudinal studies suggest that a high plasma BCAA level is predictive of the future onset of diabetes (10–13). Circulating BCAAs are also a significant prognostic marker associated with outcomes of diabetes interventions (4,10,14–16). On the other hand, elevated plasma branched-chain α-keto acids (BCKAs), the products of BCAA transamination, are also associated with IR and are potentially better biomarkers for diabetes (8,9). These observations strongly suggest a causal role of disrupted BCAA homeostasis in IR, which remains to be established experimentally.
BCAAs are essential amino acids derived from protein-containing foods. The BCAA catabolic pathway, consisting of more than 40 enzymes in mitochondria, plays a pivotal role in maintaining BCAA homeostasis in mammals (2). BCAA catabolism is initiated by branched-chain aminotransferase (BCAT), which facilitates a reversible transamination reaction generating BCKAs including α-ketoisocaproic acid (from leucine), α-keto-β-methylvaleric acid (from isoleucine), and α-ketoisovaleric acid (from valine). Mitochondrial BCAT (BCAT2) is expressed ubiquitously and plays a main role in peripheral BCAA catabolism (2,17). The subsequent irreversible decarboxylation of BCKAs by BCKA dehydrogenase (BCKD) is the rate-limiting step in BCAA catabolism, giving rise to CoA moieties that feed into the citric acid cycle after several reactions. In addition to substrate-dependent allosteric modulation, BCKD activity is also regulated by posttranslational modifications. Phosphorylation of the BCKD E1α subunit by BCKD kinase (BCKDK) inhibits BCKD, whereas dephosphorylation by protein phosphatase 2Cm (PP2Cm) activates BCKD. Loss-of-function mutations in BCAA catabolic genes encoding BCKD subunits and PP2Cm lead to BCKD deficiency, BCAA and BCKA accumulation, and maple syrup urine disease (18–21).
Given the critical role of the BCAA catabolic pathway in maintaining BCAA homeostasis and the strong association between diabetes and elevated BCAAs and BCKAs, the BCAA catabolic pathway may play an important role in IR pathogenesis. Individual genes (BCKDHA and PP2Cm) of the BCAA catabolic pathway have been genetically linked with IR (22–25). In this study, integrated genomic analyses revealed a unique association between BCAA catabolic gene expression at the pathway level and obesity-associated IR in human and mouse populations. We then examined the causal role of suppressed BCAA catabolism in IR in both ob/ob and high fat–induced obese mice and the therapeutic potential of targeting BCAA catabolism to treat type 2 diabetes.
Research Design and Methods
Human Genome-Wide Association Studies for Insulin-Related Traits
Publicly available human genome-wide association study (GWAS) data sets for fasting glucose, fasting insulin, and IR (unadjusted and adjusted for BMI) were retrieved from large meta-analysis consortia including MAGIC (the Meta-Analyses of Glucose and Insulin-related Traits Consortium) (26) and the GENESIS (Genetics of Insulin Sensitivity) Consortium (27). After retrieving summary-level statistics for all single nucleotide polymorphisms (SNPs), we removed SNPs with a weak association (<80%). The remaining SNPs with high linkage disequilibrium (r2 > 0.5) were filtered by using a previously described method (28). Linkage disequilibrium data for European ancestry was obtained from HapMap3 (29) and the 1000 Genomes Project (30). Comprehensive lists of human cis–expression quantitative trait loci (eQTL) from human adipose, liver, and muscle tissues were accessible from our Mergeomics web server (31). Cis-eQTL were defined as eQTL in which the associated SNP and gene pairs are within 1 MB of each other. Details of the eQTL data sets used in the study are listed in Supplementary Table 1. A total of 1,690 coexpression modules were constructed from adipose, liver, and muscle tissue samples generated from multiple human and mouse studies (Supplementary Table 1) by using the WGCNA package in R software (32).
Amino Acid Pathways and Other Canonical Pathways
We retrieved BCAA catabolism pathways and 11 pathways for amino acids that are not BCAAs from the Kyoto Encyclopedia of Genes and Genomes (KEGG) Database (33). PPM1K and BCKDK were manually added into the KEGG BCAA pathway, which was further categorized into groups of genes specific to the degradation of leucine, valine, and isoleucine. For non-BCAA pathways, we also removed genes overlapping with BCAA. We used the amino acid pathways, along with other pathways from the KEGG Database, to annotate all 1,690 coexpression modules; we tested the enrichment of pathway genes using the Fisher exact test. Statistical significance was indicated by Bonferroni-corrected P < 0.05, fold enrichment >5, and >2 overlapping genes.
Integrative Genomics Analysis Using the Mergeomics Pipeline
We used the Marker Set Enrichment Analysis (MSEA) library in Mergeomics to determine the association of coexpression networks with human insulin traits by leveraging human GWAS and eQTLs (34) (Fig. 1A). Specifically, coexpression modules were first mapped to adipose, liver, and muscle eQTLs in order to derive the corresponding representative expression SNP sets. The P values indicating the association of the expression SNPs with disease were then extracted from the filtered summary-level statistics as described above. We assessed the significance of enrichment for SNPs indicating moderate to strong risk for a trait within each module using a χ2 like statistic followed by multiple-testing correction in order to estimate false discovery rate (FDR) (34). Modules with an FDR <5% and P < 0.05 were considered to be significantly or suggestively associated with the respective trait.
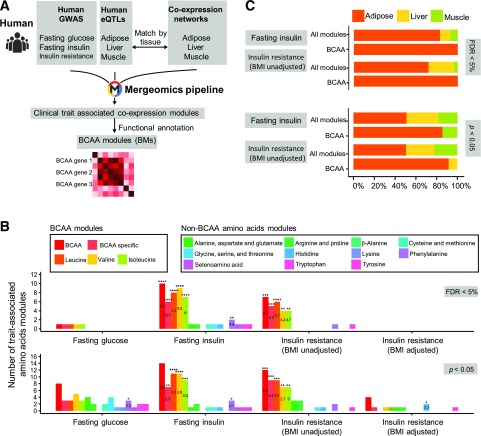
Figure 1.
Integrative genomic analyses associate the BCAA catabolic pathway with IR-related traits in human populations. A: The integrative genomics workflow we used to investigate the association of BCAAs with IR-related traits in humans. Specifically, human GWAS were integrated with eQTLs and coexpression networks matched by tissue, and then analyzed using the Mergeomics pipeline in order to identify coexpression modules that showed significant genetic association with IR-related clinical traits. Coexpression modules with significant over-representation of BCAA genes among the module genes were then annotated as BCAA modules. B: Number of BCAA modules with significant trait association (FDR <5% or P < 0.05, as assessed by using MSEA in the Mergeomics pipeline). Numbers in the bars indicate the fold enrichment of BCAA modules among all significant coexpression modules for the corresponding trait. Statistical significance of enrichment of BCAA modules among all significant modules was determined by using the Fisher exact test. Details of enrichment test are in Supplementary Table 2. C: Comparison of tissue origin distribution of BCAA modules and all coexpression modules significantly associated with fasting insulin and IR (BMI unadjusted) at FDR <5% and P < 0.05. Significance of differences in the mean correlation strength between gene categories was calculated by using the Student t test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To test whether BCAA genes play key regulatory roles in the trait-associated BCAA modules, we used the weighted Key Driver Analysis library in Mergeomics to identify key drivers of the significant modules. Key drivers of a given module were defined as genes whose neighboring subnetwork exhibited significant enrichment (FDR <1%) for member genes in that module. Adipose Bayesian networks used in key driver analysis were constructed through the use of a previously developed method (35,36).
Gene-Trait Correlation in the Hybrid Mouse Diversity Panel
Pearson correlations of the expression of BCAA genes in liver, adipose, and muscle tissues with fasting glucose, fasting insulin, and HOMA-IR measurements were retrieved from the Hybrid Mouse Diversity Panel (HMDP) (https://systems.genetics.ucla.edu/data/hmdp), which comprises ∼100 strains of mice fed a high-fat diet (HFD) (37,38). We assessed clinical traits using 8–12 mice per strain, whereas we profiled gene expression using 3 mice per strain per tissue.
Animals
Male ob/ob mice or male wild-type C57BL/6 mice were purchased from The Jackson Laboratory or SLAC Laboratory Animals Co. Ltd, Shanghai, China. Animals from The Jackson Laboratory were used for our investigation of 3,6-dichlorobenzo[b]thiophene-2-carboxylic acid (BT2), and animals from SLAC were used for other studies. All mice were housed at 22°C under a 12-h light/12-h dark cycle, with free access to water and standard chow. An isocaloric normal protein diet (NPD) (20% protein; TD.91352) and a low-protein diet (LPD) (6% protein; TD.90016) (Supplementary Table 5) were purchased from Envigo Teklad Diet. A normal BCAA diet (A11072001) and a low-BCAA diet (60% less BCAAs than the normal BCAA diet; A12030802) (Supplementary Table 6) were purchased from OpenSource Diets. Tissue samples from white adipose tissue (epididymal fat), skeletal muscle (soleus/gastrocnemius), and liver were quickly harvested and frozen in liquid nitrogen and maintained at −80°C until processed. All animal procedures were carried out in accordance with the guidelines and protocols approved by the Committee for Humane Treatment of Animals at Shanghai Jiao Tong University School of Medicine, the University of Texas Southwestern Medical Center Institutional Animal Care and Use Committee, or the University of California at Los Angeles Institutional Animal Care and Use Committee.
Western Blotting Analysis
Antisera against the BCKD E1α subunit were a gift from Dr. Yoshiharu Shimomura (Nagoya Institute of Technology, Nagoya, Japan). We purchased other antibodies against pBCKD E1α (Novus Biologicals); BCKDE2 (Thermo Fisher Scientific); and phospho-Akt (Thr308), Akt, phospho-p70S6K (Thr389), total p70S6K, tubulin, GAPDH, and β-actin (Cell Signaling Technology). Densitometry was performed with ImageJ software.
RNA Isolation and Quantitative RT-PCR
Total RNA was extracted from the various tissues by using the TRIzol Reagent (Invitrogen), according to the manufacturer’s instructions. Total RNA (2 μg) was reverse transcribed by using random primers and Maloney murine leukemia virus (Promega). Each cDNA sample was analyzed in triplicate with the Applied Biosystems Prism 7900HT Real-Time PCR System using Absolute SYBR Green (Applied Biosystems). The primer sequences are listed in Supplementary Table 4.
Metabolomic Analysis
The global metabolomic analysis was carried out by Metabolon, Inc. (Durham, NC), as described previously (39). The Welch two-sample t test was used to test whether two unknown means were different from two independent populations. The wild-type mice were fed the NPD (20% protein by weight) and ob/ob mice were fed the NPD or LPD (6% protein by weight) for 4 weeks beginning at 10 weeks of age. For the BCAA group (LPD + BCAA), drinking water was supplemented with BCAA (3 mg/mL) beginning after 2 weeks of eating the LPD; this supplementation lasted 2 weeks.
Determination of BCAA and BCKA Concentrations With Mass Spectrometry
Plasma was precleared of protein by using ethanol. The supernatant was lyophilized and resuspended. Standards and samples were diluted in butanolic HCL, heated to 60°C for 20 min, and then dried in a speed vacuum. The samples were resuspended in distilled H2O and acetonitrile (50:50) containing 0.1% formic acid, and analyzed by liquid chromatography–tandem mass spectrometry in order to measure BCAA concentrations. To determine BCKA concentrations, plasma, a solution of [13C] α-ketoisovaleric acid, and o-phenylenediamine were mixed and incubated at 80°C for 20 min. Na2SO4 and ethyl acetate were added to the cooled solutions, which were then centrifuged. The upper organic phase was re-extracted, pooled with the first, and dried under mild heat (40°C). The samples were resuspended in MeOH and 5 mmol/L NH4 acetate (50:50) and analyzed by liquid chromatography–tandem mass spectrometry.
Glucose and Insulin Tolerance Tests
Mice were deprived of food for 6 h. For the insulin tolerance test, mice were injected intraperitoneally with insulin (0.75 units/kg body weight; Sigma). For the glucose tolerance test, mice were injected intraperitoneally with d-glucose (1.5 g/kg body weight; Sigma). Blood glucose concentrations were measured in tail blood by using a portable glucometer (Johnson & Johnson) at the times indicated after injection in figures. Plasma insulin was measured with MILLIPLEX Multiplex Immunoassay Kits (MMHMAG-44K-14; Merck Millipore, Darmstadt, Germany). To allow us to examine in vivo insulin signaling, mice were deprived of food for 6 h and injected intraperitoneally with insulin (2 units/kg body weight) for 10 min.
Mouse Treatment With the BCKDK Inhibitor BT2
The animals were fed an HFD (60% kcal from fat) (catalog no. D12492; Research Diets) for ∼12 weeks starting at the age of ∼6 weeks in order to induce obesity in wild-type mice (diet-induced obesity [DIO]). For ob/ob mice, the treatment started at 8 weeks of age. Obese mice (DIO mice or ob/ob mice) were randomized into two groups and received either the vehicle or BT2 treatment. BT2 was dissolved in DMSO and diluted in 5% DMSO, 10% Cremophor EL, and 85% 0.1 mol/L sodium bicarbonate (pH 9.0) for delivery. Mice received via oral gavage daily doses of BT2 (40 mg/kg/day) or an equal volume of vehicle for 8–10 weeks. BCKD activity was analyzed as described previously (40).
Statistics
Unless otherwise specified, statistical analyses were performed with the two-sided Student t test (two groups) and one-way ANOVA (more than two groups) or two-way ANOVA (tolerance tests), followed by a Tukey post hoc test, where appropriate, using GraphPad Prism software. Data were calculated as the mean ± SD unless otherwise indicated. A P value <0.05 was considered to be statistically significant.
Results
BCAA Catabolic Pathway Is Genetically Associated With Obesity-Associated IR in Human Populations
To explore the connection between specific metabolic pathways and IR in an unbiased manner, we leveraged existing genetics and functional genomics data sets from human and mouse populations using an integrative framework (Fig. 1A).
For the human-focused analysis, we mainly used GWAS of IR from the GENESIS Consortium (27) and glycemic traits from MAGIC (26) to capture genetic association signals. Instead of focusing on individual genetic signals in individual genes, we focused on the aggregate behavior of groups of functionally related genes by integrating the GWAS data sets with tissue-specific eQTLs and coexpression network modules containing sets of coregulated genes in IR-relevant tissues—including adipose tissue, liver, and muscle (Fig. 1A)—using the MSEA library in the Mergeomics pipeline (34). Among all significant coexpression modules for fasting insulin and IR traits at either an FDR <5% (correcting for multiple testing) or P < 0.05 (uncorrected), we observed significant over-representation of modules annotated with the BCAA catabolic pathway, among other well-established IR-related processes such as glucose metabolism, oxidative phosphorylation, and inflammation (Fig. 1B and Supplementary Table 2). For instance, 57 of a total of 1,690 modules were found to be significantly associated with IR. Among these, seven modules were enriched for BCAA catabolic genes, representing a 4.2-fold enrichment for BCAA-related modules (enrichment P = 1.8e−4) (Fig. 1B and Supplementary Table 2). In contrast, the BCAA catabolic pathway was not among coexpression modules associated with fasting glucose or BMI-adjusted IR, suggesting an adiposity-dependent association between the BCAA catabolic pathway and IR (Fig. 1B). No association was observed for metabolic pathways for amino acids that are not BCAAs (Fig. 1B). Details of the BCAA catabolic genes, corresponding eQTLs, and P values for trait associations for the modules are provided in Supplementary Table 3.
After matching the tissue origin of coexpression modules and eQTLs, we found that the BCAA modules associated with IR were primarily from the adipose tissue, not skeletal muscle or liver (Fig. 1C). In addition, we used the weighted Key Driver Analysis library in the Mergeomics pipeline to pinpoint potential key drivers (28,41–43), revealing BCAA catabolic genes as key drivers for all BCAA modules. These results support BCAA metabolic genes as playing a pivotal regulatory role in the IR-associated BCAA modules (Supplementary Fig. 1). Together, these unbiased genomics analyses from human populations reveal a unique and strong association between obesity-associated IR and the BCAA catabolic pathway.
BCAA Catabolic Gene Expression at the Pathway Level Is Correlated With IR in the HMDP
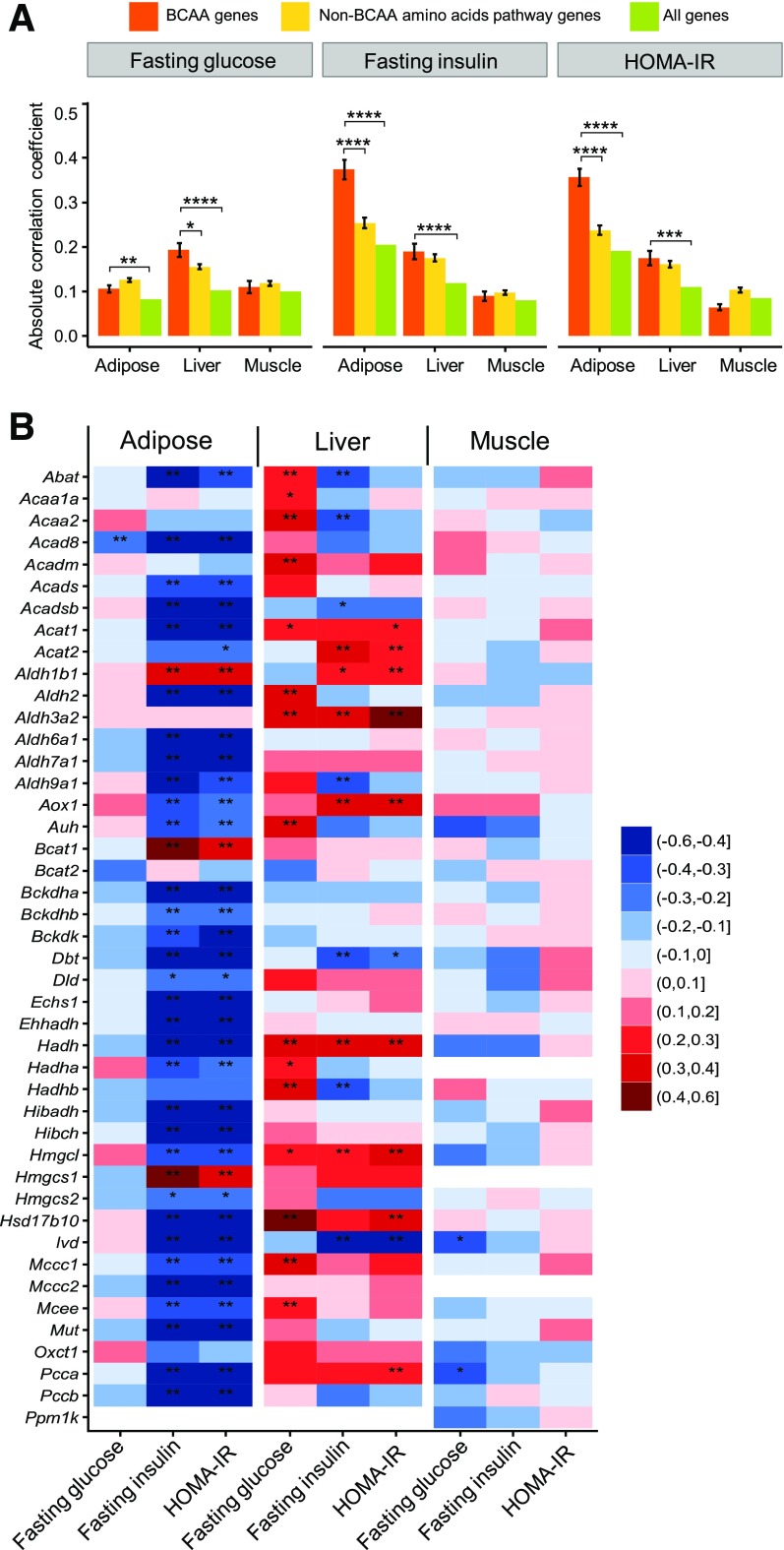
To connect the human genomic analysis results to mouse models in which IR is typically studied, we evaluated the association of the expression levels of BCAA catabolic genes in adipose, liver, and muscle with IR-relevant traits in ∼100 genetically divergent strains of mice fed with an HFD in the HMDP (37,38) (Supplementary Fig. 2). Consistent with the findings from the aforementioned human studies, the expression levels of BCAA metabolic genes in the adipose tissue exhibited a strong negative correlation with fasting insulin level and IR (HOMA-IR), but not fasting glucose level, in the HMDP cohort (Fig. 2A and B). The expression levels of a smaller set of hepatic BCAA catabolic genes showed either a positive or a negative association with fasting glucose, fasting insulin, and IR traits (Fig. 2B). The strength of the correlation in liver was weaker than that in the adipose tissue (Fig. 2B) and was not significantly different from the genes in amino acid (non-BCAA) pathways (Fig. 2A). The muscle tissue, on the other hand, did not exhibit specific correlations between BCAA catabolic gene expression and glycemic traits (Fig. 2A and B). These data from the HMDP mouse cohort study further support the association between IR and BCAA catabolic gene expression observed in the human population, substantiating a common genetic feature related to the BCAA catabolic pathway in diabetes.
Figure 2.
The BCAA catabolic pathway shows a strong correlation with IR in a mouse population. A: Comparison of correlation strengths of the expression of BCAA pathway genes, non-BCAA amino acid pathway genes, and all genes with IR-related traits in mice. The correlation data between tissue-specific expression profiling and measurements of clinical traits were extracted from the HMDP, which contains ∼100 strains of genetically divergent mice fed an HFD. The mean and SE of the absolute values of Pearson correlations between each gene within a gene category (BCAA, non-BCAA, all genes) and a trait are shown. The significance of differences in the mean correlation strength between gene categories was calculated by using the Student t test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. B: Correlation of individual BCAA catabolic genes in different tissues for fasting glucose, fasting insulin, and HOMA-IR in HMDP mice fed an HFD. Red indicates a positive correlation, whereas blue indicates a negative correlation. *P < 0.05 and **P < 0.05 after Bonferroni correction for the number of genes and traits.
BCKD Deficiency and BCAA/BCKA Accumulation Characterize BCAA Catabolic Defect in Obese ob/ob Mice
We next investigated the relationship between the BCAA catabolic pathway and obesity-associated IR in a well-established experimental obese/diabetic animal model, leptin-deficient (ob/ob) mice (Supplementary Fig. 3). Examination of gene expression revealed systemic downregulation of the BCAA catabolic pathway in white adipose tissue and liver, but not in skeletal muscle, in ob/ob mice (Fig. 3A). These expression patterns corresponded to the genetic association between IR and the BCAA catabolic pathway (particularly the negative correlation in adipose tissue) identified by the genomic analyses in human and mouse populations (Figs. 1 and 2).
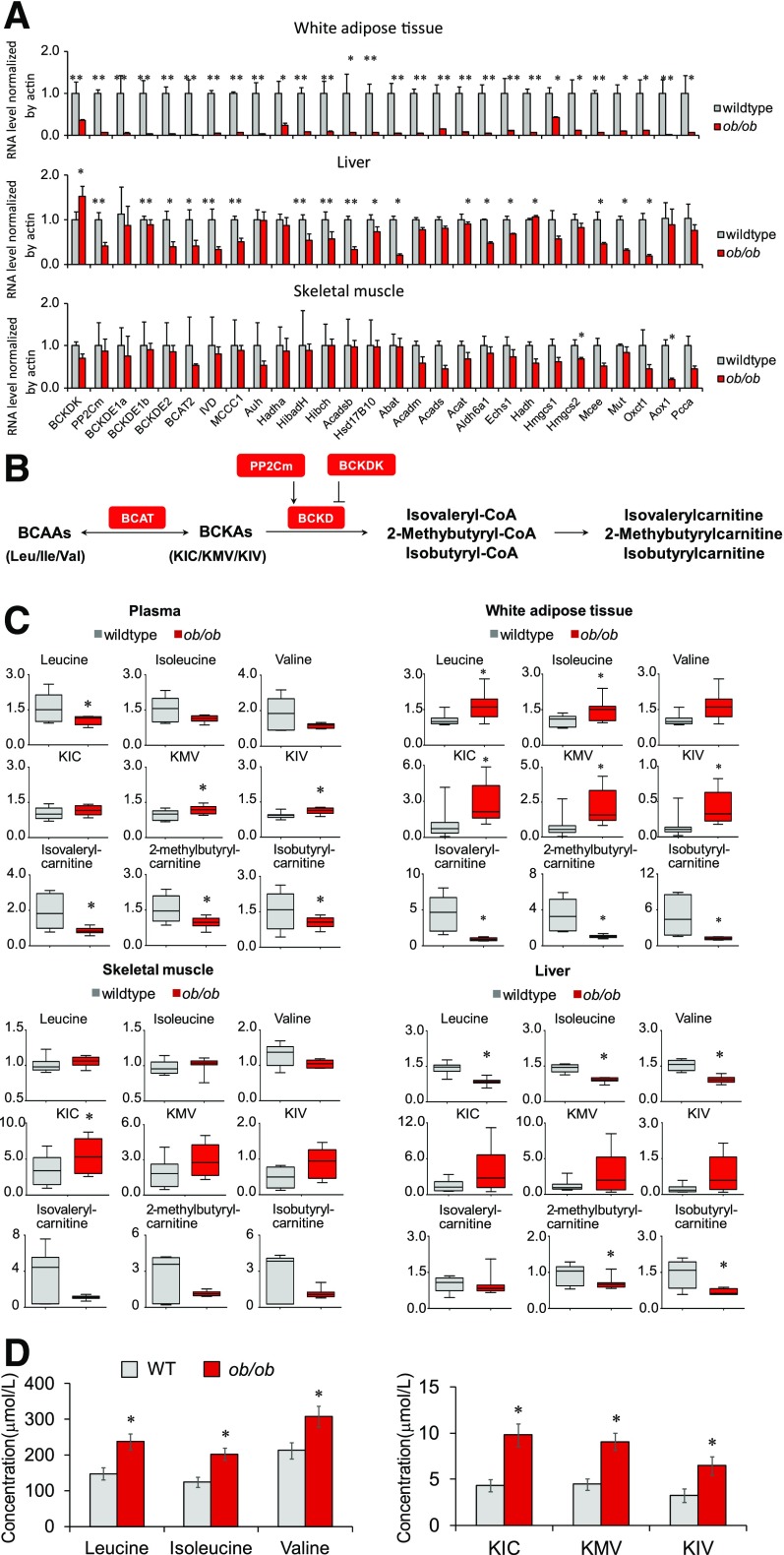
Figure 3.
Systematic downregulation of the BCAA catabolic pathway leads to a BCAA catabolic defect in ob/ob mice. A: Quantitative PCR results of BCAA catabolic genes in white adipose tissue, skeletal muscle, and liver in lean wild-type (WT) mice (n = 4) and ob/ob mice (n = 4) deprived of food for 6 h. B: Illustration of the partial BCAA catabolic process with enzymes, intermediates, and derivatives. C: Relative levels of BCAAs and their metabolites in plasma and tissues of lean WT mice (n = 6–8) and ob/ob mice (n = 8). Male mice, age 14 weeks, were deprived of food for 6 h before being sacrificed. D: Serum concentrations of BCAAs and BCKAs in lean WT mice (n = 8) and ob/ob mice (n = 10). Male mice, age 8 weeks, were deprived of food from 8:00 a.m. to 5:00 p.m. and then supplied with chow diet for 1 hour. Blood (100 μL) was collected at 6:00 p.m. from the orbital sinus by using capillary tubes for serum collection. *P < 0.05 and **P < 0.01 vs. WT mice. ΚΙC, α-ketoisocaproic acid; KIV, α-ketoisovaleric acid; KMV, α-keto-β-methylvaleric acid.
We performed a targeted metabolomics analysis to characterize BCAA catabolism in ob/ob mice by examining the abundance of BCAAs and their metabolites in plasma and various tissues (Fig. 3B). Fasting plasma levels of BCAAs were unexpectedly not higher in obese mice (Fig. 3C). Mass spectrometry also detected similar specific measurements of plasma BCAA levels in food-deprived wild-type and ob/ob mice (Supplementary Fig. 4). BCAA abundances were, however, higher in white adipose tissue, lower in liver, and unchanged in skeletal muscle in food-deprived obese mice relative to lean mice (Fig. 3C). The fasting plasma BCKA concentrations were significantly higher, in accordance with previous reports (8,9). Significant elevation of BCKAs in white adipose tissue and trends of elevation in liver and skeletal muscle were also detected in food-deprived ob/ob mice (Fig. 3C). On the other hand, the concentrations of BCKD metabolites were significantly lower in plasma, liver, white adipose tissue, and probably skeletal muscle of food-deprived ob/ob mice (Fig. 3C).
The elevation of plasma BCAA levels has been viewed as a metabolic hallmark of IR in obesity (2,4–9). The absolute increases are usually moderate, however, and they become smaller or even disappear under fasting conditions (6,44,45). Our data show that, in fed animals, plasma BCAA levels are significantly higher in ob/ob obese mice than in lean mice (Fig. 3D), and the plasma BCKA levels were even higher in ob/ob obese mice (Fig. 3C and D). The magnitudes of BCKA elevations were more pronounced than those of BCAAs in different settings, reflecting BCKD deficiency.
Therefore, although BCAA catabolic genes are suppressed at the pathway level in various tissues, systemic BCAA catabolism in obese ob/ob mice is characterized by a deficiency of the rate-limiting enzyme BCKD, accompanied by reduced catabolic flux and by BCAA/BCKA accumulation. Diminished BCKD protein expression and increased BCKD E1α phosphorylation further support BCKD deficiency in obese ob/ob mice (Supplementary Fig. 5), in agreement with previous studies (8,9,45–49).
A BCAA Catabolic Defect Contributes to the Pathogenesis of IR in ob/ob Mice
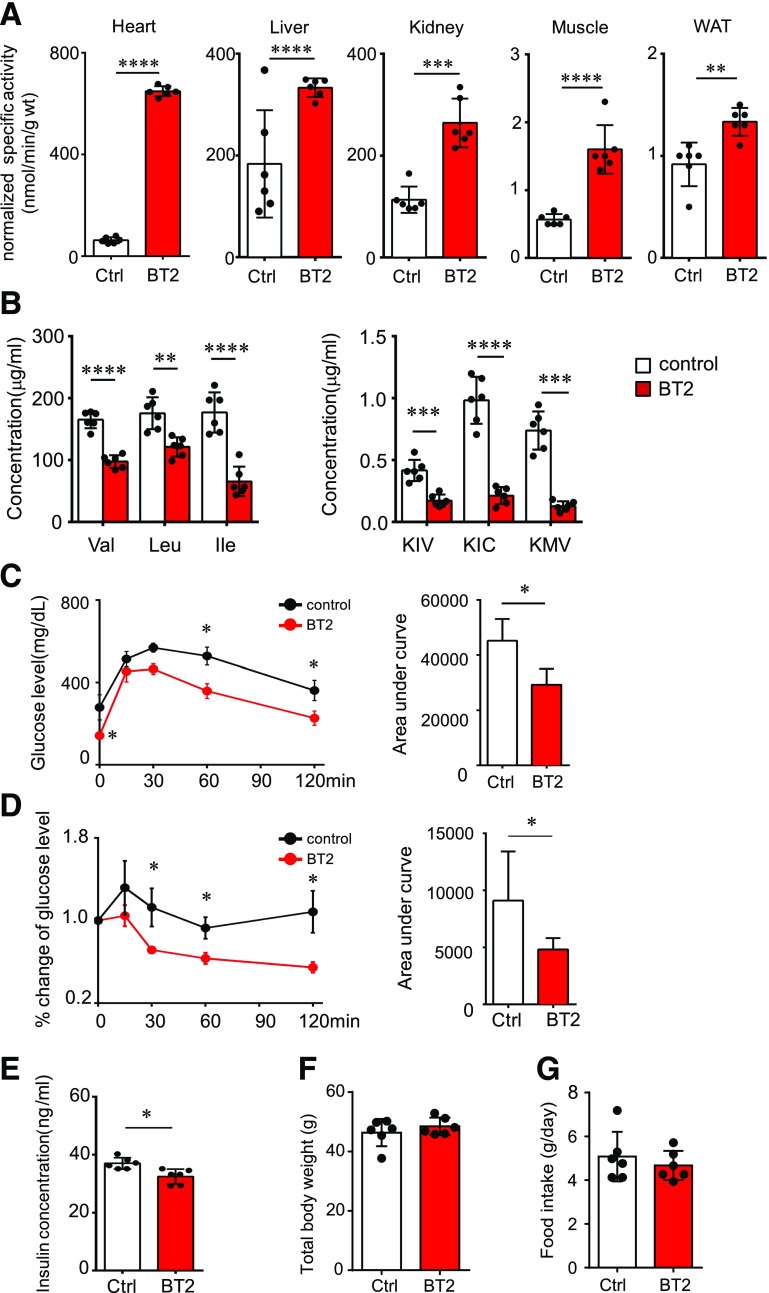
BCKDK phosphorylates the E1α subunit of BCKD and inhibits its activity (2). BT2 is a specific and potent inhibitor of BCKDK (39,40). We next assessed whether the BCAA catabolic defect played a causal role in obesity-associated IR by using BT2 to restore BCAA catabolism in obese mice. BT2 administration significantly enhanced BCKD activities in various tissues of ob/ob mice, including liver, skeletal muscle, and white adipose tissue (Fig. 4A). BT2 treatment significantly reduced plasma BCAA levels in the obese ob/ob mice, but the magnitudes of reductions in plasma BCKA concentrations were far more pronounced (Fig. 4B). Importantly, BT2-treated mice displayed significantly improved glucose tolerance and insulin sensitivity (Fig. 4C and D), accompanied by the attenuation of hyperinsulinemia (Fig. 4E). BT2 treatment reduced the phosphorylation of BCKD E1α in liver and induced the expression of BCKD E1α and BCKDK protein in skeletal muscle (Supplementary Fig. 6). BT2 also increased the expression of numerous distal genes in the BCAA catabolic pathway in skeletal muscle and adipose tissue (Supplementary Fig. 7). Among the 17 amino acids that are not BCAAs, tryptophan was the only one to show a reduced concentration in plasma in the BT2-treated mice (Supplementary Fig. 8); this reduction is potentially due to increased tryptophan transport into tissues when BCAA levels are reduced (21). BT2 treatment did not affect body weight or food intake in the ob/ob mice (Fig. 4F and G). These results strongly support a causal role for the BCAA catabolic defect in the onset of IR in obese ob/ob mice.
Figure 4.
The BCKDK inhibitor restores BCAA catabolism and attenuates IR in ob/ob mice. ob/ob mice were treated with the vehicle (Ctrl) or BT2 (at 10 weeks [A, B, and E] or 4–6 weeks [C and D]) by oral gavage (n = 6 mice in each group). We analyzed BCKD activity in various tissues (A), fasting plasma levels of BCAAs (B, left) and BCKAs (B, right), glucose tolerance test results (C), insulin tolerance test results (D), fasting plasma insulin level (E), body weight (F), and food intake (G). *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001 vs. Ctrl. ΚΙC, α-ketoisocaproic acid; KIV, α-ketoisovaleric acid; KMV, α-keto-β-methylvaleric acid; WAT, white adipose tissue.
Elevated BCAAs and BCKAs Are Pathogenic Factors for IR in ob/ob Mice
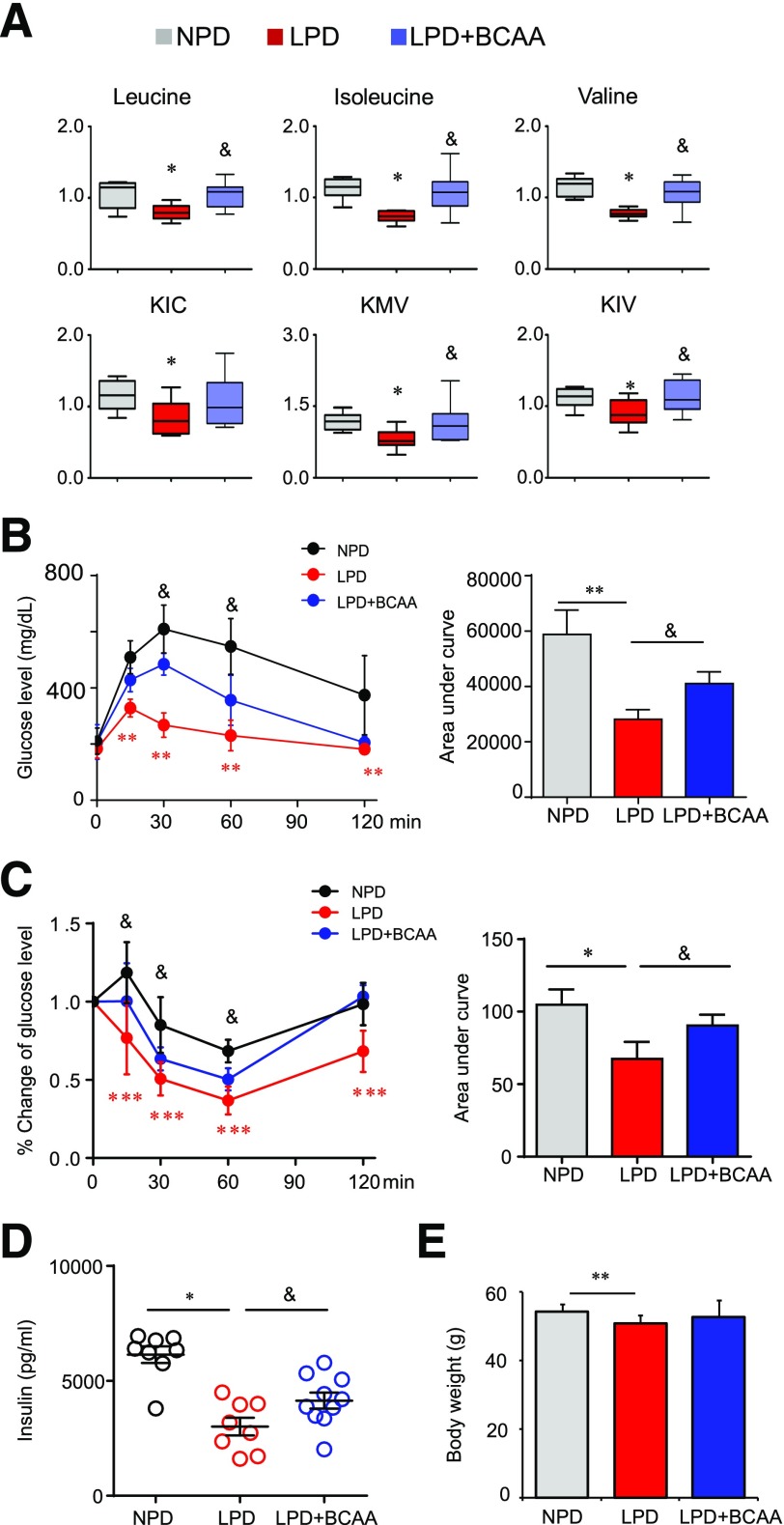
The BCAA catabolic defect results in BCAA/BCKA accumulation and impaired BCAA catabolic flux. BCAA can enhance mammalian target of rapamycin complex 1 (mTORC1) activity, and the catabolic flux may affect glucose and lipid oxidation, both of which have been implicated as possible contributors to IR (2,50). We showed that BT2 treatment reduced BCAA levels while enhancing catabolic flux. To assess further which of these two changes contributes to the improved insulin sensitivity in ob/ob mice, we examined the influences of various dietary manipulations that alter BCAA abundance and the catabolic flux in different ways. We used an isocaloric LPD (6% protein by weight), instead of an NPD (20% protein by weight), to reduce BCAA intake and consequently the load to the catabolic flux. The LPD significantly reduced the plasma levels of BCAAs and BCKAs (Fig. 5A) and markedly improved systemic glucose tolerance (Fig. 5B). In contrast, increasing dietary intake of BCAAs by administering them in water increased plasma levels of BCAAs/BCKAs (Fig. 5A) and significantly exacerbated the systemic glucose tolerance of ob/ob mice being fed the LPD (Fig. 5B). The insulin tolerance test showed that the LPD dramatically improved systemic insulin sensitivity in ob/ob mice, whereas BCAA supplementation significantly impaired insulin sensitivity in mice fed the LPD (Fig. 5C). The hyperinsulinemia in ob/ob mice was significantly attenuated by the LPD but aggravated by BCAA supplementation (Fig. 5D). Fasting plasma glucose levels were not affected by the LPD or BCAA supplementation (Fig. 5B). The body weight of ob/ob mice was slightly reduced by the LPD, whereas BCAA supplementation did not affect food intake or body weight (Fig. 5E and Supplementary Fig. 9). The LPD significantly reduced plasma levels of multiple amino acids; BCAAs were the only ones reversed by BCAA supplementation (Supplementary Fig. 10). These results show that the BT2 treatment and reducing BCAA intake both attenuate IR through a smaller abundance of BCAAs and BCKAs. On the other hand, BT2 treatment enhanced the BCAA catabolic flux, whereas reducing BCAA intake probably decreased the flux. In addition, increasing BCAA intake promoted IR with more BCAAs/BCKAs and higher catabolic flux. Collectively, all these data are consistent with the notion that more BCAAs/BCKAs, rather than the status of catabolic flux, plays a key role in the onset of IR in obese ob/ob mice.
Figure 5.
Altering BCAA intake affects IR in ob/ob mice. ob/ob mice were fed an NPD (20% protein by weight) or an LPD (6% protein by weight) for 4 weeks beginning at 10 weeks of age. For the BCAA group (LPD + BCAA), supplementation of BCAAs in drinking water (3 mg/mL) was started after mice had eaten the LPD for 2 weeks and lasted 2 weeks. We analyzed fasting plasma levels of BCAAs and metabolites (n = 8 mice; *P < 0.05 vs. NPD; &P < 0.05 vs. LPD) (A), glucose tolerance test results (n = 6–13 mice; **P < 0.01, LPD vs. NPD; &P < 0.05, LPD vs. LPD + BCAA) (B), insulin tolerance test results (n = 6 mice in each group; *P < 0.05, ***P < 0.001, LPD vs. NPD; &P < 0.05, LPD vs. LPD + BCAA) (C), fasting plasma insulin levels (n = 8–12 mice; *P < 0.05; &P < 0.05) (D), and body weight (n = 14 mice in each group; **P < 0.01).
Both BCAAs and BCKAs Can Impair Insulin Signaling
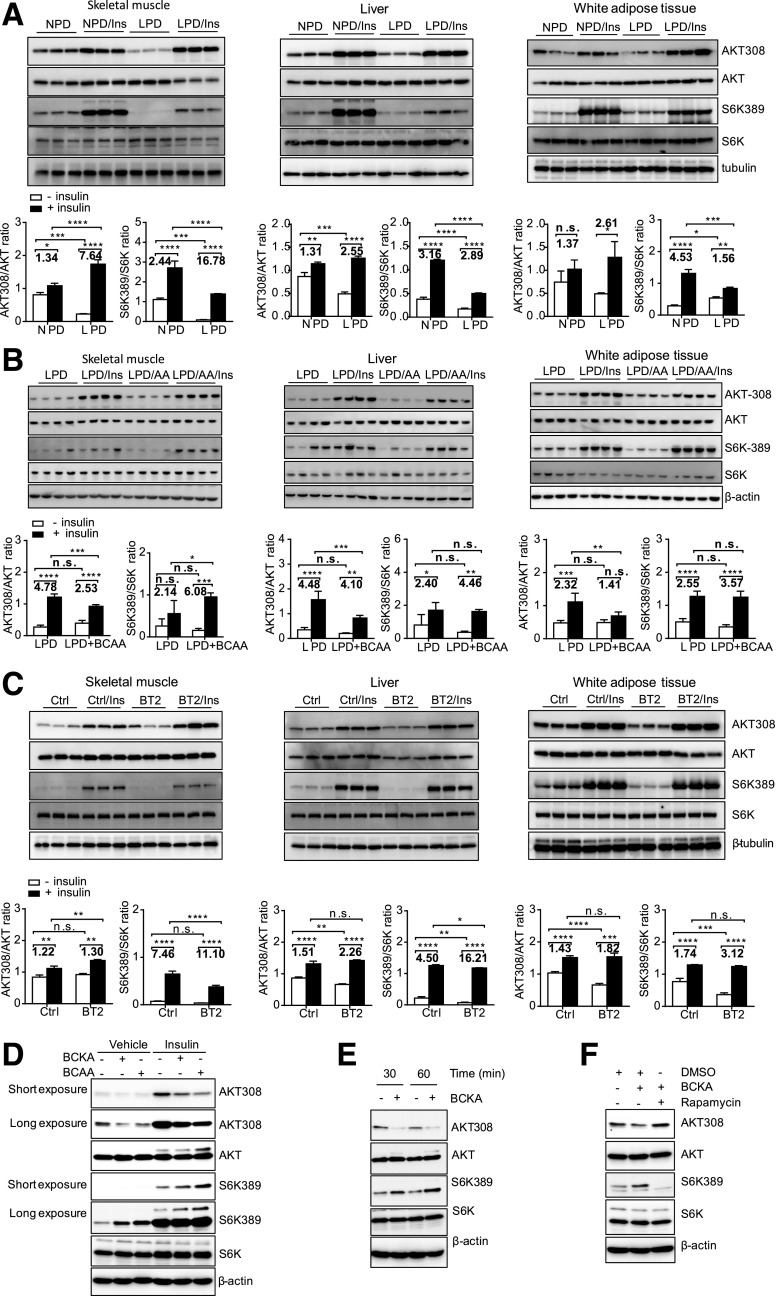
We next examined insulin signaling in food-deprived mice in the aforementioned experimental settings. Compared with the response (indicated by the fold induction of pAKT-Thr308) in ob/ob mice fed the NPD, insulin signaling in ob/ob mice fed the LPD was significantly higher in skeletal muscle (7.6 vs. 1.34 fold), liver (2.55 vs. 1.31 fold), and white adipose tissue (2.61 vs. 1.37 fold), indicating enhanced insulin sensitivity (Fig. 6A). In contrast, BCAA supplementation impaired insulin response in liver, white adipose tissue, and, to a much lesser extent, skeletal muscle in ob/ob mice fed the LPD (Fig. 6B). Similar to the LPD, BT2 treatment significantly enhanced AKT signaling in response to insulin, indicated by the higher fold induction of pAKT-Thr308 in various tissues (Fig. 6C). The enhanced response to insulin in the LPD and BT2 groups was primarily attributable to the lower basal AKT activity, consistent with the lower fasting insulin levels in the same groups (Figs. 4E and 5D).
Figure 6.
BCAAs and BCKAs contribute to impaired insulin signaling in ob/ob mice. A–C: Representative immunoblots for specific proteins, created by using tissue lysates from skeletal muscle, liver, and white adipose tissue of mice without or with insulin (Ins) injection for 10 min following 6 h of food deprivation. ob/ob mice were fed an NPD (20% protein by weight) or an LPD (6% protein by weight) for 4 weeks beginning at 10 weeks of age (A). BCAA supplementation (LPD + BCAA or LPD/amino acids) in drinking water (3 mg/mL) was started after mice had consumed the LPD for 2 weeks and lasted 2 weeks (B). ob/ob mice were treated with the vehicle (Ctrl) or BT2 by oral gavage for 5 weeks (C). The graphs below the immunoblots in A–C present densitometric values of the bands. D–F: Representative immunoblots for specific proteins, created by using cell lysates. 3T3-L1 cells were treated with FBS- and BCAA-free DMEM for 1–2 h before BCAA (500 μmol/L), BCKA (500 μmol/L), or rapamycin (100 nmol/L) treatment for 1 h (D and F) or various times (E), followed by insulin treatment (10 nmol/L) for 1 h (D). *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.0001.
BCAA-enhanced activation of mTORC1 has been implicated as an important mechanism underlying the impairment of insulin signaling (1). The LPD and BT2 treatment reduced the basal or insulin-stimulated mTORC1 activity (as indicated by p70S6K phosphorylation on Thr389), or both, in all three tissues, consistent with an enhanced response to insulin (Fig. 6A and C) and reduced BCAA levels (Figs. 4B and 5A). On the other hand, BCAA supplementation increased the amount of BCAAs in ob/ob mice fed the LPD (Fig. 5A) and enhanced the insulin-stimulated mTORC1 activity in skeletal muscle and probably adipose tissue (Fig. 6B). Of note, BCAA supplementation impaired insulin signaling without enhancing mTORC1 activity in liver in ob/ob mice fed the LPD.
As the abundances of BCKAs and BCAAs changed concordantly (Figs. 4B and 5A), we also examined the direct influence of BCKAs on insulin signaling in cultured cells. Interestingly, similar to BCAAs, BCKAs impaired insulin signaling while increasing insulin-stimulated mTORC1 activity (Fig. 6D). Further examination showed that BCKAs suppressed basal AKT phosphorylation while augmenting mTORC1 activity in the absence of BCAAs in culture medium (Fig. 6E). The mTORC1 inhibitor rapamycin abolished BCKA-repressed AKT phosphorylation, indicating an mTORC1-dependent action (Fig. 6F). These data suggest that BCKAs, in addition to BCAAs, may also contribute to impaired insulin signaling in obese ob/ob mice.
The Pharmacological Inhibitor of BCKDK Attenuates IR in DIO Mice
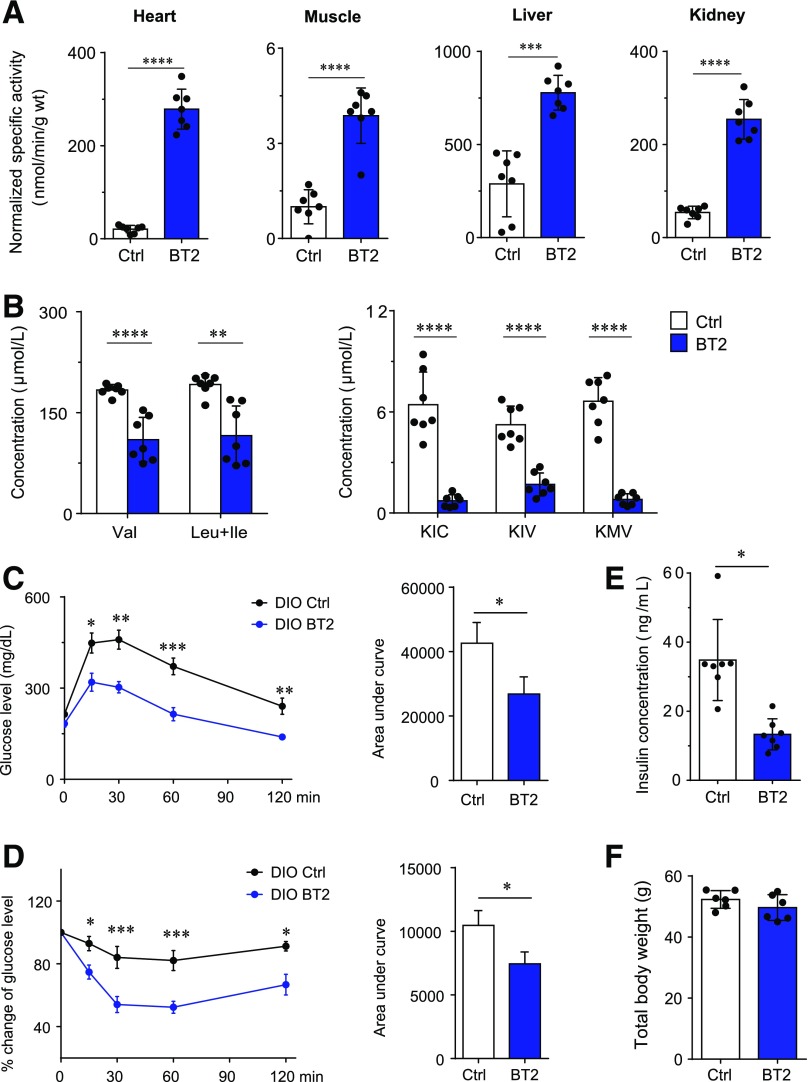
Given the importance of the BCAA catabolic defect in the pathogenesis of obesity-associated IR, enhancing BCKD activity through small-molecule treatment may represent a new pharmacological approach to treating diabetes. We assessed the effect of the BCKDK inhibitor BT2 on insulin sensitivity in wild-type mice with DIO, a model frequently used to mimic the metabolic impairments of obesity in humans. As expected, BT2 treatment augmented BCKD activities in various tissues of DIO mice and reduced plasma BCAA and BCKA levels (Fig. 7A and B). More importantly, BT2 treatment improved glucose tolerance, insulin sensitivity, and hyperinsulinemia in DIO mice (Fig. 7C–E) without affecting body weight (Fig. 7F). These results demonstrate that restoring BCAA catabolic activity with a small-molecule BCKDK inhibitor is effective to improve insulin sensitivity in DIO mice. This in vivo proof-of-concept demonstration validates targeting the BCAA catabolic defect as a potential therapeutic approach to attenuating obesity-associated IR and diabetes.
Figure 7.
The BCKDK inhibitor enhances BCAA catabolism and attenuates IR in DIO mice. DIO mice were treated with the vehicle (Ctrl) or BT2 by oral gavage for 8 weeks (n = 7 mice in each group). We analyzed BCKD activity in various tissues (A), fasting plasma levels of BCAAs (B, left) and BCKAs (B, right), glucose tolerance test results (C), insulin tolerance test results (D), fasting plasma insulin level (E), and body weight (F). *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001, all vs. Ctrl. ΚΙC, α-ketoisocaproic acid; KIV, α-ketoisovaleric acid; KMV, α-keto-β-methylvaleric acid.
Discussion
This study examines the causal role of disrupted BCAA homeostasis in obesity-associated IR and the therapeutic potential of targeting the elevated amounts of BCAAs and BCKAs. The unbiased integrative genomic analyses reveal a specific and robust association between the BCAA catabolic pathway and obesity-related IR in both human and mouse populations. In genetically obese mice, characteristic BCKD deficiency and BCAA/BCKA buildup accompany the suppression of BCAA catabolic genes at the pathway level. Correcting this BCAA catabolic defect abolishes BCAA/BCKA elevation and attenuates IR in obese ob/ob mice, supporting a causal role for the BCAA catabolic defect in IR onset in obese mice. Furthermore, reducing protein (and thus BCAA) intake effectively reduces BCAA/BCKA abundances and improves insulin sensitivity, whereas BCAA supplementation increases BCAA/BCKA abundances and promotes IR in ob/ob mice. These results together demonstrate the pathogenic role of elevated BCAAs/BCKAs in IR in obese mice. Similar to BCAAs, BCKAs also can impair insulin signaling. Finally, directly targeting the BCAA catabolic defect with a small-molecule BCKDK inhibitor reduces BCAA/BCKA abundances and attenuates IR in DIO mice. These findings suggest that the BCAA catabolic defect and elevated BCAAs/BCKAs play causal roles in the onset of obesity-associated IR and provide a valid target for dietary or pharmacological interventions to treat diabetes.
Several studies have established genetic links between individual BCAA catabolic genes (BCKHA or PP2Cm) and dysfunctional glucose metabolism (9,22–25). Instead of individual genes, the comprehensive genomic analyses in this study identified a genetic link between IR and the entire BCAA catabolic pathway. The significant and unique genetic association is based on information about both genetics and gene expression. The latter is of particular interest because nutritional status in obesity profoundly affects BCAA catabolic gene expression. Numerous studies have shown aberrant BCAA catabolic gene expression in animals and humans that are obese and have diabetes (4,8,9,45–49). Interestingly, a study of monozygotic twins discordant for BMI, which aimed to profile obesity-altered biological pathways in identical genetic backgrounds, revealed significant downregulation of the BCAA adipose catabolic pathway in obese cotwins, which correlated closely with IR and hyperinsulinemia (46). Thus, BCAA catabolism can be impaired by obesity status and linked with IR. In the bioinformatics study, we performed both BMI-adjusted and BMI-unadjusted analyses to look for IR-associated pathways that are independent of BMI (based on BMI-adjusted analysis) and that can be dependent on or independent of BMI (based on unadjusted analysis). That the genetic association between the BCAA catabolic pathway and IR is mainly observed in the unadjusted analysis and disappeared in the BMI-adjusted analysis strongly supports an obesity-dependent relationship. Our study, together with other data, indicates that BCAA catabolism is suppressed in obesity, which contributes to IR.
The special link between the adipose BCAA catabolic pathway and IR is intriguing. Studies in humans and rodents have indicated that an impaired adipose BCAA catabolic pathway contributes to the rise of plasma BCAAs/BCKAs and is associated with IR in obesity (4,8,9,45–49). In this study, the genetic association between the BCAA catabolic pathway and obesity-associated IR is mainly observed when gene expression in adipose tissue is considered in the genomics analyses. In ob/ob mice, adipose tissue shows the most dramatic loss of BCAA catabolic genes and the most significant accumulation of intratissue BCAAs and BCKAs, when compared with the liver and skeletal muscle. Therefore, BCAA catabolic activity in adipose tissue seems to play a unique role in regulating systemic BCAA homeostasis and IR in obesity. However, it remains unclear how adipose tissue contributes significantly to systemic BCAA catabolism despite its relatively low BCKD activity. It is possible that BCAA catabolic capacity of tissue is determined not only by BCKD activity but also by other factors such as BCAA/BCKA uptake from blood by tissues. On the other hand, as a main contributor to obesity-associated IR, an adipose BCAA catabolic defect may exert its effect locally or systemically. The local accumulation of BCAA/BCKA may trigger functional remodeling in adipose tissue. As adipose tissue plays a critical role in systemic lipid and glycemic metabolism, changes in adipose physiology may in turn contribute to local and systemic IR in obese mice. It is also possible, however, that an adipose BCAA catabolic defect functions as a main contributor to the systemic BCAA/BCKA accumulation that in turn promotes IR in various tissues. In either case, BT2 can enhance BCAA catabolism in heart, liver, kidney, and other tissues, relieving the systemic and adipose BCAA catabolic defect, irrespective of the site, and thus improving systemic insulin sensitivity; thus, BT2 should be considered for therapy.
Hepatic BCAA metabolism demonstrates weak and inconsistent correlations with IR. Directional discrepancies of correlations are observed between liver BCAA catabolic genes and fasting insulin level and IR traits. Some BCAA catabolic genes in liver are positively correlated with fasting glucose levels. The molecular details and functional implications for this discrepancy are unclear. We speculate that some positive correlations between BCAA genes in liver and glycemic traits in the HMDP study are likely a reactive rather than upstream causal process in the development of hyperglycemia or IR. Indeed, it is not uncommon to observe directional discrepancies in gene-trait correlations in various tissues and experimental settings because of feedback regulations and systems-level interactions between tissues. Meanwhile, the key enzymes determining the BCAA metabolic pattern are either negatively (DBT) or not significantly (BCAT2, BCKHDA, BCKDHB, BCKDK) associated with glycemic traits. In addition, our human genetics analysis does not support an enrichment of genetic signals in liver BCAA modules for any of the glycemic traits examined. Therefore, although it is possible that the BCAA catabolic pathway in liver also plays a role in glycemic regulation, the genetic evidence is not as strong as that for a role in adipose tissues and requires further investigation.
A high level of BCAAs in blood has been viewed as a metabolic hallmark of IR and diabetes in numerous studies (2,4–9). Thus, it was unexpected that the fasting plasma BCAA levels were not elevated in ob/ob mice. Plasma BCAA abundances were higher in fed obese ob/ob mice, suggesting that they are strongly affected by both the dietary intake and degradation in tissues. Similarly, it has been shown that BCAA levels are significantly increased in fed obese mice, although the differences dramatically decline or even disappear when the mice are deprived of food (45). On the other hand, the elevations of plasma BCKAs are significant in both food-deprived and fed obese ob/ob mice, in agreement with previous studies indicating higher plasma BCKA as a better biomarker for diabetes than BCAA (8,9). In addition, BCKAs are catabolites generated by tissues. Elevated BCKA levels in blood reflect a BCKD deficiency in tissues of obese mice.
BCAAs can trigger different and even opposite metabolic effects in mammals, depending on their catabolic and anabolic states (51). Thus, a causal relationship between disrupted BCAA metabolism and obesity-associated IR can be addressed only in animals that are already obese. Previous studies examining the effects of BCAA intake on metabolism in obese animals have fallen short of establishing this causal relationship. In obese New Zealand mice, reducing protein intake significantly improved glucose homeostasis, whereas BCAA repletion did not reverse it (52). In a mouse model of preexisting DIO, reducing dietary BCAAs improved glucoregulatory control while reducing body weight (53). In this study, however, the causal role of BCAAs remains unclear, because its impact on glucose regulation could be attributed to changes in body weight rather than BCAAs. In fatty Zucker rats, BCAA restriction showed no impact on systemic glucose tolerance but improved skeletal muscle insulin sensitivity (7). Similarly, we found that a low-BCAA diet did not affect systemic insulin sensitivity in ob/ob mice (Supplementary Fig. 11). However, reducing protein intake significantly improved systemic glucose tolerance in ob/ob mice. Reducing protein intake reduced the uptake of all amino acids and the plasma levels of eight amino acids, including BCAAs, phenylalanine, tryptophan, glutamine, tyrosine, and proline (Supplementary Fig. 10). Increasing BCAA intake in mice fed the LPD restored plasma BCAA levels and significantly impaired systemic insulin sensitivity, specifying the causal role of BCAAs. The impacts of non-BCAA amino acids affected by the LPD remain to be tested, whereas phenylalanine and tyrosine have been associated with IR (1). BCAA supplementation did not reduce glucose tolerance to the level of that in the NPD group, suggesting that the systemic insulin-sensitizing effect of BCAA restriction in obese ob/ob mice may need assistance from other amino acids. Importantly, BCAA supplementation did not change the body weight of ob/ob mice fed the LPD. Compared with the total energy intake (∼94% from carbohydrate and fat in the LPD), the caloric intake from BCAAs is likely very small. Thus, the caloric contribution from BCAA oxidation is unlikely to account for IR pathogenesis. Together with results showing that BT2 attenuated IR while enhancing BCAA oxidation without affecting food intake and body weight in both obese ob/ob and DIO mice (Figs. 4 and 7), these data clearly demonstrate the causal role of a BCAA catabolic defect in obesity-associated IR. On the other hand, although the effects of BT2 on ob/ob mice are similar to those recently reported in a genetic obese rat model (54) (Fig. 4), the beneficial impacts of BT2 in DIO mice (Fig. 7) add to the clinical relevance of our findings, as the DIO rodents remain the most clinically relevant model by mimicking the common cause and metabolic impairments of obesity in humans.
It has been suggested that, because of reduced catabolism by adipose tissue in obese mice, BCAA catabolism is enhanced in liver and skeletal muscle, which “clogs” lipid and glucose oxidation and promotes IR (50). A more recent study showed that obese and insulin-resistant mice shunt BCAA oxidation from the liver and fat toward muscle (55). In this study, BT2 reduced BCAA/BCKA abundances and improved insulin sensitivity while enhancing BCAA catabolic activities in various tissues including liver and skeletal muscle in both obese ob/ob and DIO mice. On the other hand, the LPD attenuated IR and BCAA/BCKA abundances while probably reducing BCAA catabolism through lowering BCAA load to the catabolic pathway in ob/ob mice. Furthermore, increasing BCAA intake promoted IR while elevating BCAA/BCKA abundances and probably the catabolic flux. Therefore, IR is consistently attenuated by reducing BCAA/BCKA abundances, rather than altering the status of catabolic flux, in obese mice. One recent independent study reported similar beneficial effects of BT2 on insulin sensitivity in genetically obese rats (54). Other reports have suggested that reducing BCAA intake improves insulin sensitivity via various mechanisms including reducing body weight or increasing energy expenditure (7,52,53). The common feature of these studies is the reduced abundances of BCAAs (and probably of BCKAs), corroborating the pathogenic roles of elevated BCAAs and BCKAs in obesity-associated IR.
BCAA-stimulated mTORC1 activation has been widely implicated in reducing insulin signaling (2,7,56–58). Our data show that BCKAs are also capable of suppressing insulin signaling. Thus, in addition to BCAAs per se, other components of the BCAA catabolic pathway may also play important roles in obesity-associated IR. Furthermore, our data suggest that mTORC1 probably mediates the suppression of AKT phosphorylation by BCAAs and BCKAs. However, disconnections have been observed between enhanced mTORC1 and impaired insulin signaling. For example, BCAA supplementation impaired insulin signaling without enhancing mTORC1 activity in liver in ob/ob mice fed the LPD. The tissue-specific impacts of manipulations of insulin signaling and mTORC1 activity by BCAA also remain puzzling. A recent study showed that BCAAs becoming replete while dietary protein is reduced restores mTORC1 signaling but does not induce IR in obese New Zealand mice (52). Thus, mTORC1 activation cannot fully explain the effects of modulating BCAA catabolism on insulin sensitivity, and additional mTORC1-independent mechanisms warrant further investigation.
The roles of BCAA catabolic flux and the distal steps of BCAA catabolism in obesity-associated IR remain to be further investigated. In addition to the “clogging” model, recent studies have indicated that elevated BCAA oxidation in skeletal muscle in obese db/db mice may lead to the production of 3-hydroxyisobutyrate from valine and promote IR (55,57). In contrast, another report suggested that defective BCAA metabolism in muscle contributes to impaired lipid metabolism and thus the development of obesity-associated IR (59). BT2 treatment enhances BCAA catabolism in muscle in healthy mice (55), obese rats (54), and obese mice (Figs. 4 and 7), while attenuating IR in obese animals, supporting the detrimental effects of impaired BCAA oxidation in muscle of obese animals. Interestingly, BT2 treatment increased BCKD activity (Figs. 4A and 7A) without reducing its phosphorylation in skeletal muscle of obese ob/ob mice (Supplementary Fig. 6) and obese rats (54). But BT2 increases the expression of BCKD subunits (Supplementary Fig. 6) and multiple distal genes in the BCAA catabolic pathway (Supplementary Fig. 7), which may contribute to enhanced BCAA catabolic flux. Quantitative measurements of BCAA catabolic flux at various steps in tissues of obese animals upon BT2 treatment or dietary manipulation will shed light on the roles of BCAA catabolic flux and the distal steps in obesity-associated IR.
Tissue-specific patterns of BCAA catabolism and responses to BT2 treatment have been observed in various experimental settings. In the bioinformatics analyses, the BCAA catabolic pathway in skeletal muscle showed a much weaker association with IR than that in adipose tissue and liver. BCAA catabolic gene expression and phosphorylation patterns vary dramatically in various tissues of ob/ob mice. BCAA abundances are elevated in adipose tissue, unchanged in skeletal muscle, and reduced in liver in ob/ob mice. Furthermore, BT2 treatment and dietary approaches alter insulin signaling differently in liver, skeletal muscle, and adipose tissue. Interestingly, BT2 treatment affects BCKD activity, BCKD phosphorylation, BCKD subunits, and distal gene expression in a tissue-specific pattern. To make it even more complicated, one recent study showed that acute BT2 treatment in healthy mice activates BCAA oxidation specifically in muscle even though BCKD phosphorylation is significantly reduced in liver, skeletal muscle, and heart (55). These intriguing patterns of BCAA catabolism and the impacts of BT2 may be attributed to the tissue-specific expression of BCAA catabolic enzymes and the pharmacokinetics of BT2. For example, although BCKDK expression is high in liver, skeletal muscle, and adipose tissue, BCKD phosphatase (PP2Cm) expression is lower in skeletal muscle and adipose tissue than in the liver. The strong dephosphorylation of BCKD by BT2 in liver might be the result of both BCKDK inhibition and PP2Cm activation. Nevertheless, despite these tissue-specific patterns of BCAA catabolism and BT2 effects, systemic BCAA catabolism is defective in obese mice but can be relieved by BT2 treatment, irrespective of the site, in order to improve systemic insulin sensitivity. Meanwhile, more mechanistic details remain to be determined.
BT2 is a potent allosteric inhibitor of BCKDK. BCKDK and pyruvate dehydrogenase kinases (PDKs) share similar structures. PDKs phosphorylate and inhibit the pyruvate dehydrogenase (PDH) complex, a key player in glucose metabolism. However, the allosteric ligand-binding pocket in BCKDK (volume 412Å3) is two- to fourfold larger than its counterparts in PDKs (90Å3–213Å3) (Supplementary Fig. 12). The larger allosteric ligand-binding volume in BCKDK allows for the occupancy of natural inhibitors like BCKAs or the artificial inhibitor BT2. By comparison, the allosteric sites in PDKs can accommodate up to 3-carbon α-keto acids such as pyruvate and its small-molecule inhibitor dichloroacetate (Supplementary Fig. 12). The structural constraints that preclude BT2 binding to PDKs are reflected by the absence of PDK inhibition and PDH activation by BT2 (data not shown). Therefore, the metabolic phenotypes resulting from BT2 treatment are unlikely to be related to PDK-regulated PDH activity; rather, they are exerted via the reprogramming of BCAA catabolism.
In summary, this study provides a compelling mechanistic basis for the strong association between elevated plasma BCAAs and the onset of IR in humans, demonstrating the causal role of a BCAA catabolic defect and the pathogenic role of elevated BCAAs/BCKAs in obesity-associated IR. The influences of BCAA intake on insulin sensitivity indicate the potential impact of dietary protein on IR and diabetes in obese people, consistent with a recent report showing that protein restriction improves glycemic control in overweight and mildly obese humans (60). BCKDK and its inhibitor represent new pharmacological targets and an approach to attenuating obesity-associated IR. Finally, the genetic link between IR and the BCAA catabolic pathway in human populations highlights the clinical significance of BCAA catabolism as a potential therapeutic target in treating obesity-associated IR and diabetes.
Supplementary Material
Article Information
Funding. This work was supported by the Ministry of Science and Technology of China (grant nos. 2012BAI02B05 and 2013YQ030923), the National International Science Cooperation Foundation (grant no. 2015DFA30560), the National Natural Science Foundation of China (grant nos. NSFC81570717 and 81522011), the National Institutes of Health (grant nos. HL108186, HL103205, HL098954, and DK62306), the National Heart, Lung, and Blood Institute (grant no. HL080111), the Laubisch Fund (to the University of California Los Angeles), the Welch Foundation (grant no. I-1286), and the Science and Technology Commission of Shanghai Municipality (grant nos. 13ZR1423300 and 16JC1404400). L.S. is supported by a China Scholarship Council scholarship, a UCLA Eureka and Hyde scholarship, and a Burroughs Wellcome Fund fellowship. X.Y. is supported by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (grant no. R01DK104363), the American Heart Association (grant no. 13SDG17290032), an American Heart Association Cardiovascular Genome-Phenome Study Pathway Grant, and a Leducq Foundation Transatlantic Networks of Excellence Grant.
Duality of Interest. Y.W. and H.S. participated in an advisory board for Ramino Bio Ltd. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.Z., J.S., C.-Y.W., L.S., W.D., Y.L., M.C., R.M.W., Jiq.W., Ji W., and W.-J.G. performed the research. M.Z., J.S., C.-Y.W., L.S., W.D., Y.L., M.C., R.M.W., Jiq.W., Ji W., W.-J.G., X.Q., A.J.L., Z.L., W.W., G.N., X.Y., D.T.C., Y.W., and H.S. prepared the manuscript. M.Z., C.-Y.W., L.S., W.D., Y.W., and H.S. analyzed the data. X.Q. performed the chemical design and synthesis. A.J.L., Z.L., W.W., G.N., X.Y., D.T.C., and Y.W. designed the overall study and analyzed the data. H.S. designed the research. H.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db18-0927/-/DC1.
References
- 1.Newgard CB, An J, Bain JR, et al. . A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 2014;10:723–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felig P, Marliss E, Cahill GFJ Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281:811–816 [DOI] [PubMed] [Google Scholar]
- 4.Lu J, Xie G, Jia W, Jia W. Insulin resistance and the metabolism of branched-chain amino acids. Front Med 2013;7:53–59 [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Wang Y, Ong C-N, et al. . Metabolic signatures and risk of type 2 diabetes in a Chinese population: an untargeted metabolomics study using both LC-MS and GC-MS. Diabetologia 2016;59:2349–2359 [DOI] [PubMed] [Google Scholar]
- 6.Chen T, Ni Y, Ma X, et al. . Branched-chain and aromatic amino acid profiles and diabetes risk in Chinese populations. Sci Rep 2016;6:20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White PJ, Lapworth AL, An J, et al. . Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol Metab 2016;5:538–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.She P, Olson KC, Kadota Y, et al. . Leucine and protein metabolism in obese Zucker rats. PLoS One 2013;8:e59443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menni C, Fauman E, Erte I, et al. . Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 2013;62:4270–4276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang TJ, Larson MG, Vasan RS, et al. . Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D, Moore SC, Matthews CE, et al. . Plasma metabolomic profiles in association with type 2 diabetes risk and prevalence in Chinese adults. Metabolomics 2016;12. pii: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Würtz P, Soininen P, Kangas AJ, et al. . Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013;36:648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floegel A, Stefan N, Yu Z, et al. . Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes 2013;62:639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melnik BC. Leucine signaling in the pathogenesis of type 2 diabetes and obesity. World J Diabetes 2012;3:38–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah SH, Crosslin DR, Haynes CS, et al. . Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 2012;55:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laferrère B, Reilly D, Arias S, et al. . Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med 2011;3:80re2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutson SM, Sweatt AJ, Lanoue KF. Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. J Nutr 2005;135(Suppl.):1557S–1564S [DOI] [PubMed] [Google Scholar]
- 18.Lu G, Sun H, She P, et al. . Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest 2009;119:1678–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burrage LC, Nagamani SC, Campeau PM, Lee BH. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Hum Mol Genet 2014;23:R1–R8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyarzabal A, Martínez-Pardo M, Merinero B, et al. . A novel regulatory defect in the branched-chain α-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum Mutat 2013;34:355–362 [DOI] [PubMed] [Google Scholar]
- 21.Zinnanti WJ, Lazovic J, Griffin K, et al. . Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain 2009;132:903–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiffin N, Adie E, Turner F, et al. . Computational disease gene identification: a concert of methods prioritizes type 2 diabetes and obesity candidate genes. Nucleic Acids Res 2006;34:3067–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taneera J, Lang S, Sharma A, et al. . A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab 2012;16:122–134 [DOI] [PubMed] [Google Scholar]
- 24.Xu M, Qi Q, Liang J, et al. . Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2013;127:1283–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goni L, Qi L, Cuervo M, et al. . Effect of the interaction between diet composition and the PPM1K genetic variant on insulin resistance and β cell function markers during weight loss: results from the Nutrient Gene Interactions in Human Obesity: implications for dietary guidelines (NUGENOB) randomized trial. Am J Clin Nutr 2017;106:902–908 [DOI] [PubMed] [Google Scholar]
- 26.Dupuis J, Langenberg C, Prokopenko I, et al.; DIAGRAM Consortium; GIANT Consortium; Global BPgen Consortium; Anders Hamsten on behalf of Procardis Consortium; MAGIC investigators . New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 2010;42:105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knowles JW, Xie W, Zhang Z, et al.; RISC (Relationship between Insulin Sensitivity and Cardiovascular Disease) Consortium; EUGENE2 (European Network on Functional Genomics of Type 2 Diabetes) Study; GUARDIAN (Genetics UndeRlying DIAbetes in HispaNics) Consortium; SAPPHIRe (Stanford Asian and Pacific Program for Hypertension and Insulin Resistance) Study . Identification and validation of N-acetyltransferase 2 as an insulin sensitivity gene [published correction appears in J Clin Invest 2016;126:403]. J Clin Invest 2015;125:1739–175125798622 [Google Scholar]
- 28.Mäkinen VP, Civelek M, Meng Q, et al.; Coronary Artery Disease Genome-Wide Replication and Meta-Analysis (CARDIoGRAM) Consortium . Integrative genomics reveals novel molecular pathways and gene networks for coronary artery disease. PLoS Genet 2014;10:e1004502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.International HapMap Consortium The International HapMap Project. Nature 2003;426:789–796 [DOI] [PubMed] [Google Scholar]
- 30.1000 Genomes Project Consortium; Abecasis GR, Auton A, Brooks LD, et al. . An integrated map of genetic variation from 1,092 human genomes. Nature 2012;491:56–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arneson D, Bhattacharya A, Shu L, Mäkinen VP, Yang X. Mergeomics: a web server for identifying pathological pathways, networks, and key regulators via multidimensional data integration. BMC Genomics 2016;17:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langfelder P, Horvath S.. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 2008;9:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000;28:27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shu L, Zhao Y, Kurt Z, et al. . Mergeomics: multidimensional data integration to identify pathogenic perturbations to biological systems. BMC Genomics 2016;17:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Wiener MC, Zhang C, et al. . Increasing the power to detect causal associations by combining genotypic and expression data in segregating populations. PLoS Comput Biol 2007;3:e69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu J, Zhang B, Smith EN, et al. . Integrating large-scale functional genomic data to dissect the complexity of yeast regulatory networks. Nat Genet 2008;40:854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett BJ, Farber CR, Orozco L, et al. . A high-resolution association mapping panel for the dissection of complex traits in mice. Genome Res 2010;20:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parks BW, Nam E, Org E, et al. . Genetic control of obesity and gut microbiota composition in response to high-fat, high-sucrose diet in mice. Cell Metab 2013;17:141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun H, Olson KC, Gao C, et al. . Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 2016;133:2038–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tso S-C, Gui W-J, Wu C-Y, et al. . Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain α-ketoacid dehydrogenase kinase. J Biol Chem 2014;289:20583–20593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Zhang B, Molony C, et al. . Systematic genetic and genomic analysis of cytochrome P450 enzyme activities in human liver. Genome Res 2010;20:1020–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang IM, Zhang B, Yang X, et al. . Systems analysis of eleven rodent disease models reveals an inflammatome signature and key drivers. Mol Syst Biol 2012;8:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meng Q, Ying Z, Noble E, et al. . Systems nutrigenomics reveals brain gene networks linking metabolic and brain disorders. EBioMedicine 2016;7:157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tai ES, Tan MLS, Stevens RD, et al. . Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010;53:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol Endocrinol Metab 2007;293:E1552–E1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pietiläinen KH, Naukkarinen J, Rissanen A, et al. . Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med 2008;5:e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lackey DE, Lynch CJ, Olson KC, et al. . Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab 2013;304:E1175–E1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem 2010;285:11348–11356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmerman HA, Olson KC, Chen G, Lynch CJ. Adipose transplant for inborn errors of branched chain amino acid metabolism in mice. Mol Genet Metab 2013;109:345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 2012;15:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bifari F, Nisoli E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: a pharmacological point of view. Br J Pharmacol 2017;174:1366–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maida A, Chan JSK, Sjøberg KA, et al. . Repletion of branched chain amino acids reverses mTORC1 signaling but not improved metabolism during dietary protein dilution. Mol Metab 2017;6:873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cummings NE, Williams EM, Kasza I, et al. . Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol 2018;596:623–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White PJ, McGarrah RW, Grimsrud PA, et al. . The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Cell Metab 2018;27:1281–1293.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neinast MD, Jang C, Hui S, et al. . Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab 2019;29:417–429.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao F, Huang Z, Li H, et al. . Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 2011;60:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang C, Oh SF, Wada S, et al. . A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med 2016;22:421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xiao F, Yu J, Guo Y, et al. . Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Metabolism 2014;63:841–850 [DOI] [PubMed] [Google Scholar]
- 59.Lerin C, Goldfine AB, Boes T, et al. . Defects in muscle branched-chain amino acid oxidation contribute to impaired lipid metabolism. Mol Metab 2016;5:926–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fontana L, Cummings NE, Arriola Apelo SI, et al. . Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep 2016;16:520–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.