Abstract
We investigated whether the composition of modified forms of LDL in circulating immune complexes (LDL-ICs) was associated with cardiovascular disease (CVD) outcomes, including any CVD, major adverse cardiac and cerebrovascular events (MACCE), myocardial infarction (MI), and coronary artery disease, in type 1 diabetes (T1D). Our results demonstrate that the baseline levels of oxidized LDL (oxLDL), MDA-modified LDL (MDA-LDL), and advanced glycosylation–modified LDL (AGE-LDL) in circulating ICs were associated with the four CVD outcomes in unadjusted models, and adjustment by age and mean HbA1c only resulted in minimal reduction of these associations. After adjustments were made for other cardiovascular risk factors, particularly LDL cholesterol, oxLDL-IC and MDA-LDL-IC remained independently associated with the risk of CVD, and oxLDL-IC was independently associated with the risk of MACCE and MI. In the majority of cases, the baseline levels of modified LDL-IC (measured many years before the occurrence of any CVD event) were associated with the risk of CVD over a 25-year period even after adjustment for other risk factors (including LDL cholesterol). Therefore, modified LDL biomarkers may help identify patients with T1D at high risk for MACCE and CVD events very early in the evolution of the disease, before other signals of disease are apparent.
Introduction
There is considerable interest in identifying predictive biomarkers for acute cardiovascular events. In the last decades, markers of inflammation and modified LDL were added to the classical risk factors to predict progression of atherosclerosis and eventually acute events (1–4). It has been established that modified forms of LDL are immunogenic and induce the production of autoantibodies leading to the formation of immune complexes (ICs). Modified forms of LDL in ICs have been shown to be strongly proinflammatory and proatherosclerotic (5–7). ICs containing modified LDL can be detected in peripheral blood (8,9) and in the arterial wall (10,11). We, and others, have found that high levels of modified LDL-ICs, assessed by the measurement of cholesterol or apolipoprotein B (apoB) in circulating ICs, had predictive value for the development of atherosclerosis and coronary heart disease (CHD) (12–15). We then developed capture assays for determining the concentration of different forms of modified LDL: oxidized LDL (oxLDL), malondialdehyde-modified LDL (MDA-LDL), and advanced glycosylation–modified LDL (AGE-LDL) in isolated ICs (16). Studies performed in participants with type 1 diabetes (T1D) from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort revealed that ICs containing high levels of oxLDL measured in serum samples collected at DCCT baseline were strongly associated with coronary calcification (17). High levels of both oxLDL-IC and AGE-LDL-IC were highly predictive of increased progression of CHD, assessed by two measurements of carotid intima-media thickness (IMT) in the same DCCT/EDIC cohort (18). Increased progression of atherosclerosis was further validated by examining the progression of IMT in the same participants over a 12-year follow-up period (19). In this study, oxLDL, AGE-LDL, and MDA-LDL were all predictors of carotid IMT progression, but oxLDL and AGE-LDL levels were stronger predictors of IMT progression than MDA-LDL (19). In all of these studies, the levels of modified LDL in ICs were stronger predictors of cardiovascular disease (CVD) than any other conventional markers of atherosclerosis progression, including LDL cholesterol, blood pressure, albumin excretion rate (AER), and HbA1c levels.
Interestingly, in participants with T2D enrolled in the Veterans Affairs Diabetes Trial (VADT), high levels of MDA-LDL in ICs were predictive of future acute cardiovascular events (20). The hazard ratios (HRs) were particularly strong for myocardial infarction (MI) (20) even after adjustments for all conventional risk factors. No association was observed between high levels of oxLDL in ICs, AGE-LDL in ICs, LDL cholesterol, HbA1c, or elevated blood pressure and acute CVD events.
In the current study, we examined whether the levels of modified LDL in circulating ICs, previously shown to be associated with progression of CHD in participants from the DCCT/EDIC cohort, were also associated with CVD outcomes in the same cohort over an extended follow-up period (10–25 years). Our goal was to investigate whether high levels of different forms of modified LDL in circulating ICs were predictors of not only CHD progression but also CVD events, above and beyond traditional risk factors.
Research Design and Methods
DCCT/EDIC Study Design and Participants
The methods of DCCT/EDIC have been previously described (21,22). In brief, the DCCT was a controlled clinical trial that randomly assigned 1,441 participants (age 13–39 years) with T1D to receive either intensive (n = 711) or conventional (n = 730) diabetes therapy with the goal of evaluating whether reducing glycemia resulted in lower risks of microvascular complications. At baseline, the DCCT cohort consisted of a primary prevention cohort (1–5 years of diabetes duration, no retinopathy based on fundus photography, and AER <40 mg/24 h) and a secondary intervention cohort (1–15 years of diabetes duration, minimal to moderate nonproliferative retinopathy, and AER <200 mg/24 h).
After an average of 6.5 years of follow-up, the DCCT ended in 1993, and all participants were instructed in intensive therapy and referred to their health care providers for subsequent diabetes care. In 1994, 98% of the surviving DCCT cohort was enrolled in the EDIC follow-up study, with annual assessments for risk factors and complications. Ninety-four percent of the cohort survivors were still actively participating after >20 years of additional follow-up. The DCCT and EDIC protocols were approved by the institutional review boards of all participating centers, and all participants provided written informed consent.
Three IC biomarkers (AGE, MDA-LDL-IC, and oxLDL-IC) were evaluated in 518 DCCT/EDIC participants at up to four time points (DCCT baseline, DCCT closeout, EDIC years 4–6, and EDIC years 8–11), for a total of 1,944 evaluations. The 518 participants were selected based on their retinopathy, nephropathy, and carotid IMT status as previously described (19).
DCCT/EDIC visits consist of a detailed medical history, including demographic and behavioral risk factors, medical outcomes, and a physical examination, which included measurements of height, weight, sitting blood pressure, and pulse rate (21,22). Blood samples were collected at each visit, and HbA1c was assayed quarterly during DCCT and annually during EDIC. Fasting lipids (triglycerides and LDL and HDL cholesterol) were determined annually during DCCT and every other year during EDIC, alternating with AER (23). To account for the difference in measurement frequency during DCCT and EDIC, the updated time-weighted mean of a risk factor (such as HbA1c) was computed, weighting each value by the interval between measurements.
IC Biomarkers
In brief, serum samples obtained after an overnight fast were stored at −80°C until the assays were performed as described in previous publications (16–18). In brief, circulating ICs were first precipitated with 4% polyethyleneglycol 8000, and the precipitates were equilibrated in 0.02 mol/L sodium phosphate buffer containing 0.5 mol/L NaCl, previously shown to be able to dissociate LDL-ICs, and fractionated by protein G affinity chromatography in protein G–Sepharose columns equilibrated with the same buffer. Whereas IgG, the predominant isotype of the antibodies against modified lipoproteins, was retained in the column, modified LDL was eluted and could be measured without the interference of ICs. The reactivity of modified LDL eluted from LDL-ICs was assayed by capture assays developed in our laboratory using rabbit antibodies specific for the different LDL (oxLDL, MDA-LDL, and AGE-LDL) modifications (16). The characteristics of the antibodies used in the assay and the specificity and reproducibility of the capture assays have been previously reported (16,24). Interassay coefficient of variation was 5.2% for oxLDL, 5.5% for MDA-LDL, and 8.3% for AGE-LDL. The development of standards for calibration of the oxLDL, MDA-LDL, and AGE-LDL assays, as well as sensitivity, reproducibility, and recovery data for the capture assays, has been reported elsewhere (16). The levels of the different LDL modifications in human circulating ICs were calculated per milligram of apoB contained in the ICs, using serum total apoB/L to calculate the final serum concentration. The final values reported represent the concentration in mg/L serum.
Cardiovascular Outcomes
CVD events were ascertained based on a medical history, electrocardiogram, and available medical records and were adjudicated by a committee masked to DCCT treatment group and HbA1c levels. The primary CVD outcome (“any CVD”) was defined as the time to the first occurrence of CVD death, nonfatal MI, nonfatal stroke, subclinical MI on electrocardiogram, angina confirmed by ischemic changes with exercise tolerance testing or by clinically significant obstruction on coronary angiography, revascularization (with angioplasty or coronary artery bypass), or congestive heart failure (paroxysmal nocturnal dyspnea, orthopnea, or marked limitation of physical activity caused by heart disease) (25). The second CVD outcome, major adverse cardiac and cerebrovascular events (MACCE), included only the time to cardiovascular death, nonfatal MI, or nonfatal stroke, whichever occurred first. The third CVD outcome, MI, included fatal or nonfatal events. The fourth CVD outcome considered, coronary artery disease (CAD), was obtained by removing the fatal and nonfatal stroke events from the any CVD composite outcome. Participants free of a CVD event were administratively censored as of 31 December 2013 (the date of the last CVD data lock).
Statistical Analyses
All statistical analyses used inverse probability weighting (using the sampling weights) to obtain results representative of the full cohort (n = 1,441). Sampling weights were calculated for each stratum defined by the possible combinations of retinopathy, nephropathy, and carotid stenosis status. The weights were based on the numbers in the subcohort (n = 518) and the full DCCT/EDIC cohort (n = 1,441) and were calibrated to the number of males and females in the full cohort and, likewise, to the number of participants in the primary prevention cohort and the secondary intervention cohort.
Summary statistics (percentages for binary variables and medians and quartiles for continuous variables) were used to describe the baseline characteristics of the DCCT participants and to assess whether, after adjustment for the sampling weights, the 518 participants with available IC measurements included in these analyses were representative of the entire DCCT/EDIC cohort.
Kaplan-Meier survival curves were used to describe the time-to-event outcomes (i.e., any CVD, MACCE, and MI) from the date of randomization into the DCCT. Cox proportional hazards models (separately for any CVD, MACCE, MI, and CAD) with robust SEs were used to assess the association between IC biomarker values and the subsequent risk of CVD. The power to detect associations in time-to-event analyses is dictated by the number of events. Given the relatively small number of incident CVD events among the 518 participants in our study, a set of prespecified models were considered: 1) unadjusted models, 2) models adjusted for age and mean updated HbA1c, and 3) models adjusted for age, mean HbA1c, cohort, sex, mean systolic blood pressure (SBP), pulse, (log) triglycerides, mean LDL, (log) AER, and duration of T1D. Given the relatively low number of MI events, the fully adjusted models (3) for MI only included age, mean HbA1c, mean LDL, and (log) AER. Each of these models was separately employed using 1) the baseline IC biomarker values as a fixed covariate, 2) DCCT closeout IC biomarker values as a fixed covariate, and 3) all four IC measurements as a time-dependent covariate. Subjects with any outcome CVD event prior to the IC measurement used in the analysis were excluded.
The IC biomarkers had distributions skewed to the right and were analyzed on the log scale. The HRs in the Cox models were reported per 1 unit increase on the log scale in the biomarker values. The functional form of the association between the IC biomarkers and the log hazards of the four CVD outcomes was investigated using smoothing splines (26). All analyses were performed using R, and P values ≤0.05 were considered nominally significant.
Results
Table 1 presents the DCCT baseline characteristics of the 518 participants with IC biomarker values after adjustment for sampling weights, along with the characteristics of the full DCCT/EDIC cohort. The weighted subcohort is representative of the full cohort.
Table 1.
Baseline characteristics (unweighted and weighted) of the DCCT subcohort (n = 518) versus the full DCCT cohort (n = 1,441), and the baseline summaries of the three biomarkers
| Unweighted | Weighted* | Full cohort | |
|---|---|---|---|
| Age (years) | 27 (22, 32) | 27 (21, 32) | 27 (22, 32) |
| Diabetes duration (months) | 55 (30, 115) | 50 (29, 107) | 51 (28, 109) |
| AER (mg/24 h) | 11.5 (7.2, 18.7) | 11.5 (7.2, 18.7) | 11.5 (7.2, 18.7) |
| eGFR (mL/min/1.73 m2) | 124.8 (117.9, 133.1) | 124.9 (117.9, 133.9) | 125 (118, 134) |
| BMI (kg/m2) | 23.4 (21.6, 25.3) | 23.3 (21.5, 25.3) | 23.3 (21.5, 25.2) |
| Triglycerides (mg/dL) | 70 (54, 93) | 69 (54, 91) | 70 (55, 94) |
| LDL (mg/dL) | 105 (88, 126) | 104 (87, 124) | 107 (91, 127) |
| HDL (mg/dL) | 48 (42, 57) | 50 (42, 57) | 49 (42, 57) |
| SBP (mmHg) | 114 (108, 122) | 114 (108, 122) | 114 (108, 122) |
| Diastolic blood pressure (mmHg) | 74 (68, 80) | 74 (68, 80) | 72 (68, 80) |
| Pulse (bpm) | 76 (68, 84) | 76 (68, 84) | 76 (68, 84) |
| HbA1c (%; mmol/mol) | 8.6 (7.6, 9.8); 70 (60, 84) | 8.5 (7.6, 9.8); 69 (60, 84) | 8.7 (7.8, 9.9); 72 (62, 85) |
| Treatment group (% intensive) | 46.3 | 48.7 | 49.3 |
| Cohort (% primary) | 45.2 | 50.0 | 50.4 |
| Sex (% male) | 52.3 | 52.9 | 52.8 |
| Smoking (%) | 19.3 | 18.4 | 18.5 |
| AGE-LDL-IC (mg/L) | 6.4 (2.6, 12) | 5.8 (2.5, 10.8) | |
| MDA-LDL-IC (mg/L) | 107.3 (42.7, 195.9) | 98.5 (37.4, 185.9) | |
| oxLDL-IC (mg/L) | 161.7 (88.6, 295.3) | 149.4 (80.8, 265.9) |
Data are median (quartile 1, quartile 3) unless otherwise indicated.
*With weights based on the joint distribution of the complications and further adjusted for sex and cohort.
At DCCT baseline, 53% of the participants were males, 49% were randomized to the intensive group, and 18% were current smokers (Table 1). In addition, participants had a median (first and third quartiles) age of 27 years (21, 32), median duration of diabetes of 50 months (29, 107), and a median HbA1c of 8.5% (7.6, 9.8) (69 mmol/mol [60, 84]).
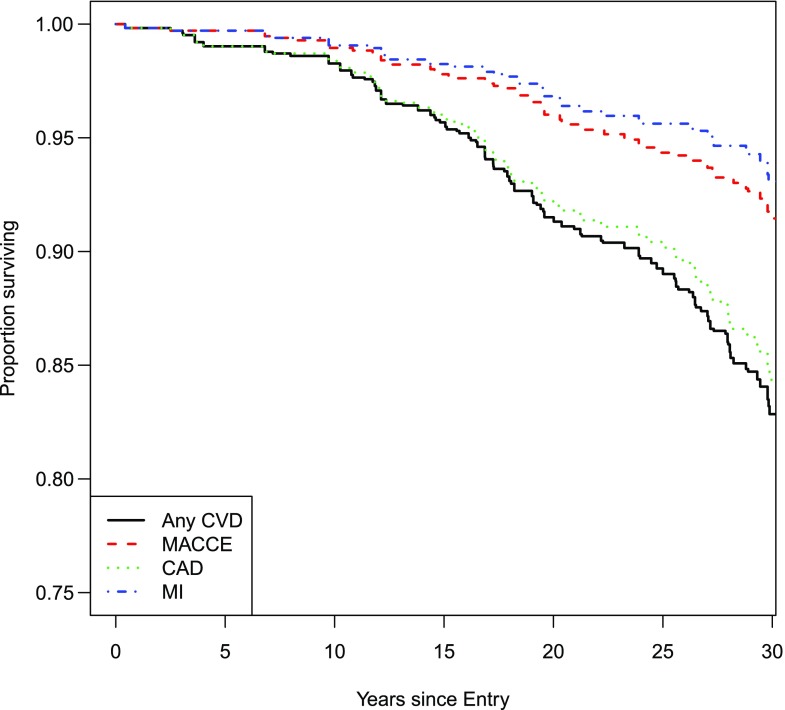
Among the 518 participants in this study, 100 had a CVD event over a total follow-up of 14,391 person-years (rate = 6.9 events per 1,000 individuals at risk for 1 year), 46 had a MACCE event over a total follow-up of 14,931 person-years (rate = 3.1 events per 1,000 individuals at risk for 1 year), 36 had an MI event over a follow-up of 15,023 person-years (rate = 2.4 events per 1,000 individuals at risk), and 91 had a CAD event over a total follow-up of 14,470 person-years (rate = 6.3 events per 1,000 individuals at risk for 1 year). Figure 1 depicts the Kaplan-Meier survival curves for the four CVD outcomes.
Figure 1.
Kaplan-Meier survival (event-free) curves for the CVD outcomes.
The risk gradients for the association between the log IC biomarkers and the log hazard of CVD outcomes were linear (Supplementary Fig. 1). Tables 2–4 and Supplementary Table 1 describe the associations between the three IC biomarkers and the risk of a subsequent CVD event.
Table 2.
Association between the IC biomarkers and the risk of any CVD in unadjusted, minimally adjusted, and final adjusted Cox models
| Unadjusted |
Adjusted for age and HbA1c |
Final models* |
||||
|---|---|---|---|---|---|---|
| HR (95% CI)** | P value | HR (95% CI)** | P value | HR (95% CI)** | P value | |
| DCCT baseline (100 events, n = 518) | ||||||
| AGE-LDL-IC (mg/L) | 1.31 (1.06, 1.60) | 0.011 | 1.28 (1.04, 1.57) | 0.019 | 1.21 (0.98, 1.50) | 0.083 |
| MDA-LDL-IC (mg/L) | 1.28 (1.05, 1.56) | 0.015 | 1.29 (1.06, 1.58) | 0.012 | 1.24 (1.01, 1.52) | 0.045 |
| oxLDL-IC (mg/L) | 1.46 (1.14, 1.87) | 0.003 | 1.45 (1.14, 1.84) | 0.002 | 1.33 (1.04, 1.72) | 0.025 |
| DCCT closeout (93 events, n = 500) | ||||||
| AGE-LDL-IC (mg/L) | 1.28 (1.05, 1.57) | 0.016 | 1.22 (1.01, 1.49) | 0.045 | 1.02 (0.81, 1.28) | 0.901 |
| MDA-LDL-IC (mg/L) | 1.32 (1.07, 1.63) | 0.010 | 1.31 (1.06, 1.63) | 0.012 | 1.16 (0.93, 1.44) | 0.191 |
| oxLDL-IC (mg/L) | 1.58 (1.24, 2.01) | <0.001 | 1.49 (1.17, 1.88) | 0.001 | 1.21 (0.93, 1.58) | 0.150 |
| All measurements (100 events, n = 518) | ||||||
| AGE-LDL-IC (mg/L) | 1.37 (1.14, 1.65) | 0.001 | 1.31 (1.08, 1.58) | 0.007 | 1.17 (0.96, 1.44) | 0.120 |
| MDA-LDL-IC (mg/L) | 1.33 (1.09, 1.62) | 0.005 | 1.32 (1.07, 1.63) | 0.011 | 1.20 (0.97, 1.48) | 0.093 |
| oxLDL-IC (mg/L) | 1.56 (1.26, 1.93) | <0.001 | 1.47 (1.18, 1.81) | <0.001 | 1.27 (1.03, 1.57) | 0.028 |
P values <0.05 are presented in boldface type.
*Adjusted for age, mean HbA1c, cohort, sex, mean SBP, pulse, (log) triglycerides, mean LDL, (log) AER, and duration of T1D.
**Per 1 unit increase in the log-transformed biomarker value. The HR per (for example) a 25% increase in the biomarker (or equivalently, a 1.25-fold change) is given by HR^log(1.25), where “^” denotes “to the power of.” Biomarkers are amount per milligram of apoB contained in the IC, using serum total apoB/L.
Table 4.
Association between the IC biomarkers and the risk of MI events in unadjusted, minimally adjusted, and final adjusted Cox models
| Unadjusted |
Adjusted for age and HbA1c |
Final models* |
||||
|---|---|---|---|---|---|---|
| HR (95% CI)** | P value | HR (95% CI)** | P value | HR (95% CI)** | P value | |
| DCCT baseline (36 events, n = 518) | ||||||
| AGE-LDL-IC (mg/L) | 1.53 (1.12, 2.11) | 0.008 | 1.48 (1.07, 2.04) | 0.018 | 1.42 (1.02, 1.99) | 0.040 |
| MDA-LDL-IC (mg/L) | 1.35 (1.04, 1.76) | 0.027 | 1.36 (1.02, 1.80) | 0.035 | 1.32 (0.98, 1.77) | 0.069 |
| oxLDL-IC (mg/L) | 1.94 (1.33, 2.83) | 0.001 | 1.86 (1.27, 2.72) | 0.001 | 1.75 (1.18, 2.58) | 0.005 |
| DCCT closeout (33 events, n = 504) | ||||||
| AGE-LDL-IC (mg/L) | 1.47 (1.04, 2.07) | 0.028 | 1.40 (0.99, 2.00) | 0.061 | 1.27 (0.88, 1.83) | 0.210 |
| MDA-LDL-IC (mg/L) | 1.40 (1.06, 1.86) | 0.019 | 1.40 (1.03, 1.90) | 0.034 | 1.26 (0.92, 1.71) | 0.150 |
| oxLDL-IC (mg/L) | 1.97 (1.31, 2.95) | 0.001 | 1.83 (1.21, 2.75) | 0.004 | 1.65 (1.09, 2.49) | 0.018 |
| All measurements (36 events, n = 518) | ||||||
| AGE-LDL-IC (mg/L) | 1.45 (1.03, 2.05) | 0.035 | 1.34 (0.94, 1.92) | 0.113 | 1.26 (0.87, 1.81) | 0.223 |
| MDA-LDL-IC (mg/L) | 1.34 (0.97, 1.84) | 0.073 | 1.28 (0.92, 1.77) | 0.146 | 1.20 (0.87, 1.64) | 0.263 |
| oxLDL-IC (mg/L) | 1.77 (1.16, 2.70) | 0.008 | 1.56 (1.03, 2.36) | 0.038 | 1.45 (0.96, 2.19) | 0.079 |
P values <0.05 are presented in boldface type.
*Adjusted for age, mean HbA1c, mean LDL, and (log) AER. The number of covariates was reduced in the final models due to the small number of events (see research design and methods).
**Per 1 unit increase in the log-transformed biomarker value. The HR per (for example) a 25% increase in the biomarker (or, equivalently, a 1.25-fold change) is given by HR^log(1.25), where “^” denotes “to the power of.” Biomarkers are noted amount per milligram of apoB contained in the IC, using serum total apoB/L.
For any CVD (Table 2), all three biomarkers were statistically significant in the unadjusted models and in models adjusted for age and mean HbA1c, whether using the baseline values, the DCCT closeout values, or all (up to four) time points. In the final models, MDA-LDL-IC at baseline (HR = 1.24 per 1 unit increase in biomarker on the log scale, P = 0.045), oxLDL-IC at baseline (HR = 1.33 per 1 unit increase in biomarker on the log scale, P = 0.025), and oxLDL-IC using all measurements (HR = 1.27 per 1 unit increase in biomarker on the log scale, P = 0.028) remained significantly associated with the risk of any CVD (Table 2). Findings similar to those for any CVD were observed for CAD (Supplementary Table 1).
For MACCE (Table 3), all three biomarkers were statistically significant in the unadjusted models, whether using the biomarker values from baseline, DCCT closeout, or all (up to four) time points. With the exception of AGE-LDL-IC at DCCT closeout and AGE-LDL-IC and MDA-LDL-IC using all four measurements, the associations remained significant after adjustment for age and mean HbA1c. In the final models, oxLDL-IC at baseline (HR = 1.57 per 1 unit increase in biomarker on the log scale, P = 0.014) and oxLDL-IC at DCCT closeout (HR = 1.56 per 1 unit increase in biomarker on the log scale, P = 0.028) remained significantly associated with the risk of MACCE (Table 3).
Table 3.
Association between the IC biomarkers and the risk of MACCE in unadjusted, minimally adjusted, and final adjusted Cox models
| Unadjusted |
Adjusted for age and HbA1c |
Final models* |
||||
|---|---|---|---|---|---|---|
| HR (95% CI)** | P value | HR (95% CI)** | P value | HR (95% CI)** | P value | |
| DCCT baseline (46 events, n = 518) | ||||||
| AGE-LDL-IC (mg/L) | 1.47 (1.13, 1.93) | 0.005 | 1.42 (1.01, 1.87) | 0.012 | 1.34 (0.99, 1.80) | 0.051 |
| MDA-LDL-IC (mg/L) | 1.30 (1.04, 1.63) | 0.021 | 1.30 (1.03, 1.65) | 0.029 | 1.23 (0.95, 1.60) | 0.109 |
| oxLDL-IC (mg/L) | 1.78 (1.26, 2.51) | 0.001 | 1.72 (1.22, 2.42) | 0.002 | 1.57 (1.10, 2.25) | 0.014 |
| DCCT closeout (43 events, n = 504) | ||||||
| AGE-LDL-IC (mg/L) | 1.37 (1.01, 1.86) | 0.045 | 1.29 (0.95, 1.76) | 0.108 | 1.16 (0.81, 1.66) | 0.419 |
| MDA-LDL-IC (mg/L) | 1.34 (1.05, 1.71) | 0.018 | 1.33 (1.02, 1.73) | 0.035 | 1.23 (0.93, 1.64) | 0.150 |
| oxLDL-IC (mg/L) | 1.87 (1.29, 2.70) | 0.001 | 1.73 (1.19, 2.51) | 0.004 | 1.56 (1.05, 2.31) | 0.028 |
| All measurements (46 events, n = 518) | ||||||
| AGE-LDL-IC (mg/L) | 1.44 (1.08, 1.91) | 0.012 | 1.33 (0.98, 1.79) | 0.064 | 1.21 (0.90, 1.65) | 0.224 |
| MDA-LDL-IC (mg/L) | 1.34 (1.03, 1.75) | 0.029 | 1.28 (0.97, 1.68) | 0.078 | 1.18 (0.90, 1.55) | 0.238 |
| oxLDL-IC (mg/L) | 1.71 (1.20, 2.44) | 0.003 | 1.50 (1.06, 2.13) | 0.022 | 1.34 (0.94, 1.90) | 0.106 |
P values <0.05 are presented in boldface type.
*Adjusted for age, mean HbA1c, cohort, sex, mean SBP, pulse, (log) triglycerides, mean LDL, (log) AER, and duration of T1D.
**Per 1 unit increase in the log-transformed biomarker value. The HR per (for example) a 25% increase in the biomarker (or equivalently, a 1.25-fold change) is given by HR^log(1.25), where “^” denotes “to the power of.” Biomarkers are amount per milligram of apoB contained in the IC, using serum total apoB/L.
For MI (Table 4), results were similar to those for MACCE with point estimates generally being slightly higher, but statistical significance in some cases decreased slightly. In the final models, AGE-LDL-IC at DCCT baseline (HR = 1.42 per 1 unit increase in biomarker on the log scale, P = 0.040), oxLDL-IC at DCCT baseline (HR = 1.75 per 1 unit increase in biomarker on the log scale, P = 0.005), and oxLDL-IC at closeout (HR = 1.65 per 1 unit increase in biomarker on the log scale, P = 0.018) remained significantly associated with the risk of MI (Table 4).
Areas under the curve (AUCs) were compared for the various DCCT baseline models to determine the relative contribution to the predictive ability of the model of oxLDL-IC and LDL cholesterol. Comprehensive results are presented in Supplementary Table 2. In brief, for the end point MI, the AUC for the model with standard cardiovascular risk factors excluding LDL cholesterol (i.e., age, HbA1c, AER, sex, SBP, duration of T1D, DCCT cohort, and triglycerides) was 0.714, and adding LDL cholesterol to the model increased the AUC only slightly (0.718), as did adding oxLDL-IC (0.734), with neither biomarker resulting in a statistically significant change in the AUC value.
Discussion
Our results demonstrate that the baseline levels of all three modified LDL-IC biomarkers examined (AGE-LDL, oxLDL, and MDA-LDL in circulating ICs) were associated with the four CVD outcomes in unadjusted models, and adjustment by age and mean HbA1c only resulted in minimal reduction of these associations. However, after adjustments were made for other cardiovascular risk factors, in particular LDL cholesterol, only oxLDL-IC and MDA-LDL-IC (HR 1.333 [95% CI 1.036, 1.717] and HR 1.235 [1.005, 1,517], respectively) remained independently associated with the risk of CVD, and only oxLDL-IC was independently associated with the risk of MACCE (HR 1.569 [95% CI 1.096, 2.245]), MI (HR 1.745 [95% CI 1.179, 2.583]), and CAD (HR 1.35 [95% CI 1.03, 1.77]).
It is important to note that in the majority of the cases, the baseline levels of the three modified LDL-ICs were measured many years before the occurrence of any CVD event. However, the baseline levels of the three biomarkers were associated with the risk of CVD over a 25-year period even after adjustment for other risk factors, including LDL cholesterol. Interestingly, when subsequent measurements of oxLDL-IC were incorporated into the models, effectively reducing the time between the modified LDL-IC measurements and incident events, the HRs for the association with the risk of CVD, MACCE, and MI events were attenuated in the fully adjusted models. One possible explanation for these results could be that in T1D, the biomarkers predictive of incident CVD events are those with a causal association with the development and progression of atherosclerosis.
Some of our previous studies have clearly shown that the levels of oxLDL in ICs are very strong predictors of atherosclerosis and the development/progression of atherosclerosis. Two of these studies used repeated measurements of carotid IMT to assess CHD progression (18,19), and both showed that oxLDL-IC in the same group of participants was a much stronger predictor of IMT progression than LDL cholesterol. The third study (17) showed that oxLDL-IC could, independently of other CVD risk factors, predict coronary artery calcification, assessed by computed tomography, in the same group of patients.
The results presented in this manuscript in T1D differ from those obtained in the VADT T2D cohort in which the MDA-LDL content of isolated LDL-ICs was predictive of acute MI but neither oxLDL-IC nor AGE-LDL-IC was significantly associated with acute CVD events (20). A possible explanation for the differences between these cohorts with T1D and T2D is the fact that while development of atherosclerosis is connected with CVD events in both diseases, the close association of T2D with metabolic syndrome, higher degrees of oxidative stress, and inflammation may diversify the triggers for acute CVD events in T2D. The significant association of increased levels of MDA-LDL-IC with acute CVD events, mainly MI, in T2D may be secondary not to the MDA-LDL-IC–induced progression of atherosclerosis but, rather, to its high proapoptotic effect and increased release of TNF and matrix metalloproteinase 1 (MMP-1) (27). Both cell apoptosis and increased TNF and MMP-1 have been shown to be associated with plaque destabilization. Reinforcing this postulate is the fact that high levels of circulating MDA-LDL have been shown to be associated with acute CVD events and unstable angina in several independent studies (28–33). The close association of strong markers of atherosclerosis progression and CVD events in T1D and the dissociation between these markers in T2D are of considerable interest.
We have clearly shown in T2D that statin therapy with simvastatin leads not only to a decrease in LDL cholesterol but also to a marked decrease of modified LDL cholesterol and of anti-oxLDL IgG antibody (34), therefore influencing the formation of ICs by decreasing not only modified LDL but also the formation of antibodies. Recently, Hörl et al. (35) demonstrated that simvastatin lowers small LDL-IgG IC levels in subjects with atherosclerosis to a greater extent than it lowers LDL cholesterol and LDL-apoB. Whether this is a drug class–associated characteristic has yet to be determined. These data, together with our present findings showing that it is possible to clearly identify patients at high risk for MI or any CVD events very early in the evolution of the disease, could create a new paradigm of treatment in which an aggressive and early treatment with statins should be initiated in patients with increased levels of oxLDL-IC before any signals of disease are apparent. That would require the development of a commercial assay of oxLDL-IC that is adequately validated for routine use. As we have previously shown, the levels of oxLDL in ICs are a much stronger predictor for progression of atherosclerosis in T1D than LDL cholesterol. Early intervention in these patients may markedly impact the main cause of death in T1D.
Supplementary Material
Article Information
Funding. The DCCT/EDIC has been supported by cooperative agreement grants (1982–1993 and 2012–2017) and contracts (1982–2012) with the Division of Diabetes Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (current grant numbers U01-DK-094176 and U01-DK-094157) and through support by the National Eye Institute, the National Institute of Neurologic Disorders and Stroke, the General Clinical Research Centers Program (1993–2007), and the Clinical Translational Science Center Program (2006 to present), Bethesda, MD. Industry contributors have had no role in the DCCT/EDIC study but have provided free or discounted supplies or equipment to support participants’ adherence to the study: Abbott Diabetes Care (Alameda, CA), Animas (West Chester, PA), Bayer Diabetes Care (North America Headquarters, Tarrytown, NY), Becton Dickinson (Franklin Lakes, NJ), Eli Lilly and Company (Indianapolis, IN), Extend Nutrition (St. Louis, MO), Insulet Corporation (Bedford, MA), LifeScan (Milpitas, CA), Medtronic Diabetes (Minneapolis, MN), Nipro Home Diagnostics (Fort Lauderdale, FL), Nova Diabetes Care (Billerica, MA), Omron (Shelton, CT), Perrigo Diabetes Care (Allegan, MI), Roche Diabetes Care (Indianapolis, IN), and Sanofi (Bridgewater, NJ).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.F.L.-V. wrote the initial draft of the manuscript. M.F.L.-V., I.B., K.J.H., G.V., N.L.B., B.B., X.G., and J.M.L. revised the manuscript and approved the final content. I.B. conducted statistical analyses. I.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data and Resource Availability. Access to the DCCT/EDIC data is available through the NIDDK repository (https://repository.niddk.nih.gov/studies/edic/).
Footnotes
Clinical trial reg. nos. NCT00360893 and NCT00360815, clinicaltrials.gov
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0358/-/DC1.
A complete list of participants in the DCCT/EDIC Research Group is presented in the Supplementary Material published online for the article in N Engl J Med 2017;376:1507–1516.
References
- 1.Ehara S, Ueda M, Naruko T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001;103:1955–1960 [DOI] [PubMed] [Google Scholar]
- 2.Hulthe J, Fagerberg B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arterioscler Thromb Vasc Biol 2002;22:1162–1167 [DOI] [PubMed] [Google Scholar]
- 3.Jenkins AJ, Rowley KG, Lyons TJ, Best JD, Hill MA, Klein RL. Lipoproteins and diabetic microvascular complications. Curr Pharm Des 2004;10:3395–3418 [DOI] [PubMed] [Google Scholar]
- 4.Koukkunen H, Penttilä K, Kemppainen A, et al. C-reactive protein, fibrinogen, interleukin-6 and tumour necrosis factor-alpha in the prognostic classification of unstable angina pectoris. Ann Med 2001;33:37–47 [DOI] [PubMed] [Google Scholar]
- 5.Griffith RL, Virella GT, Stevenson HC, Lopes-Virella MF. Low density lipoprotein metabolism by human macrophages activated with low density lipoprotein immune complexes. A possible mechanism of foam cell formation. J Exp Med 1988;168:1041–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saad AF, Virella G, Chassereau C, Boackle RJ, Lopes-Virella MF. OxLDL immune complexes activate complement and induce cytokine production by MonoMac 6 cells and human macrophages. J Lipid Res 2006;47:1975–1983 [DOI] [PubMed] [Google Scholar]
- 7.Virella G, Atchley D, Koskinen S, Zheng D, Lopes-Virella MF; DCCT/EDIC Research Group . Proatherogenic and proinflammatory properties of immune complexes prepared with purified human oxLDL antibodies and human oxLDL. Clin Immunol 2002;105:81–92 [DOI] [PubMed] [Google Scholar]
- 8.Virella G, Carter RE, Saad A, Crosswell EG, Game BA, Lopes-Virella MF; DCCT/EDIC Study Group . Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clin Immunol 2008;127:394–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Virella G, Colglazier J, Chassereau C, Hunt KJ, Baker NL, Lopes-Virella MF. Immunoassay of modified forms of human low density lipoprotein in isolated circulating immune complexes. J Immunoassay Immunochem 2013;34:61–74 [DOI] [PubMed] [Google Scholar]
- 10.Ylä-Herttuala S, Palinski W, Rosenfeld ME, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 1989;84:1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ylä-Herttuala S, Palinski W, Butler SW, Picard S, Steinberg D, Witztum JL. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb 1994;14:32–40 [DOI] [PubMed] [Google Scholar]
- 12.Lopes-Virella MF, Virella G, Orchard TJ, et al. Antibodies to oxidized LDL and LDL-containing immune complexes as risk factors for coronary artery disease in diabetes mellitus. Clin Immunol 1999;90:165–172 [DOI] [PubMed] [Google Scholar]
- 13.Orekhov AN, Kalenich OS, Tetov VV, Novikov ID, Vorobeva EG. Cholesterol level in circulating immune complexes as a marker of coronary atherosclerosis. In Hypercholesterolemia, Hypocholesterolemia, Hypertriglyceridemia, In Vivo Kinetics. Malmendier CL, Ed. New York, Plenum Press, 1990, p. 393–397 [Google Scholar]
- 14.Orekhov AN, Kalenich OS, Tertov VV, et al. Diagnostic value of immune cholesterol as a marker for atherosclerosis. J Cardiovasc Risk 1995;2:459–466 [DOI] [PubMed] [Google Scholar]
- 15.Orekhov AN, Bobryshev YV, Sobenin IA, Melnichenko AA, Chistiakov DA. Modified low density lipoprotein and lipoprotein-containing circulating immune complexes as diagnostic and prognostic biomarkers of atherosclerosis and type 1 diabetes macrovascular disease. Int J Mol Sci 2014;15:12807–12841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virella G, Derrick MB, Pate V, Chassereau C, Thorpe SR, Lopes-Virella MF. Development of capture assays for different modifications of human low-density lipoprotein. Clin Diagn Lab Immunol 2005;12:68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes-Virella MF, Baker NL, Hunt KJ, Lachin J, Nathan D, Virella G; DCCT/EDIC Research Group . Oxidized LDL immune complexes and coronary artery calcification in type 1 diabetes. Atherosclerosis 2011;214:462–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes-Virella MF, Hunt KJ, Baker NL, Lachin J, Nathan DM, Virella G; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Levels of oxidized LDL and advanced glycation end products-modified LDL in circulating immune complexes are strongly associated with increased levels of carotid intima-media thickness and its progression in type 1 diabetes. Diabetes 2011;60:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt KJ, Baker N, Cleary P, et al.; DCCT/EDIC Research Group . Oxidized LDL and AGE-LDL in circulating immune complexes strongly predict progression of carotid artery IMT in type 1 diabetes. Atherosclerosis 2013;231:315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes-Virella MF, Hunt KJ, Baker NL, Virella G, Moritz T; VADT Investigators . The levels of MDA-LDL in circulating immune complexes predict myocardial infarction in the VADT study. Atherosclerosis 2012;224:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT). Design and methodologic considerations for the feasibility phase. Diabetes 1986;35:530–545 [PubMed] [Google Scholar]
- 22.Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The DCCT Research Group Lipid and lipoprotein levels in patients with IDDM diabetes control and complication. Trial experience. Diabetes Care 1992;15:886–894 [DOI] [PubMed] [Google Scholar]
- 24.Virella G, Thorpe SR, Alderson NL, et al. Definition of the immunogenic forms of modified human LDL recognized by human autoantibodies and by rabbit hyperimmune antibodies. J Lipid Res 2004;45:1859–1867 [DOI] [PubMed] [Google Scholar]
- 25.Lachin JM, Orchard TJ, Nathan DM; DCCT/EDIC Research Group . Update on cardiovascular outcomes at 30 years of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care 2014;37:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therneau T, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, Springer-Verlag, 2000 [Google Scholar]
- 27.Virella G, Wilson K, Elkes J, et al. Immune complexes containing malondialdehyde (MDA) LDL induce apoptosis in human macrophages. Clin Immunol 2018;187:1–9 [DOI] [PubMed] [Google Scholar]
- 28.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation 1998;98:1487–1494 [DOI] [PubMed] [Google Scholar]
- 29.Prasad A, Clopton P, Ayers C, et al. Relationship of autoantibodies to MDA-LDL and ApoB-immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler Thromb Vasc Biol 2017;37:1213–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Hu B, Meng Y, Zhang C, Li K, Hui C. The level of malondialdehyde-modified LDL and LDL immune complexes in patients with rheumatoid arthritis. Clin Biochem 2009;42:1352–1357 [DOI] [PubMed] [Google Scholar]
- 31.Tanaga K, Bujo H, Inoue M, et al. Increased circulating malondialdehyde-modified LDL levels in patients with coronary artery diseases and their association with peak sizes of LDL particles. Arterioscler Thromb Vasc Biol 2002;22:662–666 [DOI] [PubMed] [Google Scholar]
- 32.Holvoet P, Perez G, Zhao Z, Brouwers E, Bernar H, Collen D. Malondialdehyde-modified low density lipoproteins in patients with atherosclerotic disease. J Clin Invest 1995;95:2611–2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amaki T, Suzuki T, Nakamura F, et al. Circulating malondialdehyde modified LDL is a biochemical risk marker for coronary artery disease. Heart 2004;90:1211–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes-Virella MF, Mironova M, Stephan E, Durazo-Arvizu R, Virella G. Role of simvastatin as an immunomodulator in type 2 diabetes. Diabetes Care 2004;27:908–913 [DOI] [PubMed] [Google Scholar]
- 35.Hörl G, Froehlich H, Fersti U, et al. Simvastatin efficiently lowers small LDL-IgG immune complex levels: a therapeutic quality beyond the lipid-lowering effect. PLoS One 2016;11:e0148210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.