Abstract
Mungbean yellow mosaic India virus (MYMIV) belonging to the family Geminiviridae and the genus Begomovirus is a severe pathogen of tropical legumes including soybean. The absence of genetically mapped loci conferring resistance together with the genetic diversity of begomoviruses infecting soybean warrants the utilization of RNA interference (RNAi) technology to develop virus resistance. However, viral suppressors of RNAi (VSRs) reduce the effectiveness of RNA silencing. Here, we report the effectiveness of Agrobacterium-mediated transient expression of shRNA, targeting a conserved region of AC2 ORF (a VSR) of MYMIV, in conferring virus resistance in soybean. Transient expression of shRNA showed progressive reduction of the viral titre estimated by the MYMIV-derived AC2 gene copy numbers from the initial inoculum by approximately 80-fold 20 days post-application. In addition, the newly emerging leaves exhibited symptom recovery. Thus, this study proves that AC2 of MYMIV is a potent target gene for obtaining RNAi-mediated virus resistance in soybean. Agro-infiltration-based delivery of shRNA was an efficient means of gene silencing and could pave way for the development of transgenic virus-resistant soybean genotype.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1865-7) contains supplementary material, which is available to authorized users.
Keywords: Begomovirus, MYMIV, RNAi, shRNAs, Soybean, Virus resistance
Introduction
RNA interference (RNAi) is a sequence-specific gene regulatory mechanism that has been exploited to engineer antiviral resistance in plants (Ding and Voinnet 2007). RNA-based gene silencing functions on the principle of nucleotide sequence complementarity. Hence, meticulous identification and expression of a target viral region, such as self-complementary RNA, could potentially downregulate cognate viral gene and provide immunity to the plants. Geminiviridae is a family of phytopathogenic viruses that has characteristic geminate virion particles encompassing single-stranded DNA molecules as genomic components. Numerous strategies have been adopted to develop geminivirus resistance in plants utilizing virus-derived nucleic acids. Initially, viral-derived complete, truncated or mutated coat protein (Kunik et al. 1994), movement protein, nuclear shuttle protein (Hou et al. 2000), and replication-associated protein (Antignus et al. 2004; Shivaprasad et al. 2006) were ectopically expressed in plants to develop geminivirus resistance. Later, antisense RNA-based virus resistance strategy was exploited to confer geminivirus resistance (Bendahmane and Gronenborn 1997; Haq et al. 2010). RNAi has been exploited by the plant virologists to express small interfering RNAs (siRNAs) (Vanitharani et al. 2003), intron-spliced hairpin RNAs (Ramesh et al. 2007; Praveen et al. 2010) and artificial microRNAs (miRNAs) (Vu et al. 2013) to target various geminiviruses.
Legume yellow mosaic viruses (LYMVs) of family Geminiviridae cause severe yellow mosaic disease (YMD) of legumes (Qazi et al. 2007; Briddon et al. 2010). LYMVs comprise species of begomoviruses, viz., Mungbean yellow mosaic virus (MYMV), Mungbean yellow mosaic India virus (MYMIV), Dolichos yellow mosaic virus (DoYMV) and Horsegram yellow mosaic virus (HgYMV), Kudzu mosaic virus (KuMV), Rhynchosia yellow mosaic virus (RhYMV), and Rhynchosia yellow mosaic India virus (RhYMIV). LYMVs cause severe YMD and greatly hinder the production of soybean (Glycine max L. Merr.) (Qazi et al. 2007; Briddon et al. 2010; Fauquet et al. 2008). Among the LYMVs, MYMIV and MYMV are bipartite begomoviruses that cause yield losses of $ 300 million/annum in grain legumes including soybean (Qazi et al. 2007; Girish and Usha 2005; Varma and Malathi 2003). YMD infection during the seedling stage could cause yield loss up to 85–100% (Nene 1973). MYMIV has been characterized as the causal agent of YMD of soybean cultivated in central India (Girish and Usha 2005; Ramesh et al. 2013, 2017a). Seven different viruses were found to be naturally infecting soybean, and MYMIV was a predominant virus among them infecting 71 of 77 soybean accessions (Sharma et al. 2016).
MYMIV infecting soybean has two ssDNA genomic components (DNA A and DNA B) each ~ 2.7 kb long. Genome of MYMIV encodes proteins in virion-sense and complementary-sense strands. Among the seven overlapping open reading frames (ORFs) in DNA A, coat protein (AV1) and pre-coat protein (AV2) genes are in the virion-sense, whereas replication-associated protein (AC1), transcriptional activator protein (AC2), replication enhancer protein (AC3), AC4 and AC5 are in complementary-sense strand. DNA A of MYMIV encodes for proteins involved in virus encapsidation, insect transmission, replication and gene expression. DNA B of MYMIV encodes for nuclear shuttle protein (BC1) involved in intracellular transport of viral ssDNA in complementary-sense strand and movement protein (MP) for intercellular movement of viral nucleoproteins (BV1) in virion-sense strand.
Until recently, MYMIV infection was confined to the plains of north India; however, the disease had spread to central India causing a severe reduction in soybean productivity (Anonymous: Soybean News 2015). Development of MYMIV-resistant soybean cultivar is indispensable to protect the soybean cultivation in central India. Soybean varieties developed in the plains of north India, through breeding approaches, are resistant to MYMIV, nevertheless are not adapted to central Indian conditions. Molecular breeding and genetics tools could not dissect the genetics of YMD resistance; nonetheless, a molecular marker tightly linked to disease resistance has been characterized in soybean (Rani et al. 2017). Furthermore, Begomovirus species causing the disease are diverse as MYMV, DoYMV, HgYMV and MYMIV are known to cause disease in the field (Qazi et al. 2007; Girish and Usha 2005). In this context, RNA silencing-mediated virus resistance would be an efficient alternative for engineering MYMIV resistance in soybean. However, the proteins encoded by plant viruses called viral suppressors of RNAi (VSRs) effectively counteract the RNA silencing. MYMV-encoded AC2 protein [Transcriptional activator protein (TrAP)] suppresses RNA silencing (Trinks et al. 2005) by interacting with host adenosine kinase (ADK) and reduces viral DNA methylation (Wang et al. 2005). Molecular basis of the suppressor activity of MYMIV-derived AC2 showed that the AC2 protein inhibits both the RDR6 and AGO—the key players of host RNA silencing (Kumar et al. 2015). RNA silencing of MYMV-encoded TrAP or AC2 reduced viral titre in transgenic tobacco plants (Shanmugapriya et al. 2015). Almost 100% reduction in MYMIV titre and disease symptoms was documented by targeting AC2 and AC4 genes through RNAi-mediated transgenic resistance in cowpea (Kumar et al. 2017). Kumari et al. (2018) have demonstrated that a conserved region of MYMIV-derived coat protein (CP) gene is a suitable target to devise a broad-spectrum resistance against begomoviruses infecting soybean. In this study, we developed an intron-spliced short hairpin RNA gene construct to target conserved region of MYMIV AC2 gene and examined its silencing potential in soybean. This strategy involved the expression of potent sense and antisense viral DNA region with an intervening intron so that upon transcription, dsRNA formed is diced in vivo into small interfering RNAs (siRNAs) by the concerted activity of host RNA-silencing machinery. Thus, the host RNAi mechanism mounts antiviral resistance against the invading MYMIV.
Materials and methods
Biological materials
Soybean cultivar JS335 (susceptible to MYMIV) was used for the transient expression of short hairpin RNA and agro-inoculation assays. Seeds of soybean cultivar (JS335) were germinated in a plastic pot containing vermiculite under controlled conditions of appropriate photoperiod (16 h light/8 h dark) and constant temperature (28 °C). Genomic components of MYMIV (GenBank Accessions: DNA A KC852204 and DNA B KP828155) infecting soybean were used. MYMIV-sb infectious clones developed in-house were used to inoculate the virus and incite YMD in soybean (Ramesh et al. 2017b, 2019).
Target amplification, RNAi plasmid construction and Agrobacterium transformation
Plasmid DNA containing a full-length DNA A genomic component of MYMIV-sb was used as a template for the amplification of the viral target region. A 160-bp-long conserved region of AC2 gene [KC852204: nucleotide position 1584–1743 in complementary-sense strand] was amplified using specific primers (AC2-Ihp-F 5′CATGCCATGGTCTAGAGTGGTATCCCCACCATCTTT 3′/and AC2-Ihp-R 5′ CGGGCGCGCCGGATCCGA ACCTTGATTGACGGAGGA 3′) with appropriate restriction enzyme recognition sites in the forward (NcoI/XbaI) and reverse primers (BamHI/AscI). The amplicon was cloned in pGEM®-T EASY TA cloning vector to aid the directional cloning in the binary vector pFGC5941 (Sambrook and Russell 2001) producing AC2-Ihp. Briefly, the amplicon was cloned in the sense orientation (using enzymes NcoI and AscI) followed by cloning in the antisense orientation (using BamHI and XbaI) with an intervening chalcone synthase intron. The recombinant vector pFGC5941 carrying AC2-Ihp gene-silencing construct (pFGC5941::AC2-IHP) was mobilized into Agrobacterium tumefaciens strain LBA 4404 by the freeze–thaw method (Chen et al. 1994).
MYMIV inoculation of soybean
Soybean seedlings, 3–4 weeks old, were inoculated with A. tumefaciens strain LBA 4404 harbouring binary plasmids NRCS-A2X-7 and NRCS-B2X-1 following the stem inoculation method. Wounds were generated around the stem node of soybean plants using a 30-gauge needle to inoculate MYMIV and inoculated plants were maintained in an insect-free growth chamber (Ramesh et al. 2017b, 2019). Soybean seedlings agro-inoculated with empty vector pFGC5941-ΔCHSA were the uninoculated control (Ramesh et al. 2019).
Agro-infiltration of the RNAi plasmid in soybean seedlings
A. tumefaciens strain LBA 4404 harbouring pFGC5941::AC2-ihp plasmid (comprising the AC2 gene silencing construct) was cultured in LB broth with Kanamycin selection at 28 °C and 200 rpm for a period of 30 h. Bacterial cells were harvested when the liquid culture reached OD600 value of 1.0. Harvested cells were resuspended in agro-infiltration medium to obtain a final OD600 of 0.2 (Patil and Fauquet 2010). After incubation at room temperature for 3 h, agro-infiltration was performed in leaves 2 days post-MYMIV inoculation using a syringe without a needle. Soybean seedlings were inoculated with MYMIV infectious clones (NRCS-A2X-7 and NRCS-B2X-1); agro-infiltrated soybeans with A. tumefaciens LBA4404 harbouring empty pFGC5941 served as inoculated control for monitoring MYMIV disease development.
Detection of AC2 gene-derived siRNAs
Total RNA was extracted from the co-infiltrated area and MYMIV-inoculated control of the soybean leaves at various time intervals [5 days post-agro-infiltration (dpa) of RNAi plasmid, 10 dpa, 15 dpa, 20 dpa] as well as from the new emerging leaf using the mirVana miRNA isolation kit (Ambion, Austin, TX, USA). For Northern blot analysis, 20 µg of total RNA was separated by electrophoresis on 15% denatured polyacrylamide gels containing 7 M urea and blotted to a Nytran® SuPer Charge membrane (Roche Applied Science, Germany). Hybridization, washing and detection were performed with a non-radioactive DIG-labelled AC2-antisense probe spanning 160 nucleotides according to the manufacturer’s instructions. The labelled single-stranded RNA probes were produced by in vitro transcription from the linearized AC2-pGEMT plasmid DNA templates in the presence of digoxigenin-UTP and T7 RNA polymerase. In vitro-obtained transcripts were hydrolyzed before using as probes for siRNA detection. The siRNA band intensities were quantified using the ImageJ software (https://imagej.nih.gov/ij/index.html).
Quantification of MYMIV titre and viral gene silencing
Total DNA extracted from the soybean leaves and primers (qPCR-v-Forward—ATGTGGGATCCATTGTTGAACG/qPCR-v-Reverse—TCAATCTCCTCCGTGCATTCG) that detect conserved coat protein (AV1) region were used in qPCR to estimate the viral load. Quantification of viral titre was carried out in Light Cycler R 480 II system (Roche) and the calculations were performed as described previously (Ramesh et al. 2019). Similarly, downregulation of MYMIV-derived AC2 gene was studied using gene-specific primers (q-AC2-RT-F—GATTTTCGC ACAGGGGAGTA and q-AC2-RT-R—GCTTCAAGTCCAGGAAGCAC). One-way analysis of variance (ANOVA) was performed to determine if the differences in the viral titres between control plants and treated plants are statistically significant.
Results
Potent target region and RNAi construct
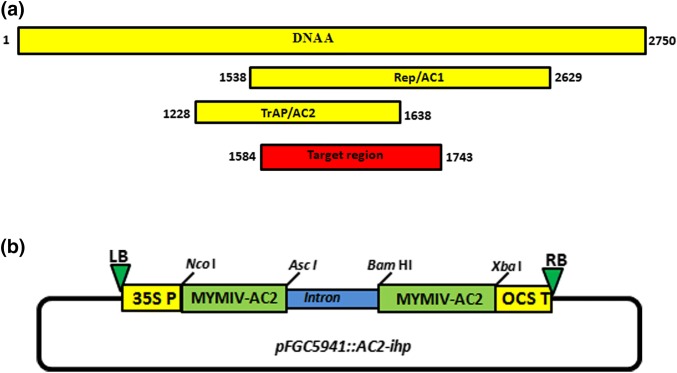
A 160-bp-long region of MYMIV AC2 gene (160 bp) [KC852204 (nucleotide position 1584–1743 in its complementary-sense strand)] was identified as a potent target region to induce RNA silencing against MYMIV (Fig. 1a). Particularly, this region also spans replication-associated protein gene (ORF AC1 encoding the rep gene) (nucleotide position 887–1046) of MYMIV-sb. Thus, short hairpin RNA designed to target the AC2 gene of MYMIV-sb (AC2-ihp) could potentially target AC1 mRNA. The target region was sequentially cloned into a generic RNAi vector to develop pFGC5941::AC2-ihp construct (Fig. 1b). The orientation of the hairpin arms in the recombinant vector was verified through PCR using AC2 and CHSA intron-specific oligonucleotides (Table 1). It was followed by restriction enzyme digestion of putative recombinant RNAi vector with XbaI. Consequently, upon digestion, the positive clone having the whole RNAi cassette (pFGC5941::AC2-ihp) released the expected size of DNA fragment (~ 1.7 kb).
Fig. 1.
a Target MYMIV-AC2 region selected for the development of shRNA-based gene-silencing vector. b Schematic map of the RNA-silencing construct pFGC5941::AC2-ihp developed to downregulate the expression of MYMIV-sb-encoded AC2 gene (restriction enzymes NcoI and AscI were used to clone the selected AC2 DNA piece in sense direction, whereas BamHI and XbaI were used to clone antisense direction, respectively; the two AC2 DNA pieces are separated by CHSA intron)
Table 1.
List of primers used in this investigation (sequence details, target region for amplification and the purpose oligonucleotides used are mentioned)
| S. no. | Primers | Primer sequences (5′–3′) | Viral genomic region | Purpose |
|---|---|---|---|---|
| 1 | AC2-Ihp-F | CATGCCATGGTCTAGAGT GGTATCCCCACCATCTTT | AC2 ORF (transcriptional activator protein—TrAP) | Generation of inverted repeats (RNAi vector) |
| 2 | AC2-Ihp-R | CGGGCGCGCCGGATCCGA ACCTTGATTGACGGAGGA | ||
| 5 | qPCR-v-F | ATGTGGGATCCATTGTTG AACG | AV1 ORF (coat protein) | MYMIV titre quantification |
| 6 | qPCR-v-R | TCAATCTCCTCCGTGCAT TCG | ||
| 7 | q-AC2-RT-F | GATTTTCGCACAGGGGAG TA | AC2 ORF (transcriptional activator protein—TrAP) | Quantification of MYMIV AC2 gene silencing |
| 8 | q-AC2-RT-R | GCTTCAAGTCCAGGAAGCAC | ||
| 9 | F-CHSA | ACTAACTTTGTGGAACTAAA | Complementary to chalcone synthase intron in pFGC5941 | PCR verification of inverted repeats (RNAi) vector construction |
MYMIV inoculation of soybean and viral titre quantification
We have already shown that the characteristic yellow mosaic symptoms, associated with the YMD, start appearing in soybean leaves 9 days post-agro-inoculation (Ramesh et al. 2017b; Ramesh et al.2019). MYMIV titre was monitored since the first dpi by detecting the copy numbers of viral gene AV1. In control plants, MYMIV titre showed an increase from 3 dpi (45 × 105 DNA molecules) reaching a peak at 15 dpi (315 × 105 molecules) and the virus accumulation was maintained thereafter (Supplementary file 1a).
Transient expression of AC2 shRNA in soybean leaves
Following agro-infiltration of A. tumefaciens strain LBA 4404 harbouring pFGC5941::AC2-ihp gene construct in soybean leaves, the expression of AC2-specific hairpin RNA was confirmed through reverse transcription quantitative PCR (RT-qPCR). Quantification revealed that 3 days post-agro-inoculation, the expression levels of AC2 hairpin RNA increased 13-fold when compared to the house-keeping gene (GmEF1A-eukaryotic elongation factor 1-alpha) in soybean. Control soybean plants that were agro-inoculated with empty pFGC5941 A. tumefaciens cells did not show AC2 hpRNA expression. The relative expression level of hairpin RNA targeting AC2 remained constant since the 3rd day of agro-inoculation.
shRNA expression alleviates MYMIV-specific symptoms
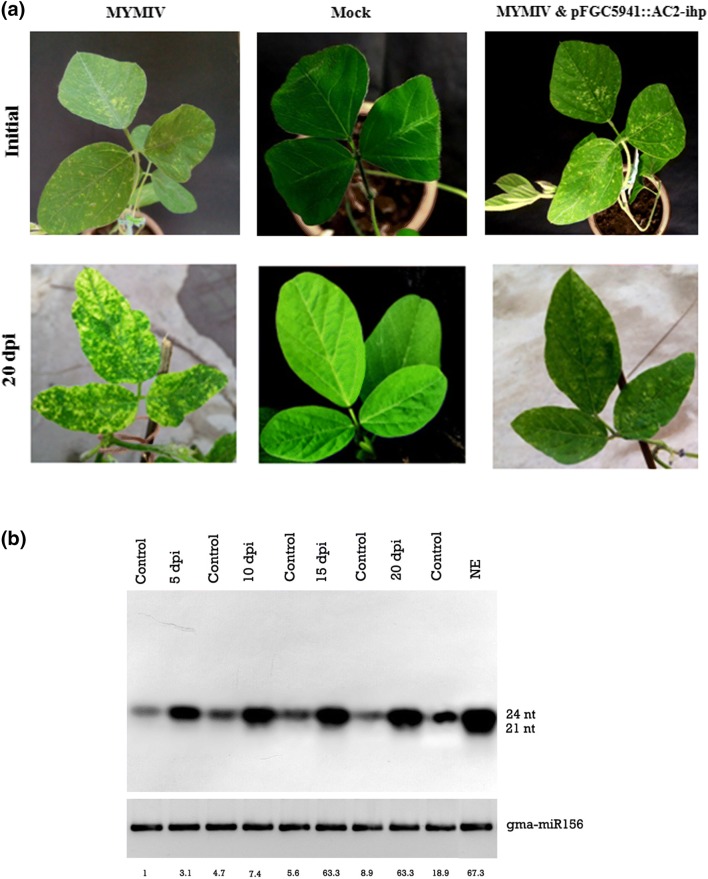
Transient gene silencing assay was performed in soybean seedlings to ascertain the efficiency of RNAi gene constructs in suppressing viral copy number. Soybean seedlings agro-inoculated with MYMIV were agro-infiltrated with A. tumefaciens strain LBA 4404 (pFGC5941::AC2-ihp) 2 days post-MYMIV inoculation. Plants that were agro-infiltrated with A. tumefaciens (pFGC5941::AC2-ihp) showed alleviation of YMD symptoms (Fig. 2a). It was also found that the newly emerging leaves were relatively free of viral symptoms when the control (MYMIV agro-inoculated only) plants exhibited the characteristic YMD symptoms.
Fig. 2.
a Phenotyping of soybean plants inoculated with MYMIV-sb, mock-inoculated plants (uninoculated control) and plants super-infiltrated with Agrobacterium tumefaciens strain LBA 4404 harbouring pFGC5941::AC2-ihp plasmid. Soybean plants inoculated with MYMIV and super-infiltrated with pFGC5941:AC2-ihp showed reduced viral disease symptoms. b Detection of siRNA expression in leaves of soybean plants inoculated with MYMIV and super-infiltrated with pFGC5941::AC2-ihp at different time points (5, 10, 15, 20 days post-inoculation; control (mock inoculated), NE—new emerging leaves). The relative band intensities of siRNAs were estimated using ImageJ software (https://imagej.nih.gov/ij/index.html) and normalized with reference to the loading control (gma-miR156)
Accumulation of AC2-derived siRNAs
Accumulation of AC2-derived siRNAs following double infiltration of MYMIV-sb and ihp-RNA targeting AC2 mRNA was detected using Northern blotting. siRNA detection analysis at various time points, namely 5, 10, 15 and 20 dpi (days post-inoculation) was conducted to assess the production of siRNAs and silencing efficiency of the RNAi-inducing construct. The production of siRNAs corresponding to AC2 viral gene transcript was found to gradually increase with time (Fig. 2b). Among the siRNA species, 24-nt size small RNAs were predominant compared to the 21-nt size class. Newly emerging soybean leaves following MYMIV inoculation and hairpin RNA expression were found to be free of disease symptoms. Typical YMD symptoms were observed in control plants where hairpin RNA targeting AC2 was not applied (Fig. 2a).
Decline of MYMIV titre in AC2 shRNA-treated soybean
Quantitative PCR assay was employed to estimate the absolute copy number of the MYMIV genome in the co-infiltrated area of the soybean leaf. qPCR-based analysis revealed that the virus titre was reduced in MYMIV-inoculated leaves upon agro-infiltration with pFGC5941::AC2-ihp (Fig. 3). The reduction in viral titre was observed even at 5 dpi where MYMIV copy number dropped from 378.52 × 105 to 238.51 × 105 molecules. Progressively viral titre decreased to 185.57 × 105, 49.22 × 105 and 4.78 × 105 copies during 10, 15, 20 dpi, respectively. In the newly emerging soybean leaves, where visible symptoms were not observed, the viral copy number was estimated to be 3.58 × 105 (Fig. 3). Reduction in viral titres showed statistically significant differences between both the control and treated soybean plants (f ratio value = 34.83549 and p value 0.004123). It appears that transient expression of hairpin RNA targeting AC2 gene has effectively downregulated AC2 transcripts, and decreased the viral titre. Hence, a drastic reduction in symptoms associated with the disease was observed in the newly emerging leaves.
Fig. 3.
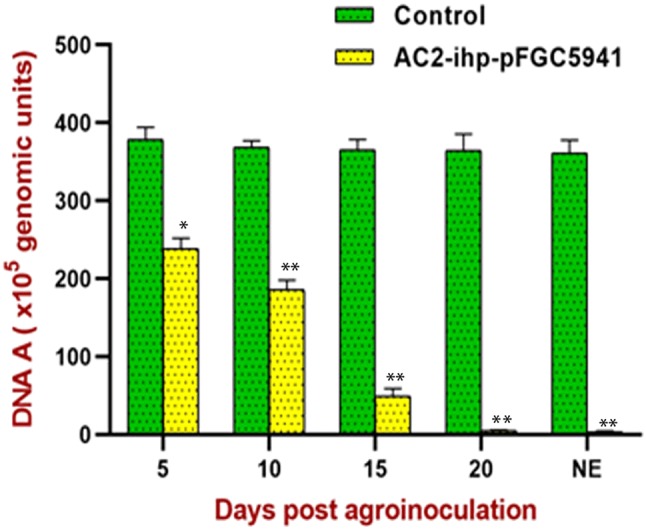
Virus titre estimation in the leaves of MYMIV-inoculated control soybean plants and leaves MYMIV-inoculated followed by agro-infiltration with pFGC5941:: AC2-ihp. Statistical significance of viral titres at various dpi compared to the plants agro-infiltrated with pFGC5941::AC2-ihp was determined by one-way analysis of variance (ANOVA) and is denoted by asterisks (*P < 0.05; **P < 0.001)
Discussion
MYMIV is a serious threat to the cultivation of soybean and hence development of MYMIV-resistant soybean cultivars is a main research focus. Recently, genetic mapping identified soybean genomic loci linked to MYMIV resistance (Rani et al. 2017). However, screening of genotypes in segregating populations aiming at developing resistant soybean variety suitable for Central India is a time-consuming process. In addition, begomoviruses (family Geminiviridae) that cause YMD of soybean and other grain legumes are diverse. The present study sought to achieve efficient control of MYMIV in soybean through RNA-silencing strategy using a short hairpin RNA construct derived from AC2 (and AC1) genes of the virus.
RNAi has opened up avenues to provide virus resistance in crop plants by manipulating viral-derived nucleic acids (Vanderschuren et al. 2007). In general, the attempts to achieve geminivirus resistance in plants have used replication-associated protein (AC1 ORF) and expressed siRNAs targeting the rep gene (Ramesh et al. 2007; Praveen et al. 2010; Vanderschuren et al. 2007; Bonfim et al. 2007; Fuentes et al. 2006). However, VSRs counteract both the host defence and transgene-induced RNAi. MYMV-encoded transcriptional activator protein (TrAP) (AC2) interacts with host adenosine kinase (ADK) and impairs host methylation-mediated defence response in transcriptional gene silencing (TGS) process. Hence, AC2 (TrAP) was chosen as the target region for RNA silencing.
We have shown that hairpin RNA-mediated downregulation of AC2 gene has effectively reduced MYMIV titre (Fig. 3). It was in concurrence with the findings of Shanmugapriya et al. (2015) who demonstrated that RNA silencing of TrAP has effectively reduced the MYMIV DNA accumulation in N. tabacum plants. The TrAP is involved in transactivation of the viral (Rajeswaran et al. 2007) and host genes (Trinks et al. 2005), influencing the host methyl cycle by interacting with SNF1 kinase genes, and functions as a VSR (Trinks et al. 2005). Hence, downregulation of MYMIV-encoded TrAP through the expression of shRNA might have deprived the infecting virus of its vital protein that is indispensable for multiple functions. Also, AC2 gene sequence overlaps with an AC1 that encodes for replication-associated protein. Hence, shRNA-mediated silencing of AC2 could have concomitantly affected the expression levels of replication-associated protein, thereby causing the reduced virus titre levels. The relative abundance of 24-nt size class viral-derived siRNAs (vsiRNAs) warrants investigating the effect on transcriptional gene silencing. Presumably, 24-nt vsiRNA-mediated viral genome methylation could also be a major defence mechanism acting against the MYMIV infection.
Interestingly, the time course study post-agro-infiltration showed a constant state of AC2 mRNA expression since 3 dpa. Ago-infiltration of MYMIV-sb, inciting viral infection in soybean leaves, increased the accumulation of AC2-derived siRNAs overtime that correlated with YMD symptom exhibition (Fig. 2b). It could be ascribed to the activation of host or virus genes and other defence-related proteins following MYMIV infection (Trinks et al. 2005). The newly emerging soybean leaves showed little disease-associated symptoms concomitant with the increased levels of AC2-derived siRNAs in the inoculated leaves, which could be partly attributed to the mobile nature of siRNAs. Also, siRNA production could have been induced due to MYMIV agro-inoculation and activation of the endogenous RNAi pathway.
Agro-infiltration of soybean plants has been effectively utilized for the transient expression of hairpin RNAs targeting coatomer subunit alpha (COPA) and aquaporin 9 (AQ9) genes of the spider mite (Tetranychus urticae) (Dubey et al. 2017). Similarly, transient expression of sense and antisense siRNAs targeting various ORFs of DNA A and DNA B of African cassava mosaic virus-Cameroon has demonstrated the utility of agro-infiltration to screen suitable target genes for incorporating virus resistance (Patil et al. 2016). Besides evaluating the efficacy of the siRNA-mediated viral gene silencing, agro-infiltration assay was effectively applied to identify the suitable artificial miRNAs (amiRNAs) for developing resistance against Tomato spotted wilt virus (TSWV) (Mitter et al. 2016). Considering the labour-, cost- and time-intensive process of development of transgenics in recalcitrant species such as soybean, the agro-infiltration-based evaluation of gene silencing efficiency would help in identifying suitable viral target gene sequences for further genetic manipulations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Authors gratefully acknowledge Indian Council of Agricultural Research (ICAR) (DSR 1.24/12) for funding this investigation.
Author contributions
SVR conceived and designed the study; SVR and BSC performed the experiments; MS and SC contributed through intellectual inputs and reagents; SP designed viral quantification experiments; SVR, MS, and SP wrote the manuscript; all authors have read and approved the manuscript.
Funding
This work was supported by the Indian Council of Agricultural Research (ICAR) (Grant number DSR 1.24/12).
Compliance with ethical standards
Conflict of interest
This article does not contain any studies with human or animal subjects performed by any of the authors. Authors declare that they have no competing interests.
References
- Anonymous. Soybean News 2015. ICAR-Indian Institute of Soybean Research, Indore. https://iisrindore.icar.gov.in/
- Antignus Y, Vunish R, Lachman O, Pearlsman M, Maslenin L, Hananya U, Rosner A. Truncated rep gene originated from Tomato yellow leaf curl virus-Israel [mild] confers strain-specific resistance in transgenic tomato. Ann App Biol. 2004;44:39–44. doi: 10.1111/j.1744-7348.2004.tb00314.x. [DOI] [Google Scholar]
- Bendahmane M, Gronenborn B. Engineering resistance against tomato yellow leaf curl virus (TYLCV) using antisense RNA. Plant Mol Biol. 1997;33:351–357. doi: 10.1023/A:1005715805000. [DOI] [PubMed] [Google Scholar]
- Bonfim K, Faria JC, Nogueira OPL, Mendes EA, Aragao FJL. RNAi-mediated resistance to Bean golden mosaic virus in genetically engineered common bean (Phaseolus vulgaris) Mol Plant-Microbe Interact. 2007;20:717–726. doi: 10.1094/MPMI-20-6-0717. [DOI] [PubMed] [Google Scholar]
- Briddon RW, Patil BL, Bagewadi B, Nawaz-ul-Rehman MS, Fauquet CM. Distinct evolutionary histories of the DNA-A and DNA-B components of bipartite begomoviruses. BMC Evol Biol. 2010;10:97. doi: 10.1186/1471-2148-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Nelson RS, Sherwood JL. Enhanced recovery of transformants of Agrobacterium tumefaciens after freeze-thaw transformation and drug selection. Biotechniques. 1994;16:664–670. [PubMed] [Google Scholar]
- Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey VK, Lee UG, Kwon D, Lee SH. Agroinfiltration-based expression of hairpin RNA in soybean plants for RNA interference against Tetranychus urticae. Pest Biochem Physiol. 2017;10:1016. doi: 10.1016/j.pestbp.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Fauquet C, Briddon R, Brown J, Moriones E, Stanley J, Zerbini M, Zhou X. Geminivirus strain demarcation and nomenclature. Arch Virol. 2008;153:783–821. doi: 10.1007/s00705-008-0037-6. [DOI] [PubMed] [Google Scholar]
- Fuentes A, Ramos PL, Fiallo E, Callard D, Sanchez Y, Peral R, Rodriguez R, Pujol M. Intron–hairpin RNA derived from replication associated protein C1 gene confers immunity to Tomato Yellow Leaf Curl Virus infection in transgenic tomato plants. Transgenic Res. 2006;15:291–304. doi: 10.1007/s11248-005-5238-0. [DOI] [PubMed] [Google Scholar]
- Girish KR, Usha R. Molecular characterization of two soybean-infecting Begomoviruses from India and evidence for recombination among legume-infecting Begomoviruses from South-East Asia. Virus Res. 2005;108:167–176. doi: 10.1016/j.virusres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Haq QMI, Ali A, Malathi VG. Engineering resistance against Mungbean yellow mosaic India virus using antisense RNA. Indian J Virol. 2010;21:82–85. doi: 10.1007/s13337-010-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YM, Saunders R, Ursin VM, Gilbertson RL. Transgenic plants expressing geminivirus movement proteins: abnormal phenotypes and delayed infection by Tomato mottle virus in transgenic tomatoes expressing the Bean dwarf mosaic virus BV1 or BC1 proteins. Mol Plant Microbe Interact. 2000;13:297–308. doi: 10.1094/MPMI.2000.13.3.297. [DOI] [PubMed] [Google Scholar]
- Kumar V, Mishra SK, Rahman J, Taneja J, Sundaresan G, Mishra NS, Mukherjee SK. Mungbean yellow mosaic Indian virus encoded AC2 protein suppresses RNA silencing by inhibiting Arabidopsis RDR6 and AGO1 activities. Virology. 2015;486:158–172. doi: 10.1016/j.virol.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Kumar S, Tanti B, Patil BL, Mukherjee SK, Sahoo L. RNAi-derived transgenic resistance to Mungbean yellow mosaic India virus in cowpea. PLoS One. 2017;12(10):e0186786. doi: 10.1371/journal.pone.0186786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Hada A, Subramanyam K, Theboral J, Misra S, Ganapathi A, Malathi VG. RNAi-mediated resistance to yellow mosaic viruses in soybean targeting coat protein gene. Acta Physiol Plant. 2018;40(2):32. doi: 10.1007/s11738-018-2608-9. [DOI] [Google Scholar]
- Kunik T, Salomon R, Zamir D, Navot N, Zeidan M, Michelson I, Gafni Y, Czosnek H. Transgenic tomato plants expressing the tomato yellow leaf curl virus capsid protein are resistant to the virus. Nat Biotech. 1994;12:500–504. doi: 10.1038/nbt0594-500. [DOI] [PubMed] [Google Scholar]
- Mitter N, Zhai Y, Bai AX, Chua K, Eid S, Constantin M, Mitchell R, Pappu HR. Evaluation and identification of candidate genes for artificial microRNA-mediated resistance to tomato spotted wilt virus. Virus Res. 2016;211:151–158. doi: 10.1016/j.virusres.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Nene YL. Viral disease of some warm weather pulse crops in India. Plant Dis Rep. 1973;57:463–467. [Google Scholar]
- Patil BL, Fauquet C. Differential interaction between cassava mosaic geminiviruses and geminivirus satellites. J Gen Virol. 2010;91:1871–1882. doi: 10.1099/vir.0.019513-0. [DOI] [PubMed] [Google Scholar]
- Patil BL, Bagewadi B, Yadav JS, Fauquet CM. Mapping and identification of cassava mosaic geminivirus genome sequences for efficient siRNA expression and RNAi based virus resistance by transient agro-infiltration studies. Virus Res. 2016;213:109–115. doi: 10.1016/j.virusres.2015.11.011. [DOI] [PubMed] [Google Scholar]
- Praveen S, Ramesh SV, Mishra AK, Koundal V, Palukaitis P. Silencing potential of viral derived RNAi constructs in tomato leaf curl virus-AC4 gene suppression in tomato. Transgenic Res. 2010;19:45–55. doi: 10.1007/s11248-009-9291-y. [DOI] [PubMed] [Google Scholar]
- Qazi J, Ilyas M, Mansoor S, Briddon W. Legume yellow mosaic viruses: genetically isolated begomoviruses. Mol Plant Pathol. 2007;8:343–348. doi: 10.1111/j.1364-3703.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- Rajeswaran R, Sunitha S, Shivaprasad PV, Pooggin MM, Hohn T, Veluthambi K. The mungbean yellow mosaic begomovirus transcriptional activator protein transactivates the viral promoter driven transgene and causes toxicity in transgenic tobacco plants. Mol Plant Microbe Interact. 2007;20:1545–1554. doi: 10.1094/MPMI-20-12-1545. [DOI] [PubMed] [Google Scholar]
- Ramesh SV, Mishra AK, Praveen S. Hairpin RNA-mediated strategies for silencing of tomato leaf curl virus AC1 and AC4 genes for effective resistance in plants. Oligonucleotides. 2007;17:251–257. doi: 10.1089/oli.2006.0063. [DOI] [PubMed] [Google Scholar]
- Ramesh SV, Bhaskale R, Admane N, Gupta GK, Husain SM (2013) Multiply Primed Rolling Circle Amplification (MPRCA) of Yellow mosaic virus genome from infected soybean in central Indian region divulges it as Mungbean yellow mosaic Indian virus-[sb] and its implications for RNAi mediated virus resistance. Paper Presented at the IXth world soybean research conference
- Ramesh SV, Chouhan BS, Gaurav K, Praveen S, Chand S. Expression dynamics of Glycine max (L.) Merrill derived microRNAs (miRNAs) and their targets during Mungbean yellow mosaic India virus (MYMIV) infection. Physiol Mol Plant Pathol. 2017;10:13–22. doi: 10.1016/j.pmpp.2017.05.001. [DOI] [Google Scholar]
- Ramesh SV, Chouhan BS, Gupta GK, Husain SM, Chand S. Genomic sequence characterization of Begomovirus infecting soybean and molecular evolutionary genomics of legume yellow mosaic viruses (LYMVs) Plant Omics J. 2017;10:88–96. doi: 10.21475/poj.10.02.17.pne391. [DOI] [Google Scholar]
- Ramesh SV, Shivakumar M, Ramteke R, Bhatia VS, Chouhan BS, Goyal S, Singh A, Praveen S, Gill BS, Chand S. Quantification of a legume begomovirus to evaluate soybean genotypes for yellow mosaic disease (YMD) resistance. J Virol Methods. 2019;268:24–31. doi: 10.1016/j.jviromet.2019.03.002. [DOI] [PubMed] [Google Scholar]
- Rani A, Kumar V, Gill BS, Rathi P, Shukla S, Singh RK, Husain SM. Linkage mapping of Mungbean yellow mosaic India virus (MYMIV) resistance gene in soybean. Breeding Sci. 2017;67:95–100. doi: 10.1270/jsbbs.16115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring-Harbour Laboratory Press; 2001. [Google Scholar]
- Shanmugapriya G, Das SS, Veluthambi K. Transgenic tobacco plants expressing siRNA targeted against the Mungbean yellow mosaic virus transcriptional activator protein gene efficiently block the viral DNA accumulation. Virus Dis. 2015;26:55–61. doi: 10.1007/s13337-015-0251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Sharma S, Gautam I, Baranwal VK. Natural infection of Glycine max by seven viruses belonging to different genera in India. J Plant Pathol. 2016;98(3):569–575. [Google Scholar]
- Shivaprasad PV, Thillaichidambaram P, Balaji V, Veluthambi K. Expression of full-length and truncated Rep genes from Mungbean yellow mosaic virus-Vigna inhibits viral replication in transgenic tobacco. Virus Genes. 2006;33:365–374. doi: 10.1007/s11262-006-0077-5. [DOI] [PubMed] [Google Scholar]
- Trinks D, Rajeswaran R, Shivaprasad PV, Akbergenov R, Oakeley EJ, Veluthambi K, Hohn T, Pooggin M. Suppression of RNA silencing by a geminivirus nuclear protein AC2 correlates with transactivation of host genes. J Virol. 2005;79:2517–2527. doi: 10.1128/JVI.79.4.2517-2527.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren H, Akbergenov R, Pooggin MM, Hohn T, Gruissem W, Zhang P. Transgenic cassava resistance to African cassava mosaic virus is enhanced by viral DNA-A bidirectional promoter-derived siRNAs. Plant Mol Biol. 2007;64:549–557. doi: 10.1007/s11103-007-9175-6. [DOI] [PubMed] [Google Scholar]
- Vanitharani R, Chellappan P, Fauquet CM. Short interfering RNA-mediated interference of gene expression and viral DNA accumulation in cultured plant cells. Proc Nat Acad Sci USA. 2003;100:9632–9636. doi: 10.1073/pnas.1733874100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A, Malathi VG. Emerging geminivirus problems: a serious threat to crop production. Ann Appl Biol. 2003;142:145–164. doi: 10.1111/j.1744-7348.2003.tb00240.x. [DOI] [Google Scholar]
- Vu TV, Choudhury NR, Mukherjee SK. Transgenic tomato plants expressing artificial microRNAs for silencing the pre-coat and coat proteins of a begomovirus, Tomato leaf curl New Delhi virus, show tolerance to virus infection. Virus Res. 2013;172:35–45. doi: 10.1016/j.virusres.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Wang H, Buckley KH, Yang X, Buchmann RC, Bisaro DM. Adenosine kinase inhibition and suppression of RNA silencing by Geminivirus AL2 and L2 proteins. J Virol. 2005;79:7410–7418. doi: 10.1128/JVI.79.12.7410-7418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.