Abstract
Introduction:
The adenosine pathway plays a key role in modulating immune responses in health and in disease. In health, anti-inflammatory effects of adenosine balance pro-inflammatory ATP, limiting tissue destruction by activated immune cells. In disease, this balance is disturbed.
Areas covered:
This review focuses on cancer and explains how in the microenvironment, the ATP-adenosine balance shifts towards an excess of extracellular adenosine
Expert commentary:
The CD73-adenosine axis plays a key role in the inhibition of anti-tumor functions of immune effector cells. Today, adenosine emerges as one of the immune checkpoints that are implicated in the tumor escape from the host immune system. The adenosine pathway is currently viewed as a significant barrier to the effectiveness of immune therapies and becomes an important therapeutic target in cancer. Pharmacologic inhibitors or antibodies specific for the components of the adenosine pathways or adenosine receptors show efficacy in pre-clinical studies and are entering the clinical arena.
Keywords: Cancer, adenosine, ATP, adenosine receptors, CD73-ADO axis
1. Introduction
Adenosine (ADO) and its biological effects have been intensively investigated in the last decade. Earlier studies have defined the adenosine pathway, including adenosine receptors (ADOR) as well as ligands and enzymes mediating ADO synthesis and degradation [1]. Subsequently, in vivo experiments demonstrated the pathophysiological role of ADO and its receptors in a variety of biological reactions, including inflammation and immune responses to cancer. More recent studies focusing on molecular and cellular mechanisms associated with ADO effects on tumor progression suggested that ADO might serve as a potential immune checkpoint, similar to e.g. CTLA-4, in mediating cancer-associated immune suppression. Further, the possibility of pharmacologic or immunologic blockade of the ADO pathway as a clinically useful therapy in cancer was advanced and is currently being translated to clinical trials. Emerging evidence suggests that ADO and also other components of the ADO pathway play a role in tumor escape from the immune system and represent potential therapeutic targets in cancer immunotherapy.
2. The ADO pathway
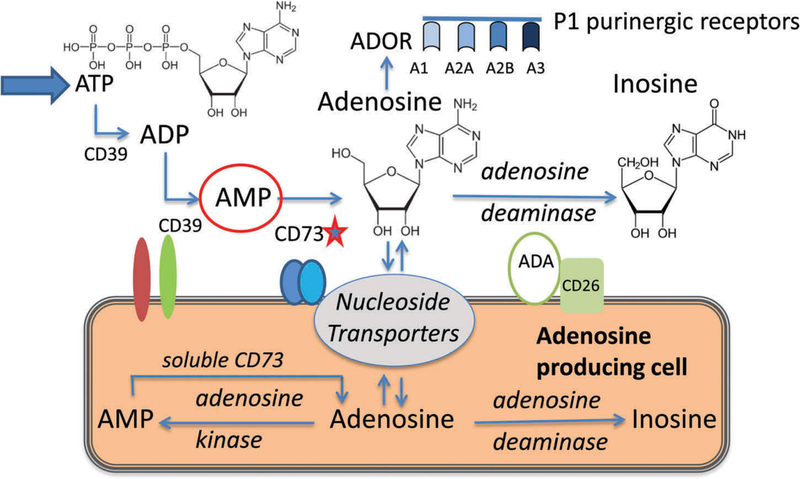
ADO is an extracellular and intracellular metabolite (Figure 1), and its levels in tissues are calibrated by activities of several key enzymes.
Figure 1.
Extracellular and intracellular adenosine pathways. CD73, a 5′-ectonucleotidase, is a rate controlling enzyme of the extracellular ADO pathway. CD73, as soluble or exosomal 5′–nucleotidase, also produces intracellular ADO from AMP. ADO signals via four P1 purinergic receptors expressed on a wide variety of cell types. The figure is modified and reproduced with permission from ref. 17.
Extracellular ADO is a product of the enzymatic breakdown of adenosine 5′-triphosphate (ATP) in the sequential steps catalyzed by two ectonucleotidases, CD39 and CD73 [2]. First, ATP dephosphorylation to adenosine diphosphate (ADP) and to adenosine monophosphate (AMP) is mediated by ectonucleoside triphosphate dephosphohydrolase-1 (ENTPD-1 or CD39)). Next, AMP is dephosphorylated to ADO by 5′-ectonucleotidase (CD73). The bioavailability of extracellular ADO is regulated by adenosine deaminase (ADA) which degrades ADO to inosine or by ADO transport into cells by nucleoside transporters residing in the cell membrane. ATP is either actively released from stressed cells (e.g. during inflammation, hypoxia, apoptosis) via vesicle exocytosis and via transporters or it passively leaks out from necrotic cells into the pericellular space [3]. Extracellular ATP is sensed by a large array of P2X and P2Y purinergic receptors, which are expressed by many different cells and play a key role in autocrine signaling and immune cell activation [4]. Extracellular ATP regulates immune responses and is largely proinflammatory [5].
Intracellular ADO is produced by hydrolysis of 3′5′-cAMP by phosphodiesterases (PDEs) from AMP through intracellular 5′ nucleotidase (CD73). Intracellular ADO can be also produced de novo by hydrolysis of S-adenosyl homocysteine. Intracellular ADO levels are strictly controlled and maintained at physiological concentrations by ADO transport out of the cell, ADO phosphorylation to AMP by salvage kinases or ADO deamination by ADA to inosine [6].
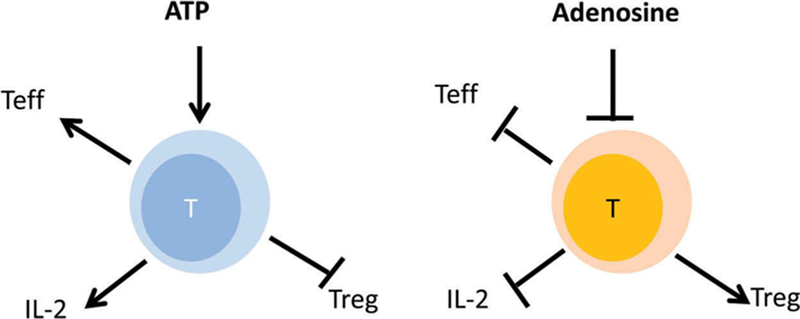
Once released by cells, ADO signals via the P1-type purinergic receptors (A1R, A2AR, A2BR and A3R), and it mainly mediates anti-inflammatory effects. ADORs belong to the family of G protein-coupled receptors that are expressed by many different cell types, including immune cells [7]. By engaging these receptors, ADO activates or inactivates adenylyl cyclase (AC) and modulates levels and activity of 3′5′-cAMP. A2AR and A2BR stimulate AC and increase 3′5′-cAMP levels, while A1R and A3R inhibit AC and downregulate cAMP [8]. Recently, inosine, in addition to ADO, was shown to be a ligand of A2AR [9] and suppress immunity [10]. The overall result of increased extracellular ADO levels in tissues is the downregulation of immune responses. Extracellular ADO and ATP tend to have opposite effects on immune cell responses (Figure 2). Acting as Yin and Yang, ADO and ATP maintain the fine balance between pro- and anti-inflammatory mediators in tissues that is essential for health. In pathological situations such as cancer, this balance is disturbed in favor of ADO with consequences that impact the disease progression and outcome.
Figure 2.
The opposite effects of ATP (pro-inflammatory) and ADO (anti-inflammatory) on immune cells in tissues. The ATP-ADO balance is maintained in health to prevent tissue damage by activated immune cells. In the tumor microenvironment, ADO present in excess inhibits anti-tumor immune responses.
3. The ADO pathway in cancer
The ADO pathway is one of the major inhibitory pathways operating in the tumor microenvironment (TME). Three factors are responsible for ADO prominence in the TME. First, the presence of cancer in tissues is accompanied by accumulation of exogenous (e) ATP in the extracellular space. Second, arrival in the TME of inflammatory cells creates conditions akin to local inflammation and upregulates expression of ATP dephosphorylating enzymes, presumably to prevent tissue destruction by the unfolding inflammatory cascade. Third, hypoxia is the major ADO-inducing factor in the TME [11]. As the production of ADO increases in the TME, ADO, acting as an anti-inflammatory mediator, downregulates functions of infiltrating immune cells thus preventing tissue destruction. In the presence of tumor, however, this normally beneficial response for the host becomes converted into chronic suppression of antitumor immune responses benefiting the growing tumor [12]. The tumor reprograms the TME so that inflammatory cells acquire capabilities to produce a new portfolio of cytokines which promote tumor growth and inhibit antitumor activity of immune cells newly recruited to the TME. As the tumor is a rich source of both ATP and ADO, reprogramming of the TME is largely driven by the tumor. When maintained for a prolonged period of time this situation becomes chronic, favors tumor growth, and inhibits the local immune response. Immune cells fail to exercise antitumor activities in the profoundly immunosuppressive milieu. The failure to balance and regulate the ATP-ADO interface in the presence of progressing tumor enables ADO to maintain its anti-inflammatory activity. Ectonucleotidases, CD39 and CD73, are the key enzymes regulating this ATP-ADO balance in the TME.
4. Cellular mechanisms responsible for ado-mediated polarization in the TME
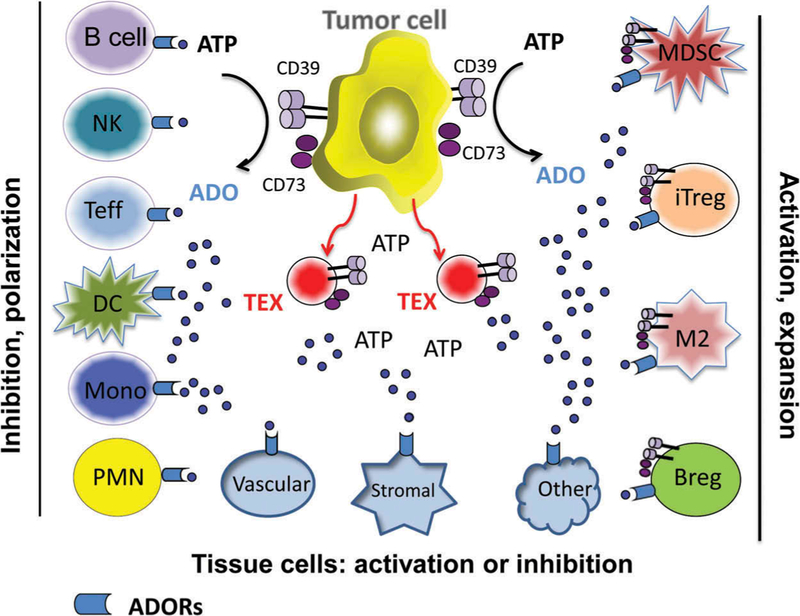
In the TME, virtually all tissue and infiltrating immune cells express ADOR and can be variably modulated by ADO. Many of these cells also express CD39 and CD73 that are responsible for ADO production, with CD73 playing the gatekeeping role as the rate controlling enzyme [13]. Tumors are often avid ADO producers [11]. The ability of the tumor cells to secrete ADO is critical for the tumor vascularization and stroma reprogramming [14]. Expression of CD73 in murine and human tumors has been well documented [15]. Coexpression of CD39 and CD73 in tumor cells has also been reported [16]. Utilization of ADO by tumor cells has been variously reported to result in promotion or suppression of its growth and seems to depend on the type and numbers of ADORs it expresses. Figure 3 illustrates the stimulatory or inhibitory effects of ADO on different types of cells present in the TME. Notably, these cells can be either activated or inhibited by ADO, depending on the ADORs they utilize. Immune cells are divided into two categories of responders to ADO: (a) in effector immune cells (B cells, Teff, NK cells, DC, monocytes and PMNs) ADO suppresses functions and (b) in regulatory immune cells (Treg, Breg, M2, and MDSC), ADO stimulates activation, proliferation and suppressor functions [17,18].
Figure 3.
Differential effects of ADO on immune cells present in the tumor microenvironment. ADO shapes both the innate and adaptive arms of anti-tumor immunity. The figure is reproduced with permission from ref. 17.
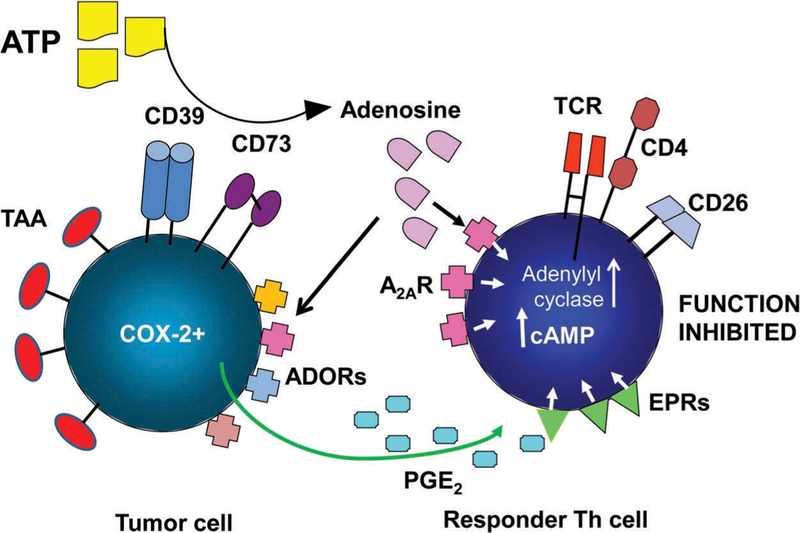
The mechanisms responsible for ADO-mediated suppression of effector T cells (Figure 4) have been evaluated through the use of small-molecule pharmacologic agents which selectively either activate or antagonize enzymes or receptors of the adenosinergic pathway, allowing for in vivo or in vitro measurements of the functional alterations mediated by these agents [18,19]. ADO signaling via the A2AR in effector T cells upregulates AC-7 (isoform # 7) activity and increases the levels of 3′5′-cAMP [19]. High levels of cAMP result in suppression of T effector cells at various stages of their maturation, differentiation, activation, migration or effector functions [17,20]. Specifically, the A2AR activation was reported to inhibit Th1 and Th2 cell development and effector functions [21]. Interestingly, conditional deletion of the A2AR in some mice strains was reported to result in depletion of CD8 + T cells by activation-induced cell death (AICD) and increased tumor growth [22]. In NK cells, ADO signals via A2AR suppressing their cytotoxicity and cytokine production. In monocytes, ADO signaling via the A2BR skews macrophage differentiation from M1 to M2, alters the cytokine profile and inhibits phagocytosis [23–26]. The available data suggest the A2AR on T cells functions in a way that resembles signaling via the checkpoint inhibitory receptors such as CTLA-4 or PD-1 operating in activated T cells.
Figure 4.
ADO produced in the tumor microenvironment mediates immune suppression via A2AR expressed on immune effector (Th) cells. The tumor expresses tumor-associated antigens (TAA) and produces other inhibitory factors such as prostaglandin E2 (PGE2). Signaling via ADOR or EPRs (PGE2 receptors) expressed on immune cells, ADO and PGE2 collaborate in up-regulating levels of cAMP in a T effector cell, leading to suppression of its functions.
5. ADO and immune cell regulation
Regulatory T cells (Treg) are a small subset of CD4 + T lymphocytes (~5%) in the peripheral circulation, which specialize in suppressing responses of other immune cells. Commonly identified in humans as CD3+CD4+FOXP3+CD25high cells, Treg are a heterogeneous T cell subset, comprising at least two different subsets of thymus-derived natural nTreg and of induced iTreg [27]. In contrast to conventional CD4 + T cells, Treg expand in the presence of ADO signaling via the A2AR and upregulate their immunosuppressive functions [28]. In 2009, two groups of investigators reported that murine and human Treg express CD39 and CD73 [29,30]. This suggested that Treg not only utilized ADO to promote their suppressive functions but also produced ADO and used it for mediating suppression by increasing intracellular cAMP levels in responder cells. We have confirmed this hypothesis by demonstrating that suppressive activity of Treg was in part mediated by the secretion of ADO [18,31,32]. This was also confirmed by Stagg and colleagues, who reported that CD73 expression on the surface of Treg is required for Treg-mediated suppression of antitumor immune responses [33]. Thus, in Treg, A2AR-mediated signaling translates into a gain of suppressive functions by upregulation of cAMP levels. The molecular underpinning of this result remains unclear, and it contrasts with the data reported above for T effector cells, where A2AR signaling results in a loss of effector functions.
Not only ADO production but also its degradation to inosine plays an important role in immune suppression mediated by Treg. Adenosine deaminase (ADA, CD26) is responsible for deamination of ADO to inosine. We showed that Treg are negative for CD26, while conventional T cells (Tconv) are CD26+ [34]. The absence of ADA in Treg suggests that pericellular ADO levels driving autocrine ADO signaling are high in these cells, potentially contributing to upregulation of suppressive activity mediated by Treg. On the other hand, in CD26 + Tconv converting ADO to inosine immune dysfunction rather than upregulation of effector functions is seen. Newer evidence suggests that inosine, a product of ADO deamination, binds to A2AR on T cells and functions as a potent agonist of this receptor [9]. Similar to ADO, inosine-mediated A2AR activation leads to cAMP production and functional immunosuppression. However, unlike ADO which has short half-life, inosine is a stable metabolite and its prolonged occupation of A2AR results in strong and sustained signaling and cAMP accumulation [9,10]. In addition, downstream inosine signaling is ERK1/2 biased, while ADO produces cAMP-based signaling [9]. Thus, inosine emerges as a functional A2AR agonist that mediates anti-inflammatory effects long after ADO is catabolized [35–37]. In CD26 + T conv, which can catabolize eADO in the pericellular space, persistent autocrine inosine signaling is likely to contribute to and drive immune suppression.
In the TME, ADO may operate in synergy with other immunosuppressive pathways, for example, the PGE2 pathway, which is known to be active in many solid tumors [38]. The suppressive PGE2 pathway has been linked to tumor progression and poor outcome [39]. PGE2, a product of COX-2 activity, binds to four G-protein-coupled receptors (EpR1-EpR4) on responder cells. Like ADOR, these receptors following ligation signal via AC-7 and 3′5′-cAMP, leading to suppression of immune cell functions [40]. Our data showed that iTreg generated in cultures (referred to as Tr1 cells) under conditions simulating the TME not only co-expressed CD39 and CD73 producing an excess of ADO but were also COX2+ and PGE2 + [34]. This finding called attention to the possibility that the ADO and PGE2 pathways may be collaborating in inducing suppression in the TME and that Treg utilize both pathways to mediate suppression [34,40]. Further studies, involving pharmacologic antagonists of A2AR and Ep2R, showed that these receptors on responder lymphocytes mediate signaling via cAMP and that the collaboration between ADO and PGE2 results in highly effective suppression of Tconv by Treg [17,34,40].
B lymphocytes are also present in immune infiltrates in solid tumors, often as distinct lymph node-like structures [41]. Since B cells are known to express CD39 and CD73 and to produce ample ADO [42], we hypothesized that autocrine signaling via ADOR might downregulate functions of these cells. We showed that human CD19 + B cells expressed mRNA for A1R, A2AR and A3R but not for A2BR and that autocrine ADO signaling suppressed B cell activation, proliferation and cytokine production [43]. Pharmacologic inhibition of ADOR in these experiments showed that ADO mediated autocrine suppression of B cell functions via A3R. This was a counterintuitive finding, as A3R signaling was previously reported to reduce cAMP levels and to upregulate lymphocyte functions [43]. It is possible, however, that autocrine signaling by ADO engages ADOR other than A2AR, for example, A3R in highly activated B cells which produce very high levels of ADO.
Among B cells a small subset of regulatory B cells (Breg) was shown to control CD4 + T cell responses by producing IL-10 or TGF-β or by promoting Treg expansion [43,44]. We observed that in vitro activated B cells, selectively upregulated expression of CD39 and downregulated CD73 expression levels. Further, activated B cells with the highest CD39 expression co-incubated with autologous T cells mediated the strongest suppression of T cell functions. This suppression was associated with excess production of 5′-AMP by activated B cells. Given that T cells express A1R and, as recently reported, 5′-AMP is an agonist of A1R with affinity equal to or greater than that of ADO [45], we surmised that activated B cells suppressed T cell functions by using 5′-AMP signaling via A1R on responder T cells. Our more recent data show that B cells with the highest CD39 expression function as Breg by producing immunosuppressive factors such as ADO, 5′-AMP and IL-10 and downregulating functions of T cells [44]. In aggregate, these data identified B cell-derived 5′-AMP as yet another catabolic product of the ADO pathway with immunosuppressive activity that is mediated by activated Breg. The frequency and regulatory functions of Breg are increased in the TME.
6. The CD73-ADO axis
The CD73 (ecto-5′-nucleotidase) is a rate-controlling enzyme in the ADO pathway, and in that capacity it regulates ADO production presumably in response to environmental stimuli. Enzymatic activity of CD73 results in conversion of 5′-AMP to ADO and by regulating ADO production, CD73 determines the extent of immune dysfunction in the TME [17,46]. CD73 is a dimer of two identical 70 KDa subunits anchored to the cell membrane via a C-terminal serine residue linked to glycosyl phosphatidylinositol (GPI) [13]. CD73 is also found in the soluble form in plasma as a result of the proteolytic cleavage of GPI. CD73 plasma levels are increased in cancer [47,48]. CD73 is found to be variably expressed in various types of solid tumors as well as in normal cells such as endothelial cells and immune cells present in the TME [48]. High levels of CD73 expression in the TME appear to correlate with poor outcome, and studies are in progress to establish its value as an independent prognostic biomarker in cancer [48–50]. To date, the levels of CD73 expression in tumor tissues were reported to be associated with poor prognosis in prostate cancer [49], triple-negative breast cancer (TNBC) [50] and high-grade serous ovarian cancer [51]; with worse disease-free survival in glioblastoma [48]; and with prognostically significant impairments of antitumor immunity in high-grade serous ovarian cancer [51]. In the TME, the CD73-ADO pathway is driven by tissue hypoxia, and activation of HIF-1α promotes expression of CD73 and upregulates its enzymatic activity [52]. The presence of inflammatory cytokines found in the TME, including type I interferons, TNF-α, IL-1β, TGF-β, PGE2 can also induce CD73 expression [48]. In studies with human tumor cell lines, elevated CD73 expression promoted proliferation, survival, migration, and invasion of tumor cells [48]. Expression of CD73 on immune cells is highly variable in contrast to CD39, which shows stable and consistent expression on regulatory T and B lymphocytes [53–55]. It is likely that the high rate of CD73 turnover accounts for CD73 paucity on the cell surface of activated lymphocytes [54]. Nevertheless, CD73 activity was shown to be responsible, at least in part, for promoting suppressive functions of Treg, Breg, and MDSC, as discussed above [17]. Numerous in vitro and in vivo experiments with pharmacologic or biologic inhibitors of CD73 activity have demonstrated significant recovery of antitumor functions in tumor-bearing mice and have correlated this recovery with the tumor rejection. Overall, the available in vitro and in vivo data provide a strong rationale for the development of therapeutic strategies to block activity of the CD73-ADO axis in subjects with cancer.
7. The ADO pathway and exosomes
The recent emergence of exosomes as vehicles of intercellular communication has focused attention on their involvement in the ADO pathway. Exosomes are membrane-bound vesicles ranging in size from 30 to 150 nm (i.e. are virus size) that are produced by all cells [56]. Stressed cells, including tumor cells and activated cells, including immune cells produce large quantities of exosomes [57]. As exosomes isolated from supernatants of cell lines or from human plasma carry enzymatically active CD39 and CD73 proteins [58] and can hydrolyze eATP to adenosine in a test tube, they are expected to modulate the ADO pathway. Indeed, we have reported that tumor-derived exosomes co-incubated with Treg or Breg obtained from human peripheral blood upregulated expansion and suppressor functions, including ADO as well as inosine production, by these regulatory lymphocytes [54,59]. Further, as exosomes are ubiquitous in body fluids and tissues, we have suggested that they could deliver membrane tethered CD73 to the surface of immune and non-immune cells in which this ectonucleotidase is only transiently expressed or absent, perhaps depending on the state of cellular activation. A good example of this type of cellular cooperation is provided by circulating CD4+CD39+ Treg, which are rich in CD73+ cytoplasmic granules but express little or no CD73 on the surface. This is because once delivered to the surface, CD73 readily aggregates forming characteristic caps and is shed from the surface or internalized. Nevertheless, these CD4+CD39+ Treg transiently devoid of CD73 produce ADO [54]. We have suggested that exosomes carrying enzymatically active CD73 deliver it to the cells and, serving as a source of membrane-bound CD73, ensure continuous ADO production. Tumor-derived exosomes, shown to carry a cargo enriched in CD39 and CD73 enzymes, may be especially programmed to perform this role in the TME.
8. Targeting the ADO pathway in cancer therapy
Recent in vivo and in vitro preclinical studies indicate that the ADO pathway components can be targeted with antibodies, pharmacologic inhibitors, or siRNAs to reduce or silence their pro-tumor activities [60–62]. The available strategies for blocking the ADO pathway can be divided into three categories: (a) blocking of ADO synthesis by tumor, tissue, or immune cells; (b) blocking or preventing ADO signaling via ADOR on the surface of responder cells; and (c) inhibiting ADO effects inside the target cell [17]. While these therapeutic strategies are being actively evaluated in preclinical models, they are also being translated to the clinic. So far, clinical trials are in progress for only few of these strategies, largely for treatment of diseases other than cancer, notably cardiovascular, inflammatory and autoimmune conditions [see the website ClinicalTrials.gov]. Nevertheless, there is increasing interest in blocking the ADO pathway in cancer, and several phase I clinical trials in cancer are in progress. For example, results of a trial using the MEDI9447 MoAb which targets ADO synthesis by blocking CD73 were recently reported [63]. The trial designed to block ADO signaling using an antagonist of the A2AR, CPI-444 sponsored by Corvus Pharmaceuticals is ongoing, based on preclinical studies demonstrating antitumor benefits of the strategy [64,65]. Novartis/Palobiofarma is testing its A2AR antagonists PFB509 in phase I trial, and several other pharmaceutical companies have disclosed plans for developing anti-CD73 mAbs.
CD73 utilizes multiple mechanisms for mediating antitumor activities, including: the upregulation of inhibitory functions of Treg, Breg, and/or MDSC as well as the promotion of tumor growth, angiogenesis, and metastatic spread. Molecular inhibition of CD73 with pharmacologic inhibitors or Abs in vivo in murine cancer models was shown to inhibit tumor formation, growth, and metastasis [66]. MEDI9447 anti-CD73 MoAb is a high-affinity therapeutic Ab that blocks conversion of CD73 from inactive to catalytically active enzyme [63]. It reverses ADO-mediated CD4 + T cell suppression, upregulates antigen presentation by DC, enhances lymphocyte activation, promotes release of proinflammatory cytokines (IFN-γ, IL-1β, TNF-α) and thus alters the composition of lymphoid and myeloid cells infiltrating the TME. In vivo, it inhibits growth of syngeneic tumors in mice. More recently, it has been found to exert synergistic in vivo effects upon combined delivery with anti-PD-1 Abs to tumor-bearing mice [63]. These recently described attributes of MEDI9447 and, especially its potential for altering the TME, confirm its role as an attractive candidate for cancer monotherapy or combinatorial immunotherapies with checkpoints inhibitors, vaccines, or immune adoptive cell transfers. In addition to anti-CD73 Abs, a variety of pharmacological inhibitors of CD73 as well as siRNA have been used in mice to inhibit tumor growth [66] and may be considered as promising candidates for future therapeutic applications. CD39 is also being evaluated as a therapeutic target in cancer [55,67]. Experiments with KO cells or pharmacological and antibody-based blocking of CD39 activities support antitumor effects of CD39 inhibition [55,67]. Importantly, therapies involving inhibition of CD39 target not only tumor cells, but also immune suppressor cells such as Treg or Breg via selective inhibition of suppressor functions these cells mediate without inducing their death or depletion [55].
Blocking of the ADOR signaling has also been a therapeutic target. However, since autocrine and paracrine signals may be processed by different ADOR expressed on the same cells, and these receptors could either promote or inhibit antitumor activity, only A2AR and A2BR are currently targeted for therapy. Signaling via these receptors on immune cells inhibits effector functions of these cells and has pro-tumor effects. Antagonism of A2AR and A2BR signaling represents potentially promising targeted therapy, and several preclinical studies have already confirmed antitumor effects of pharmacologic or antibody antagonists of these receptors [60,64,68]. The promising preclinical data have encouraged the design and implementation several clinical trials for cancer patients, including the trial with CPI-444 antagonist of the A2AR mentioned above. CPI-444 is currently evaluated alone or in combination with anti-PDL-1 Ab in a multicenter phase I clinical trial designed for several different types of advanced cancer.
Blocking ADO effects inside the target cell could be implemented by regulating the levels of AC or phosphodiesterases. With respect to AC, the key enzyme responsible for 3′5′-cAMP synthesis, blocking of ADO signaling via the A2AR is expected to restore antitumor functions of immune cells and thus is considered to be an attractive therapeutic strategy in cancer [69]. The development of selective AC inhibitors, specifically inhibitors of AC-7 activity, the enzyme isoform present in immune cells, could offer a promising approach to restoration of immune antitumor activity in effector cells. In another potentially promising approach, the blockade of AC-7 could be combined with activation of the PDE isoform 4 shown to operate in immune cells [40]. This enzyme mediates intracellular hydrolysis of 3′5′-cAMP to 5′-AMP [70]. Enzymatic downregulation of 3′5′-cAMP activity through the simultaneous engagement of its synthetase (AC-7) and activation of its hydrolysis via PDE4 in immune responder cells represents a novel albeit challenging therapeutic strategy for protecting immune effector cells from ADO-mediated suppression of antitumor functions. The restoration of immune cell effector functions and their protection from suppressive ADO effects are the objectives of this form of immunotherapy. When combined with other immunotherapies, for example, antibodies targeting immune checkpoint inhibitors, ADO antagonism, which can be addressed at several different levels, as illustrated in Figure 4, might become an important therapeutic option to consider in the near future.
9. Expert commentary
The ADO pathway has emerged as one of the major immunoinhibitory and tumor growth promoting mechanisms operating in the TME of many human solid tumors. The engagement of this pathway in inflammatory events and tumor progression might be more evident in some solid tumors than others. Nevertheless, all cells present in the TME, including the tumor and immune cells, are subject to the ATP-ADO-mediated regulation. Tumor cells, immune cells, tissue cells and their products and exosomes present in the TME express CD39 and CD73 ectonucleotidases and contribute to converting ATP to ADO and inosine. The latter has been recently recognized as an important contributor to ADO-mediated immune suppression, although inosine binds to A2AR with lower affinity than ADO, its receptor occupancy is longer.
Given that ADO and inosine suppress antitumor functions of immune effector cells on one hand and promote tumor growth on the other hand, there exists a strong rationale for blocking their anti-immune as well as pro-tumor activities. Efficacy of this strategy has been amply demonstrated in numerous studies performed in tumor-bearing mice. Translation of these strategies to the clinic based on promising preclinical studies is being actively pursued, and the first phase I clinical trials testing safety of various antagonists of the ADO pathway in cancer patients are in progress. Based on promising results of preclinical in vivo studies in animal tumor models [71,72], it is expected that co-blockade of immune checkpoints and A2AR will be effective in arresting tumor metastases in the future combinatorial therapies.
10. Five-year view
As immune therapy for cancer gathers momentum and our insights into the TME become more informed, questions concerning the frequency and length of therapeutic responses vs. therapeutic failures will increasingly frequently arise. It has already become clear that a considerable number of cancer patients do not respond to checkpoint inhibition for reasons that are not yet understood. At the same time, increasing awareness of the existence in the TME of numerous immunoinhibitory pathways, including the ubiquitous adenosine pathway currently emerging as another immune checkpoint in cancer, indicates a need for combinatorial therapies. By combining therapies selectively directed at blocking those immunoinhibitory signals that are most prominent in the TME of individual cancer patients, it may be possible to restore an immune equilibrium disturbed by cancer. This type of personalized approach to cancer biotherapy, made possible by rapid progress in defining genetic and immune signatures of tumors, is likely to grow. Immune and noncancer immune biomarkers will be identified to facilitate selection of therapies and to predict response to therapy and outcome. In the years to come, pharmacologic or immunologic blockade of the adenosine pathway (and other molecular pathways as needed) is likely to become a component of cancer biotherapies used alone or in combination with conventional therapies. In the next five years, using personalized therapies and novel biomarkers, it will be possible to effectively restore antitumor immune competence in patients with cancer and improve outcome.
Key points.
The adenosine pathway is a major immunosuppressive component of many human tumors
Adenosine and inosine emerge as critical immune checkpoints in cancer
Cooperation of the adenosine and PGE2 pathways in the tumor microenvironment contributes to suppression of anti-tumor immune effector cells
Targeting of the adenosine pathway with pharmacologic inhibitors or Abs emerges as a promising therapeutic strategy in cancer
Blocking activities of ectonucleotidases or of adenosine receptor signaling in preclinical in vivo studies has been successful in inhibiting tumor growth and metastasis
The adenosine pathway blockade alone or in combination with other immune therapies, including checkpoint inhibitors, is currently being implemented in initial phase I clinical trials for patients with advanced malignancies
Acknowledgments
This study was supported in part by NIH grants R01 CA168628 and R21 CA205644 to TLW.
Funding
National Institutes of Health grants R01 CA 168,628 and R21 CA205644
Footnotes
Conflict of interest
The author has no conflict of interest.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) to readers.
- 1.Burnstock G Introduction to the special issue on purinergic receptors. Adv Exp Med Biol 2017. [Epub ahead of print]. [DOI] [PubMed]
- 2.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 2008;1783:673–694. [DOI] [PubMed] [Google Scholar]
- 3.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol 2001;13:114–119. [DOI] [PubMed] [Google Scholar]
- 4.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 2014;509:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacob F, Perez Novo C, Bachert C, et al. Purinergic signaling in inflammatory cells: P2 receptor expression, functional effects, and modulation of inflammatory responses. Purinergic Signal 2013;9:285–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonioli L, Colucci R, La Motta C, et al. Adenosine deaminase in the modulation of immune system and its potential as a novel target for treatment of inflammatory disorders. Curr Drug Targets 2012;13:842–862. [DOI] [PubMed] [Google Scholar]
- 7.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 2004;25:33–39.• A nice, brief but comprehensive review of how adenosine influences functions of innate immune cells.
- 8.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998;50:413–492. [PubMed] [Google Scholar]
- 9.Welihinda AA, Kaur M, Greene K, et al. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal 2016;28:552–560.• This paper reports that inosine binds to A2AR with greater stability than adenosine and functionality amplifies and prolongs A2AR activation.
- 10.Hasko G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci 2004;25:152–157.• This paper calls attention to effects inosine exerts on the immune system.
- 11.Vaupel P, Mayer A. Hypoxia-driven adenosine accumulation: a crucial microenvironmental factor promoting tumor progression. Adv Exp Med Biol 2016;876:177–183. [DOI] [PubMed] [Google Scholar]
- 12.Borea PA, Gessi S, Merighi S, et al. Adenosine as a multi-signalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci 2016;37:419–434. [DOI] [PubMed] [Google Scholar]
- 13.Strater N Ecto-5′-nucleotidase: structure function relationships. Purinergic Signal 2006;2:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allard B, Turcotte M, Stagg J. CD73-generated adenosine: orchestrating the tumor-stroma interplay to promote cancer growth. J Biomed Biotechnol 2012;2012:485156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stagg J, Thompson LF, Dwyer KM. Ectonucleotidases in cancer and inflammation. J Biomed Biotechnol 2012;2012:951423.• This paper describes the key roles CD39 and CD73 play in regulating inflammation and antitumor immunity.
- 16.Aliagas E, Vidal A, Texido L, et al. High expression of ecto-nucleotidases CD39 and CD73 in human endometrial tumors. Mediators Inflamm 2014;2014:509027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller-Haegele S, Muller L, Whiteside TL. Immunoregulatory activity of adenosine and its role in human cancer progression. Expert Rev Clin Immunol 2014;10:897–914. [DOI] [PubMed] [Google Scholar]
- 18.Ohta A A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol 2016;7:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryzhov S, Zaynagetdinov R, Goldstein AE, et al. Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J Immunol 2008;180:7212–7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarek PE, Powell JD. Adenosine and anergy. Autoimmunity 2007;40:425–432. [DOI] [PubMed] [Google Scholar]
- 21.Csoka B, Himer L, Selmeczy Z, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. Faseb J 2008;22:3491–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cekic C, Linden J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res 2014;74:7239–7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csoka B, Selmeczy Z, Koscso B, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. Faseb J 2012;26:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasko G, Kuhel DG, Chen JF, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. Faseb J 2000;14:2065–2074. [DOI] [PubMed] [Google Scholar]
- 25.Nemeth ZH, Lutz CS, Csoka B, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol 2005;175:8260–8270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csoka B, Koscso B, Toro G, et al. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes 2014;63:850–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther 2015;4:159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4 +CD25highFOXP3+ regulatory T cells. J Biol Chem 2010;285:7176–7186.• One of the first reports on how human Treg utilize adenosine to suppress functions of other T lymphocytes.
- 29.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007;110:1225–1232. [DOI] [PubMed] [Google Scholar]
- 30.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandapathil M, Szczepanski MJ, Szajnik M, et al. Adenosine and prostaglandin E2 cooperate in the suppression of immune responses mediated by adaptive regulatory T cells. J Biol Chem 2010;285:27571–27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta A, Kini R, Ohta A, et al. The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front Immunol 2012;3:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stagg J, Divisekera U, Duret H, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 2011;71:2892–2900. [DOI] [PubMed] [Google Scholar]
- 34.Mandapathil M, Szczepanski M, Harasymczuk M, et al. CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4(+) T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology 2012;1:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasko G, Kuhel DG, Nemeth ZH, et al. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol 2000;164:1013–1019. [DOI] [PubMed] [Google Scholar]
- 36.Marton A, Pacher P, Murthy KG, et al. Anti-inflammatory effects of inosine in human monocytes, neutrophils and epithelial cells in vitro. Int J Mol Med 2001;8:617–621. [PubMed] [Google Scholar]
- 37.Mabley JG, Pacher P, Liaudet L, et al. Inosine reduces inflammation and improves survival in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol 2003;284:G138–144. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, DuBois RN. The role of anti-inflammatory drugs in colorectal cancer. Annu Rev Med 2013;64:131–144. [DOI] [PubMed] [Google Scholar]
- 39.Pang LY, Hurst EA, Argyle DJ. Cyclooxygenase-2: a role in cancer stem cell survival and repopulation of cancer cells during therapy. Stem Cells Int 2016;2016:2048731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whiteside TL, Jackson EK. Adenosine and prostaglandin e2 production by human inducible regulatory T cells in health and disease. Front Immunol 2013;4:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen JS, Nelson BH. Tumor-infiltrating B cells and T cells: working together to promote patient survival. Oncoimmunology 2012;1:1623–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minguet S, Huber M, Rosenkranz L, et al. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur J Immunol 2005;35:31–41. [DOI] [PubMed] [Google Scholar]
- 43.Saze Z, Schuler PJ, Hong CS, et al. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood 2013;122:9–18.• The paper provides evidence that ADO is utilized by human Breg to suppress functions of other immune cells.
- 44.Figueiro F, Muller L, Funk S, et al. Phenotypic and functional characteristics of CD39high human regulatory B cells (Breg). Oncoimmunology 2016;5:e1082703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rittiner JE, Korboukh I, Hull-Ryde EA, et al. AMP is an adenosine A1 receptor agonist. J Biol Chem 2012;287:5301–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hausler SF, Montalban Del Barrio I, Strohschein J, et al. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother 2011;60:1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longhi MS, Robson SC, Bernstein SH, et al. Biological functions of ecto-enzymes in regulating extracellular adenosine levels in neoplastic and inflammatory disease states. J Mol Med (Berl) 2013;91:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allard D, Allard B, Gaudreau PO, et al. CD73-adenosine: a next-generation target in immuno-oncology. Immunotherapy 2016;8:145–163. [DOI] [PubMed] [Google Scholar]
- 49.Leclerc BG, Charlebois R, Chouinard G, et al. CD73 expression is an independent prognostic factor in prostate cancer. Clin Cancer Res 2016;22:158–166. [DOI] [PubMed] [Google Scholar]
- 50.Loi S, Pommey S, Haibe-Kains B, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013;110:11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turcotte M, Spring K, Pommey S, et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res 2015;75:4494–4503. [DOI] [PubMed] [Google Scholar]
- 52.Sitkovsky MV, Hatfield S, Abbott R, et al. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res 2014;2:598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beavis PA, Milenkovski N, Stagg J, et al. A2A blockade enhances anti-metastatic immune responses. Oncoimmunology 2013;2: e26705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuler PJ, Saze Z, Hong CS, et al. Human CD4(+) CD39(+) regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73(+) exosomes or CD73(+) cells. Clin Exp Immunol 2014;177:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bastid J, Cottalorda-Regairaz A, Alberici G, et al. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 2013;32:1743–1751. [DOI] [PubMed] [Google Scholar]
- 56.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest 2016;126:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atay S, Godwin AK. Tumor-derived exosomes: A message delivery system for tumor progression. Commun Integr Biol 2014;7:e28231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clayton A, Al-Taei S, Webber J, et al. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol 2011;187:676–683. [DOI] [PubMed] [Google Scholar]
- 59.Muller L, Mitsuhashi M, Simms P, et al. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep 2016;6:20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Antonioli L, Blandizzi C, Pacher P, et al. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 2013;13:842–857. [DOI] [PubMed] [Google Scholar]
- 61.Antonioli L, Pacher P, Vizi ES, et al. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013;19:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young A, Mittal D, Stagg J, et al. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov 2014;4:879–888. [DOI] [PubMed] [Google Scholar]
- 63.Hay CM, Sult E, Huang Q, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology 2016;5: e1208875.• This is an excellent summary of preclinical studies showing that targeting of CD73 with an antibody reprograms the tumor microenvironment and relieves ADO-mediated immune suppression.
- 64.Leone RD, Lo YC, Powell JD. A2aR antagonists: next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J 2015;13:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beavis PA, Divisekera U, Paget C, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A 2013;110:14711–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stagg J, Divisekera U, McLaughlin N, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 2010;107:1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bonnefoy N, Bastid J, Alberici G, et al. CD39: A complementary target to immune checkpoints to counteract tumor-mediated immunosuppression. Oncoimmunology 2015;4:e1003015.• A report showing that treatment with CD39-blocking antibody alleviates tumor-mediated immune suppression.
- 68.Cekic C, Sag D, Li Y, et al. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol 2012;188:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iwatsubo K, Okumura S, Ishikawa Y. Drug therapy aimed at adenylyl cyclase to regulate cyclic nucleotide signaling. Endocr Metab Immune Disord Drug Targets 2006;6:239–247. [DOI] [PubMed] [Google Scholar]
- 70.Saldou N, Obernolte R, Huber A, et al. Comparison of recombinant human PDE4 isoforms: interaction with substrate and inhibitors. Cell Signal 1998;10:427–440. [DOI] [PubMed] [Google Scholar]
- 71.Mittal D, Young A, Stannard K, et al. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res 2014;74:3652–3658. [DOI] [PubMed] [Google Scholar]
- 72.Young A, Mittal D, Stannard K, et al. Co-blockade of immune checkpoints and adenosine A2A receptor suppresses metastasis. Oncoimmunology 2014;3:e958952. [DOI] [PMC free article] [PubMed] [Google Scholar]