Abstract
Background & objectives:
Seeding density is one of the major parameters affecting the quality of tissue-engineered cartilage. The objective of this study was to evaluate different seeding densities of osteoarthritis chondrocytes (OACs) to obtain the highest quality cartilage.
Methods:
The OACs were expanded from passage 0 (P0) to P3, and cells in each passage were analyzed for gross morphology, growth rate, RNA expression and immunochemistry (IHC). The harvested OACs were assigned into two groups: low (1×107 cells/ml) and high (3×107 cells/ml) cell density. Three-dimensional (3D) constructs for each group were created using polymerised fibrin and cultured for 7, 14 and 21 days in vitro using chondrocyte growth medium. OAC constructs were analyzed with gross assessments and microscopic evaluation using standard histology, IHC and immunofluorescence staining, in addition to gene expression and biochemical analyses to evaluate tissue development.
Results:
Constructs with a high seeding density of 3×107 cells/ml were associated with better quality cartilage-like tissue than those seeded with 1×107 cells/ml based on overall tissue formation, cell association and extracellular matrix distribution. The chondrogenic properties of the constructs were further confirmed by the expression of genes encoding aggrecan core protein and collagen type II.
Interpretation & conclusions:
Our results confirmed that cell density was a significant factor affecting cell behaviour and aggregate production, and this was important for establishing good quality cartilage.
Keywords: Cartilage, chondrocytes, collagen-fibrin, osteoarthritis, seeding density
Chondrocytes in articular cartilage are embedded within an extracellular matrix (ECM) containing collagen, proteoglycan and non-collagenous proteins. Normally, articular cartilage undergoes continuous remodelling to replace degraded matrix. However, ageing and cartilage injury cause overexpression of matrix metalloproteinase which shifts the balance towards matrix degradation1 and ultimately leads to osteoarthritis (OA)2. The first human chondrocyte transplantation was performed in 1994 to induce regeneration3,4. However, expansion of chondrocytes in two-dimensional (2D) environment leads to dedifferentiation evident by morphological change, increased expression of collagen type I and reduced expression of collagen type II and aggrecan5. Interestingly, dedifferentiated chondrocytes were able to re-differentiate in three-dimensional (3D) culture environment6.
The success of 3D constructs for cartilage tissue regeneration depends on the composition of the culture medium, surrounding environment during expansion and tissue construction7. It is well known that chondrogenic properties of chondrocytes can be maintained in 3D cell spheroid4. Collagen and alginate scaffolds have been extensively used in tissue engineering of cartilage. However, collagen was reported to induce chondrocyte dedifferentiation, while the formation of alginate scaffolds is difficult to control and inconsistently stimulates the ECM production2. In contrast, fibrin is biocompatible and non-toxic and can be autologous, thus suitable for tissue reconstruction8,9. Fibrin was known to promote the migration and proliferation of cells10. Thus, this study was aimed to evaluate the effect of the seeding density of osteoarthritis chondrocytes (OACs) on fibrin construct.
Material & Methods
Isolation and culture of chondrocytes: Following approval by the Universiti Kebangsaan Malaysia Research and Ethics Committee (UKM 1.5.3.5/244/FF-2014-215), and obtaining written informed consent six human OA cartilage samples were collected from Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia, from consenting patients (54-69 yr) undergoing total knee arthroplasty surgery. Cartilages were collected from the lateral and medial parts of femoral condyle of OA patients’ varus knee (Fig. 1). Cartilage samples were diced and digested using collagenase type II (Gibco, USA). The isolated cells were resuspended in chondrocyte growth medium (CGM) with an initial seeding density of 5000 cells/ml. The CGM was prepared according to the previous method11,12. CGM was replaced every 2-3 days. The total cell number and viability were recorded until passage 3 to determine the growth rate (cells/day/cm2).
Fig. 1.
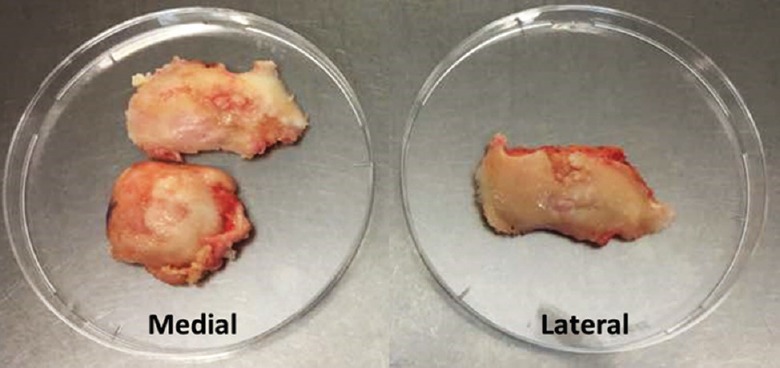
Gross view of the collected samples.
Cell cycle analysis of monolayer cultured chondrocytes: Flow cytometric analysis was performed using FACScan (Becton Dickinson, San Jose, CA, USA), and CELLQuest software (Becton Dickinson) with doublet discrimination was used to analyze the DNA content of freshly isolated and cultured OAC. Cell suspensions (n=6) were centrifuged at 400×g for five minutes, and pellet was washed and incubated with propidium iodide according to a previous method11. For each sample, 12,000 gated events were collected, and cell cycle analysis was performed using Modfit LT software (Verity Software House, Topsham, ME, USA).
Preparation of plasma-derived fibrin (PDF): Blood (5 ml) was collected from human volunteers (N=6) into vacutainer tubes containing sodium citrate, and the blood and anticoagulant were carefully mixed followed by centrifugation at 2500 × g for 10 min. The plasma layer was aspirated and centrifuged to remove the remaining insoluble matter. Plasma-derived fibrin (PDF) was stored at −80°C for future use.
Formation of the osteoarthritis chondrocyte-fibrin (OACF) constructs: The OACs at 1×107 and 3×107 cells/ml were added to 1 ml PDF, and the mixture was polymerized with 250 mM calcium chloride (CaCl2). The resulting gel-like osteoarthritis chondrocyte-fibrin (OACF) constructs were cultured for 7, 14 or 21 days. The constructs were used for histological and biochemical analyses.
RNA extraction and real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR): Total RNA was extracted from OAC monolayer and OACF construct using TRI Reagent (Molecular Research Centre, Cincinnati, USA) according to the previous method12. The yield and purity of the extracted RNA were determined by a NanoDrop™ 2000 spectrophotometer (Thermo Scientific, Wilmington, DE). Two-step qRT-PCR for chondrocytes specific genes; aggrecan, type I and type II collagen were performed using iScript™ cDNA Synthesis Kit followed by amplification using iQ™ SYBR Green® Supermix (BioRad, Hercules, CA, USA) on MyiQ™ iCycler Real-Time PCR Detection System (BioRad) as per kit instructions. Relative quantification of the gene expression was performed using the ΔCt method13. Housekeeping gene glyceraldehyde phosphate dehydrogenase (GAPDH) was used for internal normalization. The primer sequences (Biobasic, Markham, Ontario, Canada) are shown in the Table.
Table.
List of primers for real-time polymerase chain reaction
| Gene | Sequence |
|---|---|
| GAPDH | F: TCCCTGAGCTGAACGGGAAG |
| R: GGAGGAGTGGGTGTCGCTGT | |
| Type I collagen | F: AGGGCTCCAACGAGATCGAGA |
| R: TACAGGAAGCAGACAGGGCCA | |
| Type II collagen | F: CTATCTGGACGAAGCAGCTGGCA |
| R: ATGGGTGCAATGTCAATGATGG | |
| Aggrecan | F: CACTGTTACCGCCACTTCCC |
| R: ACCAGCGGAAGTCCCCTTCG |
GAPDH, glyceraldehyde phosphate dehydrogenase; F, forward; R, reverse
Histological and immunochemistry (IHC) analyses: Histological and immunochemistry (IHC) analyses of the OACF construct were performed at 7, 14 and 21 days. Gross observation was conducted prior to fixation. The constructs were fixed with 10 per cent formalin, embedded in paraffin and cut into 5 μm slices. For histological analysis, samples were stained with haematoxylin (Sigma-Aldrich, St. Louis, Mo., USA), fast green (Sigma-Aldrich) and Safranin O (Sigma-Aldrich) to visualize collagen deposition. For IHC, the sections were pre-treated with proteinase K (Dako Cytomation, Ely, UK) and peroxidase blocker (Dako Cytomation) prior to incubation with the primary antibodies (mouse anti-human collagen type I and II; Abcam, Cambridge, MA, USA), horseradish peroxidase-labelled polymer conjugated to goat anti-mouse Ig (DAKO Cytomation) and peroxidase substrate 3,3'-diaminobenzidine (DAB; DAKO Cytomation) sequentially. The sections were counterstained with haematoxylin and mounted in a glycerol gel (DAKO Cytomation).
Sulphated glycosaminoglycan (sGAG) production assay: All OACF constructs were digested with a papain digestion solution (125 μg/ml papain, 5 mM L-cystein, 100 mM Na2HPO4 and 5 mM EDTA; pH 6.8) at 60°C for 16 h. Sulphated glycosaminoglycan content was analyzed using a 1,9-dimethylmethylene blue (DMMB) assay (Biocolor, Belfast, UK). A 20 μl aliquot of each sample was pipetted into the microplate reader and added with 200 μl DMMB. Samples were analyzed immediately by measuring the absorbance at 525 nm.
Statistical analysis:The data were analyzed using two-way ANOVA and Tukey's multiple comparison tests.
Results
The characteristics of OACs were evaluated by their morphology, growth rate and expression of matrix proteins. OAC cultured in CGM preserved the small polygonal morphology throughout the passage (Fig. 2A). The OAC growth rate was significantly (P<0.05) increased from P0 (7144±786.6 cells/day/cm2) to P1 (14363±1279 cells/day/cm2), and then subsequently decreased until P3. The growth rate of OAC at P3 (8548±968.2 cells/day/cm2) was significantly (P<0.05) lower than that of P1. Comparison of growth rate between patients in each passage showed no significant difference (Fig. 2B). Results from the qRT-PCR showed that OAC expressed aggrecan and type I and II collagen during the subculture (Fig. 2C). Type II collagen was highly expressed by OAC in all passages relative to type I collagen and aggrecan. The expression of type II collagen was significantly higher at P0 (1.82±0.10) compared to that of subsequent passages. A similar trend was observed for type I collagen expression; however, type I collagen expression at P0 showed no significant differences with any other passages except P3 (0.70±0.02). There were no significant differences for aggrecan expression between the passages. Gene expression for patients in each passage also showed no significant differences. The changes in the expression of type I and type II collagen were further confirmed by results from the immunostaining analysis (Fig. 2D). Prominent staining for type II collagen was observed at P0 but showed weaker staining in subsequent passages until P3. In contrast, type I collagen staining was high for all the passages.
Fig. 2.
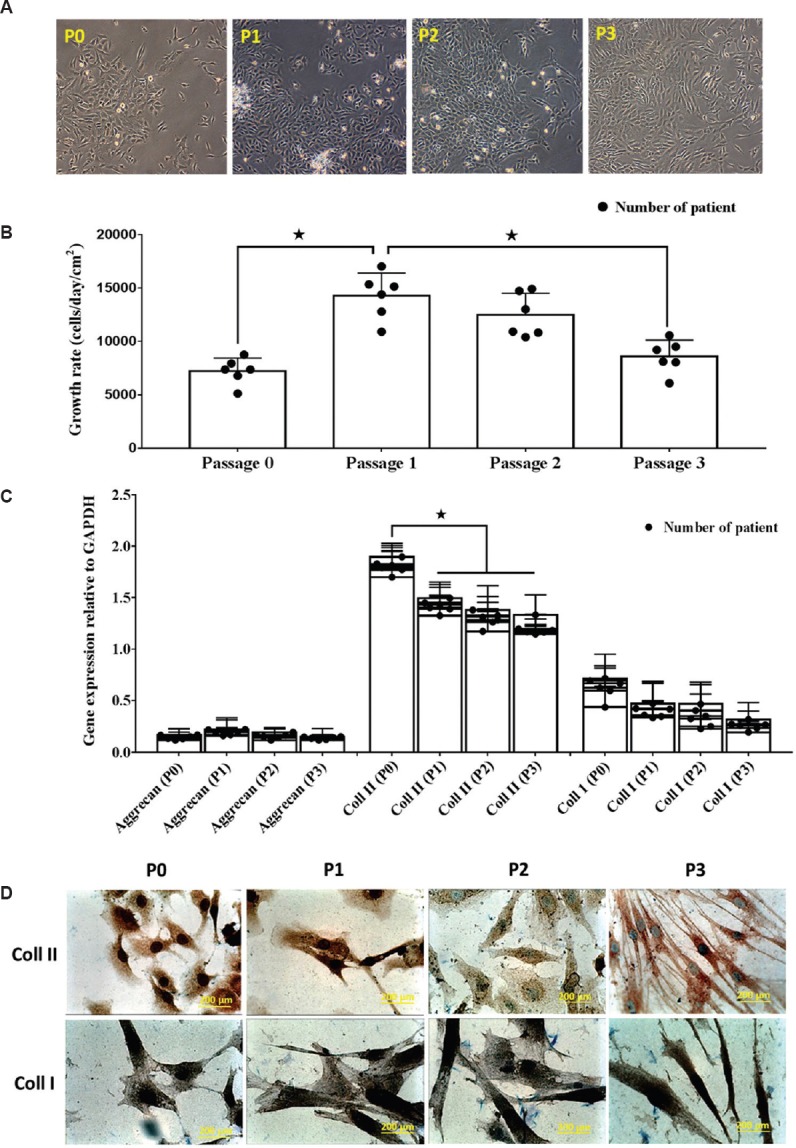
(A). Photomicrographs (×40) of human osteoarthritis chondrocytes at day 7 from P0, P1, P2 and P3. After seven days of in vitro culture, all chondrocytes demonstrated polygonal morphology. (B) The growth rate of cultured chondrocytes decreased over successive passages *P<0.05. (C) Gene expression for collagen types I and II reduced after several passages, whereas aggrecan gene expression was consistently expressed. (D) Prominent staining of collagen type II in P0 detected by immunocytochemistry, which became weaker by P3. Collagen type I staining was observed for all passages.
Cell cycle analysis: The results from the cell cycle analysis suggested that cultured OAC maintained 100 per cent diploidy with no evidence of aneuploidy, haploidy or tetraploidy (Fig. 3A). The proportion of cells in G1 at P0 was 96.53±0.67 per cent, which significantly decreased to 88.1±0.80 per cent at P3 (P<0.05). The G2 phase index significantly increased from 1.53 per cent at P0 to 5.2 per cent at P3 (P<0.05). The index for the S phase was slightly increased at P3 compared to other passages; however, this was not significant (Fig. 3B).
Fig. 3.

(A) Cytofluorometry with propidium iodide staining showing diploid DNA content in cultured chondrocytes, suggesting that cultured chondrocytes maintained 100 per cent diploidy in the defined chondrocyte growth medium, with no evidence of aneuploidy, haploidy or tetraploidy. The X-axis represents the relative fluorescence intensity proportional to the DNA content. (B) Percentage of cells in the G1, G2 and S phases over successive passages. S phase index indicated that chondrocytes were actively proliferating during monolayer cultivation P<0.05 compared to P0.
Effect of cell density on in vitro OACF constructs: The OACF constructs were prepared by culturing 1×107 or 3×107 cells/ml with PDF. Gross observation revealed that all constructs formed stable 3D cartilage-like structures, with a smooth, white and glossy appearance (Fig. 4A and 4B). However, the texture, size and shape of the constructs changed as culture progressed. As shown in Fig. 4C, the diameter of the OACF constructs decreased with time for both cell densities, but the value was comparatively higher for high-density constructs than low-density constructs. The diameter of high-density OACF constructs was significantly (P<0.05) higher at days 14 (8.85±0.09 mm) and 21 (6.98±0.06 mm) compared to that of low-density constructs. There were no significant differences of diameter from patient to patient in each evaluated time point. In vitro Safranin O staining of both OACF constructs at day 7 demonstrated a homogeneous distribution of cells, with no sign of lacunae and minor accumulation of proteoglycan (Fig. 4A and B). At days 14 and 21, however, good distribution of cells embedded within the basophilic ground substance was detected, with matrix protein accumulation observed in both groups. To confirm the spatial distribution of cells in the constructs, fluorescence-labelled chondrocytes were used to create low- and high-density OACF constructs. In both OACF constructs, the number of cells increased with time, also demonstrating homogeneous distribution (Fig. 4D and E). In low-density OACF constructs, cells were found to form loose aggregates. In contrast, chondrocytes in high-density OACF constructs exhibited dense aggregates at day 21, consisting of both rounded and spindle-shaped cells. In addition, the chondrocytes formed a thick layer at the periphery of the OACF constructs.
Fig. 4.
(A&B) In vitro osteoarthritis chondrocyte-fibrin constructs derived from cells seeded at low and high densities at days 7, 14 and 21, showing cells of various shapes and sizes. Positive Safranin O (magnification: 200μm) staining confirmed the presence of cartilage-specific proteoglycan (C). The diameter of the high-density seeding construct was significantly bigger compared to that of the low-density construct at days 14 and 21 (*P<0.05), with a difference of 2.1 and 1.6 mm, respectively (D&E). Image showing the cell distribution and aggregates in the fibrin construct seeded with chondrocytes at low and high densities at different time points (day 7, 14 and 21).
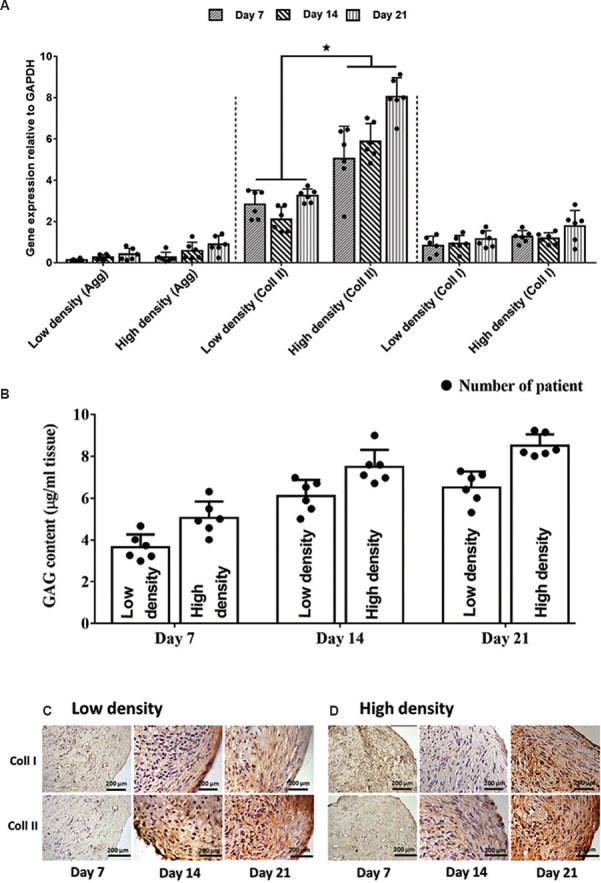
Production of ECM by OACF constructs: The production of type I and II collagen and aggrecan was evaluated to determine the quality of the in vitro OACF constructs. Low- and high-density OACF constructs demonstrated the expression of aggrecan and type I and II collagen (Fig. 5A). However, the expression of type II collagen was comparatively higher in both low- and high-density constructs compared to that of type I collagen and aggrecan. In addition, the high-density OACF constructs showed significantly higher expression of type II collagen compared to low-density OACF constructs at each time point (P<0.05). The expression of type II collagen in high-density OACF constructs showed a significant increase with increasing culture time, with type II collagen expression at day 21 being 1.71- and 1.44-times higher than that at days 7 and 14, respectively. In the low-density seeded OACF constructs, type II collagen was expressed almost equally at each time point. In contrast, the expression for aggrecan and collagen type I showed no significant differences between the low- and high-density OACF constructs. No significant difference was found between patients for gene expression either within time point or seeding density. Immunostaining observation revealed that, at day 7, both groups (Fig. 5B and C) demonstrated low expression of type II collagen. However, by days 14 and 21, higher type II collagen expression was observed in both OACF constructs. Unlike type II collagen, the expression of type I collagen did not differ throughout the culture period. In addition, as shown in Fig. 5B proteoglycan synthesis gradually increased with increasing cultured periods for both OACF constructs. High-density OACF constructs produced more proteoglycan compared to that of low-density constructs; however, no significant differences were observed.
Fig. 5.
(A) Significant (*P<0.05) expression of collagen type II (coll II), particularly in high-density seeded cells. All evaluated genes showed progressive expression throughout the culture period. Constructs showed strong expression of collagen type II in the pericellular matrix area and throughout the extracellular matrix, confirming an immature cartilage phenotype at days 14 and 21 for both groups glyceraldehyde phosphate dehydrogenase (GAPDH) and glycosaminoglycan (GAG) (B). Proteoglycan deposition showed no significant differences between constructs (C&D). Immunohistochemistry staining. Collagen type I and II (coll I & II) deposition was also detected in both constructs at day 7, 14 and 21 in low and high seeding chondrocytes fibrin construct.
Discussion
Several studies have demonstrated that OACs have similar characteristics as normal cartilage cells and proven to have more mesenchymal progenitor cell (MPC) properties2,14,15. One study has shown a significant increase of CD105+/CD166+ MPC markers in OAC15. However, no concrete evidence was reported on the effect of cell density of abnormal chondrocytes on cartilage formation. Thus, the present study was aimed to evaluate the effect of OAC seeding densities in the fibrin scaffold for cartilage regeneration.
In cartilage tissue engineering, the major challenge is to produce enough chondrocytes with limited starting materials and dedifferentiation of cultured chondrocytes. A previous study showed that combination of transforming growth factor-β2 (TGF-β2), insulin-like growth factor-1 (IGF-1) and basic fibroblast growth factor (bFGF) effectively improved the proliferation of chondrocytes and cartilage matrix production16. The use of IGF-1 and bFGF induced cell proliferation but was less effective without TGF-β. The use of a defined CGM containing ITS, bFGF, IGF-1 and TGF-2 supports the growth and chondrogenic properties of OAC. Based on the cell cycle analysis, it was found that aneuploidy or tetraploidy of cultured OAC was consistent with that of previous cytofluorometry studies that used normal auricular chondrocytes17. Although rapid cell expansion was observed, the results of the cell cycle analysis suggested that there were no changes in the cell cycle properties.
In this study, fibrin derived from human plasma was used as a scaffold to form stable constructs. It fulfils the criteria of being controllable degradation rate, non-toxic, biocompatible and stimulates cell proliferation and ECM production9. As shown in histology and immunofluorescent staining, fibrin held the embedded cells together, in addition to promoting cell attachment, proliferation and ECM secretion. It was similarly observed during the earlier evaluation of fibrin as a natural 3D scaffold that promotes cartilage regeneration18,19,20. It was hypothesized that the cell density would facilitate cell-cell and cell-matrix interactions, in addition to promoting chondrogenesis within the transplanted cells. In this study, two different densities, 1×107 and 3×107 cells/ml, of OAC were encapsulated within a fibrin gel. The in vitro OACF constructs for both high- and low-seeding densities demonstrated a similar gene expression pattern to that of chondrocytes following serial passage. Type II collagen was highly expressed in the high-density OACF constructs when compared to the low-density constructs, suggesting that the high-density seeding constructs favoured the formation of a cartilage-specific phenotype. Although both low- and high-density constructs were of the same size, histological analysis showed that the high-density OACF constructs were superior, displaying histologic architectural features consistent with normal cartilage, and the results were consistent with a previous study21.
The expression of aggrecan and collagen type I in low- and high-density seeding constructs may be due to the accumulation of protein during the development of articular cartilage. In this study, the engineered cartilage was newly formed, and therefore showed characteristics of immature tissue. Chondrocytes adopt a fibroblast-like morphology in monolayer culture, accompanied by increased proliferation and an altered phenotype22. This might have occurred to a certain degree but was not sufficient to affect the properties of chondrocytes used as the cell source. This could explain why the constructs were stained positive for Safranin O. After 21 days of in vitro culture, there was no difference in GAG quality between constructs with a cell seeding density of 3×107 and 1×107 cells/ml. The diameter was slightly larger for the high-density constructs compared to the low-density constructs.
Overall, our results support the hypothesis that chondrogenic process starts in the areas of cell condensation. Cell-scaffold construct showed homogeneous cell distribution, and chondrogenic activity was found to be higher with a seeding density of 3×107 cells/ml. In vitro experiments with OAC seeded to human fibrin showed a linear relationship between biological activity and the number of seeding cells, and it was similarly claimed by other researchers23,24. In conclusion, higher seeding density (3×107 cells/ml) significantly enhanced ECM production and biochemical properties of human OAC on fibrin scaffolds compared to that of lower seeding density.
Footnotes
Financial support & sponsorship: This work was funded by grants from Universiti Kebangsaan Malaysia (AP-2013-015) and the Malaysian Ministry of Science and Technology Science Fund (02-01-02-SF0961).
Conflicts of Interest: None.
References
- 1.Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4:269–85. doi: 10.1177/1759720X12448454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li H, Davison N, Moroni L, Feng F, Crist J, Salter E, et al. Evaluating osteoarthritic chondrocytes through a novel 3-dimensional in vitro system for cartilage tissue engineering and regeneration. Cartilage. 2012;3:128–40. doi: 10.1177/1947603511429698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brittberg M LA. Treatment of deep cartilage defects in the knee autologous chondrocytes transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 4.Freed LE, Vunjak-Novakovic G. Culture of organized cell communities. Adv Drug Deliv Rev. 1998;33:15–30. doi: 10.1016/s0169-409x(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 5.Phull AR, Eo SH, Abbas Q, Ahmed M, Kim SJ. Applications of chondrocyte-based cartilage engineering: An overview. Biomed Res Int. 2016;2016:1879837. doi: 10.1155/2016/1879837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170–8. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 7.Tallheden T, Bengtsson C, Brantsing C, Sjögren-Jansson E, Carlsson L, Peterson L, et al. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther. 2005;7:R560–8. doi: 10.1186/ar1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bensaïd W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Meng H, Liu Y, Lee BP. Fibrin gel as an injectable biodegradable scaffold and cell carrier for tissue engineering. Sci World J. 2015;2015:685690. doi: 10.1155/2015/685690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amable PR Teixeira MV, da Cruz Pacheco I, Correa do Amaral RJ, Granjeiro JM, Borojevic R CRB. Platelet-rich paslam preparation for regnerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther. 2013;4:1–13. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan KK, Tan GH, Shamsul BS, Chua KH, Ng MHA, Ruszymah BHI, et al. Bone graft substitute using hydroxyapatite scaffold seeded with tissue engineered autologous osteoprogenitor cells in spinal fusion: early result in a sheep model. Med J Malaysia. 2005;60(Suppl C):53–8. [PubMed] [Google Scholar]
- 12.Ude CC, Shamsul Bin Sulaiman, Min-Hwei N, Hui-Cheng C, Ahmad J, Yahaya NM, et al. Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS One. 2014;9:e98770. doi: 10.1371/journal.pone.0098770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y, Tuan RS. Origin and function of cartilage stem/progenitor cells in osteoarthritis. Nat Rev Rheumatol. 2015;11:206–12. doi: 10.1038/nrrheum.2014.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50:1522–32. doi: 10.1002/art.20269. [DOI] [PubMed] [Google Scholar]
- 16.Oda T, Sakai T, Hiraiwa H, Hamada T, Nakashima M, Matsukawa T, et al. Orthopaedic Research Society (ORS 2014) Annual Meeting; 2014 March 15 - 18. New Orleans, Louisiana: Orthopaedic Research Society. (Poster No. 1285); Osteoarthritis-derived chondrocytes are promising source of cartilage-tissue engineering as the multipotent progenitors. [Google Scholar]
- 17.Talukdar S, Nguyen QT, Chen AC, Sah RL, Kundu SC. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials. 2011;32:8927–37. doi: 10.1016/j.biomaterials.2011.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khoshfetrat AB, Kino-Oka M, Takezawa Y, Yamamoto T, Sugawara K, Taya M. Seeding density modulates migration and morphology of rabbit chondrocytes cultured in collagen gels. Biotechnol Bioeng. 2009;102:294–302. doi: 10.1002/bit.22029. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi S, Meir A, Urban J. Effect of cell density on the rate of glycosaminoglycan accumulation by disc and cartilage cells in vitro . J Orthop Res. 2008;26:493–503. doi: 10.1002/jor.20507. [DOI] [PubMed] [Google Scholar]
- 20.Kamil SH, Woda M, Bonassar LJ, Novitsky YW, Vacanti CA, Eavey RD, et al. Normal features of tissue-engineered auricular cartilage by flow cytometry and histology: Patient safety. Otolaryngol Head Neck Surg. 2003;129:390–6. doi: 10.1016/S0194-59980300710-1. [DOI] [PubMed] [Google Scholar]
- 21.Munirah S, Samsudin OC, Aminuddin BS, Ruszymah BHI. Expansion of human articular chondrocytes and formation of tissue-engineered cartilage: A step towards exploring a potential use of matrix-induced cell therapy. Tissue Cell. 2010;42:282–92. doi: 10.1016/j.tice.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Dua R, Comella K, Butler R, Castellanos G, Brazille B, Claude A, et al. Integration of stem cell to chondrocyte-derived cartilage matrix in healthy and osteoarthritic states in the presence of hydroxyapatite nanoparticles. PLoS One. 2016;11:e0149121. doi: 10.1371/journal.pone.0149121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noriega SE, Hasanova GI, Schneider MJ, Larsen GF, Subramanian A. Effect of fiber diameter on the spreading, proliferation and differentiation of chondrocytes on electrospun chitosan matrices. Cells Tissues Organs. 2012;195:207–21. doi: 10.1159/000325144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruszymah BHI, Chua KH, Mazlyzam AL, Aminuddin BS. Formation of tissue engineered composite construct of cartilage and skin using high density polyethylene as inner scaffold in the shape of human helix. Int J Pediatr Otorhinolaryngol. 2011;75:805–10. doi: 10.1016/j.ijporl.2011.03.012. [DOI] [PubMed] [Google Scholar]