Abstract
This study examines and compares the walking function in contusion and distraction spinal cord injury (SCI) mechanisms. Moderate contusion and distraction SCIs were surgically induced between C5 and C6 in Sprague-Dawley male rats. The CatWalk system was used to perform gait analysis of walkway walking. The ladder rung walking test was used to quantify skilled locomotor movements of ladder rung walking. It was found that the inter-paw coordination, paw support, front paw kinematics, hind paw kinematics, and skilled movements were significantly different before and after contusion and distraction. Step sequence duration, diagonal support, forelimb intensity, forelimb duty cycle, forelimb paw angle, and forelimb swing speed were more greatly affected in distraction than in contusion at 2 weeks post-injury, whereas hindlimb stand was more greatly affected in contusion than in distraction at 8 weeks post-injury. After 8 weeks post-injury, diagonal coupling—variation, girdle coupling—variation, ipsilateral coupling—mean, forelimb maximum contact at, forelimb intensity, forelimb paw angle, and number of forelimb misplacements recovered to normal in contusion but not in distraction, whereas step sequence duration, ipsilateral coupling—variation, forelimb stand, forelimb duty cycle, hindlimb swing duration, hindlimb swing speed, and number of forelimb slips recovered to normal in distraction but not in contusion. Some of the behavioral outcomes, but not the others, were linearly correlated with the histological outcomes. In conclusion, walking deficits and recovery can be affected by the type of common traumatic SCI.
Keywords: Ambulation, locomotion, behavior, CatWalk, ladder rung
Introduction
“Can I walk?” is a frequent question from patients after spinal cord injury (SCI).1,2 The walking recovery is currently known to be associated with many factors such as the severity of damage (eg, amount of intramedullary edema), the level of spinal cord lesion, the severity of sensory and motor function deficits, age, sex, and the presence of abnormal reflexes (eg, delayed plantar response) and syndromes (eg, central cord syndrome).2,3 The immobilization can lead to other health problems including bone loss and muscle atrophy.4 Walking is one of the top priorities of individuals with SCI.5 It is relatively easy to check and quantify, and we now have a fairly good understanding of the neuronal mechanisms generating the motor pattern.6,7 Consequently, walking function is commonly used to evaluate the neurological and behavioral deficits and test the efficacy of treatments for SCI.2,6,8,9
At present, we know very little about the effect of cause of the lesion on walking. Few studies have reported that the walking deficits and recovery between traumatic and non-traumatic SCIs are comparable.2 Moreover, a study has shown that prolonged compression following contusion increases tissue damage and worsens functional recovery when compared with contusion alone.10 However, to the authors’ knowledge, no study has critically investigated the effect of different types of common traumatic SCI on walking.
Contusion and distraction are 2 common types of traumatic SCI.11-16 They are also 2 morphologically distinct SCI mechanisms; in comparison, contusion is a focal injury that causes localized membrane compromise, node of Ranvier deformation, and lesion cavity, more severe loss of myelinated axons in the dorsal and ventral white matter, less uniform neuronal death in the dorsal horn, and cord atrophy, whereas distraction is a dispersed injury that causes widespread membrane compromise, node of Ranvier deformation, and lesion cavity, less severe loss of myelinated axons in the dorsal and ventral white matter, more uniform neuronal death in the dorsal horn, and tissue structural alternation17-20 Some of their behavioral outcomes (ie, open-field activity, forelimb locomotion, grooming function, grip strength) have been analyzed, and difference was also observed; the grip strength after C5-C6 contusion was initially weakened followed by a gradual recovery to normal, whereas the grip strength after C5-C6 distraction was weakened with no sign of recovery.19 It will benefit the patients, physicians, and researchers to know whether these 2 types of SCI lead to different walking deficits and recovery.
The aim of this study is to examine and compare the walking function after cervical contusion and distraction SCIs in rats.
Materials and Methods
Animals
The study was approved by the UBC Committee on Animal Care in accordance with the Guide to the Care and Use of Experimental Animals by the Canadian Council on Animal Care, and the raw data were generously provided by ICORD. The study was also approved by the Ethics Committee of the School of Biological Science and Medical Engineering of the BUAA. A total of 12 16-day-old male Sprague-Dawley rats were acquired for the experiment (n = 6 for contusion and distraction). They were housed 4 per cage on a 12-h reverse light cycle at 19°C to 21°C with 30% to 50% humidity, fed standard chow and water ad libitum, handled daily, and allowed to acclimate to the housing facilities for 5 days.
Behavioral tests
Rats were trained to perform 2 behavioral tasks for the next 17 days. Gait analysis of rats walking across a walkway was performed using the CatWalk system (Noldus Information Technology, Wageningen, The Netherlands).21 Skilled locomotor movements of rats walking across a custom ladder rung walking test apparatus (Figure 7) were quantified using the ladder rung walking test.22 Each task was administered at the same time of day during the dark cycle. During each training session, rats were placed at one end of the CatWalk walkway or the ladder rung walking test apparatus to learn to reach the other end, which was connected to a custom ramp with a 1-way door enabling normal and injured rats to return to their home cage safely and preventing them from coming back. In addition to the cagemates, food reward (puffed wheat) was available in the cage. Rung pattern was changed from session to session to prevent rats from learning the pattern as described in the literature.22 A total of 4 training sessions were completed for both tasks, and 5 runs were completed for each rat in each session. Their task performance was then recorded for the following 4 days and also 2, 4, 6, and 8 weeks post-injury.
Figure 7.
Representative behavior during walking on the ladder rungs. Images were extracted from the captured videos while analyzing a normal rat (A-G), a rat at 8 weeks after contusion (H-O), and a rat at 8 weeks after distraction (P-Y). The starting and finish lines were marked by the white stickers with a written arrow below the ladder rungs. Time measurement began after a limb passed the starting line and it ended after all 4 limbs passed the finish line.
Surgery
After baseline assessment of walking function, rats were allowed to rest for 3 days with puffed wheat and nutrition shake. They were 46 days old at the time of surgery. A standard surgical procedure for inducing moderate bilateral cervical contusion and distraction SCIs was performed in rats.19 A dorsal midline incision was made from approximately C2 to C7. For contusion, the spinal cord between C5 and C6 was exposed by laminectomy. Custom clamps held the transverse processes at C4-C7 on a stereotaxic frame. A linear actuator applied a small preload (0.03 N) to the surface of the dura mater with a 2-mm-diameter semi-spherical impactor between C5 and C6, retracted 6 mm above the dura mater, and accelerated into the spinal cord to 1.6 mm with a peak velocity of 1.2 m/s. For distraction, the posterior ligaments between C5 and C6 were transected, and a C5/6 facetectomy was performed. A pair of custom clamps, respectively, held the transverse processes at C4-C5 on the stereotaxic frame and the transverse processes at C6-C7 on the linear actuator. The linear actuator applied a small preload (2 N) to the spinal column and acutely translated C6-C7 to 5.6 mm caudal from C4-C5 with a peak velocity of 1.3 m/s. A custom implant was then used to hold the transverse processes at C5-C6.
Rats were caged individually with oats as bedding and injected daily with 2 doses of buprenorphine (0.05 mg/kg, SC) and Ringer’s lactate (10 mL, SC) for the first 2 days after surgery. Nutrition shake was provided to them for the rest of the experiment. Additional Ringer’s lactate injection, hand-feeding with nutrition shake, and manual bladder expression were performed when necessary.
Data processing
The recorded behavioral data were processed by 2 observers who were blinded to the study design. Paw prints acquired by the CatWalk system were assigned labels (Figure 2). The first and last paw prints that triggered and terminated the data acquisition were neglected to avoid incomplete paw prints. Labeling started with the second paw print and continued until each of the 4 paws had been used at least 3 times for stepping. A run was not used for the analysis when it had less than 3 consecutive step cycles, a labeled paw touched the edge, or the rat slowed down or stopped. Videos captured in the ladder rung walking test were observed frame by frame at 30 frames/s using Adobe Premiere Pro (Adobe Systems, San Jose, CA, USA) to count the number of forelimb misplacements, forelimb slips, and hindlimb slips and measure the crossing time. Count values from the left and right sides were averaged. Any run with a supporting limb retreating backward was not used for the analysis. Only 3 of the 5 runs from each recording session were analyzed in both tasks.
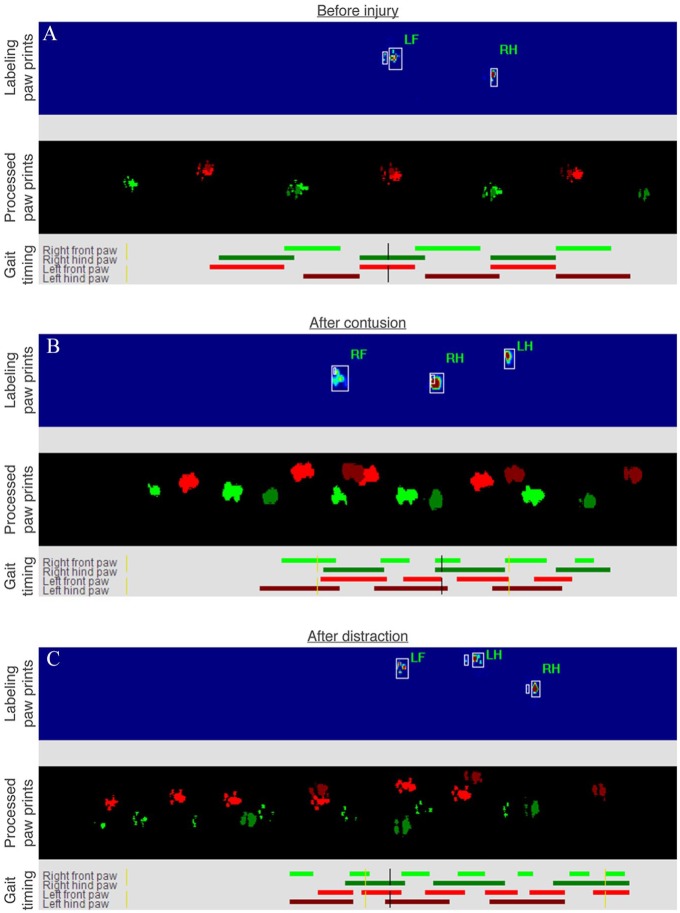
Figure 2.
Representative labeled paw prints and gait timing during walking on the walkway. Images were extracted from the CatWalk system while analyzing a normal rat (A), a rat at 8 weeks after contusion (B), and a rat at 8 weeks after distraction (C). Note that the labeling paw prints and the processed paw prints show the animals moving from right to left and the gait timing shows the animals moving from left to right by default.
Statistical analysis
The obtained behavioral parameters before and after contusion as well as distraction were compared using the Friedman test (P < .05) followed by the Wilcoxon signed rank test (2-tailed, P < .05). The differences between the 2 SCI mechanisms were analyzed using the Kruskal-Wallis test (P < .05) followed by the Mann-Whitney U-test (2-tailed, P < .05). Moreover, the correlations between the presently analyzed behavioral parameters and the previously published histological data from the same experiments were assessed using the Pearson correlation coefficient (r) (2-tailed, P < .05).19 Analysis was performed using SPSS (IBM, Chicago, IL, USA).
Results
Coordination
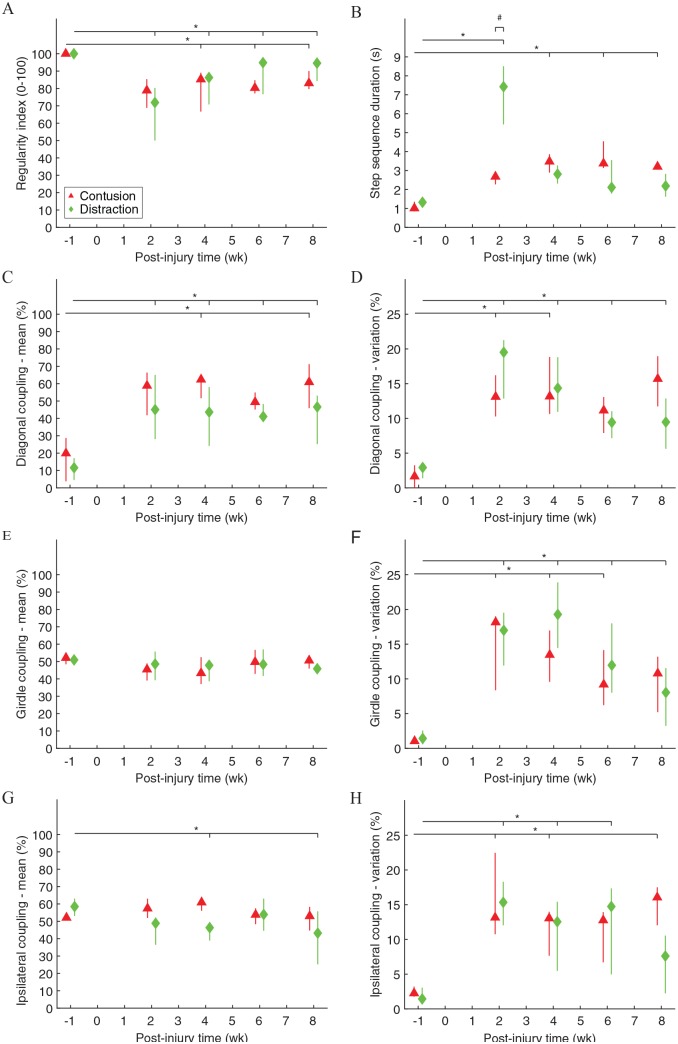
Inter-paw coordination of rats walking across the CatWalk walkway was significantly different before and after contusion and distraction (Figure 1). The step sequence patterns were fully normal before injury, but they were significantly irregular after the SCIs (Figure 1A). The step sequence duration became significantly longer after the SCIs, although it recovered to normal after 4 weeks post-injury in distraction (Figure 1B). The inter-paw coupling characteristics had significantly changed after the SCIs (Figure 1C to H). Noticeably, post-injury, the timing of initial contact of a front paw followed by initial contact of the hind paw on the opposite side of the body was dramatically delayed (Figure 1C), and the coupling among step cycles was highly variable (Figure 1D, F, and H). Changes in inter-paw coordination could be clearly observed by comparing the gait timing before and after the SCIs (Figure 2A vs B and C). Furthermore, the step sequence duration was significantly different between the 2 SCI mechanisms, but it was only temporary at 2 weeks post-injury (Figure 1B).
Figure 1.
Inter-paw coordination during walking on the walkway. Regularity index expressed the number of normal step sequence patterns (ie, each of the 4 paws was used exactly once in a step cycle) relative to the total number of paw placements (A). Step sequence duration was the time needed to complete a step cycle (ie, each of the 4 paws was used at least once) (B). Coupling expressed the temporal relationship between the placement of 2 paws within a step cycle (C, E, G) and among step cycles (D, F, H). Diagonal, girdle, and ipsilateral couplings were measured from the LF-RH, LF-RF, and LF-LH relationships, respectively. Data are presented as median with quartiles and offset horizontally for clarity. LF indicates left front paw; LH, left hind paw; RF, right front paw; RH, right hind paw.
*Significantly different from normal (P < .05); #significantly different between injury types (P < .05).
Support
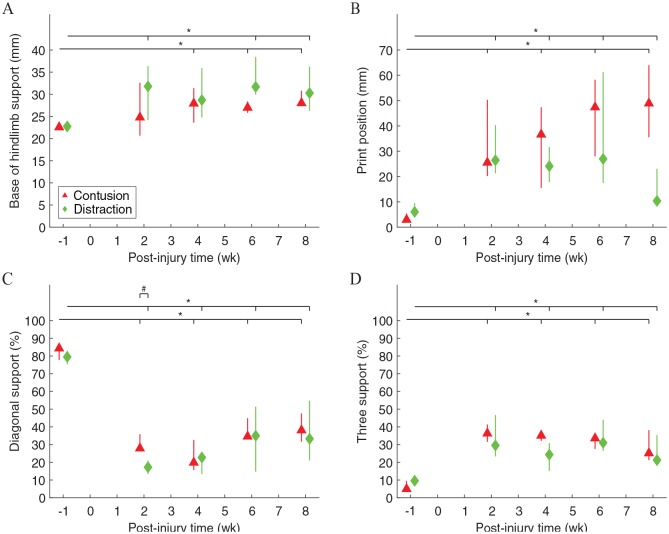
Paw support of rats walking across the CatWalk walkway was significantly different before and after contusion and distraction (Figure 3). The base of support between the hind paws became significantly wider after the SCIs (Figure 3A). Similarly, the print positions between the ipsilateral front and hind paws were significantly wider after the SCIs (Figure 3B). Changes in inter-paw support width could be clearly observed by comparing the paw prints before and after the SCIs (Figure 2A vs B and C). Paw support was mostly performed diagonally with a small chance of using 3 paws at the same time before injury, but diagonal support and three support significantly decreased and increased, respectively, after the SCIs (Figure 3C and D). These changes could be clearly observed by comparing the gait timing before and after the SCIs (Figure 2A vs B and C), and the three support appeared to be more frequently performed by the hind paws and a front paw than by the front paws and a hind paw (Figure 2B and C). Furthermore, the diagonal support was significantly different between the 2 SCI mechanisms, but it was only temporary at 2 weeks post-injury (Figure 3C).
Figure 3.
Paw support during walking on the walkway. Base of hindlimb support was the average width between the hind paws (A). Print positions were the average distance between the position of a hind paw and the position of the previously placed front paw on the same side of the body in a step cycle (B). Diagonal support was the percentage of using 2 diagonal paws (LF-RH, RF-LH) simultaneously for support (C). Three support was the percentage of using 3 paws (LF-LH-RH, RF-LH-RH, LF-LH-RH, RF-LH-RH) simultaneously for support (D). Data are presented as median with quartiles and offset horizontally for clarity. LF indicates left front paw; LH, left hind paw; RF, right front paw; RH, right hind paw.
*Significantly different from normal (P < .05); #significantly different between injury types (P < .05).
Front paw movements
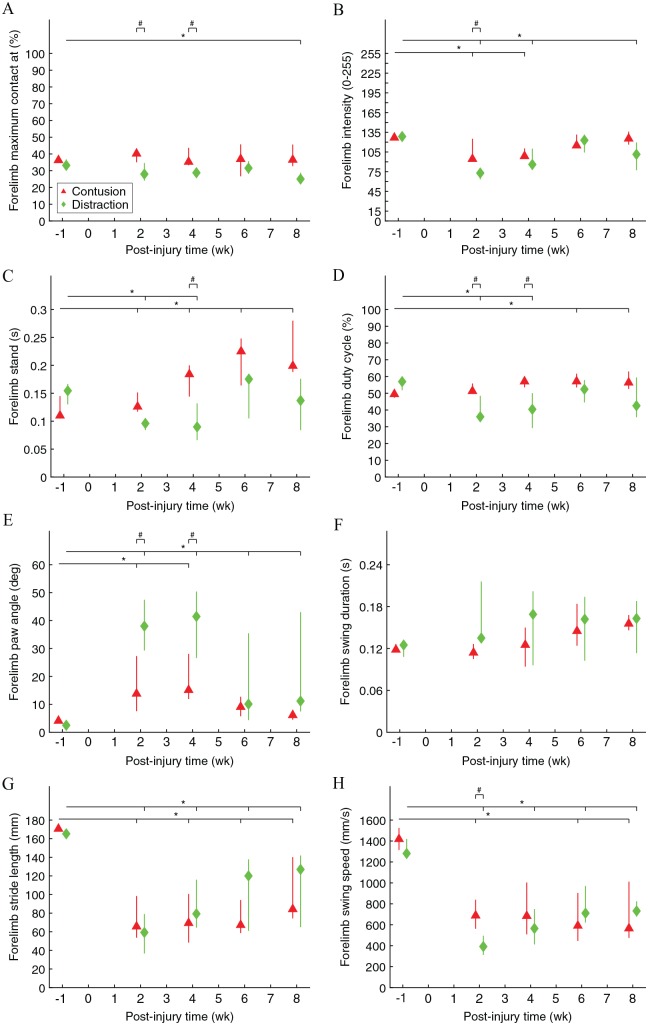
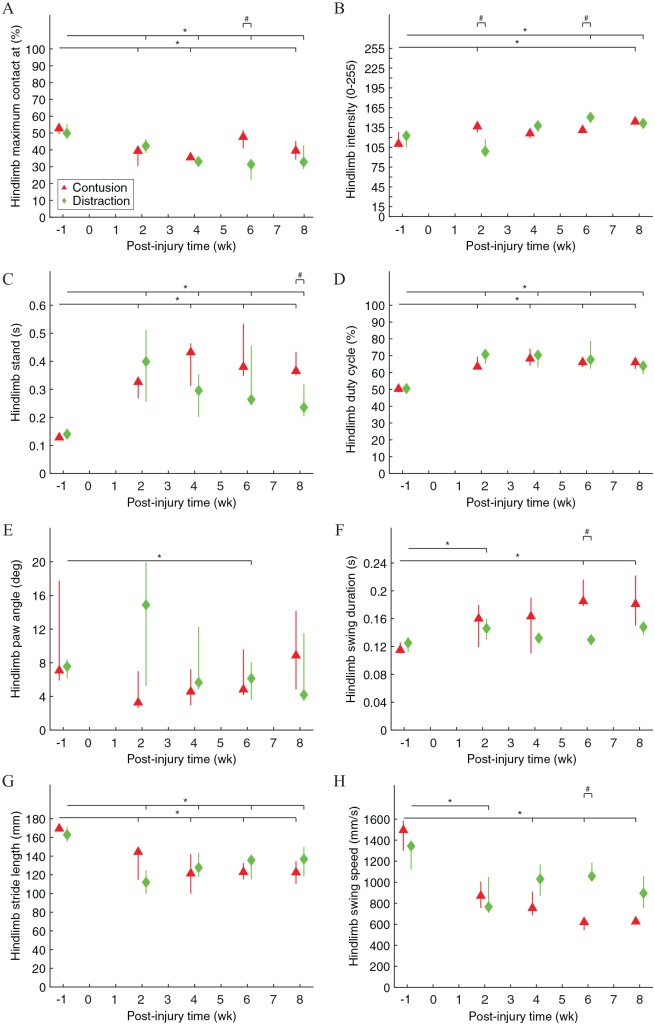
Front paw kinematics of rats walking across the CatWalk walkway were significantly different before and after contusion and distraction (Figure 4). The relative time that the front paws reached maximum contact area with the floor during a stance phase was significantly earlier after distraction (Figure 4A). The pressure that the front paws applied to the floor as they reached maximum contact area was significantly lower after the SCIs, although it recovered to normal after 6 weeks post-injury in contusion (Figure 4B). The forelimb stand time and duty cycle were significantly higher after the SCIs, although it recovered to normal after 6 weeks post-injury in distraction (Figure 4C and D). The front paw angle at initial contact and lift-off was significantly rotated from parallel after the SCIs, although it recovered to normal after 6 weeks post-injury in contusion (Figure 4E). The forelimb swing duration was similar, whereas the forelimb stride length was significantly shorter after the SCIs, and, as a result, the forelimb swing speed was significantly slower after the SCIs (Figure 4F to H). Furthermore, differences in front paw kinematics existed between the 2 SCI mechanisms, but they were only temporary at 2 and 4 weeks post-injury (Figure 4A to E and H).
Figure 4.
Front paw movements during walking on the walkway. “Forelimb maximum contact at” was the average percent time that a front paw spent to achieve maximum contact area with the floor during a stance phase (A). Forelimb intensity was the average brightness that a front paw generated in the system as it achieved maximum contact area with the floor (B). Forelimb stand was the average standing time of a front paw during a stance phase (C). Forelimb duty cycle was the average percent time that a front paw spent on the stance phase in a step cycle (D). Forelimb paw angle was the average rotated angle with respect to the parallel orientation of a front paw at initial contact and lift-off (E). Forelimb swing duration was the average travel time of a front paw during a swing phase (F). Forelimb stride length was the average travel distance of a front paw during a swing phase (G). Forelimb swing speed was the average travel speed of a front paw during a swing phase (H). Data are presented as median with quartiles and offset horizontally for clarity.
*Significantly different from normal (P < .05); #significantly different between injury types (P < .05).
Hind paw movements
Hind paw kinematics of rats walking across the CatWalk walkway were significantly different before and after contusion and distraction (Figure 5). The relative time that the hind paws reached maximum contact area with the floor during a stance phase was significantly earlier after the SCIs (Figure 5A). The pressure that the hind paws applied to the floor as they reached maximum contact area was significantly higher after the SCIs (Figure 5B). The hindlimb stand time and duty cycle were significantly higher after the SCIs (Figure 5C and D). The hind paw angle at initial contact and lift-off was significantly rotated from parallel particularly at 6 weeks after distraction (Figure 5E). The hindlimb swing duration and stride length were significantly longer and shorter, respectively, after the SCIs, and, as a result, the hindlimb swing speed was significantly slower after the SCIs, although the hindlimb swing duration and speed recovered to normal after 4 weeks post-injury in distraction (Figure 5F to H). Furthermore, differences in hind paw kinematics existed between the 2 SCI mechanisms and, particularly, hindlimb stand time was the only difference at 8 weeks post-injury (Figure 5A to C, F, and H).
Figure 5.
Hind paw movements during walking on the walkway. “Hindlimb maximum contact at” was the average percent time that a hind paw spent to achieve maximum contact area with the floor during a stance phase (A). Hindlimb intensity was the average brightness that a hind paw generated in the system as it achieved maximum contact area with the floor (B). Hindlimb stand was the average standing time of a hind paw during a stance phase (C). Hindlimb duty cycle was the average percent time that a hind paw spent on the stance phase in a step cycle (D). Hindlimb paw angle was the average rotated angle with respect to the parallel orientation of a hind paw at initial contact and lift-off (E). Hindlimb swing duration was the average travel time of a hind paw during a swing phase (F). Hindlimb stride length was the average travel distance of a hind paw during a swing phase (G). Hindlimb swing speed was the average travel speed of a hind paw during a swing phase (H). Data are presented as median with quartiles and offset horizontally for clarity.
*Significantly different from normal (P < .05); #significantly different between injury types (P < .05).
Skills
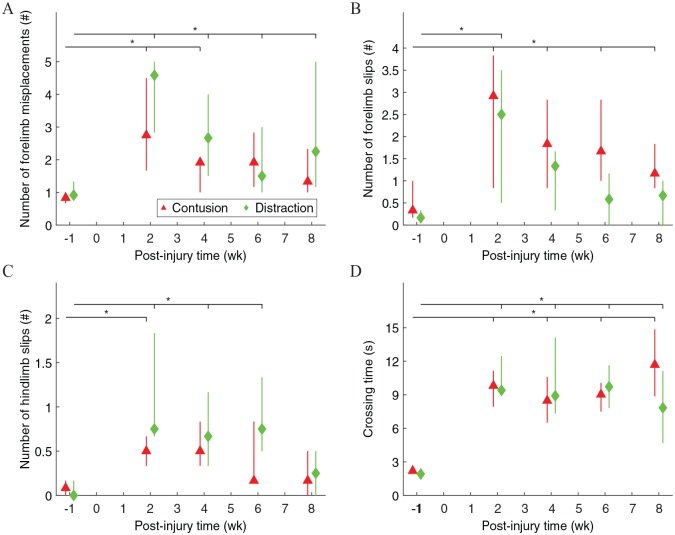
Skilled locomotor movements of rats walking across the ladder rung walking test apparatus were significantly different before and after contusion and distraction (Figure 6). The number of forelimb misplacements was significantly higher after the SCIs, although they recovered to normal after 6 weeks post-injury in contusion (Figure 6A). The number of forelimb slips was significantly higher after the SCIs, although they recovered to normal after 4 weeks post-injury in distraction (Figure 6B). The number of hindlimb slips was significantly higher after the SCIs, but the effects were only temporary (Figure 6C). The crossing time was significantly longer after the SCIs (Figure 6D).
Figure 6.
Skilled locomotor movements during walking on the ladder rungs. A misplacement was counted when a forelimb was placed on a rung with any part of the limb other than the midportion of the palm for support (A). A slip was counted when a limb fell between rungs (B, C). Measurement of the crossing time began after a limb passed the starting line and it ended after all 4 limbs passed the finish line (D). Data are presented as median with quartiles and offset horizontally for clarity.
*Significantly different from normal (P < .05); #significantly different between injury types (P < .05).
The approach that rats walked across the ladder rung walking test apparatus was noticeably different before injury, after contusion, and after distraction (Figure 7). Before injury, when rats were placed at one end of the apparatus, they immediately moved to pass the starting line (Figure 7A and B). They walked quickly, and it took a small amount of time for the 4 limbs to completely pass the starting line (Figure 7C). The step sequence patterns were almost always normal and the paw support was mostly performed diagonally. It can be seen from Figure 7D that the right forelimb swing was followed by the left hindlimb swing during the stance phase of the other 2 limbs, and it can be seen from Figure 7E that the left forelimb swing was followed by the right hindlimb swing during the stance phase of the other 2 limbs. As the rats reached the finish line, they jumped to pass it (Figure 7F and G). After contusion, when rats were placed at one end of the apparatus, they sometimes stayed just behind the starting line for a while before they decided to move (Figure 7H and I). They walked carefully, and it took a large amount of time for the 4 limbs to completely pass the starting line (Figure 7J). The step sequence patterns were almost always abnormal and the paw support was mostly performed non-diagonally; usually, both forelimbs reached out while both hindlimbs were standing until the forelimbs held onto a rung, and then the hindlimbs moved forward one at a time (like using the forelimbs as a walker; Figure 7K to M). As the rats reached the finish line, they walked past it with the same speed (Figure 7N and O). After distraction, when the rats were placed at one end of the apparatus, they sometimes stayed just behind the starting line for a short time before they decided to move (Figure 7P and Q). They also walked carefully, and it took a large amount of time for the 4 limbs to completely pass the starting line (Figure 7R). The step sequence patterns were sometimes normal and the paw support was occasionally diagonal, because the forelimbs reached out more frequently than normal. Figure 7S shows the beginning of a normal step sequence pattern, Figure 7T shows that the right forelimb swing was followed by the left hindlimb swing during the stance phase of the other 2 limbs, Figure 7U shows the left forelimb swing during the stance phase of the other 3 limbs, Figure 7V shows the right hindlimb swing during the stance phase of the other 3 limbs, and Figure 7W shows an additional left forelimb swing that made the next step sequence pattern abnormal. As the rats reached the finish line, they often walked past it with an increasing speed (Figure 7X and Y).
Histological correlations
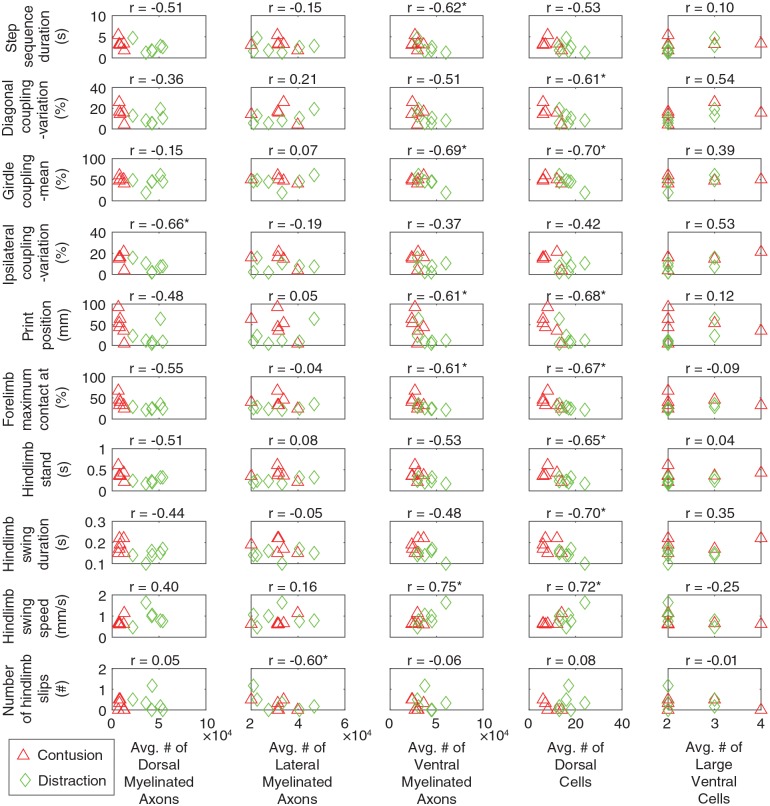
Among the 32 behavioral parameters that were obtained in this study for walking function after the SCIs (Figures 1 and 3 to 6), 10 were significantly linearly correlated with the histological parameters (Figure 8). Interestingly, the number of surviving large cells in the ventral horn had no linear correlation with any of these behavioral parameters.
Figure 8.
Scatterplots showing the relation between the behavioral and histological parameters evaluated by Pearson correlation coefficient. The behavioral parameters were obtained at 8 weeks after the SCIs. Only the behavioral parameters that were significantly correlated with at least one of the histological parameters are shown. In other words, the other behavioral parameters in Figures 1 and 3 to 6 that are not listed here had no strong correlation with any of the histological parameters. The histological parameters have been published previously.19 The average numbers of dorsal, lateral, and ventral myelinated axons were the average values between 5 mm rostral and caudal in the various white matter regions. The average numbers of dorsal and large ventral cells were the average values, respectively, between 1.0 mm rostral and 0.6 mm caudal and between 2.2 mm rostral and 1.6 mm caudal to the epicenter in the horn areas. These ranges to the epicenter were where histological differences existed across the SCI mechanisms. SCI indicates spinal cord injury.
*Significantly correlated (P < .05).
Discussion
In this study, differences in walking deficits were found between the contusion and distraction SCI mechanisms, and they changed over time. For example, the walking deficits tended to be more severe in distraction than in contusion at 2 weeks post-injury, as the behavioral parameters (ie, step sequence duration, diagonal support, forelimb intensity, forelimb duty cycle, forelimb paw angle, forelimb swing speed) were more greatly affected in distraction than in contusion during that time. Reduced intensity and duty cycle could be a sign of neuropathic pain that occurred for a short time after SCI.23 In contrast, the walking deficits tended to be more severe in contusion than in distraction at 8 weeks post-injury, as a behavioral parameter (ie, hindlimb stand) was more greatly affected in contusion than in distraction during that time. Stand duration of the hindlimbs was previously shown to increase after moderate SCI in rats.24 These results indicate that walking deficits can be affected by the type of SCI and the time post-injury.
In addition, differences in walking recovery existed between the contusion and distraction SCI mechanisms. After 8 weeks post-injury, some behavioral parameters (ie, diagonal coupling—variation, girdle coupling—variation, ipsilateral coupling—mean, forelimb maximum contact at, forelimb intensity, forelimb paw angle, number of forelimb misplacements) recovered to normal in contusion but not in distraction, whereas some behavioral parameters (ie, step sequence duration, ipsilateral coupling—variation, forelimb stand, forelimb duty cycle, hindlimb swing duration, hindlimb swing speed, number of forelimb slips) recovered to normal in distraction but not in contusion. Increased support is generally needed for walking after SCI, which was reflected in the decreased diagonal support and increased three support after contusion and distraction. Not surprisingly, rats with contusion SCI had an increased stand duration and duty cycle of the forelimbs to improve support, which provided more time for the hindlimbs to swing slowly, and, as a result, step sequence duration was increased. However, rats with distraction SCI were previously shown to have weak forelimb grip strength.19 Therefore, their forelimbs were likely only able to achieve early maximum contact with low applied pressure and unable to maintain parallel paw angle. The 2 different walking approaches might then lead to different recovery outcomes for the inter-paw coupling and ladder rung crossing skills. These results indicate that walking recovery can be affected by the type of SCI.
Moreover, it was found that some behavioral and histological parameters were linearly correlated with each other, but many of them had no linear correlation. Walking is normally governed by a highly complex system involving the central pattern generators, descending pathways, and sensory feedback in many animals.6 SCI disrupts the system, and even though a therapy is not intentionally given to rats, they can train themselves in the cage that further modifies the system.25 These results reinforce the idea that accurate and precise relationships of the behavioral and histological outcomes after different SCIs will require a much larger study with multivariate statistical analysis for better understanding.19
In conclusion, walking deficits and recovery can be affected by the type of common traumatic SCI, which should be taken into consideration when using walking function for medical evaluation and treatment development.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (project no. 81771347). The raw data was provided by ICORD, UBC.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: YG, HH, and KC conceived and led the project. KC performed the experiments. All authors processed the data. YG, HH, and KC analyzed the data. All authors wrote the paper.
ORCID iD: Kinon Chen  https://orcid.org/0000-0003-4539-3285
https://orcid.org/0000-0003-4539-3285
References
- 1. Subbarao JV. Walking after spinal cord injury. Goal or Wish? West J Med. 1991;154:612-614. [PMC free article] [PubMed] [Google Scholar]
- 2. Scivoletto G, Tamburella F, Laurenza L, Torre M, Molinari M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front Hum Neurosci. 2014;8:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dietz V, Nakazawa K, Wirz M, Erni T. Level of spinal cord lesion determines locomotor activity in spinal man. Exp Brain Res. 1999;128:405-409. [DOI] [PubMed] [Google Scholar]
- 4. Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simpson LA, Eng JJ, Hsieh JT, Wolfe DL. The health and life priorities of individuals with spinal cord injury: a systematic review. J Neurotrauma. 2012;29:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fouad K, Pearson K. Restoring walking after spinal cord injury. Prog Neurobiol. 2004;73:107-126. [DOI] [PubMed] [Google Scholar]
- 7. Lam T, Noonan VK, Eng JJ. A systematic review of functional ambulation outcome measures in spinal cord injury. Spinal Cord. 2008;46:246-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barbeau H, Ladouceur M, Norman KE, Pepin A, Leroux A. Walking after spinal cord injury: evaluation, treatment, and functional recovery. Arch Phys Med Rehabil. 1999;80:225-235. [DOI] [PubMed] [Google Scholar]
- 9. Domingo A, Al-Yahya AA, Asiri Y, Eng JJ, Lam T. A systematic review of the effects of pharmacological agents on walking function in people with spinal cord injury. J Neurotrauma. 2012;29:865-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orr MB, Simkin J, Bailey WMet al. Compression decreases anatomical and functional recovery and alters inflammation after contusive spinal cord injury. J Neurotrauma. 2017;34:2342-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ergun A, Oder W. Pediatric care report of spinal cord injury without radiographic abnormality (SCIWORA): case report and literature review. Spinal Cord. 2003;41:249-253. [DOI] [PubMed] [Google Scholar]
- 12. Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21:1355-1370. [DOI] [PubMed] [Google Scholar]
- 13. Dumont RJ, Okonkwo DO, Verma Set al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254-264. [DOI] [PubMed] [Google Scholar]
- 14. Kriss VM, Kriss TC. SCIWORA (spinal cord injury without radiographic abnormality) in infants and children. Clin Pediatr. 1996;35:119-124. [DOI] [PubMed] [Google Scholar]
- 15. Marinier M, Rodts MF, Connolly M. Spinal cord injury without radiographic abnormality (SCIWORA). Orthopedic Nursing. 1997;16:57-63; quiz 64-55. [DOI] [PubMed] [Google Scholar]
- 16. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001;26:S2-S12. [DOI] [PubMed] [Google Scholar]
- 17. Choo AM, Liu J, Lam CK, Dvorak M, Tetzlaff W, Oxland TR. Contusion, dislocation, and distraction: primary hemorrhage and membrane permeability in distinct mechanisms of spinal cord injury. J Neurosurg Spine. 2007;6:255-266. [DOI] [PubMed] [Google Scholar]
- 18. Choo AM, Liu J, Liu Z, Dvorak M, Tetzlaff W, Oxland TR. Modeling spinal cord contusion, dislocation, and distraction: characterization of vertebral clamps, injury severities, and node of Ranvier deformations. J Neurosci Methods. 2009;181:6-17. [DOI] [PubMed] [Google Scholar]
- 19. Chen K, Liu J, Assinck Pet al. Differential histopathological and behavioral outcomes eight weeks after rat spinal cord injury by contusion, dislocation, and distraction mechanisms. J Neurotrauma. 2016;33:1667-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Zhang M, Guo Yet al. Quantification of surviving neurons after contusion, dislocation, and distraction spinal cord injuries using automated methods. Exp Neurol. 2019;13:1-9.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, Gispen WH. Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma. 2001;18:187-201. [DOI] [PubMed] [Google Scholar]
- 22. Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. J Neurosci Methods. 2002;115:169-179. [DOI] [PubMed] [Google Scholar]
- 23. Vrinten DH, Hamers FF. “CatWalk” automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102:203-209. [DOI] [PubMed] [Google Scholar]
- 24. Kloos AD, Fisher LC, Detloff MR, Hassenzahl DL, Basso DM. Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Exp Neurol. 2005;191:251-265. [DOI] [PubMed] [Google Scholar]
- 25. Chen K, Al’Joboori YD, Gigout Set al. Recovery mechanisms of anti-Nogo-A antibody treatment, epidural stimulation, and treadmill training after severe contusion spinal cord injury in rats, Exp Neurol. 2017;292:135-144. [DOI] [PubMed] [Google Scholar]