Abstract
Liver fibrosis is a common consequence of chronic liver disease. Over time, liver fibrosis can develop into liver cirrhosis. Current therapies for liver fibrosis are limited, and liver transplant is the only curative therapy for patients who progress to end-stage disease. A potential approach to treat chronic liver disease with increasing interest is cell-based therapy. Among the multiple cell types which have been proposed for therapeutic uses, human amnion epithelial cells and amniotic fluid-derived mesenchymal cells are promising. These cells are highly abundant, and their use poses no ethical concern. Furthermore, they exert potent anti-inflammatory and antifibrotic effects in animal models of liver injury. This review highlights the therapeutic characteristics and discusses how human amnion epithelial cells can be utilised as a therapeutic tool for chronic liver disease.
1. Introduction
Chronic liver disease (CLD) results in the development of chronic hepatic wound healing, characterised by persistent liver inflammation and the accumulation of extracellular matrix proteins (ECM), collectively described as fibrosis [1, 2]. Crucial to the pathogenesis of liver fibrosis are hepatic stellate cells (HSCs) and macrophages [3, 4]. HSCs propagate fibrosis by secreting ECM proteins and profibrotic factors while macrophages, consisting of resident Kupffer cells and those derived from infiltrating monocytes, perform a diversity of functions during hepatic wound healing [5, 6]. These include secreting a myriad of proinflammatory and profibrotic factors, recruiting other immune cell populations and clearing cell debris by phagocytosis. These activities perpetuate fibrosis which can develop into liver cirrhosis.
Cirrhosis is associated with reduced liver function and an increased risk of developing liver cancers. Currently, a liver transplant is the only cure for patients who progress to end-stage liver cirrhosis. However, transplantation is complicated by low organ availability, high cost, and long-term immunosuppression [7]. Consequently, it is imperative that a novel antifibrotic therapy for CLD be developed.
Research has highlighted the potential of stem cell-based therapies for CLD [8, 9]. For instance, mesenchymal embryonic and induced pluripotent stem cells have been recognised as possessing therapeutic properties relevant to CLD. However, the clinical realisation of these candidates is hindered by safety, cost, availability, and ethical considerations [9]. These issues have led scientists to seek alternative cell sources, of which perinatal stem cells are one of the most promising.
Perinatal stem cells are derived from extraembryonic tissues, such as the foetal membrane and umbilical cord [10, 11]. They share a unique ontogenetic relationship to the developing foetus, with some even arising prior to gastrulation. Their unique origin is thought to be the reason why perinatal cells combine the therapeutic qualities of adult stem cells, such as mesenchymal stem cells, with the differentiation potential of embryonic stem cells [12, 13]. Additionally, perinatal stem cells are immune privileged and genetically stable, meaning they do elicit an inflammatory immune response or form teratomas following transplantation in animal models [12, 14]. Finally, perinatal stem cells are isolated in abundance from material that is normally discarded after birth, so their use poses less ethical concern. Combined, these advantages make a strong case that perinatal stem cells are more practical for clinical use compared to other cell therapy candidates.
Over the decades, multiple cell types have been isolated from extraembryonic material, including the foetal membrane, umbilical cord, and amniotic fluid. Of these sources, one of the most extensively investigated is the amniotic component of the foetal membrane [15, 16]. The amniotic membrane is currently used in clinical ophthalmology and skin grafting due to its ability to reduce fibrosis and promote tissue repair [17–20]. It contains an epithelial and a mesenchymal cell population which, have been isolated and investigated as a therapeutic tool [11, 21, 22]. Both cell types demonstrate similar therapeutic properties; however, only the epithelial population can be isolated in a clinically compliant manner at numbers sufficient for clinical use [23]. As a result, the therapeutic properties of human amnion epithelial cells (hAECs) have been explored in animal models of the liver, lung, cardiac, epidermal, and neurological [22, 24–31]. These preclinical studies show that systemic infusion of hAECs attenuates inflammation and reduces fibrosis suggesting that they may be able to ameliorate chronic hepatic wound healing in patients with CLD.
2. Human Amnion Epithelial Cells Modulate Chronic Wound Healing
Generally, chronic wound healing involves a highly complex and dynamic interplay between injured parenchymal cells, myofibroblasts, inflammatory cells, and tissue-specific stem/progenitor cells (Figure 1) [32, 33]. It has been suggested that due to this complexity, a multifactorial approach is needed to slow or reverse the progression of tissue fibrosis [34, 35]. Herein lies the value of hAECs; as studies show they suppress and modulate multiple aspects of chronic wound healing. Specifically, hAECs have been reported to (i) attenuate myofibroblast activation [24, 36, 37], (ii) suppress monocyte/macrophage recruitment [36, 38, 39], (iii) promote macrophage polarisation toward a reparative phenotype [36, 40, 41], and (iv) induce regulatory T-cell differentiation [42]. These effects are largely mediated by the paracrine factors secreted by hAECs [24, 36, 37, 43] as demonstrated in studies demonstrating that the benefits of hAEC therapy occur independently of cell engraftment [37, 44]. Furthermore, hAEC-conditioned medium is able to reduce liver fibrosis in a mouse model of chronic liver injury [24, 45]. Accordingly, identifying the trophic factors secreted by hAECs and how they affect chronic wound healing is an active area of research.
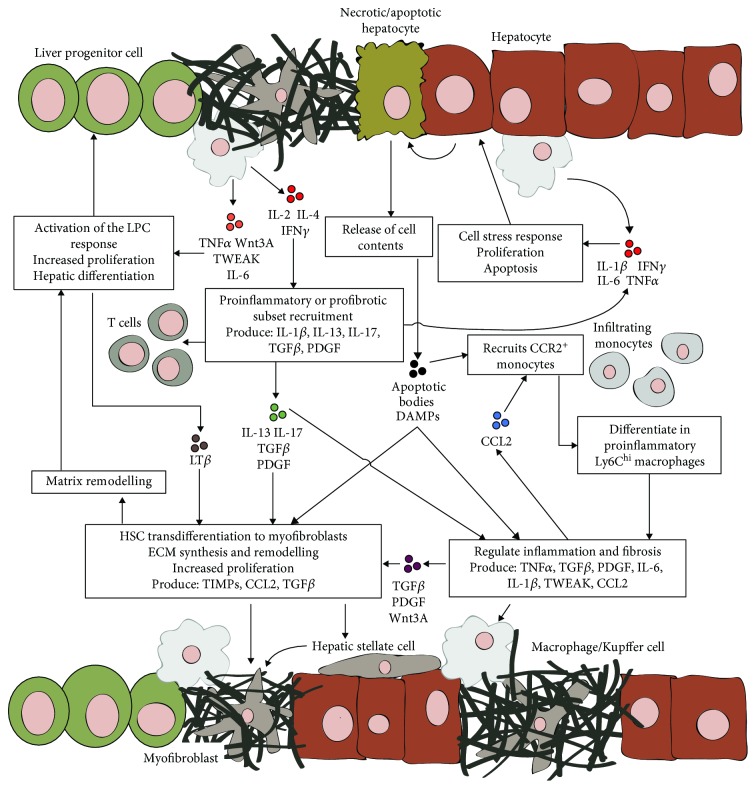
Figure 1.
Overview of chronic hepatic wound healing and fibrosis. Necrotic and/or apoptotic hepatocytes release their cell content stimulating monocyte infiltration and hepatic stellate cell activation. Upon infiltration, monocytes differentiate into proinflammatory Ly6Chi macrophages which, along with resident Kupffer cells, release a myriad of proinflammatory and profibrotic cytokines. These factors promote additional inflammatory cell recruitment, regulate tissue repair, and activate hepatic stellate cells. Activated stellate cells transdifferentiate into ECM-producing myofibroblasts. Myofibroblasts also augment ECM remodelling by producing TIMPs and regulate tissue repair by secreting inflammatory and profibrotic factors. As a consequence of liver inflammation, T cells are recruited which further promote inflammation and/or stellate cell activation through cytokine production. Finally, persistent hepatic inflammation, ECM remodelling, and hepatocyte injury activate the liver progenitor cell compartment. Liver progenitor cells proliferate and differentiate into hepatocytes to assist liver regeneration during CLD. CLD perpetuates this wound healing response resulting in persistent liver inflammation and the development of fibrosis.
3. hAECs Inhibit Myofibroblast Activation
A hallmark of tissue fibrosis is the accumulation of ECM-producing myofibroblasts [1, 46]. Myofibroblasts are a crucial component of tissue repair as they secrete ECM proteins, regulate ECM remodelling, and produce inflammatory and fibrotic cytokines and chemokines [3]. Typically, these cells are cleared once injury and inflammation subside; however, during CLD, persistent inflammation perpetuates myofibroblast activation resulting in the progressive accumulation of ECM in the liver [47].
Myofibroblasts can be derived from a variety of precursor cells type; however, liver myofibroblasts are almost exclusively derived from hepatic stellate cells (HSCs) [4]. These normally quiescent perisinusoidal cells found in the Space of Disse activate during liver injury and transdifferentiate into liver myofibroblasts. To this end, suppressing HSC activation and promoting the clearance of myofibroblasts is a major goal in the development of antifibrotic therapies.
Preclinical studies in animal models of the liver, lung, and skin fibrosis report that hAEC therapy reduces the number of myofibroblasts within injured tissue [24, 31, 36, 40, 48]. Furthermore, in the murine models of chronic carbon tetrachloride- (CCl4-) induced liver injury and bleomycin-induced lung injury, hAEC therapies are reported to reduce the levels of profibrotic factors, namely, transforming growth factor-β (TGFβ) and platelet-derived growth factor (PDGF) [24, 36, 40]. TGFβ stimulates HSC activation, maintains myofibroblast survival, and promotes ECM synthesis. Furthermore, TGFβ reduces the activity of matrix-degrading matrix metalloproteinases (MMPs) by upregulating the expression of tissue inhibitor of metalloproteinases (TIMPs) by myofibroblasts [49]. PDGF is a potent mitogen for myofibroblasts and is upregulated by liver injury [50]. Thus, by downregulating the production of these factors, hAEC therapy attenuates HSC activation and ECM synthesis.
In addition to reducing TGFβ and PDGF activity, hAECs directly suppress the fibrotic activity of HSCs and myofibroblasts through paracrine signalling. For instance, Hodge et al. demonstrated that when cultured in hAEC-conditioned medium, HSCs adopt an antifibrotic phenotype characterised by a reduction in proliferation, activation, and ECM production [51]. Furthermore, Zhao et al. demonstrated that hAECs block TGFβ signalling in myofibroblasts by secreting soluble human leukocyte antigen G5 [48]. Other antifibrotic factors inducing prostaglandin E2, bone morphogenetic protein-7, and interleukin-10 are also secreted by hAECs [51]. Overall, these studies indicate that hAECs attenuate the profibrotic activity of HSCs and myofibroblasts, through both direct and indirect mechanisms.
4. hAECs Modulate Macrophage Recruitment
CLD is closely associated with the enrichment of liver macrophages [52]. Macrophages promote inflammation and fibrosis by secreting a host of cytokines including TGFβ, PDGF, interleukin- (IL-) 1, IL-6, tumor necrosis factor alpha (TNFα), and TNF-related weak inducer of apoptosis (TWEAK) [5, 53]. These factors perpetuate inflammation, stimulate and maintain myofibroblast activation, and induce immune-mediated tissue injury [54–57]. Consequently, macrophages have become an attractive target for antifibrotic therapies.
The liver houses a specialised macrophage population known as Kupffer cells; however, following injury, the liver's macrophage population expands dramatically through monocyte recruitment. These monocytes differentiate into monocyte-derived macrophages (MDMs) upon infiltration into the injured liver [58]. Research suggests that Kupffer cells are imperative for maintaining liver homeostasis and the early response to liver injury [59]. In comparison, MDMs are crucial for inflammation and tissue repair following liver injury [60]. In fact, during liver repair, the number of Kupffer cells decreases while there is a substantial increase in the number of MDMs [61, 62]. Furthermore, the development of inflammation and fibrosis in other tissues such as the lung and kidney is associated with the recruitment of MDMs [63]. Therefore, suppressing MDM recruitment could alleviate liver inflammation and fibrosis.
The benefits of hAEC therapy are associated with reduced macrophage recruitment. Studies using bleomycin-induced lung injury and liver injury caused by CCl4 or a high fat diet show a reduction in macrophage numbers when hAECs are infused systemically [36, 38–40, 45]. Accordingly, the expression levels of their associated factors including TGFβ, PDGF, IL-1, IL-6, and TNFα are reduced [22, 36, 38–40]. This outcome may be related to a decrease in the expression levels of the chemokine CCL2 [36, 39]. CCL2 recruits CCR2-expressing monocytes and is considered a key mediator of MDM recruitment following injury [58, 64]. In fact, attenuation of CCL2-CCR2 signalling either by genetic or by pharmacological means reduces MDM recruitment and hepatic fibrosis in murine models [58, 65]. Furthermore, both serum levels and liver expression of CCL2 are reported to correlate with the severity of CLD [66]. Hence, suppression of CCL2/CCR2 MDM recruitment by hAECs may be of therapeutic value to patients with CLD.
5. hAECs Alter Macrophage Polarisation
Animal models of macrophage depletion have shown that macrophages can both promote and resolve tissue fibrosis. For example, Duffield et al., using the CD11b-DTR transgenic mouse, established that macrophage depletion during liver injury and inflammation prevents the development of liver fibrosis. Conversely, depletion during recovery attenuates the degradation of matrix proteins [67]. These contrasting roles highlight the plasticity of macrophages. Macrophages will adapt their phenotype in response to signals from their microenvironment. In general, these phenotypes are classified as classical (M1) or alternatively (M2) activated [68].
M1 macrophages are predominantly proinflammatory, releasing cytokines such as TNFα, IL-1β, and IL-6 [68, 69]. In contrast, M2 macrophages are associated with immunomodulation and tissue repair [68, 69]. M2 macrophages display a higher capacity for phagocytosis and secrete factors including IL-10, TGFβ, and MMPs [68, 70] (Figure 2). It is important to highlight that the M1/M2 classification system oversimplifies the heterogeneity of macrophage phenotypes in disease conditions. Macrophages often express M1 and M2 activation markers simultaneously, so rather than two distinct subpopulations, the M1/M2 paradigm signifies a spectrum of activation states. Nonetheless, the transition from predominantly M1 to M2 during wound healing is associated with the resolution of inflammation and initiation of tissue repair [68, 71]. This transition becomes dysregulated during CLD resulting in chronic inflammation, dysfunctional wound healing, and fibrosis. Therefore, modulating macrophage polarisation to limit chronic inflammation and fibrosis in patients with CLD is an attractive strategy.
Figure 2.
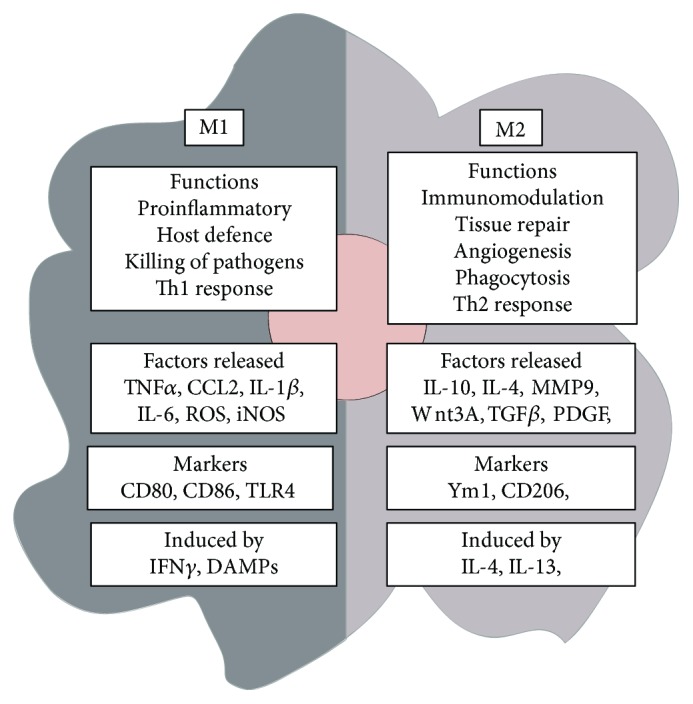
M1/M2 paradigm of macrophage polarisation. The M1/M2 paradigm describes alternative states of macrophage polarisation with each exerting different functions in inflammation and fibrosis. The activation state adopted by macrophages is dependent on signalling molecules from their microenvironment.
Evidence suggests that hAEC therapy augments macrophage polarisation toward an M2 phenotype [24, 36–38, 72]. The expression of M2-associated markers including CD206, IL-10, and MMP9 is increased by hAEC therapy in murine models of CCl4-induced liver and bleomycin-induced lung injuries [24, 36, 40, 41]. IL-10 is a potent anti-inflammatory cytokine known to suppress monocyte infiltration, synthesis of proinflammatory mediators, and collagen synthesis by myofibroblasts [73, 74]. In fact, a clinical trial of IL-10 in patients with chronic hepatitis C reported a reduction in fibrosis and improvements in liver histology and function [75]. Similarly, MMP9, which breaks down collagen, is reported to exert an antifibrotic effect in murine models of chronic liver injury [6, 76]. It is important to highlight that hAEC therapy does not promote the profibrotic functions of M2 macrophages through their secretion of TGFβ and PDGF. In fact, the M2 functions that are induced by hAEC therapy are associated with immunosuppression and breakdown of ECM.
It is likely that soluble factors secreted by hAECs play an important role in their modulation of macrophages. For instance, Tan et al. demonstrated that hAECs secrete lipoxin A4 which promotes macrophage phagocytosis in culture [43]. Interestingly, lipoxin A4 has been shown to reduce inflammation and fibrosis by promoting M2 polarisation in a mouse model of obesity-induced hepatic injury [77]. Similarly, the production of soluble human leukocyte antigen G5 by hAECs may induce M2 polarisation [78]. Furthermore, hAECs are reported to produce the chemokine CX3CL1 which induces MDMs to differentiate into the Ly6Clow phenotype [79, 80] (Figure 3). This macrophage subpopulation protects against liver fibrosis and is critical for its resolution [61]. However, while hAEC therapy does increase hepatic expression of CX3CL1 during chronic CCl4-induced liver injury, it is currently unknown whether this translates to an increase in Ly6Clow MDMs in this context [36]. Nevertheless, these studies suggest that multiple soluble factors secreted by hAECs may promote a macrophage phenotype that resolves liver fibrosis.
Figure 3.
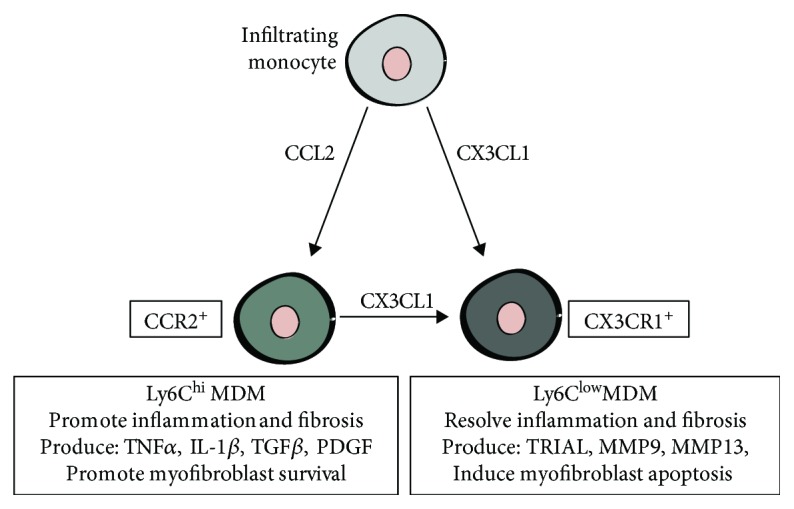
Differential expression of Ly6C distinguishes MDMs with opposing functions in tissue repair. During liver repair, monocytes recruited by the CCL2/CCR2 axis differentiate into profibrotic Ly6Chi-expressing MDMs. In contrast, monocytes recruited by the CX3CL1/CX3CR1 axis give rise to Ly6Clow MDMs. This subpopulation promotes the resolution of tissue repair and regression of fibrosis. CX3CL1 can induce a phenotypic switch of Ly6Chi MDMs to a Ly6Clow phenotype.
6. hAECs Promote Regulatory T Cells
Regulatory T cells (Tregs) are important regulators of inflammation and fibrosis. This subset of CD4+ T helper cells suppresses the immune response by secreting immunosuppressive factors including IL-10 and TGFβ. Furthermore, Tregs promote M2 macrophage polarisation and counteract the activity of other CD4+ T-cell subsets (Figure 4). An imbalance between T-cell subsets is an important factor in the progression of CLD. For example, an excessive Th1 and/or Th17 response is associated with increased severity of chronic hepatitis B [81]. In fact, Gu et al. demonstrated that rapamycin ameliorates liver inflammation and fibrosis by upregulating Tregs and downregulating Th17 cells in murine models [82]. Therefore, promoting Treg maturation has the potential to reduce liver fibrosis in patients with CLD.
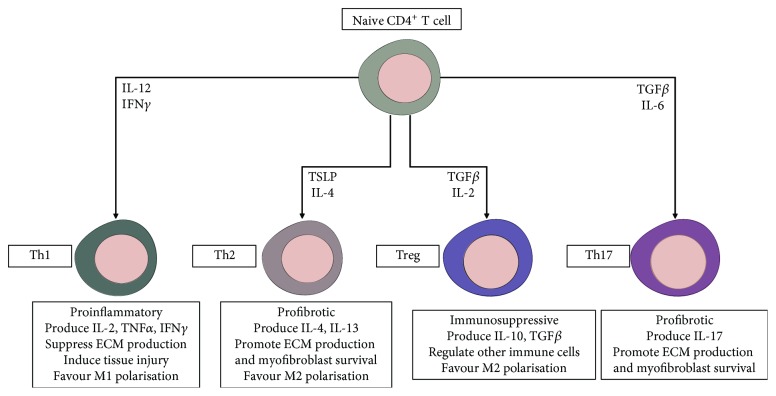
Figure 4.
Functions of CD4+ T-cell subsets in liver fibrosis. The differentiation of naive CD4+ T cells into distinct functional subtypes is driven by factors produced by injured parenchymal cells and other inflammatory cells, namely, macrophages. Tregs suppress other T-cell subsets and limit the magnitude of inflammation and fibrosis during tissue repair.
The induction and expansion of Tregs is a crucial component of hAEC therapy. hAECs increase the number of Tregs in the lung following bleomycin-induced injury [42]. Additionally, hAEC therapy prevents bleomycin-induced lung damage in Rag1−/− mice, only when an adoptive transfer of either Tregs or naive T cells is coadministered [42]. Importantly, research suggests that enhancing Treg activity ameliorates inflammation and fibrosis in a mouse model of chronic liver injury [83]. Conversely, Treg depletion during chronic liver injury exacerbates liver inflammation and fibrosis [84]. Therefore, the upregulation of Treg activity by hAECs may achieve beneficial outcomes in patients with CLD.
7. hAECs May Support Endogenous Liver Regeneration
Most studies investigating hAEC-based therapies for liver pathologies focus on the inflammatory and fibrotic aspects. Currently, it is unknown whether hAEC therapy enhances hepatocyte regeneration. Regardless, as hAEC therapy improves markers of tissue function in models of chronic liver and lung injury, it is likely that tissue regeneration is occurring [24, 36, 37].
hAEC therapy may support liver regeneration through multiple mechanisms. Firstly, hAECs secrete growth factors such as EFG and IGF2 which are known to induce hepatocyte proliferation [24, 85, 86]. Secondly, since the fibrotic matrix is a major inhibitor of hepatocyte proliferation [87], the antifibrotic properties of hAECs, particularly their ability to suppress HSC activation, should assist liver regeneration. In addition, the ability of hAECs to upregulate MMP and downregulate TIMP expression may encourage regeneration by promoting ECM degradation [36, 51, 87]. Finally, hAEC therapy reduces hepatocyte apoptosis during CCl4-indcued liver injury [22, 36]. This is likely mediated by their immunomodulatory effects, as factors including TNFα, IL-1β, and TGFβ are known to stimulate hepatocyte apoptosis [88, 89]. Excessive hepatocyte apoptosis is a common feature of CLD and directly contributes to the progressive loss of liver parenchyma [88, 89]. Therefore, by tempering hepatocyte apoptosis, hAECs may be able to mitigate the progressive loss of the liver parenchyma in patients with CLD and promote regeneration. Collectively, these studies suggest that through a combination of both direct and indirect mechanisms, hAEC therapy may augment liver repair to favour hepatocyte regeneration (Figure 5).
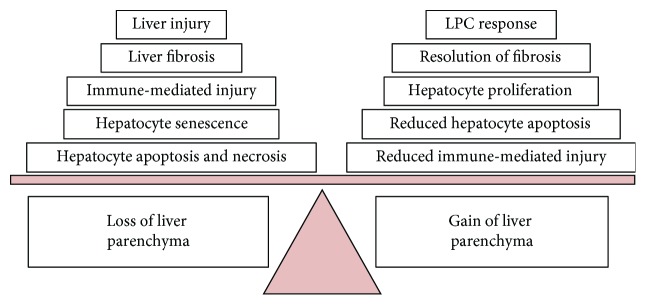
Figure 5.
Mechanisms that contribute to the loss or gain of liver parenchyma during CLD. Liver repair following injury involves a balancing act between mechanisms that result in either loss or gain of the liver parenchyma. Accordingly, the progression of CLD can be framed as liver repair that favours parenchyma loss over gain. Flipping this balance in favour of parenchyma gain is the ultimate goal of regenerative therapies for CLD.
hAEC therapy may also promote hepatocyte regeneration by stimulating the proliferation and differentiation of liver progenitor cells (LPCs). LPCs are a population of bipotential epithelial cells that reside in the canals of Hering, located within the biliary tree [90, 91]. LPCs are rare in a healthy liver; however, during persistent liver injury, especially where there is chronic inflammation, fibrosis, and hepatocyte senescence, LPCs emerge from the bile canaliculi, proliferate, and differentiate into hepatocytes or cholangiocytes. This response is frequently referred to as the ductular reaction and is observed in patients with viral hepatitis, alcoholic liver disease, and fatty liver disease [35]. Notably, LPCs only assist with liver regeneration when hepatocyte regeneration is impaired, a common occurrence in human CLD [92, 93]. However, the overall contribution of LPCs to liver regeneration is still debated [93, 94]. Regardless, LPCs can contribute to liver regeneration during chronic liver injury; therefore, understanding how hAEC therapy impacts their biology is warranted.
It is difficult to discern the exact impact hAEC therapy will have on LPCs. On the one hand, several of the factors such as IGF2 and galectin 3 which are secreted by hAECs may stimulate LPC proliferation [95–97]. Conversely, the anti-inflammatory effects of hAECs would indirectly suppress the LPC response as MDMs and their associated inflammatory factors are important stimulators of LPC activity [98–100]. Additionally, LPC expansion is closely associated with the progression of liver fibrosis; hence, the antifibrotic effects of hAECs would likely dampen the LPC response [101, 102]. Consequently, hAEC-based therapies have the potential to both promote and inhibit liver regeneration by LPCs. To this end, characterisation of the effects hAECs have on LPC behaviour is warranted to fully understand the mechanism underpinning hAEC therapy.
8. Conclusion
Preclinical research over the last decade has illustrated the potential of hAEC-based therapies as a treatment for CLD (Table 1). These studies consistently show that the infusion of hAECs or their secretome reduces hepatic inflammation and fibrosis by modulating the activity of HSCs, macrophages, and other inflammatory cells. However, there are still many unanswered questions regarding the mechanisms behind these effects. Nevertheless, hAECs have progressed to clinical trials. A phase I pilot study aimed at evaluating the safety of intravenously administered hAECs in patients with end-stage CLD is currently underway [103]. Furthermore, outcomes from a phase I trial of allogeneic hAEC therapy in preterm infants with established bronchopulmonary dysplasia demonstrated no adverse effects, suggesting that hAECs will be safe to use in patients with CLD [104]. Looking ahead, research should aim to identify the factors secreted by hAECs that exert their beneficial effects. This will assist in designing future clinical trials and may lead to the development of an antifibrotic therapy based on the hAEC secretome. This cell-free approach could potentially be simpler, safer, and more commercially viable than whole-cell therapy.
Table 1.
Summary of results from studies using hAEC-based therapies in models of liver disease.
| Study | Injury model | hAEC treatment | Main results |
|---|---|---|---|
| Manuelpillai et al. [22] | C57BL/6 mice administered CCl4 twice weekly for 4 weeks | Intraperitoneal injection of whole hAECs | Decreased liver injury, inflammation, and fibrosis |
| Sant'Anna et al. [105] | Bile duct ligation in Wistar rats for 6 weeks | Amniotic membrane place over ligation site | Reduced liver fibrosis |
| Manuelpillai et al. [36] | C57BL/6 mice administered CCl4 twice weekly for 12 weeks | Single and double dose of whole hAECs by intraperitoneal injection | Decrease fibrosis, decreased macrophage infiltration, increased M2 polarisation, and reduced T-cell infiltration |
| Ricci et al. [106] | Bile duct ligation in Sprague Dawley rats for 6 weeks | Fresh or cryopreserved amniotic membrane place over ligation site | Fresh and cryopreserved amniotic membrane produced the same antifibrotic effects |
| Alhomrani et al. [24] | C57BL/6 mice administered CCl4 twice weekly for 12 weeks | Tail vein injection of hAEC-CM or hAEC-derived exosomes | hAEC-CM and exosomes derived from hAECs reduce liver inflammation and fibrosis |
| Kuk et al. [45] | C57BL/6J mice on a Western fast food diet for 42 weeks | Multiple intraperitoneal injections of either whole hAECs or hAEC-CM | hAEC-CM reduces inflammation and fibrosis |
CCl4: carbon tetrachloride; hAEC: human amnion epithelial cell; hAEC-CM: human amnion epithelial cell-conditioned medium.
Acknowledgments
We would like to thank Associate Professor Rebecca Lim, from the Hudson Institute of Medical Research, Melbourne, Australia; Dr. Adam Passman from the Barts Cancer Institute, Queen Mary University, London, UK; and Dr. Robyn Strauss from the University of Western Australia, Perth, Australia, for their advice on this article.
Conflicts of Interest
All authors have no conflicts of interest relevant to this article.
References
- 1.Bataller R., Brenner D. A. Liver fibrosis. Journal of Clinical Investigation. 2005;115(2):209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson N. C., Iredale J. P. Liver fibrosis: cellular mechanisms of progression and resolution. Clinical Science. 2007;112(5):265–280. doi: 10.1042/CS20060242. [DOI] [PubMed] [Google Scholar]
- 3.Friedman S. L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiological Reviews. 2008;88(1):125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira R. K. Hepatic stellate cells and liver fibrosis. Archives of Pathology & Laboratory Medicine. 2007;143(11):1728–1734. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- 5.Li H., You H., Fan X., Jia J. Hepatic macrophages in liver fibrosis: pathogenesis and potential therapeutic targets. BMJ Open Gastroenterology. 2016;3(1, article e000079) doi: 10.1136/bmjgast-2016-000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas J. A., Pope C., Wojtacha D., et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53(6):2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 7.Prakoso E., Verran D., Dilworth P., et al. Increasing liver transplantation waiting list mortality: a report from the Australian National Liver Transplantation Unit, Sydney. Internal Medicine Journal. 2010;40(9):619–625. doi: 10.1111/j.1445-5994.2010.02277.x. [DOI] [PubMed] [Google Scholar]
- 8.Forbes S. J., Gupta S., Dhawan A. Cell therapy for liver disease: from liver transplantation to cell factory. Journal of Hepatology. 2015;62(1):S157–S169. doi: 10.1016/j.jhep.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 9.Fagoonee S., Famulari E. S., Silengo L., Camussi G., Altruda F. Prospects for adult stem cells in the treatment of liver diseases. Stem Cells and Development. 2016;25(20):1471–1482. doi: 10.1089/scd.2016.0144. [DOI] [PubMed] [Google Scholar]
- 10.Witkowska-Zimny M., Wrobel E. Perinatal sources of mesenchymal stem cells: Wharton’s jelly, amnion and chorion. Cellular & Molecular Biology Letters. 2011;16(3):493–514. doi: 10.2478/s11658-011-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim R. Concise review: fetal membranes in regenerative medicine: new tricks from an old dog? Stem Cells Translational Medicine. 2017;6(9):1767–1776. doi: 10.1002/sctm.16-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim M. J., Shin K. S., Jeon J. H., et al. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: a comparative analysis of their potential as placenta-derived stem cells. Cell and Tissue Research. 2011;346(1):53–64. doi: 10.1007/s00441-011-1249-8. [DOI] [PubMed] [Google Scholar]
- 13.Toda A., Okabe M., Yoshida T., Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. Journal of Pharmacological Sciences. 2007;105(3):215–228. doi: 10.1254/jphs.CR0070034. [DOI] [PubMed] [Google Scholar]
- 14.Miki T., Lehmann T., Cai H., Stolz D. B., Strom S. C. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23(10):1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 15.Ilancheran S., Moodley Y., Manuelpillai U. Human fetal membranes: a source of stem cells for tissue regeneration and repair? Placenta. 2009;30(1):2–10. doi: 10.1016/j.placenta.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Kmiecik G., Niklińska W., Kuć P., et al. Fetal membranes as a source of stem cells. Advances in Medical Sciences. 2013;58(2):185–195. doi: 10.2478/ams-2013-0007. [DOI] [PubMed] [Google Scholar]
- 17.Meller D., Pauklin M., Thomasen H., Westekemper H., Steuhl K. P. Amniotic membrane transplantation in the human eye. Deutsches Ärzteblatt International. 2011;108(14):243–248. doi: 10.3238/arztebl.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insausti C. L., Alcaraz A., García-Vizcaíno E. M., et al. Amniotic membrane induces epithelialization in massive posttraumatic wounds. Wound Repair and Regeneration. 2010;18(4):368–377. doi: 10.1111/j.1524-475X.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- 19.Rahman M. S., Akhtar N., Hasan M. Z., Asaduzzaman S. M. Human tissue banking in Bangladesh: hope for the patients of massive burns, surgical wound and bone associated complications. International Journal of Burns and Trauma. 2019;9(2):23–27. [PMC free article] [PubMed] [Google Scholar]
- 20.Akhtar N., Rahman M. S., Jamil H. M., Arifuzzaman M., Miah M. M., Asaduzzaman S. M. Tissue banking in Bangladesh: 12 years of experience (2003–2014) Cell and Tissue Banking. 2016;17(2):189–197. doi: 10.1007/s10561-016-9549-6. [DOI] [PubMed] [Google Scholar]
- 21.Lee P.-H., Tu C. T., Hsiao C. C., et al. Antifibrotic activity of human placental amnion membrane-derived CD34+ mesenchymal stem/progenitor cell transplantation in mice with thioacetamide-induced liver injury. Stem Cells Translational Medicine. 2016;5(11):1473–1484. doi: 10.5966/sctm.2015-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manuelpillai U., Tchongue J., Lourensz D., et al. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4-treated mice. Cell Transplantation. 2010;19(9):1157–1168. doi: 10.3727/096368910X504496. [DOI] [PubMed] [Google Scholar]
- 23.Murphy S., Rosli S., Acharya R., et al. Amnion epithelial cell isolation and characterization for clinical use. Current Protocols in Stem Cell Biology. 2010;13(1):1E.6.1–1E.6.25. doi: 10.1002/9780470151808.sc01e06s13. [DOI] [PubMed] [Google Scholar]
- 24.Alhomrani M., Correia J., Zavou M., et al. The human amnion epithelial cell secretome decreases hepatic fibrosis in mice with chronic liver fibrosis. Frontiers in Pharmacology. 2017;8(748) doi: 10.3389/fphar.2017.00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu D., Tan J., Maleken A. S., et al. Human amnion cells reverse acute and chronic pulmonary damage in experimental neonatal lung injury. Stem Cell Research & Therapy. 2017;8(1):p. 257. doi: 10.1186/s13287-017-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melville J. M., McDonald C. A., Bischof R. J., et al. Human amnion epithelial cells modulate the inflammatory response to ventilation in preterm lambs. PLoS One. 2017;12(3, article e0173572) doi: 10.1371/journal.pone.0173572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu T., Wu J., Huang Q., et al. Human amniotic epithelial cells ameliorate behavioral dysfunction and reduce infarct size in the rat middle cerebral artery occlusion model. Shock. 2008;29(5):603–611. doi: 10.1097/shk.0b013e318157e845. [DOI] [PubMed] [Google Scholar]
- 28.Song Y. S., Joo H. W., Park I. H., et al. Transplanted human amniotic epithelial cells secrete paracrine proangiogenic cytokines in rat model of myocardial infarction. Cell Transplantation. 2015;24(10):2055–2064. doi: 10.3727/096368914X685609. [DOI] [PubMed] [Google Scholar]
- 29.Zhao B., Liu J. Q., Zheng Z., et al. Human amniotic epithelial stem cells promote wound healing by facilitating migration and proliferation of keratinocytes via ERK, JNK and AKT signaling pathways. Cell and Tissue Research. 2016;365(1):85–99. doi: 10.1007/s00441-016-2366-1. [DOI] [PubMed] [Google Scholar]
- 30.Yawno T., Schuilwerve J., Moss T. J. M., et al. Human amnion epithelial cells reduce fetal brain injury in response to intrauterine inflammation. Developmental Neuroscience. 2013;35(2-3):272–282. doi: 10.1159/000346683. [DOI] [PubMed] [Google Scholar]
- 31.Murphy S., Lim R., Dickinson H., et al. Human amnion epithelial cells prevent bleomycin-induced lung injury and preserve lung function. Cell Transplantation. 2011;20(6):909–924. doi: 10.3727/096368910X543385. [DOI] [PubMed] [Google Scholar]
- 32.Wynn T. A., Ramalingam T. R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nature Medicine. 2012;18(7):1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forbes S. J., Newsome P. N. Liver regeneration - mechanisms and models to clinical application. Nature Reviews Gastroenterology & Hepatology. 2016;13(8):473–485. doi: 10.1038/nrgastro.2016.97. [DOI] [PubMed] [Google Scholar]
- 34.Schuppan D., Kim Y. O. Evolving therapies for liver fibrosis. The Journal of Clinical Investigation. 2013;123(5):1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39(6):1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 36.Manuelpillai U., Lourensz D., Vaghjiani V., et al. Human amniotic epithelial cell transplantation induces markers of alternative macrophage activation and reduces established hepatic fibrosis. PLoS One. 2012;7(6, article e38631) doi: 10.1371/journal.pone.0038631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vosdoganes P., Wallace E. M., Chan S. T., Acharya R., Moss T. J. M., Lim R. Human amnion epithelial cells repair established lung injury. Cell Transplantation. 2013;22(8):1337–1349. doi: 10.3727/096368912X657657. [DOI] [PubMed] [Google Scholar]
- 38.Murphy S. V., Shiyun S. C., Tan J. L., et al. Human amnion epithelial cells do not abrogate pulmonary fibrosis in mice with impaired macrophage function. Cell Transplantation. 2012;21(7):1477–1492. doi: 10.3727/096368911X601028. [DOI] [PubMed] [Google Scholar]
- 39.Moodley Y., Ilancheran S., Samuel C., et al. Human amnion epithelial cell transplantation abrogates lung fibrosis and augments repair. American Journal of Respiratory and Critical Care Medicine. 2010;182(5):643–651. doi: 10.1164/rccm.201001-0014OC. [DOI] [PubMed] [Google Scholar]
- 40.Tan J. L., Chan S. T., Wallace E. M., Lim R. Human amnion epithelial cells mediate lung repair by directly modulating macrophage recruitment and polarization. Cell Transplantation. 2014;23(3):319–328. doi: 10.3727/096368912X661409. [DOI] [PubMed] [Google Scholar]
- 41.Magatti M., Vertua E., de Munari S., et al. Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. Journal of Tissue Engineering and Regenerative Medicine. 2017;11(10):2895–2911. doi: 10.1002/term.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan J. L., Chan S. T., Lo C. Y., et al. Amnion cell-mediated immune modulation following bleomycin challenge: controlling the regulatory T cell response. Stem Cell Research & Therapy. 2015;6(1):p. 8. doi: 10.1186/scrt542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan J. L., Tan Y. Z., Muljadi R., et al. Amnion epithelial cells promote lung repair via lipoxin A4. Stem Cells Translational Medicine. 2017;6(4):1085–1095. doi: 10.5966/sctm.2016-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vosdoganes P., Hodges R. J., Lim R., et al. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. American Journal of Obstetrics and Gynecology. 2011;205(2):156.e26–156.e33. doi: 10.1016/j.ajog.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 45.Kuk N., Hodge A., Sun Y., et al. Human amnion epithelial cells and their soluble factors reduce liver fibrosis in murine non-alcoholic steatohepatitis. Journal of Gastroenterology and Hepatology. 2019 doi: 10.1111/jgh.14643. [DOI] [PubMed] [Google Scholar]
- 46.Bataller R., Brenner D. A. Hepatic stellate cells as a target for the treatment of liver fibrosis. Seminars in Liver Disease. 2001;21(3):437–452. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- 47.Novo E., Cannito S., Morello E., et al. Hepatic myofibroblasts and fibrogenic progression of chronic liver diseases. Histology and Histopathology. 2015;30(9):1011–1032. doi: 10.14670/HH-11-623. [DOI] [PubMed] [Google Scholar]
- 48.Zhao B., Liu J. Q., Yang C., et al. Human amniotic epithelial cells attenuate TGF-β1-induced human dermal fibroblast transformation to myofibroblasts via TGF-β1/Smad3 pathway. Cytotherapy. 2016;18(8):1012–1024. doi: 10.1016/j.jcyt.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Edwards D. R., Murphy G., Reynolds J. J., et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. The EMBO Journal. 1987;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borkham-Kamphorst E., Weiskirchen R. The PDGF system and its antagonists in liver fibrosis. Cytokine & Growth Factor Reviews. 2016;28:53–61. doi: 10.1016/j.cytogfr.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Hodge A., Lourensz D., Vaghjiani V., et al. Soluble factors derived from human amniotic epithelial cells suppress collagen production in human hepatic stellate cells. Cytotherapy. 2014;16(8):1132–1144. doi: 10.1016/j.jcyt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann H. W., Seidler S., Nattermann J., et al. Functional contribution of elevated circulating and hepatic non-classical CD14+CD16+ monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5(6, article e11049) doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dwyer B. J., Olynyk J. K., Ramm G. A., Tirnitz-Parker J. E. E. TWEAK and LTβ signaling during chronic liver disease. Frontiers in Immunology. 2014;5:p. 39. doi: 10.3389/fimmu.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y. M., Seki E. TNFα in liver fibrosis. Current Pathobiology Reports. 2015;3(4):253–261. doi: 10.1007/s40139-015-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsutsui H., Cai X., Hayashi S. Interleukin-1 family cytokines in liver diseases. Mediators of Inflammation. 2015;2015:19. doi: 10.1155/2015/630265.630265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gieling R. G., Wallace K., Han Y. P. Interleukin-1 participates in the progression from liver injury to fibrosis. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296(6):G1324–G1331. doi: 10.1152/ajpgi.90564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fielding C. A., Jones G. W., McLoughlin R. M., et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40(1):40–50. doi: 10.1016/j.immuni.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karlmark K. R., Weiskirchen R., Zimmermann H. W., et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50(1):261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 59.Kolios G., Valatas V., Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World Journal of Gastroenterology. 2006;12(46):7413–7420. doi: 10.3748/wjg.v12.i46.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tacke F., Zimmermann H. W. Macrophage heterogeneity in liver injury and fibrosis. Journal of Hepatology. 2014;60(5):1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 61.Ramachandran P., Pellicoro A., Vernon M. A., et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(46):E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holt M. P., Cheng L., Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. Journal of Leukocyte Biology. 2008;84(6):1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wynn T. A. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. The Journal of Clinical Investigation. 2007;117(3):524–529. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seki E., de Minicis S., Inokuchi S., et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50(1):185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baeck C., Wehr A., Karlmark K. R., et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut. 2012;61(3):416–426. doi: 10.1136/gutjnl-2011-300304. [DOI] [PubMed] [Google Scholar]
- 66.Degré D., Lemmers A., Gustot T., et al. Hepatic expression of CCL2 in alcoholic liver disease is associated with disease severity and neutrophil infiltrates. Clinical and Experimental Immunology. 2012;169(3):302–310. doi: 10.1111/j.1365-2249.2012.04609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duffield J. S., Forbes S. J., Constandinou C. M., et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. Journal of Clinical Investigation. 2005;115(1):56–65. doi: 10.1172/JCI200522675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stout R. D., Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. Journal of Leukocyte Biology. 2004;76(3):509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mantovani A., Sica A., Locati M. Macrophage polarization comes of age. Immunity. 2005;23(4):344–346. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Shapouri-Moghaddam A., Mohammadian S., Vazini H., et al. Macrophage plasticity, polarization, and function in health and disease. Journal of Cellular Physiology. 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 71.Wermuth P. J., Jimenez S. A. The significance of macrophage polarization subtypes for animal models of tissue fibrosis and human fibrotic diseases. Clinical and Translational Medicine. 2015;4(1):p. 2. doi: 10.1186/s40169-015-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leaw B., Zhu D., Tan J., et al. Human amnion epithelial cells rescue cell death via immunomodulation of microglia in a mouse model of perinatal brain injury. Stem Cell Research & Therapy. 2017;8(1):p. 46. doi: 10.1186/s13287-017-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson K., Maltby J., Fallowfield J., McAulay M., Millward-Sadler H., Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28(6):1597–1606. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- 74.Tilg H., Kaser A., Moschen A. R. How to modulate inflammatory cytokines in liver diseases. Liver International. 2006;26(9):1029–1039. doi: 10.1111/j.1478-3231.2006.01339.x. [DOI] [PubMed] [Google Scholar]
- 75.Nelson D. R., Lauwers G. Y., Lau J. Y. N., Davis G. L. Interleukin 10 treatment reduces fibrosis in patients with chronic hepatitis C: a pilot trial of interferon nonresponders. Gastroenterology. 2000;118(4):655–660. doi: 10.1016/S0016-5085(00)70134-X. [DOI] [PubMed] [Google Scholar]
- 76.Duarte S., Baber J., Fujii T., Coito A. J. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biology. 2015;44-46:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Börgeson E., Johnson A. M. F., Lee Y. S., et al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metabolism. 2015;22(1):125–137. doi: 10.1016/j.cmet.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee C. L., Guo Y. F., So K. H., et al. Soluble human leukocyte antigen G5 polarizes differentiation of macrophages toward a decidual macrophage-like phenotype. Human Reproduction. 2015;30(10):2263–2274. doi: 10.1093/humrep/dev196. [DOI] [PubMed] [Google Scholar]
- 79.Szukiewicz D., Kochanowski J., Mittal T., Pyzlak M., Szewczyk G., Cendrowski K. Chorioamnionitis (ChA) modifies CX3CL1 (fractalkine) production by human amniotic epithelial cells (HAEC) under normoxic and hypoxic conditions. Journal of Inflammation. 2014;11(1):p. 12. doi: 10.1186/1476-9255-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karlmark K. R., Zimmermann H. W., Roderburg C., et al. The fractalkine receptor CX3CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology. 2010;52(5):1769–1782. doi: 10.1002/hep.23894. [DOI] [PubMed] [Google Scholar]
- 81.Ye Y., Xie X., Yu J., et al. Involvement of Th17 and Th1 effector responses in patients with hepatitis B. Journal of Clinical Immunology. 2010;30(4):546–555. doi: 10.1007/s10875-010-9416-3. [DOI] [PubMed] [Google Scholar]
- 82.Gu L., Deng W. S., Sun X. F., Zhou H., Xu Q. Rapamycin ameliorates CCl4-induced liver fibrosis in mice through reciprocal regulation of the Th17/Treg cell balance. Molecular Medicine Reports. 2016;14(2):1153–1161. doi: 10.3892/mmr.2016.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuttkat N., Mohs A., Ohl K., et al. Hepatic overexpression of cAMP-responsive element modulator α induces a regulatory T-cell response in a murine model of chronic liver disease. Gut. 2017;66(5):908–919. doi: 10.1136/gutjnl-2015-311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katz S. C., Ryan K., Ahmed N., et al. Obstructive jaundice expands intrahepatic regulatory T cells, which impair liver T lymphocyte function but modulate liver cholestasis and fibrosis. Journal of Immunology. 2011;187(3):1150–1156. doi: 10.4049/jimmunol.1004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J., Hu X., Chen J., et al. Pericentral hepatocytes produce insulin-like growth factor-2 to promote liver regeneration during selected injuries in mice. Hepatology. 2017;66(6):2002–2015. doi: 10.1002/hep.29340. [DOI] [PubMed] [Google Scholar]
- 86.Natarajan A., Wagner B., Sibilia M. The EGF receptor is required for efficient liver regeneration. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(43):17081–17086. doi: 10.1073/pnas.0704126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Issa R., Zhou X., Trim N., et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. The FASEB Journal. 2003;17(1):47–49. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- 88.Guicciardi M. E., Gores G. J. Apoptosis: a mechanism of acute and chronic liver injury. Gut. 2005;54(7):1024–1033. doi: 10.1136/gut.2004.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guicciardi M. E., Malhi H., Mott J. L., Gores G. J. Apoptosis and necrosis in the liver. Comprehensive Physiology. 2013;3(2) doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Passman A. M., Strauss R. P., McSpadden S. B., et al. A modified choline-deficient, ethionine-supplemented diet reduces morbidity and retains a liver progenitor cell response in mice. Disease Models & Mechanisms. 2015;8(12):1635–1641. doi: 10.1242/dmm.022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaur S., Siddiqui H., Bhat M. H. Hepatic progenitor cells in action: liver regeneration or fibrosis? The American Journal of Pathology. 2015;185(9):2342–2350. doi: 10.1016/j.ajpath.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 92.Boulter L., Lu W.-Y., Forbes S. J. Differentiation of progenitors in the liver: a matter of local choice. The Journal of Clinical Investigation. 2013;123(5):1867–1873. doi: 10.1172/JCI66026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raven A., Lu W. Y., Man T. Y., et al. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547(7663):350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu W.-Y., Bird T. G., Boulter L., et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nature Cell Biology. 2015;17(8):971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Russell J. O., Monga S. P. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annual Review of Pathology. 2018;13(1):351–378. doi: 10.1146/annurev-pathol-020117-044010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang M.-J., Chen F., Liu Q. G., et al. Insulin-like growth factor 2 is a key mitogen driving liver repopulation in mice. Cell Death & Disease. 2018;9(2):p. 26. doi: 10.1038/s41419-017-0186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hsieh W.-C., Mackinnon A. C., Lu W. Y., et al. Galectin-3 regulates hepatic progenitor cell expansion during liver injury. Gut. 2015;64(2):312–321. doi: 10.1136/gutjnl-2013-306290. [DOI] [PubMed] [Google Scholar]
- 98.Yeoh G. C. T., Ernst M., Rose-John S., et al. Opposing roles of gp130-mediated STAT-3 and ERK-1/2 signaling in liver progenitor cell migration and proliferation. Hepatology. 2007;45(2):486–494. doi: 10.1002/hep.21535. [DOI] [PubMed] [Google Scholar]
- 99.Knight B., Yeoh G. C. T., Husk K. L., et al. Impaired preneoplastic changes and liver tumor formation in tumor necrosis factor receptor type 1 knockout mice. The Journal of Experimental Medicine. 2000;192(12):1809–1818. doi: 10.1084/jem.192.12.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Viebahn C. S., Benseler V., Holz L. E., et al. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. Journal of Hepatology. 2010;53(3):500–507. doi: 10.1016/j.jhep.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 101.Lowes K. N., Brennan B. A., Yeoh G. C., Olynyk J. K. Oval cell numbers in human chronic liver diseases are directly related to disease severity. American Journal of Pathology. 1999;154(2):537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Viebahn C. S., Yeoh G. C. T. What fires Prometheus?: The link between inflammation and regeneration following chronic liver injury. The International Journal of Biochemistry & Cell Biology. 2008;40(5):855–873. doi: 10.1016/j.biocel.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 103.Lim R., Hodge A., Moore G., Wallace E. M., Sievert W. A pilot study evaluating the safety of intravenously administered human amnion epithelial cells for the treatment of hepatic fibrosis. Frontiers in Pharmacology. 2017;8:549–549. doi: 10.3389/fphar.2017.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lim R., Malhotra A., Tan J., et al. First-in-human administration of allogeneic amnion cells in premature infants with bronchopulmonary dysplasia: a safety study. Stem Cells Translational Medicine. 2018;7(9):628–635. doi: 10.1002/sctm.18-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sant'anna L. B., Cargnoni A., Ressel L., Vanosi G., Parolini O. Amniotic membrane application reduces liver fibrosis in a bile duct ligation rat model. Cell Transplantation. 2011;20(3):441–453. doi: 10.3727/096368910X522252. [DOI] [PubMed] [Google Scholar]
- 106.Ricci E., Vanosi G., Lindenmair A., et al. Anti-fibrotic effects of fresh and cryopreserved human amniotic membrane in a rat liver fibrosis model. Cell and Tissue Banking. 2013;14(3):475–488. doi: 10.1007/s10561-012-9337-x. [DOI] [PubMed] [Google Scholar]