Abstract
Objective:
The present study was aimed to evaluate dose-dependent effects of phytobiotic (Galibiotic) supplements in feed on growth performance, hematological parameters, intestinal pH, and gut bacterial population in broiler chick.
Materials and Methods:
A total of 50 ten day old broiler chicks were divided into five groups, namely, Group A as control (without galibiotic), Group B (galibiotic at 1 gm/kg feed), Group C (galibiotic at 2 gm/kg feed), Group D (galibiotic at 5 gm/kg feed), and Group E (galibiotic at 10 gm/kg feed). All the birds were reared for 42 days and samples were collected before and after sacrifice.
Results:
Live body weights showed no significant differences between the groups but overall feed conversion ratios (FCRs) of treatment groups were significantly low in Group E having the lowest. Blood samples collected for hematology differed significantly (p < 0.01) among the different groups. Intestinal pH was lower in treatment groups with Group E having the lowest. Cecal total viable count was highest in Group A and lowest in Group E. The cecal coliform count was low in all the treatment groups.
Conclusion:
Along with previously published report, it may conclude that the phytobiotic could be used as an alternative to antibiotics due to positive growth performance, lower FCR, carcass quality, and improved gut health of broiler chicks.
Keywords: Growth promoter, phytobiotic, hematology, intestinal pH, gut bacteria, broiler
Introduction
Antibiotics use in livestock production as growth promoters have paved the way for the emergence of resistant bacteria many of which are pathogenic to humans [1]. Cross-resistance and co-resistance of antibiotics in pathogenic bacteria have been strongly linked to therapeutic and/or prophylactic uses of antibiotics in human and veterinary practice [2]. Due to possible negative consequences to human and animal health as well as food safety, several countries have banned the use of antibiotics as growth promoters in recent years [3]. There is an urgent need to develop alternatives to antibiotics due to public health concerns and the demand from farmers to prevent the economic losses and to control infectious organisms like Salmonella, Escherichia coli at feed level.
Poultry industry has seen an unparalleled growth in the last three decades and is now recognized as one of the fastest growing subsectors of agriculture due to increased consumption of eggs, meat, ease of access, relatively low cost, and rich in most essential nutrients [4]. The gut health of poultry is very much important site where nutrient uptake takes place. Therefore, in recent years, gut health of poultry has been the area of intense studies for demanding increase poultry production [5]. Impaired gut function causes poor digestion, absorption of nutrients, and finally poor performance which ultimately affect the economics of poultry industries. Beneficial effects of using antibiotics like thickening of intestine which leads to more nutrient absorption is being questioned, owing to increase antibiotic resistance [6]. Use of antibiotics as sub-therapeutic doses in food animals eliminates the essential sensitive bacterial population leaving the variants with unusual resistant traits. The resistant population transmits the resistance gene which is genetically defined to subsequent progeny and also to other bacterial strains via mutation or plasmid mediated transfer [7]. Humans may get exposed to such resistant bacteria population through consumption and handling of meat and eggs contaminated with such pathogens [8]. Once these are acquired, such resistant bacteria can colonize the intestinal tract of human and the genes coding for resistance to antibiotics in these bacteria can be transferred to other bacteria belonging to the endogenous microflora of humans, thus causing delay in treatment of bacterial infections.
Phytobiotics are secondary plant metabolites. They are now seen as antioxidants, digestive enhancers, nutraceuticals, and health promoting substances [9]. They are used as growth promoters in food animal production, especially monogastrics [10]. Phytobitics have been also found to modify the gut microflora positively by reducing the number of pathogenic organisms [11]. Galibiotic (phytobiotic) is a mixture of medium chain fatty acids derived from coconut oil and palm kernel meal. It is seen as a good alternative to nutritional antibiotics in poultry, due to the high antibacterial activity of the medium chain fatty acids. Research in poultry has proven medium chain fatty acids as good alternatives to antibiotic growth promoters [12]. The present study was focused on to evaluate the effects of different doses of phytobiotic (galibiotic) on hematology, growth performance, intestinal pH modification, and gut bacterial population in broiler.
Materials and Methods
All the experimental procedures were performed according to the guidelines for the care and use of animals as established by Animal Welfare and Ethical Committee, Bangladesh Agricultural University, Mymensingh [Approval number: AWEEC/BAU/2018(11)]. The experimental shade, housing Feeders, drinkers, buckets, and all other equipments were properly washed and disinfected. According to the manufacturer’s instruction, the inclusion rate of the phytobiotic in commercial broiler feed was 250 gm/100 kg feed. The broiler chicks were fed with broiler starter for 14 days and broiler grower from 15 to 42 days of age, feeds were the products of Quality Feeds Ltd., Bangladesh. A total of 50 commercial broiler chicks (day old) of either sex were collected from Kazi Farms Ltd., Bangladesh. After 1 week of brooding, birds were divided into five groups of 10 each. Birds of Group A were supplied only commercial diet, Group B commercial diet with galibiotic at 1 gm/kg feed, Group C commercial diet with galibiotic 2 gm/kg feed, Group D commercial diet with galibiotic at 5 gm/kg feed, and Group E commercial diet with galibiotic at 10 gm/kg feed. Body weights were recorded every week. On day 21 and 42, five birds from each group were sacrificed for intestinal pH measurement and bacteriological analysis of both feces and intestinal content. Standard vaccination program for broilers was maintained, and strict biosecurity inside and outside of the research shed was followed. For bacteriological analysis, plate count agar (PCA) media was used for total viable count (TVC); Eosin Methylene Blue (EMB) agar media was used for total coliform count (TCC) and Salmonella-Shigella agar (SS agar) media was used for total Salmonella count.
Immunization by vaccination
The following vaccination schedule was maintained during the experimental period to prevent the birds from common viral diseases. Vaccines were purchased from FnF Pharmaceuticals Ltd. Bangladesh and were administered as per the manufacturer’s instructions. On seventh day of age, Baby Chicks Ranikhet Disease Vaccine (BCRDV) was administered as eye drop followed by booster doses were given on 21st and 24th days of age. On 11th day of age, Gumboro vaccine was administered as eye drop.
Hematology tests
Hemoglobin concentration (Hb), packed cell volume (PCV), erythrocyte sedimentation rate (ESR), and total erythrocyte count (TEC) were determined using approximately 3 ml of blood collected from the wing vein of birds in each group within 2 h of collection.
Measurement of pH
The pH test of small and large intestinal contents was performed with Adwa AD 1020 pH Meter. Samples of large and small intestines were collected in petri dishes immediately after slaughter. The intestines were opened with sterile scissors and pH was measured by inserting a glass pH electrode probe.
Enumeration of TVC, TCC, and Total Salmonella Count (TSC)
TVC, TCC, and TSC were determined by single plate dropping method described by Thomas et al. [13]. Shortly, 900-ml phosphate buffer solution containing eight Eppendorf tubes were taken. Nearly, 100 μl of suspension was used to prepare 10 serial fold dilution of each content. PCA plate, EMB agar plate, and SS agar plates were divided into eight parts using marker. Three drops (each drop was 10 μl) from each dilution was placed on each part carefully. After completion of dropping from each dilution, the plate was allowed for drying off at room temperature. After drying off, all the plates were transferred to bacteriological incubator and incubated at 37°C for overnight for the development of countable colonies. The countable colonies at particular dilution were counted and TVC, TCC, and TSC were determined by the calculation.
Body weights and feed conversion ratio
Body weights of the experimental birds were recorded using electric balance weekly. Feed consumption was calculated as the total feed consumed in each group divided by the number of birds in each group. The amount of feed consumed per unit of weight gain was calculated and shown as feed conversion ratio (FCR). At the end of the experiment, birds were slaughtered to determine the growth performance, such as carcass weight and dressing weight.
Statistical analysis
During the study period, body weights were measured weekly for 6 weeks. Samples were collected and hematological and microbiological parameters were studied. Data were analyzed with the help of Graph Pad Prism 6. The mean differences among the treatment groups were determined using one way analysis of variance followed by Bonfferoni post hoc test.
Results
Higher TEC was observed in phytobiotic treated groups compared to control (p < 0.01) (Table 1). Group E having the highest TEC and lowest in Group A by 6 weeks. Highest hemoglobin concentration was seen in Group E and lowest in Group A with significance between Groups C, E and control (p < 0.01). However, Group C had the highest PCV with control group having the lowest. Significant difference was observed between Groups C, E and control on day 42 (p < 0.05). Group E had the best hematology results but Group C was the most cost effective.
Table 1. Average hematological parameters in different groups of broilers at days 21 and 42 (n = 5).
| Day 21 | Day 42 | |||||||
|---|---|---|---|---|---|---|---|---|
| Group | TEC (m/mm3) | Hb (gm/dl) | PCV (%) | ESR | TEC (m/mm3) | Hb (gm/dl) | PCV (%) | ESR |
| A | 2.53 ± 0.1c | 6.68 ± 0.2c | 22.00 ± 2.0b | 5.40 ± 0.6a | 2.72 ± 0.1b | 6.88 ± 0.2c | 24.60 ± 1.1ce | 5.40 ± 0.1 |
| B | 2.64 ± 0.1bc | 6.78 ± 0.1c | 22.80 ± 3.0b | 4.00 ± 0.7b | 2.79 ± 0.1b | 7.08 ± 0.1b | 25.20 ± 1.2b | 4.60 ± 0.1 |
| C | 2.73 ± 0.1b | 7.44 ± 0.2b | 26.20 ± 1.6a | 4.40 ± 0.6b | 3.06 ± 0.1a | 7.92 ± 0.1a | 28.00 ± 1.2a | 5.20 ± 1.1 |
| D | 2.53 ± 0.1c | 6.88 ± 0.1c | 23.00 ± 1.6b | 4.00 ± 0.7b | 2.80 ± 0.1b | 6.96 ± 0.1c | 25.80 ± 1.5b | 4.40 ± 0.9 |
| E | 2.87 ± 0.1a | 7.92 ± 0.3a | 27.40 ± 2.5a | 4.40 ± 0.9b | 3.12 ± 0.1a | 8.04 ± 0.1a | 26.80 ± 2.1ab | 5.00 ± 1.4 |
Values with different superscripts in the same column differ significantly (p < 0.05).
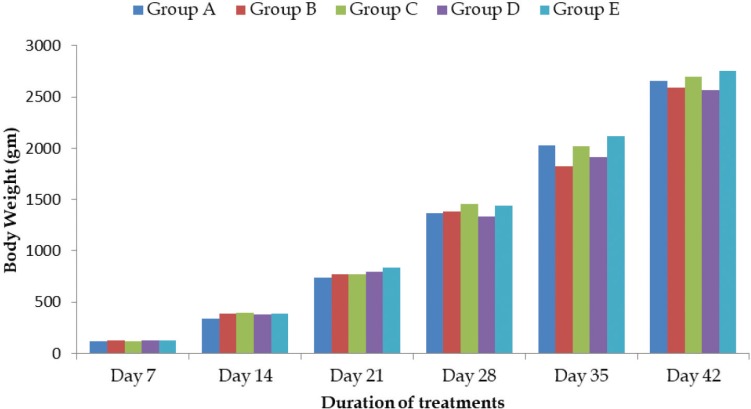
With respect to weight gain, there was no significant difference (p > 0.05) among the groups (Fig. 1). The highest body weight and gain values were recorded in Group E and lowest in Group D at 6 weeks of age. The most cost effective production was observed when galibiotic was given at 2 gm/kg feed (Group C). These results suggested that the use of galibiotic at 10 gm/kg feed and galibiotic at 2 gm/kg feed in broilers is useful in helping them to record higher gain in weight compared to all other groups. The highest cost of production was recorded in control group (Table 2). No statistical difference in body weight was recorded among the groups during experimental period.
Figure 1. Effect of different doses of phytobiotic on average body weights in treatment groups of broilers.
Table 2. Feed intake and FCR in birds of different experimental groups.
| Group | IW (gm) | FWG (gm) | TWG (gm) | FI (gm) | FCR | Cost/kg production |
|---|---|---|---|---|---|---|
| A | 115 ± 3.78 | 2658 ± 148.1 | 2,543 | 4,806b | 1.89b | 94.5 |
| B | 124.3 ± 5.04 | 2591 ± 121.2 | 2,466.7 | 4,242a | 1.72a | 86.8 |
| C | 121.3 ± 1.5 | 2693 ± 227.3 | 2,571.7 | 4,320a | 1.68a | 85.6 |
| D | 123.0 ± 1.3 | 2569 ± 60.75 | 2,446 | 4,256a | 1.74a | 91 |
| E | 122.5 ± 2.87 | 2754 ± 193.9 | 2,631.5 | 4,263a | 1.62a | 89 |
Values with different superscript letter(s) in the same column differ significantly (p < 0.05).
On day 21, the pH of all the treatment groups was significantly decreased compare to control Group A (p < 0.05). The lowest (5.450 ± 0.0173) intestinal pH was recorded in Group E (galibiotic at 10 gm/kg feed) and highest (5.747 ± 0.01453) in control Group A. On day 42, the pH of all the treatment groups was significantly decreased comparing to control group (p < 0.05). The lowest (5.437 ± 0.0176) intestinal pH was recorded in Group E (galibiotic at 10 gm/kg feed) and highest (5.637 ± 0.0088) in control Group A (Table 3).
Table 3. Intestinal pH (mean ± SE) in different groups on different days (n = 5).
| Group | 21 days | 42 days |
|---|---|---|
| A | 5.747 ± 0.01453b | 5.637 ± 0.008819b |
| B | 5.573 ± 0.02333a | 5.513 ± 0.008819a |
| C | 5.527 ± 0.006667a | 5.493 ± 0.008819a |
| D | 5.547 ± 0.01202a | 5.500 ± 0.01155a |
| E | 5.450 ± 0.01732a | 5.437 ± 0.01764a |
Values with different superscript letter(s) in the same column differ significantly (p < 0.05).
The TVC, TCC, and TSC were 1.2 × 108, 5 × 107, and 2 × 103, respectively, in birds before treatment. TVC and TCC count were higher in control group than the treatment groups by 6 weeks (Table 4). TSC count was nil in all the groups. This could suggest that rearing birds for longer time (here 42 days) can completely suppress Salmonella growth. On day 21, TVC, TCC, and TSC counts were not statistically significant between treated groups and control (p > 0.05). Although TSC count was nil in intestinal contents, we found positive count of TSC in faces of broilers. Group E had the lowest TVC, TCC, and TSC count and best growth performances with least maintenance energy for intestinal microflora.
Table 4. Average colony forming units in different groups of broilers.
| Group | 21 days | 42 days | ||||
|---|---|---|---|---|---|---|
| TVC (CFU/gm sample) | TCC (CFU/gm sample) | TSC (CFU/gm sample) | TVC (CFU/gm sample) | TCC (CFU/gm sample) | TSC (CFU/gm sample) | |
| A | 2.8 × 109 | 1.0 × 105 | 5.02 × 104 | 1.6 × 109 | 1.4 × 106 | 0 |
| B | 5.3 × 108 | 4.5 × 104 | 4.65 × 103 | 1.5 × 108 | 6.5 × 105 | 0 |
| C | 1.5 × 109 | 2.5 × 104 | 3.25 × 103 | 9.61 × 107 | 4.25 × 105 | 0 |
| D | 9.3 × 108 | 7.5 × 104 | 1.5 × 104 | 1.4 × 108 | 9.75 × 105 | 0 |
| E | 9.5 × 108 | 9.75 × 103 | 1.1 × 103 | 2.4 × 107 | 6.5 × 104 | 0 |
Discussion
Beyond the beneficial effects, maximum residual limit of drugs residues in poultry meat and eggs has led to the development of antibiotic resistance pathogens. The results of the present study suggested that the phytobiotic (Galibiotic) might be an alternative to antibiotics as growth promoter in broiler industry. Our findings of increase in body weight gain in treated groups is in agreement with Shahram et al. [14]. Bhujbal et al. [15] observed more weight gain in phytobiotic treated groups than their control group though no statistical significance. However, it differed from Fasanmi et al. [16], Ertas et al. [17], and Cross et al. [18] who reported significant difference in weight gain between phytobiotic fed broiler groups and control group. This is probably because of difference in sources of phytobiotics, dose variation, management, and environmental factors.
On day 21, pH levels of all the treatment groups were significantly decreased compared to control Group A (p < 0.05). The lowest (5.450 ± 0.0173) intestinal pH was recorded in group E (galibiotic at 10 gm/kg feed) and highest (5.747 ± 0.01453) in control group. Again on day 42, the pH levels of treatment groups significantly decreased compared to control group (p < 0.05). The lowest intestinal pH was recorded in Group E (5.437 ± 0.0176) and highest in control group (5.637 ± 0.0088). Lower pH in the treatment groups might be due to medium chain fatty acids present in galibiotics, health of the chicken, kind of nutrients, and more important increase essential microflora content in the gastrointestinal tract (GIT). Previous study reported that there was a mutual correlation exists between the pH, micorflora, and nutrients [19]. The pH level in specific areas of the GIT is a factor which establishes a specific microbial population and also affects the digestibility and absorptive value of most nutrients. Most of the pathogens grow in a pH close to 7 or slightly higher. In contrast, beneficial microorganisms live in a lower pH and compete with pathogens [20]. In addition, lowering the pH by phytobiotic improves nutrient absorption [21], thus improve growth performances. Our findings agree with this statement. These results are in harmony with the result of the Al-Tarazi and Alshawabkeh [22] who reported that adding phytobiotic to broiler diet reduced crop and cecal pH significantly. However, Hernandez et al. [23] and Al-Natour and Alshawabkeh [24] found insignificant reduction in the intestinal pH in broilers.
As shown in Table 4, on day 21, TVC, TCC, and TSC count were higher in control group than the treatment groups. This is probably because galibiotic containing medium chain fatty acids can penetrate inside the bacteria in a non-dissociated form. Once in the protoplasm, the bacteria dissociate them, which in turn lead to an increase in concentrations of hydrogen ions [25], thus lower intracellular pH may promote inactivation of bacterial enzymes leading to cell death [26–28]. Higher TVC count suggests that the nutrients were more utilized by microflora in control group than all the treatment groups resulting in less nutrients utilization by the bird itself. Higher TCC and TSC count suggest that more pathogenic bacteria inhabit the intestinal content which is detrimental for beneficial microflora and also responsible for disease production and immune suppression. As Group E (galibiotic at 10 gm/kg feed) had the lowest TVC, TCC, and TSC count, it caused best growth performances with least maintenance energy for intestinal microflora. This is in agreement with Bhujbal et al. [15] who found lower mean TVC count in phytobiotic treatment groups than the control group resulting to best growth performances. On day 42, TVC and TCC count were higher in control group than the treatment groups. TSC count was totally nil in all the experimental groups. This could suggest that rearing birds for longer time (here 42 days) can completely suppress Salmonella growth. Group E (galibiotic at 10 gm/kg feed) had the lowest TVC and TCC count with best growth performances. This is in agreement with Guo et al. [29–31], Dierick et al. [32], and Bhujbal et al. [15] who found that phytobiotics and their extracts could improve the growth performance by reducing the population of harmful bacterial microflora and enhancing both cellular and humoral immune responses of chickens [26].
On day 42, similar trend of data were found as observed in day 21 that is higher (p > 0.05) TVC, TCC, and TSC counts were recorded in control group than the treatment groups. Although TSC count was nil from intestinal content, we found positive count of TSC in faces of the broilers. This could be because of environmental contamination. Along with previously published report, it indicates that dietary supplementation of phytobiotic improved the gut health by decreasing the cecal TCC [33,34]. Further investigations are needed to determine the molecular mechanical effects of phytobiotic as regards their compositional analysis.
Conclusions
Although galibiotic at 10 gm/kg in feed showed a significantly lowest intestinal pH, lower total viable count and lowest total coliform count and the best growth performances among all the groups; however, we got most cost effective performance with galibiotic at 2 gm/kg in feed. Finally, it may be concluded that phytobiotic in feed may be used as a growth promoter in broiler production.
Acknowledgments
This work was supported by Special Allocation Project for the Year 2015–2016 under the Ministry of Science & Technology to Kazi Rafiqul Islam (Number and date of sanction order: 39.00.0000.009.002.057.2015-2016/BS-17/946, Date: 08-12-2015).
Conflict of Interests
Md. Mustafijur Rahman Ripon and Md. Harunur Rashid have equal contribution to this work. Beside that all authors declare that they have no other conflict of interest.
Authors’ contribution
Md. Mustafijur Rahman Ripon and Md. Harunur Rashid performed all the experiments and drafted the manuscript. Kazi Rafiq, Muhammad Tofazzal Hossain, and Muslah Uddin Ahammad designed and supervised the research work. Kazi Rafiq prepared and finalized the manuscript. Aminatu Abubakar Sani, Md. Moshiur Rahman, Md. Faisal Ferdous, and Md. Shafiul Arefin did the statistical analyses. All authors read and approved the final version of the manuscript.
References
- [1].Miranda JM, Guarddon M, Vázquez BI, Fente CA, Barros-Velázquez J, Cepeda A, et al. Antimicrobial resistance in Enterobacteriaceae strains isolated from organic chicken, conventional chicken and conventional turkey meat: a comparative survey. Food Control. 2008;19(4):412–6. doi: 10.4315/0362-028x-70.4.1021. [DOI] [PubMed] [Google Scholar]
- [2].Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–12. doi: 10.1093/jn/125.6.1401. https://doi.org/10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- [3].Fernando U, Biswas D, Allan B, Wilson P, Potter AA. Influence of Campylobacter jejuni fliA, rpoN and flgK genes on colonization of the chicken gut. Int J Food Microbiol. 2007;118:194–200. doi: 10.1016/j.ijfoodmicro.2007.07.038. https://doi.org/10.1016/j.ijfoodmicro.2007.07.038. [DOI] [PubMed] [Google Scholar]
- [4].Dhama K, Latheef SK, Saminathan M, Samad HA, Karthik K, Tiwari R, et al. Multiple beneficial applications and modes of action of herbs in poultry health and production—a review. Int J Pharmacol. 2015;11:152–76. https://doi.org/10.3923/ijp.2015.152.176. [Google Scholar]
- [5].Rinttila T, Apajalahti J. Intestinal microbiota and metabolites—implications for broiler chicken health and performance. J Appl Poult Res. 2013;22:647–58. https://doi.org/10.3382/japr.2013-00742. [Google Scholar]
- [6].Tiwari R, Chakraborty S, Saminathan M, Dhama K, Singh SV. Ashwagandha (Withania somnifera): role in safeguarding health, immunomodulatory effects, combating infections and therapeutic applications: a review. J Biol Sci. 2014;14:77–94. https://doi.org/10.3923/jbs.2014.77.94. [Google Scholar]
- [7].Catry B, Laevens H, Devriese LA, Opsomer G, De Kruif A. Antimicrobial resistance in livestock. J Vet Pharmacol Ther. 2003;26:81–93. doi: 10.1046/j.1365-2885.2003.00463.x. https://doi.org/10.1046/j.1365-2885.2003.00463.x. [DOI] [PubMed] [Google Scholar]
- [8].Van den Bogaard AE, Stobberingh EE. Epidemiology of resistance to antibiotics. Links between animals and humans. Int J Antimicrob Agents. 2000;14:327–35. doi: 10.1016/s0924-8579(00)00145-x. https://doi.org/10.1016/S0924-8579(00)00145-X. [DOI] [PubMed] [Google Scholar]
- [9].Narimani-Rad M, Nobakht A, Shahryar HA, Kamani J, Lotfi A. Influence of dietary supplemented medicinal plants mixture (ziziphora, oregano and peppermint) on performance and carcass characterization of broiler chickens. J Med Plants Res. 2011;5:5626–9. [Google Scholar]
- [10].Karangiya VK, Savsani HH, Patil SS, Garg DD, Murthy KS, Ribadiya NK, et al. Effect of dietary supplementation of garlic, ginger and their combination on feed intake, growth performance and economics in commercial broilers. Vet World. 2016;9:245–50. doi: 10.14202/vetworld.2016.245-250. https://doi.org/10.14202/vetworld.2016.245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salim AB. Effect of some plant extracts on fungal and aflatoxin production. Int J Acad Res. 2011;3:116–20. [Google Scholar]
- [12].Mathis GF. New research into feed additives to control necrotic enteritis. Poult World. 2006;22:3–5. [Google Scholar]
- [13].Thomas P, Sekhar AC, Upreti R, Mujawar MM, Pasha SS. Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast CFU enumeration and single colony isolation from diverse samples. Biotechnol Rep. 2015;8:45–55. doi: 10.1016/j.btre.2015.08.003. https://doi.org/10.1016/j.btre.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shahram M, Habib AS, Yahya E, Alireza A, Behnam T. Effect of different levels of medium chain fatty acids on performance, and some of microbial population of gastro in broiler chicks. Res J Chem Environ Sci. 2013;1(4):5–7. [Google Scholar]
- [15].Bhujbal RN, Patil MB, Kank VD, Ranade AS, Gadegaonkar GM, Karambele NR, et al. Effects of phytobiotics on the performance of broilers. J Bombay Vet Coll. 2009;17:33–5. [Google Scholar]
- [16].Fasanmi OG, Oladele-Bukola MO, Balogun FA, Olona JF, Okuneye OJ. Growth performance characteristics haematology and serum biochemistry of finisher broilers fed diet containing aromabiotic and neobacin. Global Sci Res J. 2015;3:132–7. [Google Scholar]
- [17].Ertas ON, Güler T, Çiftçi M, Dalkiliç B, Simsek ÜG. The effect of an essential oil mix derived from oregano, clove and anise on broiler performance. Int J Poult Sci. 2005;4:879–84. https://doi.org/10.3923/ijps.2005.879.884. [Google Scholar]
- [18].Cross DE, Mcdevitt RM, Hillman K, Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br Poult Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. https://doi.org/10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- [19].Sarra PG, Dellaglio F, Bottazzi V. Taxonomy of lactobacilli isolated from the alimentary tract of chickens. Syst Appl Microbiol. 1985;6:86–9. https://doi.org/10.1016/S0723-2020(85)80017-5. [Google Scholar]
- [20].Ferd DJ. The effect of microflora on gasrrointestinal pH in the chick. Poult Sci. 1974;53:115–31. https://doi.org/10.1080/00071667408416086. [Google Scholar]
- [21].Boling SD, Snow JL, Parsons CM, Baker DH. The effect of citric acid on calcium and phosphorus requirements of chicks fed corn-soybean meal diets. Poult Sci. 2001;80:783–8. doi: 10.1093/ps/80.6.783. https://doi.org/10.1093/ps/80.6.783. [DOI] [PubMed] [Google Scholar]
- [22].Al-Tarazi YH, Alshawabkeh K. Effect of dietary formic and propionic acids on Salmonella pullorum shedding and mortality inlayer chicks after experimental infection. J Vet Med. 2003;50:112–7. doi: 10.1046/j.1439-0450.2003.00644.x. https://doi.org/10.1046/j.1439-0450.2003.00644.x. [DOI] [PubMed] [Google Scholar]
- [23].Hernandez F, Garcia V, Madrid J, Orengo J, Catala P, Megias MD. Effect of formic acid on performance, digestibility, intestinal histomorphology and plasma metabolite levels of broiler chickens. Br Poult Sci. 2006;47:50–6. doi: 10.1080/00071660500475574. https://doi.org/10.1080/00071660500475574. [DOI] [PubMed] [Google Scholar]
- [24].AL-Natour M, AL-Shawabkeh KM. Using varying levels of formic acid to limit growth of Salmonellagallinarum in contaminated broiler food. Asian Australas J Anim Sci. 2005;18:390–5. https://doi.org/10.5713/ajas.2005.390. [Google Scholar]
- [25].Sun CQ, O’connor CJ, Turner SJ, Lewis GD, Stanley RA, Roberton AM. The effect of pH on the inhibition of bacterial growth by physiological concentrations of butyric acid: implications for neonates fed on suckled milk. Chem Biol Interact. 1998;113:117–31. doi: 10.1016/s0009-2797(98)00025-8. https://doi.org/10.1016/S0009-2797(98)00025-8. [DOI] [PubMed] [Google Scholar]
- [26].Chen Y, Wen C, Zhou Y. Dietary synbiotic incorporation as an alternative to antibiotic improves growth performance, intestinal morphology, immunity and antioxidant capacity of broilers. J Sci Food Agric. 2018;98(9):3343–50. doi: 10.1002/jsfa.8838. https://doi.org/10.1002/jsfa.8838. [DOI] [PubMed] [Google Scholar]
- [27].Olukosi OA, Dono ND. Modification of digesta pH and intestinal morphology with the use of benzoic acid or phytobiotics and the effects on broiler chicken growth performance and energy and nutrient utilization. J Anim Sci. 2014;92(9):3945–53. doi: 10.2527/jas.2013-6368. https://doi.org/10.2527/jas.2013-6368. [DOI] [PubMed] [Google Scholar]
- [28].Viegas CA, Sa-correia I. Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J Gen Microbiol. 1991;137:645–51. doi: 10.1099/00221287-137-3-645. https://doi.org/10.1099/00221287-137-3-645. [DOI] [PubMed] [Google Scholar]
- [29].Guo FC, Kwakkel RP, Williams BA, Li WK, Li HS, Luo JY, et al. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on growth performance of broiler. Science. 2004a;45:684–94. doi: 10.1080/00071660400006214. https://doi.org/10.1080/00071660400006214. [DOI] [PubMed] [Google Scholar]
- [30].Guo FC, Williams BA, Kwakkel R.P, Li WK. Effect of mushroom and herb polysaccharides , as alternatives for an antibiotic, on the caecal microbial ecosystem in broiler chicken. Poult Sci. 2004b;83:175–82. doi: 10.1093/ps/83.2.175. https://doi.org/10.1093/ps/83.2.175. [DOI] [PubMed] [Google Scholar]
- [31].Guo FC, Kwakkel RP, Williams BA. Effect of mushroom and poly saccherides on cellular and humoral immune response of Eimeriatenella infected chickens. Poult Sci. 2004c;83:1124–32. doi: 10.1093/ps/83.7.1124. https://doi.org/10.1093/ps/83.7.1124. [DOI] [PubMed] [Google Scholar]
- [32].Dierick N, Decuypere J, Molly K, Van Beek E, Vanderbeke E. The combined use of triacylglycerols (TAGs) containing medium chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative for nutritional antibiotics in piglet nutrition. II. In vivo release of MCFAs in gastric cannulated and slaughtered piglets by endogenous and exogenous lipases; effects on the luminal gut flora and growth performance. Livest Prod Sci. 2002;76:1–16. https://doi.org/10.1016/S0301-6226(01)00331-1. [Google Scholar]
- [33].Salah AS, Ahmed-Farid OA, El-Tarabany MS. Carcass yields, muscle amino acid and fatty acid profiles, and antioxidant indices of broilers supplemented with synbiotic and/or organic acids. J Anim Physiol Anim Nutr. 2018;103(1):41–52. doi: 10.1111/jpn.12994. https://doi.org/10.1111/jpn.12994. [DOI] [PubMed] [Google Scholar]
- [34].Michael G. Use of phytobiotics in broiler nutrition—an alternative to infeed antibiotics? J Anim Feed Sci. 2010l;19:50–8. https://doi.org/10.22358/jafs/66297/2010. [Google Scholar]