Abstract
Objective:
The study was carried out to detect the carriage of methicillin-resistant Staphylococcus aureus (MRSA) and their antimicrobial susceptibilities in village chickens sold at Maiduguri poultry markets using phenotypic characterization.
Materials and Methods:
This was a cross-sectional study where 120 samples comprised 50% each of Nasal and cloacal swabs, were, respectively, collected from live village chickens sold at Maiduguri poultry markets and examined for the presence MRSA based on bacterial culture, biochemical characteristics, growth on oxacillin resistant screening agar base, and antimicrobial susceptibility tests.
Results:
The study revealed an overall occurrence of 38.33% S. aureus and 32.60% MRSA, respectively. Antimicrobial susceptibility test was carried out on MRSA positive isolates against seven antimicrobials. All the isolates (100%) exhibited resistance against cefoxitin, whereas the least antimicrobial resistance was recorded against erythromycin and streptomycin each with 26.6%, respectively. In the same way, the highest antibiotic susceptibility in this study was observed against erythromycin (60%) and least susceptibility was against vancomycin and streptomycin with 20% each. A varying intermediate antibiotic susceptibility ranging from 13.33% to 53.33% was observed. Multiple-drug resistance patterns were exhibited by MRSA isolates from this study with 73.3% of the isolates exhibiting resistance to two or more antibiotics.
Conclusion:
This study has shown the carriage of MRSA by village chickens which calls for serious public health concern and concludes that these birds might have acquired these pathogens from the environment or infected humans since they normally receive no less medical attention.
Keywords: Antibiotics, Maiduguri, MRSA, village chickens
Introduction
The development and acquisition of antimicrobial resistance (AMR) in bacteria is a world-wide problem confronting both animal and human health [1]. The greatest concern for this is poised by the heightened use of antimicrobials in animal husbandry which could lead to increased development of resistance by human and animal pathogens [2]. The high antimicrobial use as a result of intensive farming had made the transmission of AMR pathogens from livestock to humans, and/or vice-versa a major risk today [1]. Staphylococcus aureus is a known commensal and pathogen of a wide range of animal species including humans. This pathogen is known to be associated with different forms of infections including superficial skin and soft-tissue infections, and life threatening conditions such as endocarditis, toxic shock syndrome, and necrotizing pneumonia [3,4]. The common carriage site for the bacterium is the skin, anterior nares, and oral cavity of man and animals [5–7].
Methicillin-resistant S. aureus (MRSA) was first reported in 1961 when it emerged as a major pathogen of public health importance in both nosocomial and community settings [8,9]. MRSA has the ability to colonize and cause different forms of infections in a wide variety of host species including livestock, wildlife, and companion animals and humans [10]. The presence of MRSA in animals can serve as an important reservoir of zoonotic infections in humans beside the impacts they poses on the health and welfare of animals [10,11].
In poultry, S. aureus is associated with the skeleton and skeletal muscle infections, and this represents a burden for the poultry industry, particularly broilers [11]. Several studies have reported the presence of MRSA in poultry and poultry products with special attention given to exotic birds such as broilers and layers [12–16]. However, there is a paucity of information on the carriage of these pathogens in village chickens in which the most cases are reared under the extensive management systems with no specialize housing, and allowed to feed as scavengers. Even though village chickens are given no serious medical attention, it is important to study and determine the carriage of antibiotic resistant pathogens in these birds since they could serve as important reservoirs of these pathogens. Therefore, this study was carried out to determine the carriage and antibiotic susceptibilities of MRSA in village chickens sold at Maiduguri poultry market.
Materials and Methods
Study design and sampling
This study was a cross-sectional one carried out on local chickens sold at major poultry markets (Monday market and custom market) in Maiduguri. A total of 120 (60 nasal and 60 cloacal swabs) samples were aseptically collected from 60 local chickens. The samples were collected by the gentle rotation of sterile swab-sticks dipped in peptone within the sampling sites (nares and cloacae). These were tightly closed, labeled, and packed on ice packs in polystyrene coolers for onward transportation to the Bacteriology research laboratory, Department of Veterinary Microbiology, University of Maiduguri for analysis.
Bacterial isolation and identification
Staphylococcus aureus was isolated and identified following the procedure of Ochei and Kolhatkar [17]. Briefly, each sample was inoculated into Peptone water (TITAN Biotech, Rajasthan, India) for enrichment, and incubated at 37°C for 24 h. This was sub-cultured onto freshly prepared mannitol salt agar (OXOID Basingstoke, UK) and incubated for 24 h at 37°C. The colonies presumptive of S. aureus (golden-yellow colonies) were phenotypically and biochemically confirmed using grams reaction, haemolysis on blood agar, catalase and coagulase tests.
Detection of methicillin resistance among S. aureus using ORSAB medium
Fresh S. aureus isolates were then sub-cultured onto oxacillin resistant screening agar base (ORSAB, OXOID, Basingstoke, UK) and incubated at 37°C for 18 h for preliminary screening of MRSA. The MRSA were identified by the production of deep blue colony which is an indication of mannitol fermentation by isolates that are oxacillin (methicillin) resistant [18].
Antimicrobial susceptibility test of MRSA
Antimicrobial susceptibility tests were conducted in vitro using Kirby Bauer’s disc diffusion method. The antimicrobials groups used in this study include: aminoglycosides, macrolides, tetracene, sulphonamide, phenicol, quinolones, glycopeptide, and semisynthetic cephamycin. Briefly, the turbidity of fresh (24-h broth culture) cultures of the MRSA isolates from Mueller–Hinton broth (OXOID, Basingstoke, UK) were adjusted to 0.5 McFarland standard and 1 ml of each was dispensed onto freshly prepared Mueller–Hinton agar (OXOID, Basingstoke, UK). This was evenly spread across the surface of the agar by gently tilting at different angles and allowed for 10 min. Antimicrobial sensitivity discs were then placed on the agar surface and allowed to stay for 15 min to allow for pre-diffusion of the antimicrobials. These were then incubated at 37°C. Following 24 h incubation, diameters of zones of inhibition were measured in millimeters using transparent ruler and the results interpreted based on Clinical and Laboratory Standards Institute guidelines [19]. The isolates were tested for susceptibility against cefoxitin (FOX 30 μg), erythromycin (ERY 30 μg), vancomycin (VAN 30 μg), nalidixic acid (NA 30 μg), tetracycline (TE 30 μg), sulfamethoxazole/trimethoprim (SXT 25 μg), streptomycin (S 10 μg), chloramphenicol (C 30 μg), and ciprofloxacin (CIP 5 μg) (OXOID, Basingstoke, UK).
Results presentation and data analysis
The results obtained from this study were summarized as percentages in the form of tables and charts for descriptive purposes. Fisher’s exact test was carried out to check for statistically significant association of the occurrence of MRSA in village chickens’ samples from different sample sites using GraphPad Instat (GraphPad software, 2365 Northside, Dr. Suite 560, San Diego, CA).
Results
Table 1 is a presentation of the occurrence of S. aureus and MRSA from the nares and cloaca of local chickens sampled in Maiduguri poultry market. A total occurrence 38.3% (46/120) of S. aureus was obtained with the highest occurrence of 40.0% (24/60) from the nares. However, there is no statistically significant association in S. aureus occurrence with respect to site of sample collection (p > 0.05, RR = 0.9167). The occurrence of MRSA among S. aureus isolated from chickens in this study is 32.6% (15/46). An occurrence of 41.1% (10/24) and 22.7% (5/22) were recorded from the nares and cloacae of birds, respectively. There is also no statistically significant association observed with the occurrence of MRSA in chickens with respect to sampling sites (p > 0.05, RR = 0.5455) (Table 1). Figure 1 shows the antibiotic susceptibility of MRSA isolates from local chickens in Maiduguri poultry markets. The highest susceptibility was recorded against erythromycin 60% (9/15) followed by sulfamethoxazole/trimethoprim 46.67% (10/15) and tetracycline 33.33% (5/15), while the least susceptibility was recorded against vancomycin and streptomycin having 20% (3/15), respectively. However, all the MRSA isolates were resistant to cefoxitin 100% (15/15). The least resistance was recorded against erythromycin 26.67% (4/15) and streptomycin 26.67% (4/15). The multi-drug resistance pattern of MRSA isolates from village chickens is shown on Table 2. Out of the 15 isolates, 80% (12/15) exhibited multiple-drug resistance to at least two different classes of antimicrobials. Nearly, 26% (4/15) resisted six antibiotics reflecting the highest multi-drug resistance by the isolates in the study. This is followed by 6.67% (1/15) that resisted five antibiotics, 20% (3/15) resisted four antibiotics, and 13.33% (2/15) displayed resistance against two antibiotics. It is important to note that all the isolates exhibited resistance against cefoxitin which is surrogate for testing for oxacillin resistance.
Table 1. Occurrence of S. aureus and MRSA in village chickens from poultry market in Maiduguri.
| Isolates | Sample sites | No. of sample | No. (%) positive | p value | RR | CI |
|---|---|---|---|---|---|---|
| S. aureus | Cloacae | 60 | 22 (36.7) | REF | NA | NA |
| Nares | 60 | 24 (36.7) | 0.7073 | 0.9167 | 0.5818–1.444 | |
| Total | 120 | 46 (38.3) | ||||
| MRSA | Cloacae | 22 | 5 (22.7) | REF | NA | NA |
| Nares | 24 | 10 (41.7) | 0.2919 | 0.5455 | 0.1817–1.376 | |
| Total | 46 | 15 (32.6) |
RR = relative risk; CI = confidence interval.
Figure 1. Antimicrobial susceptibility of MRSA from chickens sold at Maiduguri poultry market.
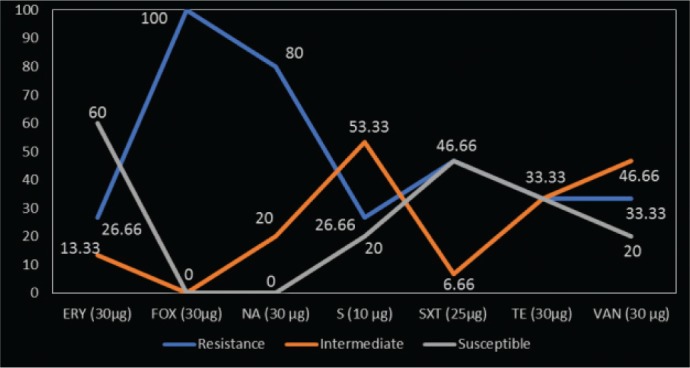
Table 2. Multi-drug resistant pattern of MRSA isolated in village chickens from Maiduguri poultry market.
| Multi-drug resistant pattern | No. of isolates (n = 15) |
|---|---|
| FOX-NA | 1 (6.67) |
| SXT-FOX | 2 (13.33) |
| TET-FOX-NA | 1 (6.67) |
| S-FOX-NA-CIP | 1 (6.67) |
| SXT-FOX-NA-ERY | 1 (6.67) |
| VAN-FOX-NA-ERY | 1 (6.67) |
| S-SXT-VAN-FOX-NA-ERY | 1 (6.67) |
| TET-SXT-VAN-FOX-NA-ERY | 3 (20.00) |
| Total | 11 (73.3) |
Discussion
Staphylococcus aureus is an important pathogen known to be associated with various forms of infections in human and economically important livestock such as large and small ruminants, poultry, and rabbit [20]. In chickens, this pathogen is associated with several disease manifestations such as comb necrosis [21], bacterial chondronecrosis, lameness [22], and septicemia which may affect a reasonable proportion of a flock [23].
The findings of this study revealed 38.3% occurrence of S. aureus in village chickens sampled at Maiduguri poultry markets which closely agrees with the work of Abdulrahman et al. [24] who reported 39.3% occurrence of this bacteria in the same target population from the same study area. However, our finding is lower than that of Suleiman et al. [25] 83%, Owuna et al. [26] 72.5%, and Kwoji et al. [14] 72.1%. This variation could be attributed to the differences in the sampled population. The occurrence of S. aureus in village chickens from this study may be due to the presence of S. aureus as a normal flora of the skin and which is often associated with poor hygiene as observed by previous studies [14,24].
The emergence of antimicrobial resistant pathogens poses serious threat for both human and veterinary medicine [27]. Studies have revealed the occurrence of MRSA as an increasingly emerging problem in Veterinary medicine, especially in small animals and poultry [28,29]. Previous reports have shown the occurrence of this “superbug” (MRSA) in a variety of poultry farms, abattoirs, carcasses, or food of poultry origin [14,16,30,31].
This study investigated the carriage of MRSA in village chickens from Maiduguri poultry markets and a total occurrence of 32.6% of MRSA was observed. The growth of the isolates on ORSAB, and a 100% drug resistance to Cefoxitin implies that these isolates are methicillin-resistant, and may harbor the mecA gene that confers methicillin resistance among S. aureus [16,18]. The finding of this work is in contrast to that of Otalu et al. [27] who reported 0% occurrence of MRSA from village chickens in Zaria, Nigeria. The detection of MRSA carriage by village chickens in this study even though they are less exposed to antibiotics compared with exotic chickens is an indication that the pathogen is becoming more spread in the environment and could pose serious threat to public health. The presence of MRSA in exotic chickens raised on commercial farms linked to frequent and uncontrolled use of antimicrobials has been reported in several studies in Nigeria with varying prevalence [14,15,27,32].
The antimicrobial susceptibility test of the MRSA isolates in this study revealed a varying degree of resistance and susceptibilities, with most isolates resisting at least two or more classes of the antimicrobials. From our result, all the isolates showed 100% resistance to cefoxitin. The high resistance to cefoxitin agrees with the result of Onanuga and Awhowho [33] who also reported the same finding in a study carried out at Yenagoa, Nigeria. This further confirmed that the isolates are methicillin resistant since cefoxitin is used as a surrogate for oxacillin when testing for MRSA [18]. Next to cefoxitin, 80% resistance to nalidixic acid which is similar to the finding of Chaalal et al. [34] was observed in this work. However, the resistance of MRSA to Erythromycin observed in this study is lower than the report of Kwoji et al. [35] who reported 91.7% resistance to erythromycin in a similar study conducted in Sokoto, Nigeria. This difference might be because the sample population in this present study is generally less exposed to antibiotics compared with what is obtainable in commercial poultry farms as positioned by Kwoji et al. [35]. The multi-drug resistance of MRSA to more than two classes of antibiotics as expressed by 73.3% of the isolates calls for serious concern and a need for an urgent intervention in other to tackle the menace of spreading of these pathogens. This is because some of these antibiotics such as vancomycin are considered drug of choice in the treatment of unresponsive human staphylococcal infections.
Conclusion
In conclusion, this study recommends an in-depth surveillance of AMR pathogens at molecular level, not only on commercial settings, but also among local indigenous species of livestock and wild species that could serve as the important reservoirs for the spread of these organisms.
Acknowledgments
The authors acknowledge the Head, Department of Veterinary Microbiology, University of Maiduguri in person of Assoc. Prof. Mustapha B. Abubakar for his support to the success of this work. We also acknowledge the efforts and commitment of the Technical staff of Bacteriology Lab, Department of Veterinary Microbiology, University of Maiduguri, Nigeria.
Conflict of Interests
The authors confirm and declare that there is no conflict of interest with respect to the research, authorship, and publication of this manuscript.
Authors’ contributions
The work was conceived and designed by Iliya Dauda Kwoji and Solomon Jauro. All the authors participated in the lab work. Jasini Athanda Musa and Yusuf Lekko Madaki analyzed the data obtained and Sabo Salihu and Hassan Abdullahi Danchuwa wrote and edited the manuscript. The manuscript was revised by all the authors.
References
- [1].Schrijver R, Stijntjes M, Rodríguez-Bano J, Tacconelli E, Rajendran BN, Voss A. Review of antimicrobial resistance surveillance programmes in livestock and meat in EU with focus on humans. Clin Microbiol Infect. 2018;24:577–90. doi: 10.1016/j.cmi.2017.09.013. https://doi.org/10.1016/j.cmi.2017.09.013. [DOI] [PubMed] [Google Scholar]
- [2].van de Sande-Bruinsma N, Grundmann H, Verloo D, Tiemersma E, Monen J, Goossens H, et al. European antimicrobial resistance surveillance system group, European surveillance of antimicrobial consumption project group (2008) Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14(11):1722–30. doi: 10.3201/eid1411.070467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Erwin V, Kluytmans J. Livestock-associated Staphylococcus aureus CC398: Animal reservoirs and human infections. Infect Genet Evol. 2014;21:523–30. doi: 10.1016/j.meegid.2013.02.013. https://doi.org/10.1016/j.meegid.2013.02.013. [DOI] [PubMed] [Google Scholar]
- [4].Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. https://doi.org/10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- [5].Crago B, Ferrato C, Drews SJ, Svenson LW, Tyrrell G, Louie M. Prevalence of Staphylococcus aureus and methicillin resistant S. aureus (MRSA) in food samples associated with foodborne illness in Alberta, Canada from 2007 to 2010. Food Microbiol. 2012;32(1):202–5. doi: 10.1016/j.fm.2012.04.012. https://doi.org/10.1016/j.fm.2012.04.012. [DOI] [PubMed] [Google Scholar]
- [6].Darwish WS, Atia AS, Reda LM, Elhelaly AE, Thompson LA, Eldin WFS. Chicken giblets and wastewater samples as possible sources of methicillin-resistant Staphylococcus aureus: Prevalence, enterotoxin production, and antibiotic susceptibility. J Food Safety. 2018:12478. https://doi.org/10.1111/jfs.12478. [Google Scholar]
- [7].Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–62. doi: 10.1016/S1473-3099(05)70295-4. https://doi.org/10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- [8].Ge B, Mukherjee S, Hsu CH, Davis JA, Tran TTT, Yang Q, et al. MRSA and multidrug-resistant Staphylococcus aureus in U.S. retail meats, 2010–2011. Food Microbiol. 2017;62:289–97. doi: 10.1016/j.fm.2016.10.029. http://doi.org/10.1016/j.fm.2016.10.029. [DOI] [PubMed] [Google Scholar]
- [9].Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6:17936. doi: 10.1371/journal.pone.0017936. https://doi.org/10.1371/journal.pone.0017936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cuny C, Witte W. MRSA in equine hospitals and its significance for infections in humans. Vet Microbiol. 2017;200:59–64. doi: 10.1016/j.vetmic.2016.01.013. https://doi.org/10.1016/j.vetmic.2016.01.013. [DOI] [PubMed] [Google Scholar]
- [11].Aires-de-Sousa M. Methicillin-resistant Staphylococcus aureus among animals: current overview. Clin Microbiol Infect. 2017;23:373–80. doi: 10.1016/j.cmi.2016.11.002. https://doi.org/10.1016/j.cmi.2016.11.002. [DOI] [PubMed] [Google Scholar]
- [12].Bakeet AM, Darwish SF. Phenotypic and genotypic detection of methicillin-resistant Staphylococcus aureus (MRSA) in broiler chickens. Assiut Vet Med J. 2014;60(143):142–51. [Google Scholar]
- [13].Britta K, Ballhausen B, Leeser D, Tenhagen B. Methicillin-resistant Staphylococcus aureus from broiler and turkey: a molecular view from farm to fork. Vet Microbiol. 2017;200:25–32. doi: 10.1016/j.vetmic.2016.05.022. https://doi.org/10.1016/j.vetmic.2016.05.022. [DOI] [PubMed] [Google Scholar]
- [14].Kwoji ID, Tambuwal FM, Abubakar MB, Yakubu Y, Bitrus AA, Jauro S. Occurrence of methicillin resistant Staphylococcus aureus in chickens and farm personnel in Sokoto, North-western Nigeria. J Adv Vet Anim Res. 2017;4(3):255–60. https://doi.org/10.5455/javar.2017.d220. [Google Scholar]
- [15].Oke AJ, Adewale AO. Incidence of methicillin resistant Staphylococcus aureus (MRSA) in a small poultry in South West, Nigeria. IOSR J Agric Vet Sci. 2013;5(3):53–5. https://doi.org/10.9790/2380-0535355. [Google Scholar]
- [16].Persoons D, Van Hoorebeke S, Hermans K, Butaye P, de Kruif A, Haesebrouck F, et al. Methicillin resistant Staphylococcus aureus in poultry. Emerg Infect Dis. 2009;15(3):452–3. doi: 10.3201/eid1503.080696. https://doi.org/10.3201/eid1503.080696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ochei J, Kolhatkar A. Theory and Practice. 2nd. Tata Mcgraw-Hill Publishing Company Limited; New Delhi, India: 2000. Medical Laboratory Science; pp. 331–49. [Google Scholar]
- [18].Fernandes CJ, Fernandes LA, Collignon P. Cefoxitin resistance as a surrogate marker for the detection of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2005;55:506–10. doi: 10.1093/jac/dki052. https://doi.org/10.1093/jac/dki052. [DOI] [PubMed] [Google Scholar]
- [19].Clinical and Laboratory Standards Institute (CLSI) 26th. Wayne, PA: 2016. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100S. [Google Scholar]
- [20].Fitzgerald JR. Livestock-associated Staphylococcus aureus: origin, evolution and public health threat. Trends Microbiol. 2012;20(4):192–8. doi: 10.1016/j.tim.2012.01.006. https://doi.org/10.1016/j.tim.2012.01.006. [DOI] [PubMed] [Google Scholar]
- [21].Nakamura K, Shirai J, Imai K, Hihara H, Tanimura N. Outbreak of comb necrosis in layer breeder chickens. Avian Dis. 1997;41:252–6. https://doi.org/10.2307/1592467. [PubMed] [Google Scholar]
- [22].McNamee PT, Smyth JA. Bacterial chondronecrosis with osteomyelitis (‘femoral head necrosis’) of broiler chickens: a review. Avian Pathol. 2000;29:477–95. doi: 10.1080/030794500750047243. https://doi.org/10.1080/030794500750047243. [DOI] [PubMed] [Google Scholar]
- [23].Fluit AC. Livestock-associated Staphylococcus aureus. Clin Microbiol Infect. 2012;18:735–44. doi: 10.1111/j.1469-0691.2012.03846.x. https://doi.org/10.1111/j.1469-0691.2012.03846.x. [DOI] [PubMed] [Google Scholar]
- [24].Abdulrahman HI, Geidam YA, Abubakar MB, Gashua MM, Gulani A, Galadima HB. Phenotypic detection and antibiogram of Staphylococcus aureus from poultry processing units in Maiduguri, Borno State, Nigeria. Asian J Res Anim Vet Sci. 2018;1(1):1–8. [Google Scholar]
- [25].Suleiman A, Zaria LT, Grema HA, Ahmadu P. Antimicrobial resistant coagulase positive Staphylococcus aureus from chickens in Maiduguri, Nigeria. Sokoto J Vet Sci. 2013;11(1):51–5. https://doi.org/10.4314/sokjvs.v11i1.8. [Google Scholar]
- [26].Owuna G, Abimiku RH, Nkene IH, Joseph GW, Ijalana O. Isolation and antibiotic susceptibility of Staphylococcus aureus from Fresh Poultry Meat Sold in Keffi Metropolis, Nigeria. Int J Res Stud Biosci. 2015;3(11):1–5. [Google Scholar]
- [27].Otalu O, Kabir JJ, Okolocha EC, Umoh VP, Kwaga JKP, Owolodun AO. Detection of methicillin resistant Staphylococcus aureus in chicken carcasses and live birds in Zaria, Nigeria. FUTA J Res Sci. 2015;11(1):132–8. [Google Scholar]
- [28].Cuny C, Strommenger B, Witte W, Stanek C. Clusters of infections in horses with MRSA ST1, ST254, and ST398 in a veterinary hospital. Microb Drug Resist. 2008;214:307–10. doi: 10.1089/mdr.2008.0845. [DOI] [PubMed] [Google Scholar]
- [29].Kwon I, Wang P, Tirrell DA. Design of a bacterial host for site-specific incorporation of p-bromophenylalanine into recombinant proteins. J Am Chem Soc. 2006;128(36):11778–83. doi: 10.1021/ja0626281. [DOI] [PubMed] [Google Scholar]
- [30].Lin MF, Yang CM, Lin CH, Huang ML, Tu CC, Liou ML. Clinical features and molecular epidemiology of multidrug-resistant Acinetobacter calcoaceticus—A baumannii complex in a regional teaching hospital in Taiwan. Am J Infect Control. 2009;37:e1–3. doi: 10.1016/j.ajic.2009.03.008. [DOI] [PubMed] [Google Scholar]
- [31].Nemati M, Hermans K, Lipinska U, Denis O, Deplano A, Struelens M, et al. Antimicrobial resistance of old and recent Staphylococcus aureus isolates from poultry: first detection of livestock-associated methicillin-resistant strain ST398. Antimicrob Agents Chemother. 2008;52:3817–9. doi: 10.1128/AAC.00613-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nworie A, Madubuko EF, Eze UA. Nasal carriage of Methicillin-resistant Staphylococcus aureus amongst meat sellers in Abakaliki Metropolis, Ebonyi State, Nigeria. Microbiol Res Int. 2013;1(3):48–53. [Google Scholar]
- [33].Onanuga A, Awhowho GO. Antimicrobial resistance of Staphylococcus aureus strains from patients with urinary tract infections in Yenagoa, Nigeria. J Pharm Bioall Sci. 2012;4(3):226–30. doi: 10.4103/0975-7406.99058. https://doi.org/10.4103/0975-7406.99058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Chaalal W, Aggad H, Zidane K, Saidi N, Kihal M. Antimicrobial susceptibility profiling of Staphylococcus aureus isolates from milk. Br Microbiol Res J. 2016;13(3):1–7. http://doi.org/10.9734/BMRJ/2016/24064. [Google Scholar]
- [35].Kwoji ID, Tambuwal FM, Abubakar MB, Yakubu Y, Musa JA, Jauro S, et al. Antibiotic sensitivity patterns of methicillin-resistant Staphylococcus aureus isolated from chickens in poultry farms in Sokoto, Nigeria. Adv Anim Vet Sci. 2018;6(1):8–11. [Google Scholar]


