Abstract
Despite the significant progress in the recent efforts toward developing an effective vaccine against toxoplasmosis, the search for new protective vaccination strategy still remains a challenge and elusive goal because it becomes the appropriate way to prevent the disease. Various experimental approaches in the past few years showed that developing a potential vaccine against the disease can be achievable. The combination of multi-epitopes expressing different stages of the parasite life cycle has become an optimal strategy for acquiring a potent, safe, and effective vaccine. Epitope-based vaccines have gained attention as alternative vaccine candidates due to their ability of inducing protective immune responses. This mini-review highlights the current status and the prospects of Toxoplasma gondii vaccine development along with the application of epitope-based vaccine in the future parasite immunization as a novel under development and evaluation strategy.
Keywords: Toxoplasma gondii, Epitope vaccine, DNA vaccines
Introduction
Toxoplasmosis is a prevalent disease caused by Toxoplasma gondii (T. gondii), which is a zoonotic parasite that infects humans, domestic, and wild mammals [1,2]. It is a significant, life-threatening disease with medical, veterinary, and economic importance worldwide [3,4]. Immunocompetent individuals infected with toxoplasmosis are usually asymptomatic or might have mild symptoms, while, this disease in immunocompromised patients can be quite severe or even fatal [5,6]. Despite several available antiparasitic chemical drugs used to prevent or cure the infection and to limit and control the spread of T. gondii parasite in an infected host, these drugs still have limited efficacy and are not absolutely safe and could cause severe side effects [7–9]. Thus, acquiring safe and effective vaccine to control the vital impact of toxoplasmosis in both humans and animals is urgently needed [10].
Intensive efforts and significant advances toward acquiring an effective vaccine are under way to control infection and limit the incidence of the disease; however, no vaccine has, thus, far been available for use in humans [11–13]. Currently, the live attenuated tachyzoites of the strain S48 (commercially named “Toxovax”) is the only approved vaccine for veterinary use. This vaccine was unfortunately shown limited efficacy [14].
Consequently, numerous studies on toxoplasmosis vaccination have been conducted and different forms of the parasite or parasitic antigens were tested, including inactivated or life attenuated vaccine, crude or recombinant antigen, subunit or multi-antigenic vaccines, and DNA vaccine [15]. Interestingly, the results indicated acquiring an effective vaccine against toxoplasmosis can be achieved.
The lack of available vaccine highlights the needs of exploring alternative reagents that can be used for immunization purposes. Further discussion was conducted to address the challenges, along with difficulties to acquire potential vaccine construct. Many suggestions were proposed and put forward in order to identify possible directions of the future research studies on the development of potential vaccines against T. gondii. In response to this scenario, the scientific suggestions were based on paying special focus on multi-epitope antigens that contain various immunoreactive epitopes of different T. gondii antigens. The potential use of the epitope-based antigen was explored to develop a new approach that expected to meet the demand of achievement of an effective vaccine. Characterization and identification of immunogenic epitopes from organism antigens could help in developing sensitive diagnostic assays, potential vaccines, as well as effective therapeutic agents [16].
The application of epitope-based vaccine in the immunization attempts of various infections has shown encouraging results and proved to stimulate protective cellular and humoral immunity [17–21]. Accordingly, epitope-based vaccine has suggested as a new potential candidates for acquiring a novel and effective T. gondii vaccine.
To date, the large number of immunization attempts highlight that future T. gondii vaccination approaches should apply antigens with potential to stimulate protective immunity against the parasite and are expressed in most of the parasite life stages [22,23]. Therefore, epitope-based vaccine features excellent immunogenicity and is valuable for acquiring new immunization strategy [22,24]. This review summarizes the approaches in developing vaccines against toxoplasmosis with emphasis on epitope-based vaccine and describes the designing and construction strategies of such vaccines, the advantages, disadvantages, and the current applications of these types of vaccines in toxoplasmosis vaccination strategies.
Approaches in Development of Vaccines Against Toxoplasmosis
The development of protective vaccines against T. gondii parasite can reduce the high incidence of the disease and prevent the clinical outcome in humans and animals [24,25]. Therefore, effective immunization is expected to reduce the shedding of the oocyst and prevent the cyst formation. Such vaccination would significantly reduce parasite transmission to intermediate hosts and definitely improve disease control [24]. Economically, the vaccine could also reduce losses in the livestock industry [26]. Thus, achieving effective vaccines against toxoplasmosis is a high priority and extremely important, given the high incidence of the disease worldwide, as well as the serious veterinary and clinical outcomes of the parasite, including chorioretinitis, abortions, mental defects, and death [26,27].
During the last 20 years, different immunization strategies in the achievement of effective T. gondii vaccine have been investigated. Consequently, the protection level has been evaluated with different types of immunogens, including the following: life-attenuated parasites, killed vaccines, native parasite antigens, recombinant antigens, and DNA vaccine; these immunogens have been tested as a new immunization strategy [22–25]. Moreover, inoculation of live parasites significantly induces effective immune protection against toxoplasmosis reinfection [28].
Despite the significant efforts in developing T. gondii vaccine, only the live attenuated tachyzoites of strain S48 (commercially named “Toxovax”) was approved and licensed in 1992 to minimize the abortion rate in sheep. Unfortunately, this vaccine cannot be used for human immunization because of the risk of reverting to a virulent form; likewise, the vaccine may be pathogenic in immunocompromised patients [24]. Toxovax has a short shelf life and entails high costs [22]. In addition, immunization with other strains (ts-4) has been widely used in T. gondii immunization studies, providing significant resistance against cyst formation but partial protection against congenital toxoplasmosis. This approach increased the survival rate during acute toxoplasmosis [29]. Mouse inoculated with the temperature-sensitive mutant strain ts-4 induced protective immunity against lethal infection after a parasite challenge. By contrast, injection of mice with killed tachyzoite lysate provided no protection, neither alone nor with an adjuvant [21]. Despite the numerous vaccination strategies studied, as well as the vast knowledge of the molecular genetics, immunology, and pathology related to the T. gondii pathogen, no safe and protective vaccine exists for both humans and animals [22].
Thus far, all information obtained from the large number of immunization attempts have indicated that future T. gondii vaccination approaches should use antigens with the potential to stimulate cell-mediated immunity against the parasite, expressed in all parasite life stages and compatible with appropriate vaccination routes [22,23].
Vaccine in Cats and Livestock
The key step in controlling T. gondii infection is the prevention of oocyst formation in the definitive host (cats and other felines). Given that cats are the only source of oocysts, and that most probably, transmission of infection to intermediate hosts occurs through contaminated feces, the development of any protective vaccines to be used in this species must be able to limit the shedding of oocyst to prevent environmental contamination by the oocysts [30]. Only, few studies have, thus, far focused on the cat vaccination. The use of a live mutant bradyzoite named T-263 was the first trial in which kittens were vaccinated [31]. After the oral inoculation, most of the kittens generated protective immunity; oocyst shedding was successfully prevented in 84% of the cats when challenged with the T. gondii parasite [31,32]. Unfortunately, T-263 has many disadvantages, including the need to use live bradyzoites and high costs [33]. Similarly, the effect of 60Co-irradiated tachyzoites on the stimulation of protective immunity against the Beverley strains of T. gondii was investigated. The vaccine induced partial resistance after infection challenge; however, the vaccine showed disadvantages, such as the need for refrigeration and high costs [34]. Oocyst shedding was not reduced when DNA vaccines encoding rhoptry protein (ROP) 2 were used [35] even though DNA vaccine currently shows potential as an immunization tool.
In pigs, live T. gondii vaccines exhibited mild protection against the parasite but still showed risks of reverting to the virulent type and cause the disease [36]. Thus, in this species, the development of killed vaccines was necessary [22]. Recently, intradermal immunization of pigs with a DNA vaccine expressing GRA1–GRA7 of Toxoplasma antigens elicited high humoral and cellular immunity. The study proved that DNA vaccine could be effectively induce strong immune protection [37]. In addition, the potential of the tachyzoites of strain S48 in reducing the number of cysts in pork, and thus, improving food safety has been highlighted [38].
Recombinant and DNA Vaccines
Among the various approaches for acquiring effective T. gondii vaccines, the recombinant DNA technology is an alternative strategy of great potential [25,39]. The immunological effects of several Toxoplasma recombinant antigens have been widely evaluated in the last few years; these include surface antigens (SAGs), micronemal proteins, dense-granule proteins (GRAs), and ROPs [9,35,40,41]. Of them, only limited antigens were capable of inducing a strong and protective immunity. Unluckily, recombinant antigens tend to lack immunogenicity, especially vaccination trails of the intracellular pathogens. Therefore, uses of appropriate adjuvants are required to enhance their potency [42]. In addition, the production of the recombinant proteins within another expression system or organism could cause an allergic reaction [43].
The establishments of DNA vaccination strategies have opened a new perspective in the future of vaccine development [34,44]. Recently, various DNA vaccines against T. gondii have been developed and evaluated; some of them have shown promise [45]. The ability of such vaccines to induce high humoral and cell-mediated immunity makes it a promising vaccination strategy against intracellular pathogens including Toxoplasma[15]. DNA vaccines exhibit several advantageous such as easy to produce, easy to administer, stable, very immunogenic, and possess the potential for long-lasting immunity. In addition, DNA vaccines show high flexibility as several types of genes can be encoded in one DNA vaccine. Moreover, DNA vaccine has little risk of reverting to a virulent form or cause secondary infection [46].
Epitope-Based Vaccines
Antigenic variation and genetic polymorphisms represent major obstacles in the attempts of acquiring a successful vaccine of any particular pathogen. Therefore, understanding of these variation and polymorphisms in the populations is crucial for proper vaccine design and evaluation. Such data might also provide invaluable insights into parasite–host interaction [21,47]. The improved knowledge in bioinformatics tools, along with the advances in recombinant DNA technology, has allowed new strategies toward the design and production of novel epitope-based vaccines [48]. Epitopes or antigenic determinants are the minimal immunogenic part of any particular antigen, which are capable of inducing specific immune responses [49]. Accordingly, various immunogenic epitopes have been identified in different infectious pathogens and cancer, and they have significantly improved the development of potential epitope-based vaccines [50].
The improved understanding of how the immune cells recognize and interact with pathogenic antigens at the molecular and cellular level has significant contribution in the development and acquiring of rationally designed epitope vaccines [7]. The concept of epitope vaccines mainly relies on the prediction of immunodominant T and B cell epitopes that can elicit specific and protective immune response [51]. The antigenic variation in most infectious agents has impeded the development of effective vaccines [17]. Therefore, the use of immunogenic peptides in trials of acquiring epitope-based vaccine has recently drawn attention [51].
The most critical requirements include the proper identification of both T and B cells epitopes, as well as the selection of a novel and powerful approach to deliver those epitopes [48]. Immunization with multi-epitope vaccine expressing T-cell or/and B-cell epitopes against different pathogens showed significant increase in both cellular and humoral immunes responses, as well as prolonged survival time [30]. Numerous studies identified potential epitope-based antigens that could effectively induce high and protective immunity against diverse pathogens. The approach has been used to develop and evaluate vaccines to various infectious agents, such as Influenza Virus [52], Human Immunodeficiency Virus [53], Epstein-Barr Virus [19], and hepatitis B virus [54].
Advantages and Disadvantages of Epitope-Based Vaccine
The potential advantages of using this vaccination strategy are as follows: it decreases the biohazard risk associated with other types of immunization; it has the ability to rationally engineer and optimize the epitope structure to increase potency in eliciting strong immunity; and it provides the opportunity to focus and generate specific immune responses to known conserved immunodominant epitopes [55]. In addition to the lack of infectious potential, epitope-based vaccine also shows chemical stability, and therefore, such kind of vaccines have been developed and tested against various infectious agents, including parasitic, bacterial, fungal, and viral infections, as well as cancers [51,56]. In the clinical trials of various cancers, peptide vaccine has entered phases I and II with satisfactory and promising clinical outcomes [57]. However, more effort is needed to eliminate the associated obstacles, including the necessity to have a better adjuvant, as well as the low or/and lack of resulting immunogenicity during antigen processing and presentation. Nonetheless, other study showed a significant progress in defying these limitations [51].
Identification of the Immunodominant Epitopes
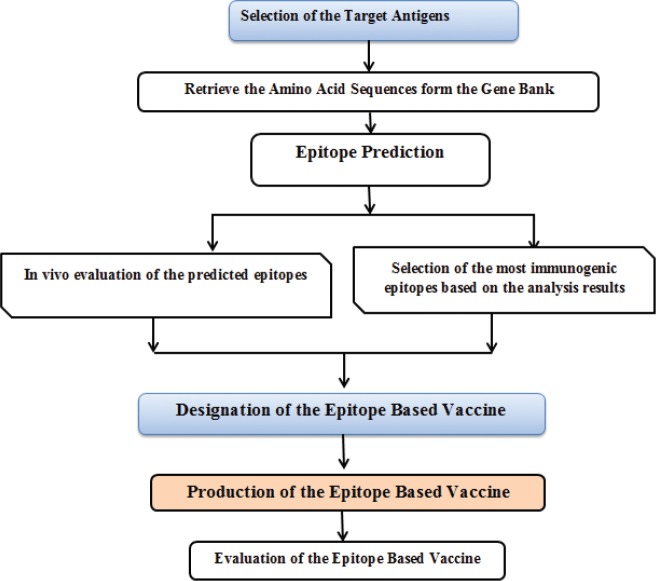
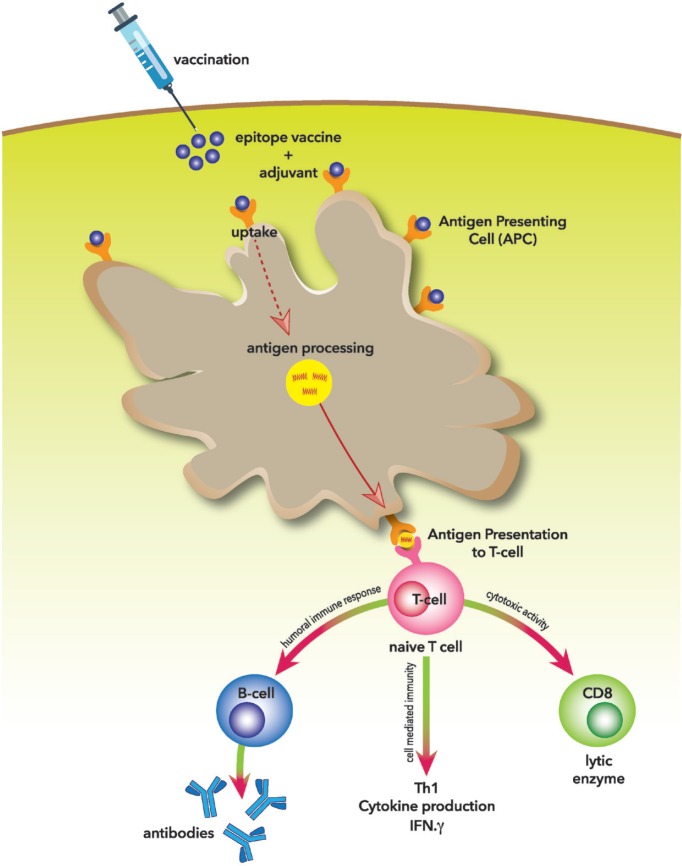
Development of any potential epitope vaccine requires proper prediction and validation of highly immunogenic epitopes that are capable of inducing protective immune response and constitutes the basis of vaccines development as shown in Figure 1 [58]. In fact, significant barrier in designing such kind of vaccine is epitope identification [59]. Therefore, predicting or identifying T and B cell epitopes significantly furthered our understanding of how the immune response against the pathogens is generated and increased the chances of developing potential vaccines. The mechanism of action and how the epitope-based vaccines generate specific immune response were illustrated in Figure 2. Bioinformatic tools remain the vital option for analyzing immunogenic epitopes with high antigenicity and immunogenicity even though the inherent complexity of microbial antigens recognition complicates the process of epitope prediction [21,60]. Yet, significant efforts have been put toward acquiring novel strategies and efficient tools for epitope analysis. Consequently, different algorithms have been developed and tested for predicting and screening of possible epitopes, and the results indicate a promising strategy for vaccine development [59].
Figure 1. Schematic illustration of the epitope-based vaccine destination and construction.
Figure 2. Mechanism of action of epitope-based vaccination.
Application of bioinformatics approaches in the analysis of conserved sequences and predicting of potential epitopes have been widely used against various pathogens. It represents a powerful alternative strategy of epitopes discovery that significantly reduces the cost, time, as well as the effort involved in the experimental approach of epitope screening. However, the variation among epitopic regions might affect the prediction process, and thus acquiring functional protective epitopes. Selection of immunogenic epitopes is crucial in designating any particular epitope-based vaccine; therefore, the differences in pathogens genotypes and subtypes should be taken into account.
Epitope-Based Vaccine Against Toxoplasmosis
Since the application of bioinformatics tools in the production of epitope-based antigen has become potential strategies to acquire a novel and powerful vaccine against infectious agents [61], considerable efforts have been made toward developing a promising epitope-based vaccine against toxoplasmosis (Table 1) [62,63]. Researcher assumes that construction of single- or multi-epitope-based antigen expressing potential B or/and T cell epitopes of both tachyzoite and bradyzoite specific antigens would greatly improve T. gondii immunization strategies [64]. Accordingly, several studies have been conducted and resulted in the identification of various promising epitopes that are capable of inducing protective immune response, and would possibly contribute to the attempts of developing a protective vaccine against T. gondii [7]. This evidenced by the significant immune protection generated in mice models [65,66].
Table 1. List of predicted Epitopes evaluated as potential Epitope-based vaccine.
| Epitope | Antigen Gene | Reference |
|---|---|---|
| LGPVKLSAEGPT, TAAKTHTVRGFKV, SYFAADRLVP |
SAG1, GRA2, GRA7 |
[41] |
| KLFETTDMY, VRQEAIARALARAAA |
Anopheles mosquito salivary proteins | [70] |
| GNIEGQWALKNHSLVSLSEQVLVSCDNIDD | CPA (Cysteine peptidase A) | [59] |
| YSNIGVCK | [71] | |
| QTLIAIHTLAIRYAN | Paracoccidioides brasiliensis gp43 antigen | [72] |
| RPPIFIRRL, sSVRDRLARL |
EBNA3 | [19] |
| Residues 137–160 and 197–211 | VP1 gene of foot-and-mouth disease virus | [73] |
| (TAKDGMEYYNKMGELYKQ, (RCLLGFKEVGGKCVPASI) | Plasmodium knowlesi merozoite surface protein-142 | [7] |
| TCPDKKSTA | SAG1 (59–67) | [9] |
| KSFKDILPK, STFWPCLLR, AVVSLLRLLK, SSAYVFSVK, AMLTAFFLR) |
SAG1, SAG2, GRA5, SRS52A, GRA6 |
[17] |
Recently, a synthetic vaccine expressing nine epitopes predicted from GRA2, GRA7, and SAG1 of T. gondii was tested in BALB/c mice. Immunization with this multi-epitope vaccine significantly generates mixed Th1/Th2 antibody response and high production of IFN-γ cytokine [67]. Similarly, significant increase in the cellular and humoral immunity was generated when the mice was immunized with a multi-epitope vaccine containing two T cell epitopes and one B cell epitope of SAG1, GRA1, and GRA4. In addition, vaccinated mice obtained long-term survival rates compared with the unvaccinated controls [12].
In contrast, epitope vaccine composed of a single B or T cell epitopes has been used previously and confirmed to elicit strong immune responses, for instance, synthetic B and T cell epitopes identified from GRA2 antigen were able to stimulate both cellular and humoral immunity and to increase the survival rate of immunized animals [64]. Similarly, mice immunized with epitopes vaccine identified form ROP19 protein induced significant T and B cell immune response and also indicated effective protection following parasite challenge with PRU strain T. gondii cysts [68]. However, effective systemic and mucosal immunity was enhanced with both single and mixed peptides with a strong lymphoproliferative response associated with significant IFN-γ, IL-2, and IL-4 production, and a high level of specific antibody responses. In addition, partial immune protection against acute and chronic toxoplasmosis was also generated [7]. Furthermore, a combination of DNA/peptide vaccine significantly reduced the formation of the brain cyst among the immunized mice [69].
This emphasized the involvement of single or mixture of epitopes has shown to remarkably induce effective humoral and cellular immune response against toxoplasmosis. This could be powerful and efficient strategy that can be considered in the production of possibly protective vaccine candidate against toxoplasmosis.
Conclusion
The development of potential vaccine against T. gondii has significantly progressed in the last few years. Numerous experimental studies of preventive immunization have explored various forms of T. gondii antigens including live-attenuated vaccines, subunit vaccines, recombinant vaccine, and DNA vaccines [13]. Accordingly, significant strides have been conducted in antigen isolation and characterization, gene cloning, and immunological techniques. In addition to all the prevention strategies, new options to produce effective vaccines are currently needed as the appropriate way to prevent the disease [15].
Previous studies on developing effective vaccines against T. gondii revealed that vaccines that express only single antigen or single stage induce partial immune protection against the parasite [64]. Thus, a vaccine that expresses multiple stages of the parasite life cycle must be synthesized. Adopting bioinformatics to identify antigenic epitopes and theoretically arranging multiple epitopes in a single antigen could aid in the achievement of potential T. gondii vaccines. The use of epitope-based antigens is highly promising in the development of potential vaccine candidates that would generate lasting protective immune reaction against T. gondii. Furthermore, the use of epitope-based antigens could be an important approach in investigating the improvement of the disease vaccination in the future. Future studies should also consider the exploration of appropriate adjuvants that can be used along with epitope-based vaccination strategies and establishing optimal immunization protocols along with evaluation criteria.
Conflict of Interest
The authors declare that they have no conflict of interest.
Authors’ contribution
All authors were equally contributed in the writing and approving this mini-review.
References
- [1].Dubey JP. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol. 2009;39(8):877–82. doi: 10.1016/j.ijpara.2009.01.005. http://dx.doi.org/10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- [2].Karakavuk M, Aldemir D, Mercier A, Şahar EA, Can H, Murat J-B, et al. Prevalence of toxoplasmosis and genetic characterization of Toxoplasma gondii strains isolated in wild birds of prey and their relation with previously isolated strains from Turkey. PLoS One. 2018;13(4):e0196159. doi: 10.1371/journal.pone.0196159. https://doi.org/10.1371/journal.pone.0196159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sun X, Mei M, Zhang X, Han F, Jia B, Wei X, et al. The extracellular matrix protein mindin as a novel adjuvant elicits stronger immune responses for rBAG1, rSRS4 and rSRS9 antigens of Toxoplasma gondii in BALB/c mice. BMC Infect Dis. 2014;14(1):429. doi: 10.1186/1471-2334-14-429. https://doi.org/10.1186/1471-2334-14-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Silva LA, Andrade RO, Carneiro ACA, Vitor RW. Overlapping Toxoplasma gondii genotypes circulating in domestic animals and humans in Southeastern Brazil. PLoS One. 2014;9(2):e90237. doi: 10.1371/journal.pone.0090237. https://doi.org/10.1371/journal.pone.0090237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hajissa K, Zakaria R, Suppian R, Mohamed Z. An evaluation of a recombinant multiepitope based antigen for detection of Toxoplasma gondii specific antibodies. BMC Infect Dis. 2017;17(1):807. doi: 10.1186/s12879-017-2920-9. https://doi.org/10.1186/s12879-017-2920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lakhrif Z, Moreau A, Hérault B, Di-Tommaso A, Juste M, Moiré N, et al. Targeted delivery of Toxoplasma gondii antigens to Dendritic cells Promote immunogenicity and Protective efficiency against Toxoplasmosis. Front Immunol. 2018;9:317. doi: 10.3389/fimmu.2018.00317. https://doi.org/10.3389/fimmu.2018.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang T-E, Yin L-T, Li R-H, Wang H-L, Meng X-L, Yin G-R. Protective immunity induced by peptides of AMA1, RON2 and RON4 containing T-and B-cell epitopes via an intranasal route against toxoplasmosis in mice. Parasites Vect. 2015;8(1):1–9. doi: 10.1186/s13071-015-0636-5. https://doi.org/10.1186/s13071-015-0636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Z, Li Y, Yao Z, Wang D, Xie Q, Wang C, et al. Immune protection of Rhoptry protein 21 (ROP21) of Toxoplasma gondii as a DNA vaccine against toxoplasmosis. Front Microbiol. 2018;9:909. doi: 10.3389/fmicb.2018.00909. https://doi.org/10.3389/fmicb.2018.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu Y, Cao A, Li Y, Li X, Cong H, He S, et al. Immunization with a DNA vaccine encoding Toxoplasma gondii Superoxide dismutase (TgSOD) induces partial immune protection against acute toxoplasmosis in BALB/c mice. BMC Infect Dis. 2017;17(1):403. doi: 10.1186/s12879-017-2507-5. https://doi.org/10.1186/s12879-017-2507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grzybowski MM, Dziadek B, Gatkowska JM, Dzitko K, Długońska H. Towards vaccine against toxoplasmosis: evaluation of the immunogenic and protective activity of recombinant ROP5 and ROP18 Toxoplasma gondii proteins. Parasitol Res. 2015;114(12):4553–63. doi: 10.1007/s00436-015-4701-y. https://doi.org/10.1007/s00436-015-4701-y. [DOI] [PubMed] [Google Scholar]
- [11].Zhou C-X, Zhou D-H, Liu G-X, Suo X, Zhu X-Q. Transcriptomic analysis of porcine PBMCs infected with Toxoplasma gondii RH strain. Acta Trop. 2016;154:82–8. doi: 10.1016/j.actatropica.2015.11.009. https://doi.org/10.1016/j.actatropica.2015.11.009. [DOI] [PubMed] [Google Scholar]
- [12].Cong H, Yuan Q, Zhao Q, Zhao L, Yin H, Zhou H, et al. Comparative efficacy of a multi-epitope DNA vaccine via intranasal, peroral, and intramuscular delivery against lethal Toxoplasma gondii infection in mice. Parasit Vectors. 2014;7:145. doi: 10.1186/1756-3305-7-145. https://doi.org/10.1186/1756-3305-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Foroutan M, Ghaffarifar F. Calcium-dependent protein kinases are potential targets for Toxoplasma gondii vaccine. Clin Exp Vaccine Res. 2018;7(1):24–36. doi: 10.7774/cevr.2018.7.1.24. https://doi.org/10.7774/cevr.2018.7.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pinzan CF, Sardinha-Silva A, Almeida F, Lai L, Lopes CD, Lourenço EV, et al. Vaccination with recombinant microneme proteins confers protection against experimental toxoplasmosis in mice. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0143087. https://doi.org/10.1371/journal.pone.0143087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Li Y, Zhou H. Moving towards improved vaccines for Toxoplasma gondii. Expert Opin Biol Ther. 2018;18(3):273–80. doi: 10.1080/14712598.2018.1413086. https://doi.org/10.1080/14712598.2018.1413086. [DOI] [PubMed] [Google Scholar]
- [16].Cheong FW, Fong MY, Lau YL. Identification and characterization of epitopes on Plasmodium knowlesi merozoite surface protein-1 42 (MSP-1 42) using synthetic peptide library and phage display library. Acta Trop. 2016;154:89–94. doi: 10.1016/j.actatropica.2015.11.005. http://dx.doi.org/10.1016/j.actatropica.2015.11.005. [DOI] [PubMed] [Google Scholar]
- [17].Liu Z, Chen Y-H. Design and construction of a recombinant epitope-peptide gene as a universal epitope-vaccine strategy. J Immunol Methods. 2004;285(1):93–7. doi: 10.1016/j.jim.2003.10.018. https://doi.org/10.1016/j.jim.2003.10.018. [DOI] [PubMed] [Google Scholar]
- [18].Stoloff GA, Caparros-Wanderley W. Synthetic multi-epitope peptides identified in silico induce protective immunity against multiple influenza serotypes. Eur J Immunol. 2007;37(9):2441–9. doi: 10.1002/eji.200737254. https://doi.org/10.1002/eji.200737254. [DOI] [PubMed] [Google Scholar]
- [19].Alonso-Padilla J, Lafuente EM, Reche PA. Computer-aided design of an Epitope-based vaccine against Epstein-Barr virus. J Immunol Res. 2017 doi: 10.1155/2017/9363750. Article ID 9363750; https://doi.org/10.1155/2017/9363750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cong H, Gu QM, Yin HE, Wang JW, Zhao QL, Zhou HY, et al. Multi epitope DNA vaccine linked to the A2/B subunit of cholera toxin protect mice against Toxoplasma gondii. Vaccine. 2008a;26(31):3913–21. doi: 10.1016/j.vaccine.2008.04.046. https://doi.org/10.1016/j.vaccine.2008.04.046. [DOI] [PubMed] [Google Scholar]
- [21].Thompson CP, Lourenço J, Walters AA, Obolski U, Edmans M, Palmer DS, et al. A naturally protective epitope of limited variability as an influenza vaccine target. Nat Commun. 2018;9(1):3859. doi: 10.1038/s41467-018-06228-8. https://doi.org/10.1038/s41467-018-06228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Palatnik-De-Sousa CB, Soares IS, Rosa DS. Epitope discovery and Synthetic Vaccine design. Front Immunol. 2018;9:826. doi: 10.3389/fimmu.2018.00826. https://doi.org/10.3389/fimmu.2018.00826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fereig RM, Abdelbaky HH, Mohamed AEA, Nishikawa Y. Recombinant subunit vaccines against Toxoplasma gondii: Successful experimental trials using recombinant DNA and proteins in mice in a period from 2006 to 2018. J Vet Med Anim Sci. 2018;1:1005. [Google Scholar]
- [24].Huang S-Y, Jensen MR, Rosenberg CA, Zhu X-Q, Petersen E, Vorup-Jensen T. In silico and in vivo analysis of Toxoplasma gondii epitopes by correlating survival data with peptide–MHC-I binding affinities. Int J Infect Dis. 2016;48:14–9. doi: 10.1016/j.ijid.2016.04.014. https://doi.org/10.1016/j.ijid.2016.04.014. [DOI] [PubMed] [Google Scholar]
- [25].Gao Q, Zhang N-Z, Zhang F-K, Wang M, Hu L-Y, Zhu X-Q. Immune response and protective effect against chronic Toxoplasma gondii infection induced by vaccination with a DNA vaccine encoding profilin. BMC Infect Dis. 2018;18(1):117. doi: 10.1186/s12879-018-3022-z. https://doi.org/10.1186/s12879-018-3022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mbabazi P, Hopkins H, Osilo E, Kalungu M, Byakika-Kibwika P, Kamya MR. Accuracy of two malaria rapid diagnostic tests (RDTS) for initial diagnosis and treatment monitoring in a high transmission setting in Uganda. Am J Trop Med Hyg. 2015;92(3):530–6. doi: 10.4269/ajtmh.14-0180. https://doi.org/10.4269/ajtmh.14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Picchio MS, Sánchez VR, Arcon N, Soto AS, Sibilia MP, de los Angeles Aldirico M, et al. Vaccine potential of antigen cocktails composed of recombinant Toxoplasma gondii TgPI-1, ROP2 and GRA4 proteins against chronic toxoplasmosis in C3H mice. Exp Parasitol. 2018;185:62–70. doi: 10.1016/j.exppara.2018.01.006. https://doi.org/10.1016/j.exppara.2018.01.006. [DOI] [PubMed] [Google Scholar]
- [28].Waldeland H, Frenkel J. Live and killed vaccines against toxoplasmosis in mice. J Parasitol. 1983;69(1):60–5. https://doi.org/10.2307/3281275. [PubMed] [Google Scholar]
- [29].Jongert E, Roberts CW, Gargano N, Förster-Waldl E, Petersen E. Vaccines against Toxoplasma gondii: challenges and opportunities. Memórias Instituto Oswaldo Cruz. 2009;104(2):252–66. doi: 10.1590/s0074-02762009000200019. http://dx.doi.org/10.1590/S0074-02762009000200019. [DOI] [PubMed] [Google Scholar]
- [30].Zhang N-Z, Chen J, Wang M, Petersen E, Zhu X-Q. Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev Vaccines. 2013;12(11):1287–99. doi: 10.1586/14760584.2013.844652. https://doi.org/10.1586/14760584.2013.844652. [DOI] [PubMed] [Google Scholar]
- [31].Lu G, Zhou J, Zhao YH, Wang L. DNA vaccine ROP 29 from Toxoplasma gondii containing R848 enhances protective immunity in mice. Parasite Immunol. 2018;40(10):e12578. doi: 10.1111/pim.12578. https://doi.org/10.1111/pim.12578. [DOI] [PubMed] [Google Scholar]
- [32].Freyre A, Choromanski L, Fishback J, Popiel I. Immunization of cats with tissue cysts, bradyzoites, and tachyzoites of the T-263 strain of Toxoplasma gondii. J Parasitol. 1993;79(5):716–9. https://doi.org/10.2307/3283610. [PubMed] [Google Scholar]
- [33].Li Z-Y, Lu J, Zhang N-Z, Chen J, Zhu X-Q. Immune responses induced by HSP60 DNA vaccine against Toxoplasma gondii infection in Kunming mice. Korean J Parasitol. 2018;56(3):237. doi: 10.3347/kjp.2018.56.3.237. https://doi.org/10.3347/kjp.2018.56.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Foroutan M, Zaki L, Ghaffarifar F. Recent progress in microneme-based vaccines development against Toxoplasma gondii. Clin Exp Vaccine Res. 2018;7(2):93–103. doi: 10.7774/cevr.2018.7.2.93. https://doi.org/10.7774/cevr.2018.7.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee S-H, Kang H-J, Lee D-H, Kang S-M, Quan F-S. Virus-like particle vaccines expressing Toxoplasma gondii rhoptry protein 18 and microneme protein 8 provide enhanced protection. Vaccine. 2018;36(38):5692–700. doi: 10.1016/j.vaccine.2018.08.016. https://doi.org/10.1016/j.vaccine.2018.08.016. [DOI] [PubMed] [Google Scholar]
- [36].Supply P, Sutton P, Coughlan SN, Bilo K, Saman E, Trees AJ, et al. Immunogenicity of recombinant BCG producing the GRA1 antigen from Toxoplasma gondii. Vaccine. 1999;17(7):705–14. doi: 10.1016/s0264-410x(98)00255-2. https://doi.org/10.1016/S0264-410X(98)00255-2. [DOI] [PubMed] [Google Scholar]
- [37].Suschak JJ, Williams JA, Schmaljohn CS. Advancements in DNA vaccine vectors, non-mechanical delivery methods, and molecular adjuvants to increase immunogenicity. Hum Vaccin Immunother. 2017;13(12):2837–48. doi: 10.1080/21645515.2017.1330236. https://doi.org/10.1080/21645515.2017.1330236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Burrells A, Benavides J, Cantón G, Garcia JL, Bartley PM, Nath M, et al. Vaccination of pigs with the S48 strain of Toxoplasma gondii–safer meat for human consumption. Vet Res. 2015;46(1):47. doi: 10.1186/s13567-015-0177-0. https://doi.org/10.1186/s13567-015-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mendes ÉA, Fonseca FG, Casério BM, Colina JP, Gazzinelli RT, Caetano BC. Recombinant vaccines against T. gondii: comparison between homologous and heterologous vaccination protocols using two viral vectors expressing SAG1. PLoS One. 2013;8(5):e63201. doi: 10.1371/journal.pone.0063201. https://doi.org/10.1371/journal.pone.0063201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang Y, Yin H. Research progress on surface antigen 1 (SAG1) of Toxoplasma gondii. Parasites Vect. 2014;7(1):180. doi: 10.1186/1756-3305-7-180. https://doi.org/10.1186/1756-3305-7-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cao A, Liu Y, Wang J, Li X, Wang S, Zhao Q, et al. Toxoplasma gondii: Vaccination with a DNA vaccine encoding T-and B-cell epitopes of SAG1, GRA2, GRA7 and ROP16 elicits protection against acute toxoplasmosis in mice. Vaccine. 2015;33(48):6757–62. doi: 10.1016/j.vaccine.2015.10.077. https://doi.org/10.1016/j.vaccine.2015.10.077. [DOI] [PubMed] [Google Scholar]
- [42].Didierlaurent AM, Collignon C, Bourguignon P, Wouters S, Fierens K, Fochesato M, et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol. 2014:1400948. doi: 10.4049/jimmunol.1400948. https://doi.org/10.4049/jimmunol.1400948. [DOI] [PubMed] [Google Scholar]
- [43].Liu Q, Singla LD, Zhou H. Vaccines against Toxoplasma gondii: status, challenges and future directions. Hum Vaccin Immunother. 2012;8(9):1305–8. doi: 10.4161/hv.21006. https://doi.org/10.4161/hv.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dalmo R. DNA vaccines for fish: review and perspectives on correlates of protection. J Fish Dis. 2018;41(1):1–9. doi: 10.1111/jfd.12727. https://doi.org/10.1111/jfd.12727. [DOI] [PubMed] [Google Scholar]
- [45].Chen Y, Yu M, Hemandez J, Li J, Yuan Z-G, Yan H. Immuno-efficacy of DNA vaccines encoding PLP1 and ROP18 against experimental Toxoplasma gondii infection in mice. Exp Parasitol. 2018;188:73–8. doi: 10.1016/j.exppara.2018.04.003. https://doi.org/10.1016/j.exppara.2018.04.003. [DOI] [PubMed] [Google Scholar]
- [46].Kim J-H, Lee S-H, Sohn H-J, Lee J, Chwae Y-J, Park S, et al. The immune response induced by DNA vaccine expressing nfa1 gene against Naegleria fowleri. Parasitol Res. 2012;111(6):2377–84. doi: 10.1007/s00436-012-3093-5. https://doi.org/10.1007/s00436-012-3093-5. [DOI] [PubMed] [Google Scholar]
- [47].Jalloh A, Jalloh M, Matsuoka H. T-cell epitope polymorphisms of the Plasmodium falciparum circumsporozoite protein among field isolates from Sierra Leone: age-dependent haplotype distribution. Malaria J. 2009;8(120):1475–2875. doi: 10.1186/1475-2875-8-120. https://doi.org/10.1186/1475-2875-8-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Khan AM, Miotto O, Heiny A, Salmon J, Srinivasan K, Nascimento EJ, et al. A systematic bioinformatics approach for selection of epitope-based vaccine targets. Cell Immunol. 2006;244(2):141–7. doi: 10.1016/j.cellimm.2007.02.005. https://doi.org/10.1016/j.cellimm.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Shi J, Zhang J, Li S, Sun J, Teng Y, Wu M, et al. Epitope-based vaccine target screening against highly pathogenic MERS-CoV: an in silico approach applied to emerging infectious diseases. PLoS One. 2015;10(12):e0144475. doi: 10.1371/journal.pone.0144475. https://doi.org/10.1371/journal.pone.0144475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Correia BE, Bates JT, Loomis RJ, Baneyx G, Carrico C, Jardine JG, et al. Proof of principle for epitope-focused vaccine design. Nature. 2014;507(7491):201–6. doi: 10.1038/nature12966. https://doi.org/10.1038/nature12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Patronov A, Doytchinova I. T-cell epitope vaccine design by immunoinformatics. Open Biol. 2013;3(1):120139. doi: 10.1098/rsob.120139. https://doi.org/10.1098/rsob.120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Muñoz-Medina JE, Sánchez-Vallejo CJ, Méndez-Tenorio A, Monroy-Muñoz IE, Angeles-Martínez J, Santos Coy-Arechavaleta A, et al. In silico identification of highly conserved epitopes of influenza A H1N1, H2N2, H3N2, and H5N1 with diagnostic and vaccination potential. BioMed Res Int. 2015 doi: 10.1155/2015/813047. Article ID 813047; http://dx.doi.org/10.1155/2015/813047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sahay B, Nguyen CQ, Yamamoto JK. Conserved HIV Epitopes for an effective HIV vaccine. J Clin Cell Immunol. 2017;8(4):518. doi: 10.4172/2155-9899.1000518. https://doi.org/10.4172/2155-9899.1000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Comber JD, Karabudak A, Shetty V, Testa JS, Huang X, Philip R. MHC class I presented T cell epitopes as potential antigens for therapeutic vaccine against HBV chronic infection. Hepatitis Res Treat. 2014 doi: 10.1155/2014/860562. Article ID 860562; http://dx.doi.org/10.1155/2014/860562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sette A, Fikes J. Epitope-based vaccines: an update on epitope identification, vaccine design and delivery. Curr Opin Immunol. 2003;15(4):461–70. doi: 10.1016/s0952-7915(03)00083-9. https://doi.org/10.1016/S0952-7915(03)00083-9. [DOI] [PubMed] [Google Scholar]
- [56].Xu Q, Ma X, Wang F, Li H, Xiao Y, Zhao X. Design and construction of a chimeric multi-epitope gene as an epitope-vaccine strategy against ALV-J. Protein Expr Purif. 2015;106:18–24. doi: 10.1016/j.pep.2014.10.007. https://doi.org/10.1016/j.pep.2014.10.007. [DOI] [PubMed] [Google Scholar]
- [57].Naz RK, Dabir P. Peptide vaccines against cancer, infectious diseases, and conception. Front Biosci J Virtual Lib. 2006;12:1833–44. doi: 10.2741/2191. http://dx.doi.org/10.2741/2191. [DOI] [PubMed] [Google Scholar]
- [58].Ip PP, Nijman HW, Daemen T. Epitope prediction assays combined with validation assays strongly narrows down putative cytotoxic T lymphocyte epitopes. Vaccines. 2015;3(2):203–20. doi: 10.3390/vaccines3020203. https://doi.org/10.3390/vaccines3020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Agallou M, Athanasiou E, Koutsoni O, Dotsika E, Karagouni E. Experimental validation of multi-epitope peptides including promising MHC class I-and II-restricted epitopes of four known Leishmania infantum proteins. Front Immunol. 2014;5:268. doi: 10.3389/fimmu.2014.00268. https://doi.org/10.3389/fimmu.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Saha S, Raghava G. Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins Struct Func Bioinform. 2006;65(1):40–8. doi: 10.1002/prot.21078. https://doi.org/10.1002/prot.21078. [DOI] [PubMed] [Google Scholar]
- [61].Cong H, Yuan Q, Li Y, Zhao Q. The construction, bioinformatic analysis and vitro expression of multi-Epitope based gene from Toxoplasma gondii. 2011 5th International Conference on Paper presented at the Bioinformatics and Biomedical Engineering (iCBBE); http://doi.org/10.1109/icbbe.2011.5780050. [Google Scholar]
- [62].El Bissati K, Chentoufi AA, Krishack PA, Zhou Y, Woods S, Dubey JP, et al. Adjuvanted multi-epitope vaccines protect HLA-A* 11: 01 transgenic mice against Toxoplasma gondii. JCI Insight. 2016;1(15) doi: 10.1172/jci.insight.85955. http://dx.doi.org/10.1172/jci.insight.85955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].El Bissati K, Zhou Y, Dasgupta D, Cobb D, Dubey JP, Burkhard P, et al. Effectiveness of a novel immunogenic nanoparticle platform for Toxoplasma peptide vaccine in HLA transgenic mice. Vaccine. 2014;32(26):3243–8. doi: 10.1016/j.vaccine.2014.03.092. https://doi.org/10.1016/j.vaccine.2014.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bastos LM, Macêdo AG, Jr, Silva MV, Santiago FM, Ramos EL, Santos FA, et al. Toxoplasma gondii-derived synthetic peptides containing B-and T-cell epitopes from GRA2 protein are able to enhance mice survival in a model of experimental toxoplasmosis. Front Cell Infect Microbiol. 2016;6:59. doi: 10.3389/fcimb.2016.00059. https://doi.org/10.3389/fcimb.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Darcy F, Maes P, Gras-Masse H, Auriault C, Bossus M, Deslee D, et al. Protection of mice and nude rats against toxoplasmosis by a multiple antigenic peptide construction derived from Toxoplasma gondii P30 antigen. J Immunol. 1992;149(11):3636–41. [PubMed] [Google Scholar]
- [66].Wang Y, Wang M, Wang G, Pang A, Fu B, Yin H, et al. Increased survival time in mice vaccinated with a branched lysine multiple antigenic peptide containing B-and T-cell epitopes from T. gondii antigens. Vaccine. 2011;29(47):8619–23. doi: 10.1016/j.vaccine.2011.09.016. https://doi.org/10.1016/j.vaccine.2011.09.016. [DOI] [PubMed] [Google Scholar]
- [67].Hajissa K, Zakaria R, Suppian R, Mohamed Z. Immunogenicity of Multi-epitope Vaccine Candidate against Toxoplasma gondii Infection in BALB/c Mice. Iran J Parasitol. 2018;13(2):215–24. [PMC free article] [PubMed] [Google Scholar]
- [68].Zhou J, Wang L, Lu G, Zhou A, Zhu M, Li Q, et al. Epitope analysis and protection by a ROP19 DNA vaccine against Toxoplasma gondii. Parasite. 2016;23:17. doi: 10.1051/parasite/2016017. https://doi.org/10.1051/parasite/2016017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lu G, Wang L, Zhou A, Han Y, Guo J, Song P, et al. Epitope analysis, expression and protection of SAG5A vaccine against Toxoplasma gondii. Acta Trop. 2015;146:66–72. doi: 10.1016/j.actatropica.2015.03.013. https://doi.org/10.1016/j.actatropica.2015.03.013. [DOI] [PubMed] [Google Scholar]
- [70].Pandey RK, Bhatt TK, Prajapati VK. Novel immunoinformatics approaches to design multi-epitope subunit vaccine for malaria by investigating anopheles salivary protein. Sci Rep. 2018;8(1):1125. doi: 10.1038/s41598-018-19456-1. https://doi.org/10.1038/s41598-018-19456-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gillam F, Zhang C. Epitope selection and their placement for increased virus neutralization in a novel vaccination strategy for porcine epidemic diarrhea virus utilizing the Hepatitis B virus core antigen. Vaccine. 2018;36(30):4507–16. doi: 10.1016/j.vaccine.2018.06.015. https://doi.org/10.1016/j.vaccine.2018.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Holanda RA, Muñoz JE, Dias LS, Silva LBR, Santos JRA, Pagliari S, et al. Recombinant vaccines of a CD4+ T-cell epitope promote efficient control of Paracoccidioides brasiliensis burden by restraining primary organ infection. PLoS Negl Trop Dis. 2017;11(9):e0005927. doi: 10.1371/journal.pntd.0005927. http://doi.org/10.1371/journal.pntd.0005927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shao J-J, Wang J-F, Chang H-Y, Liu J-X. Immune potential of a novel multiple-epitope vaccine to FMDV type Asia 1 in guinea pigs and sheep. Virol Sin. 2011;26(3):190–7. doi: 10.1007/s12250-011-3174-0. https://doi.org/0.1007/s12250-011-3174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]