Abstract
Objective:
The objective of this study was to determine the prevalence, geographical distribution, and main risk factors for peste des petits ruminants (PPR) in the Republic of Chad.
Materials and methods:
A total of 3,546 sera collected from unvaccinated small ruminants including 1,699 goats and 1,847 sheep in 19 of the 23 regions in Chad were randomly sampled. The competitive enzyme-linked immunosorbent assay technics were used for serological analysis.
Results:
The overall seroprevalence at the individual level was 52.9%±1.6% (48.9% for goats and 56.2% for sheep). Seroprevalence observed in the Chari Baguirmi, Ouaddaï, and N’Djamena regions was significantly higher than those in the other regions. Transhumant herds are the most exposed than the sedentary ones. Older animals were more affected than the young ones. Kababich sheep are the most affected than other breeds.
Conclusion:
This study has shown that the PPR virus is circulating in the Republic of Chad. In view of the results obtained, the disease is enzootic in the country. Epidemiological information obtained including seroprevalence rate, risk factors (sex, breed, age, and mode of rearing), and geographical distribution will help to define an appropriate strategy for PPR control in the Republic of Chad.
Keywords: ELISA, goats, Kababich sheep, PPR, seroprevalence
Introduction
The Republic of Chad is a vast country in the heart of Africa with an area of 1,284,000 km2. It is bordered on the North by Libya, on the East by Sudan, on the West by Niger, on the South by the Central African Republic, and on the Southwest by Cameroon and Nigeria (Fig. 1). Administratively, Chad is divided into 23 regions where the departments, districts, township, and villages are included.
Figure 1. Map of the Republic of Chad showing the study area and bordering countries.
After oil, the economy of the Republic of Chad is based mainly on agriculture and livestock. The livestock sector in the country occupies an essential place in the national economy [1]. The livestock gross domestic product (GDP) accounts for over 35% of agricultural GDP and 19% of national GDP. At the social level, livestock provides a source of incomes for the most disadvantaged populations. It provides a livelihood for 40% of the rural population and employs 80% of the workforce, of whom, more than half are women [1].
Chad has about 94 million head of livestock according to the latest census. Small ruminants account for 61% of this number, including 32.5% goats and 28.2% sheep [2]. Several breeds of sheep and goats exist in Chad [3]. Because of their high level of adaptation, ease of maintenance, and socio-cultural role, they are raised in almost all regions of the country. However, several factors limit the development of this sector, particularly peste des petits ruminant (PPR), which is one of the main priority diseases whose mortality rate remains high in the Republic of Chad [4,5].
In fact, PPR is an infectious, highly contagious disease that affects mainly small domestic ruminants (sheep and goats) and wild ruminants (gazelles and antelopes). Cattle and pigs present a subclinical form of the disease [6] but do not excrete the virus. In Africa, the disease is recorded in several countries and is an impediment to the development of small ruminants [7,8]. In Chad, PPR virus was isolated for the first time in 1995 [9] suspected cases recorded in some areas of the country were also confirmed [10]. Although it is one of the priority diseases monitored by the National Disease Surveillance System in Chad [11], its epidemiology at the national level is still poorly understood. No national mass vaccination campaign against this disease was conducted before 2017.
In view of its socio-economic importance, PPR is targeted to be eradicated from the planet by 2030. To this end, global, continental, regional, and national strategies for its control and eradication have been developed [12,13]. The Republic of Chad, like other countries, has joined this line with the ambition of eradicating the disease by 2025 [14]. The success of this fight requires a better knowledge of the epidemiology of the disease including its prevalence and its geographical distribution. The present study aims at determining the seroprevalence of PPR in the Republic of Chad and knowing its geographical distribution that can be used to define appropriate control strategies.
Materials and methods
Sampling and sample collection
A total of 3,546 small ruminants including 1,699 goats and 1,847 sheep distributed in 19 of the 23 regions in Chad were sampled. For financial reasons, the four regions located in the north of the country were not affected by this study. The sampled animals are over 4 months old and presumed unvaccinated against PPR. They were randomly chosen regardless of their gender and race. Samples collection took place from June to July 2017 in the flock. Blood was removed from the jugular vein using Vacutainer needles and sterile 5 ml dry tubes. Sera, obtained after clot extraction in the field, were kept cool (under ice) until it was sent to the virology laboratory of Livestock Research Institute for Development based in N’Djamena. Centrifugation of the sera was done at 3,000 rpm for 5 min. The information on the flock and the relevant samples has been recorded in the sheets designed for this purpose.
Serological analysis
The competitive enzyme-linked immunosorbent assay (c-ELISA) test was used for the detection of anti-PPR Virus antibodies [15]. This kit is sold by IDvet from France. The Instructions of the manufacturer were applied. The optical densities (ODs) were read using a Magellan PR 4100 BIORAD ELISA reader at a wavelength of 450 nm.
All sera whose ODs are:
Less or equal to 50% are considered as positive
Greater than 50% and less than or equal to 60% are considered doubtful
More than 60% are considered negative.
All sera, whose results were doubtful, were retested.
Statistical analysis
Collected data were entered into a relational database designed on the Access® software. Data processing and analysis were performed using Excel®, R®, and Quantum GIS® software. The chi-square test was used for the comparison of proportions.
Results
Of the 3,546 sera of small ruminants tested, the overall individual seroprevalence rate was 52.9%±1.6%. As shown in Table 1, seroprevalence observed in sheep is significantly higher than in goats (p = 0.0001).
Table 1. Seroprevalence rate according to species.
| Species | No. of samples | Positive samples | Seroprevalence % | 95% C.I. |
|---|---|---|---|---|
| Goats | 1,699 | 821 | 48.3 | [45.9–50.7] |
| Sheep | 1,847 | 1,055 | 57.1 | [54.9–59.4] |
| Total | 3,546 | 1,876 | 52.9 | [51.3–54.5] |
Table 2 gives the seroprevalence rate of small ruminants according to risk factors. Statistically and independently of species, seroprevalence in females is higher than in males (p < 0.0001). According to age, the seroprevalence rate in small ruminants older than 3 years is higher than those under 3 years of age (p < 0.0002). It is also noted that the seroprevalence rate in animals aged between 1 and 3 years is higher than those less than 1 year old. The seroprevalence rate in transhumant animals is more dominant than in sedentary (p = 0.01529). Regarding breed, seroprevalence rate is higher in Kababich sheep than in Fulani sheep (p < 0.05) compare to other breeds.
Table 2. Seroprevalence by species of small ruminants according to risk factors.
| Risk factors | Sheep | Goats | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of samples | Positive samples | Seroprevalence % | 95% C.I. | No. of samples | Positive samples | Seroprevalence % | 95% C.I. | ||
| Sex | Female | 1,470 | 894 | 60.8 | [58.3–63.3] | 1,498 | 746 | 49.8 | [47.3–52.3] |
| Male | 377 | 161 | 42.7 | [37.7–47.7] | 201 | 75 | 37.3 | [30.6–44.0] | |
| Total | 1,847 | 1,055 | 57.1 | [54.9–59.4] | 1,699 | 821 | 48.3 | [45.9–50.7] | |
| Age | < 1 an | 182 | 67 | 36.8 | [29.8–43.8] | 151 | 61 | 40.4 | [32.6–48.2] |
| > 1 an < 3 ans | 1,000 | 538 | 53.8 | [50.7–56.9] | 1,019 | 416 | 40.8 | [37.8–43.8] | |
| >3 ans | 665 | 450 | 67.7 | [64.1–71.2] | 529 | 344 | 65 | [61.0–69.1] | |
| Total | 1,847 | 1,055 | 57.1 | [54.9–59.4] | 1,699 | 821 | 48.3 | [45.9–50.7] | |
| Breeding type | Sedentary | 1,415 | 793 | 56.0 | [53.5–58.6] | 1,487 | 714 | 48.0 | [45.5–50.6] |
| Transhumant | 432 | 262 | 60.6 | [56.0–65.3] | 212 | 107 | 50.5 | [43.7–57.2] | |
| Total | 1,847 | 1,055 | 57.1 | [54.9–59.4] | 1,699 | 821 | 48.3 | [45.9–50.7] | |
| Breed | Arabe | 340 | 147 | 43.2 | [38.0–48.5] | – | – | – | – |
| Kababich | 331 | 225 | 68.0 | [62.9–73.0] | – | – | – | – | |
| Kirdimi | 726 | 389 | 53.6 | [50.0–57.2] | – | – | – | – | |
| Fulani | 450 | 294 | 65.3 | [60.9–69.7] | – | – | – | – | |
| Kirdimi | – | – | – | – | 1,097 | 525 | 47.9 | [44.9–50.8] | |
| Sahelian | – | – | – | – | 602 | 296 | 49.2 | [45.2–53.2] | |
| Total | 1,847 | 1,055 | 57.1 | [54.9–59.4] | 1,699 | 821 | 48.3 | [45.9–50.7] | |
: not concerned
According to goats breed, no significant difference between the seroprevalence rate of Kirdimi and Sahelian goats (p = 0.6038).
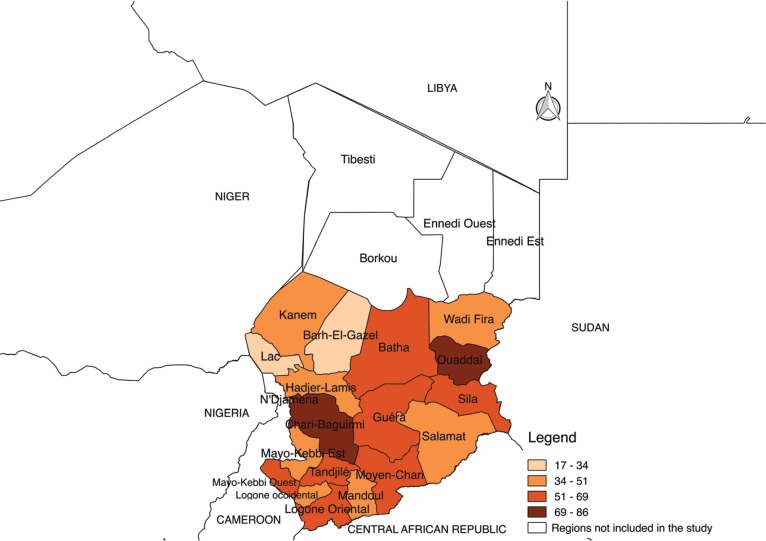
Fig. 2 below shows the distribution of seroprevalence of PPR according to regions. It is noted that the distribution of seroprevalence is heterogeneous. It varied from 29.3% to 85.8%. Higher seroprevalences rate are observed in Chari Baguirmi, Ouaddaï, and N’djamena regions.
Figure 2. Geographical distribution map of the seroprevalence of PPR in the Republic of Chad.
Discussion
The results of this study gave an overall PPR seroprevalence rate at the individual level of 52.9% ± 1.6% (48.9% for goats and 56.2% for sheep). This figure highlights the importance of the circulation of the PPR virus in the Republic of Chad. This seroprevalence rate is approximately similar to the results obtained in the neighboring countries of Chad, particularly in Nigeria where seroprevalence rate was 55% [16]; 51% in Sudan [17]; 45% in Niger [18]; 46.7% in Libya [19]; and 30% in Cameroon [20].
The heterogeneity of the seroprevalence rates observed in the regions of Chad can be explained by the specificity of these regions in relation to the risk factors. The high seroprevalence rate observed in the region of Chari Baguirmi can be explained by its geographical position. This region constitutes a zone of concentration of transhumants and markets of cattle and small ruminants. This area is a crossroad for most transhumants, thus promoting the spread of the virus. On the other hand, in the Ouaddaï region, Kababich sheep [3] would probably be a factor in favor of the higher seroprevalence rates observed in this breed. In fact, Kababich sheep are sheep breeds that would be introduced into Chad from Sudan, a country bordering the Ouaddai region. Because of its outstanding zootechnical performance, this breed has spread throughout the country. Special attention should be paid to this breed, which seems to be very sensitive to several diseases and the role it could play in spreading the PPR virus.
Regarding goats, the study showed that there is no significant difference between Kirdimi and Sahelian breeds. In Cameroon and Chad, the study showed that Sahelian goats are less sensitive than Kirdimi breeds [20,21]. This divergence in the results at the breed level shows the importance of the breed of small ruminants in the epidemiology of PPR.
Statistically, the analysis of the results showed that seroprevalence rate in sheep is significantly higher than in goats. This trend is also observed in similar studies conducted in Nigeria, Burkina Faso, Kenya, and Saudi Arabia [22–25]. On the other hand, other studies have revealed the reverse as in Ethiopia, Cameroon, and India [20,26,27]. These results highlight the influence of the small ruminant species in the epidemiology of PPR virus. This difference in receptivity may be due to the intrinsic factors of the species and probably to the sensitivity of certain animals to the lineages of the virus in question.
This study also showed that whatever the species, seroprevalence rate in females is higher than in males. These results corroborate those of other studies [18,23,28,29]. The dominance of the seroprevalence rate in females is attributable to the herd operating system. In Chad, as in most other country breedings, females are generally kept longer than males, as shown by the relatively large numbers of females compared to males in this survey. Males are the most destocked for the vital needs of the breeder (food, parties, ceremonies, and household income).
The results of this study showed that, in general, the seroprevalence rate is higher in older animals than in younger. Beyond the logical nature of this finding, the older the animal, the more likely it is to have contracted PPR virus in view of the importance of the circulation of this virus in the Republic of Chad. This is probably reinforced by livestock management. Typically, young animals graze around the camp while adults are driven at long distances in search of pastures and drink in pools or rivers where animals from the area converge, which promote their exposure to the virus.
The type of breeding is a factor to consider. As shown by the results, seroprevalence rates observed among transhumants are highest compared to sedentary livestock. Indeed, the exposure of animals of this type of breeding to the PPR virus is higher for the distribution of this disease in all regions of Chad.
Conclusion
This study has shown that the PPR virus is circulating in the Republic of Chad. In view of the results obtained, the disease is enzootic in the country. Epidemiological information obtained including seroprevalence rate, risk factors (sex, breed, age, and mode of rearing), and geographical distribution will help to define an appropriate strategy for PPR control in the Republic of Chad.
Acknowledgments
The authors are grateful to the Sahel Regional Pastoral Support Project (PRAPS-TD), funded by the World Bank and CILSS, which made this work possible.
Conflict of Interest
The authors do not declare any conflict of interest.
Authors’ contribution
Ouagal Mahamat designed the study, interpreted the data and drafted the manuscript. Tchari Doungous was involved in the collection of data and contributed in the laboratory testing of the sample. Bidjeh Kebkiba took part in the drafting and critical checking of this manuscript. Hadjé Arabié Oumar was involved in the collection of data and contributed in laboratory testing of the sample in manuscript preparation. Assandi Oussiguéré was involved in the collection of data and critical checking of this manuscript. Adam Hassan Yacoub, Adoum Goudja, Mahamat Guindé, and Ahmat Hassan Moussa took part in critical checking of this manuscript.
References
- 1.Ministry of Livestock. National Plan of livestock development (PNDE, 2009–2016) [Mar 20;2018 ];2008 :82. http://www.eeas.europa.eu/archives/delegations/tchad/documents/more_info/pnde_version_20juin08_mf2_compresse_fr.pdf.
- 2.Ministry of Livestock and Animal Production. General Census of Livestock (RGE), Final results. 2018:78. [Google Scholar]
- 3.Ministry of Livestock (ME), Veterinary and zootechnical research laboratory. National report on zoogenetic ressources of Chad. [Mar 20;2018 ];2003 :78. http://www.fao.org/tempref/docrep/fao/011/a1250f/annexes/CountryReports/Chad.pdf.
- 4.Maho A, Logtene MY. Actes du colloque, 27–31 mai 2002. Garoua, Cameroun: [Mar 20;2018 ]. 2007. Pathological dominance of domestic ruminants and monogastrics in the Sudanian zone of Chad; p. 4. https://hal.archives-ouvertes.fr/hal-00139193/document. [Google Scholar]
- 5.Ouagal M, Hendrikx P, Saegerman C, Berkvens D. Comparison between active and passive surveillance within the network of epidemiological surveillance of animal diseases in Chad. [Mar 20;, 2018 ];Acta Tropica. 2010 116:147–51. doi: 10.1016/j.actatropica.2010.07.004. https://doi.org/10.1016/j.actatropica.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Shaila MS, Shamaki D, Forsyth MA, Diallo A, Goatley L, Kitching RP, et al. Geographic distribution and epidemiology of peste des petits ruminants viruses. Virus Res. 1996;43:149–53. doi: 10.1016/0168-1702(96)01312-3. https://doi.org/10.1016/0168-1702(96)01312-3. [DOI] [PubMed] [Google Scholar]
- 7.Diallo A, Minet C, Le Goff C, Berhe G, Albina E, Libeau G, et al. The threat of peste des petits ruminants: progress in vaccine development for disease control. Vaccine. 2007;25:5591–7. doi: 10.1016/j.vaccine.2007.02.013. https://doi.org/10.1016/j.vaccine.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Lefèvre PC, Diallo A. Peste des petits ruminants. Revue scientifique et technique (International Office of Epizootics). 1990;9:935–81. doi: 10.20506/rst.9.4.532. https://doi.org/10.20506/rst.9.4.532. [DOI] [PubMed] [Google Scholar]
- 9.Bidjeh K, Bornarel P, Imadine M, Lancelot R. First isolation of PPR virus in the Republic of Chad and experimental reproduction of the disease. Revue d’élevage et de médecine vétérinaire des pays tropicaux. 1995;48:295–300. https://doi.org/10.11648/j.avs.20150303.13. [PubMed] [Google Scholar]
- 10.Bidjeh K, Ban-Bo BA, Abakar MF, Oussiguéré A, Kembé A, Doungous T, et al. Distribution and seroprevalence rate of PPR virus in the Republic of Chad during 2004–2014. Anim Vet Sci. 2015;3:89–93. [Google Scholar]
- 11.Ouagal M, Hendrikx P, Berkvens D, Nchare A, Cisse B, Akpeli PY, et al. Epidemiosurveillance networks in west francophone and central Africa. [Mar 20;2018 ];Revue scientifique et technique (International Office of Epizootics) 2008 27:689–702. https://doi.org/10.20506/rst.27.3.1828. [PubMed] [Google Scholar]
- 12.FAO/OIE. Food and Agriculture Organization of the United Nations and the World Organisation for Animal Health Rome; 2016. [Mar 20;2018 ]. Peste des petits ruminants: Global Eradication Programme. Contributing to food security, poverty alleviation and resilience five years (2017–2021) p. 80. http://www.fao.org/3/a-i6316e.pdf. [Google Scholar]
- 13.FAO/OIE. Global strategy for the control and eradication of PPR. [Mar 20;2018 ];2015 :83. http://www.fao.org/3/a-i4460e.pdf.
- 14.General Directorate of Veterinary Services. Control and eradication strategy for peste des petits ruminants in Chad. 2017:85. [Google Scholar]
- 15.Libeau G, Rehaud C, Lancelot R, Colas F, Guerre L, Bishop DH, et al. Development of a competitive ELISA for detecting antibodies to the peste des petits ruminants virus using a recombinant nucleoprotein. Res Vet Sci. 1995;58:50–5. doi: 10.1016/0034-5288(95)90088-8. [DOI] [PubMed] [Google Scholar]
- 16.El-Yuguda AD, Baba SS, Ambali AG, Egwu GO. Seroprevalence of peste des petits ruminants among domestic small and large ruminants in semi-arid region of north-eastern Nigeria. Vet World. 2013;10:807–11. doi:10.14202/vetworld.2013.807-811 https://doi.org/10.1016/0034-5288(95)90088-8. [Google Scholar]
- 17.Osman NA, Ali AS, Me AR, Fadol MA. Antibody seroprevalences against Peste des Petits Ruminants (PPR) virus in sheep and goats in Sudan. Trop Anim Health Prod. 2009;41:1449–53. doi: 10.1007/s11250-009-9333-8. https://doi.org/10.1007/s11250-009-9333-8. [DOI] [PubMed] [Google Scholar]
- 18.Farougou S, Gagara M, Mensah GA. Prevalence of peste des petits ruminants in the arid zone in the Republic of Niger. Onderstepoort J Vet Res. 2013;80:6. doi: 10.4102/ojvr.v80i1.544. [DOI] [PubMed] [Google Scholar]
- 19.Almeshay MD, Gusbi A, Eldaghayes I, Mansouri R, Bengoumi M, Dayhum AS. An epidemological study on Peste des petits ruminants in Tripoli Region, Lybia. Veterinaria Italiana. 2017;53:235–42. doi: 10.12834/vetit.964.5025.3. [DOI] [PubMed] [Google Scholar]
- 20.Awa DN, Ngagnou A, Tefiang E, Yaya D, Joyan A. Post vaccination and colostral peste des petits ruminants antibody dynamics in research flocks of north Cameroon. Prev Vet Med. 2002;55:267–71. doi: 10.1016/s0167-5877(02)00013-2. https://doi.org/10.1016/S0167-5877(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 21.Akpavie SO, Orkpeh JMT, Durojaiye OA, Olowu TA, Alli RO. Maternal antibody to peste des petits ruminants and rinderpest viruses in kids and lambs and antibody responses in vaccinated adult small ruminants. Trop Vet. 1997;5:55–64. [Google Scholar]
- 22.Al-Afaleq A, Abu-Elzein E, Al-Naeem A, Amin M. Serosurveillance for PPR and rinderpest antibodies in naturally exposed Saudi sheep and goats. Veterinarski arhiv. 2004;74:459–65. [Google Scholar]
- 23.Dahiru A, Saheed S, Egwu A. Seroprevalence of peste des petits ruminants among domestic small and large ruminants in the semi-arid region of North-eastern Nigeria. Veterinary World. 2013;6:807–11. [Google Scholar]
- 24.Kihu SM. Sero-epidemiology of Peste des petits ruminants virus infection in Turkana County, Kenya. BMC Vet Res. 2015;87:1–13. doi: 10.1186/s12917-015-0401-1. https://doi.org/10.1186/s12917-015-0401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sow A, Ouattara L, Compaore Z, Doulkom BR, Pare M, Poda G, et al. Serologic prevalence of Peste des Petits Ruminants in Soum province, North of Burkina Faso. Revue d'élevage et de médecine vétérinaire des pays tropicaux. 2008;61:5–9. https://doi.org/10.19182/remvt.10012. [Google Scholar]
- 26.Abraham G, Sintayehu A, Libeau G, Albina E, Roger F, Laeakemariam Y, et al. Antibody seroprevalence against Peste des petits ruminants (PPR) virus in camel, cattle, Goats and Sheep in Ethiopia. Prev Vet Med. 2005;70:51–7. doi: 10.1016/j.prevetmed.2005.02.011. https://doi.org/10.1016/j.prevetmed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Singh RP, Saravanan P, Sreenivasa B P, Singh RK, Bandyopadhyay SK. Prevalence and distribution of peste des petits ruminants virus infection in small ruminants in India. Revue scientifique et technique (International Office of Epizootics) 2004;23:807–19. doi: 10.20506/rst.23.3.1522. [DOI] [PubMed] [Google Scholar]
- 28.Kamissoko B, Sidibé CAK, Niang M, Samaké K, Traoré A, Diakité A, et al. Seroprevalence of peste des petits ruminants in sheep and goats in Mali. Revue d’élevage et de médecine vétérinaire des pays tropicaux. 2013;66:5–10. [Google Scholar]
- 29.Senthilkumar A, Balamurugan P, Sribalaji N, Srinivasan G, Murugesan S. Outbreak of PPR in an Organised Goat Farm in Theni District of Tamilnadu. Res J Chem Environ Sci. 2018;6:64–7. [Google Scholar]