Abstract
Objectives:
This study was conducted to assess the prevalence and characterization of Staphylococcus aureus from chicken and quail eggshells and to study the antibiogram of the isolates.
Materials and methods:
A total of 300 eggs (220 chicken eggs and 80 quail eggs) were collected from different retail shops and farms in Mymensingh district. Swabs taken from the egg surfaces were cultured on Mannitol Salt Agar for the isolation of S. aureus. Polymerase chain reaction was conducted for confirmatory identification of the bacterial species targeting nuc gene, followed by confirmation of methicillin-resistant S. aureus by targeting the mecA gene. Antibiotic sensitivity test of the isolated bacteria was done against commonly used antibiotics by the disk diffusion method.
Results:
The prevalence of Staphylococcus spp. and S. aureus in the chicken eggshell surface was 20.45% and 10.45%, respectively. Similarly, the prevalence of Staphylococcus spp. and S. aureus in quail eggshell surface was 16.25% and 5%, respectively. Overall, 27 isolates were identified as S. aureus, of which 23 were from the chicken eggshell surface and four from quail eggshell surface. Among the seven isolates tested, overall four (57.14%) were positive for the nuc gene. On the other hand, the mecA gene could be detected in three (50%) S. aureus out of six oxacillin resistant isolates. The antibiogram study indicated that most of the isolates were resistant to the antibiotics under β-lactam group.
Conclusion:
The present study concludes that chicken and quail egg surface harbor multidrug-resistant bacteria which may cause public health hazards, if these antibiotic-resistant bacteria are transferred to a human.
Keywords: MRSA, antibiogram, mecA, public, health
Introduction
Table eggs are devoured worldwide in varied forms and are viewed as a very nutritious and cheap source of protein. Staphylococci comprise an imperative part of the microflora which can be segregated from the table egg surface and its contents. They can possibly cause deterioration and infection in consumers through entering the food channel pathway [1]. The shell can be contaminated when going across the vent, but many researchers recommend that contamination mainly happens immediately after laying due to attachment with infected surfaces [2]. It has been estimated that after laying, bacteria deposited on egg surface can infiltrate the shell and subsequently infect egg contents [3].
Eggshell contains several microorganisms, including Staphylococcus aureus, Salmonella spp., Streptococcus spp., Escherichia. coli, Bacillus spp., and Listeria monocytogenes [4]. Several diseases occurred in poultry are caused by Staphylococcus spp. [5]. Nearly, 50% of S. aureus produce enterotoxins which create food poisoning in consumers [6]. Among all foodborne diseases in the world, Staphylococcal food poisoning is ranked as the third [7]. Animal originating Staphylococcus strains can potentially be harmful to humans. Most of the strains of Staphylococcus show resistance to antibiotics and cause zoonoses [8]. Eggs are the potential source of transmitting antibiotic-resistant Staphylococcus strains to human causing food-borne infection [9]. Methicillin-resistant S. aureus (MRSA) is considered as one of the important bacterium among the Staphylococci, which is genetically different from other strains. The MRSA is developed through horizontal gene transfer and natural selection. As a result, multidrug resistance (MDR) in the bacteria may develop [10].
Along with the chicken eggs, quail rearing and retail sale of its eggs are getting popular day by day in Bangladesh. Thus, there is a chance of transmitting the MRSA to human through egg consumption. However, to our knowledge, limited research has been conducted on MRSA bearing resistance gene in chicken and quail eggshell. Considering the above fact, the present experiment was conducted to isolate and characterize the S. aureus and/or MRSA from chicken and quail eggshell surface.
Materials and Methods
Sample collection
A total of 220 fresh chicken eggs and 80 quail eggs were collected for sampling from different farms and retail shops in Mymensingh during the period from January to June 2017. The eggs were transported to the Bacteriology Laboratory, Department of Microbiology and Hygiene, Bangladesh Agricultural University, Mymensingh.
Isolation and identification of Staphylococcus aureus
The collected eggs were swabbed with sterile cotton buds and dispersed in nutrient broth for enrichment overnight at 37°C in bacteriological incubator (FIEM, Italy). After enrichment, the samples were streaked on to Mannitol Salt Agar (MSA). The colonies showing typical cultural characteristics of S. aureus were further inoculated on blood agar for further isolation and pure culture. Gram stain and sugar fermentation test, indole, coagulase, catalase, Methyl Red Voges Proskauer test, and motility test were performed for confirmation of the isolates [11].
Detection of nuc and mecA genes in the S. aureus
The genomic DNA was extracted from the isolated organisms by boiling method [12]. Polymerase chain reaction (PCR) was conducted to amplify the nuc and mecA genes following the methods described by Kalorey et al. [13] and Hussain et al. [14]. Oligonucleotide primers targeting nuc and mecA genes of S. aureus are mentioned in Table 1. For the amplification of both the genes, a final reaction volume of 25 μl for PCR was used consisting of 2 μl of each primer (10 pmol/μl), 12.5 μl 2X PCR master mixture, 3 μl of template DNA (about 10 ng), and 5.5 μl of nuclease-free water. Thermocycler machine (Applied Biosystem, Singapore) was used for amplification of the genes, and the thermal profile used for both nuc and mecA genes consisted of an initial denaturation for 5 min at 95°C, followed by 30 cycles of denaturation at 95°C for 1 min, annealing at 55°C for 45 sec, and extension at 72°C for 1 min. The final extension was set at 72°C for 10 min. An amount of 5 μl PCR products were separated in 1.5% agarose gel and was visualized using UV trans-illuminator (Biometra, Germany) after staining with ethidium bromide.
Table 1. Oligonucleotide primers used in this study.
Antibiotic sensitivity test
Antibiogram of S. aureus was done against nine commonly used antibiotics, including amoxicillin (30 μg), ciprofloxacin (5 μg), erythromycin (5 μg), gentamycin (5 μg), nalidixic acid (μg), oxacillin (1 μg), penicillin (10 μg), tetracycline (30 μg), and vancomycin (30 μg). The antibiotics disks were purchased from Himedia, India. The antibiotic sensitivity test was performed by the disk diffusion method [15]. The zone of inhibition produced by S. aureus was interpreted according to the standards of the Clinical and Laboratory Standards Institute [16].
Results
Overall prevalence of Staphylococcus spp. and Staphylococcus aureus
On the basis of cultural and biochemical characteristics, 20.45% (n = 45/220) chicken egg samples were found to be associated with Staphylococcus spp. Among these 45 isolates, 23 (10.45%) were identified as S. aureus on the basis of the coagulase test (Table 2). Similarly, 13 (16.25%) out of 80 quail eggs were associated with Staphylococcus spp., of which four (5%) were S. aureus (Table 3).
Table 2. Overall prevalence of Staphylococcus spp. and S. aureus in chicken egg sample.
| Sample source | Sample(N) | Staphylococcus spp. positive# | Prevalence of Staphylococcus spp. (%) | S. aureus positive* | S. aureus(%) |
|---|---|---|---|---|---|
| BAU poultry farm | 50 | 15 | 30 | 10 | 20 |
| Janota poultry Farm | 50 | 2 | 4 | 0 | 0 |
| KR market | 20 | 4 | 20 | 2 | 10 |
| Shesh more | 20 | 6 | 30 | 3 | 15 |
| Wapda more | 20 | 5 | 25 | 2 | 10 |
| Kewatkhali Bazar | 20 | 3 | 15 | 1 | 5 |
| Poultry more | 20 | 4 | 20 | 1 | 5 |
| Mesua bazar | 20 | 6 | 30 | 4 | 20 |
| Total | 220 | 45 | 20.45 | 23 | 10.45 |
On the basis of cultural and biochemical properties,
On the basis of coagulase test.
Table 3. Overall prevalence of Staphylococcus spp. and S. aureus in quail egg sample.
| Sample source | Sample(N) | Staphylococcus spp. positive# | Prevalence of Staphylococcus spp. (%) | S. aureus positive* | S. aureus(%) |
|---|---|---|---|---|---|
| BAU poultry farm | 20 | 4 | 20 | 2 | 10 |
| KR market | 20 | 2 | 10 | 1 | 5 |
| Shesh more | 20 | 3 | 15 | 0 | 0 |
| Mesua bazar | 20 | 4 | 20 | 1 | 5 |
| Total | 80 | 13 | 16.25 | 4 | 5 |
On the basis of cultural and biochemical properties,
On the basis of coagulase test.
Molecular detection of nuc and mecA
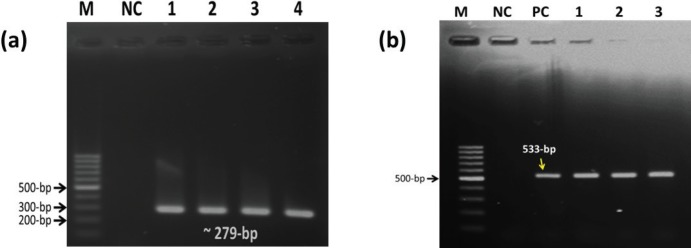
From the coagulase positive isolates, seven (randomly selected) were used for PCR amplification targeting the nuc gene, of which four (one from quail and three from chicken eggshell) were positive (Table 4, Fig. 1a). Among the coagulase positive isolates (n = 27), six isolates originated from chicken eggs were resistance to oxacillin, of which three were found positive for the mecA gene (Table 4, Fig. 1b).
Table 4. Molecular detection of nuc and mecA genes in S. aureus.
| Gene | Total sample | Coagulase positive | Coagulase positive used for PCR | PCR positive |
|---|---|---|---|---|
| nuc | 300 | 27 | 7 | 4 |
| mecA | 300 | 27 | 6 | 3 |
Figure 1. PCR amplification of nuc (a) and mecA (b) genes of S. aureus. (a) M = marker, NC = negative control, Lane 1–4 = test samples, (b) M = marker, NC = negative control, PC = positive control, Lane 1–3 = test samples.
Antibiotic sensitivity test
All the 23 isolates of S. aureus from chicken egg sample were subjected to antibiotic sensitivity test against nine commonly used antibiotics (Table 5). The results showed that vancomycin was sensitive to 73.91% isolates. Besides, 91.30% isolates were found to be resistant to amoxicillin (Table 5). On the other hand, all the four S. aureus isolates from quail egg samples were highly susceptible (75%) to vancomycin, oxacillin, and tetracycline. In contrast, 75% isolates were resistant to amoxicillin, nalidixic acid, and penicillin (Table 5).
Table 5. Antibiotic sensitivity profile of S. aureus isolated from chicken and quail eggs.
| Antimicrobial agents | Group | No. of isolates (%) | |||||
|---|---|---|---|---|---|---|---|
| Chicken egg isolates | Quail egg isolates | ||||||
| R | I | S | R | I | S | ||
| Amoxicillin | β-lactam | 21 (91.30) | 2 (8.69) | 0 (0.0) | 3 (75.0) | 1 (25.0) | 0 (0.0) |
| Oxacillin | β-lactam | 6 (26.08) | 2 (8.69) | 15 (65.21) | 1 (25.0) | 0 (0.0) | 3 (75.0) |
| Penicillin | β-lactam | 19 (82.60) | 4 (34.78) | 0 (0.0) | 3 (75.0) | 1 (25.0) | 0 (0.0) |
| Ciprofloxacin | Quinolone | 6 (26.08) | 4 (17.39) | 13 (56.52) | 2 (50.0) | 1 (25.0) | 1 (25.0) |
| Nalidixic acid | Quinolone | 19 (82.60) | 2 (8.69) | 2 (8.69) | 3 (75.0) | 1 (25.0) | 0 (0.0) |
| Erythromycin | Macrolide | 9 (39.13) | 6 (26.08) | 8 (34.78) | 1 (25.0) | 1 (25.0) | 2 (50.0) |
| Gentamycin | Aminoglycoside | 8 (34.78) | 5 (21.73) | 10 (43.47) | 1 (25.0) | 2 (50.0) | 1 ( 25 ) |
| Tetracycline | Tetracycline | 9 (39.23) | 2 (8.69) | 12 (52.17) | 1 (25.0) | 0 (0.0) | 3 (75.0) |
| Vancomycin | Glycopeptide | 4 (17.39) | 2 (8.69) | 17 (73.91) | 0 (0.0) | 1 (25.0) | 3 (75.0) |
R = Resistant, I = Intermediate, S = Sensitive.
Discussion
Out of 300 eggs, 58 (19.33%) eggshells yielded growth of Staphylococcus spp., which was supported by Syed et al. [17] in Pakistan, who reported 21.3% prevalence of Staphylococcus spp. However, the prevalence found in our study was comparatively higher than the findings of Parveen et al. [18], Eid et al. [19], Chaemsanit et al. [20], Pyzik et al. [21], and Pyzik and Marek [22] who observed the prevalence was 5.5%, 14.5%, 18%, 7.61%, and 15.6% in Dinajpur (Bangladesh), Sharkia (Egypt), Thailand, and Lubin city, respectively. The prevalence of Staphylococcus spp. (25%) in table eggs collected from different markets of Dhaka city, as reported by Fardows et al. [23], was higher as compared with our study. In developing countries like Bangladesh, an increased percentage of bacterial contamination have been found on egg surface due to inappropriate refrigeration and even no refrigeration during the market storing. Thus, variation in the prevalence of Staphylococcus spp. in eggshell might be due to inappropriate storage condition at market level.
In this study, Staphylococcus spp. showed golden-yellow colonies on MSA due to fermentation of mannitol, as reported by Konuku et al. [24] and Kwoji et al. [25]. Microscopically, Staphylococcus spp. was Gram-positive cocci arranged in a grape-like cluster [11]. The isolation of coagulase positive Staphylococcus spp. in this study warns that this organism may cause human infection elicited by toxins produced by them. In this study, 58 isolates were catalase positive, of which 27 were coagulase positive indicating that the isolates were S. aureus, as described by Kumar et al. [26].
Out of seven coagulase-positive S. aureus, nuc gene was confirmed to be present in four (57.14%) isolates. Six isolates were oxacillin resistant, of which three (50%) contained the mecA gene. In another study, Sadeghi and Mansouri [27] reported that 162 S. aureus isolates were confirmed to be present with the nuc gene, of which 56.8% were MRSA. Similar report was also reported by Pyzik et al. [21].
Nowadays, MDR is an emerging issue worldwide in treating infectious diseases. Here, Staphylococcus spp. originated from chicken eggs showed varying degrees of resistance to amoxicillin (91.30%) and oxacillin (26.08%). Similar results reported by Eid et al. [24] indicated that 87% isolates of Staphylococcus spp. were resistant to amoxicillin. However, slightly lower resistant to amoxicillin (73.3%) was recorded by Lee [28]. In another study, Nam et al. [29] reported that only 6.2% Staphylococcus spp. were resistant to oxacillin. The eggs collected directly from the farms and the grocery stores were not washed before being sold. Though isolation of S. aureus was performed, enumeration was not conducted from these samples. This study also limits on the characterization of bacteria on the eggshell surface rather than the inner content. So, it would be difficult to interpret the public health significance in its present form. However, this study described the presence of MRSA on eggshell in Bangladesh, and it would be interesting to continue working on the same track and develop the knowledge of the hygienic status in the egg production.
Conclusion
Our results reveal that eggshells are contaminated with Staphylococcus spp. at a higher proportion, and MDR S. aureus are recorded. This research confers a risk of being affected by MDR S. aureus from eggs of retail shops and farms unless they are properly washed and stored at the collection to marketing stage. So, it is important to establish proper hygienic practice and awareness among the people regarding the risk of MDR bacteria in consumers.
Acknowledgments
The research work was supported with the grants from the Ministry of Education and Ministry of Science and Technology, Bangladesh.
Conflict of Interest
The authors declare that there is no conflict of interest towards the publication of this article.
Authors’ Contribution
Amrita Pondit, Zobayda Farzana Haque, and Abdullah Al Momen Sabuj carried out the experiments, analyzed the data, and wrote the initial draft of the manuscript. Sukumar Saha and Md. Shahidur Rahman Khan designed and supervised research work, revised, and finalized the manuscript. All authors read and approved the manuscript before submission.
References
- 1.Salihu MD, Garba B, Isah Y. Evaluation of microbial contents of table eggs at retail outlets in Sokoto metropolis, Nigeria. Sokoto J Vet Sci. 2015;13(1):22–8. [Google Scholar]
- 2.Smith A, Rose SP, Wells RG, Pirgozliev V. The effect of changing the excreta moisture of caged laying hens on the excreta and microbial contamination of their egg shells. Br Poult Sci. 2000;41(2):168–73. doi: 10.1080/713654903. https://doi.org/10.1080/713654903. [DOI] [PubMed] [Google Scholar]
- 3.Bahrouz M, Al-Jaff A. The risk of bacterial contamination in hen eggs of Sulaimani poultries. J Zankoy Sulaimani. 2005;8:63–71. [Google Scholar]
- 4.Mahdavi M, Jalali M, Safaei HG, Shamloo E. Microbial quality and prevalence of Salmonella and Listeria in eggs. Int J Environ Health Eng. 2012;1(1):48. https://doi.org/10.4103/2277-9183.105347. [Google Scholar]
- 5.Stepien-Pysniak D, Marek A, Rzedzicki J. Occurrence of bacteria of the genus Staphylococcus in table eggs descended from different sources. Pol J Vet Sci. 2009;1(4):481. [PubMed] [Google Scholar]
- 6.Abdullah IN. Isolation and identification of some bacterial isolates from table egg. Al-Anbar J Vet Sci. 2010;3(2):59–67. [Google Scholar]
- 7.Boerema A, Clemens R, Brightwell G. Evaluation of molecular methods to determine enterotoxigenic status and molecular genotype of bovine, ovine, human and food isolates of Staphylococcus aureus. Int J Food Microbiol. 2006;107(2):192–201. doi: 10.1016/j.ijfoodmicro.2005.07.008. https://doi.org/10.1016/j.ijfoodmicro.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton PD, Taylor PW. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci Prog. 2002;15(2):57–72. doi: 10.3184/003685002783238870. https://doi.org/10.3184/003685002783238870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abulreesh HH, Organji SR. The prevalence of multidrug-resistant Staphylococci in food and the environment of Makkas, Saudi Arabia. Res J Microbiol. 2011;6(6):510–23. https://doi.org/10.3923/jm.2011.510.523. [Google Scholar]
- 10.Gurusamy KS, Koti R, Toon CD, Wilson P, Davidson BR. Antibiotic therapy for the treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in surgical wounds. Cochrane Database Syst Rev. 2013;(8):CD009726. doi: 10.1002/14651858.CD009726.pub2. https://doi.org/10.1002/14651858.CD009726.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheesbrough M. Microbiology. 1st. English Language Book Society; London: 1985. Medical laboratory manual for tropical countries; pp. 400–80. [Google Scholar]
- 12.Dashti AA, Jadaon MM, Abdulsamad AM, Dashti HM. Heat treatment of bacteria: a simple method of DNA extraction for molecular techniques. Kuwait Med J. 2009;41(2):117–22. [Google Scholar]
- 13.Kalorey DR, Shanmugam Y, Kurkure NV, Chousalkar KK, Barbuddhe SB. PCR-based detection of genes encoding virulence determinants in Staphylococcus aureus from bovine subclinical mastitis cases. J Vet Sci. 2007;8(2):151–4. doi: 10.4142/jvs.2007.8.2.151. https://doi.org/10.4142/jvs.2007.8.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain K, Rahman M, Nazir KHMNH, Rahman H, Khair A. Methicillin resistant Staphylococcus aureus (MRSA) in patients of Community Based Medical College Hospital, Mymensingh, Bangladesh. Am J Biomed Life Sci. 2016;4(3):26–9. https://doi.org/10.11648/j.ajbls.20160403.11. [Google Scholar]
- 15.Bauer A, Kirby W, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. https://doi.org/10.1093/ajcp/45.4_ts.493. [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute (CLSI) CLSI supplement M100s. 26th. Clinical and Laboratory Standards Institute; Wayne, Pennsylvania: 2016. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 17.Syed MA, Shah SHH, Sherafzal Y, Shafi-ur-Rehman S, Khan MA, Barrett JB, et al. Detection and molecular characterization of methicillin-resistant Staphylococcus aureus from table eggs in Haripur, Pakistan. Foodborne Pathog Dis. 2018;15(2):86–93. doi: 10.1089/fpd.2017.2336. https://doi.org/10.1089/fpd.2017.2336. [DOI] [PubMed] [Google Scholar]
- 18.Parveen A, Rahman MM, Fakhruzzaman M, Akter MR, Islam MS. Characterization of bacterial pathogens from egg shell, egg yolk, feed and air samples of poultry houses. Asian J Med Biol Res. 2017;3(2):168–74. https://doi.org/10.3329/ajmbr.v3i2.33564. [Google Scholar]
- 19.Eid S, Nasef SA, Erfan AM. Multidrug resistant bacterial pathogens in eggs collected from backyard chickens. Assiut Vet Med J. 2015;61(144):87–103. [Google Scholar]
- 20.Chaemsanit S, Akbar A, Anal AK. Isolation of total aerobic and pathogenic bacteria from table eggs and its contents. Food Appl Biosci J. 2015;3(1):1–9. [Google Scholar]
- 21.Pyzik E, Marek A, Hauschild T. Characterisation of Staphylococcus aureus and Staphylococcus aureus like strains isolated from table eggs. Bull Vet Inst Pulawy. 2014;58(1):57–63. https://doi.org/10.2478/bvip-2014-0009. [Google Scholar]
- 22.Pyzik E, Marek A. Characterization of bacteria of the genus Staphylococcus isolated from the eggs of Japanese quail (Coturnixcoturnix japonica) Pol J Vet Sci. 2012;15(4):767–72. doi: 10.2478/v10181-012-0116-1. https://doi.org/10.2478/v10181-012-0116-1. [DOI] [PubMed] [Google Scholar]
- 23.Fardows J, Siddique AB, Moureen A, Islam TAB, Farhana N, Naheen CR. Isolation and identification of pathogenic Gram-positive bacteria from egg shell of hen and to see their antimicrobial susceptibility pattern. J Enam Med Coll. 2016;6(1):15–8. https://doi.org/10.3329/jemc.v6i1.26374. [Google Scholar]
- 24.Konuku S, Rajan MM, Muruhan S. Morphological and biochemical characteristics and antibiotic resistance pattern of Staphylococcus aureus isolated from grapes. Int J Nutr Pharmacol Neurol Dis. 2012;2(1):70–3. https://doi.org/10.4103/2231-0738.93135. [Google Scholar]
- 25.Kwoji ID, Tambuwal FM, Abubakar MB, Yakubu Y, Bitrus AA, Jauro S. Occurrence of methicillin resistant Staphylococcus aureus in chickens and farm personnel in Sokoto, North-western Nigeria. J Adv Vet Anim Res. 2017;4(3):255–60. http://doi.org/10.5455/javar.2017.d220. [Google Scholar]
- 26.Kumar R, Yadav BR, Singh RS. Antibiotic resistance and pathogenicity factors in Staphylococcus aureus isolated from mastitic Sahiwal cattle. J Biosci. 2011;36(1):175–88. doi: 10.1007/s12038-011-9004-6. https://doi.org/10.1007/s12038-011-9004-6. [DOI] [PubMed] [Google Scholar]
- 27.Sadeghi J, Mansouri S. Molecular characterization and antibiotic resistance of clinical isolates of methicillin-resistant Staphylococcus aureus obtained from Southeast of Iran (Kerman) Acta Pathol Microbiol Immunol Scand. 2014;122(5):405–11. doi: 10.1111/apm.12158. https://doi.org/10.1111/apm.12158. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH. Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl Environ Microbiol. 2003;69(11):6489–94. doi: 10.1128/AEM.69.11.6489-6494.2003. https://doi.org/10.1128/AEM.69.11.6489-6494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nam HM, Lee AL, Jung SC, Kim MN, Jang GC, Wee SH, et al. Antimicrobial susceptibility of Staphylococcus aureus and characterization of methicillin-resistant Staphylococcus aureus isolated from bovine mastitis in Korea. Foodborne Pathog Dis. 2011;8(2):231–8. doi: 10.1089/fpd.2010.0661. https://doi.org/10.1089/fpd.2010.0661. [DOI] [PubMed] [Google Scholar]