Abstract
Objective:
The current research was carried out to evaluate the use of magnesium hydroxide as buffer to control acidosis in rumen culture fermenting carbohydrates in vitro.
Materials and Methods:
The experiments were carried out in the chemostat system in which the reactor used was a 200 ml of working volume. A series of fed-batch trials were carried out in fed-batch system with hydraulic retention time of 4 days. All digesters were completely mixed with the rotation of 55 rpm, and the temperature was controlled at 39°C ± 0.5°C.
Results:
Results showed that the supplementation of magnesium hydroxide (50 mM/day) to the corn starch feed (12.5 gm/l per day) for the rumen culture could prevent acidosis while at the same concentration of sodium bicarbonate addition to rumen culture, acidosis cannot be prevented in which lactic acid accumulated up to 200 mM. Supplementing magnesium hydroxide to the mixture of starch and sugar feeds prevented acidosis in which the major fermentation end product formed was acetate. A daily feeding with the ratio of 4.5:1 [starch: Mg(OH)2] was feasible to prevent rumen acidosis.
Conclusion:
Magnesium hydroxide added to the rumen culture could prevent lactic acid accumulation while sodium bicarbonate supplementation did not prevent acidosis and had lactic acid accumulation.
Keywords: Rumen acidosis, carbohydrates, magnesium hydroxide
Introduction
Acidosis is a fermentation disorder occurred in the rumen caused by organic acid accumulation [1]. This metabolic disorder happened as the ruminants consumed large amount of fermentable carbohydrates in the purpose of enhancing their energy, weight, and, milk production [1–3]. A critical rumen acidosis is normally caused by lactic acid build up, and at this condition rumen pH fell into less than 5.0 that may lead to the mortality of rumen animals [4–7]. Acidic condition in the rumen leads to an imbalance rumen microbiota [5]. Thus, maintaining ruminal pH at the normal level (5.8–7.2) is important factor to balance the rumen microbes between acid producers and consumers [5,8,9].
Some studies had been carried out by some researchers to control acidosis by using buffers and neutralizing agents [10]. In the cattle industry, buffers used for preventing rumen acidosis include sodium bicarbonate, magnesium carbonate, calcium carbonate, and potassium bicarbonate [11]. Some neutralizing agents such as magnesium oxide, sodium carbonate, and potassium carbonate are normally used for preventing acidosis [12]. Sodium bicarbonate is a buffer that is widely known and used by cattle growers as a feed additive for preventing lactic acidosis on starch-based feeds [11]. However, a problem was found when sodium bicarbonate was added to the rumen for preventing acidosis in which carbon dioxide gas was built up. Overproduction of carbon dioxide in the rumen caused by sodium bicarbonate supplementation may harm the host of rumen [13]. Thus, study on the use of buffer addition for controlling acidosis without generating any side effects to the ruminants is still necessary.
Study on the supplementation of magnesium hydroxide to prevent acidosis is still limited. Some studies reported that both magnesium hydroxide and magnesium oxide are similar supplements that can be used safely for cows [14,15]. For neutralizing acid accumulation in the rumen, some researchers usually added a neutralizing agent such as magnesium oxide [3,16]. Acidosis caused by the onset of lactic acid accumulation sometimes is quicker than the buffering system available in the rumen [11,16]. Thus, the use of magnesium oxide would be infeasible because it has slow buffering effect. For the purpose of preventing acidosis, this current study used magnesium hydroxide as a buffer to prevent acidosis in the rumen culture fermenting polymeric carbohydrates.
The purposes of the current research were to assess the potential role of Mg(OH)2 as a buffer to control acidosis. As sodium bicarbonate has been widely known and used by cow growers to prevent acidosis, the current study also include the study on the effect of sodium bicarbonate addition for preventing acidosis in the rumen culture fermenting carbohydrate. This is important as the current study would also investigate and evaluate the effectiveness of sodium bicarbonate addition for preventing rumen acidosis as some studies have revealed that sodium bicarbonate addition may also cause sickness on ruminants [17], and thereby this current study would explore the side effect of using sodium bicarbonate for preventing acidosis. The comparison of magnesium hydroxide versus sodium bicarbonate as buffer for preventing acidosis had been evaluated in vitro. The effects of magnesium hydroxide supplementation to rumen culture fermenting different composition of carbohydrates, and the different concentration of magnesium hydroxide supplemented to rumen culture were also investigated in vitro experiments.
Materials and Methods
Collection of samples
All methods for collecting rumen fluid had been examined and agreed by the Animal Ethics Committee at Murdoch University. Rumen fluid was collected from a previously prepared cow with a surgically created rumen fistula at the School of Veterinary and Life Science, Murdoch University, Perth, Western Australia. The cow was 3-year-old female and fed on irrigated pasture of Kikuyu grass.
Medium composition
The compositions of the medium used for the rumen fermentation experiment were 44 mg/l KH2PO4, 160 mg/l NH4Cl, 25 mg/l MgSO4.7H2O and 1 gm/l Bacto-yeast extract, 1 gm/l Bacto-peptone, 125 mg/l NaHCO3, and 1.25 ml/l of trace element solution. The trace element was composed of ethylenediaminetetraacetic acid 15 gm/l, ZnSO4.7H2O 0.43 gm/l, CoCl2.6H2O 0.24 gm/l, MnCl2.4H2O 0.99 gm/l, CuSO4.5H2O 0.25 gm/l, NaMoO4.2H2O 0.22 gm/l, NiCl2.6H2O 0.19 gm/l, NaSeO4.10H2O 0.21 gm/l, H3BO4 0.014 gm/l, and NaWO4.2H2O 0.050 gm/l.
Experimental design and procedure
The two identical computer-controlled glass reactors were used for the chemostat system with the working volume of 200 ml. The reactors were mixed continuously with 55 rpm. The temperature was maintained at 39°C ± 0.5°C. To monitor the change of pH during the rumen fermentation, pH probes were attached to the reactors. All the data measured were automatically recorded into a spreadsheet. The process of feeding and discharging were automatically operated using a particular logic in the computer program using the software LabVIEW™ (version7.1; National Instrument).
Effects of buffers addition to the rumen culture fermenting polymeric carbohydrate
These experiments were carried out in fed-batch system with the hydraulic retention time (HRT) of 4 days indicating that there was 50 ml/day of influent and effluent. The feeding system was established with the feeding operation of four times a day meaning that the substrate fed to the rumen culture was every 6 h. The substrate used for this experiment was corn flour (90.2% of total solids). Each reactor was filled up with a 200 ml fresh rumen fluid and fed with a 50 gm/l corn starch with medium solution. To test the effects of buffer addition to the rumen culture fermenting starch, the first reactor was supplemented with sodium bicarbonate while the second digester was added with magnesium hydroxide. The buffer concentration applied was a 200 mmol/l. Another control reactor was operated by feeding a 50 gm/l corn starch with medium solution without any buffer addition.
Rumen fermentation on different composition of carbohydrates added with Mg(OH)2
These experiments were carried out in the chemostat system in which the reactor used was a 200 ml of working volume. A series of fed-batch trials were carried out in fed-batch system with 4 days of HRT. A 200 ml fresh rumen fluid was used for the experiment. The first digester was fed with a 100% corn starch (50 gm/l). Another digester was added with a 50% corn starch (25 gm/l) and 50% wheat starch (25 gm/l). The third reactor was fed with a 50% wheat starch (25 gm/l), 25% corn starch (12.5 gm/l) and 25% sucrose (12.5 gm/l). The forth digester was fed with a 50% corn starch (25 gm/l), 25% wheat starch (12.5 gm/l), 25% sucrose (12.5 gm/l). Each substrate supplied was mixed with medium solution and 200 mM magnesium hydroxide. The control reactor was fed with corn starch only (50 gm/l) with medium solution without buffer addition. All processes were operated with the computer program.
Different concentration of Mg(OH)2 added to the rumen culture fermenting starch
In order to monitor the effectiveness of magnesium hydroxide addition to the rumen culture fermenting starch, different concentration of this buffer was supplemented to prevent acidosis. This test was carried out in fed-batch system with a 200 ml working volume and 4 days of HRT. Each reactor was filled up with a 200 ml fresh rumen fluid. A 50 gm/l corn starch with medium was fed to the rumen culture. For the test reactor, initially the substrate fed was supplemented with 100 mM magnesium hydroxide. Then, an increasing 50 mM magnesium hydroxide was applied every 48 h in order to avoid a long exposure of acidic condition to the rumen bacteria that may affect their life. The maximum concentration of buffer applied was 200 mM. The operational procedures were controlled by the computer program. For the control reactor, the rumen culture was loaded with corn starch (50 gm/l) only with medium solution without buffer supplementation.
Analytical methods
Lactic and volatile fatty acids were determined by using gas chromatography. The analytical procedures were done according to the method developed by Darwin et al. [18].
Results
Influence of buffers addition to the rumen culture fermenting starch
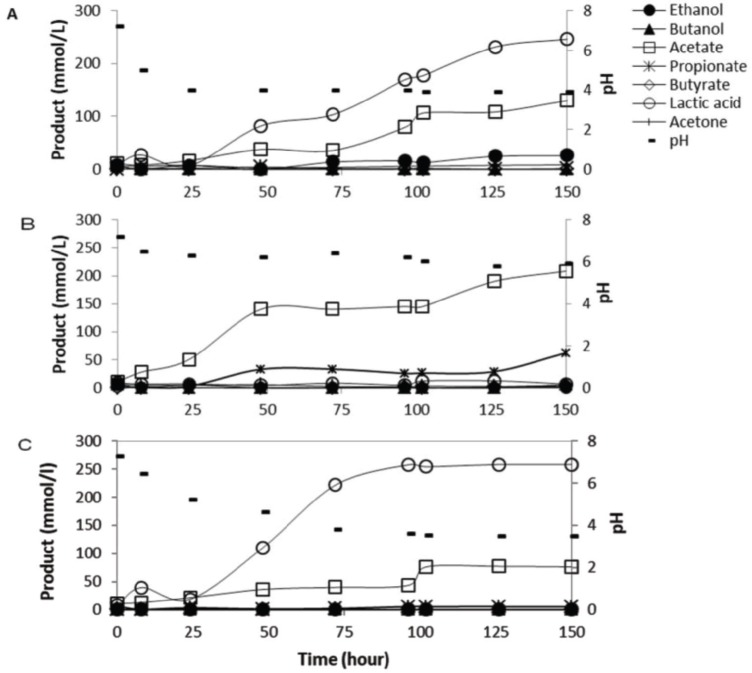
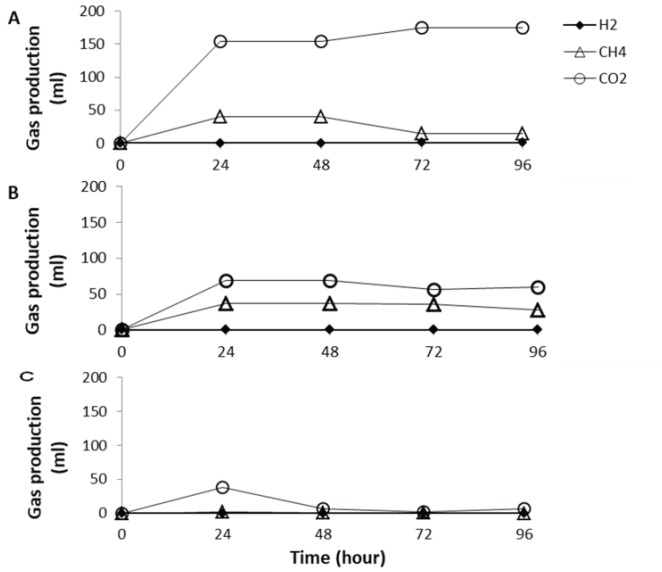
In order to prevent lactic acid build up in the rumen culture fermenting carbohydrates, two types of buffers including magnesium hydroxide and sodium bicarbonate were used. In this test, sodium bicarbonate was used due to the fact that this buffer is widely known and used for neutralizing the acid accumulation in the rumen [11,12,19]. Each buffer (200 mmol/l) used was mixed with the corn starch feed (50 gm/l). This test was carried out in the chemostat system under fed-batch mode with the rate of 50 ml/day (HRT of 4 days) indicating that a 50 gm/l of corn starch concentration fed to the rumen was distributed into a 12.5 gm/l per day, and the buffer supplemented was also distributed into a 50 mmol/l per day. Results showed that the rumen culture fermenting corn starch added with sodium bicarbonate cannot prevent acidosis while the rumen culture supplemented with magnesium hydroxide can prevent acidosis (Fig. 1). Furthermore, results from this current study also showed that feeding the ruminants with the mixture of corn starch (12.5 gm/l per day) and sodium bicarbonate (50 mmol/l per day) produced a lot of gas, which was mainly carbon dioxide (Fig. 2).
Figure 1. Profiles of rumen fermentation end products from the fed-batch fermentation of corn starch using different type of buffers: (A) sodium bicarbonate, (B) magnesium hydroxide, and (C) control reactor with no buffer addition. The buffer concentration used was 200 mmol/l. The substrate concentration used was 50 gm/l.
Figure 2. Profiles of the gas production from the rumen culture fermenting corn starch using different type of buffers: (A) NaHCO3, (B) Mg(OH)2, and (C) control reactor with no buffer addition. The buffer concentration used was 200 mmol/l. The substrate concentration used was 50 gm/l.
Different composition of carbohydrates with the addition of magnesium hydroxide
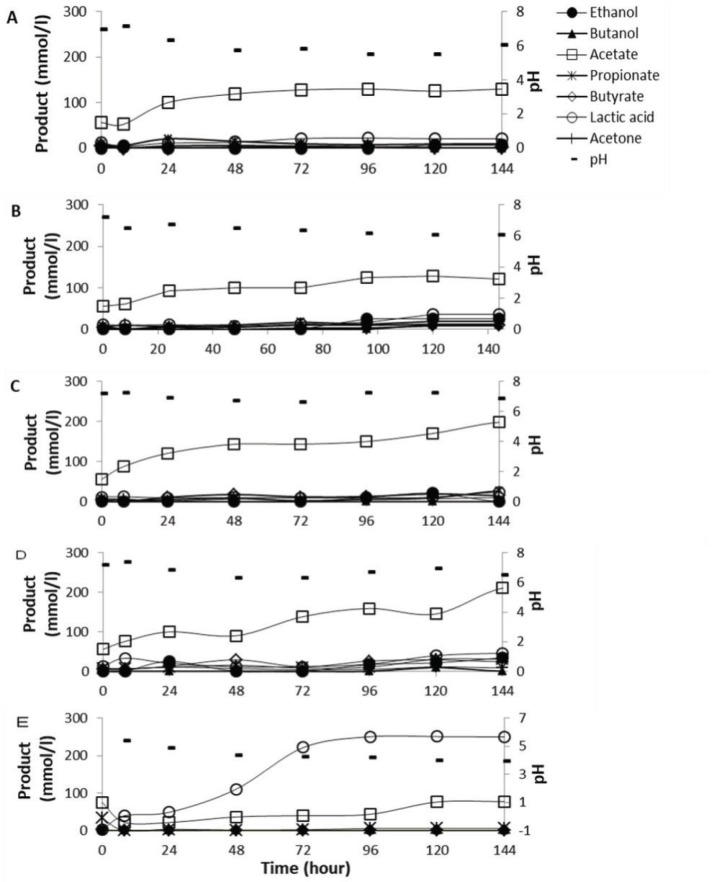
The previous tests showed that a 200 mmol/l Mg(OH)2 was able to control lactic acid build up in the rumen cultures that fermented corn starch. As corn starch is not the only grain component in ruminant feeds, the Mg(OH)2 amended fermentation of other carbohydrates by the rumen culture was tested by a fed-batch operation using various concentration of corn starch, wheat starch, and sucrose. In this test, sucrose addition was used as this type of sugar is normally preferred additive in the dairy cow feeds to stimulate the feed consumption of dairy cows in the early lactation [20]. Furthermore, sucrose used as a sweetened additive in dairy cow feeds also can enhance the yield of milk fat [21]. Thus, this test was conducted in order to evaluate whether magnesium hydroxide added in the starch feeds containing sugar can prevent lactic acidosis in the rumen culture.
Results showed that in all mixtures of starch and sucrose amended with magnesium hydroxide lactic acid accumulation was avoided and that acetate was the major end product with the concentration from 110 to 210 mM (Fig. 3). The pH in each sample was maintained between 6.25 and 7.25. Results suggested that sucrose addition in the starch feeds produced more metabolites than starch feed only as sucrose was a soluble carbohydrate that can be easily taken by the rumen bacteria to produce fermentation end products (Fig. 3C and D).
Figure 3. Profiles of rumen fermentation end products of fed-batch digesters under the different composition of substrates with the addition of a 200 mmol/l Mg(OH)2: (A) 100% corn, (B) 50% corn and 50% wheat, (C) 50% wheat, 25% corn, and 25% sucrose, (D) 50% corn, 25% wheat, and 25% sucrose, and (E) control corn starch without buffer addition. Total carbohydrates concentration applied was 50 gm/l.
Different concentration of magnesium hydroxide added to the rumen culture
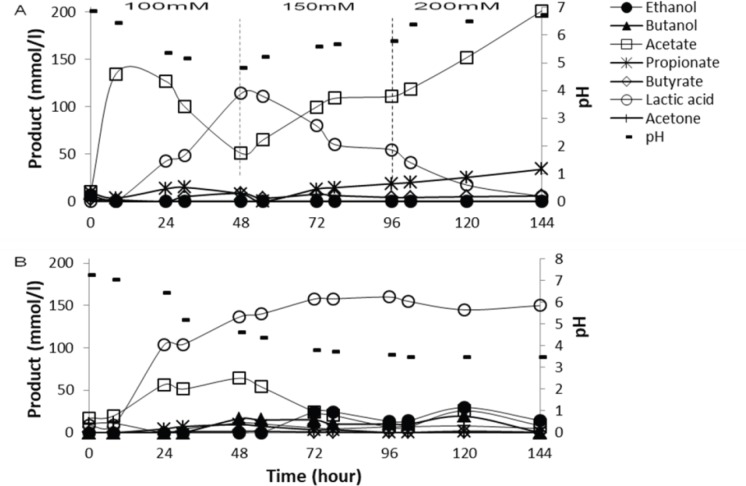
The previous tests showed that the lactic acid build up in the rumen fermentation could be prevented by adding a 200 mmol/l magnesium hydroxide to the feeds of a 50 gm/l corn starch as well as the mixture of starch and sugar. In this test, step changes from the different concentration of magnesium hydroxide addition in the rumen fed-batch test fermentation were carried out. The changing of different concentration of buffer was applied every 48 h. This technique was applied in order to avoid the ruminal bacteria becoming inactive due to the long exposure to the acidic condition [22–24]. Thus, an increasing of 50 mmol/l magnesium hydroxide was applied every 48 h to assess the feasible concentration for the rumen culture fermenting starch. In this test, initially, 100 mmol/l magnesium hydroxide was added to the feeds (50 gm/l corn starch) operated for 48 h fermentation. Then, the buffer concentration was increased to 150 and 200 mmol/l subsequently.
Results showed that the addition of 100 mmol/l magnesium hydroxide to the 50 gm/l corn starch feeds cannot prevent the lactic acid build up in the rumen culture, and the pH dropped significantly from 6.84 to 4.82 within 48 h (Fig. 4A). At this pH level (4.86), the lactic acid producing bacteria may dominate the starch fermentation in the rumen, and the extreme low pH (<5.0) in the rumen culture was regarded as critical rumen acidosis [5]. In this condition, the lactic acid production was in the rate of a 2.375 mmol/l per hour.
Figure 4. Profiles of metabolites from the fed-batch fermentation of corn starch using different concentration of buffer: (A) Mg(OH)2, (B) control reactor with no buffer addition. The substrate concentration used was a 50 gm/l corn starch.
When the concentration of magnesium hydroxide was increased from 100 to 150 mmol/l, then the pH in the rumen cultures increased from 4.82 to 5.78. Although pH in the rumen cultures increased, lactic acid concentration in the rumen culture was still high (54.5 mM). This result is in agreement with the study by Olson [9] describing that a ruminal pH from 5.5 to 5.8 is still considered as a marginal or developing ruminal acidosis that still can produce lactic acid as the major fermentation end product.
Ruminal acidosis induced by the lactic acid build up in the rumen culture fermenting starch could be prevented once the concentration of magnesium hydroxide added was increased to a 200 mmol/l. At this stage, the rumen pH increased from 5.78 to 6.72. In this condition, lactic acid production reduced while the acetate increased and reached to a 201.32 mmol/l. At this pH level (>6), interestingly propionate was also produced (33.95 mmol/l) as the second major end product. These results suggested that 200 mM magnesium hydroxide was a sufficient concentration for the rumen culture fermenting 50 gm/l corn starch.
Discussion
The rumen culture fed with corn starch supplemented with sodium bicarbonate (Fig. 1A) cannot maintain pH at a tolerable level (≥5.5) [25,26] in which the pH dropped to lower than 5.0 leading to a severe ruminal acidosis [5]. In this condition, the rumen microbiota was deeply disturbed by the onset of lactic acid accumulation [27]. The rumen culture fermenting starch added with magnesium hydroxide was able to maintain pH at 6, and could prevent lactic acid accumulation (Fig. 1B). At this condition, the major fermentation end product was acetate (150–200 mM) and a small amount of propionate (25–60 mM).
These results indicated that magnesium hydroxide had buffer capacity higher than Sodium bicarbonate. This current study is in agreement with some studies revealing that rumen supplemented with sodium bicarbonate had less buffering capacity compared to the rumen receiving magnesium oxide as well as magnesium hydroxide [15,28]. This is because Eq. (1) in theory showed that magnesium hydroxide has at least twice the buffer capacity than an equal molar of sodium bicarbonate [Eqs. (1 and 2)]. Furthermore, the addition of sodium bicarbonate to the rumen culture in the anaerobic condition could result in the production of carbonic acid. This acid may also contribute to lower pH in the rumen culture [Eq. (2)].
Mg(OH)2 + 2CH3CHOHCOOH → (CH3CHOHCOO)2Mg + 2H2O (1)
NaHCO3 + CH3CH(OH)COOH → CH3CH(OH)COONa + H2CO3 (2)
As sodium bicarbonate has lower buffer capacity than magnesium hydroxide, a higher concentration of sodium bicarbonate is required to prevent lactic accumulation in the rumen culture fermenting carbohydrates [15,28]. However, a negative effect may appear when high concentration of sodium bicarbonate is supplemented to the rumen culture fermenting starch. This is because in theory an addition of sodium bicarbonate for buffering lactic acid accumulation could generate carbon dioxide gas [Eq. (3)]. If the carbon dioxide could be released from the anaerobic system, the rumen culture could be alkalinized [29]. Thus, supplementing high concentration of sodium bicarbonate for the purpose of preventing lactic acidosis should be avoided. As the digestion process occurred in the rumen is under a closed system, an accumulation of carbon dioxide may harm the host of rumen [13].
NaHCO3 + CH3CH(OH)COOH → CH3CH(OH)COONa + H2O + CO2 (3)
It should be noted that the addition of magnesium hydroxide to the rumen culture should be carefully monitored because if it is added too much or at high concentration, it will generate alkalosis in the rumen that may harm the host of rumen [30,31]. This may occur because magnesium hydroxide has higher buffering effect than sodium bicarbonate [15,28], and also has a potent alkalinizing effect on the rumen pH that may inhibit the growth of ruminal bacteria [30,32]. Supplementation of high concentration of magnesium hydroxide can reduce rumen protozoa and microbial activity [32]. In this current test, magnesium hydroxide is supplemented with the corn starch feed to the rumen culture under a fed-batch system in which the feed was loaded every 6 h. Thus, the tendency of lactic acid accumulation may occur due to high amount of starch received by the rumen culture (Fig. 4B).
For this current study, as the system was set up with 4 days of HRT, the addition of feeds and buffer supplementation was distributed into 50 mM/day (2.92 gm/l per day) Mg(OH)2 and 12.5 gm/l per day corn starch. This indicated that for a daily feeding, the ratio of 4.5:1 [starch: Mg(OH)2] was feasible to prevent acidosis caused by the lactic accumulation in rumen culture fermenting corn starch. Magnesium hydroxide is classified as a strong base [33], and thereby have a strong effect in reducing proton concentration in the fermentation culture. Riis et al. [33] mentioned that the addition of water insoluble salts of a strong base (e.g., magnesium hydroxide) could effectively increase pH and create alkaline condition. Therefore, the use of this chemical for preventing rumen acidosis should be monitored carefully. This is important because the addition of this supplement with high concentration to prevent acidosis may generate another side effect called alkalosis [32]. Alkalosis that occurs in rumen is caused by a decrease in hydrogen ion concentration, and an increase of pH in the rumen culture [34].
Results of this current experiment showed that no alkalosis occurred by supplementing a 50 mM per day magnesium hydroxide to the corn starch feed of 12.5 gm/l per day in which the ruminal pH is still in the range of optimal level (5.8–7) for the rumen [5,24–26]. This result is quite different from the study conducted by Smith and Correa [32] revealing that magnesium hydroxide supplementation to the rumen led to alkalosis in which the rumen pH reached to 8.01. The different results occurred as they supplemented a four times higher concentration (800 mol/l) of magnesium hydroxide to rumen.
Conclusion
Supplementation of magnesium hydroxide can be applied to prevent acidosis in the rumen culture fermenting carbohydrates. The experiment conducted in the comparison of magnesium hydroxide versus sodium bicarbonate supplementation to the rumen culture fermenting starch, showed that the supplementation of magnesium hydroxide could prevent lactic acid accumulation while sodium bicarbonate supplementation did not prevent acidosis and had lactic acid accumulation. The novelty of this current study is that the use of magnesium hydroxide for preventing rumen acidosis is more effective than using bicarbonate. This is due to the fact that magnesium hydroxide supplementation would not generate gas build up that may harm the ruminants.
Acknowledgments
The authors would like to thank Anne Barnes and Ralf Cord-Ruwisch for their advice in the rumen fermentation. Also, the authors would like to thank Syiah Kuala University for the financial support.
Conflict of Interest
The authors have no conflict of interest to declare.
Authors’ contribution
This manuscript is completely prepared by Darwin (literature review, experimental design, data collection, and English writing) while David Blignaut is a co-author contributed on discussion part and collecting rumen sample from ruminant.
References
- 1.Hernández J, Benedito JL, Abuelo A, Castillo C. Ruminal acidosis in feedlot: from aetiology to prevention. Sci World J. 2014;1:1–8. doi: 10.1155/2014/702572. https://doi.org/10.1155/2014/702572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gozho GN, Mutsvangwa T. Influence of carbohydrate source on ruminal fermentation characteristics, performance, and microbial protein synthesis in dairy cows. J Dairy Sci. 2008;91:2726–35. doi: 10.3168/jds.2007-0809. https://doi.org/10.3168/jds.2007-0809. [DOI] [PubMed] [Google Scholar]
- 3.Erdman RA, Botts RL, Hemken RW, Bull LS. Effect of dietary sodium bicarbonate and magnesium oxide on production and physiology in early lactation. J Dairy Sci. 1980;63:923–30. doi: 10.3168/jds.S0022-0302(80)83027-X. https://doi.org/10.3168/jds.S0022-0302(80)83027-X. [DOI] [PubMed] [Google Scholar]
- 4.Cullen AJ, Harmon DL, Nagaraja TG. In vitro fermentation of sugars, grains, and by-product feeds in relation to initiation of ruminal lactate production. J Dairy Sci. 1986;69:2616–21. doi: 10.3168/jds.S0022-0302(86)80709-3. https://doi.org/10.3168/jds.S0022-0302(86)80709-3. [DOI] [PubMed] [Google Scholar]
- 5.Owens FN, Secrist DS, Hill WJ, Gill DR. Acidosis in cattle: a review. J Anim Sci. 1998;76:275–86. doi: 10.2527/1998.761275x. https://doi.org/10.2527/1998.761275x. [DOI] [PubMed] [Google Scholar]
- 6.Nagaraja TG, Lechtenberg KF. Acidosis in feedlot cattle. Vet Clin Food Anim Pract. 2007;23:333–50. doi: 10.1016/j.cvfa.2007.04.002. https://doi.org/10.1016/j.cvfa.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Darwin, Barnes A, Cord-Ruwisch R. In vitro rumen fermentation of soluble and non-soluble polymeric carbohydrates in relation to ruminal acidosis. Ann Microbiol. 2018a;68:1–8. https://doi.org/10.1007/s13213-017-1307-x. [Google Scholar]
- 8.Nocek J. Bovine acidosis: implications on laminitis. J Dairy Sci. 1997;80:1005–28. doi: 10.3168/jds.S0022-0302(97)76026-0. https://doi.org/10.3168/jds.S0022-0302(97)76026-0. [DOI] [PubMed] [Google Scholar]
- 9.Olson JD. The relationship between nutrition and management to lameness in dairy cattle. Bovine Pract. 1997;31:65–8. [Google Scholar]
- 10.Calsamiglia S, Blanch M, Ferret A, Moya D. Is subacute ruminal acidosis a pH related problem? Causes and tools for its control. Anim Feed Sci Technol. 2012;172:42–50. https://doi.org/10.1016/j.anifeedsci.2011.12.007. [Google Scholar]
- 11.Erdman RA. Dietary buffering requirements of the lactating dairy cow: a review. J Dairy Sci. 1988;71:3246–66. https://doi.org/10.3168/jds.S0022-0302(88)79930-0. [Google Scholar]
- 12.Staples CR, Lough DS. Efficacy of supplemental dietary neutralizing agents for lactating dairy cows. A review. Anim Feed Sci Technol. 1989;23:277–303. https://doi.org/10.1016/0377-8401(89)90050-3. [Google Scholar]
- 13.Hindman BJ. Sodium bicarbonate in the treatment of subtypes of acute lactic acidosis: physiologic considerations. Anesthesiology. 1990;72:1064–76. https://doi.org/10.1097/00000542-199006000-00018. [PubMed] [Google Scholar]
- 14.Davenport GM, Boling JA, Gay N. Bioavailability of magnesium in beef cattle fed magnesium oxide or magnesium hydroxide. J Anim Sci. 1990;68:3765–72. doi: 10.2527/1990.68113765x. https://doi.org/10.2527/1990.68113765x. [DOI] [PubMed] [Google Scholar]
- 15.Thomas JW, Emery RS, Breaux JK, Liesman JS. Response of milking cows fed a high concentrate, low roughage diet plus sodium bicarbonate, magnesium oxide, or magnesium hydroxide. J Dairy Sci. 1984;67:2532–45. doi: 10.3168/jds.S0022-0302(84)81610-0. https://doi.org/10.3168/jds.S0022-0302(84)81610-0. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer DM, Wheeler LJ, Noller CH, Keyser RB, White JL. Neutralization of acid in the rumen by magnesium oxide and magnesium carbonate. J Dairy Sci. 1982;65:732–9. doi: 10.3168/jds.S0022-0302(82)82260-1. https://doi.org/10.3168/jds.S0022-0302(82)82260-1. [DOI] [PubMed] [Google Scholar]
- 17.Plaizier JC, Krause DO, Gozho GN, McBride BW. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet J. 2008;176:21–31. doi: 10.1016/j.tvjl.2007.12.016. https://doi.org/10.1016/j.tvjl.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Darwin, Charles W, Cord-Ruwisch R. Concurrent lactic and volatile fatty acid analysis of microbial fermentation samples by gas chromatography with heat pre-treatment. J Chromatogr Sci. 2018b;56:1–5. doi: 10.1093/chromsci/bmx086. https://doi.org/10.1093/chromsci/bmx086. [DOI] [PubMed] [Google Scholar]
- 19.Solorzano LC, Armentano LE, Grummer RR, Dentine MR. Effects of sodium bicarbonate or sodium sesquicarbonate on lactating Holsteins fed a high grain diet. J Dairy Sci. 1989;72:453–61. doi: 10.3168/jds.S0022-0302(89)79127-X. https://doi.org/10.3168/jds.S0022-0302(89)79127-X. [DOI] [PubMed] [Google Scholar]
- 20.Nombekela SW, Murphy MR. Sucrose supplementation and feed of dairy cows in early lactation. J Dairy Sci. 1995;78:880–5. doi: 10.3168/jds.s0022-0302(95)76701-7. https://doi.org/10.3168/jds.S0022-0302(95)76701-7. [DOI] [PubMed] [Google Scholar]
- 21.Penner GB, Oba M. Increasing dietary sugar concentration may improve dry matter intake, ruminal fermentation, and productivity of dairy cows in the postpartum phase of the transition period. J Dairy Sci. 2009;92:3341–53. doi: 10.3168/jds.2008-1977. https://doi.org/10.3168/jds.2008-1977. [DOI] [PubMed] [Google Scholar]
- 22.Russell JB, Hino T. Regulation of lactate production in Streptococcus bovis: a spiraling effect that contributes to rumen acidosis. J Dairy Sci. 1985;68:1712–21. doi: 10.3168/jds.s0022-0302(85)81017-1. https://doi.org/10.3168/jds.S0022-0302(85)81017-1. [DOI] [PubMed] [Google Scholar]
- 23.Russell JB, Wilson DB. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J Dairy Sci. 1996;79:1503–9. doi: 10.3168/jds.S0022-0302(96)76510-4. https://doi.org/10.3168/jds.S0022-0302(96)76510-4. [DOI] [PubMed] [Google Scholar]
- 24.Mould FL, Ørskov ER, Mann SO. Associative effects of mixed feeds. I. Effects of type and level of supplementation and the influence of the rumen fluid pH on cellulolysis in vivo and dry matter digestion of various roughages. Anim Feed Sci Technol. 1983;10:15–30. https://doi.org/10.1016/0377-8401(83)90003-2. [Google Scholar]
- 25.Kolver ES, De Veth MJ. Prediction of ruminal pH from pasture-based diets. J Dairy Sci. 2002;85:1255–66. doi: 10.3168/jds.S0022-0302(02)74190-8. https://doi.org/10.3168/jds.S0022-0302(02)74190-8. [DOI] [PubMed] [Google Scholar]
- 26.Garrett EF, Pereira MN, Nordlund KV, Armentano LE, Goodger WJ, Oetzel GR. Diagnostic methods for the detection of subacute ruminal acidosis in dairy cows. J Dairy Sci. 1999;82:1170–8. doi: 10.3168/jds.S0022-0302(99)75340-3. https://doi.org/10.3168/jds.S0022-0302(99)75340-3. [DOI] [PubMed] [Google Scholar]
- 27.Lettat A, Nozière P, Silberberg M, Morgavi DP, Berger C, Martin C. Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiol. 2012;12:1–12. doi: 10.1186/1471-2180-12-142. https://doi.org/10.1186/1471-2180-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stout JD, Bush LJ, Morrison RD. Palatability of buffered concentrate mixtures for dairy cows. J Dairy Sci. 1972;55:130–3. https://doi.org/10.3168/jds.S0022-0302(72)85443-2. [Google Scholar]
- 29.Ostrea EM, Odell GB. The influence of bicarbonate administration on blood pH in a “closed system”: clinical implications. J Pediatr. 1972;80:671–80. doi: 10.1016/s0022-3476(72)80073-8. https://doi.org/10.1016/S0022-3476(72)80073-8. [DOI] [PubMed] [Google Scholar]
- 30.Kasari TR, Woodbury AH, Morcom-Kasari E. Adverse effect of orally administered magnesium hydroxide on serum magnesium concentration and systemic acid-base balance in adult cattle. J Am Vet Med Assoc. 1990;196:735–42. [PubMed] [Google Scholar]
- 31.Ogilvie TH, Butler DG, Gartley CJ, Dohoo IR. Magnesium oxide induced metabolic alkalosis in cattle. Can J Comp Med. 1983;47:108–11. [PMC free article] [PubMed] [Google Scholar]
- 32.Smith GW, Correa MT. The effects of oral magnesium hydroxide administration on rumen fluid in cattle. J Vet Intern Med. 2004;18:109–12. doi: 10.1892/0891-6640(2004)18<109:teoomh>2.0.co;2. https://doi.org/10.1111/j.1939-1676.2004.tb00143.x. [DOI] [PubMed] [Google Scholar]
- 33.Riis T, Bauer-Brandl A, Wagner T, Kranz H. pH-independent drug release of an extremely poorly soluble weakly acidic drug from multiparticulate extended release formulations. Eur J Pharm Biopharm. 2007;65:78–84. doi: 10.1016/j.ejpb.2006.07.001. https://doi.org/10.1016/j.ejpb.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Dehkordi AJ, Dehkordi ZK. Occurrence of metabolic alkalosis in rumen lactic acidosis: a review article. Comp Clin Pathol. 2011;20:1–3. https://doi.org/10.1007/s00580-010-1129-8. [Google Scholar]