Abstract
Background:
CYP21A2 defects result in congenital adrenal hyperplasia (CAH), an autosomal recessive disorder characterized by impaired adrenal steroidogenesis. CYP21A2 lies within the major histocompatibility complex in an area of the genome highly susceptible to genetic variation. Alterations in the neighboring complement component 4 isotypes C4A and C4B have been associated with psychiatric and autoimmune disease. The purpose of this study was to evaluate C4A and C4B in patients with CAH in relation to CYP21A2 genotype and psychiatric and autoimmune comorbidity.
Methods:
We determined the copy numbers of C4A and C4B in 145 patients with CAH (median age: 15.5 yrs, IQR: 16.8) and 108 carrier relatives (median age: 41.5 yrs, IQR: 12.0) and evaluated serum C4 concentrations. Comorbidity was determined by medical record review.
Results:
Only 30% of subjects had the expected two copies each of the two C4 genes. C4B copy number determined total C4 copy number and serum C4 concentration, negatively correlated with carriership of a 30-kb deletion (P<10−5), and positively correlated with carriership of p.V281L (P<10−5). High C4A copy number (≥3) was associated with increased risk of having an externalizing psychiatric condition (relative risk: 2.67, 95% CI: 1.03–6.89, P=0.04). No association was found between C4 copy number and autoimmune disease.
Conclusion:
Mutation specific C4 structural variations commonly occur in patients with CAH and may have important clinical consequences, including increased risk of psychiatric morbidity.
Trial registration:
(November 7, 2005)
Keywords: Complement component 4, C4, congenital adrenal hyperplasia, CAH, CYP21A2
INTRODUCTION
Congenital Adrenal Hyperplasia (CAH) due to 21-hydroxylase deficiency (21-OHD; CYP21A2; OMIM201910) is an autosomal recessive disease of the adrenal cortex resulting in altered steroidogenesis and manifests as a broad spectrum of phenotypes. CAH is classified based on clinical severity: the classic (severe) form includes two subtypes, salt-wasting (SW) and simple virilizing (SV), and affects approximately 1 in 15,000 live births (Speiser et al. 2018); the mild but more common nonclassic (NC) form is estimated to occur in 1 in 200 to 1 in 1,000 Caucasians.(Hannah-Shmouni et al. 2017) Twelve common mutations and large (30-kb) deletions account for the majority of CYP21A2 defects (Finkielstain et al. 2011).
The CYP21A2 gene is mapped at a locus of low copy repeats composed of RP-C4-CYP21-TNX (RCCX) module(s), in the human histocompatibility complex on chromosome 6 (p21.33) (supplemental Fig. S1) (Blanchong et al. 2000). RP signifies RP1 (synonym STK19) encoding a serine/threonine nuclear protein kinase of unknown function and pseudogene RP2 (STK19P); C4 signifies C4A and C4B encoding two isotopes of complement component 4; CYP21 signifies CYP21A2 and pseudogene CYP21A1P; TNX signifies TNXB encoding tenascin-X and pseudogene TNXA. The locus is error prone during meiosis sometimes leading to structural variations in daughter alleles. These variations are mainly caused by unequal crossovers between highly homologous gene pairs, resulting in gene deletion or duplication. Amongst CYP21A2 pathogenic alleles are 30-kb deletions resulting in CYP21A1P/CYP21A2 or CYP21A1P-TNXA/TNXB chimeras and accounting for over 30% of CYP21A2 mutations associated with the classic form (Burch et al. 1997; Chen et al. 2012b; Finkielstain et al. 2011; Merke et al. 2013; Morissette et al. 2015). A point mutation in exon 7, p.V281L, accounts for the majority of NC alleles worldwide (Falhammar and Nordenstrom 2015; Hannah-Shmouni et al. 2017).
In the general population, a bimodular RCCX with one copy each of C4A and C4B is the most common haplotype with an allele frequency of approximately 75 percent (Banlaki et al. 2012; Blanchong et al. 2000; Sekar et al. 2016; Wu et al. 2008). C4 plays an important role in the activation of immune defenses and the clearance of immune complexes. The two isotypes of C4 have different chemical reactivities. C4A is more reactive to amino group containing antigens, while C4B primarily binds to antigens with hydroxyl groups (Blanchong et al. 2001; Law et al. 1984). These isotypes have been implicated as playing a role in a variety of diseases. High C4A gene dosage has been identified as a risk factor for schizophrenia and high copy number of either C4A or C4B has been found to be a risk factor for Alzheimer’s disease (Sekar et al. 2016; Zorzetto et al. 2017). Low C4 copy number likely contributes to the development of autoimmune diseases such as systemic lupus erythematosus (Li et al. 2017; Wu et al. 2008). Patients with CAH suffer from multiple comorbidities including mental illness (Engberg et al. 2015; Falhammar et al. 2014). The purpose of this study was to evaluate C4A and C4B copy number in patients with CAH and to evaluate the clinical implications of C4 copy number variation, specifically in relation to psychiatric morbidity and autoimmunity.
MATERIALS AND METHODS
We evaluated 145 patients with CAH due to 21-OHD and their 108 carrier relatives. (Table 1). All subjects were enrolled in an ongoing Natural History Study at the NIH Clinical Center in Bethesda, MD, USA (). All subjects (and parents of patients < 18 years old) gave written informed consent and the study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. Serum C4 concentrations were determined with a Vista analyzer (NIH Clinical Center Laboratory, Bethesda, MD). Individuals were selected based on the availability of high quality genomic DNA samples at the time of study.
Table 1.
Demographics and serum complement levels of patients with congenital adrenal hyperplasia and related subjects.
| Female | Male | subtotal | Age (years) | Serum C4 (mg/L) | |
|---|---|---|---|---|---|
| Salt-wasting | 42(2) | 42(1) | 84(3) | 18.0±12.5 | 20.2±7.7** |
| Simple virilizing | 9 | 27 | 36 | 21.0±15.6 | 21.2±6.2 |
| Nonclassic | 16 | 6 | 22 | 23.9±18.4 | 27.6±7.7* |
| CAH subtotal | 67(2) | 75(1) | 142(3) | 19.6±14.4 | 21.6±7.9 |
| CAH carriers | 68(3) | 36(1) | 104(4) | 42.0±10.2 | 22.9±7.3 |
| cohort | 135(5) | 111(2) | 246(7) | 29.0±16.9 | 22.1±7.6 |
Numbers in parentheses are those with missing serum C4 concentration and thus were excluded in the analyses related to serum C4 level. Age refers to the age when the serum C4 was tested.
P<0.05 vs carriers;
P<0.05 vs all other groups.
All subjects had previously completed CYP21A2 genotyping (Finkielstain et al. 2011). C4A and C4B copy numbers were determined by a droplet digital PCR (ddPCR) method described by Sekar et al with minor modifications in primers and probes (supplemental Table S1) (Sekar et al. 2016).
Psychiatric morbidity and autoimmunity were determined by retrospective chart review. Clinical data was not available for carriers. A mental health condition was recorded if the patient was treated with a psychotropic medication for a diagnosed condition. A psychiatrist (ME), who was blinded to the genetic data, reviewed all psychiatric data and categorized the condition as “internalizing” representing disorders such as prominent anxiety, depressive, and somatic symptoms or “externalizing” disorders representing disorders such as prominent impulsive, disruptive conduct, and substance use symptoms according to a widely accepted clinical guidelines (American Psychiatric Association 2013).
Raw C4 copy number calls (supplemental Fig.S2), were rounded to integers. Statistical analysis was performed using KaleidaGraph version 4.1 (Synergy Software, PA). Associations between total C4, C4A and C4B copy number and CYP21A2 genotype and psychiatric and autoimmune comorbidity were evaluated. ANOVA was used for comparison among groups and average values were shown as mean ± standard deviation. Open sourced R program was used for chi-square, Pearson correlation, Spearman rank correlation and relative risk analyses (R Core Team 2017). A P value of <0.05 was considered significant.
RESULTS
Copy number variations in the two C4 genes were commonly observed with a broad range of configurations (Fig. 1). Only 30% of the cohort (43/145 of CAH patients; 33/108 of carriers) had two copies of each C4 gene in diploid, a criteria for having the bimodular RCCX with C4A-C4B haplotype in both alleles.
Fig. 1.
Frequency of various C4A and C4B copy number configurations in the three clinical subtypes of congenital adrenal hyperplasia. SW, salt-wasting; SV, simple virilizing; NC, nonclassic.
The copy number of C4B (P<10−11), but not C4A (P=0.20), was associated with total serum C4 level (Fig 2). In addition, the total number of C4 genes was more strongly influenced by the C4B copy number than by the C4A copy number (rp=0.81, P<10−5 and rp=0.35, P=1.9×10−4 respectively). Similar associations were also found in carriers (supplemental Fig.S3).
Fig. 2.
Copy number of C4B, but not C4A, was associated with the serum C4 level in patients with congenital adrenal hyperplasia. (A) Total C4 copy number, (B) C4A copy number, and (C) C4B copy number are shown in relation to serum C4 levels. Spearman Rank Correlation test results are shown on the top of each panel.
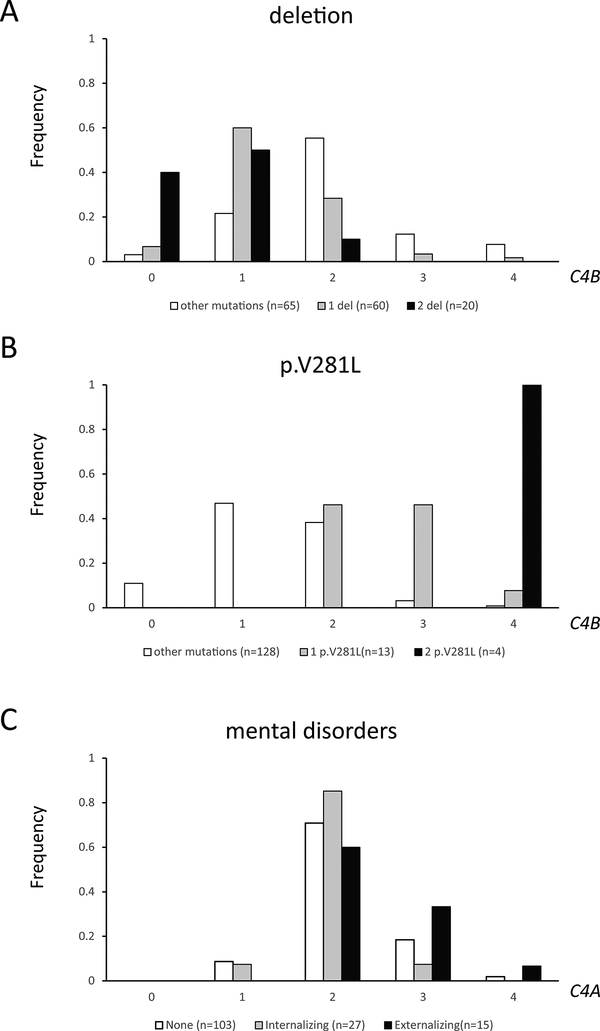
The C4B copy number was negatively associated (rp=−0.50, P<10−5) with carrying a 30-kb deletion (Fig 3A). The majority or 66.7% (40/60) of patients with one 30-kb deletion allele and 90% (18/20) of patients with two 30-kb deletion alleles had a C4B copy number ≤1, compared to 24% (16/65) of patients carrying other mutations (P<10−5). The mean C4B copy number varied in relation to the number of 30-kb deletion alleles (0, 1, 2: 2.00 ± 0.88 vs. 1.33 ± 0.73 vs. 0.7 ± 0.66; P<10−4). No association was observed between C4A copy number and the number of 30-kb deletion alleles.
Fig. 3.
Frequency of C4B copy number groups according to CYP21A2 mutations and psychiatric comorbidity. (A) Copy number of C4B was negatively associated with carriership of a 30-kb deletion and (B) positively associated with carriership of p.V281L mutation. (C) Copy number of C4A was positively associated with having an externalizing psychiatric disorder. Del, deletion.
A positive correlation (rp=0.60, P<10−5) was observed between copy numbers of C4B and p.V281L. Fifty three percent (7/13) of patients with one p.V281L allele and all four patients with two p.V281L alleles had high C4B copy number (≥3), compared to 4% (5/128) of patients with other mutations (P<10−5) (Fig. 3B). The mean C4B copy number varied in relation to the number of p.V281L alleles (0, 1 and 2: 1.36 ± 0.75 vs. 2.62 ± 0.65 vs. 4.00 ± 0.00; P<10−4). No correlation between C4A copy number and p.V281L was observed.
Forty-two of our patients with CAH suffered from mental disorders including depression, anxiety, oppositional/defiant disorder, anorexia nervosa, attention deficit hyperactivity disorder and bipolar mood disorder categorized into internalizing (n=27) and externalizing (n=15) subgroups. Forty percent (6/15) of patients with externalizing conditions had high C4A copy number (≥3), significantly more frequent than patients with internalizing conditions (7% or 2/27) or without mental disorders (20% or 21/103) (P=0.04) (Fig. 3C). The C4A copy number was highest in patients with an externalizing disorder (externalizing, internalizing, no mental disorder: 2.47 ± 0.64 vs. 2.00 ± 0.40 vs. 2.13 ± 0.58; P=0.03). The relative risk of having an externalizing mental condition with C4A copy number ≥3 was 2.67, 95% CI: 1.03–6.89, P=0.04. No association was observed between mental conditions and C4B, total C4 copy number, total serum level or the subtypes of CAH. Overall, female CAH patients were more likely to suffer from a mental disorder compared to their male counterparts (26/69 vs. 16/76, P=0.03).
Five patients suffered from autoimmune diseases including ulcerative colitis (n=2), juvenile rheumatoid arthritis (n=1), Grave’s disease (n=1), and Hashimoto’s thyroiditis (n=1). The prevalence of autoimmune disease was not associated with C4 copy number or serum C4 level.
It is notable that our CAH patient cohort is relatively young (median age of 15.5 yrs with an IQR of 16.8). Many psychiatric or autoimmune diseases develop with time, therefore our data may be underestimating specific risks.
Discussion
In our study, we confirm that individuals affected by CAH, including both patients and carriers, have unusual copy number variations of the C4 genes, which play an important role in immunity, health and disease. In this population, we demonstrate that the variations in C4 copy number and serum C4 level, as well as CYP21A2 mutation specific associations, are determined by the copy number of C4B rather than C4A. We also show for the first time an association at the genomic level between C4 copy number and susceptibility to psychopathology in patients with CAH. Although CAH is a monogenic disorder, CYP21A2 defects may influence contiguous genes, expanding the phenotypic spectrum.
We found that C4B copy number was the determinant of C4 serum levels in CAH patients. C4B copy number varied in those carrying 30-kb deletions and NC patients carrying the p.V281L mutation. Carrrying a 30-kb deletion signifies the presence of a CYP21A2P/CYP21A2 or CYP21A2P-TNXA/TNXB chimera caused by unequal crossovers in which a copy of C4 is lost. Thus, the presence of a 30-kb deletion is expected to be associated with a lower C4 copy number. These results are in agreement with the human genome assembly in that C4B rather than C4A is more likely to conjugate with CYP21A2; therefore C4B and CYP21A2 are vulnerable to be lost together in the event of a 30-kb deletion. Conversely, the CYP21A2 p.V281L mutation that causes NC CAH was previously correlated with high total C4 copy number (Chen et al. 2012a), and we found that this association is due to an increase in C4B, not C4A. The clinical implications are uncertain; however, distinctions between the C4A and C4B proteins are known. C4A has an important role in immunoclearance; while C4B is important in propagating the complement activation pathways (Yang et al. 2004). Deficiencies of both have been related to infectious and/or autoimmune diseases.
A recent study found high C4A expression due to high C4A gene dosages (e.g. haplotypes of C4A-C4A) as a risk factor for schizophrenia (Sekar et al. 2016), suggesting the importance of the C4 genes in mental health. Although our patients displayed a range of mental disorders, the one patient in our cohort with early childhood onset of psychosis had high C4 copy number, predominantly C4A. Patients with CAH are at higher risk for mental illness than the general population, most notably substance abuse, adjustment disorders and suicidality, with multiple confounders at play (Engberg et al. 2015; Falhammar et al. 2014). In Sweden, high risk of psychotic disorder was observed in CAH patients with the P30L genotype and SV phenotype (Falhammar et al. 2014). We did not find an association between psychiatric disorders and CYP21A2 genotype, but did find an association between C4A copy number and externalizing psychiatric comorbidity. The stress of having a chronic disease with multiple hormonal imbalances is likely a stronger determinant of mental health status than C4 gene dosage, as implicated by a recent Swedish study showing CAH carriers have even lower risk of psychiatric morbidity compared to the general population (Nordenstrom et al. 2017). However, we show that genotype as it relates to the neighboring C4 genes may play a role in the development of psychiatric disorders in CAH patients and even carriers of CAH.
Low total C4 gene copy number (<4) has been identified as a risk factor for autoimmune disease (Li et al. 2017). We did not find this association in our CAH cohort despite the large number of patients (52/145) having low total C4 copy number (<4). It is possible that long term glucocorticoid therapy had some protective effects. The young age of our CAH cohort may also have underestimated the lifetime risk of autoimmune disease.
As expected, RCCX structural variations were commonly observed. Only 30% of our CAH patients or carriers had two copies each of C4A and C4B resulting in a low odds of having a haplotype match the genome assembly reference of both alleles. This is in contrast to an estimated 50% chance of matching the reference genome in the general population (calculated from 70% frequency of C4A-C4B haplotype). Thus, the use of next generation sequencing platforms in testing the RCCX genes is challenging; highly homologous pseudogene originated reads and great copy number variations remain important obstacles (Mueller et al. 2013).
The strengths of our study are the large cohort size, availability of longitudinal medical records, and the comprehensive CYP21A2 genotyping that included phase configuration. Nevertheless, due to the lack of allele frequency reference for the RCCX locus in the CAH population, it was impossible to conclusively determine the haplotype configuration of the families with ≥4 C4 copies. We therefore analyzed and presented our C4 copy number in diploid without haplotype specifications. However, based on the diploid data, we did observe notable patterns implicating CAH genotype specific C4 haplotypes. For example, all 17 patients carrying p.V281L likely had at least one allele of C4A-C4B-C4B. Limitations of our study include the relatively young age of the cohort and the lack of clinical data for carriers.
The study of CYP21A2 in relation to the C4 neighboring genes provides insight into the complex genetics of the human histocompatibility complex, an important immune modulator affecting a spectrum of disorders, and provides evidence that RCCX genotype as it relates to C4 may be an additional risk factor for psychiatric comorbidity in patients with CAH. Large population based studies are needed to better evaluate the impact of C4 gene dosage on mental health in relation to CYP21A2 genotype.
Supplementary Material
Acknowledgements:
This research was supported by the Intramural Research Program at the National Institutes of Health (NIH), Bethesda, Maryland. We would like to thank Dr. Ninet Sinaii for her statistical advice.
Reference
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edn American Psychiatric Publishing, Arlington, VA [Google Scholar]
- Banlaki Z, Doleschall M, Rajczy K, Fust G, Szilagyi A (2012) Fine-tuned characterization of RCCX copy number variants and their relationship with extended MHC haplotypes. Genes Immun 13: 530–5. doi: 10.1038/gene.2012.29 [DOI] [PubMed] [Google Scholar]
- Blanchong CA, Chung EK, Rupert KL, Yang Y, Yang Z, Zhou B, Moulds JM, Yu CY (2001) Genetic, structural and functional diversities of human complement components C4A and C4B and their mouse homologues, Slp and C4. Int Immunopharmacol 1: 365–92. [DOI] [PubMed] [Google Scholar]
- Blanchong CA, Zhou B, Rupert KL, Chung EK, Jones KN, Sotos JF, Zipf WB, Rennebohm RM, Yung Yu C (2000) Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. J Exp Med 191: 2183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch GH, Gong Y, Liu W, Dettman RW, Curry CJ, Smith L, Miller WL, Bristow J (1997) Tenascin-X deficiency is associated with Ehlers-Danlos syndrome. Nat Genet 17: 104–8. doi: 10.1038/ng0997-104 [DOI] [PubMed] [Google Scholar]
- Chen W, Xu Z, Nishitani M, Van Ryzin C, McDonnell NB, Merke DP (2012a) Complement component 4 copy number variation and CYP21A2 genotype associations in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Genet 131: 1889–94. doi: 10.1007/s00439-012-1217-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Xu Z, Sullivan A, Finkielstain GP, Van Ryzin C, Merke DP, McDonnell NB (2012b) Junction site analysis of chimeric CYP21A1P/CYP21A2 genes in 21-hydroxylase deficiency. Clin Chem 58: 421–30. doi: 10.1373/clinchem.2011.174037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg H, Butwicka A, Nordenstrom A, Hirschberg AL, Falhammar H, Lichtenstein P, Nordenskjold A, Frisen L, Landen M (2015) Congenital adrenal hyperplasia and risk for psychiatric disorders in girls and women born between 1915 and 2010: A total population study. Psychoneuroendocrinology 60: 195–205. doi: 10.1016/j.psyneuen.2015.06.017 [DOI] [PubMed] [Google Scholar]
- Falhammar H, Butwicka A, Landen M, Lichtenstein P, Nordenskjold A, Nordenstrom A, Frisen L (2014) Increased psychiatric morbidity in men with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 99: E554–60. doi: 10.1210/jc.2013-3707 [DOI] [PubMed] [Google Scholar]
- Falhammar H, Nordenstrom A (2015) Nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency: clinical presentation, diagnosis, treatment, and outcome. Endocrine 50: 32–50. doi: 10.1007/s12020-015-0656-0 [DOI] [PubMed] [Google Scholar]
- Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, McDonnell NB, Merke DP (2011) Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab 96: E161–72. doi: 10.1210/jc.2010-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah-Shmouni F, Morissette R, Sinaii N, Elman M, Prezant TR, Chen W, Pulver A, Merke DP (2017) Revisiting the prevalence of nonclassic congenital adrenal hyperplasia in US Ashkenazi Jews and Caucasians. Genet Med 19: 1276–1279. doi: 10.1038/gim.2017.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law SK, Dodds AW, Porter RR (1984) A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. Embo j 3: 1819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang J, Liao D, Yang L, Wang Y, Hou S (2017) Association between C4, C4A, and C4B copy number variations and susceptibility to autoimmune diseases: a meta-analysis. Sci Rep 7: 42628. doi: 10.1038/srep42628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merke DP, Chen W, Morissette R, Xu Z, Van Ryzin C, Sachdev V, Hannoush H, Shanbhag SM, Acevedo AT, Nishitani M, Arai AE, McDonnell NB (2013) Tenascin-X haploinsufficiency associated with Ehlers-Danlos syndrome in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab 98: E379–87. doi: 10.1210/jc.2012-3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette R, Chen W, Perritt AF, Dreiling JL, Arai AE, Sachdev V, Hannoush H, Mallappa A, Xu Z, McDonnell NB, Quezado M, Merke DP (2015) Broadening the Spectrum of Ehlers Danlos Syndrome in Patients With Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab 100: E1143–52. doi: 10.1210/jc.2015-2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller PW, Lyons J, Kerr G, Haase CP, Isett RB (2013) Standard enrichment methods for targeted next-generation sequencing in high-repeat genomic regions. Genet Med 15: 910–1. doi: 10.1038/gim.2013.119 [DOI] [PubMed] [Google Scholar]
- Nordenstrom A, Butwicka A, Linden Hirschberg A, Almqvist C, Nordenskjold A, Falhammar H, Frisen L (2017) Are carriers of CYP21A2 mutations less vulnerable to psychological stress? A population-based national cohort study. Clin Endocrinol (Oxf) 86: 317–324. doi: 10.1111/cen.13242 [DOI] [PubMed] [Google Scholar]
- R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, Genovese G, Rose SA, Handsaker RE, Daly MJ, Carroll MC, Stevens B, McCarroll SA (2016) Schizophrenia risk from complex variation of complement component 4. Nature 530: 177–83. doi: 10.1038/nature16549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, White PC (2018) Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society* Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism 103: 4043–4088. doi: 10.1210/jc.2018-01865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YL, Yang Y, Chung EK, Zhou B, Kitzmiller KJ, Savelli SL, Nagaraja HN, Birmingham DJ, Tsao BP, Rovin BH, Hebert LA, Yu CY (2008) Phenotypes, genotypes and disease susceptibility associated with gene copy number variations: complement C4 CNVs in European American healthy subjects and those with systemic lupus erythematosus. Cytogenet Genome Res 123: 131–41. doi: 10.1159/000184700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lhotta K, Chung EK, Eder P, Neumair F, Yu CY (2004) Complete complement components C4A and C4B deficiencies in human kidney diseases and systemic lupus erythematosus. J Immunol 173: 2803–14. [DOI] [PubMed] [Google Scholar]
- Zorzetto M, Datturi F, Divizia L, Pistono C, Campo I, De Silvestri A, Cuccia M, Ricevuti G (2017) Complement C4A and C4B Gene Copy Number Study in Alzheimer’s Disease Patients. Curr Alzheimer Res 14: 303–308. doi: 10.2174/1567205013666161013091934 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.