Abstract
Background
Current guidelines recommend that sexually active men who have sex with men (MSM) be screened at least annually for bacterial sexually transmitted infections (STIs) at sites of sexual contact regardless of condom use. Extragenital (rectal and pharyngeal) STI are common in MSM and associated with an increased risk of HIV. We describe the prevalence of MSM who reported any STI test and an extragenital STI test in the past 12 months (p12m) in the United States.
Methods
Data were obtained from the 2017 American Men’s Internet Survey (AMIS), an annual cross-sectional behavioral internet survey of MSM in the United States. We examined the prevalence of MSM who reported any STI test and an extragenital STI test in the p12m and compared the prevalence across demographic, clinical, and behavioral factors.
Results
Of 10, 049 sexually-active MSM who participated in AMIS 2017, 42% reported any STI test in the p12m and 16% reported an extragenital (rectal or pharyngeal) STI test in the p12m. Among those who reported getting an extragenital STI test in the p12m, 19% reported providing a throat swab only, 14% reported providing a rectal swab only, and 68% reported providing both a rectal and throat swab for STI testing.
Conclusion
In a large sample of internet-using MSM in the United States, levels of STI screening were sub-optimal, with fewer than half (42%) of MSM reporting any STI test and even fewer reporting an extragenital STI test in the p12m. Increased efforts are needed to ensure annual STI screening guidelines among MSM are implemented.
Keywords: extragenital, screening, bacterial, sexually transmitted infection, MSM
In Brief:
Data from an internet-based survey indicate that only 16% of men who have sex with men had an extragenital (rectal or pharyngeal) STI test in the past 12 months.
Introduction
In 2017, nearly 2.3 million cases of chlamydia, gonorrhea, and syphilis were diagnosed in the United States, marking the fourth consecutive year of sharp increases in sexually transmitted infections (STI).1 Gay, bisexual and other men who have sex with men (collectively referred to as MSM) have an increased incidence of many STI, including syphilis and anti-microbial resistant gonorrhea, compared to women and men who have sex with women only (MSW)1. Among MSM, extragenital infections (pharyngeal or rectal) with Neisseria gonorrhoeae (GC) or Chlamydia trachomatis (CT) are common and these infections tend to be asymptomatic2, potentially serving as reservoirs of infection and contributing to the development of antimicrobial resistance.3 Extragenital STIs have also been shown to increase the risk of HIV transmission and acquisition, particularly among MSM with repeat gonococcal and chlamydial rectal infections.4,5
STI screening — testing for an STI in the absence of any clinical signs and symptoms — is crucial to detecting and treating asymptomatic STI. Current CDC screening guidelines for CT and GC recommend that MSM be screened at least annually for both infections at exposed anatomic sites, regardless of condom use, including the urethra and rectum for CT, and the urethra, rectum, and pharynx for GC. Extragenital STI screening cannot only aid in identifying MSM at high risk of HIV, but in many cases, an extragenital infection is the only indication that an individual has an STI. Most MSM with extragenital STI do not have a concurrent urogenital infection.2,6 Adherence to these screening guidelines is important to control further increases in the rates of bacterial STI, including CT and GC.
Data on the prevalence of extragenital STI screening among MSM are limited. A 2010 medical record review of HIV-positive MSM accessing care in HIV clinics found that only 2–9% of MSM had been tested for rectal CT/GC in the past 12 months.7 Among MSM attending STD clinics, more than half were tested for pharyngeal or rectal gonorrhea in the past 12 months, with a smaller proportion being tested for pharyngeal or rectal CT in the past 12 months6. Examining how frequently bacterial STI screening occurs is important to evaluate adherence to CDC screening guidelines across demographic and behavioral categories and to help with interpreting trends in rates of diagnosed STI over time. STI screening guidelines for users of HIV pre-exposure prophylaxis recommend more frequent than annual STI screening (every 6 months)8. As HIV PrEP access expands and bacterial STI screening becomes more frequent among PrEP users, monitoring the prevalence of STI screening will be helpful in understanding how trends in STI screening are changing over time. HIV PrEP implementation could reduce STIs, but this relies on adherence to STI screening guidelines.9 The objective of this analysis was to determine the proportion of MSM who reported receiving any STI test and the proportion who reported receiving an extragenital STI test in the past 12 months among an internet-recruited sample of MSM in the United States.
Methods
Data used for this analysis were collected from the 2017 American Men’s Internet Survey (AMIS). AMIS is an annual, cross-sectional internet survey conducted to assess the behaviors of MSM in the United States.10,11 MSM are recruited to participate in AMIS through convenience sampling from a variety of websites or geospatial social networking applications using banner advertisements or email blasts. Men were eligible to participate if they were ≥ 15 years of age, identified as male, lived in the United States, and reported at least one lifetime sex act (oral or anal) with a male partner. The analytical sample was further limited to MSM who had completed the survey, who were not duplicate respondents, who had sex with a male in the past 12 months and who provided a valid U.S. ZIP code. For this analysis, the objectives were to (1) determine the prevalence of MSM reporting any STI test in the past 12 months, and (2) to determine the prevalence of MSM reporting an extragenital STI test in the past 12 months. The prevalence of any STI testing in the past 12 months was determined by positive responses to two questions. Men were first asked “Have you ever been tested for sexually transmitted infections gonorrhea, chlamydia, or syphilis?” and if they answered “Yes” to this question they were asked “In the past 12 months, that is, since [MONTH/YEAR], were you tested by a doctor or other health care provider for a sexually transmitted infection like gonorrhea, chlamydia, or syphilis?”. If men reported getting tested for a STI in the past 12 months, they were asked “In the past 12 months, when you were tested by a doctor or other health care provider for a sexually transmitted infection like gonorrhea, chlamydia, or syphilis, what samples did you provide?”. Men were allowed to check more than one of the response options provided — “I had my blood drawn”, “I gave a urine sample”, “I had my rectum (butt) swabbed”, “I had my throat swabbed”, “I prefer not to answer”, “Don’t know”. Extragenital screening in the past 12 months was defined as men selecting either “I had my rectum (butt) swabbed” or “I had my throat swabbed” regardless of which other response options were also selected. Bivariate analyses were conducted to explore differences by demographic, clinical, and behavioral characteristics for the two outcomes of interest. Prevalence ratios (PRs) and 95% confidence intervals (CIs) were estimated from generalized linear models to determine factors associated with reporting any STI and extragenital STI testing in the past 12 months. We also examined demographic, clinical, and behavioral characteristics of MSM reporting an extragenital STI test in the past 12 months stratified by the participants’ self-reported HIV status (HIV-positive or HIV-negative). Race/ethnicity was defined as self-identification as black non-Hispanic, Hispanic, or white non-Hispanic. Due to small sample sizes, MSM who reported other or multiple race/ethnicities were combined into a single group (hereafter referred to as “Other” race/ethnicity). Sexual behavioral categories included reporting any condomless anal sex with a man in the past 12 months and reporting any female sex partners in the past 12 months. The number of male sex partners reported in the past 12 months were categorized as 1 partner, 2–6 partners, and ≥ 7 partners. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
All procedures performed as part of AMIS involving human participants were conducted in accordance with the ethical standards of the Emory Institutional Review Board (IRB). Incentives or compensation for study participation were not provided. For this secondary analysis of de-identified data, formal consent was not required.
Results
Participant Characteristics
In 2017, a total of 21,731 eligible and consenting men participated in the 2017 AMIS survey. After excluding those with duplicate and incomplete surveys, those with an invalid zip code, and limiting to men who had sex with another male in the past 12 months, 10,049 (46%) remained in the analysis sample (Figure 1). Respondents were mostly 40 years and older (45%), non-Hispanic White (71%), resided in urban areas (42%), and had a college or postgraduate degree (54%). A large majority had health insurance coverage and had visited a healthcare provider (HCP) in the previous 12 months (Table 1). More than two-thirds of respondents had ever disclosed their same sex behavior to a HCP (72%). Among HIV-negative MSM, 21% had used HIV pre-exposure prophylaxis (PrEP) in the last 12 months. Two-thirds of respondents had ever been tested for a sexually transmitted infection (STI) such as gonorrhea, chlamydia or syphilis and 42% reported being tested for an STI in the past 12 months. Information on the types of specimens provided for STI testing was available for 3285 survey respondents. Of these, 42% provided blood, urine, and extragenital (rectal or pharyngeal) specimens, 15% only had their blood drawn, 1% provided only extragenital specimens, and 5% provided only a urine sample (Figure 2). Among those who reported getting an extragenital STI test in the previous 12 months, 19% reported providing a throat swab only, 14% reported providing a rectal swab only, and 68% reported providing both a rectal and throat swab for STI testing.
Figure 1.
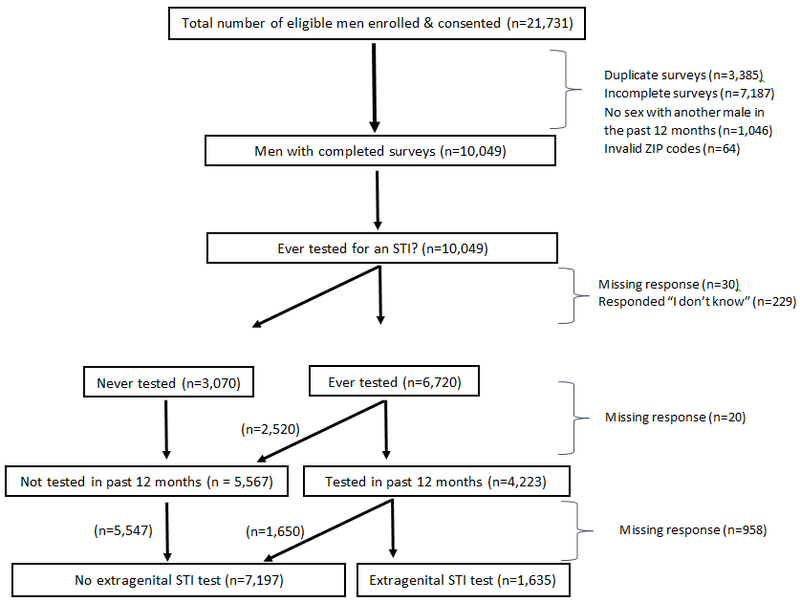
Flowchart outlining participants included in the analytical sample — American Men’s Internet Survey, 2017.
Table 1.
Demographic and behavioral characteristics of sexually active men who have sex with men participating in the American Men’s Internet Survey — United States, 2017
| STI testing in past 12 months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Any STI testing | Any extragenitala STI testing | ||||||||
| n | col % | n | row % | PR | 95% CI | n | row % | PR | 95% CI | |
| Total | 10,049 | 100.0 | 4223 | 42.0 | 1635 | 16.2 | ||||
| Age (years) | ||||||||||
| 15–24 | 2726 | 27.1 | 893 | 33.9 | Ref. | --- | 339 | 13.9 | Ref. | --- |
| 25–29 | 1246 | 12.4 | 668 | 54.5 | 1.69 | (1.54–1.84) | 293 | 27.3 | 1.96 | (1.70–2.25) |
| 30–39 | 1592 | 15.8 | 840 | 53.7 | 1.63 | (1.50–1.78) | 345 | 25.5 | 1.83 | (1.60–209.) |
| 40+ | 4485 | 44.6 | 1822 | 41.9 | 1.28 | (1.18–1.38) | 658 | 16.6 | 1.19 | (1.06–1.34) |
| Race/ethnicity | ||||||||||
| White, non-Hispanic | 6955 | 70.7 | 2801 | 41.5 | Ref. | --- | 1051 | 17.2 | Ref. | --- |
| Black, non-Hispanic | 654 | 6.7 | 338 | 52.8 | 1.27 | (1.18–1.38) | 132 | 23.2 | 1.35 | (1.15–1.59) |
| Hispanic | 1538 | 15.6 | 702 | 46.9 | 1.13 | (1.07–1.20) | 290 | 21.5 | 1.25 | (1.11–1.41) |
| Other/multiple | 687 | 7.0 | 299 | 44.5 | 1.07 | (0.98–1.17) | 135 | 22.1 | 1.29 | (1.10–1.51) |
| Highest level of education | ||||||||||
| Did not graduate high school | 419 | 4.2 | 68 | 17.1 | Ref. | --- | 23 | 6.1 | Ref. | --- |
| High school graduate/GED | 1149 | 11.6 | 325 | 29.3 | 1.71 | (1.35–2.17) | 122 | 11.7 | 1.93 | (1.25–2.96) |
| Some college/technical degree | 2954 | 29.8 | 1261 | 44.3 | 2.59 | (2.08–3.23) | 455 | 17.7 | 2.91 | (1.94–4.36) |
| College/postgraduate education | 5391 | 54.4 | 2526 | 47.9 | 2.80 | (2.25–3.48) | 1028 | 21.8 | 3.57 | (2.40–5.33) |
| Region | ||||||||||
| Northeast | 1875 | 18.7 | 811 | 44.5 | Ref. | --- | 358 | Ref. | --- | |
| Midwest | 1917 | 19.1 | 730 | 39.1 | 0.88 | (0.81–0.95) | 266 | 0.72 | (0.62–0.83) | |
| South | 3849 | 38.3 | 1525 | 40.9 | 0.92 | (0.86–0.98) | 478 | 0.65 | (0.57–0.73) | |
| West | 2398 | 23.9 | 1151 | 49.2 | 1.10 | (1.03–1.18) | 533 | 1.15 | (1.02–1.29) | |
| U.S. dependent areas | 10 | 0.1 | 6 | 60.0 | 1.35 | (0.82–2.24) | 0 | --- | --- | |
| NCHS rural-urban category | ||||||||||
| Urban | 4230 | 42.1 | 2124 | 51.3 | Ref. | --- | 942 | 25.6 | Ref. | --- |
| Suburban | 2181 | 21.7 | 832 | 39.4 | 0.77 | (0.72–0.82) | 298 | 15.4 | 0.60 | (0.53–0.67) |
| Small/medium metro | 2821 | 28.1 | 1030 | 37.8 | 0.74 | (0.70–0.78) | 340 | 13.7 | 0.54 | (0.48–0.60) |
| Rural | 806 | 8.0 | 230 | 29.5 | 0.58 | (0.51–0.64) | 55 | 7.5 | 0.29 | (0.23–0.38) |
| Current health insurance | ||||||||||
| No | 763 | 8.0 | 280 | 37.9 | Ref. | --- | 124 | 18.6 | Ref. | --- |
| Yes | 8791 | 92.0 | 3831 | 44.7 | 1.18 | (1.07–1.30) | 1480 | 19.1 | 1.03 | (0.87–1.22) |
| Visited HCP in last 12 months | ||||||||||
| No | 1010 | 11.6 | 168 | 17.2 | Ref. | --- | 58 | 6.2 | Ref. | --- |
| Yes | 7692 | 88.2 | 3469 | 46.3 | 2.69 | (2.34–3.09) | 1385 | 20.4 | 3.27 | (2.54–4.21) |
| Ever disclosed same sex behavior to HCP | ||||||||||
| No | 6005 | 59.8 | 2074 | 35.7 | Ref. | --- | 654 | 12.7 | Ref. | --- |
| Yes | 4044 | 40.2 | 2149 | 54.4 | 1.52 | (1.46–1.59) | 981 | 26.6 | 2.09 | (1.91–2.29) |
| Self-reported HIV status | ||||||||||
| Negative | 7180 | 71.5 | 3410 | 48.8 | Ref. | --- | 1321 | 21.2 | Ref. | --- |
| Positive | 964 | 9.6 | 638 | 66.9 | 1.37 | (1.30–1.44) | 256 | 31.6 | 1.49 | (1.33–1.67) |
| Unknown (includes never tested) | 1905 | 19.0 | 175 | 9.6 | 0.20 | (0.17–0.23) | 58 | 3.3 | 0.15 | (0.12–0.20) |
| Had receptive anal sex at last sex | ||||||||||
| No | 4099 | 48.5 | 1852 | 46.3 | Ref. | --- | 704 | 19.8 | Ref. | --- |
| Yes | 4358 | 51.5 | 2024 | 47.7 | 1.03 | (0.98–1.08) | 847 | 22.1 | 1.11 | (1.02–1.22) |
| In the past 12 months: | ||||||||||
| No. sex partners | ||||||||||
| 1 | 1742 | 21.7 | 341 | 20.3 | Ref. | --- | 97 | 6.0 | Ref. | --- |
| 2–6 | 3497 | 43.5 | 1289 | 37.9 | 1.87 | (1.68–2.07) | 431 | 13.7 | 2.27 | (1.84–2.80) |
| 7+ | 2795 | 34.8 | 1695 | 61.7 | 3.03 | (2.75–3.35) | 809 | 33.8 | 5.61 | (4.59–6.86) |
| Any female sex partner(s) | ||||||||||
| No | 8298 | 84.3 | 3567 | 44.2 | Ref. | --- | 1396 | 19.2 | Ref. | --- |
| Yes | 1551 | 15.8 | 579 | 38.5 | 0.87 | (0.81–0.93) | 212 | 15.3 | 0.80 | (0.70–0.91) |
| Condomless anal sex | ||||||||||
| No | 3288 | 32.7 | 946 | 29.8 | Ref. | --- | 280 | 9.4 | Ref. | --- |
| Yes | 6761 | 67.3 | 3277 | 49.7 | 1.67 | (1.57–1.77) | 1355 | 23.1 | 2.45 | (2.17–2.76) |
| Used PrEPb | ||||||||||
| No | 6043 | 84.2 | 2431 | 41.5 | Ref. | --- | 778 | 14.7 | Ref. | --- |
| Yes | 1137 | 15.8 | 979 | 86.5 | 2.08 | (2.01–2.17) | 543 | 58.1 | 3.96 | (3.64–4.31) |
Extragenital refers to rectal or pharyngeal STI tests (does not include serological tests);
Limited to those self-reporting a negative HIV status.
Figure 2.
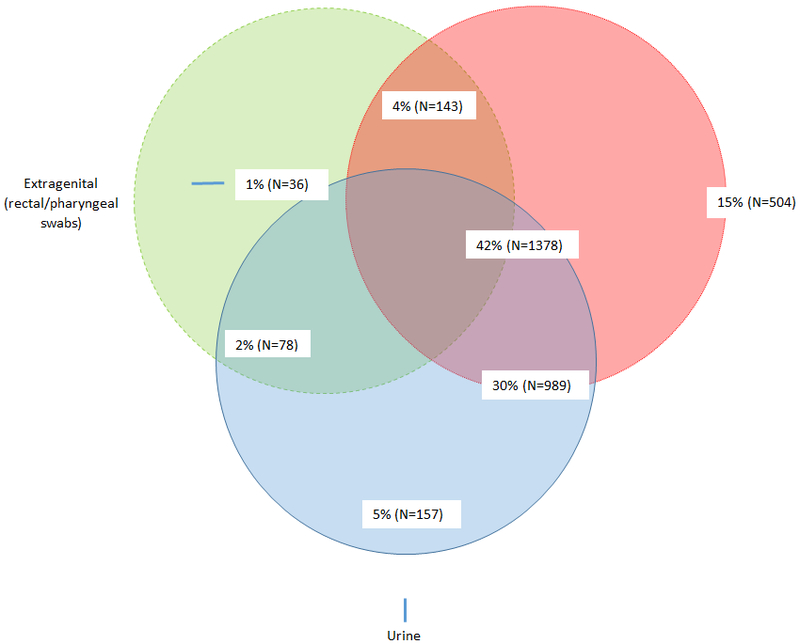
Venn diagram showing the distribution of sample types provided (total number = 3258) by participants for STI testing in the past 12 months — American Men’s Internet Survey, 2017.
Any STI testing by demographic, clinical, and behavioral characteristics
Compared to MSM aged 15–24 years, older MSM were more likely to report being screened for any STI in the past 12 months (Table 1). Across categories of race, black non-Hispanic MSM and Hispanic MSM were more likely to report being screened for an STI in the past 12 months compared to white non-Hispanic MSM. MSM living in non-urban areas (suburban, small/medium metro areas, and rural areas) were less likely to report being tested for any STI in the past 12 months compared to MSM living in urban areas. MSM who reported no health insurance coverage were less likely to report an STI test in the past 12 months compared to MSM who reported having health insurance. Visiting a HCP in the past 12 months and having ever disclosed same-sex behavior to a HCP were both associated with a higher prevalence of an STI test in the past 12 months compared to MSM who had not visited a HCP or had never disclosed same-sex behavior to a HCP in the past 12 months respectively. More than 80% of HIV-negative MSM who used PrEP in the past 12 months had an STI test and greater than half had an extragenital STI test in the past 12 months.
Extragenital STI testing by demographic, clinical, and behavioral characteristics
Compared to MSM aged 15–24 years, older MSM were more likely to report being screened for an extragenital STI in the past 12 months (Table 1). Compared to white MSM, black non-Hispanic MSM, Hispanic MSM, and MSM reporting an other race were also more likely to report extragenital STI testing in the past 12 months. MSM who reported having health insurance coverage did not have a significantly different prevalence of reporting extragenital STI testing compared to MSM who did not report health insurance coverage. MSM who had visited an HCP in the past 12 months and who had ever disclosed their same sex behavior to an HCP had a higher prevalence of reporting extragenital STI testing in the last 12 months compared to those who had not. Among HIV-negative MSM, men who reported using PrEP in the past 12 months had a higher prevalence of self-reported extragenital STI testing compared to men who did not report PrEP use.
Extragenital STI testing stratified by HIV status
Among HIV-positive MSM, there was no significant variation in the prevalence of reporting an extragenital STI test by health insurance status, having visited an HCP in the past 12 months, or disclosure of same sex behavior to an HCP (Table 2). The prevalence of reporting an extragenital STI test among HIV-positive MSM increased as the number of sex partners reported in the past 12 months increased — those reporting ≥ 7 sex partners were more likely to report an extragenital STI test compared to HIV-positive MSM reporting 1 partner in the past 12 months. HIV-positive MSM who reported condomless anal sex in the past 12 months were more likely to report extragenital STI testing in the past 12 months compared to those who did not report condomless sex (Table 2). HIV-negative MSM who visited an HCP in the past 12 months were significantly more likely to report an extragenital STI test in the past 12 months compared to MSM who did not visit an HCP in the past 12 months. HIV-negative MSM who had ever disclosed engaging in same sex behavior to an HCP were also significantly more likely to report extragenital STI testing in the past 12 months compared to HIV-negative MSM who had never disclosed (Table 2).
Table 2.
Prevalence of self-reported extragenitala STI testing in the past 12 months by HIV status among men who have sex with men participating in the American Men’s Internet Survey — United States, 2017.
| HIV+ | HIV− | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Row % | PR | 95% CI | Total | Row % | PR | 95% CI | |||
| Overall | 810 | 256 | 31.6 | 6238 | 1321 | 21.2 | ||||
| Age (years) | ||||||||||
| 15–24 | 30 | 10 | 33.3 | Ref. | --- | 1228 | 313 | 25.5 | Ref. | --- |
| 25–29 | 67 | 32 | 47.8 | 1.43 | (0.81–2.52) | 867 | 252 | 29.1 | 1.14 | (0.99–1.31) |
| 30–39 | 135 | 58 | 43.0 | 1.29 | (0.75–2.22) | 1099 | 279 | 25.4 | 1.00 | (0.87–1.14) |
| 40+ | 578 | 156 | 27.0 | 0.81 | (0.48–1.37) | 3044 | 477 | 15.7 | 0.61 | (0.54–0.70) |
| Race | ||||||||||
| White non-Hispanic | 516 | 157 | 30.4 | Ref. | --- | 4486 | 860 | 19.2 | Ref. | --- |
| Black, non-Hispanic | 115 | 34 | 29.6 | 0.97 | (0.71–1.33) | 381 | 94 | 24.7 | 1.29 | (1.07–1.55) |
| Hispanic | 119 | 40 | 33.6 | 1.10 | (0.83–1.47) | 831 | 237 | 28.5 | 1.49 | (1.32–1.68) |
| Other/multiple | 46 | 20 | 43.5 | 1.43 | (1.00–2.04) | 414 | 109 | 26.3 | 1.37 | (1.16–1.63) |
| NCHS rural-urban category | ||||||||||
| Urban | 403 | 150 | 37.2 | Ref. | --- | 2718 | 761 | 28.0 | Ref. | --- |
| Suburban | 159 | 46 | 28.9 | 0.78 | (0.59–1.02) | 1313 | 243 | 18.5 | 0.66 | (0.58–0.75) |
| Small/medium metro | 196 | 54 | 27.6 | 0.74 | (0.57–0.96) | 1731 | 270 | 15.6 | 0.56 | (0.49–0.63) |
| Rural | 49 | 6 | 12.2 | 0.33 | (0.15–0.70) | 469 | 47 | 10.0 | 0.36 | (0.27–0.47) |
| Current health insurance | ||||||||||
| No | 38 | 17 | 44.7 | Ref. | --- | 473 | 101 | 21.4 | Ref. | --- |
| Yes | 764 | 236 | 30.9 | 0.69 | (0.48–1.00) | 5606 | 1194 | 21.3 | 1.00 | (0.83–1.19) |
| Visited HCP in last 12 months | ||||||||||
| No | 12 | 3 | 25.0 | Ref. | --- | 590 | 51 | 8.6 | Ref. | --- |
| Yes | 684 | 220 | 32.2 | 1.29 | (0.48–3.45) | 4876 | 1119 | 23.0 | 2.65 | (2.03–3.47) |
| Ever disclosed same sex behavior to HCP | ||||||||||
| No | 297 | 93 | 31.3 | Ref. | --- | 3341 | 537 | 16.1 | Ref. | --- |
| Yes | 513 | 163 | 31.8 | 1.01 | (0.82–1.25) | 2897 | 784 | 27.1 | 1.68 | (1.53–1.86) |
| In the past 12 months: | ||||||||||
| No. sex partners | ||||||||||
| 1 | 100 | 18 | 18.0 | Ref. | --- | 990 | 75 | 7.58 | Ref. | --- |
| 2–6 | 222 | 51 | 23.0 | 1.28 | (0.79–2.07) | 2207 | 363 | 16.45 | 2.17 | (1.71–2.75) |
| 7+ | 297 | 126 | 42.4 | 2.36 | (1.52–3.66) | 1840 | 656 | 35.65 | 4.71 | (3.75–5.90) |
| Any female sex partner(s) | ||||||||||
| No | 727 | 231 | 31.8 | Ref. | --- | 5161 | 1118 | 21.66 | Ref. | --- |
| Yes | 67 | 21 | 31.3 | 0.99 | (0.68–1.43) | 970 | 181 | 18.66 | 0.86 | (0.75–0.99) |
| Condomless anal sex | ||||||||||
| No | 155 | 37 | 23.9 | Ref. | --- | 1972 | 233 | 11.8 | Ref. | --- |
| Yes | 655 | 219 | 33.4 | 1.40 | (1.04–1.89) | 4266 | 1088 | 25.5 | 2.16 | (1.89–2.46) |
Extragenital refers to rectal or pharyngeal STI tests (does not include serological tests). PR = prevalence ratio; HCP = healthcare provider
Discussion
Current STD screening guidelines recommend that sexually active MSM be tested at least annually for gonorrhea and chlamydia at sites of sexual contact regardless of condom use. In a large sample of internet-using MSM in the United States, we found that levels of STI screening were sub-optimal, with fewer than half (42%) of MSM reporting an STI test in the past 12 months and only 16% reporting an extragenital STI test in the past 12 months. The prevalence of self-reported STI testing in the past 12 months was higher among certain demographic subgroups — older MSM, Black, non-Hispanic and Hispanic MSM compared to White, non-Hispanic MSM, MSM who reside in urban areas compared to residents in suburban or rural areas, and college-educated MSM compared to MSM with less than a college degree. A number of population-based studies have reported higher levels of STI screening among younger MSM12,13, however we found the lowest prevalence of STI screening among those aged 15–24 years old. This is concerning given that incidence and prevalence estimates suggest that those aged 15–24 years acquire half of all new STDs.1,14 Younger age has also been associated with a higher likelihood of an unknown HIV status among a similar population, suggesting a gap in STI healthcare in this particular subgroup.15 The higher prevalence of STI screening among Black, non-Hispanic and Hispanic MSM compared to White, non-Hispanic MSM has been reported before12, but is notable given the high proportion of White, non-Hispanic participants in this sample.
Similar associations between participant characteristics (demographic, clinical, and behavioral) and the report of an extragenital STI test in the past 12 months were seen as with the report of any STI test in the past 12 months. However, while MSM with health insurance coverage were more likely to report any STI test compared to MSM without health insurance coverage, there was no significant difference in the prevalence of reporting an extragenital STI test by health insurance coverage. This finding may reflect differences in where STI care is being sought. Public STD clinics have been shown to have higher levels of extragenital STI screening6,16 and are utilized by individuals historically underserved in the traditional health care system17 including uninsured individuals. Efforts to improve STI screening rates should focus on both private and public healthcare settings.
Visiting an HCP in the past 12 months and ever disclosing same-sex behavior to an HCP was significantly associated with the reporting of any STI test in the past 12 months in the past 12 months. The comfort level of HCPs in eliciting sexual behaviors, particularly same-sex behaviors, can serve as a barrier to appropriate STI screening.18 This finding underscores the importance of routine sexual histories in guiding appropriate clinical care. Furthermore, current guidelines for more frequent STI screening (every 3–6 months) are based on the presence of sexual risk behaviors that cannot be implemented without conducting a sexual risk assessment. MSM may also be reticent to disclose their same-sex behavior to an HCP because of concerns about confidentiality, discrimination, or stigma19 and may have discomfort around communicating about same-sex behavior.20 It is important to create healthcare environments that foster more supportive and open communication between MSM and their HCPs around same-sex behavior and sexual risk behaviors. Among HIV-positive MSM, disclosure of same-sex behavior to an HCP did not have a significant association with reporting an extragenital STI test in the past 12 months. This finding may reflect differences in risk perceptions of STI acquisition by the HCP. STI screening may also be more routinized in the setting of HIV medical care and not dependent on the elicitation of sexual risk behaviors by the HCP or self-report of these behaviors by the patient.
Among HIV-negative MSM, men who used PrEP in the past 12 months had a higher prevalence of any STI testing and a higher prevalence of extragenital STI testing, in the past 12 months, compared to men overall. While it is reassuring to see a higher prevalence of STI screening among PrEP users, given the more frequent recommended STI screening intervals, it is concerning to see less than two-thirds being screened for an extragenital STI in the past 12 months. PrEP visits as part of routine care present opportunities for users to complete preventive health care recommendations including STI screening. While there have been concerns in the public health community about PrEP use being associated with decreased condom use21,22 and higher STD transmission23, PrEP-associated care may lead to higher levels of STI screenings among a population at risk of HIV and STI acquisition. Mathematical modeling of NG and CT transmission dynamics among MSM in the United States suggests that the implementation of biannual STI screening recommendations outlined in the CDC PrEP guidelines, while scaling up PrEP coverage, could result in a decline in STI incidence among MSM 9.
This sample of internet-recruited MSM is predominantly White, non-Hispanic, and college-educated limiting the generalizability of our findings to other MSM populations. The prevalence of any STI screening in the past 12 months in this population is lower than that reported by a sample of community venue-attending MSM in five U.S. cities24. The prevalence of extragenital STI screening in the past 12 months in this population is higher than that reported for HIV-positive MSM accessing HIV care7, but considerably lower than the prevalence of extragenital STI screening reported for STD clinic-attending MSM6. Data are self-reported and so may be subject to respondent biases, such as underreporting of sexual risk behaviors, and recall bias. Given the phrasing of the question assessing any extragenital STI screening, participants may not have reported provider-collected specimens, which could have resulted in an underreporting of STI screening. While the AMIS 2017 survey does collect sexual behavioral information, there is limited information on the specific anatomic sites exposed — we do not know the percentage of men who engaged in receptive anal sex in the past 12 months and/or the percentage of men who engaged in receptive oral sex in the past 12 months. However, 51% of MSM reported engaging in receptive anal sex during the last time they had sex, indicating that while not everyone may have been indicated for extragenital STI testing in the past 12 months, the prevalence of extragenital STI screening was suboptimal. Despite these limitations, the American Men’s Internet Survey is the largest ongoing survey of gay, bisexual, and other MSM in the United States allowing for robust statistical analyses assessing risk behaviors and STI/HIV outcomes among internet-using MSM.
STI screening, followed by prompt and effective treatment, is a crucial public health intervention to disrupt further disease transmission. STI screening is particularly important for extragenital infections, since these are common among MSM, are mostly asymptomatic, and as a result can remain undiagnosed and untreated for longer.25,26 Additionally, many patients with extragenital STI do not have concurrent urethral infections and therefore the extragenital infection(s) would not be identified with urogenital screening alone.2,6,27 Extragenital STI have been associated with a significantly increased risk of HIV transmission among MSM4 and can serve as a reservoir of disease and contribute to the development of reduced antimicrobial susceptibility28, further underscoring the importance of extragenital STI screening. In summary, STI testing in the past 12 months was low. Enhanced efforts to improve compliance with STI screening guidelines are warranted.
Acknowledgments
Financial support. The data for this analysis were collected through funding received from the National Institutes of Health (Emory Center for AIDS Research, P30AI050409) and the MAC AIDS Fund.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest. The authors declare no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017 Atlanta: U.S. Department of Health and Human Services;2018. [Google Scholar]
- 2.Kent CK, Chaw JK, Wong W, et al. Prevalence of rectal urethral and pharyngeal chlamydia and gonorrhea detected in 2 clinical settings among men who have sex with men: San Francisco, California, 2003. Clin Infect Dis. 2005;41:8. [DOI] [PubMed] [Google Scholar]
- 3.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014;27(3):587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein KT, Marcus JL, Nieri G, et al. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. J Acquir Immune Defic Syndr 2010;53(4):537–543. [DOI] [PubMed] [Google Scholar]
- 5.Jin F, Prestage GP, Imrie J, et al. Anal sexually transmitted infections and risk of HIV infection in homosexual men. J Acquir Immune Defic Syndr 2010;53(1):144–149. [DOI] [PubMed] [Google Scholar]
- 6.Patton ME, Kidd S, Llata E, et al. Extragenital gonorrhea and chlamydia testing and infection among men who have sex with men--STD Surveillance Network, United States, 2010–2012. Clin Infect Dis 2014;58(11):1564–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoover KW, Butler M, Workowski K, et al. STD screening of HIV-infected MSM in HIV clinics. Sex Transm Dis 2010;37(12):771–776. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention: US Public Health Service. Preexposure prophylaxis for the prevention of HIV infection in the United States−-2017 Update: a clinical practice guideline 2018. [Google Scholar]
- 9.Jenness SM, Weiss KM, Goodreau SM, et al. Incidence of Gonorrhea and Chlamydia Following Human Immunodeficiency Virus Preexposure Prophylaxis Among Men Who Have Sex With Men: A Modeling Study. Clin Infect Dis 2017;65(5):712–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez TH, Sineath RC, Kahle EM, et al. The Annual American Men’s Internet Survey of Behaviors of Men Who Have Sex With Men in the United States: Protocol and Key Indicators Report 2013. JMIR Public Health Surveill. 2015;1(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zlotorzynska M, Sullivan P, Sanchez T The Annual American Men’s Internet Survey of Behaviors of Men Who Have Sex With Men in the United States: 2016 Key Indicators Report. JMIR Public Health Surveill 2019;5(1):e11313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoots BE, Torrone EA, Bernstein KT, et al. Self-Reported Chlamydia and Gonorrhea Testing and Diagnosis Among Men Who Have Sex With Men-20 US Cities, 2011 and 2014. Sex Transm Dis 2018;45(7):469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai E, Sanchez T, Lansky A, et al. Self-reported syphilis and gonorrhoea testing among men who have sex with men: national HIV behavioural surveillance system, 2003–5. Sex Transm Infect 2008;84(6):478–482. [DOI] [PubMed] [Google Scholar]
- 14.Satterwhite CL, Torrone E, Meites E, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis 2013;40(3):187–193. [DOI] [PubMed] [Google Scholar]
- 15.Traynor SM, Brincks AM, Feaster DJ. Correlates of Unknown HIV Status Among MSM Participating in the 2014 American Men’s Internet Survey (AMIS). AIDS Behav 2018;22(7):2113–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leichliter JS, Heyer K, Peterman TA, et al. US Public Sexually Transmitted Disease Clinical Services in an Era of Declining Public Health Funding: 2013–14. Sex Transm Dis 2017;44(8):505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiller JS CT, Norris T. Early release of selected estimates based on data from the January-September 2017 National Health Interview Survey 2018. [Google Scholar]
- 18.Ford JV, Barnes R, Rompalo A, et al. Sexual health training and education in the U.S. Public Health Rep. 2013;128 Suppl 1:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bird JD, Voisin DR. “You’re an open target to be abused”: a qualitative study of stigma and HIV self-disclosure among Black men who have sex with men. Am J Public Health. 2013;103(12):2193–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klitzman RL, Greenberg JD. Patterns of communication between gay and lesbian patients and their health care providers. J Homosex 2002;42(4):65–75. [DOI] [PubMed] [Google Scholar]
- 21.Paz-Bailey G, Mendoza MC, Finlayson T, et al. Trends in condom use among MSM in the United States: the role of antiretroviral therapy and seroadaptive strategies. AIDS. 2016;30(12):1985–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss BB, Greene GJ, Phillips G 2nd, et al. Exploring Patterns of Awareness and Use of HIV Pre-Exposure Prophylaxis Among Young Men Who Have Sex with Men. AIDS Behav. 2017;21(5):1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montano MA, Dombrowski JC, Dasgupta S, et al. Changes in Sexual Behavior and STI Diagnoses Among MSM Initiating PrEP in a Clinic Setting. AIDS Behav. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones Johnson ML, Chapin-Bardales J, Bizune D, et al. Extragenital Chlamydia and Gonorrhea Among Community Venue-Attending Men Who Have Sex with Men — Five Cities, United States, 2017. Morbidity and Mortality Weekly Report 2019;68(14):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters RPH VS, Nijsten N, Ouburg S, Mutsaers J, Jansen CL, van Leeuwen AP, Morre SA. Evaluation of sexual history-based screening of anatomic sites for chlamydia trachomatis and neisseria gonorrhoeae infection in men having sex with men in routine practice. BMC Infectious Diseases. 2011;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Workowski KA. Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clin Infect Dis. 2015;61 Suppl 8:S759–762. [DOI] [PubMed] [Google Scholar]
- 27.Marcus JL, Bernstein KT, Kohn RP, et al. Infections missed by urethral-only screening for chlamydia or gonorrhea detection among men who have sex with men. Sex Transm Dis 2011;38(10):922–924. [DOI] [PubMed] [Google Scholar]
- 28.Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 2012;7(12):1401–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]


