Abstract
Background and Purpose:
The aim of this study was to investigate the efficacy of thrombus density on noninvasive computed tomography (CT) neuroimaging for predicting thrombus pathology and patient outcome after mechanical thrombectomy in acute ischemic stroke.
Materials and Methods:
This retrospective chart and imaging review included patients that were treated by mechanical thrombectomy at Siriraj Hospital according to the American Heart Association/American Stroke Association guidelines for the early management of patients with acute ischemic stroke from March 2010 to February 2015 study period. Preintervention noncontrast CT (NCCT), CT angiography (CTA), and/or contrast-enhanced CT (CECT) images were interpreted using CT densitometry. Pathology results were classified as white, red, or mixed thrombi. The result of treatment was evaluated by the modified Rankin Scale at 90 days after treatment.
Results:
From 97 included patients – 97 NCCT images, 48 CTA images, 48 CECT images, and 54 pathologic results of cerebral thrombi were included in the final analysis. Mean clot Hounsfield unit values on NCCT, CTA, and CECT were significantly different between red and white thrombus (P = 0.001 on NCCT, P = 0.03 on CTA, and P = 0.001 on CECT), and between red and mixed thrombus (P = 0.043 on NCCT and P = 0.002 on CTA). However, no significant difference was observed between white thrombus and mixed thrombus (P = 0.09 on NCCT, P = 1.00 on CTA, and P = 0.054 on CECT). There was no significant correlation between type of cerebral thrombus or clot density and the result of treatment.
Conclusion:
Thrombus density on CT was found to be a significant predictor of thrombus pathology; however, no significant association was observed between thrombus type or clot density and patient outcome after mechanical thrombectomy.
Keywords: Acute ischemic stroke, Hounsfield units, mechanical thrombectomy, noninvasive computed tomography neuroimaging, Thailand, thrombus pathology
Introduction
The American Heart Association/American Stroke Association (AHA/ASA) guidelines for early management of acute ischemic stroke recommend intra-arterial therapy combined with intravenous recombinant tissue plasminogen activator (rt-PA) in patients with no contraindications that arrive at the hospital within 3–4.5 h after suffering a stroke. Computed tomography (CT) imaging is routinely performed to guide treatment in patients with acute ischemic stroke. Mechanical thrombectomy should be considered as an alternative treatment in patients with large vessel occlusion that have one or more contraindications for rt-PA.[1] However, the cost and risks associated with mechanical thrombectomy are very high. Therefore, criteria are needed that help to identify the most appropriate reperfusion strategy on a case-by-case basis in this patient population.
Previous study reported a correlation between thrombus density on preintervention noncontrast CT (NCCT) and postintervention outcome after mechanical treatment, with thrombi with a lower number of Hounsfield units (HUs) being found to be more resistant to mechanical thrombectomy.[2] In contrast, two other studies reported no association between thrombus density and treatment outcome.[3,4] Accordingly, the aim of this study was to investigate the efficacy of thrombus density on noninvasive CT neuroimaging for predicting thrombus pathology[5,6,7,8] and patient outcome after mechanical thrombectomy in acute ischemic stroke.
Materials and Methods
Patient
This retrospective chart and imaging review included patients that were treated by mechanical thrombectomy at our hospital according to AHA/ASA guidelines for the early management of patients with acute ischemic stroke from March 2010 to February 2015 study period. Our hospital is Thailand's largest national tertiary referral center. The protocol for this study was approved by the Hospital Institutional Review Board (COA No. Si 093/2017).
Preintervention NCCT, CT angiography (CTA), and contrast-enhanced CT (CECT) images were interpreted using CT densitometry. Mechanical thrombectomy is a preferred treatment modality for patients with acute ischemic stroke who do not qualify for intravenous rt-PA, who do not improve after treatment with intravenous rt-PA,[9] or who have an acute severe stroke with large vessel occlusion. Candidacy for mechanical thrombectomy is determined by CT imaging; National Institutes of Health Stroke Scale score; and time of onset within 8 h in cases of anterior circulation large vessel occlusion and up to 12 h in cases of posterior circulation large vessel occlusion stroke. Functional outcome was assessed by modified Rankin scale (mRS) at 90-days after thrombectomy. mRS grading was, as follows: 0–2 = good clinical outcome, 3–4 = moderate clinical outcome, and >4 = poor clinical outcome.
Imaging protocol
All included patients underwent 1.25 mm (GE Discovery; GE Healthcare, Little Chalfont, UK) or 1.50 mm (GE Light Speed VCT; GE Healthcare) axial NCCT; scan CECT; and/or, CTA (acquired at 0.625 mm and reformatted to 1.25 mm). Patients receive at least one of the three imaging studies.
Computed tomography image analysis
Authors retrospectively reviewed the CT images and measured the HU values for the presence of large vessel thrombus. A region of interest was drawn within the thrombus on NCCT, CECT, and CTA studies to obtain the HU value [Figures 1 and 2]. With an aim to measure and localize the entire thrombus, we compared imaging findings among NCCT, CECT, and CTA studies. Any region that included vessel wall calcification was excluded (HU value >100 on NCCT image).
Figure 1.
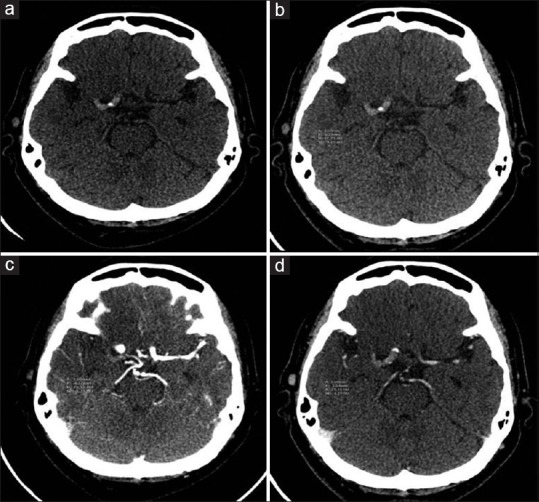
Computed tomography imaging of a 61-year-old female with underlying diabetes, hypertension, and coronary artery disease who presented with left hemiparesis and left facial palsy for 3 h. (a) Noncontrast computed tomography showed hyperdense clot sign at proximal M1 of right internal carotid artery. (b-d) Hounsfield unit value was approximately 78, 74, and 76 on noncontrast computed tomography, computed tomography angiography, and contrast-enhanced computed tomography image, respectively. The patient was sent for mechanical thrombectomy, the histopathologic results showed red thrombus, and the 90-day mRS was 5
Figure 2.
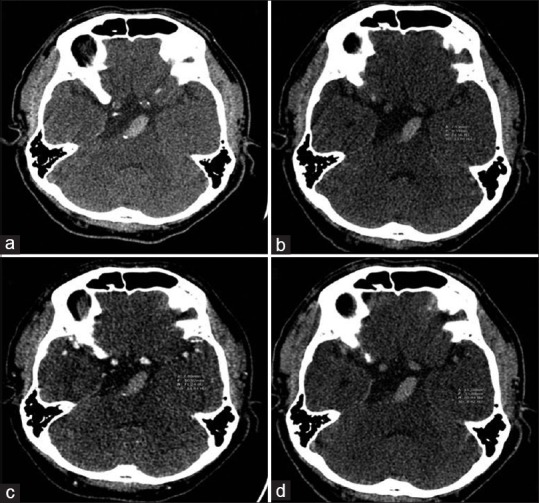
Computed tomography imaging of a 51-year-old male with underlying hypertension and hyperlipidemia who presented with dysarthria and right facial palsy. (a) Initial computed tomography scan showed hyperdensity and dolichoectasia of the basilar artery. (b-d) Hounsfield unit value was approximately 75, 71, and 70 on noncontrast computed tomography, computed tomography angiography, and contrast-enhanced computed tomography, respectively. The patient was sent for mechanical thrombectomy, the histopathologic results showed mixed thrombus, and the 90-day modified Rankin scale was 4
Histopathologic examination of thrombi
After mechanical thrombectomy was performed, recovered thrombi were fixed in formalin and sent to the pathology department. Routine staining was performed. Sections stained with hematoxylin and eosin were evaluated by a pathologist who was blinded to the patient's clinical status. The thrombi were then classified into white, mixed, or red categories according to their fibrin and erythrocyte content.[7] Thrombi in which the difference of estimated proportion of erythrocytes and fibrin lesser or more than 20% were classified as red thrombi or white thrombi in order. Thrombi not meeting either of these two criteria were classified as mixed thrombi [Figure 3].
Figure 3.
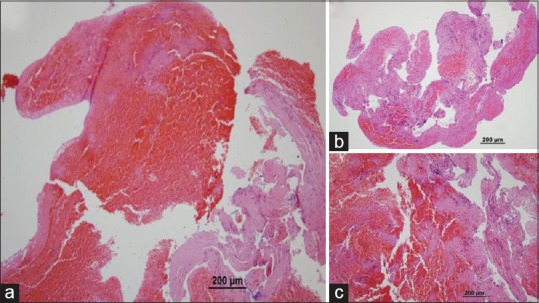
Pathologic section of clot was evaluated for percentage of fibrin and erythrocytes (HE, ×100), shows here are examples of red thrombus which erythrocytes component outnumbers fibrin by more than 20% (a). When the fibrin component outnumbers erythrocytes by more than 20%, classified as white thrombus (b). If this was not the case, thrombi were classified as mixed (c)
Statistical analysis
Data were analyzed using SPSS Statistics version 21.0 (SPSS, Inc., Chicago, IL, USA). CT studies, pathologic results, and clinical outcomes were analyzed using descriptive statistics to characterize the type of thrombus, thrombus measurements, and clinical outcomes. Kruskal–Wallis test was used to differentiate the type of cerebral thrombus among the three types using median and 25th and 75th percentile of HU measurement on NCCT, CTA, and CECT [Table 1]. One-way ANOVA was used to determine significant association between mean HU value on NCCT, CTA, and CECT and clinical outcome [Table 2]. Fisher's exact test was used to identify a significant association between type of cerebral thrombus and clinical outcome [Table 2]. A P < 0.05 was regarded as being statistically significant.
Table 1.
Relation between computed tomography number of cerebral thrombus on noncontrast computed tomography, computed tomography angiography, and contrast-enhanced computed tomography imaging and type of cerebral thrombus on pathological specimen
| Median (P25, P75) | P# | |||||
|---|---|---|---|---|---|---|
| White (n=34) | Mixed (n=22) | Red (n=4) | White-red | Mixed-red | White-mixed | |
| NCCT | 55 (50.5, 59.0) | 60 (56.0, 63.0) | 75.5 (73.5, 77.5) | 0.001 | 0.043 | 0.090 |
| CTA | 58 (52.0, 66.5) | 61 (52.0, 68.0) | 76.5 (75.25, 81.50) | 0.014 | 0.002 | 1.000 |
| CECT | 54 (50.0, 60.5) | 61.5 (54.75, 65.75) | 76 (73.75, 77.50) | 0.001 | 0.068 | 0.054 |
P<0.05 indicates statistical significance; #Kruskal-Wallis test. CT – Computed tomography; NCCT – Noncontrast computed tomography; CTA – Computed tomography angiography; CECT – Contrast-enhanced computed tomography
Table 2.
Association between computed tomography image type and clinical outcome, and type of thrombus and clinical outcome in acute ischemic stroke patients who underwent mechanical thrombectomy
| Mean±SD or n (%) | P | ||||
|---|---|---|---|---|---|
| Good (n=34) | Moderate (n=22) | Poor (n=41) | |||
| NCCT | 58.4±7.0 | 60.4±9.0 | 58.0±7.5 | 0.485* | |
| CTA | 58.5±10.6 | 63.8±8.8 | 59.8±10.2 | 0.203* | |
| CECT | 60.7±12.8 | 61.7±10.5 | 57.9±8.4 | 0.441* | |
| Pathologic result | |||||
| White | 14 (50.0) | 4 (14.3) | 10 (35.7) | 0.104# | |
| Mixed | 4 (19.0) | 7 (33.3) | 10 (47.6) | ||
| Red | 1 (25.0) | 2 (50.0) | 1 (25.0) | ||
P<0.05 indicates statistical significance. *One-way ANOVA; #Fisher’s exact test. CT – Computed tomography; SD – Standard deviation; NCCT – Noncontrast computed tomography; CTA – Computed tomography angiography; CECT – Contrast-enhanced computed tomography; ANOVA – Analysis of variance
Results
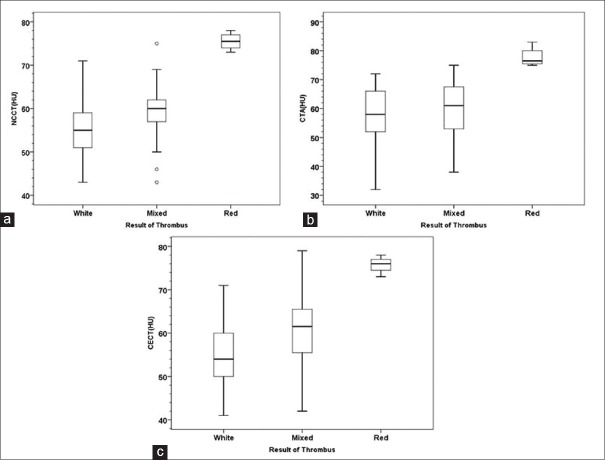
From 97 included patients – 97 NCCT images, 48 CTA images, 48 CECT images, and 54 pathologic results of cerebral thrombi were included in the final analysis. Using HU value data, we were able to significantly differentiate white cerebral thrombus from red cerebral thrombus on NCCT, CTA, and CECT (P = 0.001, P < 0.005, and P = 0.001, respectively). Moreover, mixed cerebral thrombus could be differentiated from red cerebral thrombus on NCCT and CTA images (P < 0.05 and P < 0.05, respectively). However, this method was not able to differentiate mixed cerebral thrombus from red cerebral thrombus on CECT image, nor between white cerebral thrombus and mixed cerebral thrombus on NCCT, CTA, or CECT. The median (range) HU value for each type of cerebral thrombus is shown in Figure 4. The cut point to yield higher sensitivity and specificity for detection of red thrombus on NCCT image is approximately 70 HU (sensitivity 100%, specificity 96%). Similarly, the cut point to yield higher sensitivity and specificity for detection of red thrombus on CTA and CECT is 71.5 (sensitivity 100% and specificity 95%) and 70.5 (sensitivity 100% and specificity 95%), respectively. Good outcome (mRS 0–2) occured in 45% of our patient's population. However, no significant correlation was observed between type of cerebral thrombus or clot density and the result of treatment.
Figure 4.
Forest plot of mean and range of Hounsfield unit value for each type of cerebral thrombus on NCCT (a), CTA (b), and CECT (c). HU – Hounsfield units; NCCT – Noncontrast computed tomography; CTA – Computed tomography angiography; CECT – Contrast-enhanced computed tomography
Discussion
The Presence of thrombus in acute ischemic stroke patients can be observed through the hyperdense artery sign on NCCT scan and through blooming artifact on susceptibility-weighted image or gradient-related echo magnetic resonance image sequences of the brain. However, thin-section CT-based thrombus imaging study can provide more precise information about thrombus characteristics using density measurement. CTA of the brain has higher sensitivity than NCCT for detecting a filling defect in a vessel that is caused by true arterial thrombosis.[9] The density of nonoccluded blood vessels is in the range of 40–43 HU (mean 41.3) whereas occlusive thrombus has a density that ranges from 47 to 61 HU (mean 54.0).[8] Previous studies recommended using NCCT and CTA brain to determine thrombus density. Thrombus type can be categorized as red (red blood cell [RBC] fibrin-rich), white (platelet fibrin-rich), or mixed according to the density observed on CT scan and/or from histopathology.[10,11] This character of thrombus is also predicted that the etiology, for example:-cardioembolism and atherosclerosis, of red and white thrombi may differ.[12,13] With thin slide CT and awareness of possible residual flow within the clot on baseline CTA and clot permeability mentioned earlier as factors making error in measurement of thrombus density,[14,15,16,17] this study demonstrated that red thrombus could be differentiated from white and mixed thrombus using CT densitometry and HU values. Red thrombi had significantly higher HU than both white and mixed thrombi, and mixed thrombi tended to have higher HU value than white thrombi. Moreover and importantly, this is the first study to successfully identify and report cut points (with high sensitivity and specificity) on all three CT images that indicate the presence of red thrombus. The reason for this variation in results is due to the different HU values of clot components including fibrin, platelets, RBC components, and cholesterol.
To be classified as red thrombi, the RBC component should comprise at least 20% of the clot volume, and the clot should have a platelet-rich fibrin outside coating. In contrast, white thrombi classification is assigned when the total volume of the clot is made up of at least 20% fibrin. Type of thrombus may predict the success rate of recanalization after mechanical thrombectomy. Successful recanalization was achieved more frequently after endovascular treatment in patients with high HU thrombi than in patients with low HU thrombi.[18,19] Few studies have investigated association between thrombus characteristics and recanalization rate.[19,20] Clot friction that caused resistance to thrombectomy was reported, especially in white thrombi.[21,22] As a result, complications during manipulation of mechanical thrombectomy procedure-related devices may develop more often in cases with white thrombus than in those with red thrombus. However and in contrast to the preceding hypothesis, the present study found no significant association between clinical outcome and both type of thrombus or HU value. Thrombus type was found to be associated with increased risk of peri-procedural thrombus fragmentation.[23]
The findings of this study support the predictive value of thrombus pathology by CT scan relative to treatment decision and risk management in patients with acute ischemic stroke. Although this study was unable to demonstrate a significant association between patient outcome and both HU value on CT image and thrombus type, thrombus density was reported to be associated with success of recanalization procedure in two previous studies.[19,24] In a future study, we will investigate the effect of time to recanalization, thrombolysis in cerebral infarction grade and stage of collateral circulation[25,26] on the result of mechanical thrombectomy in a larger study population.
Limitations
This study has some mentionable limitations. First and consistent with the retrospective nature of this study, some patient data were missing or incomplete. Second, the size of the study population was relatively small. As a result, our study may have lacked sufficient power to identify all significant differences and associations. Third, the patients enrolled in this study were from a single center. Fourth, our center is Thailand's largest tertiary referral hospital, which means that we are often referred patients with complicated and intransigent conditions. As such, it is possible that our findings may not be generalizable to patients with the same condition in other settings. Finally, the measured HU value in thrombin this study may not accurately represent the HU value in an entire thrombus due to difficulty measuring inhomogeneous components, hypodensity, and hyperdensity in each of the three types of CT images.
Conclusion
Thrombus density on CT was found to be a significant predictor of thrombus pathology; however, no significant association was observed between thrombus type or clot density and patient outcome after mechanical thrombectomy. These findings may improve decision-making relative to choice of treatment in patients with acute ischemic stroke.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors gratefully acknowledge Asst. Prof. Chulaluk Kolmoltri of the Division of Clinical Epidemiology, Department of Research and Development, Faculty of Medicine Siriraj Hospital for assistance with statistical analysis.
References
- 1.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 2.Moftakhar P, English JD, Cooke DL, Kim WT, Stout C, Smith WS, et al. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44:243–5. doi: 10.1161/STROKEAHA.112.674127. [DOI] [PubMed] [Google Scholar]
- 3.Puig J, Pedraza S, Demchuk A, Daunis-I-Estadella J, Termes H, Blasco G, et al. Quantification of thrombus hounsfield units on noncontrast CT predicts stroke subtype and early recanalization after intravenous recombinant tissue plasminogen activator. AJNR Am J Neuroradiol. 2012;33:90–6. doi: 10.3174/ajnr.A2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiotta AM, Vargas J, Hawk H, Turner R, Chaudry MI, Battenhouse H, et al. Hounsfield unit value and clot length in the acutely occluded vessel and time required to achieve thrombectomy, complications and outcome. J Neurointerv Surg. 2014;6:423–7. doi: 10.1136/neurintsurg-2013-010765. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhof K, Welzel T, Mecke C, Zoubaa S, Sartor K. Differentiation of white, mixed, and red thrombi: Value of CT in estimation of the prognosis of thrombolysis phantom study. Radiology. 2003;228:126–30. doi: 10.1148/radiol.2273020530. [DOI] [PubMed] [Google Scholar]
- 6.Horie N, Morikawa M, Ishizaka S, Takeshita T, So G, Hayashi K, et al. Assessment of carotid plaque stability based on the dynamic enhancement pattern in plaque components with multidetector CT angiography. Stroke. 2012;43:393–8. doi: 10.1161/STROKEAHA.111.635953. [DOI] [PubMed] [Google Scholar]
- 7.Minnerup J, Kleinschnitz C. Visualization of clot composition in ischemic stroke: Do we get what we see? Stroke. 2011;42:1193–4. doi: 10.1161/STROKEAHA.110.612150. [DOI] [PubMed] [Google Scholar]
- 8.Koo CK, Teasdale E, Muir KW. What constitutes a true hyperdense middle cerebral artery sign? Cerebrovasc Dis. 2000;10:419–23. doi: 10.1159/000016101. [DOI] [PubMed] [Google Scholar]
- 9.de Lucas EM, Sánchez E, Gutiérrez A, Mandly AG, Ruiz E, Flórez AF, et al. CT protocol for acute stroke: Tips and tricks for general radiologists. Radiographics. 2008;28:1673–87. doi: 10.1148/rg.286085502. [DOI] [PubMed] [Google Scholar]
- 10.Liebeskind DS, Sanossian N, Yong WH, Starkman S, Tsang MP, Moya AL, et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke. 2011;42:1237–43. doi: 10.1161/STROKEAHA.110.605576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinjikji W, Duffy S, Burrows A, Hacke W, Liebeskind D, Majoie CB, et al. Correlation of imaging and histopathology of thrombi in acute ischemic stroke with etiology and outcome: A systematic review. J Neurointerv Surg. 2017;9:529–34. doi: 10.1136/neurintsurg-2016-012391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato Y, Ishibashi-Ueda H, Iwakiri T, Ikeda Y, Matsuyama T, Hatakeyama K, et al. Thrombus components in cardioembolic and atherothrombotic strokes. Thromb Res. 2012;130:278–80. doi: 10.1016/j.thromres.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Kim SK, Yoon W, Kim TS, Kim HS, Heo TW, Park MS, et al. Histologic analysis of retrieved clots in acute ischemic stroke: Correlation with stroke etiology and gradient-echo MRI. AJNR Am J Neuroradiol. 2015;36:1756–62. doi: 10.3174/ajnr.A4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riedel CH, Zoubie J, Ulmer S, Gierthmuehlen J, Jansen O. Thin-slice reconstructions of nonenhanced CT images allow for detection of thrombus in acute stroke. Stroke. 2012;43:2319–23. doi: 10.1161/STROKEAHA.112.649921. [DOI] [PubMed] [Google Scholar]
- 15.Mishra SM, Dykeman J, Sajobi TT, Trivedi A, Almekhlafi M, Sohn SI, et al. Early reperfusion rates with IV tPA are determined by CTA clot characteristics. AJNR Am J Neuroradiol. 2014;35:2265–72. doi: 10.3174/ajnr.A4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ajlan FS, Qazi E, Kim CK, Venkatesan EP, Wilson L, Menon BK. Multimodality CT based imaging to determine clot characteristics and recanalization with intravenous tPA in patients with acute ischemic stroke. Neurovasc Imaging. 2017;3:2. [Google Scholar]
- 17.Qazi E, Al-Ajlan FS, Najm M, Menon BK. The role of vascular imaging in the initial assessment of patients with acute ischemic stroke. Curr Neurol Neurosci Rep. 2016;16:32. doi: 10.1007/s11910-016-0632-y. [DOI] [PubMed] [Google Scholar]
- 18.Kim EY, Heo JH, Lee SK, Kim DJ, Suh SH, Kim J, et al. Prediction of thrombolytic efficacy in acute ischemic stroke using thin-section noncontrast CT. Neurology. 2006;67:1846–8. doi: 10.1212/01.wnl.0000244492.99737.a8. [DOI] [PubMed] [Google Scholar]
- 19.Mokin M, Morr S, Natarajan SK, Lin N, Snyder KV, Hopkins LN, et al. Thrombus density predicts successful recanalization with solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2015;7:104–7. doi: 10.1136/neurintsurg-2013-011017. [DOI] [PubMed] [Google Scholar]
- 20.Linfante I, Starosciak AK, Walker GR, Dabus G, Castonguay AC, Gupta R, et al. Predictors of poor outcome despite recanalization: A multiple regression analysis of the NASA registry. J Neurointerv Surg. 2016;8:224–9. doi: 10.1136/neurintsurg-2014-011525. [DOI] [PubMed] [Google Scholar]
- 21.Niesten JM, van der Schaaf IC, van Dam L, Vink A, Vos JA, Schonewille WJ, et al. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS One. 2014;9:e88882. doi: 10.1371/journal.pone.0088882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs.t-PA alone in stroke. N Engl J Med. 2015;372:2285–95. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 23.Kaesmacher J, Boeckh-Behrens T, Simon S, Maegerlein C, Kleine JF, Zimmer C, et al. Risk of thrombus fragmentation during endovascular stroke treatment. AJNR Am J Neuroradiol. 2017;38:991–8. doi: 10.3174/ajnr.A5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA, et al. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2018;10:34–8. doi: 10.1136/neurintsurg-2016-012721. [DOI] [PubMed] [Google Scholar]
- 25.Park JH, Han YM, Jang KS, Yoon WS, Jang DK, Park SK, et al. Angiographic and clinical factors related with good functional outcome after mechanical thrombectomy in acute cerebral artery occlusion. J Korean Neurosurg Soc. 2015;58:192–6. doi: 10.3340/jkns.2015.58.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raoult H, Eugène F, Ferré JC, Gentric JC, Ronzière T, Stamm A, et al. Prognostic factors for outcomes after mechanical thrombectomy with solitaire stent. J Neuroradiol. 2013;40:252–9. doi: 10.1016/j.neurad.2013.04.001. [DOI] [PubMed] [Google Scholar]