Abstract
To evaluate whether transcranial Doppler (TCD) monitoring plays a role as a prognostic indicator, by being both a diagnostic as well as a monitoring tool for increased intracranial pressure and cerebral vasospasm (VSP), in traumatic brain injury (TBI). Electronic databases and gray literature (unpublished articles) were searched under different MeSH terms from 1990 to the present. Randomized control trials, case–control studies, and prospective cohort studies on TCD in TBI (>18 years old). Clinical outcome measures included Glasgow Coma Outcome Scale (GCOS) and Extended GCOS and mortality. Data were extracted to Review Manager Software. Twenty-five articles that met the inclusion criteria were retrieved and analyzed. Ultimately, five studies were included in our meta-analysis, which revealed that patients with TBI with abnormal TCD (mean flow velocity [MFV] >120 cm/sec or MFV <35 cm/sec and Pulsatility Index >1.2) have a >3-fold higher likelihood of having poor clinical outcome in comparison to patients with TBI and normal TCD monitoring (odds ratio [OR]: 3.87; 95% confidence interval [CI]: 2.97–5.04; P < 0.00001). Subgroup analysis revealed that abnormal TCD has a 9-fold higher likelihood of mortality (OR: 9.96; 95% CI: 4.41–22.47; P < 0.00001). Further, subgroup analysis based on TCD findings revealed that the presence of hypoperfusion on TCD (middle cerebral artery [MCA] <35 cm/s) is associated with a three-fold higher likelihood of having poor functional outcome (OR: 3.72; 95% CI: 1.97–7.0; P < 0.0001). The presence of VSP (MCA >120 cm/s) is associated with three-fold higher likelihood of poor functional outcome (OR: 3.64; 95% CI: 1.55–8.52; P = 0.003). TCD is an evolving diagnostic tool that might play a role in determining the prognosis of patients with TBI. Further prospective study is needed to prove the role of TCD in TBI.
Keywords: Intracranial pressure, transcranial Doppler ultrasound, traumatic brain injury, vasospasm
Introduction
Traumatic brain injury (TBI) either blunt or penetrating leads to disruption of normal function of the brain.[1] TBI can be classified into mild, moderate, and severe based on the severity of injury, neurological status, and clinical presentation. Due to its irreversible and chronic effects on health, TBI has been categorized into a disease process, rather than a discrete event.[2]
About 70%–90% of brain injuries as per the World Health Organization are mild.[3,4,5] The yearly incidence of TBI varies in different countries with the highest estimated to be in England.[6,7,8,9,10]
As a result of direct mechanical forces, there is deformation involving both the anatomical and functional component of the brain, ultimately leading to primary brain injury.[11] Following it is the secondary brain injury, which occurs as a complication of primary brain injury, and includes ischemic and hypoxic damage, cerebral edema, raised intracranial pressure (ICP), hydrocephalus, and infection,[12] both can lead to physiological,[13,14,15,16] emotional,[15,16] and neurocognitive disorders.[16,17,18] The immediate effect of TBI on blood flow can range from ultra-early hypoperfusion (day 0), to early hyperemia (days 1–3), to delayed cerebral vasospasm (VSP) (days 4–15), as well as raised ICP.[19,20]
There are multimodal monitors involved in the assessment of patients in neurocritical care setting that varies from invasive to less invasive devices.[21] Therefore, ICP can be measured from direct intraventricular catheter to less invasive devices that include intraparenchymal, subdural, subarachnoid, and extradural probes. In addition, oxygen extraction in the brain can be determined by either jugular venous oxygen saturation for global blood flow or intraparenchymal Clark electrode (LiCox) for focal brain parenchymal flow.[21]
Transcranial Doppler (TCD) allows a noninvasive assessment of real-time monitoring of ICP and cerebral perfusion pressure (CPP). TCD ultrasonography can be performed at the patient's bedside to determine and monitor cerebral blood flow (CBF), measured by mean blood-flow velocity (MFV), and ICP can be measured by Pulsatility Index (PI) values of the middle cerebral artery (MCA), and the rest of major intracranial vessels, regardless of patient having an altered level of consciousness or being sedated.[22]
In addition, TCD may play a role in monitoring the early development of cerebral VSP after traumatic subarachnoid hemorrhage (SAH) and the early or delayed development of increase ICP following TBI.[23] This is achieved by daily noninvasive monitoring of CBF, MFV, and ICP of the intracranial vessels.[24]
Even though TCD is a relatively low-cost, risk-free, bedside available, and high temporal resolution device, it is suitable for the emergency setting; significant limitations to the clinical utility of TCD in TBI include limited spatial resolution, assumptions made regarding the vessel diameter on TCD, operator dependency, and in patients who lack an adequate acoustic temporal window for insonation.[22,25,26,27,28]
The diagnostic and prognostic role of TCD in TBI has not been fully established since available data are limited due to unavailability of any randomized controlled trials, and a small number of prospective cohort study.
The aim of our study is to evaluate whether TCD has a role as a prognostic tool by being both a diagnostic and monitoring tool for ICP and VSP in TBI. We performed a literature review and meta-analysis to attempt to answer the above question. Our a priori hypothesis was that TCD does have a role as a prognostic tool in patients with TBI, by early detection of increased ICP and cerebral VSP.
Methods
Database search
The following databases were reviewed – Cochrane Library, Medline, Embase, Web of Science, Google Scholar, Scopus, and PubMed. In addition, we reviewed the following gray literature – unpublished abstracts from European and American Neuro-Ultrasonography conferences over the last 10 years related to TCD in TBI as shown in Figure 1. Articles published between 1990 and present were searched. Two reviewers MS and NF completed the entire review process.
Figure 1.
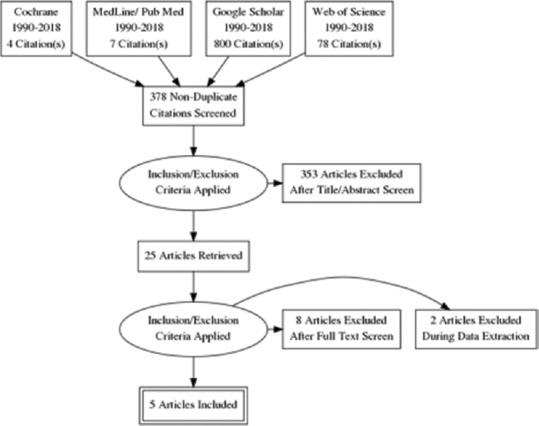
Search strategy
Literature search
Following MeSH headings were searched: Ultrasound or Doppler or transcranial or TBI or ICP monitoring or prognostic tools in TBI or cerebrodynamics monitoring. We did not define any limitation in language. A total of 378 articles were retrieved based on the MeSH headings mentioned above. The titles of articles were then reviewed and duplicates were deleted.
The titles and abstracts of the studies identified by the literature search were screened for eligibility based on the inclusion criteria. Manuscripts that met the inclusion criteria were selected.
Study selection and data extraction
Primary research question
Does TCD play a role in predicting clinical outcome in TBI by monitoring and identifying increased ICP and VSP in the TBI population. Based on this, the following Population, Intervention, Control, and Outcome questions were developed:
Population
It includes adult patients with TBI due to motor vehicle injury, blows, fall, and penetrating head injury.
Intervention
Daily TCD monitoring with diagnostic criteria for increase ICP when PI >1.2, or Resistivity Index >0.8, and VSP when MFV (MCA >120) with Lindegaard ratio >3 and hypoperfusion (MFV <35 cm/s).
Control
Patients with TBI either with normal TCD or without TCD monitoring.
Outcome
Clinical outcome dichotomized to good and bad outcome based on the Glasgow Coma Outcome Scale (GCOS) (favorable outcome: 4–5 and unfavorable outcome 1–3) or extended GCOS (EGCOS) (favorable outcome >5–8 and unfavorable outcome 1–4).
We applied stringent inclusion criteria. The following study's types were selected – randomized controlled trials, case–control studies, and prospective cohort studies in adult populations with TBI who received TCD. Retrospective cohort, case series, and case report studies were excluded from our systematic review.
The reviewer was not blind to the author's name and institutions, journals of publication, or study results.
Outcome measures
Outcome measures – the following outcomes were selected for our meta-analysis.
Primary outcome measures – dichotomized to good outcome (EGCOS 5–8 and GCOS 4–5) and poor outcome (EGCOS 1–4 and GCOS 1–3).
Measures of intervention effect – intervention efficacy was dichotomized as “Good” or “Poor” clinical functional outcome.
The GCOS and EGCOS were dichotomized to good functional outcome and poor functional outcome. In order for the TCD ultrasound to be effective as a prognostic tool, we required the threshold of distribution between good and poor outcome to be clinically and statistically significant (P < 0.05)
The odds ratio (OR) was calculated for normal versus abnormal TCD in TBI, good and poor outcome, mortality, hypoperfusion, and VSP.
Statistical analysis
Review Manager Program version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, Denmark) as provided by Cochrane Library was used to perform statistical analyses. Data from each study were extracted from the articles and placed in this software to perform a pooled meta-analysis and subgroup analysis.
First, the TCD and good and poor outcomes computed across the different ultrasound modalities used in the different prospective cohort studies were analyzed. The OR for experimental abnormal and control normal TCD, associated with good and poor outcomes were calculated in all individual studies, with available data comparing the various outcomes among different studies. The OR from separate studies was combined by the fixed-effect meta-analysis according to the Mantel–Haenszel method, which is also valid for paired OR. Heterogeneity between studies was assessed by the Breslow–Day Chi-square test and I2 statistic. The I2 statistic describes the percentage of total variation across studies that are attributable to heterogeneity rather than chance. Compared with the classical Breslow–Day Chi-square test, its interpretation is more intuitive and the value does not depend on the number of studies. There is no simple categorization of values of I2, although values >75% are usually considered as meaning high heterogeneity.
Results
Description of studies
A total of 378 tiles were reviewed from the above-mentioned electronic literature. Twenty-five studies were retrieved and analyzed; ultimately five articles, which met the inclusion criteria, were included in our meta-analysis. The baseline characteristics and the outcome of the prospective cohort studies are summarized in Tables 1 and 2.
Table 1.
Baseline characteristics of prospective studies
| Study | Prospective cohort/case control study | n (treatment/control) | Intervention (treatment/control) | Time since admission | TCD cutoff values | Initial GCS | Age (mean±SD) | Outcome measures |
|---|---|---|---|---|---|---|---|---|
| Bouzat et al. (2016)[29] | Prospective | 369: 12 excluded Normal TCD: 257 Abnormal TCD: 86 |
TCD: Echo Doppler 1- to 5-MHz 283 (80%) TCD 2 MHz: 73 (20%) |
Postinjury: <8 h | 2 MCA PI: <1.25 FVd: >25 cm/s |
9-15 | 39 (27-54)/62 (34-73) | 1. SND with 2 point decrease in GCS score on day 7 2. DRS on day 28 |
| Prasad et al. (2017)[30] | Prospective | 75 Normal TCD: 36 Abnormal TCD: 39 (18 hypoperfusion; 21 vasospasm) |
2 MHz TCD | Postinjury: <24 h | MCA Hypoperfusion: FVd <20 cm/s Vm <35 cm/s PI >1.2 Vasospasm: Vm >120 cm/s |
Normal: 7 Hypoperfusion: 5 Vasospasm: 5 |
30-50 | 1. GCS 2. GOSE |
| Zaytoun et al. (2017)[31] | Prospective | 120 Normal TCD: 68 Abnormal TCD: 52 (hypoperfusion 41, vasospasm 11) |
2 MHz TCD | Postinjury: 24 h | Hypoperfusion: MFV <35 cm/s; EDV <20 cm/s, PI >1.4 Vasospasm MFV >120 cm/s |
All patients GCS 6 (5-7) Normal 7 (6-8) Abnormal Hypoperfusion 5 (4-5) Vasospasm 5 (5-6) |
37 Normal TCD 36.5, abnormal hypoperfusion 38, vasospasm 35 |
1. GOSE at 3 months 2. FOUR score 3. APACHE-11 score 4. Rotterdam CT score 5. New ISS 6. PI |
| Ract et al. (2007)[32] | Prospective | 24 Normal TCD: 13 Abnormal TCD: 11 |
2 MHz TCD | As soon as possible on admission | Vm <30 cm/s, Vd <20 cm/s, PI >1.4 | GCS equal and <8 | Normal TCD 35±12 Abnormal TCD 33±12 |
1. GOSE at 3 months |
| Moreno et al. (2000)[33] | Prospective | 125: 67/58 | 2 MHz TCD | Postinjury: <24 h | Mean blood flow: 45±10 cm/s, normal PI less and equal to 1 |
GCS <9 | 24.14±19.16 | 1. GCOS at 6 months 2. APACHE 11 at 6 months 3. ICP and CPP 4. Blood-flow velocity at 6 months 5. PI at 6 months |
TCD – Transcranial Doppler; MCA – Middle cerebral artery; SND – Secondary neurological deficit; GCS – Glasgow Coma Scale; DRS – Disability Rating Scale; GCOS – Glasgow Coma Outcome Scale; GOSE – Extended GCOS; FOUR – Full Outline of Unresponsiveness; APACHE – Acute physiologic assessment and chronic health evaluation; ISS – Injury Severity Scale; PI – Pulsatility index; ICP – Intracranial pressure; CPP – Cerebral perfusion pressure; FVd – Diastolic blood-flow velocity; Vm – Measured flow velocity; MFV – Mean blood-flow velocity; EDV – End-diastolic velocity; Vd – End-diastolic flow velocity; SD – Standard deviation; CT – Computed tomography
Table 2.
Good and poor outcomes of prospective cohort studies
| Trial or study | Good outcome | Poor outcome | Glasgow Outcome Scale/extended | Admission TCD | Definition |
|---|---|---|---|---|---|
| Bouzat et al. (2016)[29] | N: 265/269 A: 71/87 |
N: 4/269 A: 20/87 |
N: Unavailable A: Unavailable Severe DRS (>7) on 28 days N: 257/336 A: 28/336 |
N: 269/336 A: 87/336 |
Decrease in GCS of >2 points from the initial GCS in the absence of pharmacologic sedation Deterioration in neurologic status sufficient to warrant intervention, that is, mechanical ventilation, sedation, osmotherapy, transfer to the ICU, or neurosurgical intervention |
| Prasad et al. (2016)[30] | N: 24/36 A: 4/39 |
N: 12/36 A: 35/39 |
GOSE 5-8 N: 24/36 A: 14/39 1-4 N: 7/36 A: 11/39 |
N: 36/75 A: 39/75 |
Glasgow outcomes scale extended with in-hospital mortality with poor outcome <4 and good outcome >5 |
| Zaytoun et al. (2017)[31] | N: 57/68 A: 11/52 |
N: 11/68 A: 41/52 |
GOSE at 90 days 5-8 N: 57/68 A: 11/52 1-4 N: 11/68 A: 41/52 |
N: 68/120 A: 52/120 |
GOSE, with questions covering the following aspects (1) Consciousness (2) Independence inside and outside the house (3) Resumption of normal social roles and (4) Residual symptoms interfering with daily life GOSE was dichotomized as unfavorable (score 1-4) versus favorable (score 5-8) |
| Ract et al. (2007)[32] | N: 12/13 A: 5/11 |
N: 1/13 A: 6/11 |
Glasgow Outcome Score at 3 months 3-4 N: 1/13 A: 3/11 1-2 N: 12/13 A: 5/11 |
N: 13/24 A: 11/24 |
GCOS measured at 3 months with>3 indicating unfavorable outcome |
| Moreno et al. (2000)[33] | N: 48/65 A: 19/60 |
N: 17/65 A: 41/60 |
Glasgow Outcome Score at 6 months 3-4 N: 17/65 A: 41/60 |
N: 65/125 A: 60/125 |
Glasgow Outcome Scale. Moderate disability and complete recovery were considered “good” outcome; death, vegetative state, and severe disability were considered “poor” |
ICU – Intensive care unit; DRS – Disability Rating Scale; GCOS – Glasgow Coma Outcome Scale; GOSE – Extended GCOS; GCS – Glasgow Coma Scale; TCD – Transcranial Doppler
Risk of bias in the prospective cohort studies
None of the prospective cohort trials followed double blindness. This is understandable in this type of prospective cohort study, in which a procedure is evaluated, and it may be difficult to blind the investigator or the patient to procedure allocation. However, blindness could have been achieved for functional outcome, and this was not the case in any of the studies.
Effects of interventions
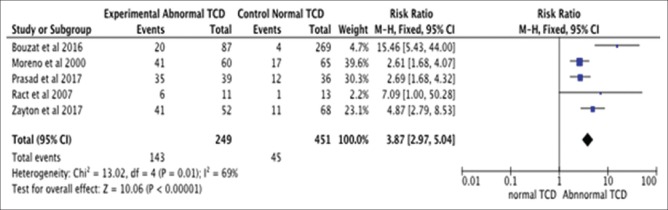
Pooled analysis for all five studies [Figure 2] – patients with TBI who underwent diagnostic TCD monitoring for increase ICP or VSP and had abnormal TCD (MFV >120 cm/s or MFV <35 cm/s, PI >1.2) had a >3-fold higher likelihood of having poor outcome in comparison to patients with TBI and normal TCD monitoring (OR of poor outcome: 3.87; 95% confidence interval [CI]: 2.97–5.04; P < 0.00001).
Figure 2.
Pooled analysis for all five studies: Patients with traumatic brain injury having abnormal and normal transcranial Doppler monitoring. (Odds ratio of poor outcome: 3.87; 95% confidence interval: 2.97–5.04; P < 0.00001)
Furthermore, subgroup analysis reveals that:
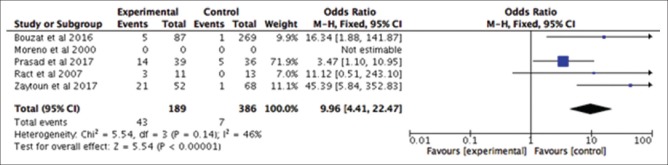
Subgroup analysis based on the role of TCD monitoring in TBI in predicting mortality – it revealed that patients with abnormal TCD had a 9-fold higher likelihood of mortality in comparison to patients with TBI who have normal TCD (OR: 9.96; 95% CI: 4.41–22.47; P < 0.00001) as indicated in Figure 3
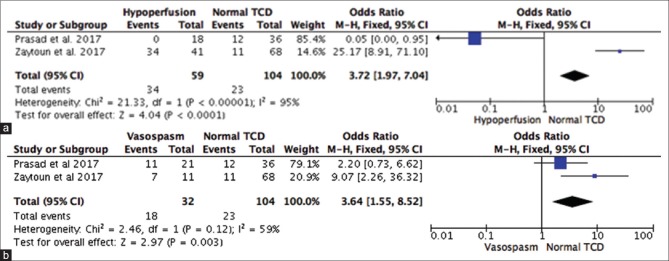
Subgroup analysis based on the TCD findings of hypoperfusion (MCA <35 cm/s) in predicting poor outcome in TBI [Figure 3a] – the TCD finding of hypoperfusion (MCA <35 cm/s) is associated with a 3-fold higher likelihood of having poor functional outcome as compared to normal TCD (OR: 3.72; 95%CI: 1.97–7.0; P < 0.0001) as indicated in Figure 4a
Subgroup analysis based on the TCD findings of VSP (MCA >120 cm/s) in predicting poor outcome in TBI [Figure 3b] – the TCD findings of VSP are associated with a 3-fold higher likelihood of having poor functional outcome as compared to normal TCD (OR: 3.64; 95% CI: 1.55–8.52; P = 0.003). However, there were insufficient data to comment on the OR of VSP on the prognosis of the patient as indicated in Figure 4b
Subgroup analysis for TCD based on PI could not be performed due to lack of studies looking at the comparison of high PI versus normal PI in TBI.
Figure 3.
Traumatic brain injury in predicting mortality: Abnormal transcranial Doppler versus normal transcranial Doppler; odds ratio: 9.96; 95% confidence interval: 4.41–22.47; P < 0.00001
Figure 4.
Subgroup analysis based on the transcranial Doppler findings of hypoperfusion (middle cerebral artery <35 cm/s) in predicting poor outcome in traumatic brain injury (a). Subgroup analysis based on the transcranial Doppler findings of vasospasm (middle cerebral artery >120 cm/s) in predicting poor outcome in traumatic brain injury (b)
Discussion
Our study is the first to evaluate whether TCD has a diagnostic or prognostic role in the TBI population. Our meta-analysis revealed that TCD plays role as a diagnostic and prognostic tool in terms of determining the functional outcome in patients with TBI. However, due to several limitations in the prospective cohort studies (lack of randomized control trials [RCTs], lack of adequate sequence generation, blindness in randomization and clinical follow-up, and the small sample size in all of the studies, and future double-blinded RCTs with large sample size are needed to prove the hypothesis of this novel noninvasive intervention. In addition, more evidence is required regarding the blood FV and PI correlation with the diagnosis and prognosis of patients with TBI.
Our meta-analysis findings are in line with the previous findings published.[34,35,36,37] In addition, our meta-analysis represents a further step in evaluating the efficacy of TCD in TBI. A low flow velocity defined as MCA MFV of <35 cm/s within 72 h of head injury has been shown to predict unfavorable outcome at 6 months (Glasgow outcome score [GOS] score 1–3: death, vegetative state, or severe disability) with an OR of 3.9 (CI 1.2–13).[34] Christou et al. 2001[35] suggested that the MCA PI correlated with outcome in TBI. The presence of high PI ≥1.56 predicted over 83% rate of poor outcome at 6 months, whereas a low PI ≤1 identified 71% of patients with a good outcome (GOS 4–5).[35] The severity of VSP may also predict the outcome on the GOS; in a study of 116 SAH patients, moderate BA VSP (MFV >60 cm/s) was associated with permanent neurological deficit, whereas the presence of severe BA VSP (MFV >85 cm/s) was associated with a vegetative state.[36] In another study, it was found that patients with TBI who had VSP or hyperemia were more prone to poor outcome (GOS: 1–3) as compared to those without significant FV change.[37]
Although the underlying brain changes after TBI are not fully understood, it has been proposed that TCD is the stethoscope of the brain.[38] Disturbances of cerebral circulation play a key role in the pathophysiology of head injury. Cerebral hemodynamics has significant implications for the monitoring and treatment of patients with head injury. In a prospective study of 125 patients,[20] the MCA and extracranial (EC) internal carotid artery (ICA) were insonated using a 2-MHz pulsed TCD device through the transtemporal and submandibular methods, respectively. This prospective study has confirmed the reported time-dependent changes in the CBF, MCA velocity, and Hemispheric Index (also referred as Lindegaard ratio, VMCA/VEC–ICA); which, considered together defined three discrete hemodynamic phases. Phase 1 (hypoperfusion phase) occurs on the day of injury (Day 0), followed by the Phase 2 (hyperemia phase, Day 1–3) and finally the Phase 3 (VSP phase, Day 4–15).
TCD is a tool that has been increasingly used in cerebrovascular hemodynamic monitoring since 1982. It measures different hemodynamic parameters such as (1) brain FV, (2) estimation of vascular brain resistance, and (3) brain perfusion pressure.[39] These parameters on TCD can be used to detect and monitor VSP in patients with aneurysmal and traumatic SAH and indirectly estimate ICP and CPP in patients with severe TBI.[40]
As per Kirkpatrick et al.[41] 1994, TCD is able to detect transient changes in the relative CBF with high resolution. Kirkpatrick et al., 1995,[42] described that increase in the ICP is depicted by a relative decrease in the CBF and FV. This is associated with synchronous desaturation (Sj02 and HBO2 signal changes) and cerebral hyperemia.
The most sensitive index of falling CPP is the increase in FV amplitude due to the divergence of FVs and FVd.[43] Czosnyka et al.,[44] 1994e, described that at high CPP values, PI remains constant whereas, at low CPP values, PI increases rapidly.
As per one study conducted by Homburg et al.,[45] 10 head-injured patients were investigated and demonstrated a positive exponential correlation between PI and epidural pressure. Moreno et al. found that an elevated PI predicts poor outcome (PI 1.56), and further correlation between ICP and PI was found.[33] As per Bellner et al.,[24] there exists a highly significant correlation between ICP and PI, independent of intracranial pathology. In a systematic review conducted by Ziegler et al.,[46] the evidence for the predictive value of PI was strong, unlike the prognostic value of blood-flow velocities where the evidence was inconclusive.
In addition, more evidence is required regarding the use of the TCD in patients with TBI, thus improving the diagnostic evidence and decreasing the complications occurring with the invasive cerebral monitors in place. Besides, the early detection of underlying brain pathology after TBI with TCD as compared to the computed tomography scan head and invasive cerebral monitors will help in early medical and neurosurgical intervention, thus improving the functional outcomes of the patient.
Our study has several limitations. First, there is a possibility of selection and publication bias in our systematic review since only two reviewers carried out this part of the process and they are part of the largest trial in this systematic review. They might, therefore, be more influenced by the positive trial results than by the negative ones. However, we tried to limit such bias using the following steps: a gray literature review, in which we reviewed the abstracts from several meetings in order to capture any RCT that was presented as an abstract but not published because of a negative result. Indeed, one abstract was found with a negative result, and it was included in the meta-analysis. Second, the lack of access to individual patient's data is one of the limitations. Third, the number of patients who are ineligible to this form of treatment due to poor temporal window has not been mentioned in all the trials, which makes it difficult to know the proportion of patients that are illegible to this form of treatment in general. Finally, our meta-analysis results cannot be generalized to all forms of TBI since we restricted mostly to moderate and severe TBI.
Conclusion
Our data point to a possible signal of efficacy of TCD as a diagnostic and prognostic role in patients with TBI and provide a basis to design a RCT with less bias and determine the sample size, mean CBF velocities, and the PI of future randomized trials of TCD in patients with traumatic brain injuries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Editors of Asian Journal of Neurosurgery.
References
- 1.Marr AL, Coronado VG, editors. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2004. Central Nervous System Injury Surveillance data Submission Standards-2002. [Google Scholar]
- 2.Masel BE, DeWitt DS. Traumatic brain injury: A disease process, not an event. J Neurotrauma. 2010;27:1529–40. doi: 10.1089/neu.2010.1358. [DOI] [PubMed] [Google Scholar]
- 3.D’Ambrosio R, Perucca E. Epilepsy after head injury. Curr Opin Neurol. 2004;17:731–5. doi: 10.1097/00019052-200412000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassidy JD, Carroll LJ, Peloso PM, Borg J, von Holst H, Holm L, et al. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO collaborating centre task force on mild traumatic brain injury. J Rehabil Med. 2004;43(Suppl):28–60. doi: 10.1080/16501960410023732. [DOI] [PubMed] [Google Scholar]
- 5.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–57. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicoletto HA, Burkman MH. Transcranial Doppler series part II: Performing a transcranial Doppler. Am J Electroneurodiagnostic Technol. 2009;49:14–27. [PubMed] [Google Scholar]
- 7.Hannay HJ, Howieson DB, Loring DW, Fischer JS, Lezak MD. Neuropathology for neuropsychologists. In: Lezak MD, Howieson DB, Loring DW, editors. Neuropsychological Assessment. Oxford: Oxford University Press; 2004. pp. 158–62. [Google Scholar]
- 8.Comper P, Bisschop SM, Carnide N, Tricco A. A systematic review of treatments for mild traumatic brain injury. Brain Inj. 2005;19:863–80. doi: 10.1080/02699050400025042. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald BD, Burnett DM, Miller MA. Congenital and acquired brain injury 1. Brain injury: Epidemiology and pathophysiology. Arch Phys Med Rehabil. 2003;84:S3–7. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. TBI: Get the Facts. Centers for Disease Control and Prevention. [Last updated on 2017 Apr 27; Last accessed on 2018 Jan 31]. Available from: https://www.cdc.gov/traumaticbraininjury/get_the_facts.html .
- 11.Mckee AC, Daneshvar DH. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham DI, Gennarelli TA, McIntosh TK. Trauma. In: Graham DI, Lantos PL, editors. Greenfield's Neuropathology. London: Arnold; 2002. pp. 823–98. [Google Scholar]
- 13.Schiff ND, Plum F, Rezai AR. Developing prosthetics to treat cognitive disabilities resulting from acquired brain injuries. Neurol Res. 2002;24:116–24. doi: 10.1179/016164102101199576. [DOI] [PubMed] [Google Scholar]
- 14.Kwasnica C, Brown AW, Elovic EP, Kothari S, Flanagan SR. Congenital and acquired brain injury 3. Spectrum of the acquired brain injury population. Arch Phys Med Rehabil. 2008;89:S15–20. doi: 10.1016/j.apmr.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Office of Communications and Public Liaison. Traumatic Brain Injury: Hope Through Research. NIH Publication No. 02-2478. National Institute of Neurological Disorders and Stroke, National Institutes of Health. 2002. Feb, [Last retrieved on 2008 Aug 17]. Available from: https://www.scribd.com/document/96346210/Traumatic-Brain-Injury-Hope-Through-Research-National-Institute-of-Neurological-Disorders-and-Stroke-NINDS .
- 16.Valadka AB. Injury to the cranium. In: Moore EJ, Feliciano DV, Mattox KL, editors. Trauma. New York: McGraw-Hill, Medical Pub. Division; 2004. pp. 385–406. [Google Scholar]
- 17.Aimaretti G, Ghigo E. Should every patient with traumatic brain injury be referred to an endocrinologist? Nat Clin Pract Endocrinol Metab. 2007;3:318–9. doi: 10.1038/ncpendmet0460. [DOI] [PubMed] [Google Scholar]
- 18.Mendez MF. The neuropsychiatric aspects of boxing. Int J Psychiatry Med. 1995;25:249–62. doi: 10.2190/CUMK-THT1-X98M-WB4C. [DOI] [PubMed] [Google Scholar]
- 19.Arlinghaus KA, Shoaib AM, Price TR. Neuropsychiatric assessment. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of Traumatic Brain Injury. Washington, D.C: American Psychiatric Association; 2005. pp. 59–62. [Google Scholar]
- 20.Martin NA, Patwardhan RV, Alexander MJ, Africk CZ, Lee JH, Shalmon E, et al. Characterization of cerebral hemodynamic phases following severe head trauma: Hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- 21.Vidgeon SD, Strong AJ. Multimodal cerebral monitoring in traumatic brain injury. J Intensive Care Soc. 2011;12:2. [Google Scholar]
- 22.Moreno JA, Mesalles E, Gener J, Tomasa A, Ley A, Roca J, et al. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. 2000;8:e8. doi: 10.3171/foc.2000.8.1.1702. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien NF, Maa T, Reuter-Rice K. Noninvasive screening for intracranial hypertension in children with acute, severe traumatic brain injury. J Neurosurg Pediatr. 2015;16:420–5. doi: 10.3171/2015.3.PEDS14521. [DOI] [PubMed] [Google Scholar]
- 24.Bellner J, Romner B, Reinstrup P, Kristiansson KA, Ryding E, Brandt L, et al. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP) Surg Neurol. 2004;62:45–51. doi: 10.1016/j.surneu.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler ultrasound: A review of the physical principles and major applications in critical care. Int J Vasc Med. 2013;2013:629378. doi: 10.1155/2013/629378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis JR, Sauro J. When 100% really isn’t 100%: Improving the accuracy of small-sample estimates of completion rates. J Usability Stud. 2006;1:136–150. [Google Scholar]
- 27.Armitage P, Berry G, Matthews JN. 4th ed. Oxford: Blackwell; 2002. Statistical Methods in Medical Research. [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouzat P, Almeras L, Manhes P, Sanders L, Levrat A, David JS, et al. Transcranial Doppler to predict neurologic outcome after mild to moderate traumatic brain injury. Anesthesiology. 2016;125:346–54. doi: 10.1097/ALN.0000000000001165. [DOI] [PubMed] [Google Scholar]
- 30.Prasad BK, Chamarthi M, Isireddy P. Role of transcranial Doppler as a predictor of prognosis in patients with traumatic brain injury. Int J Contemp Med Surg Radiol. 2017;2:139–42. [Google Scholar]
- 31.Zaytoun T, Fayed A, Elbeheiry A, Elsefi T. Role of transcranial doppler ultrasound as a predictor of outcome in severe traumatic brain injury and its correlation with glagsow coma scale and full outline of unresponsiveness score. J Med Sci Clin Res. 2017;5:5Y20135–50. [doi: 10.18535/jmscr/v5i4.66] [Google Scholar]
- 32.Ract C, Le Moigno S, Bruder N, Vigué B. Transcranial Doppler ultrasound goal-directed therapy for the early management of severe traumatic brain injury. Intensive Care Med. 2007;33:645–51. doi: 10.1007/s00134-007-0558-6. [DOI] [PubMed] [Google Scholar]
- 33.Moreno JA, Mesalles E, Gener J, Tomasa A, Ley A, Roca J, et al. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. 2000;8:1–7. doi: 10.3171/foc.2000.8.1.1702. [DOI] [PubMed] [Google Scholar]
- 34.van Santbrink H, Schouten JW, Steyerberg EW, Avezaat CJ, Maas AI. Serial transcranial Doppler measurements in traumatic brain injury with special focus on the early posttraumatic period. Acta Neurochir (Wien) 2002;144:1141–9. doi: 10.1007/s00701-002-1012-8. [DOI] [PubMed] [Google Scholar]
- 35.Christou I, Felberg RA, Demchuk AM, Grotta JC, Burgin WS, Malkoff M, et al. A broad diagnostic battery for bedside transcranial Doppler to detect flow changes with internal carotid artery stenosis or occlusion. J Neuroimaging. 2001;11:236–42. doi: 10.1111/j.1552-6569.2001.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 36.Soustiel JF, Shik V, Feinsod M. Basilar vasospasm following spontaneous and traumatic subarachnoid haemorrhage: Clinical implications. Acta Neurochir (Wien) 2002;144:137–44. doi: 10.1007/s007010200016. [DOI] [PubMed] [Google Scholar]
- 37.Zurynski YA, Dorsch NW, Fearnside MR. Incidence and effects of increased cerebral blood flow velocity after severe head injury: A transcranial Doppler ultrasound study II. Effect of vasospasm and hyperemia on outcome. J Neurol Sci. 1995;134:41–6. doi: 10.1016/0022-510x(95)00178-x. [DOI] [PubMed] [Google Scholar]
- 38.Robba C, Cardim D, Sekhon M, Budohoski K, Czosnyka M. Transcranial Doppler: A stethoscope for the brain-neurocritical care use. J Neurosci Res. 2018;96:720–30. doi: 10.1002/jnr.24148. [DOI] [PubMed] [Google Scholar]
- 39.Chacón-Lozsán F, Rodríguez-Torres M, Pacheco C. Hemodynamic neuromonitoring by ultrasound in the critical patient: Transcranial ultrasound. Acta Colomb Cuaidado Intensive. 2018;18:164–74. [Google Scholar]
- 40.Rosenfeld JV, Maas AI, Bragge P, Morganti-Kossmann MC, Manley GT, Gruen RL, et al. Early management of severe traumatic brain injury. Lancet. 2012;380:1088–98. doi: 10.1016/S0140-6736(12)60864-2. [DOI] [PubMed] [Google Scholar]
- 41.Kirkpatrick PJ, Smielewski P, Czosnyka M, Pickard JD. Continuous monitoring of cortical perfusion by laser Doppler flowmetry in ventilated patients with head injury. J Neurol Neurosurg Psychiatry. 1994;57:1382–8. doi: 10.1136/jnnp.57.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkpatrick PJ, Smielewski P, Czosnyka M, Menon DK, Pickard JD. Provisional observations with near infrared spectroscopy in head injured patients. J Neurosurg. 1995;83:963–70. doi: 10.3171/jns.1995.83.6.0963. [DOI] [PubMed] [Google Scholar]
- 43.Czosnyka M, Guazzo E, Kirkpatrick P. Clinical significance of simultaneous transcranial Doppler and ICP waveform analysis following head injury. J Neurotrauma. 1995;12:402. [Google Scholar]
- 44.Czosnyka M, Kirkpatrick P, Guazzo E, Smielewski P, Whitehouse H, Pickard JD, et al. Can TCD pulsatility indices be used for a non-invasive assessment of cerebral perfusion pressure in head injured patients? In: Nagai H, Kamiya K, Ishii S, editors. Intracranial Pressure IX. Berlin: Springer-Verlag; 1994e. pp. 146–9. [Google Scholar]
- 45.Homburg AM, Jakobsen M, Enevoldsen E. Transcranial Doppler recordings in raised intracranial pressure. Acta Neurol Scand. 1993;87:488–93. doi: 10.1111/j.1600-0404.1993.tb04142.x. [DOI] [PubMed] [Google Scholar]
- 46.Ziegler D, Cravens G, Poche G, Gandhi R, Tellez M. Use of transcranial Doppler in patients with severe traumatic brain injuries. J Neurotrauma. 2017;34:121–7. doi: 10.1089/neu.2015.3967. [DOI] [PubMed] [Google Scholar]