Abstract
The association of cavernous malformations and developmental venous anomalies (DVA) is well known, but the presence of arterial fistulous connection with the main venous collector has been reported in the literature only once. We report the unusual case of a hemorrhagic cavernous angioma associated with DVA characterized by a fine arterial supply to the main venous collector. During surgery, after the excision of the cavernous angioma, few small arterial feeders were found entering the main channel of the venous developmental anomaly. The presence of an arterial fistulous connection with the main venous collector of a DVA may be a possible mechanism involved in a higher bleeding potential of cavernous angioma.
Keywords: Cavernous malformation, cerebral hemorrhage, developmental venous anomalies, developmental venous anomaly, venous angioma
Introduction
The association of cavernous malformation (CM) and developmental venous anomaly (DVA) is well known. In patients with hemorrhagic DVAs a CM is generally considered as the source of bleeding. The rate of bleeding of CMs associated with DVAs is higher than that of CMs alone, but the way in which variations in venous malformation hemodynamics might increase the chance of hemorrhage is unclear. Stenosis of the main venous collector and secondary hypertension is often considered as an important factor in the formation and bleeding of cavernous malformations. However, the finding of a venous stricture is quite rare. We report a case of “micro-fistulous cavernoma-venous angioma complex,” which may represent, in some patients, the origin, growth, and bleeding of cavernomas associated with DVAs. The arterialization may produce overflow and hypertension in the venous anomaly and trigger microhemorrhages and angiogenesis cascade leading to the origin of a CM. Only one case of the cavernous malformation-DVA complex with fistulous arterial connection within the main venous collector has been reported to date in the literature by Nakase et al.[1] Our case represents the first supratentorial one.
Case Report
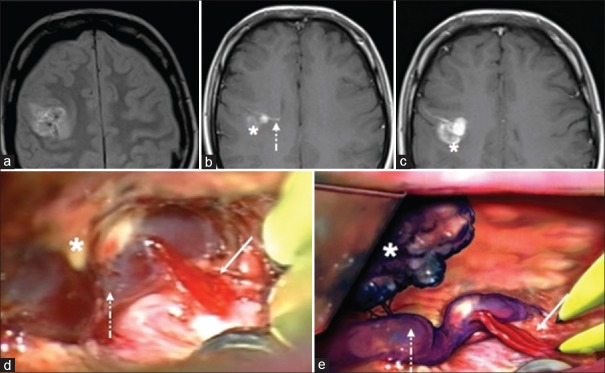
A 24-year-old woman was admitted to our hospital for seizure. Computed tomography (CT) scan demonstrated a hemorrhagic lesion in the right frontoparietal region. On neurological examination, a slight left hemiparesis was present. Magnetic resonance imaging (MRI) showed a mass with heterogeneous signal intensity and hypointense peripheral rim in the right frontoparietal region, associated with perifocal edema [Figure 1]. After contrast medium administration, a DVA adjacent to the lesion was also demonstrated [Figure 1a–c]. Interestingly, an MRI without gadolinium performed 5 years before for recurrent headaches was considered normal. Further neurological deterioration occurred 3 days later and a CT scan confirmed some enlargement of the hemorrhagic mass. The patient was immediately operated on. A fresh hemorrhage was aspirated, and a mulberry-like lesion consistent with a CM was excised [Figure 1d and e]. The exploration of the venous anomaly revealed a micro arteriovenous fistula in the main venous collector without evident reddish discoloration. The arterial radicles were coagulated leaving the vein intact [Figure 1d and e]. The pathological diagnosis was cavernous malformation. The postoperative course was uneventful and the left hemiparesis almost completely recovered. The absence of pre- and post-operative cerebral arteriography represents an important limit of our study. However, we thought the clear intraoperative finding could overcome this lack.
Figure 1.
Magnetic resonance imaging. (a) Axial DP sequence. (b and c) Axial SE T1-weighted after contrast administration. (d) Operative photograph showing the cavernous angioma (asterisk) fine arterial radicles (continuous arrow) entering the main venous collector of the developmental venous anomalies (dotted arrow). (e) The artistic drawing representing the cavernous angioma (asterisk) and the arterialization (continuous arrow) of the main venous channel of the developmental venous anomalies (dotted arrow)
Discussion
Changes in the hemodynamics of venous angiomas are generally due to hypertension secondary to stenosis of the main collector; alternatively, overflow and hypertension due to arterialization of the venous anomaly have been recently observed in some hemorrhagic DVAs.[2,3,4,5,6] The peculiarity of DVAs with the higher flow has been addressed by some authors[1,3,4] who suggested that high-flow DVAs represent mixed vascular malformations with arteriovenous shunting.[7] The common angiographic finding of this entity is the presence of early-filling prominent medullary veins, capillary blush, or early venous drainage instead of the normal flow of ordinary DVAs. Our case provides the first supratentorial surgical finding of the micro-arteriovenous shunt in a DVA associated with a CM. It supports the necessity of a careful inspection of the main venous collector in selected cases. Although the association CM-DVA is common, its interface is not well defined. Probably, the DVA freely communicates with the CM through the terminal radicles of the venous anomaly and extravasation of blood occurs across the endothelium of the sinusoidal channels corresponding to changes of venous pressure. The pathophysiological mechanism underlying the rupture of CMs is obscure.[2] Recently, Lekovic et al.[5] have described a case of intraoperative bleeding of a cavernous angioma after coagulation of a remote drainage vein and suggested that alterations in venous hemodynamics may precipitate the rupture of cavernomas. Our case supports the putative role of the venous system in the genesis and rupture of CMs[8] and introduces the concept of hypertensive “CM-DVA complex” and the problem of its diagnosis and treatment. It is known that increased venous pressure promotes increased angiogenic activity, which may lead to the formation of arteriovenous dural fistulas.[9] The de novo occurrence of the CM in our case may validate the role of venous hypertension and arterialization in the genesis of CMs associated with DVA. A very important issue is how this microfistula and at large, high-flow DVAs can be identified non-invasively, in view of selective use of cerebral angiography in these cases. According to Fushimi et al.,[3] a relatively higher flow reduces the amount of deoxyhemoglobin inside the DVA. in these cases, MR susceptibility-weighted imaging shows isointensity of the DVA compared with the surrounding brain, instead of the usual low-signal intensity of normal flow malformations. Perifocal edema may be an important indicator of venous engorgement in a high-flow venous malformation with aggressive hemorrhagic propensity. Some authors have emphasized the necessity of excision of venous malformations associated with cavernomas[10] owing to possible recurrence related to the hemodynamic effects of the venous anomaly. Although most neurosurgeons would warn against such a policy, the DVA exerts an active role in the pathophysiology of CMs and surgical exploration may be warranted. In selected cases of aggressive CMs (repeated hemorrhagic presentation, de novo occurrence, perifocal edema),[11] it seems necessary to closely inspect the venous malformation in search of occult A/V shunts, and it is enough to coagulate the arterial supply leaving the DVA intact.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Nakase K, Motoyama Y, Nakai T, Takeshima Y, Nakagawa I, Park YS, et al. Cavernous malformation associated with arterialized developmental venous anomaly: A case report. Neurosurgery. 2017;80:E257–62. doi: 10.1093/neuros/nyx065. [DOI] [PubMed] [Google Scholar]
- 2.Abdulrauf SI, Kaynar MY, Awad IA. A comparison of the clinical profile of cavernous malformations with and without associated venous malformations. Neurosurgery. 1999;44:41–6. doi: 10.1097/00006123-199901000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Fushimi Y, Miki Y, Togashi K, Kikuta K, Hashimoto N, Fukuyama H, et al. A developmental venous anomaly presenting atypical findings on susceptibility-weighted imaging. AJNR Am J Neuroradiol. 2008;29:E56. doi: 10.3174/ajnr.A1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Im SH, Han MH, Kwon BJ, Ahn JY, Jung C, Park SH, et al. Venous-predominant parenchymal arteriovenous malformation: A rare subtype with a venous drainage pattern mimicking developmental venous anomaly. J Neurosurg. 2008;108:1142–7. doi: 10.3171/JNS/2008/108/6/1142. [DOI] [PubMed] [Google Scholar]
- 5.Lekovic GP, Gonzalez LF, Khurana VG, Spetzler RF. Intraoperative rupture of brainstem cavernous malformation. Case report. Neurosurg Focus. 2006;21:e14. doi: 10.3171/foc.2006.21.1.15. [DOI] [PubMed] [Google Scholar]
- 6.Mamourian A, Wallace R. When is an atypical DVA an AVM? AJNR Am J Neuroradiol. 2009;30:E24. doi: 10.3174/ajnr.A1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oran I, Kiroglu Y, Yurt A, Ozer FD, Acar F, Dalbasti T, et al. Developmental venous anomaly (DVA) with arterial component: A rare cause of intracranial haemorrhage. Neuroradiology. 2009;51:25–32. doi: 10.1007/s00234-008-0456-9. [DOI] [PubMed] [Google Scholar]
- 8.Aboian MS, Daniels DJ, Rammos SK, Pozzati E, Lanzino G. The putative role of the venous system in the genesis of vascular malformations. Neurosurg Focus. 2009;27:E9. doi: 10.3171/2009.8.FOCUS09161. [DOI] [PubMed] [Google Scholar]
- 9.Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg. 1997;87:267–74. doi: 10.3171/jns.1997.87.2.0267. [DOI] [PubMed] [Google Scholar]
- 10.Wurm G, Schnizer M, Fellner FA. Cerebral cavernous malformations associated with venous anomalies: Surgical considerations. Neurosurgery. 2005;57:42–58. doi: 10.1227/01.neu.0000163482.15158.5a. [DOI] [PubMed] [Google Scholar]
- 11.Pozzati E, Acciarri N, Tognetti F, Marliani F, Giangaspero F. Growth, subsequent bleeding, and de novo appearance of cerebral cavernous angiomas. Neurosurgery. 1996;38:662–9. [PubMed] [Google Scholar]