Abstract
Purpose:
To study the significance of filling cystometry with pressure flow studies and bladder electromyography (EMG) in assessment and management of neurogenic bladder with myelopathies and evaluated neurological recovery in the follow-up period.
Methods:
The study was a 3-year prospective urodynamic study in 63 patients, with traumatic and nontraumatic myelopathy. Bladder management was advised based on the cystometric findings. Neurological recovery and mode of bladder management were evaluated during follow-up after a minimum of 6 months.
Results:
Mean age was 44.6 years (range 10–80 years). Thoracolumbar area was most commonly involved. Cystometry revealed overactive detrusor in 46 patients, (17 had detrusor sphincter dyssynergia [DSD], 29 without DSD) and areflexic/underactive detrusor in 9 patients. Postvoid residual (>15% of voided urine) was significant in 27 patients. Neurological recovery was seen in 60.3%, whereas 22.2% showed no improvement (partial improvement in 4.8%) and 12.6% had normal bladder function both initially and at follow-up. Correlation between neurological recovery and bladder management was found to be insignificant (P > 0.05) using spearman's correlation coefficient.
Conclusion:
Filling cystometry with pressure flow studies and EMG study is valuable for the assessment and management of neurogenic bladder in patients with myelopathy. In neurogenic bladder management and follow-up, pressure flow studies help to prevent complications and upper urinary tract complications.
Keywords: Compressive myelopathy, filling cystometry, neurogenic bladder, radiculopathy, spinal injury, urodynamic study
Introduction
A significant association exists between the level of a spinal cord lesion and urinary bladder/sphincter behavior. Lesions above the spinal micturition center may lead to overactive detrusor and detrusor sphincter dyssynergia (DSD) inducing reflux micturition with increased detrusor leak point pressures (DLPPs), causing incontinence, and consequent renal damage if untreated.[1] The lesions at or below the sacral micturition center produce detrusor areflexia and bladder hypotonicity causing urinary retention. Urodynamic study is a valuable investigative tool in the classification and management of neurogenic bladder dysfunction, prevention of renal function deterioration, and assessment of postoperative effects after surgical decompression in patients with compressive myelopathy because lower urinary tract symptoms are often misleading.[2,3] Studies of diagnostic accuracy have repeatedly shown the superiority of urodynamic studies over clinical profile alone.[4] Neurological tests such as perianal pinprick sensation and bulbocavernosus reflex are moderately sensitive indicators of return of bladder function after the spinal cord injury, but they are not predictive of the presence or the absence of coexistent urodynamic abnormalities.[5]
The present study was undertaken to assess the significance of filling cystometry with pressure flow study and electromyography study in the assessment and management of neurogenic bladder in myelopathies and correlate neurological recovery after surgical intervention.
Methods
This prospective study was conducted from November 2011 to October 2014 for 3 years in the Departments of Neurosurgery and Urology in Sher-i-Kashmir Institute of Medical Sciences, Kashmir. The patients with clinical features of myelo- or radiculo-pathy with computed tomography/magnetic resonance imaging evidence of compression of the spinal canal were included in the study. All age groups were included in this study. The patients having concomitant brain lesion, persisting spinal shock [Figure 1], active urinary tract infection, and bladder calculus, patients who could not be weaned off from indwelling urethral catheter were also excluded from the study. The study recruited 69 patients initially; however, only 63 patients reported on follow-up, and hence, only these 63 patients are discussed. The apparatus used for urodynamic study was fully automatic computerized urodynamic machine (MMS LIBRA + 3T, Netherland). Data were analyzed by MMS software version 7.3 (Mixed model analysis Software, Netherlands, Europe) t. All the patients were evaluated for neurological recovery as per the American Spinal Injury Association (ASIA) impairment scale before discharge.[6]
Figure 1.
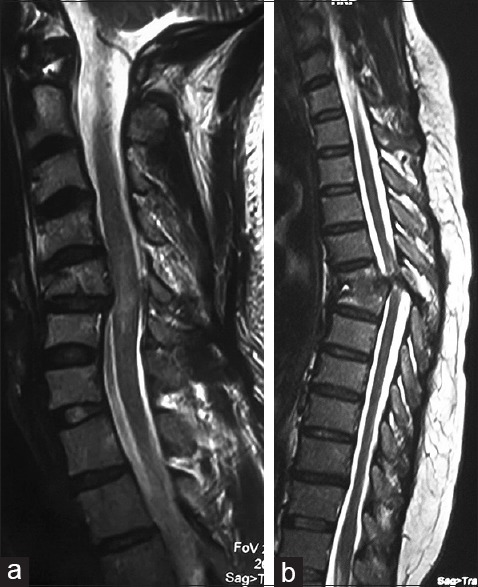
Magnetic resonance imaging T2-sagittal sections shows anterior wedge compression of C5 vertebra with cord contusion (a) and mid-dorsal fracture dislocation (b)
Procedure
Written informed consent was taken from all the patients and their attendants for their enrollment in the study and publication of this data. Prior institutional permission for the conduction and publication of study was also taken as per the institutional norms. Ambulatory and noncatheterized patients were asked to void on the commode, and spontaneous uroflowmetry recordings were obtained. Double-lumen catheter was passed into the bladder, and a rectal balloon catheter was passed into the rectum about 15 cm from the anal verge to record exact intra-abdominal pressure. Bladder inflated with saline through one of the catheter lumens. The patient was asked to cough intermittently during filling of the bladder and inhibit micturition as long as possible. Intravesical pressure was recorded through one of the lumens of double lumen catheter while as intra-abdominal pressure was recorded by means of the rectal balloon catheter. The detrusor pressure was derived by subtracting electronically the intra-abdominal pressure from the intravesical pressure. For recording pressures, symphysis pubis of the patient was considered at zero level, and all the graphs were recorded with computer-assisted video monitor [Figure 2]. Urodynamic parameters studied were maximum cystometric capacity (MCC), maximum detrusor pressure (MDP), maximum vesical pressure, first sensation to micturate, first desire to micturate, DSD, DLPP, the detrusor pressure at MCC, and postvoid residual urine volume (PVRU). A voiding velocity between 12.5 and 31.5 mL/s was taken as normal. The volume of bladder till an abrupt increase in detrusor pressure during filling phase and then dividing this volume by the detrusor pressure calculated the compliance. We defined abnormal detrusor contraction as an involuntary contraction with an increase of more than 15 cmH2O in detrusor pressure, and the normal bladder capacity was defined as bladder volume more than 300–350 ml. The patients whose compliance was higher than 15 ml/cmH2O were assigned to the normal compliance group, whereas those whose compliance was lower than 15 ml/cmH2O were assigned to the low compliance group.
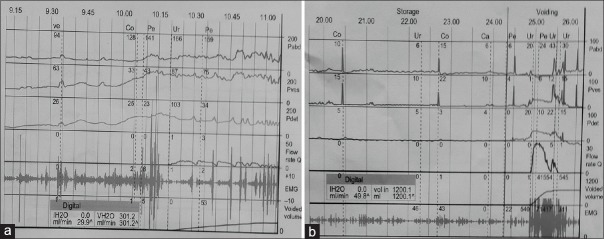
Figure 2.
Urodynamic graphs showing neurogenic detrusor overactivity and detrusor sphincter dyssynergia, (a) and poor detrusor contractility (detrusor failure) (b)
After the study, the catheter was removed, and the antibiotic cover was given for 3 days. For bedridden, patients who were already catheterized before the procedure, the catheter was kept in situ. The type of bladder dysfunction was noted. The bladder function was assessed, and function was modified with drugs, clean intermittent catheterization (CIC), and self-induced CIC. How much an operative decompression is helpful in the management of bladder dysfunction was noted. The patients with high detrusor pressures were also subjected to ultrasonography (USG) of the kidney, ureter, and bladder. An estimation of the blood urea and creatinine levels was done. A micturating cystourethrogram/intravenous urogram was performed depending on the USG findings to detect any complications requiring intervention. The patients with high bladder pressures and high residual urine volumes were put on a supervised regimen of self/attendant administered four to six hourly CIC. The use of condom catheters to avoid incontinence was actively discouraged and that of indwelling catheters was restricted. Antimuscarinic drug tolterodine 2 mg twice a day was prescribed. Combination therapy was used as indicated. These patients were periodically reviewed (every 3–6 months) with repeated urodynamic studies to measure the pressures and ensure drug compliance.
Results
The demographic profile, etiology, and presentation are given in Table 1.
Table 1.
Demographic profile and presentation of cases
| Demographic profile and presentation | n (%) |
|---|---|
| Sex | |
| Male | 42 (66.6) |
| Female | 21 (33.3) |
| Etiology | |
| Fall from height | 31 (49.2) |
| Road traffic accident | 7 (11.1) |
| Degenerative spine disease | 17 (26.9) |
| Spinal tumors | 7 (11.1) |
| Firearm injury | 1 (1.6) |
| Neurological status | |
| Normal motor power | 7 (11.1) |
| Paraparesis | 40 (63.5) |
| Paraplegia | 9 (14.3) |
| Quadriparesis | 7 (11.1) |
| Quadriplegia | 0 |
| Bladder function | |
| Urinary incontinence | 31 (49.2) |
| Retention | 24 (38.1) |
| Normal voiding pattern | 8 (12.7) |
| Level of compression | |
| Cervical | 8 (12.7) |
| Dorsal | 17 (26.9) |
| Lumbar | 33 (52.4) |
| Sacral | 5 (7.9) |
| Management | |
| Conservative | 8 (12.7) |
| Surgical | 55 (87.3) |
There were 63 patients; mean age was 44.6 years ± 16.97 (range 10–80 years) males/female ratio of 2:1. The urodynamic parameters were noted both at the time of admission and on follow-up. Pre- and post-operative values of voiding volume, PVRU, total bladder capacity (TBC), MDP, and maximum vesical pressure are given in Table 2. There was a statistically significant improvement in TBC, voided volume, and PVRU (P ≤ 0.01) [Table 2].
Table 2.
Comparison of various urodynamic parameters (mean±standard deviation) taken initially and at follow-up
| Urodynamic parameter | Preoperation | Postoperation |
|---|---|---|
| Voiding volume** | ||
| Mean (ml) | 141.6±156.9 | 277.1±144.05 |
| <200 | 63% | 0 |
| >300 | 0 | 63% |
| Postvoid residual urine** | ||
| Mean (ml) | 234.8±217.7 | 116.3±140.3 |
| >300 | 44% | 17% |
| Total bladder capacity** | ||
| Mean (ml) | 363.1±155.5 | 393.5±85.57 |
| <350 | 46% | 20% |
| 350-600 | 42% | 78% |
| >600 | 12% | 2% |
| Maximum detrusor pressure | ||
| Mean (cmH2O) | 51.3±35.18 | 58.6±30.22 |
| 0-60 | 46% | 50% |
| Low (<20) | 20% | 5% |
| High (>60) | 34% | 45% |
| Maximum vesical pressure | ||
| Mean (cmH2O) | 53.9±32.2 | 58.9±26.14 |
| <20 | 9% | 0 |
| 20-60 | 51% | 59 |
| >60 | 40% | 41% |
| Bladder compliance | 17.0±7.0 | 23.0±7.9 |
| Maximum flow rate | 10.6±5.6 | 18.7±6.5 |
**P≤0.01 which indicates significant improvement in mean voiding volume, postvoid residual urine, and total bladder capacity
There was also an improvement in bladder compliance and maximum flow rate. The mean bladder compliance improved from (17.0 ± 7.0) to (23.0 ± 7.9).
The bladder dysfunction was classified according to the detrusor activity after urodynamic study. Twenty-nine patients had overactive detrusor without detrusor sphincter dyssynergia (DSD) and 2 patients with hypoactive detrusor without nonrelaxing sphincter presented with incontinence. Totally, 17 patients with overactive detrusor with DSD and seven patients with hypoactive detrusor with nonrelaxing sphincter presented with retention [Table 3].
Table 3.
Bladder classification according to detrusor activity after urodynamic study
| Highest level of lesion | Number of cases | Overactive detrusor | Areflexic/hypoactive detrusor | Normal bladder | ||
|---|---|---|---|---|---|---|
| Without DSD | With DSD | Without nonrelaxing sphincter | With nonrelaxing sphincter | |||
| Cervical | 8 | 4 | 2 | - | - | 2 |
| Thoracic | 17 | 10 | 5 | - | 1 | 1 |
| Lumbar | 33 | 15 | 10 | 1 | 2 | 5 |
| Sacral | 5 | - | - | 1 | 4 | - |
| Total | 63 | 29 | 17 | 2 | 7 | 8 |
DSD – Detrusor sphincter dyssynergia
The bladder management initially and in the follow-up showed that in patients of myelopathy, voluntary voiding improved in all cases whatever the level of injury [Table 4]. No significant association was found between the level of spinal cord lesion and pattern of bladder recovery [Table 4].
Table 4.
Neurological status, mode of bladder management, and voluntary voiding at follow-up
| Level of lesion | Number of patients at follow-up (n=63) | Neurological improvement (ASIA score) | Neurological deterioration (ASIA score) | Mode of bladder management at follow-up | Voluntary voiding | ||
|---|---|---|---|---|---|---|---|
| Change in bladder management | No change in bladder management | Initial | Follow-Up | ||||
| Cervical | 8 | 5 | - | 2 | 4 | 2 | 4 |
| Thoracic | 17 | 12 | - | 10 | 6 | 1 | 11 |
| Lumbar | 33 | 18 | 1 | 15 | 13 | 5 | 20 |
| Sacral | 5 | 3 | 1 | 3 | 2 | 0 | 2 |
| Total | 63 | 38 | 2 | - | - | 8 | 37 |
Comparison of neurological status and mode of bladder management at follow-up shows no significant correlation using Pearson’s correlation coefficient (P=0.926). ASIA – American Spinal Injury Association
Overall, at follow-up urodynamic study, 38 (60.3%) patients showed significant improvement in bladder function, 14 (22.2%) had no improvement, 3 (4.8%) had some improvement in bladder function, whereas 8 (12.6%) patients had normal bladder function both initially and in the follow-up [Table 5].
Table 5.
The overall improvement in bladder function after the follow-up urodynamic study
| Bladder function | Number of patients (%) |
|---|---|
| Improved | 38 (60.3) |
| Nonimprovement | 14 (22.2) |
| Partial improvement | 3 (4.8) |
| Normal* | 8 (12.6) |
| Total | 63 (100) |
*Eight patients (12.6%) had normal bladder function both initially and at follow-up
Twenty-five (39%) patients showed no improvement on neurological assessment on follow-up, 36 (57%) had improvement on the ASIA scale, and two patients showed neurological deterioration. No significant association was found between neurological improvement and the pattern of bladder recovery (P = 0.925). Comparison of neurological status and mode of bladder management at follow-up shows no significant correlation using Pearson correlation coefficient.
Discussion
Bladder dysfunctions following compressive myelopathy are more precisely managed with the help of urodynamic studies.[5,7] In the present study, 63 patients with the symptoms of bladder dysfunction associated with compressive myelo- or radiculo-pathy were subjected to baseline urodynamic study and follow-up. The patients mostly presented with pain and weakness. In most of the studies, pain is more common[1,4] presentation; but in this study, 60% presented with weakness reason being majority had trauma, in which pain subsides with medication but weakness persists and also many patients present late when they develop significant motor deficits with bowel and bladder loss. In patients who presented with bladder dysfunction, incontinence was present in 49.2%, retention in 38%, and 12.7% had normal bladder function showing incontinence was more often seen than retention which is similar to other publications.[1,4]
All the patients were subjected to baseline urodynamic studies initially and repeated on follow-up after 6 months. Follow-up study showed 59% of patients who had mean vesical pressure in the range of 20–60 cmH2O, the number increased by 6%, no patients had abnormally low vesical pressure (<20), and 41% of patients had high vesical pressure (>60) suggesting that vesical pressures shifted to higher range, and all the patients with low pressures initially had improved to pressures within normal range on follow-up. Furthermore, 50% had MDP in the range of 0–60 cmH2O mean. Comparison between urodynamic study performed initially and in the follow-up showed statistically no significant improvement in maximum vesical pressure and MDP (P = 0.65) using Pearson's correlation coefficient. Cong et al. also found no statistically significant improvement in voided volume, bladder compliance, MDP, or upper urinary tract damage following treatment.[8] Wyndaele and De Taeye[9,10] et al. suggested that MDPs of 70 cmH2O or greater caused upper urinary tract damage in spinal injuries. Notably, in this study, urodynamic results were inconsistent with the clinical complaints as most of the patients presented with incontinence, urgency, and irritative symptoms, but detrusor pressures were either in the normal range or low in 66%. This emphasized the significance of performing urodynamic studies for effective bladder management in such cases as the detrusor behavior is dynamic and clinical signs/symptoms of the patients may change with time, which was shown in some other longitudinal studies.[6,11,12]
Regarding voiding phase results of TBC, 42% showed TBC of 350–600 ml which increased to 78% on follow-up, suggesting significant improvement after proper management. Initially, 63% of patients voided <200 ml of urine, but on follow-up, volume increased to >300 ml. The PVRU was initially significant in 58.4% of patients, but only 32.8% of patients showed significant residual urine in the follow-up. There was a statistically significant improvement in TBC, voided volume, and PVRU (P ≤ 0.01). There was some improvement in bladder compliance and maximum flow rate. This study results matched other studies too. Cong et al.[8] also found in their study that PVRU, MCC, and maximum flow rate improved significantly. Impaired compliance is another overlooked urodynamic parameter in cases of compressive myelopathy. Prolonged high storage pressures are known to be detrimental to renal function.[12,13,14] We concluded that patients with urodynamic parameters of reduced bladder compliance, increased maximal vesical pressures, and significant PVRU volume is potential candidates who develop renal function derangement and other related complications such as recurrent infections and stones.
Urodynamic studies performed preoperatively, and 6 months postoperatively proved that PVRU, MCC, and maximum flow rate improved significantly. There was no statistically significant improvement in voided volume, bladder compliance, MDP, and upper urinary tract damage. Our results showed significant improvement in the voided volume and some improvement in bladder compliance. Gerridzen et al.[15] found that among patients with hyperreflexic bladder, elevated voiding pressures were associated with upper tract changes, and very low cystometric capacity with high detrusor pressures was normal. Despite active bladder management, back-pressure changes were seen in 15% of cases. Chou et al.[16] have described the normal ranges for the variability of urodynamic studies in SCI neurogenic bladders, which help in determining the significance of interstudy differences.
Spinal cord lesions are known to cause neurogenic bladder dysfunction, management of which is of major importance not only for the patient but also for the caregivers. This study was found to be valuable in the diagnosis, classification, and proper bladder management according to the observations made during urodynamic studies and helps in avoiding secondary complications in the urinary tract. A significant association exists between the level of spinal cord lesion and the character of detrusor and sphincter function, which can be best elucidated by the urodynamic procedure. No significant association was found between the level of spinal cord lesion and pattern of bladder recovery in this study.
The diagnosis and the management of the neurological bladders of persons with spinal cord injury besides other deficiencies require neurological, orthopedic, and cutaneous devices because this is going to improve their quality as well as their life expectation.[17] Conservative management initially includes patient education regarding timed voiding, the Valsalva and Credé maneuvers, medications, intermittent catheterization, or persistent indwelling urinary catheter placement. Regular bladder emptying with or without anticholinergic medications is important to prevent infections, upper tract damage, and incontinence. Patients who fail conservative treatment either due to noncompliance or intolerance of side effects have various treatment options, many of which are surgical. The principle of surgical treatment is to promote storage function, decreasing outlet resistance, and achieving continence. Surgical procedures, ranging from minimally invasive to complex have been reported, attempt to relax the external sphincter with endoscopic injection of the sphincter with botulinum toxin, sphincterotomy, and stent insertion has been tried.[18,19,20,21] Literature underline the importance and the necessity to establish a protocol for the management of neurogenic bladder dysfunction and to be modified according to every patient.[22,23,24] The shortcoming with empirical bladder management for achieving a balanced bladder is that elevated intravesical pressure, responsible for the majority of urologic complications in patients with neurogenic bladder dysfunction may be clinically silent, moreover, low residual urine volume on USG cannot insure against severe urologic complications.[5,24,25,26] At the end, we concluded that urodynamic study was imperative in the diagnosis of neurogenic bladder dysfunction, prevention of renal deterioration, and assessment of postoperative effects after surgical decompression in patients with compressive myelopathy.
Conclusion
At the end we concluded that Urodynamic study was imperative in the diagnosis of neurogenic bladder dysfunction, prevention of renal deterioration, assessment of postoperative effects after surgical decompression in patients with compressive myelopathy. Filling cystometry with pressure flow studies and EMG study is valuable for assessment and management of neurogenic bladder in patients with myelopathy. In neurogenic bladder management and follow up, pressure flow studies help to prevent complications and upper tract deterioration.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Webb DR, Fitzpatrick JM, O’Flynn JD. A 15-year follow-up of 406 consecutive spinal cord injuries. Br J Urol. 1984;56:614–7. doi: 10.1111/j.1464-410x.1984.tb06129.x. [DOI] [PubMed] [Google Scholar]
- 2.Tang DH, Colayco D, Piercy J, Patel V, Globe D, Chancellor MB. Impact of urinary incontinence on health-related quality of life, daily activities, and healthcare resource utilization in patients with neurogenic detrusor overactivity. BMC Neurol. 2014;14:74. doi: 10.1186/1471-2377-14-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manack A, Motsko SP, Haag-Molkenteller C, Dmochowski RR, Goehring EL, Jr, Nguyen-Khoa BA, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn. 2011;30:395–401. doi: 10.1002/nau.21003. [DOI] [PubMed] [Google Scholar]
- 4.Dong D, Xu Z, Shi B, Chen J, Jiang X, Wang H. Urodynamic study in the neurogenic bladder dysfunction caused by intervertebral disk hernia. Neurourol Urodyn. 2006;25:446–50. doi: 10.1002/nau.20238. [DOI] [PubMed] [Google Scholar]
- 5.Shenot PJ, Rivas DA, Watanabe T, Chancellor MB. Early predictors of bladder recovery and urodynamics after spinal cord injury. Neurourol Urodyn. 1998;17:25–9. doi: 10.1002/(sici)1520-6777(1998)17:1<25::aid-nau5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 6.van Middendorp JJ, Hosman AJ, Pouw MH, Van de Meent H EM-SCI Study Group. ASIA impairment scale conversion in traumatic SCI: Is it related with the ability to walk? A descriptive comparison with functional ambulation outcome measures in 273 patients. Spinal Cord. 2009;47:555–60. doi: 10.1038/sc.2008.162. [DOI] [PubMed] [Google Scholar]
- 7.Nosseir M, Hinkel A, Pannek J. Clinical usefulness of urodynamic assessment for maintenance of bladder function in patients with spinal cord injury. Neurourol Urodyn. 2007;26:228–33. doi: 10.1002/nau.20319. [DOI] [PubMed] [Google Scholar]
- 8.Cong ML, Gong WM, Zhang QG, Sun BW, Liu SH, Li L, et al. Urodynamic study of bladder function for patients with lumbar spinal stenosis treated by surgical decompression. J Int Med Res. 2010;38:1149–55. doi: 10.1177/147323001003800344. [DOI] [PubMed] [Google Scholar]
- 9.Wyndaele JJ, De Taeye N. Early intermittent self-catheterisation after spinal cord injury. Paraplegia. 1990;28:76–80. doi: 10.1038/sc.1990.9. [DOI] [PubMed] [Google Scholar]
- 10.Wyndaele JJ, Madersbacher H, Kovindha A. Conservative treatment of the neuropathic bladder in spinal cord injured patients. Spinal Cord. 2001;39:294–300. doi: 10.1038/sj.sc.3101160. [DOI] [PubMed] [Google Scholar]
- 11.Patki P, Woodhouse J, Hamid R, Shah J, Craggs M. Lower urinary tract dysfunction in ambulatory patients with incomplete spinal cord injury. J Urol. 2006;175:1784–7. doi: 10.1016/S0022-5347(05)00979-1. [DOI] [PubMed] [Google Scholar]
- 12.Generao SE, Dall’era JP, Stone AR, Kurzrock EA. Spinal cord injury in children: Long-term urodynamic and urological outcomes. J Urol. 2004;172:1092–4. doi: 10.1097/01.ju.0000135402.56109.cf. [DOI] [PubMed] [Google Scholar]
- 13.McGuire EJ. The role of urodynamic investigation in the assessment of benign prostatic hypertrophy. J Urol. 1992;148:1133–6. doi: 10.1016/s0022-5347(17)36841-6. [DOI] [PubMed] [Google Scholar]
- 14.McGuire EJ, Woodside JR, Borden TA, Weiss RM. Prognostic value of urodynamic testing in myelodysplastic patients. J Urol. 1981;126:205–9. doi: 10.1016/s0022-5347(17)54449-3. [DOI] [PubMed] [Google Scholar]
- 15.Gerridzen RG, Thijssen AM, Dehoux E. Risk factors for upper tract deterioration in chronic spinal cord injury patients. J Urol. 1992;147:416–8. doi: 10.1016/s0022-5347(17)37254-3. [DOI] [PubMed] [Google Scholar]
- 16.Chou FH, Ho CH, Chir MB, Linsenmeyer TA. Normal ranges of variability for urodynamic studies of neurogenic bladders in spinal cord injury. J Spinal Cord Med. 2006;29:26–31. doi: 10.1080/10790268.2006.11753853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CW, Attar KH, Gall A, Shah J, Craggs M. The relationship between bladder management and health-related quality of life in patients with spinal cord injury in the UK. Spinal Cord. 2010;48:319–24. doi: 10.1038/sc.2009.132. [DOI] [PubMed] [Google Scholar]
- 18.Schurch B, Stöhrer M, Kramer G, Schmid DM, Gaul G, Hauri D. Botulinum-A toxin for treating detrusor hyperreflexia in spinal cord injured patients: A new alternative to anticholinergic drugs. Preliminary results? J Urol. 2000;164(3 Pt 1):692–7. doi: 10.1097/00005392-200009010-00018. [DOI] [PubMed] [Google Scholar]
- 19.Cruz F, Herschorn S, Aliotta P, Brin M, Thompson C, Lam W, et al. Efficacy and safety of onabotulinumtoxinA in patients with urinary incontinence due to neurogenic detrusor overactivity: A randomised, double-blind, placebo-controlled trial. Eur Urol. 2011;60:742–50. doi: 10.1016/j.eururo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Reitz A, Stöhrer M, Kramer G, Del Popolo G, Chartier-Kastler E, Pannek J, et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol. 2004;45:510–5. doi: 10.1016/j.eururo.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Schurch B, de Sèze M, Denys P, Chartier-Kastler E, Haab F, Everaert K, et al. Botulinum toxin type a is a safe and effective treatment for neurogenic urinary incontinence: Results of a single treatment, randomized, placebo controlled 6-month study. J Urol. 2005;174:196–200. doi: 10.1097/01.ju.0000162035.73977.1c. [DOI] [PubMed] [Google Scholar]
- 22.Lioyd LK. Long term follow up of neurogenic bladder. Phys Med Rehabil Clin N Am. 1993;4:391–409. [Google Scholar]
- 23.Trop CS, Bennett CJ. Autonomic dysreflexia and its urological implications: A review. J Urol. 1991;146:1461–9. doi: 10.1016/s0022-5347(17)38140-5. [DOI] [PubMed] [Google Scholar]
- 24.Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–74. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 25.Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: Uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–74. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 26.Abrams P. Urodynamic investigation led to new criteria for the selection of patients for operative treatment. J Urol. 1983;147:316. [Google Scholar]