Abstract
Background:
Surgical site infection (SSI) after instrumented spinal surgery is one of the most serious complications in spite of the routine use of prophylactic intravenous (IV) antibiotics. Many studies have suggested that intrawound vancomycin powder, applied during the intraoperative period, may decrease the incidence of SSI after surgery. However, the appropriate dose of vancomycin has not yet been reported.
Purpose:
The purpose of the study is to compare between the use of 1 g and 2 g intrawound vancomycin powder and to find out which of these two groups can reduce the rate of deep wound infection in posterior instrumented thoracic or lumbosacral spine surgery.
Materials and Methods:
The preliminary study was conducted from July 2013 to July 2015 at Lerdsin Hospital. A total of 400 patients were enrolled in the study, and their individual demographics were recorded. All patients underwent posterior instrumented thoracic or lumbosacral spine surgery. Of these, 131 patients received IV cefazolin and 2 g of vancomycin powder intrawound application, 134 patients received 1 g of intrawound vancomycin powder in addition to IV cefazolin, and 135 patients were given only IV cefazolin and were assigned as the control group.
Results:
One hundred and thirty-one patients were treated with posterior instrumented thoracic or lumbosacral fusions using IV cefazolin and adjuvant 2 g of intrawound vancomycin powder. Five patients in this group developed deep infections (3.8%). One hundred and thirty-four patients were treated with posterior instrumented thoracic or lumbosacral fusions using IV cefazolin and adjuvant 1 g of intrawound vancomycin powder. Of these, four patients developed deep infections (2.98%). One hundred and thirty-five patients in the control group were treated with posterior instrumented thoracic or lumbosacral using only IV cefazolin as prophylaxis. Of these, four patients developed deep infections (2.96%). Coagulase-negative staphylococcus was the most common isolated organism. There were no adverse clinical outcomes or wound complications due to local application of vancomycin powder.
Conclusion:
The preliminary result could not state the relation of intrawound vancomycin powder to the deep infection; further study with adequate sample size is required.
Keywords: Postoperative spinal infection, spine arthrodesis, spine surgery, vancomycin, vancomycin powder
Introduction
Surgical site infection (SSI) is one of the most serious unaccepted complications in spinal surgery, especially in spinal arthrodesis. This condition is associated with an increase in morbidity, mortality, and health care costs.[1] The incidence of SSI after spinal surgery has been reported ranging from 0.3% to 20%. Risk factors of SSI include diabetes mellitus, obesity, tobacco used, previous spinal surgery, prolonged operative time, and high blood loss.[2] Administration of intravenous (IV) antibiotics within 60 min before making a skin incision could decrease the risk of infection,[3] but some patients still have a chance of postoperative infection.
Sweet et al.[4] reported that the prophylactic application of intraoperative vancomycin powder has been shown to lower the infection risk after posterior instrumented thoracolumbar arthrodesis. Reasons for choosing vancomycin as an intrawound antibiotic application were because of comfortable use in the powder form, broad spectrum, and effectiveness in coverage against the organisms such as methicillin-resistant Staphylococcus aureus, which is a common organism in SSI in spinal surgery.
Currently, there is no obvious treatment guideline with regard to the dose of intrawound vancomycin to prevent SSI in spinal surgery. Suggested dose of vancomycin powder from previous spinal literatures was between 1 g and 2 g, but no definite dose was recommended. Therefore, our study aimed to determine the effective dose between 1 g and 2 g of intrawound vancomycin powder in posterior instrumented thoracic or lumbosacral spine surgery. We focused on deep SSI because the patients were found to be at greater risk for morbidity and had a longer length of hospital stay.
Materials and Methods
Four hundred patients, who underwent instrumented posterior thoracic or lumbosacral spine surgery in Lerdsin hospital between July 2013 and July 2015, were considered for inclusion. Patient demographics were recorded including age, gender, underlying disease, BMI, and history of alcohol, drug, or tobacco use. Patients who had open injuries, history of SSI, current infection, postoperative follow-up time <3 months, history of vancomycin allergy, and patients who rejected consent were excluded from the study. The patients who are unwilling to participate in the study were treated as usual (with or without vancomycin depending on surgeon preference). The study was approved by Institutional Review Board of Lerdsin hospital (0306/12/177).
Details of the procedure were as follows:
All patients received preoperative IV antibiotic within 60 min of the surgical incision for routine infection prophylaxis. 1 g of cefazolin was administered to all patients who did not have evidence of penicillin allergy; otherwise, clindamycin 600 mg was administered
Before incision was made, patients’ skin were prepared with povidone–iodine solutions
A standard midline incision and open approach was performed in all cases
Number of fusion levels was determined based on the quality of bone and stability
Before skin closure, the wound was irrigated with 3 L of normal saline
-
Allocation concealment was done using opaque envelopes. Allocation was assigned after performing surgery, just before skin closure. All patients were randomized into three groups, using box of six technique as follows:
- Group 1: IV antibiotic and 2 g of vancomycin powder intrawound application
- Group 2: IV antibiotic and 1 g of vancomycin powder intrawound application
- Group 3: IV antibiotic only.
Vancomycin powder was spread throughout the surgical wound [Figures 1 and 2]
Figure 1.

A half of vancomycin powder mixed with autogenous bone graft
Figure 2.
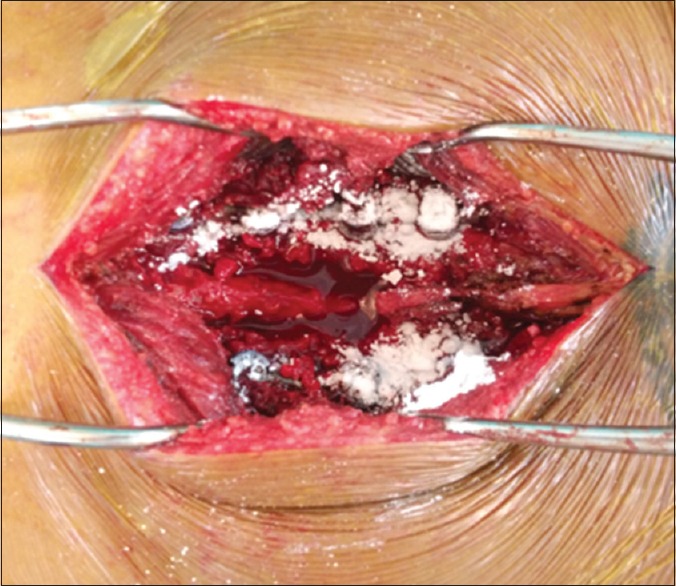
A half of vancomycin spread throughout the surgical wound in Group 1 and Group 2
Subfascial drain was applied
The wound was closed with absorbable suture in the fascia and subcutaneous layers. Skin was closed with nonabsorbable suture
The operative time, intraoperative complications, and estimated blood loss were recorded
There were no patients receiving intraoperative redosing of IV antibiotic because the duration of the surgery and the amount of blood loss did not exceed the recommendation for redosing in all cases
Standard postoperative care was performed. IV antibiotic was switched to oral antibiotic after drain removal. All patients received oral antibiotic either dicloxacillin 500 mg four times per day or clindamycin 300 mg 3 times per day for 7 days
A proper wound dressing was applied on the 3rd day of the operation
Removal of surgical drain occurred at ward under aseptic technique when drainage became <30 ml/day.
Deep wound infection was diagnosed using Guideline for Prevention of SSI; CDC 1999 Infection occurs within 30 days after the operation.[5]
Diagnosis of deep wound infection was made when infection involves deep soft tissues of the incision and at least one of the following:
Purulent drainage from the deep incision but not from the organ/space component of the surgical site.
A deep incision spontaneously dehisces or is deliberately opened by a surgeon when the patient has at least one of the following signs or symptoms: fever (>38°C), localized pain, or tenderness, unless site is culture negative
An abscess or other evidence of infection involving the deep incision is found on direct examination, during reoperation, or by histopathologic or radiologic examination
Diagnosis of a deep incisional SSI by a surgeon or attending physician.
Assessors (K. Sombat, P. Chaiwat, P. Pritsania, and P. Tinnakorn) were assessor of SSI. All agreement would be obtained before diagnosis of SSI.
Statistical analysis
The authors calculated the sample size based on the study of Sweet et al.[4] Four hundred and fifty-five patients were needed in each group with a power of 80% and an alpha error of 0.05. Statistical analysis was done using Chi-square or exact test (SPSS v. 22.0 IBM Corp, USA).
Results
Four hundred patients were included in the study. Diagnosis of the patients included trauma (e.g., spinal fracture), degenerative (e.g., cervical spondylotic myelopathy, spinal stenosis, spondylolisthesis, herniated nucleus pulposus), congenital (e.g., scoliosis), tumor (e.g., metastasis), and infection (i.e., tuberculosis). There were 131 patients who underwent posterior instrumented thoracic or lumbosacral fusions with intravenous cefazolin and adjuvant intrawound of 2 g of vancomycin. There were 134 patients in 1 g vancomycin group. The remaining patients (135 patients) underwent posterior instrumented thoracic or lumbosacral fusions with only IV cefazolin. There was no dropout patient in the study.
All groups were similar in demographic data [Table 1]. The average age at the time of surgery was 53.24 years in 2 g of vancomycin group, 51.03 years in 1 g of vancomycin group, and 55.18 years in the control group. Gender (M/F) ration in 2 g vancomycin group was 57/74, in 1 g vancomycin group was 56/78, and in the control group was 49/86. Mean BMI of patients was 27.0, 28.9, and 25.8 in 2 g, 1 g, and the control group, respectively. History of smoking and alcohol use accounted for 3% in 2 g group, 3% in 1 g group, and 5% in the control group.
Table 1.
Demographic data
| 2 g | 1 g | Control | |
|---|---|---|---|
| Mean age (range) | 53.24 (14-82) | 51.03 (11-78) | 55.18 (15-79) |
| Male | 57 | 56 | 49 |
| Female | 74 | 78 | 86 |
| BMI | 27 | 28.9 | 25.8 |
| Smoking and alcohol use (%) | 3 | 3 | 5 |
| Diabetes (%) | 6.1 | 8.2 | 8.1 |
| Hypertension (%) | 23.7 | 20.1 | 22.2 |
| Dyslipidemia (%) | 19.6 | 14.1 | 13.3 |
| Others (%) | 5.3 | 5.9 | 2.9 |
| Number of levels | 3.94 | 3.96 | 3.74 |
| Serum albumin, mean (SD) | 3.2 (1.2) | 3.2 (0.8) | 3.5 (1.3) |
| Estimated blood loss (ml) | 450 | 470 | 440 |
| Operative time (min) | 151 | 136 | 156 |
BMI - Body mass index; SD - Standard deviation
Regarding the comorbidities of the patients, in 2 g vancomycin group, the patients had diabetes 6.1%, hypertension 23.7%, and dyslipidemia 19.6%. The patients in 1 g vancomycin group had diabetes 8.2%, hypertension 20.1%, and dyslipidemia 14.1%. In the control group were diabetes 8.1%, hypertension 22.2%, and dyslipidemia 13.3%.
Average number of fusion levels in 2 g vancomycin group, 1 g vancomycin group, and the control group was 3.94, 3.96, and 3.74, respectively.
Mean serum albumin was 3.2 (standard deviation [SD] = 1.2), 3.2 (SD = 0.8), and 3.5 (SD = 1.3) in 2 g, 1 g, and the control group, respectively.
Estimated blood loss was 450 ml, 470 ml, and 440 ml in 2 g, 1 g, and the control group, respectively.
Among 400 patients, 13 patients (3.25%) developed deep SSI. Rates of infection were present in 5 patients (3.9%), 4 patients (2.98%), and 4 patients (2.96%) in 2 g of vancomycin, 1 g of vancomycin, and the control group, respectively. There was no statistical significance in rate of infection between three groups (exact test P value = 0.883). There were no adverse clinical outcomes or wound complications due to local application of vancomycin. Most organisms found in infected patients were Staphylococci spp. [Table 2], and time to diagnosis of deep wound infection was 15 days after surgery (early in 4 days and late in 31 days).
Table 2.
Details of infected cases
| Group | Age (years old) | Sex | Comorbidity | Diagnosis | Fusion levels | Organisms (from tissue culture) |
|---|---|---|---|---|---|---|
| 2 g | 61 | Male | HT | Spinal canal stenosis | 5 | P. aeruginosa |
| 2 g | 58 | Male | - | Spinal canal stenosis | 5 | NG |
| 2 g | 63 | Male | - | Spinal canal stenosis | 7 | NG |
| 2 g | 30 | Male | - | Burst fracture L2, L3 | 4 | Staphylococcus spp. |
| 2 g | 55 | Female | - | Spinal canal stenosis | 5 | Diphtheroid spp. |
| 1 g | 70 | Female | - | Spinal canal stenosis | 3 | S. aureus |
| 1 g | 69 | Female | HT, DM, DLP | Spinal canal stenosis | 6 | NG |
| 1 g | 70 | Female | - | Spinal canal stenosis | 3 | S. aureus |
| 1 g | 52 | Male | - | Spinal metastasis (lung cancer) | 5 | NG |
| Control | 63 | Male | HT | Spinal canal stenosis | 4 | NG |
| Control | 69 | Male | HT | Spinal canal stenosis | 3 | Staphylococcus spp. |
| Control | 75 | Female | HT | Spinal canal stenosis | 4 | NG |
| Control | 61 | Female | - | Spinal canal stenosis | 3 | NG |
HT - Hypertension; DM - Diabetes mellitus; DLP - Dyslipidemia; NG - No growth; P. aeruginosa - Pseudomonas aeruginosa; S. aureus - Staphylococcus aureus
In all of the three groups, pseudarthrosis occurred in one patient (1 g of vancomycin group). The patient was primary diagnosed as ankylosing spondylitis with three-column fracture at the twelfth thoracic spine. At that time, he underwent posterior spinal fusion from T10 to L3.
Discussion
From the previous studies, the postoperative infection following spinal surgery is an important complication. The incidence ranges from 0.5% to 18%.[6] The most common organism is S. aureus even though most of the patients receive prophylactic IV antibiotics before undergoing surgery. There are still postoperative infections in some patients. This is because prophylactic IV cefazolin can cover staphylococcus organisms <50%.[7]
Currently, many surgeons try to minimize the incidence of postoperative infection following spinal surgery. A widely used method is intrawound application of antibiotics mixing with polymethylmethacrylate. With regard to this method, it was first introduced in 1970 in Germany[8] and was applied among patients with infected open fractures or osteomyelitis.[9,10,11,12,13] Such treatment has been believed that the antibiotics can directly be delivered to the body without passing through the blood circulation. The advantages include attaining high dose of drug concentration as well as minimizing systemic toxicity resulting from IV administration.[14] In the recent years, it has been applied in spinal surgery. Molinari et al. reported that among 1512 consecutive spinal surgery cases, the use of 1 g of powdered intraoperative vancomycin placed in the wound before wound closure appears to be associated with a low rate deep spinal wound infection for both instrumented and uninstrumented cases.[15]
At present, there are still no standard treatment guidelines for intrawound application of vancomycin powder in terms of prevention of SSI as well as standard dose.[6,7] The previous studies have shown the results of both 1 g and 2 g of vancomycin powder in prophylactic SSI. Sweet et al. reported that intrawound application of 2 g vancomycin power for preventing SSI in 1732 consecutive thoracic and lumbar posterior instrumented spinal fusion, compared with patients who received IV cephalexin alone. The average follow-up was 2.5 years. The results showed 0.2% and 2.6% of deep wound infection in patients with intrawound application of 2 g vancomycin power and without intrawound application, respectively. They concluded that adjunctive local application of vancomycin powder decreased the postsurgical wound infection rate with statistical significance (P < 0.0001).[4]
In addition, O’Neil et al.[16] reviewed 110 patients with traumatic spine injuries treated with instrumented posterior spine fusion. A statistically significant difference in infection rate was found between the patients who received vancomycin powder in the surgical wound in addition to systemic prophylaxis (0%) and patients who received standard systemic prophylaxis only (13%, P = 0.02).
The recent study has shown that intrawound application of 2 g of vancomycin powder (n = 131), 1 g of vancomycin powder (n = 134), and without application of vancomycin powder (n = 135) resulted in no statistically significant difference of SSI (4%, 3%, and 4%, respectively).
The strength of the present study was its first prospective randomized controlled design to compare the dose of vancomycin powder in the intrawound application. However, our study has several limitations. The current study is still the preliminary report; so, a limited number of patients were included for statistical analysis. Many factors contribute to postoperative infections. There were no adverse side effects attributed to the local vancomycin powder. Additional prospective and large-volume studies are needed to further substantiate the effectiveness of this prophylactic method for minimizing postoperative spinal infection.
Conclusion
The preliminary study could not demonstrate the benefit of intrawound vancomycin in reducing the incidence of deep wound infection in patients who underwent instrumented thoracic or lumbosacral spine surgery regardless of the dose application, which may result from lack of power due to inadequacy of sample size. Further study is still in the process to analyze more data.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27:171–82. [PubMed] [Google Scholar]
- 2.Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: A survey of 850 spinal procedures. J Spinal Disord. 1998;11:124–8. [PubMed] [Google Scholar]
- 3.Milstone AM, Maragakis LL, Townsend T, Speck K, Sponseller P, Song X, et al. Timing of preoperative antibiotic prophylaxis: A modifiable risk factor for deep surgical site infections after pediatric spinal fusion. Pediatr Infect Dis J. 2008;27:704–8. doi: 10.1097/INF.0b013e31816fca72. [DOI] [PubMed] [Google Scholar]
- 4.Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: Efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976) 2011;36:2084–8. doi: 10.1097/BRS.0b013e3181ff2cb1. [DOI] [PubMed] [Google Scholar]
- 5.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital infection control practices advisory committee. Infect Control Hosp Epidemiol. 1999;20:250–78. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 6.Chahoud J, Kanafani Z, Kanj SS. Surgical site infections following spine surgery: Eliminating the controversies in the diagnosis. Front Med (Lausanne) 2014;1:7. doi: 10.3389/fmed.2014.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 8.Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with palacos resins. Chirurg. 1970;41:511–5. [PubMed] [Google Scholar]
- 9.Ostermann PA, Seligson D, Henry SL. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J Bone Joint Surg Br. 1995;77:93–7. [PubMed] [Google Scholar]
- 10.Moehring HD, Gravel C, Chapman MW, Olson SA. Comparison of antibiotic beads and intravenous antibiotics in open fractures. Clin Orthop Relat Res. 2000;(372):254–61. doi: 10.1097/00003086-200003000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Gitelis S, Brebach GT. The treatment of chronic osteomyelitis with a biodegradable antibiotic-impregnated implant. J Orthop Surg (Hong Kong) 2002;10:53–60. doi: 10.1177/230949900201000110. [DOI] [PubMed] [Google Scholar]
- 12.Klemm KW. Antibiotic bead chains. Clin Orthop Relat Res. 1993;(295):63–76. [PubMed] [Google Scholar]
- 13.Picknell B, Mizen L, Sutherland R. Antibacterial activity of antibiotics in acrylic bone cement. J Bone Joint Surg Br. 1977;59:302–7. doi: 10.1302/0301-620X.59B3.408356. [DOI] [PubMed] [Google Scholar]
- 14.Hanssen AD, Osmon DR, Patel R. Local antibiotic delivery systems: Where are we and where are we going? Clin Orthop Relat Res. 2005;(437):111–4. [PubMed] [Google Scholar]
- 15.Molinari RW, Khera OA, Molinari WJ., 3rd Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J. 2012;21(Suppl 4):S476–82. doi: 10.1007/s00586-011-2104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Neill KR, Smith JG, Abtahi AM, Archer KR, Spengler DM, McGirt MJ, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011;11:641–6. doi: 10.1016/j.spinee.2011.04.025. [DOI] [PubMed] [Google Scholar]


