Abstract
Background
Wogonin (5,7-dihydroxy-8-methoxyflavone), one of flavonoids isolated from the Scutellaria baicalensis, has been regarded as an anticancer candidate because of its maximal efficacy in cancer cells. This study aimed to explore the possible mechanism that wogonin uses to enhance the sensitivity of ovarian cancer cells to cisplatin chemotherapy.
Material/Methods
The growth inhibition rates of ovarian cancer cells SKOV3/DDP and C13* were assessed by Cell Counting Kit-8 (CCK-8) assay. The apoptosis was assessed under a fluorescence microscope following staining with Hoechst. We further analyzed the expression of Bcl-2, cleaved caspases-3, cleaved-PARP, and phospho-Akt by western blotting.
Results
In the present study, we found that wogonin reduced proliferation of ovarian cancer cells SKOV3, SKOV3/DDP, OV2008, and C13* in dose- and time-dependent manners and it sensitized cisplatin-induced cytotoxicity. Moreover, treatment with wogonin also increased cisplatin-resistant SKOV3/DDP and C13* cells to low dose cisplatin-induced cell apoptosis. Additionally, such treatment resulted in a significant decrease in phosphorylated Akt.
Conclusions
Wogonin could significantly increase the sensitivity of cisplatin-resistant ovarian cancer cells to cisplatin by downregulating the PI3K/Akt pathway.
MeSH Keywords: Antineoplastic Agents, Cisplatin, Ovarian Neoplasms
Background
Ovarian cancer is a leading cause of cancer-related deaths in gynecological malignancies. It estimates that 239 000 women were diagnosed and 152 000 women died from ovarian cancer worldwide [1]. The high mortality is due to the fact that ovarian cancer is frequently diagnosed at advanced stages because of its asymptomatic nature. The standard treatment for ovarian cancer is surgical resection of visible disease followed by platinum and paclitaxel adjuvant chemotherapy [2]. However, a major obstacle in the treatment of ovarian cancer is the intrinsic or acquired resistance to cisplatin-based chemotherapy which results in early recurrence and death. Therefore, the combination of novel therapeutic agents with cisplatin has been recognized as a promising strategy to overcome cisplatin resistance in ovarian cancer.
Wogonin (5,7-dihydroxy-8-methoxyflavone) is one of flavonoids isolated from the root of the medicinal herb Scutellaria baicalensis Georgi [3]. In addition to its anti-inflammatory, antioxidant, and antiviral activities, wogonin also has cytostatic and proapoptotic effects on several cancer cell lines, such as hepatocellular carcinoma cell line SK-HEP-1, prostate cancer cell line LNCaP, and ovarian cancer cell line A2780 [4–6]. Wogonin has been regarded as an anticancer candidate because of its maximal efficacy in cancer cells and its low toxicity to normal tissues. Besides its antitumor property, wogonin is able to sensitize cisplatin-induced apoptosis in both non-small cell lung cancer cells and the cervical cancer cells [7]. It also reverses doxorubicin resistance of breast cancer cells by regulating the IGF-1R/AKT signaling pathway [8]. In addition, it was shown to enhance the efficacy of low doses of fluorouracil (FU) without increasing toxicity in a nude mouse model of gastric cancer [9]. However, whether wogonin can increase the chemosensitivity of cisplatin in ovarian cancer is still undefined.
Activation of the oncogenic phosphatidylinositol 3-kinase (PI3K)-Akt pathway has been reported in various malignancies. In this signaling cascade, the PI3Ks phosphorylate phosphatidylinositol 4,5-bisphosphate at the 3-OH of the inositol ring to generate phosphatidylinositol 3,4,5-trisphosphate that results in the phosphorylation of Akt, a serine/threonine kinase, which in turn activates several downstream targets to regulate multiple biological processes including cell proliferation, differentiation and survival [10]. Aberrant activation of this pathway is clearly associated with tumorigenesis, cancer progression, and drug resistance. Phosphatase and tensin homologue (PTEN) and INPP4B antagonize PI3K activity by dephosphorylating PIP3 [11]. Previous studies have reported that inactivation of PI3K/AKT signaling sensitizes ovarian cancer cells to cisplatin [12]. Thus, novel agents that block PI3K/AKT signaling with proven efficacy and minimal toxicity are urgently required to overcome cisplatin resistance in ovarian cancer.
In this study, the effects of wogonin and cisplatin in a combination on cisplatin-resistant SKOV3/DDP and C13* ovarian cancer cells were investigated. The expression of phosphorylated AKT was determined in both cisplatin-resistant and primary ovarian cancer cells. The effects of wogonin, both alone and in combination with an AKT inhibitor LY294002, were observed. In addition, we further explored the mechanisms of increasing cisplatin sensitivity by wogonin, in an effort to develop novel clinical strategies against cisplatin-resistant ovarian cancer.
Material and Methods
Reagents
Wogonin, obtained from Sigma-Aldrich (St. Louis, MO, USA), was dissolved in 5% dimethylsulfoxide (DMSO) in sterile distilled H2O. Wogonin was mixed on a magnetic stirrer for at least 30 minutes prior to application. Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO, USA). Cell Counting Kit-8 (CCK-8) and Hoechst 33258 were purchased from Beyotime Biotechnology (Shanghai, China).
Cell lines and culture
The human cisplatin-sensitive ovarian cancer cells SKOV3 was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and its cisplatin-resistant counterpart SKOV3/DDP was obtained from National Infrastructure of Cell Line Resource (Beijing, China). The cisplatin-sensitive ovarian cancer cell line OV2008 and its cisplatin-resistant counterpart C13* were obtained from Chinese Type Culture Collection, Chinese Academy of Sciences. Cells were maintained in RPMI-1640 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in a humidified atmosphere with 5% CO2. All the cell lines routinely tested negative for mycoplasma contamination.
CCK-8 assay
The growth inhibition rates of ovarian cancer cells were assessed by CCK-8 assay. Briefly, cells were cultured in a 96-well plate at a density of 100 000 cells/mL. Each well contained 10 000 cells in a total volume of 100 μL. CCK-8 assay was performed after cisplatin or wogonin treatment for 24, 48, and 72 hours. Then 10 μL CCK-8 was added in each well, after incubating at 37°C for 2 hours, the absorbance was measured at 450 nm with a Multiskan™ GO microplate reader (Thermo Scientific).
IC50 of cisplatin on ovarian cancer cells
The median concentration of inhibition (IC50) values of cisplatin for SKOV3, SKOV3/DDP, OV2008, and C13* were assessed by CCK8 assay. The concentration series of cisplatin adopted were 1.25, 2.5, 5, 10, 20, 40, and 80 μg/mL. After 48 hours treatment, the inhibition rate was assessed using CCK-8 assay previously described. IC50 was calculated by probit regression (SPSS 17.0 software) according to the concentration of cisplatin and the inhibition rate.
Hoechst assay
Cells grown on coverslips in 6-well plates were exposed to cisplatin and wogonin, individually or in combination for 48 hours, then incubated with Hoechst 33258 for 30 minutes at 37°C. Fluorescence microscopy was used to observe cancer cell morphology captured from 6 random visual fields. Apoptotic cells were defined as cells showing nuclear and cytoplasmic shrinkage, chromatin condensation, and nuclear fragmentation. The ratio of apoptotic cells to total cell number was also calculated.
Western blots
Western blots were performed using primary antibodies: anti-caspase-3 (sc-7148, Santa Cruz), anti-cleaved caspase-3 (#9661, Cell Signaling Technology), anti-bcl-2 (sc-492, Santa Cruz), anti-Akt (ab32505, Abcam), anti-phospho-Akt (sc-7985-R, Santa Cruz), anti-PARP (#9542, Cell Signaling Technology) and anti-cleaved PARP (#9548, Cell Signaling Technology) antibody. The anti-β-actin (sc-47778, Santa Cruz) antibody was diluted to 1: 1000 for use as a sample loading control.
Statistical analysis
Statistical analysis was performed using the software package SPSS Version 17.0 (SPSS Inc., Chicago, IL, USA). Student’s t-tests were used to determine statistical significance of differences between experimental groups. P-value of less than 0.05 was considered significant. Graphs were created with GraphPad Prism Version 6.01 (GraphPad Software, Inc., USA).
Results
Wogonin inhibited the proliferation of ovarian cancer cells
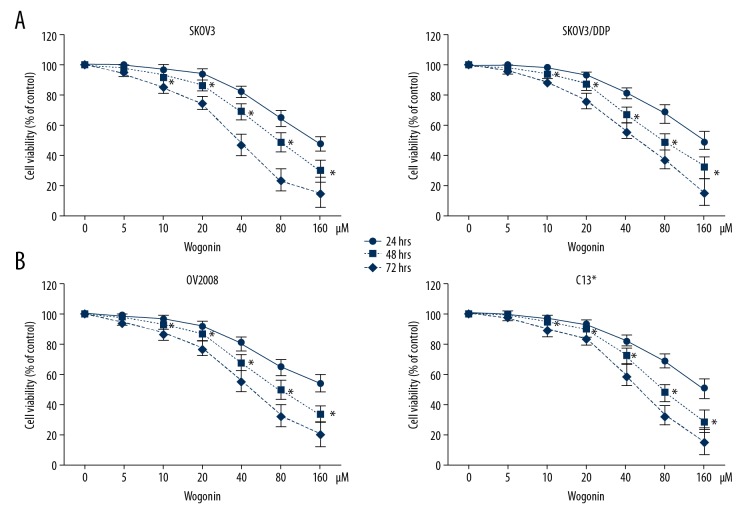
CCK-8 assay was performed to assess the antiproliferative effects of wogonin on ovarian cancer cell growth. SKOV3, SKOV3/DDP, OV2008, and C13* ovarian cancer cell lines were incubated with wogonin at different concentrations (0, 5, 10, 20, 40, 80, and 160 μM) for 24, 48, and 72 hours. Wogonin inhibited the growth of SKOV3, SKOV3/DDP, OV2008, and C13* cells in dose- and time-dependent manners. The dose-effect curve exhibited that 5 μM wogonin displayed no significant antiproliferative effects on SKOV3 and SKOV3/DDP cells (survival rate was 98.4% and 99.0%, respectively); 10 and 20 μM wogonin displayed weak antiproliferative effects (survival rate was 92.9% and 86.3% in SKOV3, and 92.3% and 87.7% in SKOV3/DDP, respectively) at 48 hours (Figure 1A). Similar phenomena were also observed in OV2008 and C13* cells. The survival rates of OV2008 were 97.9%, 92.5%, and 86.0% treated by 5, 10, and 20 μM wogonin at 48 hours, respectively. In C13* cells when treated by 5, 10, and 20 μM wogonin, the survival rates were 99.3%, 94.3%, and 89.4% at 48 hours, respectively (Figure 1B).
Figure 1.
Wogonin inhibits the proliferation of ovarian cancer cells. (A) The cell viability of SKOV3 and SKOV3/DDP cells treated with wogonin was assessed using CCK-8 assay at 24, 48, and 72 hours. The cell viability decreased with the elevated concentration of wogonin. (B) The cell viability of OV2008 and C13* cells treated with wogonin were assessed using CCK-8 assay at 24, 48, and 72 hours. The cell viability decreased with the elevated concentration of wogonin. * P<0.05, compared to 0 μM wogonin. CCK-8 – cell counting Kit-8.
Wogonin increased cisplatin cytotoxicity in cisplatin-resistant ovarian cancer cells
The IC50 values of cisplatin for SKOV3 and SKOV3/DDP cells were 7.7 and 26.8 μg/mL, and those for OV2008 and C13* cells were 9.8 and 29.6 μg/mL at 48 hours, respectively (Figure 2A, 2B; Table 1). The IC30 values of cisplatin for SKOV3/DDP (10.2 μg/mL) and C13* (11.1 μg/mL) cells were also calculated (Figure 2A, 2B). We further explored whether wogonin increased cisplatin cytotoxicity in SKOV3/DDP and C13* cells when treated by indicated concentrations of wogonin and cisplatin. The IC50 values of cisplatin for SKOV3/DDP and C13* cells were changed to 11.2 and 13.9 μg/mL treated with 20 μM wogonin at 48 hours (Table 1). The survival rates of SKOV3/DDP and C13* cells were 40.6% and 37.4% when treated by wogonin (20 μM) and cisplatin (IC50) in combination at 48 hours, respectively. Compared with SKOV3/DDP (50.4%) or C13* (58.2%) cells treated by cisplatin (IC50) alone, the proliferation rates showed no difference when treated by wogonin (20 μM) and cisplatin (IC30) in combination (Figure 2C, 2D). These results showed that the wogonin could significantly enhance the sensitive of SKOV3/DDP and C13* cells to cisplatin.
Figure 2.
Wogonin increased cisplatin cytotoxicity in cisplatin-resistant ovarian cancer cells. (A) The IC50 values of cisplatin for SKOV3 and SKOV3/DDP were assessed by CCK-8 assay. The IC50 values of cisplatin for SKOV3 and SKOV3/DDP cells were 7.7 μg/mL and 26.8 μg/mL at 48 hours, respectively. * P<0.05, compared to SKOV3. (B) The IC50 values of cisplatin for OV2008 and C13* were assessed by CCK-8 assay. The IC50 values of cisplatin for OV2008 and C13* cells were 9.8 μg/mL and 29.6 μg/mL at 48 hours, respectively. ** P<0.05, compared to OV2008. (C) Sensitizing effect of wogonin in SKOV3/DDP cells to cisplatin. Cells were treated with cisplatin (IC30, 10.2 μg/mL), cisplatin (IC50, 26.8 μg/mL), cisplatin (IC30)+wogonin (20 μM) and cisplatin (IC50)+wogonin (20 μM) for 48 hours, and cell viability was determined by the CCK-8 assay. # P<0.05, compared to cisplatin alone. (D) Sensitizing effect of wogonin in C13* cells to cisplatin. Cells were treated with cisplatin (IC30, 11.1 μg/mL), cisplatin (IC50, 29.6 μg/mL), cisplatin (IC30)+wogonin (20 μM), and cisplatin (IC50)+wogonin (20 μM) for 48 hours, and cell viability was determined by the CCK-8 assay. ## P<0.05, compared to cisplatin alone. CCK-8 – cell counting Kit-8.
Table 1.
Effects of wogonin on cisplatin cytotoxicity in cisplatin-resistant ovarian cancer cells.
| Group | Concentration (μM) | IC50 of cisplatin (μg/ml) | |
|---|---|---|---|
| SKOV3/DDP | C13* | ||
| Control | 0 | 26.8±1.6 | 29.6±2.3 |
| Wogonin | 10 | 16.4±1.2 | 19.8±1.7 |
| Wogonin | 20 | 11.2±0.6 | 13.9±1.1 |
| LY294002 | 10 | 9.8±0.4 | 10.6±1.3 |
Wogonin promoted cisplatin-induced apoptosis of cisplatin-resistant ovarian cancer cells
Apoptosis was evaluated in SKOV3/DDP and C13* cells that were exposed to wogonin alone and the combination of wogonin and cisplatin for 48 hours. The nuclei were slightly condensed in the groups pretreated with wogonin (10 μM or 20 μM) or cisplatin (5 μg/mL) alone while untreated cells displayed evenly dispersed blue fluorescence within their nuclei (Figure 3A, 3B). The cells co-treated with cisplatin (5 μg/mL or 10 μg/mL) with 20 μM wogonin showed substantial chromatin condensation or dense staining fragmentation (Figure 3B). For SKOV3/DDP, the percentage of apoptosis cells in the groups treated by wogonin+5 μg/mL cisplatin (48.2±6.5) or wogonin+10 μg/mL cisplatin (57.3±7.1) were higher than that in the control group (1.2±0.4) and the cisplatin group (5 μg/mL, 6.4±1.3). Similar phenomena were also observed in C13* cells, compared with the control group (0.8±0.2) and the cisplatin group (5 μg/mL, 4.8±0.9), the percentage of apoptosis cells were higher in the groups treated by wogonin+5 μg/mL cisplatin (55.2±7.6) or wogonin+10 μg/mL cisplatin (61.8±6.9). The apoptosis was further proven by western blot of caspase-3 and PARP protein cleavage and the expression of anti-apoptotic Bcl-2. Caspase-3 and PARP were activated and bcl-2 was reduced in the groups co-treated with cisplatin (10 μg/mL) with wogonin (20 μM) (Figure 3C). These results indicated that wogonin could increase cisplatin-resistant ovarian cancer cells to low dose cisplatin-induced cell apoptosis.
Figure 3.
Wogonin promoted cisplatin-induced apoptosis of cisplatin-resistant SKOV3/DDP and C13* cells. (A) The effect of different concentrations (10, 20, and 40 μM) of wogonin on SKOV3/DDP and C13* cell apoptosis. Nucleus cells were stained by Hoechst 33258. Fluorescence microscopy 600×. Bar 40 μm. * P<0.05, compared to control. (B) The effect of wogonin (20 μM) on cisplatin-induced apoptosis in SKOV3/DDP and C13* cells. Nucleus was stained by Hoechst 33258. Fluorescence microscopy 600×. Bar 40 μm. * P<0.05, compared to cisplatin (5 μg/mL) alone. (C) SKOV3/DDP and C13* cells were treated with PBS, cisplatin (10 μg/mL), wogonin (20 μM) and cisplatin (10 μg/mL)+wogonin (20 μM) for 48 hours, then the protein levels of bcl-2, caspase-3, cleaved caspase-3, PARP, and cleaved-PARP were detected by western blot.
Wogonin enhanced cisplatin sensitivity via downregulating the PI3K/Akt pathway
To understand whether modulation of the PI3K/Akt pathway is involved in wogonin enhanced cisplatin sensitivity in resistant cells, we treated SKOV3/DDP and C13* cells with wogonin and/or AKT inhibitor LY294002. As shown in Table 1, the IC50 value of cisplatin decreased to 11.2 μg/mL and 9.8 μg/mL in SKOV3/DDP cells with treatment with wogonin (20 μM) or LY294002 (10 μM) alone at 48 hours, respectively; and decreased to 13.9 μg/mL and 10.6 μg/mL in C13* cells, respectively. The protein levels of phosphorylated Akt in cisplatin-resistant SKOV3/DDP and C13* cells were higher than that in SKOV3 and OV2008 cells. Treatment with wogonin reduced phosphorylated Akt in SKOV3/DDP and C13* cells (Figure 4A). Additionally, when SKOV3/DDP and C13* were exposed to cisplatin (10 μg/mL), and were treated simultaneously with wogonin (20 μM) or LY294002 (10 μM) alone, notably inhibition of phosphorylated Akt occurred. A combined treatment with wogonin and LY294002 significantly reduced the level of phosphorylated Akt compared to either treatment alone (Figure 4B).
Figure 4.
Wogonin enhanced cisplatin sensitivity via downregulating the PI3K/Akt pathway. (A) The protein levels of Akt and p-Akt were detected by western blot in SKOV3, SKOV3/DDP, OV2008, and C13* cells. The effect of wogonin on Akt and p-Akt in SKOV3/DDP and C13* cells were detected by western blot. (B) The protein levels of Akt and p-Akt was determined by western blot in SKOV3/DDP and C13* cells after pretreatment with wogonin (20 μM) and/or LY294002 (10 μM). Data are presented as mean ± standard deviation, n=3, * P<0.05.
Discussion
Cisplatin (cis-diamminedichloroplatinum II, cDDP) is a major chemotherapeutic agent for the treatment of ovarian cancer. It is generally considered to be a cytotoxic drug and functions by cross-linking DNA and inhibiting DNA synthesis, resulting in cell-cycle arrest and apoptosis [13]. However, severity of the adverse side-effects of cisplatin such as nausea, hemolytic anemia, nephrotoxicity, neurotoxicity and ototoxicity, as well as cisplatin-resistance, limits treatment success, particularly at high doses [14]. It is therefore necessary to explore novel adjuvant substances to increase cisplatin sensitivity in ovarian cancer that would not only ameliorate its toxicity but also lessen the potential for cisplatin-resistance.
Phytochemicals, natural compounds derived from fruits, vegetables, and medicinal plants have gained extensive attention in recent years because of anti-cancer activities and due to their general low toxicity and availability. The therapeutic efficacies of several of these phytochemicals, combined with cisplatin, have been widely investigated. Interestingly, 2 newly characterized cranberry flavonoids, quercetin aglycone and PAC DP-9, were found to induce apoptosis and significantly increase cisplatin sensitivity in ovarian cancer cells by deactivating the MAPK-ERK pathway [15]. Oridonin has been shown to effectively reverse cisplatin-resistance in ovarian cancer cells via induction of cell apoptosis and inhibition of matrix metalloproteinase expression [16]. The combination of cisplatin and oridonin synergistically induces apoptosis of HepG2 human hepatocellular carcinoma cells by inhibition of nuclear factor κB transcription activity [17]. Curcumin reverses cisplatin resistance in cisplatin-resistant lung cancer cells by inhibiting the FA/BRCA pathway [18]. Choi et al. reported that curcumin also downregulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt pathway [19]. These findings prompted us to determine whether other phytochemicals can sensitize ovarian cancer cells to cisplatin. In the current study, we demonstrated that wogonin inhibited proliferation of ovarian cancer cells in dose- and time-dependent manners and it also sensitized cisplatin-induced cytotoxicity in cisplatin-resistant SKOV3/DDP and C13* cells. Wogonin significantly increased cisplatin-resistant ovarian cancer cells SKOV3/DDP and C13* to low dose cisplatin-induced cell death.
A number of investigations have demonstrated that wogonin attenuate chemotherapeutic drug resistance via regulation of multiple molecular pathways. Wogonin enhanced doxorubicin sensitivity by inhibition of the IGF-1R/AKT signaling pathway in breast cancer [8]. Qian et al. found that wogonin potentiated chemotherapeutic effects through inhibiting the Nrf2-ARE pathways [20]. It decreased the Nrf2 nuclear translocation and triggered intracellular reactive oxygen species (ROS) generation contributed to apoptosis. Furthermore, suppression of Nrf2 by wogonin is associated with overexpression of p53 in HepG2 cells. Wogonin induced H2O2 accumulation in A549 and HeLa cells, which substantially contributes to the sensitization of cisplatin-induced apoptosis [7]. Wogonin enhanced the effect of 5-FU cytotoxicity through inhibition of COX-2 expression and downregulation of the PI3K/Akt signaling pathway in hepatocellular carcinoma cells [21]. In our study, we found that the protein levels of phosphorylated Akt in cisplatin-resistant SKOV3/DDP and C13* cells were higher than that in SKOV3 and OV2008 cells, and the treatment with wogonin significantly reduced phosphorylated Akt in SKOV3/DDP and C13* cells.
It has been reported that Akt plays an important role in cancer therapy by promoting resistance to the apoptosis-inducing effects of chemotherapy [11]. Emerging data has revealed that constitutive activation of the PI3K/Akt pathway is responsible for acquired cisplatin resistance. Zhang et al. found that inhibition of PI3K/Akt activity in human lung cancer cells A549/DDP and H460/DDP could reverse cisplatin resistance [22]. PI3K/mTOR dual inhibitor NVP-BEZ235 can potentiate the antitumor effects of cisplatin in cisplatin-resistant bladder cancer cells via the induction of S phase cell cycle arrest and caspase-dependent apoptosis [23]. Knockout of Akt isoforms increased the cisplatin sensitivity in endometrial cancer cells [24]. Array comparative genomic hybridization studies have identified PI3K/Akt as the most frequently altered pathway in ovarian cancer [25], which confers cisplatin resistance by modulating the action of p53 on the caspase-dependent mitochondrial death pathway [26]. PI3K inhibitor LY294002 and cisplatin in combination had an additive effect on inhibiting ovarian cancer cell growth [27]. In the present study, when SKOV3/DDP and C13* were exposed to cisplatin, treatment with wogonin and/or LY294002 inhibited phosphorylated Akt. These results indicated that wogonin enhanced cisplatin sensitivity may be via downregulating the PI3K/Akt pathway.
Conclusions
Our data experimentally showed that wogonin could significantly increase the sensitivity of SKOV3/DDP and C13* cells to cisplatin through inhibiting the activation of the PI3K/Akt signaling pathway, and that the combination of wogonin and cisplatin could be a promising therapeutic strategy for the treatment of ovarian cancer cases that have developed-drug resistant to cisplatin.
Footnotes
Source of support: This project was supported by National Nature Science Foundation of China (NO.81602260)
Conflicts of interest
None.
References
- 1.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. Cancer J Clin. 2018;68(4):284–96. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santaballa A, Barretina P, Casado A, et al. SEOM Clinical guideline in ovarian cancer (2016) Clin Transl Oncol. 2016;18(12):1206–12. doi: 10.1007/s12094-016-1588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh DL, Sharma N, Kumar Singh A, et al. Anti-tumor activity of wogonin, an extract from Scutellaria baicalensis, through regulating different signaling pathways. Chin J Nat Med. 2017;15(1):15–40. doi: 10.1016/S1875-5364(17)30005-5. [DOI] [PubMed] [Google Scholar]
- 4.Chen YC, Shen SC, Lee WR, et al. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol. 2002;76(5–6):351–59. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee DH, Kim C, Zhang L, et al. Role of p53, PUMA, and Bax in wogonin-induced apoptosis in human cancer cells. Biochem Pharmacol. 2008;75(10):2020–33. doi: 10.1016/j.bcp.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen S, Cooper M, Jones M, et al. Combined activity of oridonin and wogonin in advanced-stage ovarian cancer cells: Sensitivity of ovarian cancer cells to phyto-active chemicals. Cell Biol Toxicol. 2011;27(2):133–47. doi: 10.1007/s10565-010-9176-0. [DOI] [PubMed] [Google Scholar]
- 7.He F, Wang Q, Zheng XL, et al. Wogonin potentiates cisplatin-induced cancer cell apoptosis through accumulation of intracellular reactive oxygen species. Oncol Rep. 2012;28(2):601–5. doi: 10.3892/or.2012.1841. [DOI] [PubMed] [Google Scholar]
- 8.Fu P, Du F, Liu Y, et al. Wogonin increases doxorubicin sensitivity by down-regulation of IGF-1R/AKT signaling pathway in human breast cancer. Cell Mol Biol (Noisy-le-grand) 2015;61(7):123–27. [PubMed] [Google Scholar]
- 9.Wang T, Gao J, Yu J, et al. Synergistic inhibitory effect of wogonin and low-dose paclitaxel on gastric cancer cells and tumor xenografts. Chin J Cancer Res. 2013;25(5):505–13. doi: 10.3978/j.issn.1000-9604.2013.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang F, Lee JT, Navolanic PM, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia. 2003;17(3):590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 11.Cheaib B, Auguste A, Leary A. The PI3K/Akt/mTOR pathway in ovarian cancer: Therapeutic opportunities and challenges. Chin J Cancer. 2015;34(1):4–16. doi: 10.5732/cjc.014.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mabuchi S, Kawase C, Altomare DA, et al. mTOR is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer Res. 2009;15(17):5404–13. doi: 10.1158/1078-0432.CCR-09-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manohar S, Leung N. Cisplatin nephrotoxicity: A review of the literature. J Nephrol. 2018;31(1):15–25. doi: 10.1007/s40620-017-0392-z. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Han A, Chen E, et al. The cranberry flavonoids PAC DP-9 and quercetin aglycone induce cytotoxicity and cell cycle arrest and increase cisplatin sensitivity in ovarian cancer cells. Int J Oncol. 2015;46(5):1924–34. doi: 10.3892/ijo.2015.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma S, Tan W, Du B, et al. Oridonin effectively reverses cisplatin drug resistance in human ovarian cancer cells via induction of cell apoptosis and inhibition of matrix metalloproteinase expression. Mol Med Rep. 2016;13(4):3342–48. doi: 10.3892/mmr.2016.4897. [DOI] [PubMed] [Google Scholar]
- 17.Dong X, Liu F, Li M. Inhibition of nuclear factor κB transcription activity drives a synergistic effect of cisplatin and oridonin on HepG2 human hepatocellular carcinoma cells. Anticancer Drugs. 2016;27(4):286–99. doi: 10.1097/CAD.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 18.Chen P, Li J, Jiang HG, et al. Curcumin reverses cisplatin resistance in cisplatin-resistant lung cancer cells by inhibiting FA/BRCA pathway. Tumour Biol. 2015;36(5):3591–99. doi: 10.1007/s13277-014-2996-4. [DOI] [PubMed] [Google Scholar]
- 19.Choi BH, Kim CG, Lim Y, et al. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett. 2008;259(1):111–18. doi: 10.1016/j.canlet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Qian C, Wang Y, Zhong Y, et al. Wogonin-enhanced reactive oxygen species-induced apoptosis and potentiated cytotoxic effects of chemotherapeutic agents by suppression Nrf2-mediated signaling in HepG2 cells. Free Radic Res. 2014;48(5):607–21. doi: 10.3109/10715762.2014.897342. [DOI] [PubMed] [Google Scholar]
- 21.Zhao L, Sha YY, Zhao Q, et al. Enhanced 5-fluorouracil cytotoxicity in high COX-2 expressing hepatocellular carcinoma cells by wogonin via the PI3K/Akt pathway. Biochem Cell Biol. 2013;91(4):221–29. doi: 10.1139/bcb-2012-0077. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Bao C, Mu Q, et al. Reversal of cisplatin resistance by inhibiting PI3K/Akt signal pathway in human lung cancer cells. Neoplasma. 2016;63(3):362–70. doi: 10.4149/304_150806N433. [DOI] [PubMed] [Google Scholar]
- 23.Moon du G, Lee SE, Oh MM, et al. NVP-BEZ235, a dual PI3K/mTOR inhibitor synergistically potentiates the antitumor effects of cisplatin in bladder cancer cells. Int J Oncol. 2014;45(3):1027–35. doi: 10.3892/ijo.2014.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray MJ, Mhawech-Fauceglia P, Yoo E, et al. AKT inhibition mitigates GRP78 (glucose-regulated protein) expression and contribution to chemoresistance in endometrial cancers. Int J Cancer. 2013;133(1):21–30. doi: 10.1002/ijc.27994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Zhang L, Greshock J, et al. Frequent genetic abnormalities of the PI3K/AKT pathway in primary ovarian cancer predict patient outcome. Genes Chromosomes Cancer. 2011;50(8):606–18. doi: 10.1002/gcc.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Z, Wang J, Tang J, et al. NK- and Akt-mediated Puma expression in the apoptosis of cisplatin-resistant ovarian cancer cells. Biochem J. 2012;444(2):291–301. doi: 10.1042/BJ20111855. [DOI] [PubMed] [Google Scholar]
- 27.Nonaka M, Itamochi H, Kawaguchi W, et al. Activation of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway overcomes cisplatin resistance in ovarian carcinoma cells. Int J Gynecol Cancer. 2012;22(6):922–29. doi: 10.1097/IGC.0b013e31824f0b13. [DOI] [PubMed] [Google Scholar]