Abstract
Background
Temporal bone surgery is a technically challenging and high‐risk procedure in an anatomically complex area. Safe temporal bone surgery emphasizes a consummate anatomic understanding and technique development that requires the guidance of an experienced otologic surgeon and years of practice. Temporal bone simulation can augment otologic surgical training and enable rehearsal of surgical procedures.
Objectives
The purpose of this article is to provide an updated review of temporal bone simulation platforms and their uses.
Data Sources
PubMed literature search. Search terms included temporal bone, temporal bone simulation, virtual reality (VR), and presurgical planning and rehearsal.
Discussion
Various simulation platforms such as cadaveric bone, three‐dimensional (3D) printed models, and VR simulation have been used for temporal bone surgery training. However, each simulation method has its drawbacks. There is a need to improve upon current simulation platforms to enhance surgical training and skills assessment, as well as a need to explore other clinically significant applications of simulation, such as preoperative planning and rehearsal, in otologic surgery.
Conclusions
There is no replacement for actual surgical experience, but high‐fidelity temporal bone models such as those produced with 3D printing and computer simulation have emerged as promising tools in otolaryngologic surgery. Improvements in the fidelity of both 3D printed and VR simulators as well as integration of a standardized assessment format would allow for an expansion in the use of these simulation platforms in training and assessment.
Level of Evidence
5
Keywords: Temporal bone, simulation, virtual reality, preoperative planning
INTRODUCTION
Temporal bone surgery is a technically challenging and high‐risk procedure in an anatomically complex area.1, 2 Safe temporal bone surgery emphasizes a consummate anatomic understanding and technique development that requires the guidance of an experienced otologic surgeon and years of practice.3 To mitigate the learning curve and potentially improve preoperative understanding of complex anatomy and rare pathology, a temporal bone simulation platform of sufficient fidelity to match the anatomy and pathology would be of benefit. Currently, cadaveric temporal bone is the gold standard for temporal bone surgery training, but cadaveric bones present their own limitations.
Other than cadaveric bones, the two most commonly used simulation platforms reported in the literature are three‐dimensional (3D) printed models and virtual reality (VR) simulation platforms. Most manuscripts reporting use of temporal bone simulation revolve around resident training with an emphasis on anatomic understanding and technique development.3, 4, 5 Applications related to surgical assessment, as well as those applications with more direct patient impact, have yet to be explored.3 There is a need to improve upon current simulation platforms that can complement or replace cadaveric temporal bones in surgical training and skills assessment, as well as a need to explore other clinically significant applications of simulation such as preoperative planning and rehearsal. Demonstrating an evidenced‐based impact on both surgical learning and patient outcomes has proven to be challenging. Here, we provide an updated look at temporal bone simulation platforms and their uses.
METHODS
PubMed was used to search for studies that reported the use of simulation in temporal bone surgery through August 2018. Search terms included temporal bone, temporal bone simulation, VR, and presurgical planning and rehearsal. Only articles published in the English‐language were included.
APPLICATIONS OF TEMPORAL BONE SIMULATION
Surgical Training
Previous studies have shown that simulation can be helpful in otologic skills training, but each simulation method has its drawbacks. Training on cadaveric temporal bones is the gold standard since it provides the highest fidelity, but several limitations to this approach exist. First, adequate numbers of cadaveric temporal bones are difficult to acquire.1, 6, 7, 8, 9, 10 Use of cadaveric material also requires a specialized laboratory that involves a significant amount of preparation and clean‐up after use as well as the inherent risk of infection when handling biological tissues.11 Maintaining and running a specialized laboratory is costly8, 9 and a surgical expert is still required to provide formative feedback during the learning process. Cadaveric bones can only be used once, and they cannot be used for patient‐specific rehearsal of surgical approaches for otologic pathology. Moreover, established temporal bone laboratories may be inaccessible to otolaryngology residents because of geographic distance, scheduling difficulties, and associated high costs.7
3D printed temporal bone models can provide access to a standardized and reproducible simulation and have been shown in multiple studies to be a highly rated educational tool among residents because of the models’ physical realism.1, 5, 10 However, 3D temporal bone models have yet to be perfected with respect to material selection and manufacturing techniques that allow for accurate replication of bone properties while also being anatomically accurate.10, 12 Hochman et al developed a model that is faithful in its reproduction of internal and external anatomical details and has mechanical properties similar to cadaveric bone.13 Their printing methods and materials were compared to sheep femur for bony characteristics, which may not be a similar enough model to human temporal bone because sheep femur does not contain highly aerated bone segments like human petrous and mastoid temporal bone.13 3D printed models also lack soft tissue, do not allow for simulation of bleeding during dissection,4 and like cadaveric temporal bones, the use of 3D printed models requires a specialized laboratory and significant clean‐up after use. The potential health risks associated with aerosolizing the printed material have yet to be determined. Additionally, an expert is still required to provide feedback during the learning process and the cost effectiveness of 3D printing models for each new simulation must still be evaluated.4, 5, 9
As an alternative to cadaveric temporal bones and 3D printed models, multiple VR simulators exist for temporal bone surgery and have been previously described in the literature.1, 14 Studies show that trainees exposed to VR simulation demonstrate an increase in surgical confidence throughout all levels of training and that VR training positively correlates with cadaveric dissection performance.3, 6, 8, 9, 15 VR simulation‐based training is unique because it has the potential for simulator‐integrated tutoring, guidance, and feedback that can eliminate or minimize the need for human instructors.9 The repeated, self‐directed practice made possible with VR can expedite skills acquisition prior to trainees moving on to practice on cadaveric temporal bones or supervised surgery.9, 15 Recent developments in visual rendering can produce almost photorealistic representations (Fig. 1). However, due to limitations in mechanical design, existing haptic simulations cannot yet realistically reproduce the vibration and contact forces experienced during surgery.16 Additional work is being done to further develop and increase the fidelity of vibrotactile feedback in different drilling situations.17 In recent years, the availability of VR simulation has increased due to advances in computer technology and this mode of simulation offers a solution to the time constraints encountered by human instructors whose time is limited as a result of clinical duties. Additionally, the cost of integrating VR training can be offset by reduced operating room time, a decreased need to purchase cadaveric temporal bones, and reduced costs associated with specialized laboratory maintenance.3
Figure 1.
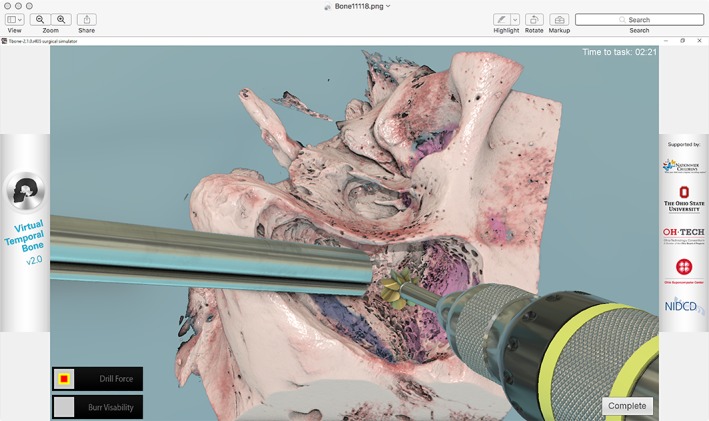
Visual rendering in virtual reality model of temporal bone and otologic instruments.
Assessment of Surgical Skills
Along with surgical training, VR simulation has been used to assess resident surgical skill and progress, but no standardized assessment format has been developed. In VR temporal bone simulation, a mixture of task‐based checklists, end‐product dissection scores, and technique grading has been used to provide performance assessment.18 Current assessment tools include the objective structured assessment of technical skill (OSATS) for assessing general surgical skill,19 a modified OSATS scale by Zirkle et al,20 an OSATS for mastoidectomy performance developed by a group at Johns Hopkins,8, 21 the Welling Scale (WS1) developed at The Ohio State University,22 and the Iowa Standardized Skill Assessment.23 Nearly all the studies reviewed by Sethia et al used differences in scores between novices and experts as evidence that the assessment was valid.24 However, this concept establishes only weak validity evidence.24 Ioannou et al also compared metrics used to differentiate between expert and resident surgeons and suggested that metrics indicative of inexperience could be used as an opportunity for an expert to provide specific feedback to the trainee.25 Most of these assessment processes are not fully automated and still require expert input, which can extend the amount of time it takes to provide feedback to the trainee.
Overall, Sethia et al determined that the Hopkins OSATS scale likely contains the most validity for mastoidectomy performance assessment of the tools described in their review because it has been the most widely validated in multiple settings by different institutions.24 Sethia et al also state that an ideal mastoidectomy assessment tool would consider dissection of all of the following 10 components: facial nerve, antrum, sigmoid/sinodural angle, ossicles/middle ear, mastoid cavity and external auditory canal wall, tegmen, facial recess, semicircular canal, digastric, and drill handling.24 However, none of the reviewed assessment tools included all 10 components.24 More work needs to be done to develop a standardized, objective assessment tool for use in temporal bone surgery. The development of this tool and integration into a VR simulator would allow for helpful, accurate feedback during training on a VR temporal bone simulator without relying on an experienced surgeon for immediate feedback.
Presurgical Planning and Rehearsal
While VR simulations can be useful in training and assessment of residents, patient‐specific presurgical planning and rehearsal is a promising additional application of temporal bone simulation. Procedural skills rehearsal has been established in other surgical procedures, such as in carotid endovascular surgery, and results suggest that case rehearsal improves surgical performance.26 Studies show that experienced surgeons found VR simulation more helpful with difficult tasks rather than simple ones3, 26 and that a strong positive correlation between operative utility ratings and cadaveric dissection scores exists among advanced surgical tasks.3 The virtual environment also provides an undo and redo function that allows surgeons to experiment with different approaches on both patient‐specific anatomy and patient‐specific pathology.3, 27
Although experienced surgeons have expressed that the virtual environment offered lower fidelity than the actual surgical experience, high fidelity is not the only goal in rehearsal.3, 26, 27 An important, clinically significant role of VR simulation is in engaging with the patient's imaging studies in the same context as would be done during surgery. Virtual surgical rehearsal allows surgeons to better understand the anatomy, extent of pathology, and difficulty of access or exposure they are likely to encounter in the operating room for a given patient,27, 28 improving both surgeon confidence and dissection performance.3, 15, 29, 30 Integration of a computational model of a clinically relevant patient outcome can allow the surgeon to assess the potential impact on patients with each simulation run.
DISCUSSION AND FUTURE DIRECTIONS
Issues in fidelity have still not been adequately resolved for simulations other than cadaveric temporal bone to be widely used in clinical training.14, 31 The realism of VR simulators is still suboptimal despite advances in haptic feedback,2, 9 and existing haptic simulations are unable to realistically reproduce the vibration and contact forces experienced during surgery.16 Additionally, VR simulations lack soft tissue and blood in dissection and cannot replicate the auditory feedback present during surgery.8, 14, 16, 26 However, simulations developed for surgical rehearsal may have different fidelity requirements than those developed for surgical training.27 Teaching technical skills may require greater realism whereas the ease of dissection and flexibility to encourage exploration become higher priorities for experienced surgeons interested in rehearsing patient‐specific cases.27
The use of VR simulation in presurgical planning and rehearsal could not only improve surgeon confidence but reduce operating room (OR) times and potentially improve patient outcomes by allowing exploration of several different approaches on patient‐specific data.3, 26, 27, 32 One particular area of potential high impact is in cochlear implantation. Despite technological advances, some patients with cochlear implants show suboptimal results in speech perception, partly due to a lack of appropriate surgical planning tools for device selection and rehearsal.33 It is known that the performance of patients with cochlear implants depends greatly on electrode placement and patient‐specific factors such as cochlear anatomy, which varies among individual patients.33, 34, 35, 36, 37, 38 Preoperative imaging may permit selection of the appropriate electrode array based on the cochlear duct length, or the desired angle of insertion based on the degree of residual acoustic hearing.39 Moreover, preoperative surgical rehearsal may permit the surgeon to practice the facial recess approach and determine whether the orientation of the basal turn of the cochlea is appropriate for a round window versus cochleostomy approach for a given patient.40 Several studies have also looked at optimization of cochlear implant positioning by using preimplant and postimplant imaging and measuring neural responses to assess cochlear implant performance.33, 34, 35, 36, 37, 38 Computational models such as those described in these studies have shown the potential to predict the performance of cochlear implant patients, provide information to guide preoperative decisions, and assist with surgical planning.33 Incorporation of VR simulation in presurgical planning and rehearsal of cochlear implant surgery (Fig. 2) would allow surgeons to try different cochlear implant placements on patient‐specific data to obtain the most favorable outcome.
Figure 2.
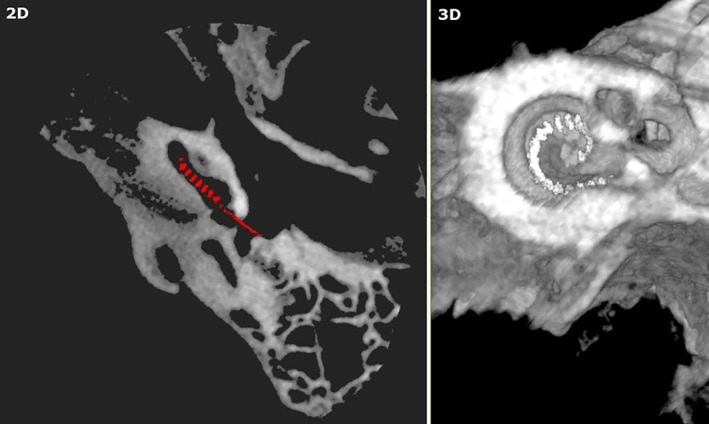
Cochlear implant in preoperative rehearsal of temporal bone surgery.
In addition to applications for cochlear implantation, the use of VR simulation in presurgical planning and rehearsal should be expanded to include additional procedures in anatomically complex areas like the petrous apex.41, 42, 43 Appropriate selection of a surgical approach, for example, the infracochlear, infralabyrinthine, or retrofacial approaches, could be enhanced by rehearsal of various trajectories, which are highly dependent on an individual patient's anatomy and mastoid/petrous aeration patterns.44 The selection of approach for lateral skull base tumors in a patient with residual hearing could also be improved through a better understanding of the bony anatomy of that patient. For instance, the choice between a middle fossa approach and a retrosigmoid approach to a small internal auditory canal tumor could be made easier if surgical rehearsal elucidated the location of the tumor relative to the labyrinth or basal turn of the cochlea. Until now, surgeons have been required to rely on general and inconsistent intraoperative anatomical landmarks to avoid transgression of the inner ear and hearing loss in these cases.45
CONCLUSION
There is no replacement for actual surgical experience, but high‐fidelity temporal bone models such as those produced with 3D printing and computer simulation have emerged as promising tools in otolaryngologic surgery.14 Currently, VR simulators have been most widely used in surgical training as a supplement to cadaveric temporal bones. Improvements in the fidelity of both 3D printed and VR simulators as well as integration of a standardized assessment format would allow for an expansion in the use of these simulation platforms in training and assessment. Specific to VR platforms, fully automated assessment features may offset the time constraints encountered by expert surgeons whose time is limited by clinical duties. Both 3D printed and VR simulations have not been regularly used in presurgical planning and rehearsal of otolaryngologic surgery, but their use could help surgeons visualize and plan the most appropriate approach for complex surgical cases. Better understanding and simulated experience with unique anatomical and pathological features of individual patients obtained with presurgical planning could lead to reduced OR time and better patient outcomes. Advances in technology will only enhance the fidelity and functionality of simulation for use in training and in more clinically relevant ways, such as presurgical planning and rehearsal. This technology is especially applicable to otologic surgery because of the wide variety of complex procedures and technical skills required for complex temporal bone surgeries.
Editor's Note: This Manuscript was accepted for publication 17 March, 2019.
Conflict of Interest: The authors declare no conflict of interest.
BIBLIOGRAPHY
- 1. Bhutta MF. A review of simulation platforms in surgery of the temporal bone. Clin Otolaryngol 2016;41:539–545. [DOI] [PubMed] [Google Scholar]
- 2. Musbahi O, Aydin A, Al Omran Y, Skilbeck CJ, Ahmed K. Current status of simulation in otolaryngology: a systematic review. J Surg Educ 2016;74:203–215. [DOI] [PubMed] [Google Scholar]
- 3. Locketz GD, Lui JT, Chan S, et al. Anatomy‐specific virtual reality simulation in temporal bone dissection: perceived utility and impact on surgeon confidence. Otolaryngol Head Neck Surg 2017;156(6):1142–1149. [DOI] [PubMed] [Google Scholar]
- 4. Hsieh T, Cervenka B, Dedhia R, Strong EB, Steele T. Assessment of a patient‐specific, 3‐dimensionally printed endoscopic sinus and skull base surgical model. JAMA Otolaryngol Head Neck Surg 2018;144(7):574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rose AS, Webster CE, Harrysson O, et al. Pre‐operative simulation of pediatric mastoid surgery with 3‐D printed temporal bone models. Int J Pediatr Otorhinolaryngol 2015;79:740–744. [DOI] [PubMed] [Google Scholar]
- 6. Al‐Noury K. Virtual reality simulation in ear microsurgery: a pilot study. Indian J Otolaryngol Head Neck Surg 2012;64(2):162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fang T, Wang P, Lui C, Su MC, Yeh SC. Evaulation of a haptics‐based virtual reality temporal bone simulator for anatomy and surgery training. Comput Methods Programs Biomed 2014;113:674–681. [DOI] [PubMed] [Google Scholar]
- 8. Francis HW, Malik MU, Varela DA, et al. Technical skills improve after practice on virtual‐reality temporal bone simulator. Laryngoscope 2012;122:1385–1391. [DOI] [PubMed] [Google Scholar]
- 9. Wiet GJ, Sorensen MS, Andersen SA. Otologic skills training. Otolaryngol Clin North Am 2017;50:933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hochman JB, Rhodes C, Wong D, et al. Comparison of cadaveric and isomorphic three‐dimensional printed models in temporal bone education. Laryngoscope 2015;125:2353–2357. [DOI] [PubMed] [Google Scholar]
- 11. Scott A, De R, Sadek SA, Garrido MC, Courteney‐Harris RG. Temporal bone dissection: a possible route for prion transmission? J Laryngol Otol 2001;115:374–375. [DOI] [PubMed] [Google Scholar]
- 12. Haffner M, Quinn A, Hsieh TY, Strong EB, Steele T. Optimization of 3D print material for the recreation of patient‐specific temporal bone models. Ann Otol Rhinol Laryngol 2018;127(5):338–345. [DOI] [PubMed] [Google Scholar]
- 13. Hochman JB, Kraut J, Kazmerik K, Unger BJ. Generation of a 3D printed temporal bone model with internal fidelity and validation of the mechanical construct. Otolaryngol Head Neck Surg 2014;150(3):448–454. [DOI] [PubMed] [Google Scholar]
- 14. Sethia R, Wiet GJ. Preoperative preparation for otologic surgery: temporal bone simulation. Curr Opin Otolaryngol Head Neck Surg 2015;23(5):355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arora A, Lau LYM, Awad Z, et al. Virtual reality simulation training in otolaryngology. Int J Surg 2014;12:87–94. [DOI] [PubMed] [Google Scholar]
- 16. Hochman JB, Sepehri N, Rampersad V, et al. Mixed reality temporal bone surgical dissector: mechanical design. Otolaryngol Head Neck Surg 2014;43:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghasemloonia A, Baxandall S, Zareinia K, et al. Evaluation of haptic interfaces for simulation of drill vibration in virtual temporal bone surgery. Comput Biol Med 2016;78:9–17. [DOI] [PubMed] [Google Scholar]
- 18. Lui JT, Hoy MY. Evaluating the effect of virtual reality temporal bone simulation on mastoidectomy performance: a meta‐analysis. Otoloaryngol Head Neck Surg 2017;156(6):1018–1024. [DOI] [PubMed] [Google Scholar]
- 19. Martin JA, Regehr G, Reznick R, et al. Objective structured assessment of technical skill (OSATS) for surgical residents. Br J Surg 1997;84(2):273–278. [DOI] [PubMed] [Google Scholar]
- 20. Zirkle M, Taplin MA, Anthony R, Dubrowski A. Objective assessment of temporal bone drilling skills. Ann Otol Rhinol Laryngol 2007;116(11):793–798. [DOI] [PubMed] [Google Scholar]
- 21. Laeeq K, Bhatti NI, Carey JP, et al. Pilot testing of an assessment tool for competency in mastoidectomy. Laryngoscope 2009;119(12):2402–2410. [DOI] [PubMed] [Google Scholar]
- 22. Butler NN, Wiet GJ. Reliability of the Welling scale (WS1) for rating temporal bone dissection performance. Laryngoscope 2007;117(10):1803–1808. [DOI] [PubMed] [Google Scholar]
- 23. Mowry SE, Hansen MR. Resident participation in cadaveric temporal bone dissection correlates with improved performance on a standardized skill assessment instrument. Otol Neurotol 2014;35(1):77–83. [DOI] [PubMed] [Google Scholar]
- 24. Sethia R, Kerwin TF, Wiet GJ. Performance assessment for mastoidectomy: state of the art review. Otolaryngol Head Neck Surg 2017;156(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ioannou I, Zhou Y, Wijewickrema S, et al. Comparison of experts and residents performing a complex procedure in a temporal bone surgery simulator. Otol Neurotol 2017;38:e85–e91. [DOI] [PubMed] [Google Scholar]
- 26. Arora A, Swords C, Khemani S, et al. Virtual reality case‐specific rehearsal in temporal bone surgery: a preliminary evaluation. Int J Surg 2014;12:141–145. [DOI] [PubMed] [Google Scholar]
- 27. Chan S, Li P, Locketz G, Salisbury K, Blevins NH. High‐fidelity haptic and visual rendering for patient‐specific simulation of temporal bone surgery. Comput Assist Surg 2016;21(1):85–101. [DOI] [PubMed] [Google Scholar]
- 28. Won TB, Hwang P, Lim JH, et al. Early experience with a patient‐specific virtual surgical simulation for rehearsal of endoscopic skull‐base surgery. Int Forum Allergy Rhinol 2018;8(1):54–63. [DOI] [PubMed] [Google Scholar]
- 29. O'Leary JD, O'Sullivan O, Barach P, Shorten GD. Improving clinical performance using rehearsal of warm‐up: an advanced literature review of randomized and observational studies. Acad Med 2014;89:1416–1422. [DOI] [PubMed] [Google Scholar]
- 30. Pike TW, Pathak S, Mushtaq F, et al. A systematic examination of preoperative surgery warm‐up routines. Surg Endosc 2017;31:2202–2214. [DOI] [PubMed] [Google Scholar]
- 31. Unger B, Sepehri N, Rampersad V, et al. Elements of virtual temporal bone surgery: manipulandum format may be more important to surgeons than haptic device force capabilities. Laryngoscope Investig Otolaryngol 2017;2(6):358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haerle SK, Daly MJ, Chan HH, et al. Virtual surgical planning in endoscopic skull base surgery. Laryngoscope 2013;123:2935–2939. [DOI] [PubMed] [Google Scholar]
- 33. Mangado N, Pons‐Prats J, Coma M, et al. Computational evaluation of cochlear implant surgery outcomes accounting for uncertainty and parameter variability. Front Physiol 2018;9:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skinner MW, Holden TA, Whiting BR, et al. In vivo estimates of the position of advanced bionics electrode arrays in the human cochlea. Ann Otol Rhinol Laryngol 2007;116(4):1–24. [PubMed] [Google Scholar]
- 35. Verbist BM, Skinner MW, Cohen LT, et al. Consensus panel on a cochlear coordinate system applicable in histologic, physiologic, and radiologic studies of the human cochlea. Otol Neurotol 2010;31:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Long CJ, Holden TA, McClelland GH, et al. Examining the electro‐neural interface of cochlear implant users using psychophysics, CT scans, and speech understanding. J Assoc Res Otolaryngol 2014;15:293–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mangado N, Ceresa M, Benav H, et al. Towards a complete in silico assessment of the outcome of cochlear implantation surgery. Mol Neurobiol 2018;55:173–186. [DOI] [PubMed] [Google Scholar]
- 38. Schuman TA, Noble JH, Wright CG, et al. Anatomic verification of a novel method of precise intrascalar localization of cochlear implant electrodes in adult temporal bones using clinically available computer tomography. Laryngoscope 2010;120:2277–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schurzig D, Timm ME, Batsoulis C, et al. A novel method for clinical cochlear duct length estimation toward patient‐specific cochlear implant selection. OTO Open 2018;2(4):2473974X18800238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mandour MF, Khalifa MA, Khalifa HA, Tomoum MO. A novel radiologic check test of round window accessibility for cochlear implantation: our experience in 198 cases. Clin Otolaryngol 2017;42(5):1108–1111. [DOI] [PubMed] [Google Scholar]
- 41. Jackson A, John NW, Thacker A, et al. Developing a virtual reality environment in petrous bone surgery: a state‐of‐the‐art review. Otol Neurotol 2002;23:111–121. [DOI] [PubMed] [Google Scholar]
- 42. Pietrantonio A, D'Andrea G, Fama I, et al. Usefulness of image guidance in the surgical treatment of petrous apex cholesterol granuloma. Case Rep Otolaryngol 2013;2013:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sweeny AD, Osetinsky LM, Carlson ML, et al. The natural history and management of petrous apex cholesterol granulomas. Otol Neurotol 2015;36:1714–1719. [DOI] [PubMed] [Google Scholar]
- 44. Grinblat G, Vashishth A, Galetti F, Caruso A, Sanna M. Petrous apex cholesterol granulomas: outcomes, complications, and hearing results from surgical and wait‐and‐scan management. Otol Neurotol 2017;38(10):e476–e485. [DOI] [PubMed] [Google Scholar]
- 45. Cueva RA, Chole RA. Maximizing exposure of the internal auditory canal via the retrosigmoid approach: an anatomical, radiological, and surgical study. Otol Neurotol 2018;39(7):916–921. [DOI] [PubMed] [Google Scholar]


