Abstract
Background
Tobacco use is estimated to kill 7 million people a year. Nicotine is highly addictive, but surveys indicate that almost 70% of US and UK smokers would like to stop smoking. Although many smokers attempt to give up on their own, advice from a health professional increases the chances of quitting. As of 2016 there were 3.5 billion Internet users worldwide, making the Internet a potential platform to help people quit smoking.
Objectives
To determine the effectiveness of Internet‐based interventions for smoking cessation, whether intervention effectiveness is altered by tailoring or interactive features, and if there is a difference in effectiveness between adolescents, young adults, and adults.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialised Register, which included searches of MEDLINE, Embase and PsycINFO (through OVID). There were no restrictions placed on language, publication status or publication date. The most recent search was conducted in August 2016.
Selection criteria
We included randomised controlled trials (RCTs). Participants were people who smoked, with no exclusions based on age, gender, ethnicity, language or health status. Any type of Internet intervention was eligible. The comparison condition could be a no‐intervention control, a different Internet intervention, or a non‐Internet intervention. To be included, studies must have measured smoking cessation at four weeks or longer.
Data collection and analysis
Two review authors independently assessed and extracted data. We extracted and, where appropriate, pooled smoking cessation outcomes of six‐month follow‐up or more, reporting short‐term outcomes narratively where longer‐term outcomes were not available. We reported study effects as a risk ratio (RR) with a 95% confidence interval (CI).
We grouped studies according to whether they (1) compared an Internet intervention with a non‐active control arm (e.g. printed self‐help guides), (2) compared an Internet intervention with an active control arm (e.g. face‐to‐face counselling), (3) evaluated the addition of behavioural support to an Internet programme, or (4) compared one Internet intervention with another. Where appropriate we grouped studies by age.
Main results
We identified 67 RCTs, including data from over 110,000 participants. We pooled data from 35,969 participants.
There were only four RCTs conducted in adolescence or young adults that were eligible for meta‐analysis.
Results for trials in adults: Eight trials compared a tailored and interactive Internet intervention to a non‐active control. Pooled results demonstrated an effect in favour of the intervention (RR 1.15, 95% CI 1.01 to 1.30, n = 6786). However, statistical heterogeneity was high (I2 = 58%) and was unexplained, and the overall quality of evidence was low according to GRADE. Five trials compared an Internet intervention to an active control. The pooled effect estimate favoured the control group, but crossed the null (RR 0.92, 95% CI 0.78 to 1.09, n = 3806, I2 = 0%); GRADE quality rating was moderate. Five studies evaluated an Internet programme plus behavioural support compared to a non‐active control (n = 2334). Pooled, these studies indicated a positive effect of the intervention (RR 1.69, 95% CI 1.30 to 2.18). Although statistical heterogeneity was substantial (I2 = 60%) and was unexplained, the GRADE rating was moderate. Four studies evaluated the Internet plus behavioural support compared to active control. None of the studies detected a difference between trial arms (RR 1.00, 95% CI 0.84 to 1.18, n = 2769, I2 = 0%); GRADE rating was moderate. Seven studies compared an interactive or tailored Internet intervention, or both, to an Internet intervention that was not tailored/interactive. Pooled results favoured the interactive or tailored programme, but the estimate crossed the null (RR 1.10, 95% CI 0.99 to 1.22, n = 14,623, I2 = 0%); GRADE rating was moderate. Three studies compared tailored with non‐tailored Internet‐based messages, compared to non‐tailored messages. The tailored messages produced higher cessation rates compared to control, but the estimate was not precise (RR 1.17, 95% CI 0.97 to 1.41, n = 4040), and there was evidence of unexplained substantial statistical heterogeneity (I2 = 57%); GRADE rating was low.
Results should be interpreted with caution as we judged some of the included studies to be at high risk of bias.
Authors' conclusions
The evidence from trials in adults suggests that interactive and tailored Internet‐based interventions with or without additional behavioural support are moderately more effective than non‐active controls at six months or longer, but there was no evidence that these interventions were better than other active smoking treatments. However some of the studies were at high risk of bias, and there was evidence of substantial statistical heterogeneity. Treatment effectiveness in younger people is unknown.
Plain language summary
Can Internet‐based interventions help people to stop smoking?
Background
Tobacco use is estimated to kill 7 million people a year. Nicotine is highly addictive, but surveys indicate that almost 70% of US and UK smokers would like to stop smoking. Although many smokers attempt to give up on their own, advice from a health professional increases the chances of quitting. As of 2016 there were 3.5 billion Internet users worldwide. The Internet is an attractive platform to help people quit smoking because of low costs per user, and it has potential to reach smokers who might not access support because of limited health care availability or stigmatisation. Internet‐based interventions could also be used to target young people who smoke, or others who may not seek traditional methods of smoking treatment.
Study Characteristics
Up to August 2016, this review found 67 trials, including data from over 110,000 participants. Smoking cessation data after six months or more were available for 35,969 participants. We examined a range of Internet interventions, from a low intensity intervention, for example providing participants with a list of websites for smoking cessation, to intensive interventions consisting of Internet‐, email‐ and mobile phone‐delivered components. We classed interventions as tailored or interactive, or both. Tailored Internet interventions differed in the amount of tailoring, from multimedia components to personalised message sources. Some interventions also included Internet‐based counselling or support from nurses, peer coaches or tobacco treatment specialists. Recent trials incorporated online social networks, such as Facebook, Twitter, and other online forums.
Key results
In combined results, Internet programmes that were interactive and tailored to individual responses led to higher quit rates than usual care or written self‐help at six months or longer.
Quality of evidence
There were not many trials conducted in younger people. More trials are needed to determine the effect on Internet‐based methods to aid quitting in youth and young adults. Results should be interpreted with caution, as we rated some of the included studies at high risk of bias, and for most outcomes the quality of evidence was moderate or low.
Summary of findings
Summary of findings for the main comparison. Internet‐based interventions for adults who want to stop smoking.
| Internet‐based interventions for adults who want to stop smoking | |||||
| Patient or population: adults who want to stop smoking Setting: Community Intervention: Internet‐based interventions | |||||
| Outcomes1 | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with Comparator | Risk with Internet‐based interventions | ||||
| Interactive and tailored versus non‐active control Self‐report or bio‐verified smoking cessation Follow‐up: 6 ‐ 12 months | 129 per 1000 | 148 per 1000 (130 to 167) | RR 1.15 (1.01 to 1.30) | 6786 (8 RCTs) | ⊕⊕⊝⊝ LOW 2, 3 |
| Internet versus active control Self‐report or bio‐verified smoking cessation Follow‐up: 6 ‐ 12 months | 129 per 1000 | 118 per 1000 (100 to 140) | RR 0.92 (0.78 to 1.09) | 3806 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 4 |
| Internet plus behavioural support versus non‐Internet‐based non‐active control Self‐report or bio‐verified smoking cessation Follow‐up: 6 ‐ 12 months | 78 per 1000 | 131 per 1000 (101 to 169) | RR 1.69 (1.30 to 2.18) | 2334 (5 RCTs) | ⊕⊕⊕⊝ MODERATE 5 |
| Internet plus behavioural support versus non‐Internet‐based active control Self‐report or bio‐verified smoking cessation Follow‐up: 6 ‐ 7 months | 157 per 1000 | 157 per 1000 (132 to 186) | RR 1.00 (0.84 to 1.18) | 2769 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 6 |
| Comparisons between Internet interventions (programmes): tailored/interactive versus not tailored/interactive Self‐report or bio‐verified smoking cessation Follow‐up: 6 ‐ 12 months | 99 per 1000 | 109 per 1000 (98 to 121) | RR 1.10 (0.99 to 1.22) | 14,623 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 4 |
| Comparisons between Internet interventions (messages): tailored/interactive versus not tailored/interactive Self‐reported smoking cessation Follow‐up: 6 months | 90 per 1000 | 106 per 1000 (88 to 128) | RR 1.17 (0.97 to 1.41) | 4040 (3 RCTs) | ⊕⊕⊝⊝ LOW 8, 9 |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1For all, outcome of interest is smoking cessation. Each row represents a different comparison.
2Downgraded one level for risk of bias: High risk of bias in one or more domains for most (five) studies. 3Downgraded one level for inconsistency: Moderate statistical heterogeneity (I2 = 53%). 4Downgraded one level for risk of bias: Unclear or high risk of bias for one or more domains for most (three) studies. 5Downgraded one level for inconsistency: Moderate statistical heterogeneity (I2 = 60%). 6Downgraded one level for risk of bias: Unclear risk of bias for two domains in two studies. 7Downgraded one level for risk of bias: High risk of attrition bias in two studies. 8Downgraded one level for inconsistency: Moderate statistical heterogeneity (I2 = 57%).
Background
Worldwide, tobacco smoking is the primary cause of preventable premature disease and death. Tobacco use is estimated to kill 7 million people a year. If current trends continue, by 2030 tobacco will contribute to the deaths of more than eight million people a year, with 80% of those deaths predicted to occur in the developing world (WHO 2017). People who smoke are more prone to developing various types of cancer, such as those of the oral cavity, larynx, bladder and particularly of the lung. Tobacco smokers are also at substantially increased risk of developing heart disease, stroke, emphysema and other fatal diseases (WHO 2004; Surgeon General 2014). Additionally, passive smoking is associated with serious morbidity (SCTH 1998; Surgeon General 2006). Smoking imposes a huge economic burden on society; maximising the delivery of smoking cessation interventions can achieve more in terms of years of life saved and economic benefits than most medical interventions for smoking‐related illnesses (Coleman 2004). To reduce the growing global burden of tobacco‐related mortality and morbidity, and the impact of tobacco use on economic indicators, tobacco control has become a worldwide public health imperative (WHO 2004).
Prevention and cessation are the two principal strategies in the battle against tobacco smoking. Nicotine is highly addictive (Benowitz 2010), but recent surveys indicate that almost 70% of US and UK smokers would like to stop smoking (Lader 2009; MMWR 2011). Although many smokers attempt to give up on their own, advice from a health professional increases quit attempts and increases the use of effective medications which can nearly double or triple rates of successful cessation (Fiore 2008).
There is good evidence for the effectiveness of brief, therapist‐delivered interventions, such as advice from a physician (Stead 2013a) in helping people to quit smoking. There appears to be additional benefit from more intensive behavioural interventions, such as group therapy (Stead 2017), individual counselling (Lancaster 2017) and telephone counselling (Stead 2013b). However, these more intensive therapies are usually dependent on a trained professional delivering or facilitating the interventions. This is both expensive and time‐consuming for the health providers, and often inconvenient to the recipient, because of lengthy waiting times and the need to take time off work. Another major limitation of these more intensive interventions is that they reach only a small proportion of those who smoke.
Potential benefits of Internet‐based interventions
It is estimated that in 2016 there were 3.5 billion Internet users worldwide (ICT 2016). The Internet has the potential to deliver behaviour change interventions (Japuntich 2006; Strecher 2006; Swartz 2006; Graham 2007). Internet‐based material is an attractive intervention platform, because of low costs for the user, resulting in high cost effectiveness for clinically‐effective interventions (Swartz 2006). Additionally, non‐consumable interventions, such as automated self‐help Internet interventions, are less expensive when delivered on a large scale (Muñoz 2012b). The Internet can be accessed in people's homes, on smart phones, in public libraries and through other public access points, such as Internet cafes and information kiosks, and is available all day every day, even in areas where there are not the resources for a smoking cessation clinic (e.g. some rural or deprived areas and low‐income countries). Internet programmes can also be highly tailored to mimic the individualisation of one‐to‐one counselling. Online treatment programmes also offer a greater level of anonymity than in‐person or phone‐based counselling, and have the potential to reach audiences who might not otherwise seek support because of limited healthcare provision or possible stigmatisation. There is some evidence suggesting that quit rates obtained by using Internet interventions for smoking cessation are comparable with quit rates reported from smoking cessation therapies or smoking cessation groups which may be more costly in terms of money, time or both (Muñoz 2012b). Internet use by young people has grown exponentially and has a powerful role in influencing youth culture, and may therefore be more effective in reaching a target population of young people who smoke than the more traditional providers. A recent review concluded that Internet use may be an effective tool for tobacco treatment interventions with college students, many of whom may not identify themselves as smokers or seek traditional methods of treatment (Brown 2013).
Potential limitations of Internet‐based interventions
Using the Internet for smoking cessation programmes may also have limitations. There are a large number of smoking cessation websites, but they do not all provide a direct intervention. Some studies of popular smoking cessation websites and their quality suggest that smokers seeking tobacco dependence treatment online may have difficulty discriminating between the many sites available (Bock 2004; Etter 2006b). In addition, websites that provide direct treatment often do not fully implement treatment guidelines and do not take full advantage of the interactive and tailoring capabilities of the Internet (Bock 2004). Furthermore, a study on rates and determinants of repeat participation in a web‐based health behaviour change programme suggested that such programmes may reach those who need them the least. For example, older individuals who had never smoked were more likely to participate repeatedly than those who currently smoke (Verheijden 2007). The Internet is also less likely to be used by people with lower incomes, who are more likely to smoke (Eysenbach 2007; Kontos 2007), and less accessed by older people (ONS 2016).
Previous version of this review
The first version of this review was published in the Cochrane Library in 2010 (Civljak 2010). The 2010 version included 20 studies, 10 of which compared an Internet‐based intervention to a non‐Internet‐based intervention or to a usual‐care control, and 10 of which compared two or more Internet‐based interventions. Due to clinical and statistical heterogeneity between the included studies, we did not conduct any meta‐analyses in the original review. Results suggested that some Internet‐based interventions can assist smoking cessation, especially where the intervention was tailored and interactive, but trials did not show consistent effects.
The second version of this review was published in 2013 (Civljak 2013), and identified 28 studies. Fifteen of these compared an active Internet intervention with a non‐Internet arm and 14 compared two Internet interventions (i.e. one study contributed to both categories); 18 were included in the meta‐analysis. All included studies were RCTs, with the exception of one study, which was quasi‐randomised (Haug 2011). We also found 13 potentially relevant ongoing or unpublished trials.
Objectives
To determine:
The effectiveness of Internet‐based interventions for smoking cessation;
Whether intervention effectiveness is altered by tailoring or interactive features;
If there is a difference in effectiveness between adolescents, young adults, and adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled trials. Examples of quasi‐random methods of assignment include alternation, date of birth, and medical record number. There were no restrictions by language.
Types of participants
Current smokers, with no exclusions by age, gender, ethnicity, language spoken or health status. We analyse studies in adolescents and young adults separately from the studies in adults, as both subgroups have particular needs which warrant separate investigation.
Types of interventions
We included studies evaluating Internet interventions in all settings and from all types of providers. There was no exclusion by intervention method or duration. We included trials where the Internet intervention was evaluated with an additional behavioural intervention/support component, or delivered alongside pharmacotherapy such as nicotine replacement therapy (NRT), bupropion or varenicline. The trials compared different types and combinations of intervention. The trials compared Internet‐based programmes to no treatment or to other forms of treatment, such as self‐help booklets. We included trials of interactive, tailored and non‐interactive interventions that focused on standard approaches to information delivery. Interactive interventions were not necessarily personalised. We defined tailored interventions as programmes that were adapted to a participant's characteristics, and interactive interventions as those which involved a two‐way flow of information between the Internet and the participant.
Personalised interventions can vary considerably, from minimal personalisation to those which have been developed based on theoretical models relevant to desired treatment outcomes, such as self‐efficacy. The interventions used in each study were fully described, illustrating the heterogeneity of the interventions (e.g. in relation to varying content, intensity, number of sessions, and duration of contact time).
We excluded trials that used the Internet solely for recruitment and not for delivery of smoking cessation treatment. We also excluded trials where Internet‐based programmes were used to remind participants of appointments for treatment that is not conducted online (e.g. face‐to‐face counselling, or pharmacotherapy). Text messaging, and smart‐phone application interventions are covered in a Cochrane Review of mobile phone interventions (Whittaker 2016), and a review of video‐based interventions is currently in progress with the Cochrane Tobacco Addiction Group (Tzelepis 2017). We therefore do not address these interventions in this review.
Types of outcome measures
The primary outcome is smoking cessation at least six months after the start of the intervention, and longer wherever the data were available. Where studies did not have follow‐up of six months or longer, we report shorter‐term outcomes narratively. We excluded trials with less than four weeks follow‐up.We preferred sustained or prolonged cessation over point prevalence abstinence, but did not exclude studies which only reported the latter. We included studies that relied on self‐reported cessation, as well as those that required biochemical validation of abstinence, but preferred biochemically‐validated rates where available.
Where reported, we extracted data on user satisfaction rates, intervention costs, adverse outcomes, use of the Internet site or programme use, self‐efficacy, use of NRT or other pharmacotherapies, reductions in the number of cigarettes or in smoking frequency, and the impact of Internet programme completion on smoking cessation.
Search methods for identification of studies
Electronic Searches
We searched the specialised register of the Cochrane Tobacco Addiction Group for records including the terms 'Internet' or 'www*' or 'web' or 'net' or 'online' , in the title, abstract or as keywords. The most recent search of the register was 23rd August 2016. At the time of the search the register included the results of searches of the Cochrane Central Register of Controlled Trials (CENTRAL), issue 7, 2016; MEDLINE (through OVID) to update 20160729; Embase (through OVID) to week 201632; PsycINFO (through OVID) to update 20160725. See the Tobacco Addiction Group Module in the Cochrane Library for full search strategies and a list of other resources searched. We also searched clinicaltrials.gov for records of relevant completed or ongoing studies.
Other Sources We searched the reference lists of identified studies for other potentially relevant trials, and contacted authors and experts in this field for unpublished work.
Data collection and analysis
Selection of studies
Two review authors independently assessed potentially relevant papers for inclusion, resolving disagreements through discussion, with each review author writing their reasons for inclusion/exclusion until a consensus was reached, and where necessary by consulting a third party. We noted reasons for exclusion.
Data extraction and management
For this update, the extraction workload was split between three review authors (GT, MD, MS). Two review authors independently extracted data, and one extractor then checked data and compared the findings. This stage included an evaluation of risks of bias (see below). We contacted study authors where outcome data were missing.
We extracted the following information from each trial:
Country and setting;
Method of selection of participants;
Study dates;
Definition of smoker used;
Population type (e.g. college students, people with chronic conditions);
Methods of randomisation (sequence generation and allocation concealment);
Demographic characteristics of participants (e.g. average age, gender, average cigarettes/day);
Intervention and control description (i.e. provider, material delivered, control intervention if any, duration, level of interactivity, etc.);
Outcomes including definition of abstinence used, and whether cessation was biochemically validated;
Proportion of participants with follow‐up data;
Any harms or adverse effects;
Sources of funding;
Conflicts of interest.
Assessment of risk of bias in included studies
Two review authors independently assessed the risks of bias for each study, using the Cochrane 'Risk of bias' tool (Higgins 2011) for each study according to the presence and quality of the randomisation process, concealment of allocation, and description of withdrawals and dropouts.
Measures of treatment effect
We produced a risk ratio (RR) for the outcome for each trial, calculated as: (number who stopped smoking in the intervention group/total number randomised to the intervention group)/(number who stopped smoking in the control group/total number randomised to the control group). A risk ratio greater than one indicates that more people stopped smoking in the intervention group than in the control group. We displayed risk ratios with 95% confidence intervals in forest plots.
We conducted an intention‐to‐treat (ITT) analysis, meaning that we include all those randomised to their original groups, whether or not they remained in the study. We treated dropouts or those lost to follow‐up as continuing smokers.
Assessment of heterogeneity
We considered clinical, statistical and methodological heterogeneity. We assessed statistical heterogeneity using the I2 statistic, which assesses the proportion of the variation between studies due to heterogeneity rather than to chance (Higgins 2003). Values over 50% suggest substantial heterogeneity, but its significance also depends upon the magnitude and direction of the effect and the strength of the evidence (as estimated by the confidence interval).
Data synthesis
We separated trials in adolescents from those in young adults and older adults. We distinguished between tailored or interactive and non‐tailored, non‐interactive interventions. In the five comparisons for which we judged meta‐analysis to be appropriate, we pooled the weighted average of risk ratios, using a Mantel‐Haenszel fixed‐effect model, with a 95% confidence interval. Where there were 10 or more of studies we planned to use funnel plots to help identify possible publication bias, but there were not enough studies reporting any individual outcome for us to do this.
Sensitivity analysis
We used sensitivity analyses to investigate the impact of using data from complete cases (i.e. including only participants who were followed up) as compared to our primary ITT analysis which assumes that those who dropped out or who were lost to follow‐up were continuing smokers.
Summary of findings table
We created a 'Summary of findings' table in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for self‐report or bio‐verified smoking cessation at 6‐months follow‐up or longer, and to draw conclusions about the quality of evidence within the text of the review.
Results
Description of studies
Across the updates we found 286 potentially relevant records through database searching, and screened them by title and abstract (this update: 135; 2013 update: 151). Some studies were spread across more than one record. We assessed 177 full‐text articles for eligibility (this update: 97; 2013 update: 80). We excluded 73 full‐text articles, with reasons (this update: 21; 2013 update: 52). A full list of these studies along with reasons for exclusion can be found in the Characteristics of excluded studies table. Sixty‐seven studies met the inclusion criteria, with 33 of them included in narrative synthesis (this update: 25; 2013 update: 8), and 34 studies were included in quantitative synthesis (meta‐analysis) (this update: 14; 2013 update: 20). Our review now includes data from over 110,000 participants, of whom 35,969 were included in the meta‐analysis. Four studies were cluster‐randomised, and one was quasi‐randomised (Haug 2011).
The Characteristics of included studies table provides further detail and 'Risk of bias' assessments for each included study. See Figure 1 for flow chart of records.
1.
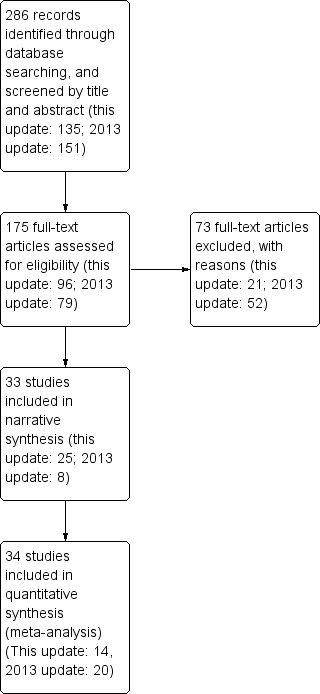
Study flow diagram. Please note that in some cases more than one article was attributable to the same study.
Recruitment and participants
Most studies were conducted in the USA and participants were therefore recruited from that population. Eleven trials were conducted in the Netherlands, five in the UK, one in the Republic of Ireland, three in Australia, two in Norway, two in Switzerland, three in Germany, one in England, one in Belgium, one in Denmark, one in Spain, one in China, and one across the USA and Canada. The studies by Muñoz and colleagues recruited from multiple countries.
In most of the studies recruitment was web‐based, with participants finding the sites through search engines and browsing. Several trials used press releases, billboards, television advertisements and flyers in addition to web‐based recruitment. As a result of these recruitment methods, participants included in these trials were motivated to quit smoking, and chose the Internet as a tool for smoking cessation support. Nineteen studies recruited smokers from healthcare settings: Clark 2004 recruited people undergoing chest computerised tomography as a screening assessment for lung cancer at their first follow‐up visits; Strecher 2008 recruited members of two health management organisations (HMOs); Swan 2010 recruited participants from a large healthcare organisation; Schulz 2014 recruited through health authorities; McClure 2014 identified people from automated healthcare records and invited participants by letter; Haug 2011 recruited participants from three German inpatient rehabilitation centres; Humfleet 2013 recruited participants from three clinics serving people with HIV; Burford 2013 recruited participants from pharmacies when presenting to collect prescriptions or purchase over‐the‐counter medications; Frederix 2015 recruited participants from cardiology departments; Harrington 2016 recruited from hospitals; two studies recruited participants from a Military Veteran Medical Centres (Dezee 2013; Calhoun 2016); Borland 2013 recruited participants during phone calls to national quit lines; Emmons 2013 recruited participants from cancer treatment centres and through websites; seven studies recruited participants from primary care settings (Dickinson 2013; Mehring 2014; Zullig 2014; Houston 2015; Voncken‐Brewster 2015; McClure 2016; Smit 2016); Voncken‐Brewster 2015 recruited through primary care and an online panel. Six studies recruited from other settings: McDonnell 2011 recruited online, sponsored links based on search terms entered into Yahoo or Google, flyers, word of mouth, a press conference, email campaign and a local television campaign; Oenema 2008 recruited from a pool of people registered with an online research agency, including non‐smokers and smokers who were not necessarily motivated to quit at baseline; Skov‐Ettrup 2016 recruited participants from two Danish health surveys; and Bannink 2014 recruited participants from educational institutions. Choi 2014 recruited employees during a regularly scheduled training session. No information was available for one study (Mananes 2014), and In Yang 2016 the source of participants was not clear.
This review includes over 110,000 participants, and 35,969 were included in the meta‐analysis. Most studies recruited a full adult age range, three studies recruited adolescents only (Patten 2006; Woodruff 2007; Bannink 2014), and seven studies recruited young adults, university or college students (An 2008; Simmons 2011; Berg 2014; Emmons 2013; An 2013; Epton 2014; Cameron 2015;). One study recruited adult participants who were childhood cancer survivors (Emmons 2013). Two studies recruited only Korean Americans (McDonnell 2011; Moskowitz 2016). Two studies recruited military veterans, or their families (Dezee 2013; Calhoun 2016). Four studies recruited participants with chronic physical conditions (Zullig 2014; Frederix 2015; Voncken‐Brewster 2015; Yang 2016), and one study recruited hospitalised patients (Harrington 2016). One study recruited pregnant smokers (Herbec 2014). Sample sizes ranged from fewer than 70 (McClure 2016) to nearly 12,000 (Etter 2005). There were more women than men (see Characteristics of included studies) and the mean age ranged from 16 years (Patten 2006; Woodruff 2007; Bannink 2014) to 63 years (Zullig 2014). In 21 studies, participants were offered financial compensation for completing assessment surveys or biochemical analysis (Muñoz 2006 Study 3; Muñoz 2006 Study 4; Woodruff 2007; An 2008; Oenema 2008; Te Poel 2009; Graham 2011; McDonnell 2011; Bricker 2013; Berg 2014; Brown 2014; Fraser 2014; Mehring 2014; Voncken‐Brewster 2015; Choi 2014; Harrington 2016; Calhoun 2016; Cobb 2016; McClure 2016; Moskowitz 2016; Smit 2016). In four studies, the participants could enter a draw to win prizes (Wangberg 2011; Elfeddali 2012; Stanczyk 2014; Borland 2015), and in four studies participants were offered financial compensation and entered into a prize draw (An 2013; Epton 2014; McClure 2014; Cameron 2015).
Interventions
A range of Internet interventions were tested in the included studies, from a very low intensity intervention providing a list of websites for smoking cessation (Clark 2004), to highly intensive interventions consisting of Internet‐, email‐ and mobile phone‐delivered components (Brendryen 2008a; Brendryen 2008b; Borland 2013). Tailored Internet interventions differed in the amount of tailoring, from a bulletin board facility (Stoddard 2008), a multimedia component (McKay 2008), tailored and personalised access (Strecher 2005; Rabius 2008; Wangberg 2011) to very high‐depth tailored stories and highly personalised message sources (Strecher 2008). Some trials also included counselling or support from nurses (Bannink 2014; Choi 2014; Smit 2016), peer coaches (An 2013) or tobacco treatment specialists (Houston 2015). Recent trials have also incorporated online social networks, such as Facebook (Cobb 2016), Twitter (Pechmann 2016), and WeChat (Yang 2016), and online forums (Dickinson 2013), chat rooms (Calhoun 2016), and support groups (Houston 2015). Two interventions were very distinct from the rest. In addition to brief smoking advice, Burford 2013 used an Internet‐based three‐dimensional face age progression simulation software package to create a stream of aged images of faces from a standard digital photograph. The resulting aged image was adjusted to compare how the participant aged as a smoker versus as a non‐smoker. In Wittekind 2015, the authors used an online version of the approach‐avoidance task, where participants used the computer mouse to pull (i.e. approach, leading to an enlarged picture) or push (i.e. avoid, leading to a reduced picture) neutral or smoking‐related pictures.
We also identified nine trials of lifestyle interventions that included a smoking cessation component. These interventions included content on a range of topics, including diet and healthy eating, physical activity and fitness, alcohol and drug use, sexual behaviour, unpleasant sexual experiences, bullying, mental health, patient‐provider relationships, and medication management (Oenema 2008; Dickinson 2013; Bannink 2014; Epton 2014; Schulz 2014; Zullig 2014; Cameron 2015; Frederix 2015; Voncken‐Brewster 2015).
More details are given in the comparisons section below, and descriptions of the main features of each study intervention are provided in the Characteristics of included studies table.
Outcomes
Cessation
Forty‐nine studies reported smoking status at least six months after the start of the intervention; the remaining 18 studies followed participants for less than six months (Etter 2005; Strecher 2005; Swartz 2006; Oenema 2008; Stoddard 2008; An 2013; Bricker 2013; Dezee 2013; Bannink 2014; Berg 2014; Herbec 2014; Mananes 2014; Mehring 2014; Shuter 2014; Zullig 2014; Wittekind 2015; Cobb 2016; Pechmann 2016).
Studies reported a range of definitions of abstinence at the time of follow‐up. Where studies reported abstinence rates for more than one definition we displayed the effect using the most conservative outcome (with the exception of An 2008, see below). For 21 studies, seven‐day smoking abstinence was the main outcome measure (Clark 2004; Japuntich 2006; Muñoz 2006 Study 3; Muñoz 2006 Study 4; Swartz 2006; McKay 2008; Stoddard 2008; Strecher 2008; Humfleet 2013; Muñoz 2009; Te Poel 2009; McDonnell 2011; Haug 2011; Wangberg 2011; Choi 2014; Fraser 2014; Shuter 2014; Houston 2015; Calhoun 2016; Cobb 2016; McClure 2016). Ten studies reported 30‐day self‐reported smoking abstinence (Patten 2006; Swan 2010; McDonnell 2011; Graham 2011; Simmons 2011; Emmons 2013; McClure 2014; Harrington 2016; Mavrot 2016; Moskowitz 2016). Mason 2012 reported three‐month prolonged abstinence at six months from baseline. Borland 2015, Stanczyk 2014, and Yang 2016 reported sustained abstinence at six months, while Borland 2013 and Brown 2014 reported six‐month sustained abstinence at seven‐month follow‐up. Bolman 2015 reported five‐month continuous abstinence at six‐month follow‐up (allowing a one‐month grace period) and Smit 2016 reported six‐month prolonged abstinence at 12‐month follow‐up. Elfeddali 2012 and Skov‐Ettrup 2016 reported continuous 12‐month abstinence. An 2008 assessed six‐month prolonged abstinence from smoking; this study also reported seven‐day and 30‐day prevalence abstinence. We used 30‐day rates as our primary outcome, because the programme did not involve setting a quit date, and the prolonged abstinence was based on self‐report of time since last cigarette rather than repeated assessments of abstinence.
Six of the 18 short‐term studies assessed self‐reported point prevalence abstinence at three‐month follow‐up only (Etter 2005; Swartz 2006; Stoddard 2008; Bricker 2013; Dezee 2013; Mananes 2014). Shuter 2014 reported biochemically‐verified point prevalence abstinence at three‐month follow‐up, Pechmann 2016 reported sustained abstinence at two‐month follow‐up, and Mehring 2014 reported continuous cessation at 12 weeks. An 2013 and Berg 2014 reported 30‐day prolonged abstinence at 12 weeks whilst Strecher 2005 assessed 28‐day continuous abstinence rates at six‐week follow‐up, and 10‐week continuous abstinence rates at 12‐week follow‐up. Herbec 2014 assessed four‐week continuous abstinence whilst Wittekind 2015 and Burford 2013 assessed point prevalence at four weeks and six months, respectively. In one study, seven‐day smoking abstinence was a secondary outcome, while time spent on the website, use of pages, cessation aids used in the past and during the study period were the main outcome measures (Stoddard 2008).
Finally, there were nine trials of lifestyle interventions that included a smoking cessation component. Zullig 2014 and Bannink 2014 assessed point prevalence abstinence at three and four months, respectively. Three studies assessed sustained cessation at six months (Epton 2014; Cameron 2015; Voncken‐Brewster 2015). Oenema 2008 measured smoking behaviour at one month, Frederix 2015 at 24 weeks, Dickinson 2013 at six months, and Schulz 2014 at 24 months, but the authors did not specify what measure of smoking cessation was used.
Due to the limited face‐to‐face contact and to data collection through Internet or telephone interviews, biochemical validation to confirm self‐reported smoking abstinence was conducted in only 18 trials. Nine measured carbon monoxide (CO) in expired air (Clark 2004; Japuntich 2006; Patten 2006; An 2008; Simmons 2011; Burford 2013; Dezee 2013; Humfleet 2013; Shuter 2014), five measured salivary cotinine (Elfeddali 2012; Harrington 2016; Brown 2014; Calhoun 2016; Smit 2016), two measured urinary cotinine (Choi 2014; Mehring 2014) and two measured nicotine and hair cotinine (Epton 2014; Cameron 2015). As Harrington 2016 only biochemically verified abstinence among a subset of self‐reported abstainers at follow‐up, we used self‐reported rates rather than validated rates.
Other outcomes
User satisfaction was measured in 21 studies (Strecher 2005; Woodruff 2007; Stoddard 2008; Muñoz 2009; Te Poel 2009; Choi 2014; Bricker 2013; Emmons 2013; Bannink 2014; Berg 2014; Brown 2014; Fraser 2014; Mananes 2014; Schulz 2014; Shuter 2014; Stanczyk 2014; Bolman 2015; Frederix 2015; Wittekind 2015; Harrington 2016; McClure 2016). Intervention cost was reported in eight studies (Etter 2005; Rabius 2008; Borland 2013; Burford 2013; Mehring 2014; Calhoun 2016; Harrington 2016; Skov‐Ettrup 2016). Few studies reported adverse events. Borland 2013 reported one case of hospitalisation at one month, while Frederix 2015 reported a new pathology in one intervention participant lost to follow‐up. Mehring 2014 reported that 26 participants experienced adverse events. In the intervention group, four participants reported weight gain, two participants reported increased perceived stress, one participant had a sleep disorder, and one participant had increased irritability. In the usual‐care group, six participants had increased perceived stress, five participants had cardiovascular problems, four participants reported fatigue, four participants reported weight gain, two participants had sweating, one participant had a sleep disorder, and one participant specified increased irritability. Three other studies reported adverse events but the interventions in these studies included smoking cessation medicines (Dezee 2013; McClure 2016; Yang 2016). Use of the Internet site or programme use was measured in 40 studies (Clark 2004; Japuntich 2006; Swartz 2006; Brendryen 2008b; McKay 2008; Oenema 2008; Rabius 2008; Strecher 2008; Muñoz 2009; Swan 2010; McDonnell 2011; Wangberg 2011; Borland 2013; An 2013; Bricker 2013; Dickinson 2013; Emmons 2013; Berg 2014; Brown 2014; Choi 2014; Epton 2014; Fraser 2014; Herbec 2014; Mananes 2014; McClure 2014; Schulz 2014; Shuter 2014; Zullig 2014; Borland 2015; Cameron 2015; Houston 2015; Voncken‐Brewster 2015; Calhoun 2016; Cobb 2016; Harrington 2016; Mavrot 2016; McClure 2016; Moskowitz 2016; Skov‐Ettrup 2016; Pechmann 2016). Smoking cessation self‐efficacy was measured in 16 trials (Haug 2011; Wangberg 2011; Emmons 2013; Choi 2014; Epton 2014; McClure 2014; Schulz 2014; Shuter 2014; Stanczyk 2014; Bolman 2015; Cameron 2015; Calhoun 2016; Harrington 2016; Mavrot 2016; Moskowitz 2016; Skov‐Ettrup 2016). Use of NRT or other pharmacotherapies was a secondary outcome measure in 12 trials (Patten 2006; Brendryen 2008b; McKay 2008; Strecher 2008; Swan 2010; Borland 2013; Emmons 2013; McClure 2014; Mehring 2014; Borland 2015; Harrington 2016; Mavrot 2016). Eleven studies assessed reductions in the number of cigarettes or in smoking frequency as secondary outcomes (Patten 2006; Woodruff 2007; Choi 2014; Berg 2014; Epton 2014; Mananes 2014; Mehring 2014; Cameron 2015; Voncken‐Brewster 2015; Wittekind 2015; Harrington 2016;). McDonnell 2011, Elfeddali 2012, Berg 2014, Mananes 2014, Shuter 2014, Zullig 2014, Voncken‐Brewster 2015, and Moskowitz 2016 also reported the impact of Internet programme completion on smoking cessation.
Comparisons
In this update, we have grouped studies according to whether they (1) compared an Internet intervention with a non‐active control arm (e.g. printed self‐help guides or usual care); (2) compared an Internet intervention with an active control arm (e.g. telephone or face‐to‐face counselling); (3) evaluated the addition of an Internet programme plus behavioural support; or (4) compared one Internet intervention to another. Where data were available, we grouped analyses by age (i.e. adults, young adults, adolescents). We treated printed self‐help materials as a non‐active control since the effect of these is typically small, although tailored materials may have more effect (Hartman‐Boyce 2014). In 15 trials, all participants were using, or were offered, pharmacotherapy (Strecher 2005; Japuntich 2006; Brendryen 2008a; Brendryen 2008b; Strecher 2008; Swan 2010; Dezee 2013; Emmons 2013; Choi 2014; Fraser 2014; Shuter 2014; Calhoun 2016; McClure 2016; Pechmann 2016; Yang 2016) and the Internet component was thus being evaluated as an adjunct to pharmacotherapy. We grouped these in comparisons based on the nature of the Internet component and the control. There were two exceptions to this: (1) Yang 2016 compared three trial arms, and one of these was not prescribed smoking cessation medication; and (2) Fraser 2014 compared variations in intervention components in which five intervention components were either "turned on or off"; one of these components was NRT, and therefore not all arms received NRT. Fraser 2014 was not eligible for meta‐analysis.
One study contributed to three comparisons (Borland 2013), and three studies each contributed to two comparisons (Simmons 2011; Skov‐Ettrup 2016; Smit 2016).
Please note that we did not include data from lifestyle interventions in the meta‐analysis, as data for smokers only were not available.
Internet intervention compared to non‐active control
Twenty‐one trials compared an Internet intervention to a non‐active control (Clark 2004; Swartz 2006; Woodruff 2007; An 2008; Oenema 2008; McDonnell 2011; Haug 2011; Elfeddali 2012; Borland 2013; Emmons 2013; Humfleet 2013; Epton 2014; Mehring 2014; Shuter 2014; Zullig 2014; Cameron 2015; Voncken‐Brewster 2015; Wittekind 2015; Harrington 2016; Smit 2016; Yang 2016).
Non‐interactive, non‐tailored
Three studies compared a non‐interactive, non‐tailored Internet programme with a non‐active control. Clark 2004 tested a very low intensity intervention for smokers having computerised tomography lung screening; a handout with a list of 10 Internet sites related to stopping smoking with a brief description of each site was compared to printed self‐help materials. Due to the low‐intensity nature of this intervention (similar to control arms in other studies), we did not include Clark 2004 in the analysis, but report results narratively. Humfleet 2013 compared an Internet‐based treatment programme to a printed self‐help guide. All participants smoking more than five cigarettes a day at study entry were offered NRT. In Wittekind 2015 participants were presented with non‐interactive/tailored smoking‐related pictures, and neutral pictures using an online platform, and the control group was sent an email explaining that participants would receive the programme after final follow‐up.
Tailored or interactive, or both
Woodruff 2007 was conducted in adolescents and evaluated an Internet‐based virtual reality world combined with motivational interviewing, conducted in real time by a smoking cessation counsellor. There was a measurement‐only control condition involving four online surveys. In An 2008 intervention group participants received USD 10 a week to visit an online college magazine that provided personalised smoking cessation messages and peer email support. The control group received only a confirmation email containing links to online health and academic resources. Both groups were informed about a campus‐wide 'Quit & Win' contest sponsored by the University Health Service. Haug 2011 evaluated a tailored and interactive Internet‐based programme for exclusive use by registered patients of participating rehabilitation hospitals. The intervention group received a complex intervention consisting of three modules (see Characteristics of included studies) and the control group received usual care. McDonnell 2011 compared a web‐based cognitive behavioural self‐help programme based on stages of change with a booklet containing the same content; material was not tailored to participants' responses. Elfeddali 2012 evaluated a programme with tailored feedback and assignments (i.e. one arm received six assignments whereas a second arm received 11) and compared this to usual care. Borland 2013 recruited smokers and recent quitters. The intervention 'QuitCoach' auto‐generated tailored cessation advice based on questionnaire responses. Participants in the control group were given contact details for web‐ and telephone‐based support. Emmons 2013 recruited childhood cancer survivors who were current adult smokers. The intervention was tailored based on participants' motivation and readiness to quit smoking, and was compared to a letter encouraging the person to quit smoking with worksheets. Free pharmacotherapy (nicotine patch or bupropion) was offered to participants and any smoking partner/spouse who wished to quit. Harrington 2016 compared 'Decide2Quit' to usual care. 'Decide2Quit' included multiple web pages on smoking and cessation‐related topics, links to other websites and interactive tools, a chat forum with a quit advisor, and tailored emails based on readiness to quit. In usual care, hospital staff would advise patients to quit and offer information about where to find support. Smit 2016 compared 'Multiple Computer Tailoring', which sent tailored feedback messages, to standard care for smoking cessation. Skov‐Ettrup 2016 'e‐quit' was a tailored and interactive Internet intervention, with optional text message support, where the website included a daily video of a person at the same stage of the smoking cessation process, exercises for increasing motivation and identifying coping strategies, tailored feedback based on level of dependence (pharmacotherapy was encouraged for those with high dependence), a blog option, and an action planning tool. The intervention was compared to usual care (sign‐posted to Danish national quitline, and callers who were ready to quit were encouraged to set a quit date and received information about pharmacotherapy if relevant). In Yang 2016 participants were randomised to an 'eChat' smoking cessation support group, which was both tailored and interactive. Information on smoking cessation was provided twice‐weekly for the first four weeks, and for the entire intervention period they could use 'WeChat' to communicate with a doctor who would answer their questions. 'WeChat' was compared to usual care. Both arms received NRT.
Lifestyle interventions
Five studies compared tailored/interactive Internet‐based lifestyle interventions to a non‐active control; we did not include these studies in the meta‐analysis as data only for smokers were not available (Oenema 2008; Epton 2014; Zullig 2014; Cameron 2015; Voncken‐Brewster 2015). Oenema 2008 tested a web‐based intervention that targeted fat intake, physical activity, and smoking. Participants who indicated that they were smokers at baseline were encouraged to complete the smoking module which was interactive and included tailored feedback. In Epton 2014 participants in the lifestyle intervention arm were directed to the 'U@Uni' website which included theory‐based messages relevant the targeted health behaviours and a planner that contained instructions to form implementation intentions. Participants were able to access information that was of interest to them, and could also download a smartphone app that was available throughout the year. The intervention was compared to a measurement‐only control. Zullig 2014 recruited participants with or at risk of cardiovascular disease. The intervention was tailored to participants' risk scores and aimed to improve multiple lifestyle behaviours (e.g. diet, exercise, smoking), and was compared to usual care. Voncken‐Brewster 2015 recruited people with or at risk of chronic obstructive pulmonary disease. In the 'Master your breath' lifestyle intervention participants received computer‐tailored feedback to promote changes in smoking cessation and physical activity, with usual care as the comparator. Cameron 2015 reported a repeat trial of Epton 2014.
Studies with follow‐up of less than six months
A further three studies were not included in the meta‐analysis because of insufficient follow‐up. Swartz 2006 compared a video‐based Internet site that presented strategies for smoking cessation and motivational materials tailored to the user’s race/ethnicity, gender and age. After follow‐up the control group had access to the programme. In Mehring 2014 the intervention website offered behavioural support and included interactive features, video clips, and quizzes; participants received feedback about their motivation and were sent corresponding short message service (SMS) messages. The control group received treatment as usual. In Shuter 2014 the intervention group received online modules designed to educate, motivate, and increase self‐efficacy to quit, and this was compared to usual smoking cessation treatment; both arms were offered a three‐month supply of nicotine patches.
Internet intervention compared to active control
Adults
Seven studies compared an Internet intervention to an active control (i.e. more intensive than usual care or self‐help only) (Swan 2010; Humfleet 2013; Borland 2013; Calhoun 2016; Skov‐Ettrup 2016). Swan 2010 was a three‐arm trial comparing an established proactive telephone counselling intervention, an interactive website based on the same programme, and a combination of phone and Internet components, all provided in conjunction with varenicline use. As well as comparing an Internet‐based intervention with a printed self‐help guide, Humfleet 2013 also included a third arm which was offered six sessions of in‐person counselling. Borland 2013 compared 'QuitCoach' which was a personalized, automated tailored cessation program based on cognitive–behavioural principles, to the 'onQ program' which was based on the same cognitive–behavioural model as QuitCoach but was delivered via a stream of SMS messages. In Calhoun 2016 Military Veterans were randomised to receive QuitNet®, a website offering personalised cessation support, access to online smoking cessation counsellors and other interactive features (i.e. forums, chat rooms, or to group or telephone counselling). In both groups interested participants received NRT. Skov‐Ettrup 2016 'e‐quit' was a tailored and interactive Internet intervention, with optional text message support, where the website included a daily video of a person at the same stage of the smoking cessation process, exercises for increasing motivation and identifying coping strategies, tailored feedback based on level of dependence (i.e. pharmacotherapy was encouraged for those with high dependence), blogging option, and an action planning tool. The intervention was compared to five sessions of telephone counselling.
Adolescents and young adults
Patten 2006 compared a home‐based, Internet‐delivered treatment for adolescent smoking cessation with a clinic‐based brief office intervention (BOI) consisting of four individual counselling sessions. Adolescents assigned to the Internet condition had access to the website for 24 weeks and abstinence was assessed at the end of this period. In Simmons 2011 university students were randomised to one of two intervention arms: (1) 'Websmoke' was a tailored and interactive Internet intervention, in which participants in the intervention were asked to create a video message about smoking to be included on the website, and participants had access to the 'Websmoke' website which included interactive components (e.g. quizzes, and a smoking cost calculator), or (2) non‐tailored and non‐interactive Internet intervention, in which participants viewed an identical web page to the 'Websmoke' condition, but the interactive features were absent and they were not instructed to create a video message. The control arm was a paper‐based version of the website, and participants were instructed to make a group video about smoking.
Lifestyle interventions
One study that compared tailored/interactive Internet‐based lifestyle interventions to an active control was not included in the meta‐analysis as data only for smokers were not available. In Frederix 2015 patients with coronary artery disease or chronic heart failure or both received an online lifestyle intervention delivered as an adjunct to non‐Internet‐based conventional centre‐based cardiac rehabilitation. The programme focused on physical activity, diet, and smoking cessation, and was compared to a centre‐based rehabilitation programme including rehabilitation sessions and exercise training sessions, 1 or more consultations with a dietician, and 1 or more consultations with a psychologist.
Studies with follow‐up of less than six months
Dezee 2013 compared GetQuit, a web‐based counselling programme with online activities, to in‐person group counselling; both arms received a standard dose of varenicline for 12 weeks.
Internet intervention plus behavioural support
Nine studies evaluated Internet programmes alongside behavioural support (Japuntich 2006; Brendryen 2008a; Brendryen 2008b; Swan 2010; Burford 2013; Borland 2013; Bannink 2014; Choi 2014; Smit 2016). Japuntich 2006 evaluated a web‐based system incorporating information, support and problem‐solving assistance which was delivered as an adjunct to bupropion and brief face‐to‐face counselling, compared to bupropion and brief face‐to‐face counselling alone. Two studies reported by Brendryen (Brendryen 2008a; Brendryen 2008b) evaluated 'Happy Endings', a one‐year programme delivered by the Internet and cell phone, consisting of more than 400 contacts by email, web pages, interactive voice response (IVR), and SMS technology, and tailored to participant responses. Brendryen 2008a recruited people attempting to quit without NRT, whilst Brendryen 2008b offered a free supply of NRT to all participants. Swan 2010 evaluated the addition of an interactive website to proactive phone counselling. In Borland 2013 integrated 'QuitCoach', which was an interactive/tailored online programme with 'QuitonQ' which involved a stream of interactive SMS messages. 'QuitonQ' included advice on quitting and motivational messages in which the user can report changes in behaviour (e.g. a quit attempt) to receive stage‐specific SMS messages. 'QuitCoach' and 'QuitonQ' were offered as a package in which users could subsequently use either or both parts. The integrated programme was compared to (1) a non‐active control arm in which participants were given brief information on web‐ and telephone‐based assistance available in Australia, and (2) SMS messaging alone. In Burford 2013 an Internet‐based three‐dimensional age progression software package was used to create aged images of the participants' faces based on a standard digital photograph, with the resulting aged image adjusted to compare how the participant aged as a smoker versus a non‐smoker. Participants also received standard two‐minute smoking cessation advice from the pharmacist. The control arm received two‐minute smoking cessation advice from the pharmacist. Choi 2014 participants were randomised to the 'Tobacco Tactics' website, which was delivered as an adjunct to telephone‐based behavioural support. The website offered tailored images and cessation feedback, with other interactive features (assessment of dependence, smoking calculator, and progress monitor, etc.). The control arm were encouraged to call 1‐800‐quit‐now. In both arms, NRT, varenicline or bupropion were available upon request. In Smit 2016 'Multiple Computer Tailoring'‐plus‐counselling participants received a tailored feedback letter, and at six weeks were offered counselling meetings with a nurse. Participants in the control arm received treatment as usual for smoking cessation.
Lifestyle interventions
In Bannink 2014 adolescents received one of two tailored and interactive lifestyle interventions as adjuncts to a behavioural component: (1) in the 'E‐health4Uth‐only' condition participants received tailored messages to reinforce healthy behavioural changes, were provided links to relevant websites and could self‐refer for face‐to‐face or email consultation with the mental health nurse; or (2) in the 'E‐health4Uth + consultation' condition participants received the same intervention as the E‐health4Uth‐only group, with those at risk of mental health problems invited for a consultation with the nurse. Interventions were compared against self‐referral to the nurse for face‐to‐face or email mental health consultation. In Frederix 2015 participants received a 'Center‐Based Cardiac Rehabilitation Program', which was a tailored and interactive Internet intervention that was delivered as an adjunct to a non‐Internet‐based behavioural intervention. The 24‐week programme focused on multiple cardiac rehabilitation components and used both physical activity telemonitoring and dietary/smoking cessation/physical activity telecoaching strategies. Participants were prescribed patient‐specific exercise training protocols, and a telecoaching system to provided them with feedback by email and SMS once weekly, encouraging them to gradually achieve predefined exercise training goals. In addition, participants received emails or SMS text messages or both (once weekly) with tailored dietary and smoking cessation recommendations. The smoking cessation telecoaching programme included information on major risks associated with smoking, the health benefits of smoking cessation, and nicotine replacement therapy. The control group was a centre‐based rehabilitation programme which was a non‐Internet‐based active control arm, and included 45 multidisciplinary rehabilitation sessions and at least two exercise training sessions a week delivered over 24 weeks. The group had at least one consultation with the dietician about healthy eating, and at least one consultation with a psychologist who aimed to improve their self‐efficacy to change prior unhealthy lifestyle behaviours, and assessed the participant's mood.
Comparisons between different Internet interventions
Thirty‐one trials compared two or more different Internet interventions.
Studies comparing tailored/interactive smoking cessation interventions to non‐tailored, non‐interactive smoking cessation interventions
Follow‐up of six months or longer
Ten studies included in the meta‐analysis compared tailored/interactive interventions to non‐tailored/interactive interventions (Rabius 2008; Te Poel 2009; Simmons 2011; Wangberg 2011; Graham 2011; Mason 2012; Brown 2014; Stanczyk 2014; Mavrot 2016; McClure 2016). Rabius 2008 compared five tailored and interactive Internet services with the targeted, minimally‐interactive American Cancer Society website providing stage‐based quitting advice and peer modelling. Graham 2011 compared an interactive tailored intervention with static information‐only content on 'QuitNet'. Te Poel 2009 compared tailored to non‐tailored messages sent after participants had completed an online survey, and information was gathered through a website. Wangberg 2011 compared a multicomponent, non‐tailored intervention for smoking cessation (control) with a version of the same intervention with tailored content delivered by website and email. Simmons 2011 compared the 'Websmoke' website in which participants had access to information about the harms of smoking and benefits of quitting, and interactive components (e.g. quizzes), and videos from peers, and were asked to upload a video about their own smoking. Participants in the control group viewed an identical smoke‐free website without interactive features and were asked to provide feedback on the website. Mason 2012 compared tailored to non‐tailored messages sent after participants had completed an online survey, and information was gathered through a website. Brown 2014 compared the website 'StopAdvisor', which included advice on quitting, and interactive features (e.g. calendar, personal progress reports, the 'StopAdvisor' Facebook page, etc.), to a one‐page static website. Participants in both arms were encouraged to use medication, and to use the NHS Stop Smoking Services. In Stanczyk 2014 the interventions were both tailored and interactive Internet interventions. Text‐ and video‐based web interventions were delivered over four months, and the content of the intervention was exactly the same across the text‐ and video‐based interventions, and was tailored to motivation to quit. Participants received tailored feedback on their smoking behaviour and how to prepare to quit. The control group received web‐based generic short text advice. Mavrot 2016 compared the 'Stop‐Tabac' website which involved a series of automatic, personalised feedback reports and emails based on the participant's answers to a tailoring questionnaire, and a personal web page with progress graphs for tobacco dependence, withdrawal symptoms, etc. to a non‐tailored, non‐interactive Internet intervention based on health behaviour theories. McClure 2016 compared the 'MyMAP' intervention, which included on‐demand adaptively‐tailored advice for managing withdrawal, a secure messaging system, and personalised reports, to the 'mHealth Self‐help Quit Guide', which included psychoeducational content for quitting smoking (the content was standardised and not tailored). Both arms received a 12‐week course of varenicline. Three studies compared tailored messages to non‐tailored messages.
Studies with follow‐up of less than six months
Six studies that compared tailored/interactive and non‐tailored/interactive interventions were not included in the meta‐analysis, due to insufficient follow‐up (Strecher 2005; Stoddard 2008; Bricker 2013; Herbec 2014; Mananes 2014; Pechmann 2016). Strecher 2005 assigned purchasers of a particular brand of nicotine patch to receive either web‐based, tailored behavioural smoking cessation materials or web‐based non‐tailored materials. Stoddard 2008 evaluated the impact of adding a bulletin board facility to the smokefree.gov cessation site. Bricker 2013 compared 'Webquit.org' which was based on acceptance and commitment therapy to smokefree.gov which was a non‐tailored and non‐interactive Internet intervention . Herbec 2014 compared the 'MumsQuit' website, which contained an interactive, personalised, and structured quit plan, to a one‐page static, non‐personalised website that provided brief standard advice for users. Mananes 2014 compared a tailored/interactive version of a web‐based smoking cessation programme based on the Clinical Guidelines for the Treatment of Smoking and cognitive behavioural therapy methods to a non‐tailored/interactive version of the web page. Pechmann 2016 compared 'Tweet2Quit', a Twitter‐based intervention which involved daily discussions, automated messages and daily engagement auto‐feedback to smokefree.gov. Both arms were provided with a 56‐day supply of nicotine patches appropriate to their baseline smoking level.
Lifestyle interventions
Two studies compared tailored/interactive and non‐tailored/interactive lifestyle interventions. Dickinson 2013 compared an enhanced site of materials designed to assist participants in behaviour change, which also included an online forum where they could post issues and discuss them with other participants working on similar behavioural changes, and an 'Ask the Expert' section, where participants could post questions for the clinical team. The enhanced site was compared to a basic version of the website that had no tailored/interactive features. In Epton 2014 participants assigned to the intervention arm were directed to the U@Uni website to view the online resources, which included theory‐based messages (i.e. text, videos and links to further information) relevant to fruit and vegetable consumption, physical activity, alcohol consumption and smoking status, and a planner that contained instructions to form implementation intentions; the intervention was compared to a measurement only control condition. In Schulz 2014 all groups received a health risk appraisal of physical activity, fruit and vegetable consumption, and alcohol and cigarette consumption. Questionnaires were used to measure the psychosocial concepts of the 'I‐Change' model. Participants were invited to change unhealthy behaviours and received feedback on all behaviours. Participants in the control arm received a "minimal intervention".
Other types of comparisons between Internet interventions
The remaining studies compared different components of Internet interventions. Etter 2005 compared the efficacy of two versions of an Internet‐based, computer‐tailored cessation programme; the control group received a shorter version modified for use by those smoking and buying NRT over the counter, although use of NRT was not a condition of enrolment.
A series of three studies by Muñoz and colleagues evaluated adjuncts to an online resource, the 'Guia', a National Cancer Institute evidence‐based intervention first developed for Spanish‐speaking smokers. In separate English language (Muñoz 2006 Study 3) and Spanish (Muñoz 2006 Study 4) studies, the control condition was the provision of access to the 'Guia' intervention and 'Individually Timed Educational Messages'. The intervention tested was the addition of an online mood management course consisting of eight weekly lessons. Muñoz 2009 also used the 'Guia' intervention as the control condition, but in a four‐arm design that evaluated the successive addition of 'Individually Timed Educational Messages', the mood management condition used in the Muñoz 2006 studies, and a 'virtual group' asynchronous bulletin board. The study recruited English‐ and Spanish‐speaking Internet users from 68 countries. Follow‐up was at 2½ months.
McKay 2008 compared the 'Quit Smoking Network', a web‐based tailored cessation programme with a multimedia component, with 'Active Lives', a web‐based programme providing tailored physical activity recommendations and goal setting in order to encourage smoking cessation.
Strecher 2008 identified active psychosocial and communication components of a web‐based smoking cessation intervention and examined the impact of increasing the tailoring depth on smoking cessation among nicotine patch users. Five components of the intervention were randomised using a fractional factorial design: high‐ versus low‐depth tailored success story, outcome expectation, and efficacy expectation messages; high‐ versus low‐personalized source; and multiple versus single exposure to the intervention components. Abstinence was assessed after six months.
In An 2013 the 'Tailored Health Message' intervention participants were required to visit the site and report on their cigarette smoking, alcohol use, exercise, and breakfast consumption. The intervention focused on building social support for healthy lifestyles, eating healthy breakfasts, increasing exercise, smoking cessation or reduction, and responsible drinking or abstinence from drinking. The 'Tailored Health Message + Peer Coach' intervention included all components of the 'Tailored Health Message' intervention but was both interactive and tailored as participants were allocated a peer coach who viewed the participants’ behavioural tracking progress charts and sent a personal video message. Both arms were compared to the 'General Lfestyle' group, which received six sessions of non‐health‐related lifestyle content over the Internet and was tailored but not interactive.
In Berg 2014 the Intervention and control arms were both tailored and interactive, but with or without emails for incentives or 'daily deals' for local businesses. Participants in both arms had access to modules that were delivered twice a week by email. Modules included short videos about smoking, advice to quit and cessation resources (e.g. pharmacotherapy options). Participants completed a timeline reporting cigarette and alcohol consumption, and time spent exercising; a graph was produced of these health behaviours over the course of the intervention. This study was not included in the meta‐analysis.
Fraser 2014 had five intervention components that were either "turned on or off" for each participant: smokefree.gov (versus a "light" website), telephone quit‐line counselling (versus none), a smoking cessation brochure (versus a "light" brochure), motivational e‐mail messages (versus none), and mini‐lozenge NRT (versus none).
McClure 2014 tested 16 variations of the 'Q2' intervention based on different stages of readiness to quit. Each participants' intervention was similar, but varied based on the randomly‐assigned experimental factor levels: 'message tone', 'navigation autonomy', 'proactive emails', and 'testimonials'.
Bolman 2015 participants received an interactive and tailored Internet‐based intervention with or without an email letter. In both arms participants received a series of tailored email letters aiming to encourage cessation. The experimental group also received tailored advice on action planning based on the participant’s response to questions about action planning at baseline.
In Borland 2015 all three arms were tailored and interactive: (1) 'QuitCoach' was a web‐based automated tailored advice programme that provided a tailored advice letter based on the participant's answers, and allowed smokers to quit to their own schedule; (2) 'QuitCoach + Rapid Implementation' included participants who had not committed to a quit date within the next two days; (3) 'QuitCoach + Structured Planning' included provision of encouragement and tools for structured planning.
Houston 2015 compared three tailored and interactive Internet interventions, with or without additional motivational messaging: (1) Decide2Quit.org was a smoking‐cessation website that included motivational information tailored to readiness to quit and other baseline factors, cessation barrier calculators and games, resources about smoking, seeking social support, and talking to a doctor about quitting; (2) The 'Messaging Group' intervention arm were allocated to Decide2Quit.org, and also received brief motivational email messages that were tailored to an individual smoker’s readiness to quit, and included messages written by smokers for other smokers; (3) The 'Personalised Group' intervention arm were allocated to Decide2Quit.org, received the same tailored motivational emails as in the 'Messaging Group', and in addition they had access to personal online support from trained tobacco treatment specialists, and a link to an online support group (BecomeAnEx.org).
Cobb 2016 compared a Facebook intervention with or without reminders for participants to use the website. The Facebook intervention was based on the '5As' model (i.e. Ask, Advise, Assess, Assist, and Arrange). Participants were asked if they smoked and were advised to quit, participant's readiness to quit was assessed and they were encouraged to plan a quit date; other interactive features were included (i.e. quit‐day countdown, savings to date). In the 'Facebook intervention with alerting' arm participants received additional online alerts to remind them to log in. This study was not included in the meta‐analysis, as variations of diffusion were compared rather than interventions.
Moskowitz 2016 compared high and low reinforcement, plus the 'QiW' programme. The intervention was a tailored/interactive cognitive‐behavioural programme based on the stages of change, and included short introductory videos using computer animations that were available in English and Korean. The high‐reinforcement condition included online interim surveys with financial incentives for these assessments and also for programme completion, and participants received reminders about the incentive with a monthly reminder to complete the interim survey.
Risk of bias in included studies
We have rated risks of bias in the following domains: (1) selection bias: random sequence generation and allocation concealment; (2) attrition bias; and (3) other potential sources of bias. While we rated most studies at low risk of bias in most or all domains, we judged a number of studies to be at high or unclear risk of bias. Over a quarter were at high or unclear risk of attrition bias, characterised by overall attrition rates greater than 50%, or more than 20% difference in attrition rates between trial arms. Several studies were at unclear risk, as there was insufficient detail to properly assess risks of bias for random sequence generation or allocation concealment or both. Figure 2 is a graphical representation of risks of bias across domains; see Characteristics of included studies for details of risk of bias assessments for each study.
2.
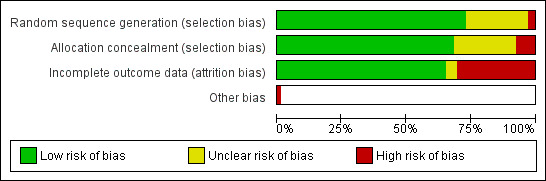
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged two studies to be at high risk of bias for both random sequence generation and allocation concealment. In Burford 2013, participants were recruited and assigned by the researcher to the different arms of the study on alternate weeks. Skov‐Ettrup 2016 allocated participants by repeatedly applying a fixed sequence of four numbers. Fourteen studies did not provide sufficient detail with which to assess methods of random sequence generation and hence we rated them as 'unclear' (Clark 2004; Strecher 2005; Japuntich 2006; Patten 2006; Woodruff 2007; Haug 2011; Dickinson 2013; Emmons 2013; Choi 2014; Fraser 2014; McClure 2014; Wittekind 2015; McClure 2016; Yang 2016).
Most studies did not explicitly describe how the randomisation sequence was generated or allocation concealed until participant enrolment. In cases where few details were reported but investigators used computerised randomisation and had minimal interaction with participants, we judged there to have been a low risk for selection bias in both domains. In 36 studies, computer randomisation was used to assign participants to intervention or control conditions and we therefore rated these studies at low risk for random sequence generation and allocation concealment (Etter 2005; Strecher 2005; Muñoz 2006 Study 3; Muñoz 2006 Study 4; Swartz 2006; Brendryen 2008a; Brendryen 2008b; Oenema 2008; Stoddard 2008; Muñoz 2009; Te Poel 2009; Swan 2010; McDonnell 2011; Simmons 2011; Elfeddali 2012; Mason 2012; Borland 2013; Bricker 2013; Humfleet 2013; Berg 2014; Brown 2014; Epton 2014; Herbec 2014; Mananes 2014; Schulz 2014; Stanczyk 2014; Bolman 2015; Borland 2015; Cameron 2015; Frederix 2015; Houston 2015; Voncken‐Brewster 2015; Cobb 2016; Moskowitz 2016; Pechmann 2016; Smit 2016). Eleven studies did not provide sufficient detail to judge risk of bias in allocation concealment and hence were rated at unclear risk (An 2013; Dickinson 2013; Emmons 2013; Bannink 2014; Choi 2014; Fraser 2014; McClure 2014; Zullig 2014; Wittekind 2015; McClure 2016; Yang 2016).
In addition to Burford 2013 and Skov‐Ettrup 2016, we judged three studies to be at high risk of bias for allocation concealment. Haug 2011 was a quasi‐randomised study with allocation by week of admission, and recruiting staff were aware of the condition to which participants would be allocated. In Humfleet 2013, although sequence generation was conducted by computer, clinic personnel were involved in recruitment, and baseline imbalances suggest some selection bias may have been introduced. Woodruff 2007 was a cluster‐randomised trial in which students were recruited after the schools were randomised, with different recruitment methods used for each. The groups differed significantly on several baseline smoking consumption‐related variables.
Incomplete outcome data
With the exception of six studies (Oenema 2008; Bricker 2013; Emmons 2013; Humfleet 2013; Cobb 2016; Yang 2016), all the included studies used ITT analyses for smoking status, reporting analyses based on the total number randomised, with dropouts and participants lost to follow‐up classified as smoking. In the case of Humfleet 2013, we were unable to establish the method used, and Oenema 2008 and Yang 2016 only provided a complete‐case analysis for the smoking outcome. Bricker 2013 chose not to impute missing data because of potential biases in effect size estimates, and Cobb 2016 did not provide details about handling of missing data. Emmons 2013 conducted outcome analyses using multiple imputation methods based on the assumption of arbitrary missing patterns and thus used Markov chain Monte Carlo methods. Wherever possible, we have noted the number of participants that completed the study in the Characteristics of included studies table.
Loss to follow‐up is often high in trials of Internet interventions (Murray 2009; Mathieu 2013). In one study (Clark 2004) there were no dropouts at one‐year follow‐up, as all study participants attended their annual review at that point. Sixteen studies ascertained smoking status for over 80% of participants at follow‐up (Japuntich 2006; An 2008; Brendryen 2008a; Brendryen 2008b; Strecher 2008; Haug 2011; Simmons 2011; Borland 2013; Humfleet 2013; Shuter 2014; Frederix 2015; Voncken‐Brewster 2015; Harrington 2016; Pechmann 2016; Skov‐Ettrup 2016; Yang 2016) and 29 studies ascertained smoking status for 50% to 80% of participants at follow‐up (Strecher 2005; Muñoz 2006 Study 4; Patten 2006; Swartz 2006; Woodruff 2007; Oenema 2008; Swan 2010; McDonnell 2011; Graham 2011; An 2013; Bricker 2013; Burford 2013; Emmons 2013; Bannink 2014; Brown 2014; Choi 2014; Epton 2014; Fraser 2014; Herbec 2014; McClure 2014; Mehring 2014; Stanczyk 2014; Borland 2015; Houston 2015; Wittekind 2015; Calhoun 2016; McClure 2016; Moskowitz 2016; Smit 2016). Eighteen studies had over 50% loss to follow‐up and were judged to be at high risk of attrition bias (Etter 2005; Muñoz 2006 Study 3; McKay 2008; Rabius 2008; Stoddard 2008; Muñoz 2009; Te Poel 2009; Wangberg 2011; Elfeddali 2012; Mason 2012; Dezee 2013; Dickinson 2013; Berg 2014; Mananes 2014; Schulz 2014; Bolman 2015; Cameron 2015; Mavrot 2016). Of these, seven trials followed up fewer than 30% of participants (McKay 2008; Wangberg 2011; Elfeddali 2012; Dickinson 2013; Mananes 2014; Schulz 2014; Bolman 2015). Most studies reported similar proportions lost to follow‐up in each group, except for two studies where survey non‐response was at least 20% higher among intervention participants then among controls (Emmons 2013; Mehring 2014) and were judged to be at high risk of attrition bias. Zullig 2014 and Cobb 2016 did not provide any attrition data and hence we judged them to be at unclear risk of bias for this domain.
Other potential sources of bias
We identified one study at high risk for other biases. In Houston 2015, while all smokers in recruited practices were eligible for referral, practices implementing the paper‐ or e‐referral implementation strategies chose which smokers to refer.
We did not use funnel plots to examine potential publication bias, as there were insufficient studies.
Effects of interventions
See: Table 1
Smoking cessation
Internet intervention compared to non‐active control
Trials in adults
We divided studies eligible for meta‐analysis into three groups:
(1) Interactive and tailored Internet‐based intervention (Haug 2011; Elfeddali 2012; Borland 2013; Emmons 2013; Harrington 2016; Skov‐Ettrup 2016; Smit 2016; Yang 2016); (2) Interactive but not tailored Internet‐based intervention (McDonnell 2011); (3) Neither interactive nor tailored Internet‐based intervention (Humfleet 2013).
Five studies were lifestyle interventions (Oenema 2008; Epton 2014; Zullig 2014; Cameron 2015; Voncken‐Brewster 2015), and four had follow‐up of less than six months (Swartz 2006; Mehring 2014; Shuter 2014; Wittekind 2015).
Interactive and tailored Internet‐based intervention
Pooled results demonstrated an effect in favour of the intervention (risk ratio (RR) 1.15, 95% confidence interval (CI) 1.01 to 1.30, Analysis 1.1, 8 studies, n = 6786). However, results should be interpreted with caution, as statistical heterogeneity was high (I2 = 58%) and was unexplained despite perceived clinical homogeneity, and we rated three trials at high risk of bias (Haug 2011; Emmons 2013; Skov‐Ettrup 2016).
1.1. Analysis.
Comparison 1 Internet versus non‐active control, Outcome 1 Smoking cessation at 6 months+ follow‐up (adults).
Not interactive/tailored Internet‐based intervention
There were no precise effects detected in the two studies that tested interventions that were not tailored based on participant responses. In McDonnell 2011, the intervention was interactive but not tailored and the point estimate marginally favoured the control group, but the confidence interval crossed the null (RR 0.87, 95% CI 0.63 to 1.20, Analysis 1.1.1, 1 study, n = 1112). Humfleet 2013 tested a non‐interactive, non‐tailored Internet intervention; results here favoured the intervention marginally, but the confidence interval crossed the null (RR 1.11, 95% CI 0.54 to 2.27, Analysis 1.1.3, 1 study, n = 140).
Studies with follow‐up of less than six months
Four studies were not eligible for meta‐analysis, as follow‐up was less than six months (Swartz 2006; Mehring 2014; Shuter 2014; Wittekind 2015). Swartz 2006 compared a video‐based Internet site that presented strategies for smoking cessation and motivational materials tailored to the user’s race/ethnicity, gender and age. After follow‐up the control group had access to the programme. At 90 days, ITT analysis showed a positive effect of the intervention (odds ratio (OR) 2.66, 95% CI 1.18 to 5.99, n = 351). Mehring 2014 compared an interactive and tailored Internet‐based intervention with SMS messaging to a non‐active control. In an ITT analysis the 'Web‐based coaching programme' produced higher quit rates compared to usual care at 12 weeks, but the confidence interval crossed the reference point (OR 1.28, 95% CI 0.38 to 4.36, n = 168). In Shuter 2014 the intervention group received online modules designed to educate, motivate, and increase self‐efficacy to quit, which was compared to usual smoking cessation treatment; both arms were offered a three‐month supply of nicotine patches. The ITT analysis using biovalidated abstinence rates favoured the intervention arm compared to standard care, but the confidence interval crossed the reference point (OR 2.49, 95% CI 0.62 to 10.10, n = 136). Wittekind 2015 found that the 'Standard Approach‐Avoidance Task' reduced the number of cigarettes smoked a day relative to wait‐list control (P = 0.026, n = 172) (note: quit rates were not reported), whereas there was no statistically significant evidence for a difference between the 'Modified Approach‐Avoidance Task' and the control group, (P = 0.822, n = 170).
Lifestyle interventions
Five studies were of lifestyle interventions and were not included in the meta‐analysis, as no data for smokers only were available (Oenema 2008; Epton 2014; Zullig 2014; Cameron 2015; Voncken‐Brewster 2015). Oenema 2008 compared an interactive and tailored lifestyle intervention to delayed treatment and found that the intervention produced higher quit rates; however the confidence interval crossed the point of reference (OR 1.41, 95% CI 0.56 to 3.55, n = 2159). Epton 2014 found that there were fewer current smokers among university students in the intervention condition compared to the control condition at six‐month follow‐up (B = 0.65 (note: B was the only effect estimate available), SE = 0.25, P = 0.01, n = 1107). In a repeat trial of Epton 2014, Cameron 2015 found that fewer students in the intervention condition reported that they had smoked since starting university compared to students in the control condition at six‐month follow‐up, but there was no statistical support for a difference (P = 0.29, n = 1454, note: number of quitters not reported). Zullig 2014 compared an intervention that was tailored to participants' risk scores to usual care; unpublished three‐month follow‐up results indicated that the control arm produced higher quit rates (RR 0.88, 95% CI 0.32 to 2.41, n = 91). Voncken‐Brewster 2015 compared a computer‐tailored feedback intervention to promote changes in smoking cessation and physical activity to usual care. Voncken‐Brewster 2015 found a small positive effect of the tailored and interactive self‐management lifestyle intervention compared to no intervention, but the confidence interval crossed the null (OR 1.12, 95% CI 0.45 to 2.77, n = 1307).
Other studies not eligible for meta‐analysis
We did not pool Clark 2004, due to the extremely low intensity of the intervention group, who only received a printed list of websites, and were compared to self‐help written materials. At one‐year follow‐up the control arm produced higher quit rates, and the confidence interval crossed the reference point (OR 0.4, 95% CI 0.1 to 1.4, n = 171).
Trials in adolescents and young adults
An 2008 was conducted In young adults (university students), and detected an effect on abstinence at 30‐week follow‐up (RR 1.95, 95% CI 1.42 to 2.69, Analysis 1.2, 1 study, n = 517). Woodruff 2007 recruited eligible adolescents based on a report of smoking in the past month; at baseline some described themselves as "former" smokers or indicated they had not smoked in the past week. Intervention participants had lower past‐week abstinence rates at baseline than controls (14% versus 29%). At 12‐month follow‐up, the two groups had similar abstinence rates, with the estimate favouring the control group, but the 95% confidence interval crossed the null (RR 0.93, 95% CI 0.60 to 1.44, Analysis 1.3, 1 study, n = 136).
1.2. Analysis.
Comparison 1 Internet versus non‐active control, Outcome 2 Smoking cessation at 6 months+ follow‐up (young adults).
1.3. Analysis.
Comparison 1 Internet versus non‐active control, Outcome 3 Smoking cessation at 6 months+ follow‐up (adolescents).
Internet intervention compared to active control
None of the seven studies comparing Internet to an active control detected statistically significant evidence for differences between the conditions (Analysis 2.1).
2.1. Analysis.
Comparison 2 Internet versus active control, Outcome 1 Smoking cessation at 6 months+ follow‐up.
Trials in adults
Five trials were conducted in adults (Swan 2010; Borland 2013; Humfleet 2013; Calhoun 2016; Skov‐Ettrup 2016). The pooled effect estimate favoured the control group, but the confidence interval crossed the null (RR 0.92, 95% CI 0.78 to 1.09, Analysis 2.1.1, 5 studies, n = 3806), and there was no evidence of statistical heterogeneity (I2 = 0%).
Trials in adolescents and young adults
Simmons 2011 conducted a trial in young adults (university students) and found that the direction of the effect favoured the intervention group. However the confidence interval crossed the null (RR 1.42, 95% CI 0.74 to 2.71, Analysis 2.1.2, 1 study, n = 168). Patten 2006, conducted in adolescents, compared an interactive Internet intervention with up to four in‐person counselling sessions, the direction of effect favoured the counselling group, however the confidence interval crossed the null: Internet versus in‐person counselling: RR 0.44, 95% CI 0.14 to 1.36, Analysis 2.1.3, 1 study, n = 139.
Studies with follow‐up of less than six months
Dezee 2013 found that the 'GetQuit' web‐based counselling programme produced higher quit rates than in‐person counselling, but the confidence interval crossed the line of no effect (RR 1.14, 95% CI 0.57 to 2.28, n = 217).
Internet intervention plus behavioural support
Internet intervention plus behavioural support versus non‐active control
Five studies evaluated Internet plus behavioural therapy (Brendryen 2008a; Brendryen 2008b; Borland 2013; Burford 2013; Smit 2016) (Analysis 3.1) compared to a non‐active control. These studies indicated a positive effect of the intervention: RR 1.69, 95% CI 1.30 to 2.18, Analysis 3.1, 5 studies, n = 2334, although statistical heterogeneity was substantial (I² = 60%) and was unexplained.
3.1. Analysis.
Comparison 3 Internet plus behavioural support, Outcome 1 Smoking cessation at 6 months+ follow‐up (adults) versus non‐Internet‐based non‐active control.
Internet intervention plus behavioural support versus active control
Four studies evaluated the Internet plus behavioural support compared to active control in adults (Japuntich 2006; Swan 2010; Borland 2013; Choi 2014) (Analysis 3.2). None of the studies detected a statistically significant difference between the Internet intervention plus behavioural therapy compared to active control: RR 1.00, 95% CI 0.84 to 1.18, Analysis 3.2, 4 studies, n = 2769. There was no evidence of statistical heterogeneity (I2 = 0%).
3.2. Analysis.
Comparison 3 Internet plus behavioural support, Outcome 2 Smoking cessation at 6 months+ follow‐up (adults) versus non‐Internet‐based active control.
Lifestyle interventions
Two studies were of lifestyle interventions and were not included in the meta‐analysis, as no data for smokers only were available. Bannink 2014 found that there was a higher proportion of self‐reported non‐smokers across the combined intervention arms (82.0%, n = 674/822), compared to the treatment‐as‐usual condition (80.8%, n = 349/434). Frederix 2015 did not record smoking status data as an outcome, and data were unavailable from the authors.
Comparisons between different Internet interventions
Interactive and/or tailored Internet intervention versus non‐tailored, non‐interactive Internet intervention
Seven studies, all conducted in adults (Rabius 2008; Graham 2011; Simmons 2011; Wangberg 2011; Brown 2014; Mavrot 2016; McClure 2016), compared an interactive and/or tailored Internet programme or website with an Internet intervention that was neither tailored nor interactive. Pooled results from studies with follow‐up of six months or longer favoured the intervention group. However, the confidence interval crossed the null: RR 1.10, 95% CI 0.99 to 1.22, Analysis 4.1, 7 studies, n = 14,623. There was no evidence of statistical heterogeneity (I2 = 0%).
4.1. Analysis.
Comparison 4 Comparisons between internet interventions: tailored/interactive versus not tailored/interactive, Outcome 1 Internet programmes: Smoking cessation at 6 months+ follow‐up (adults).
Three studies (Te Poel 2009; Mason 2012; Stanczyk 2014) conducted in adults compared tailored with non‐tailored messages, with all other website/programme characteristics being comparable. There was no evidence that interactive or tailored interventions, or both, produced higher smoking cessation rates compared to non‐interactive Internet interventions: RR 1.17, 95% CI 0.97 to 1.41, Analysis 4.2, 3 studies, n = 4040, but there was substantial statistical heterogeneity (I2 = 57%), which was attributable to Te Poel 2009.
4.2. Analysis.
Comparison 4 Comparisons between internet interventions: tailored/interactive versus not tailored/interactive, Outcome 2 Messages: Smoking cessation at 6 months+ follow‐up (adults).
Studies with follow‐up of less than six months
Seven studies reported smoking cessation at follow‐up of less than six months. Strecher 2005 found that the 10‐week abstinence rate was higher in the tailored website intervention arm compared to the non‐tailored website control arm at 12 weeks (OR 1.33, 95% CI 1.13 to 1.57, n = 3971). Stoddard 2008 found that adding an interactive 'bulletin board' to smokefree.gov did not produce higher quit rates than smokefree.gov alone at three‐month follow‐up (RR 0.95, 95% CI 0.64 to 1.40, n = 1375). Bricker 2013 reported that a tailored and interactive Internet intervention produced higher 30‐day quit rates at three‐month follow‐up (RR 2.20, 95% CI 0.90 to 5.40, n = 115), but the confidence interval crossed the null. Herbec 2014 also compared a tailored and interactive Internet intervention to a non‐tailored and non‐interactive intervention and found that the tailored/interactive website produced higher four‐week continuous abstinence quit rates, compared to the control, but the confidence interval crossed the null (OR 1.5, 95% CI 0.8 to 2.9, n = 200). Mananes 2014 reported that the tailored and interactive intervention did not produce a higher quit rate compared to the control arm at three‐month follow‐up (RR 0.59, 95% CI 0.49 to 0.73, n = 23,213). Berg 2014 compared a tailored and interactive Internet intervention to a non‐tailored and non‐interactive Internet intervention and found that quit rates were higher in the intervention arm at three‐month follow‐up, but the confidence interval crossed the null (RR 2.19, 95% CI 0.59 to 8.06, n = 122). Pechmann 2016 found that 'Tweet2Quit' doubled sustained abstinence at 60‐day follow‐up compared to a non‐interactive and non‐tailored website (OR 2.27, 95% CI 1.04 to 4.97, n = 160).
Lifestyle interventions
Dickinson 2013 did not report smoking outcome data as "there were too few subjects who were smokers to detect a difference over time", and data were not available upon request. In Schulz 2014, a tailored and non‐interactive Internet intervention was compared to a non‐tailored and non‐interactive Internet control, with the tailored and non‐interactive intervention producing "changes" in abstinence (P = 0.004, n = 3374) but the direction of the effect was not explained (effect size = 0.41).
Other comparisons between Internet interventions
Four studies, all conducted in adults, provided other comparisons between Internet interventions (Muñoz 2006 Study 3; Muñoz 2006 Study 4; McKay 2008; Muñoz 2009) and provided follow‐up at six months or longer (Analysis 5.1). Muñoz 2006 Study 3 and Muñoz 2006 Study 4 evaluated the addition of a mood management course to an Internet intervention. Whereas Muñoz 2006 Study 4 did not detect a significant difference between arms (RR 0.90, 95% CI 0.58 to 1.41, n = 288), Muñoz 2006 Study 3 detected a difference in favour of the arm that did not receive the mood management course (RR 0.51, 95% CI 0.26 to 0.97, n = 280). McKay 2008 compared an intervention with a tunnel design (i.e. a set order in which participants had to navigate the site) followed by a free path to an intervention with a tunnel design throughout, and detected no difference between the two interventions (RR 1.02, 95% CI 0.68 to 1.54, n = 2318). Muñoz 2009 compared variants of an Internet programme, evaluating the addition of automated emails, a mood management course, and a bulletin board. They found no differences for any of the additions compared with control (all three adjuncts versus programme only: RR 1.08, 95% CI 0.76 to 1.54, n = 502).
5.1. Analysis.
Comparison 5 Other comparisons between internet interventions, Outcome 1 Smoking cessation at 6 months+ follow‐up (adults).
Studies with follow‐up of less than six months
Etter 2005, was not eligible for the meta‐analysis, as it did not report follow‐up at six months or longer. This study compared two interactive Internet interventions; the control programme was a shorter and simplified version of the intervention programme. At 2½ months, results favoured the longer and more complex programme (RR 1.36, 95% CI 1.24 to 1.50, n = 11,969).
Other comparisons not eligible for meta‐analysis
Strecher 2008 compared multiple conditions in a fractional factorial design, and hence we have not presented results in forest plots. Participants could receive up to three high‐depth components, addressing efficacy expectations, outcome expectations and success stories, as part of their tailored web‐based intervention. The study found that tailoring depth was related to six‐month smoking cessation across the entire range of cumulative high‐depth components (OR 1.91, 95% CI 1.18 to 3.11, n = 943), and for each high‐depth component added (OR 1.24, 95% CI 1.06 to 1.45, n = 943).
An 2013 compared three Internet‐based lifestyle interventions: one was tailored and interactive, another was tailored and non‐interactive, and the control arm was non‐tailored but interactive. At 12‐week follow‐up 30‐day abstinence rates were 11% (n = 62/567) for the controls, 23% (n = 130/566) for the tailored and non‐interactive arm, and 31% (n = 175/565) for the tailored and interactive intervention; "differences were statistically significant, p < 0.0001" for each tailored intervention compared with the control arm. The tailored and interactive condition produced higher quit rates when compared to the tailored but non‐interactive arm (P = 0.006).
McClure 2014 randomised all participants to one of 16 variations of an Internet intervention, with different levels of the following experimental factors: message tone, navigation autonomy, proactive emails, and testimonials. They did not find strong evidence that any of the experimental factor levels altered the odds of quitting smoking at 12‐month follow‐up (NB. see study report for estimates from all eight comparisons).
Participants in Fraser 2014 were randomised to a version of an Internet intervention where a combination of five components were either turned on or off, resulting in 32 conditions: the National Cancer Institute’s (NCI) website ('smokefree.gov versus a 'lite' website), telephone quitline counselling (versus none), a smoking cessation brochure (versus a 'lite brochure'), motivational e‐mail messages (versus none), and mini‐lozenge NRT (versus none). Based on averaged outcome data across conditions where components were on or off, they found that the full website produced stronger effects relative to the 'lite website' when individuals received no messaging compared to when they receive messaging. There was a two‐way interaction between the website and messaging factors at three months on cessation. This interaction occurred because the full website produced stronger effects compared to the 'lite website' when individuals received no messaging (33.5% versus 21.8%, n = 800, P = 0.003), versus when they received messaging (25.7% versus 26.8%, n = 800, P = 0.762).
In Bolman 2015 participants had access to an interactive and tailored Internet intervention; one condition included a supplementary action‐planning component. This trial found continuous abstinence rates were higher in the experimental arm at six‐month follow‐up (OR 1.68, 95% CI 0.96 to 2.92, n = 1982).
Borland 2015 compared four arms:'QuitCoach', which was a tailored and interactive Internet intervention, with or without advice to quit as soon as possible ('rapid implementation'), and 'QuitCoach' with or without 'structured planning' to aid cessation. 'QuitCoach with rapid implementation' produced a higher quit rate of 10.7% (95% CI 8.6 to 13.0) compared to control of 9.8% (95% CI 7.9 to 12.1), n = 1601, P = 0.59.
Houston 2015 compared three interactive and tailored Internet interventions, and found that those receiving messages (standard enhanced messages with pushed personalised motivational email messages) produced higher quit rates than the standard interactive control website (OR 2.16, 95% CI 1.21 to 3.9, n = 463), and that the personalised group (i.e. features from the message and control groups, plus access to a tobacco treatment specialist, and a smoker‐to‐smoker online support group) produced higher quit rates than the control website (OR 1.58, 95% CI 0.96 to 2.60, n = 736).
Cobb 2016 gave all participants access to a smoking cessation intervention through a Facebook app, but randomised participants to different variants of the app where features related to three drivers of diffusion (i.e. time, contacts, and contagion, both active and passive) were turned on or off, resulting in 16 conditions. They found that the Facebook social network was sufficient to increase the numbers of individuals receiving treatment when using the version of the app with all features turned on (R value of 0.09, 95% CI 0.07 to 0.11, n = 756).
Moskowitz 2016 was not included in the meta‐analysis, as we judged that this trial was assessing the effectiveness of the behavioural component of the intervention (i.e. high or low incentive reinforcement) rather than the effectiveness of the Internet intervention as there was no relevant comparator. The study found that adding reinforcement to the tailored and interactive intervention did not produce higher quit rates (percentage difference 1.0%, 95% CI −4.1% to 6.6%, n = 403).
Sensitivity analysis
For this update, we report separate analyses of complete‐case data. We repeated all ITT meta‐analyses reported in Analysis 1.1 to Analysis 5.1, in studies where we had complete‐case data. We report complete‐case analyses in Analysis 6.1 to Analysis 9.1. Only five studies were not included in the complete‐case analyses because data were not available (Woodruff 2007; Rabius 2008; Muñoz 2009; Mason 2012; Yang 2016).
6.1. Analysis.
Comparison 6 Sensitivity analysis (complete cases) ‐ Internet versus non‐active control, Outcome 1 Smoking cessation at 6 months+ follow‐up (adults).
9.1. Analysis.
Comparison 9 Sensitivity analysis (complete cases) ‐ Other comparisons between Internet interventions, Outcome 1 Smoking cessation at 6 months+ follow‐up (adults).
There were two complete‐case analyses that differed slightly from their ITT counterparts. The ITT analysis of tailored/interactive Internet programmes versus non‐tailored/interactive programmes produced a small effect, and the confidence interval crossed the null (RR 1.10, 95% CI 0.99 to 1.22, Analysis 4.1, 7 studies, n = 14,623). When these studies were pooled using complete‐case data only, the estimate did not change greatly, but the confidence interval no longer crossed the null (RR 1.12, 95% CI 1.00 to 1.27, Analysis 10.1, 6 studies, n = 5111), reflecting the smaller sample size. The effect estimate from the ITT analysis of comparisons between Internet‐based tailored/interactive messages versus not tailored/interactive messages indicated a small effect, with the confidence interval crossing the null (RR 1.17, 95% CI 0.97 to 1.41, Analysis 4.2, 3 studies, n = 4040); when these studies were pooled using complete‐case data, the confidence interval no longer crossed the null (RR 1.37, 95% CI 1.10 to 1.70, Analysis 10.2, 2 studies, n = 1648).
10.1. Analysis.
Comparison 10 Sensitivity analysis (complete cases) ‐ Comparisons between Internet interventions: tailored/interactive versus not tailored/interactive, Outcome 1 Internet programmes: Smoking cessation at 6 months+ follow‐up (adults).
10.2. Analysis.
Comparison 10 Sensitivity analysis (complete cases) ‐ Comparisons between Internet interventions: tailored/interactive versus not tailored/interactive, Outcome 2 Messages: Smoking cessation at 6 months+ follow‐up (adults).
User satisfaction
We extracted data on user satisfaction as a secondary outcome, but only 21 studies reported relevant information.
In Woodruff 2007, adolescents were randomised to an Internet intervention or a non‐active control. Intervention participants completed a five‐item questionnaire assessing their satisfaction with the programme immediately after the post‐test assessment; 89% reported they would recommend the programme to another person who smoked.
Stoddard 2008 compared the publicly‐available version of smokefree.gov, designated as the control condition, to an identical‐looking website that included an asynchronous bulletin board. Satisfaction with the website was high and did not differ between conditions (control 90.2%, bulletin board 84.9%, P = 0.08).
In Te Poel 2009, participants in the intervention group rated the tailored letter significantly higher overall (mean = 7.1, SD = 1.5) compared with participants who received the non‐tailored letter (mean = 6.5, SD = 1.9).
Muñoz 2009 reported ratings of the website at one month were: “not helpful” 7.2%; “somewhat helpful” 40.0%; “quite helpful” 35.7%, and “extremely helpful” 17.0%. Similar ratings at three months were: 8.2%, 43.8%, 34.7%, and 13.3%; at six months: 11.9%, 40.7%, 34.2%, and 13.2%; and at 12 months: 10.2%, 47.9%, 29.4%, and 12.5%.
At the six‐week questionnaire, respondents in Strecher 2005 in the 'CQ PLAN' condition were more likely than those in the control condition to report the materials as being helpful and relevant, and more likely to state they would recommend the programme to others. Additionally, among smokers at the six‐week follow‐up, 'CQ PLAN' respondents were more likely to state that they would use the programme in the future.
Ninety per cent of participants from both conditions in Berg 2014 reported satisfaction with the Internet programmes, with a high proportion reporting that they would recommend the programmes to friends who smoke.
In Brown 2014, satisfaction rates were higher for participants using the interactive StopAdvisor website compared to the information‐only control website.
Participants in both groups of Bolman 2015 who received an interactive and tailored Internet‐based intervention with or without action planning evaluated the computer‐tailored email letters as comprehensible, credible, and trustworthy.
Compared to smokefree.gov, participants from Bricker 2013 randomised to WebQuit.org reported greater satisfaction with their assigned website, greater agreement that their assigned programme was a good fit, and were more likely to report that their programme’s quit plan was useful.
In Choi 2014, where the intervention condition offered a tailored and interactive Internet intervention as an adjunct to telephone‐based behavioural support, “overall helpfulness" of the phone calls was rated higher in the intervention group than the 1‐800‐quit‐now control group (P = 0.023). Participants in the intervention group reported more comfort with asking questions (P = 0.01), more satisfaction with the answers provided by the counsellors (P = 0.003), and felt more supported (P < 0.001) than those in the control group. However, the authors reported no difference between the groups' tendency to recommend the intervention to someone else (P = 0.171).
Of the tailored web‐based intervention participants in Emmons 2013 who logged in, 87.9% (n = 116/132) reported being satisfied or very satisfied with the site, with 81.1% (n = 107/132) reporting they would recommend the site to other childhood and young adult cancer survivors.
Mananes 2014 recorded user satisfaction at three‐month follow‐up. Fifteen per cent (n = 121/1085) of participants were not at all satisfied, 19.3% (n = 209/1085) were slightly satisfied, 34.1% (n = 370/1085) were somewhat satisfied, 25.8% (n = 280/1085) were very satisfied, and 9.7% (n = 105/1085) were extremely satisfied. Satisfaction differed by version of the web‐based programme (Chi2 4 = 25.4, P < 0.001, Cramer’s V = 0.153, P < 0.001), such that users of the interactive version of the programme showed higher proportions of very satisfied and extremely satisfied responses compared to the static control version.
At three‐month follow‐up in McClure 2016, 92.0% (n = 22/24) of participants receiving an interactive and tailored Internet intervention thought the programme could help people quit smoking, 97.0% (n = 17/22) thought it could help people to consistently take their stop‐smoking medication, and 87.0% (n = 20/23) would recommend the programme to others.
Wittekind 2015 reported that satisfaction was 52.6% (n = 38) for participants in the 'standard approach‐avoidance task (AAT) programme' and 42.4% (n = 33) for participants in the 'modified AAT programme' (where response times for each trial were shown), both delivered over the Internet.
In Shuter 2014, over 78.0% of respondents indicated that the interactive intervention website was positive in terms of being helpful, meeting expectations, leading to user satisfaction, and being personally relevant. Additionally, 95.2% indicated that they would recommend the intervention to family or friends who were interested in quitting.
Finally, Stanczyk 2014 reported no differences between trial arms in “appreciation of program” measures, while Fraser 2014 stated they collected satisfaction data but this was not reported in the publication. In Harrington 2016, 70 participants in the web intervention arm added text comments when reporting their perceptions of the website and emails, with 48.6% being positive (e.g. “helpful,” “useful”) and 40% being negative (e.g. “don't use computer,” “e‐mails incessant”).
Among lifestyle intervention studies, participants in the 'E‐health4Uth' condition in Bannink 2014 scored most items on the use and appreciation of the tailored messages and the programme positively, mean 6.7 (SD = 1.6) (i.e. overall satisfaction on a scale from 1 (negative) to 10 (positive)). In Frederix 2015, 44.0% (n = 30/69) of participants in the intervention group who received an Internet‐based, comprehensive telerehabilitation programme reported being very satisfied, while 51.0% (n = 35/69) reported being satisfied. At last follow‐up in Schulz 2014, 84.43% (n = 998/1182) of participants reported that the online health risk appraisal gave a good overview of their lifestyle, 77.6% (n = 917/1182) liked the use of traffic lights in the health risk appraisal, 72.3% (n = 852/1178) liked the layout, and 76.7% (n = 904/1178) experienced website use as user‐friendly. Participants in both intervention conditions evaluated the tailored advice as relevant (75.4%, n = 86/114), credible (76.5%, n = 88/115), informative (70.4%, n = 81/155), well‐arranged (84.3%, n = 97/115), clear (85.1%, n = 97/114), interesting (71.3%, n = 82/115), and with an attractive layout (70.0%, n = 77/115).
Costs
Eight studies reported information about the cost of their intervention.
While Borland 2013 described the cost of the 'QuitCoach' Internet intervention as negligible, they reported that the cost of 'onQ', an interactive automated text‐messaging programme as AUD 20 per user (n = 3530 at baseline). The exact number used to calculate cost per user was not reported.
Burford 2013 reported that the total cost of implementing their face simulation software intervention was AUD 463, or the equivalent of AUD 5.79 for each participant. They also noted that the incremental cost‐effectiveness ratio was AUD 46 per additional quitter, or the equivalent of AUD 74 per additional lifetime quitter (n = 80).
Mehring 2014 reported the cost of their web‐based smoking cessation coaching programme as EUR 79 (note: based on standard treatment cost).
Calhoun 2016 found that per‐person costs were higher for participants randomised to the Internet intervention arm, where mean costs were USD 178 (median = USD 113, n = 205), versus USD 26 (median = USD 7, n = 203) for those randomised to referral for specialty care. However, they noted that of the USD 178 in costs for the Internet participants, USD 121 (median = USD 58) were due to the price of NRT, while NRT costs on average only represented USD 12 (median = USD 0) of the USD 26 per‐person costs for specialty‐care participants. They determined that among users of NRT, the cost of NRT was much greater for Internet participants (USD 162 (median = USD 96, n = 153)) compared to specialty‐care participants (USD 60 (median = USD 52, n = 40)).
Harrington 2016 reported the average cost per quitter at six‐month follow‐up was USD 283 for their web intervention (n = 190) and USD 20 for usual care (n = 198), but noted that the cost of the intervention decreases to USD 57 per quit without programmer costs.
Skov‐Ettrup 2016 reported that the cost per user of their Internet intervention ‘e‐quit’ was GBP 4.30 (n = 225), while the cost of the self‐help booklet was GBP 1.80 (n = 451), and the cost of proactive and reactive telephone counselling was GBP 48 (n = 245) and GBP 39 (n = 30), respectively.
Etter 2005 estimated that the total cost of implementing the website, for a reach of 8000 participants in computer‐tailored programmes and for 600,000 visitors a year to the website, is comparable to the cost of running a small smoking cessation clinic which would treat about 50 smokers a month.
Rabius 2008 suggested Internet assistance for smoking cessation was cost‐effective, since four days of programming at a cost of less than USD 2000 allowed approximately 5000 additional users for services from the five tailored interactive service providers, compared with the much higher cost of serving 1000 new clients with telephone counselling (approximately USD 100,000).
Based on the results of these trials, only Burford 2013 demonstrated cost effectiveness, as they found strong evidence that more participants in the intervention group had quit at six months (confirmed by biochemical validation) than participants in the control group. The mean cost of implementing the intervention was AUD 5.79 per participant. The incremental cost‐effectiveness ratio was AUD 46 for each additional quitter (n = 80). However, Etter 2005 and Mehring 2014 followed up only to 2½ and three months, respectively. Rabius 2008, Borland 2013, Calhoun 2016, Harrington 2016, and Skov‐Ettrup 2016 found no evidence for the effectiveness of Internet interventions relative to controls, so cost effectiveness was not demonstrated by these studies.
Discussion
The Internet, with its richness of options and opportunities for communication and sharing information, has now become a regular part of daily life for most people in many countries. It is therefore appropriate to consider using it as a tool to increase choice and access to smoking cessation support. Online treatment is convenient, in that it can be accessed anywhere at any time; it also offers the option of anonymity. For healthcare providers it has the potential of being very cost‐effective if provided as an automated service. Internet interventions for smoking cessation can be provided in conjunction with other cessation support such as individual or group counselling and NRT or other pharmacotherapy.
Summary of main results
For this update we identified data from 39 new randomised trials. We now have 67 included studies, yielding data from over 110,000 participants, of whom 35,969 are included in the meta‐analyses. This is a growing area of research, with the earliest trial published in 2004. Studies included in the meta‐analysis fell into three main categories: (1) interactive and tailored interventions; (2) non‐tailored/interactive interventions; and (3) Internet interventions plus behavioural support. Studies included in the narrative review fell into one of the following categories: comparing components of Internet interventions, lifestyle interventions that included a smoking cessation component, and studies with less than six months follow‐up.
Nine studies in adults compared Internet interventions to usual care or printed self‐help, with pooled results indicating that the intervention was relatively effective when tailored and interactive, although statistical heterogeneity was high and four studies were at high or unclear risk of bias, whereas non‐tailored/interactive Internet interventions appeared no better than non‐active controls. There were only two studies of adolescents and young adults, with no evidence of an intervention effect compared to non‐active controls, and both studies were at low risk of bias. There was no evidence of an intervention effect in the five trials comparing Internet interventions with active control (i.e. phone or face‐to‐face counselling) in adults, or in the two studies conducted in adolescents or young adults.
Five studies compared tailored and/or interactive Internet intervention plus behavioural support to a non‐active control. They detected a treatment effect, but with high statistical heterogeneity. Four studies comparing tailored and/or interactive Internet intervention plus behavioural support to an active control found no intervention effect, and there was no evidence of statistical heterogeneity.
Seven studies in adults compared tailored/interactive Internet intervention programmes with non‐tailored and non‐active Internet interventions and reported data at six months or longer. There was no treatment effect and most studies (six out of seven) were at low risk of bias. Three studies compared tailored messages to a non‐tailored message; one found an effect in favour of the tailored version (Te Poel 2009), but the pooled estimate crossed the null, with moderate statistical heterogeneity.
We also found that participants were generally satisfied with the use of Internet interventions, but we could not assess the cost effectiveness of these interventions, as we did not find evidence for their effectiveness. However, only 21 studies measured user satisfaction, and only eight studies reported intervention cost. Finally, adverse events were very rarely reported and were few in number.
Quality of the evidence
Ratings for the quality of the evidence for our selected outcomes ranged from 'low' to 'high' (see Table 1). While we had nine studies (7909 participants) comparing interactive and tailored Internet interventions versus non‐active control, most of them were at high risk of bias in one or more domains and we found evidence of moderate statistical heterogeneity (I2 = 53%), resulting a rating of low quality. This suggests that our confidence in the effect estimate is limited, and that the true effect may be substantially different from our reported estimate of the effect. The quality of evidence for our outcome of comparisons between interactive or tailored versus not interactive or tailored Internet interventions that included messages was rated low for similar reasons, while the evidence for our outcome of comparisons between interactive or tailored versus not interactive or tailored Internet interventions that included programmes was rated as being of moderate quality. The evidence for our outcomes comparing Internet interventions plus behavioural support versus non‐Internet‐based non‐active control and non‐Internet‐based active control were both similarly rated as moderate, as we judged there to be an unclear or high risk of bias for one or more domains in multiple studies. Finally, the evidence for the Internet intervention compared to active control outcome was rated as high quality, suggesting that we are very confident that the true effect lies close to that of our estimate of the effect.
The trials enrolling adults generally relied on self‐reported data on smoking status. While biochemical validation of self‐reported cessation was only attempted in 18 trials in the original review, this update identified 12 more trials which used validation. This suggests a promising trend towards more Internet trials reporting biochemically‐verified quit rates, particularly as some trials have found discrepancies between reported and confirmed quit rates (although these were few and did not impact study results) (An 2008; Elfeddali 2012; Burford 2013; Mehring 2014; Calhoun 2016; Smit 2016). However, the Society for Research on Nicotine and Tobacco (SRNT) subcommittee on biochemical verification in clinical trials considers that verification is not necessary when a trial includes a large community‐based population with limited face‐to‐face contact, and where the optimal data collection methods are through the mail, telephone, or Internet (SRNT 2002). The SRNT does, however, recommend that biochemical verification be used in studies of smoking cessation in special populations, including adolescents (SRNT 2002). Despite this, half of the studies in adolescents and young people did not use biochemical verification of self‐reported abstinence (Woodruff 2007; An 2013; Emmons 2013; Bannink 2014; Berg 2014).
Conducting research through the Internet provides opportunities to generate large sample sizes, but it is also methodologically challenging, because of threats to internal and external validity such as selection bias or differential dropout rates (Feil 2003; Cobb 2005). Although there was limited detail about procedures for sequence generation and allocation concealment, we judged that the likelihood of selection bias was small in studies that recruited participants online. Rates of loss to follow‐up were varied, and were often high in some large online studies. In line with standard Cochrane Tobacco Addiction Group methodology, we regarded those lost to follow‐up as continuing smokers. However, as we identified many new trials for this update, this allowed us to conduct sensitivity analyses where we repeated all ITT meta‐analyses (in which participants lost to follow‐up were considered continuing smokers) in studies where complete‐case data were made available (all except four studies). Complete‐case analyses were broadly consistent with results from our primary ITT analyses.
A final consideration is that determining the contribution of a specific website or intervention presents a difficult challenge, since Internet users appear to access different sites when searching for information or support. For example, contamination in control groups may be difficult to prevent because of unrestricted access to the Internet, and we cannot be sure that the intervention group is using only the intended intervention (Eysenbach 2002; Feil 2003).
Agreements and disagreements with other studies or reviews
Other reviews in this area
There are seven reviews previously conducted on this area (Myung 2009; Shahab 2009; Chen 2012; Brown 2013; Hou 2014; Blankers 2016; Graham 2016).
Myung 2009 pooled data from a number of studies of both web‐ and computer‐based interventions, and concluded that there is now sufficient evidence to support the use of both categories of intervention for adult smokers. Their estimate for Internet interventions, based on nine studies and using a random‐effects model, was RR 1.40, 95% CI 1.13 to 1.72. Shahab 2009 also suggests that, based on 11 studies, interactive web‐based interventions can be effective in aiding cessation; all but one of the 11 studies is included in our review (we were able to include longer‐term data for Pike 2007, as Rabius 2008). We excluded Prochaska 2008, because we were unable to confirm missing outcome data with the authors.
Shahab 2009 pools the studies in a number of subgroups: the intervention (tailored/non tailored); length of treatment; motivation to quit; and whether the intervention was fully automated or not. They assessed interactive web‐based smoking cessation interventions to be effective compared to non‐tailored booklet or email interventions (random‐effects RR 1.8, 95% CI 1.4 to 2.3), but this was based on just three trials. They also estimated that tailored interventions increase six‐month abstinence by 17% and suggest that only interventions aimed at smokers motivated to quit were effective (RR 1.3, 95% CI 1.0 to 1.7).
A Health Technology Assessment review (Chen 2012) evaluated electronic interventions in general, and includes not only studies of Internet‐based interventions but also of interventions delivered by mobile phones, interactive voice response (IVR) systems, and computer‐based interventions that do not involve the Internet. The authors' classifications of interventions varied slightly from ours, but followed the same general structure, including separating studies based on whether or not the intervention was interactive or tailored. Their estimate for Internet interventions compared to no intervention includes two studies, both of which are included in our review but neither of which provide follow‐up at six months or longer (Swartz 2006; Oenema 2008). The pooled estimate at longest follow‐up from these two studies crossed line of no effect (RR 1.86, 95% CI 0.98 to 3.50). The authors conclude that electronic aids to smoking cessation compared with no intervention or generic self‐help can increase the rate of prolonged abstinence, although the effect is small (RR 1.32, 95% CI 1.21 to 1.45). They highlight the comparative effectiveness of different electronic interventions as the key source of uncertainty in their results.
Brown 2013 conducted a review on technology‐based interventions tobacco dependance in college students and aimed to examine methodologies used, theoretical frameworks and outcome measures for tobacco treatment to guide development of a program in college students. They included four RCTs and four cohort studies and found the theoretical frameworks most commonly used were: transtheoretical model of change, health belief model, theory of social support, and social cognitive theory. Interventions varied and included computer‐generated advice letters, web‐based cessation guides, computer‐generated text messages, and peer e‐mail support. Although some studies indicated that Internet interventions had a positive affect on quit rates, meta‐analysis was not carried out due to heterogeneity of the interventions. The authors concluded that it was not clear what types of computer‐based applications were most effective due to use of multiple components, differences in interventions and the number of contacts, small sample sizes, lack of control groups, and inconsistency in outcome measures limit the ability to provide conclusive evidence to support these interventions—but support the feasibility to use in the design of future programs.
Hou 2014 conducted a review of Internet interventions aiming to change health behaviours in the general population, which included a search for smoking cessation interventions. The review identified five studies which were not meta‐analysed and concluded that advice from a trained counsellor during the Internet intervention was associated with a stronger intervention effect. This is in line with findings from our update, confirming that Internet interventions that offered tailored and interactive components, which often involved access to live advice from a professional, were effective compared to non‐active controls.
Blankers 2016 conducted a secondary analysis of the previous update of this review (Civljak 2013), in which they compared effect estimates derived from three different imputation methods: one in which participants lost to follow‐up were assumed to be smoking (i.e. missing = smoking), a complete‐case analysis, and a simulation using multiple imputation of missing outcome data. The re‐analysis indicated that the 'missing = smoking' assumption produced estimates that did not cross the null, compared to the complete‐case and multiple‐imputation analyses which produced estimates that crossed the line of no effect. In our update we found that the estimates derived from our analyses in which those lost to follow‐up are assumed to be continuing smokers did not differ greatly from our sensitivity analyses in which we analysed data from complete cases only, except for tailored/interactive Internet programmes versus non‐tailored/interactive Internet programmes, where including complete‐case data only strengthened the effect of the intervention.
Graham 2016 conducted a review of Internet interventions for smoking cessation, with 40 RCTs of Internet interventions for smoking cessation, 24 of which were included in their meta‐analysis; this review categorised studies according to the comparator arm (i.e. wait‐list or paper‐materials control). Graham 2016 concluded that Internet interventions were superior to wait‐list controls, but not to print materials. However, in our update we have categorised studies according to the comparator arm, whether or not the intervention was tailored/interactive, and by age group. We found that tailored and interactive Internet interventions were superior to non‐active controls in adults. Graham 2016 included fewer studies than our review and combined varying age groups, and tailored/interactive, non‐tailored/interactive interventions. Graham 2016 also reported that Internet interventions were no more effective than counselling for smoking cessation, which is consistent with our findings.
Implications for research
We have identified 18 ongoing studies in this area (see Characteristics of ongoing studies). Future trials and reviews should include analyses of participants according to sociodemographic data, in order to identify the types of smokers who seek Internet assistance to quit smoking. Overall, attrition in Internet interventions is high, and many studies reported that higher levels of depression, older age, and higher levels of nicotine dependence were associated with loss to follow‐up. Future studies should also attempt to investigate these factors and how to improve Internet intervention adherence for smokers fitting these descriptions. There were few studies reporting biovalidated cessation. Future studies should biovalidate smoking status.
Authors' conclusions
Implications for practice.
Evidence in adults suggests that interactive and tailored Internet‐based interventions may be slightly more effective than usual care or printed self‐help at six months or longer. However these results should be interpreted with caution, as we judged some of the studies to be at high risk of bias, and there was evidence of substantial statistical heterogeneity. In adults there was evidence that tailored and interactive interventions delivered with additional behavioural support were more effective than non‐active controls, but with evidence of substantial statistical heterogeneity. We found no evidence that Internet interventions with or without the addition of behavioural support were better than active smoking cessation treatments. There were only 10 studies of Internet interventions in adolescents or young adults and only four of these were eligible for meta‐analysis, so treatment effectiveness in younger smokers is unknown.
Implications for research.
There remains a requirement for higher‐quality studies, adequately powered and reporting bioverified smoking cessation, with at least six months follow‐up. In this review there were only 10 studies conducted in adolescents or young adults, and only four of these were suitable for meta‐analysis. More trials of Internet interventions aimed at younger smokers (i.e. 25 years and younger) are needed to determine the effectiveness of Internet interventions for this group. Most studies were conducted in high‐income countries, which leaves a knowledge gap about the effectiveness of Internet interventions in developing countries.
What's new
| Date | Event | Description |
|---|---|---|
| 5 September 2017 | Amended | Minor amendment correcting reference to number of studies in analysis 1.1. |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 20 October 2016 | New citation required but conclusions have not changed | No change to conclusions |
| 20 October 2016 | New search has been performed | New searches run. 39 new studies included. |
| 24 April 2013 | New citation required but conclusions have not changed | Conclusions revised but largely unchanged following restructured analyses. JH‐B added as an author |
| 24 April 2013 | New search has been performed | Most recent searches April 2013. Eight new included studies. |
| 21 September 2010 | Amended | Correction to axis labels in comparison 3 |
| 27 November 2008 | New citation required and minor changes | Error in author order corrected |
| 21 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Queenie Hon, Chris Zheng Jie, Huang Zhilian, Sng Ming Keat, and Xu Xiaomeng for their help translating records. Thank you to Nicola Lindson‐Hawley for advice about methodological aspects of the study and analysis. Many thanks to Jamie Hartmann‐Boyce who assisted with data extraction, analysis, and writing of the 2013 version of this review.
Data and analyses
Comparison 1. Internet versus non‐active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6 months+ follow‐up (adults) | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Interactive and tailored | 8 | 6786 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.01, 1.30] |
| 1.2 Interactive, not tailored | 1 | 1112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.20] |
| 1.3 Not interactive or tailored | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.54, 2.27] |
| 2 Smoking cessation at 6 months+ follow‐up (young adults) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Smoking cessation at 6 months+ follow‐up (adolescents) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Internet versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6 months+ follow‐up | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Adults | 5 | 3806 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.09] |
| 1.2 Young adults | 1 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.74, 2.71] |
| 1.3 Adolescents | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.14, 1.36] |
Comparison 3. Internet plus behavioural support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6 months+ follow‐up (adults) versus non‐Internet‐based non‐active control | 5 | 2334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.30, 2.18] |
| 2 Smoking cessation at 6 months+ follow‐up (adults) versus non‐Internet‐based active control | 4 | 2769 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.84, 1.18] |
Comparison 4. Comparisons between internet interventions: tailored/interactive versus not tailored/interactive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Internet programmes: Smoking cessation at 6 months+ follow‐up (adults) | 7 | 14623 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.99, 1.22] |
| 2 Messages: Smoking cessation at 6 months+ follow‐up (adults) | 3 | 4040 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.97, 1.41] |
Comparison 5. Other comparisons between internet interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6 months+ follow‐up (adults) | 4 | 3388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.75, 1.16] |
Comparison 6. Sensitivity analysis (complete cases) ‐ Internet versus non‐active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6 months+ follow‐up (adults) | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Interactive and tailored | 7 | 4433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.09, 1.39] |
| 1.2 Interactive, not tailored | 1 | 587 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.76, 1.39] |
| 1.3 Not interactive or tailored | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.67, 2.67] |
| 2 Smoking cessation at 6 months+ follow‐up (young adults) | 1 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.40, 2.63] |
6.2. Analysis.
Comparison 6 Sensitivity analysis (complete cases) ‐ Internet versus non‐active control, Outcome 2 Smoking cessation at 6 months+ follow‐up (young adults).
Comparison 7. Sensitivity analysis (complete cases) ‐ Internet versus active control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6 months+ follow‐up | 7 | 3307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.80, 1.08] |
| 1.1 Adults | 6 | 3241 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.81, 1.10] |
| 1.2 Adolescents | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.12, 1.02] |
7.1. Analysis.
Comparison 7 Sensitivity analysis (complete cases) ‐ Internet versus active control, Outcome 1 Smoking cessation at 6 months+ follow‐up.
Comparison 8. Sensitivity analysis (complete cases) ‐ Internet plus behavioural support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6 months+ follow‐up (adults) versus non‐Internet‐based active control | 4 | 2241 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.18] |
| 2 Smoking cessation at 6 months+ follow‐up (adults) versus non‐Internet‐based non‐active control | 5 | 1846 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.28, 2.12] |
8.1. Analysis.
Comparison 8 Sensitivity analysis (complete cases) ‐ Internet plus behavioural support, Outcome 1 Smoking cessation at 6 months+ follow‐up (adults) versus non‐Internet‐based active control.
8.2. Analysis.
Comparison 8 Sensitivity analysis (complete cases) ‐ Internet plus behavioural support, Outcome 2 Smoking cessation at 6 months+ follow‐up (adults) versus non‐Internet‐based non‐active control.
Comparison 9. Sensitivity analysis (complete cases) ‐ Other comparisons between Internet interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at 6 months+ follow‐up (adults) | 3 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.09] |
Comparison 10. Sensitivity analysis (complete cases) ‐ Comparisons between Internet interventions: tailored/interactive versus not tailored/interactive.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Internet programmes: Smoking cessation at 6 months+ follow‐up (adults) | 6 | 5111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.00, 1.27] |
| 2 Messages: Smoking cessation at 6 months+ follow‐up (adults) | 2 | 1648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.10, 1.70] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
An 2008.
| Methods | Randomised controlled trial Location: University of Minnesota, Twin Cities, USA Funding: Grant from Clear Way Minnesota, and University of Minnesota Trans disciplinary Tobacco Research Centre Recruitment: by Internet health screening in October 2004 | |
| Participants | 517 (257 intervention, 260 control), aged 18 ‐ 24, smoked cigarettes in the past 30 days, and indicated that they intended to be in school for the next 2 semesters; av. age 20 years; 75.4% female control vs 70.4% intervention; 9.2% non‐white control vs 7.8% intervention, av. CPD 4.2 control vs 3.8 intervention, past year quit attempts 52.9% control vs 46.9% intervention | |
| Interventions | Intervention: 'RealU' intervention group were asked to make 20 weekly visits to the study website over a 30‐week period. At the start of each week participant received an email invitation to visit the study website to (1) report on health and lifestyle habits for the prior week (e.g. days smoking, drinking, stress etc); (2) take an interactive quiz with tailored feedback to learn about a smoking‐related or general interest topic; (3) view a student‐authored general interest online college life magazine. Smoking cessation content and messages were introduced gradually over the intervention period. Participants were invited to take week‐long 'breaks' from smoking throughout the intervention period but were not asked to quit for a longer time until the final month of intervention. The intervention site actively promoted the campus‐wide 'Quit & Win' contest and included links to the online sign‐up for this contest. Participants also received weekly emails written by 1 of 9 peer coaches and were encouraged to write back to peer coaches through the 'Question of the Week' contests (i.e. topics that encouraged participants to think about reasons for quitting) Control: received a confirmation email containing links to online health and academic resources. 'Quit & Win' contest was promoted using advertisements in the student newspaper, campus posters, direct mail and email to all university students |
|
| Outcomes | Long‐term abstinence: self‐reported 30‐day abstinence at week 30 validated by CO < 8 ppm. (Individuals who reported 30‐day abstinence at the final evaluation were offered USD 50 to complete an in‐person exit interview during which exhaled CO was measured) Short‐term abstinence: 7‐day PPA at 8 weeks. Other reported abstinence outcomes: 6‐month prolonged at 30 weeks, based on reported duration of abstinence, 7‐day PPA at 20 and 30 weeks Other reported outcomes: quit attempts. | |
| Notes | High level of incentives used to encourage adherence
30‐day abstinence with validation used as primary outcome. No details provided about conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All eligible individuals identified on the health screening were asked to complete online baseline survey prior to enrolment. Participants who completed the baseline survey and provided online consent were enrolled and randomised in real time following a blocked random number sequence generated by the study statistician |
| Allocation concealment (selection bias) | Low risk | Centralised process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No individual withdrew from the study. Follow‐up survey response rates exceeded 90% and did not differ between the groups at any time point. All randomised participants included in ITT analysis |
An 2013.
| Methods | Randomised controlled trial Location: USA Funding: National Institutes of Health (RO1 HL089491) Recruitment: Participants were recruited by an online sample provided by Survey Sampling International. Participants who completed the survey were entered into a draw for 1 of 5 USD 100 cash prizes, or an Apple iPad. Participants who completed the enrolment process received USD 10. All participants received financial incentives (USD 10 a week) to make weekly visits to the study website, and USD 20 incentive to complete the 12‐week evaluation. Study dates: not stated. |
|
| Participants | Participants (n = 567 Lifestyle web, n = 566 Tailored web, n = 565 tailored web and peer coaching) were young adults (18 ‐ 30 years old), and inclusion criteria were aged 18 to 30 years, had smoked at least 1 puff of a cigarette in the previous 30 days, had Internet access for the next 3 months, used the Internet more than twice a week, and lived in the USA. 72.44% were female, and the mean age was 24.1 years. 11.0% were Hispanic/Latino, 89.0% were non‐Hispanic/Latino, 73.9% were white, 10.4% were black, 8.6% were of other ethnicity, and 7.1% were of multiple ethnicity. Education 32.6% had high school education or less, 50.1% had some college and 2‐year degree, 17.0% had a 4‐year degree or more. On average participants smoked 19.8 cigarettes per day | |
| Interventions | The 'Tailored health message' intervention was a tailored and non‐interactive Internet intervention requiring participants to visit the site and report on their cigarette smoking, alcohol use, exercise, and eating breakfast. The intervention focused on building social support for healthy lifestyles, eating healthy breakfasts, increasing exercise, smoking cessation or reduction in smoking, responsible drinking or abstinence from drinking The 'Tailored health + peer coach' intervention included all components of the 'Tailored health message' intervention but was both interactive and tailored as participants were allocated a peer coach who viewed the participants’ behavioural tracking progress charts and sent a personal video message The 'General lifestyle' group received 6 sessions of non‐health‐related lifestyle content over the Internet |
|
| Outcomes | Outcome data were collected at 7 and 12 weeks. Outcomes were non‐bioverified 30‐day prolonged abstinence, alcohol use, eating breakfast, and exercise | |
| Notes | No details provided about conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated using a blocked random number sequence that was generated by the study statistician |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were: 24% tailored health + peer coach, 22% tailored health message, 21% general lifestyle |
Bannink 2014.
| Methods | Cluster‐randomised controlled trial. Clusters were school classes (n = 86) Location: Dordrecht and Zwijndrecht, Netherlands Funding: This study was supported by the Netherlands Organisation for Health Research and Development (ZonMw) (Grant number: 156511010). The publication of this study was supported by a grant of the Netherlands Organisation for Scientific Research (NWO). Recruitment: Secondary schools were invited by 2 youth health care organisations, and participants were not compensated for participation in the study. Study dates: September 2012 to May 2013 |
|
| Participants | Participants (n = 1702) ('E‐health4Uth' condition n = 629; 'E‐health4Uth + consultation' n = 658; control n = 702) were adolescents in the 3rd or 4th years of secondary school. 45.3% were female, mean age was 15.9 (SD = 0.69) years. 76.2% (n = 957) were Dutch. 50.5% (n = 634) had some vocational training, 49.52% (n = 622) had pre‐university education. Participants in the 'E‐health4Uth' were less likely to have used drugs in the past 4 weeks and were younger compared the control group | |
| Interventions | Participants in the 'E‐health4Uth' condition received a tailored and interactive Internet intervention as an adjunct to a behavioural intervention. Participants received tailored messaged based on responses to a health‐risk behaviour and well‐being assessment, received feedback to reinforce healthy behaviour change, were provided links to relevant websites to read more information on the topics. Participants could also self‐refer to the nurse for face‐to‐face or email consultation Participants in the 'E‐health4Uth + consultation' condition received the same intervention as that applied in the 'E‐health4Uth‐only' group, and participants at risk of mental health problems were invited for a consultation with the nurse. Adolescents in the control group completed the same questionnaire assessing health‐risk behaviours and well‐being as adolescents in the intervention groups, with the exception of the questions on unpleasant sexual experience, suicidal thoughts, and suicidal attempts, but did not receive messages afterwards based on their scores. Participants could also self‐refer to the nurse for face‐to‐face or email consultation |
|
| Outcomes | Outcome data were collected at 4 months. Outcomes were non‐bioverified PPA, alcohol consumption, drug use, condom use, mental health, health‐related quality of life | |
| Notes | The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “A computer‐generated list of random numbers was used" |
| Allocation concealment (selection bias) | Unclear risk | The randomisation list was prepared by an investigator with no involvement in the trial and was applied by the researchers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 26.5% E‐health4Uth group, 28.4% E‐health4Uth and consultation group, 29.4% control group |
Berg 2014.
| Methods | Randomised controlled trial Location: South Eastern USA Funding: National Centre for Advancing Translational Sciences (1R43TR000358‐01) and the Georgia Cancer Coalition Recruitment: Participants were recruited by email to complete an online survey, and were sent up to 3 emails to solicit their participation. Participants received a USD 10 gift card for completing the survey, and a USD 10 gift card for completing each assessment. Study dates: From January 2013, to‐date not stated |
|
| Participants | Participants (n = 122) (Intervention n = 63, Control n = 59) were college students, and inclusion criteria were daily or non‐daily smokers, aged 18 ‐ 30 years. Participants were 67.2% (n = 82) female, and mean age was 21.16 (SD = 1.74) years. 56.6% (n = 69) were white, 17.2% (n = 21) black, 7.4% (n = 9) were Asian/Pacific Island, 0.8% (n = 1) American Indian/Alaskan Native, 10.7% (n = 13) were multiracial, 7.4% (n = 9) were of other ethnicity, 9.0% (n = 11) were Hispanic. Participants smoked on average 3.86 (SD = 3.61) CPD, and 47.7% had made a past quit attempt in the past 12 months. There were more Hispanic participants in the intervention condition | |
| Interventions | The Intervention was a tailored and interactive Internet intervention and was delivered over 6 weeks. Participants received a twice‐weekly email reminder to complete a daily record of health behaviours, and that they would receive health‐related information and deals with local businesses. 12 modules were delivered by email twice a week, with email reminders to complete the module. Modules included a short video with a targeted message about: smoker identify, second‐hand smoke exposure, alcohol consumption and cigarette smoking, and likelihood of continued smoking or progression to regular smoking by graduation, tobacco industry manipulation, coping with stress, dealing with lapses and relapses, other cessation resources, e.g. pharmacotherapy options, and other topics. Twice‐weekly emails reminded participants to complete a timeline reporting cigarette and alcohol consumption, and time spent exercising; a graphical figure was produced of participants reported health behaviours over the course of the intervention. The control condition was a tailored and interactive Internet‐based intervention and was delivered over 6 weeks. The control condition involved 12 modules delivered twice‐weekly, with email contacts prompting participants to complete the module; modules contained information cited from the American Cancer Society’s 'Guide to Quitting Smoking'. In this arm participants did not receive the incentives or “daily deals” that were offered to the intervention group |
|
| Outcomes | Outcome data were collected at 6 and 12 weeks. Outcomes were non‐bioverified 30‐day prolonged abstinence, intervention adherence and retention, average CPD for non‐daily smokers, times stopped smoking for 1 day or longer during a quit attempt, readiness to quit, confidence in quitting, and motivation to quit | |
| Notes | Both groups reported high levels of satisfaction with the programme, "roughly 90%" reported that they would recommend the program to a friend. Intervention participants spent more time on the website (P = 0.04), and visited the website more frequently (P < 0.001) Authors reported no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using a random‐number generator |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned using a random‐number generator |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: Intervention 41%, control 69% |
Bolman 2015.
| Methods | Randomised controlled trial Location: Maastricht, Netherlands Funding: The Netherlands Organization for Health Research and Development (Grant number 6200000) Recruitment: Participants were recruited by the Netherlands Foundation for a Smoke‐free Future website. Study dates: From January 2014, to‐date not reported. |
|
| Participants | Participants (n = 1982) ('Action Planning' intervention n = 977, control n = 1005)were from the general population and inclusion criteria were aged 18 years or older, smoked cigarettes and/or hand‐rolling tobacco, and intended to quit smoking within one year. 67.4% were female, and mean age was 38.8 years (SD = 11.4). 13.9% had a low level of education, 49.4% had a medium level of education, 36.7% had a high level of education. 85.7% reported a past quit attempt. There were no differences between arms baseline characteristics | |
| Interventions | The 'Action Planning' arm was a interactive and tailored Internet‐based intervention. Participants received a series of tailored email letters which addressed participants’ perceptions of the pros and cons of quitting, advice on how to deal with a social environment, and aimed to enhance self‐efficacy. Letters for the experimental group also included tailored advice on action planning based on the participant’s response to questions about action planning at baseline. The 'Computer tailored' arm was a interactive and tailored Internet‐based intervention, in which participants received the same email letters as the 'Action Planning' group, except the letters did not include advice on action planning. |
|
| Outcomes | Outcome data were collected at 1 and 6 months. Outcomes were non‐bioverified continued abstinence for 5 months, smoking‐related disease, nicotine dependence, action planning and execution of plans, attitude, social modelling, self‐efficacy, readiness to quit smoking, quit plan generation, and completion of planning sheet | |
| Notes | Participants in both groups evaluated the computer‐tailored email letters as comprehensible, credible, and trustworthy. Participants lost to follow‐up were younger, more likely to be male, less educated, had a higher addiction level, no children, or no partner The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by a computer |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by a computer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: 80% intervention group, 79% control |
Borland 2013.
| Methods | Randomised controlled trial Location: Victoria and South Australia Funding: This study was supported by a National Health and Medical Research Council (Australia) Project Grant no. 396405 Recruitment: Participants were recruited during calls made to the Victorian or South Australian Quitlines, and from 2 Internet survey panels maintained by a Melbourne‐based market and social research company. No details about inducements were provided. Study dates, recruited between November 2008 and November 2009. Study end date not reported. |
|
| Participants | Participants (n = 3530) ('QuitCoach' n = 809; 'Integrated' n = 785; 'onQ program' n = 756; 'Choice' n = 758; control n = 422) were from the general population, and smokers, and recent quitters were included. Participants were: Female 60% (n = 2118), and mean was 42.1 years. Average CPD was 16.9 | |
| Interventions | Intervention arm 1: 'QuitCoach' only was tailored and interactive Internet intervention. QuitCoach is a personalised, automated tailored cessation programme based on cognitive–behavioural principles that generates 2‐ to 4‐page letters of advice with suggestions about strategy, both actions and ways of thinking, and encouragement to persist. The advice is based on answers to an assessment questionnaire and is complemented by some untailored additional resources. The QuitCoach is designed to be used many times, as the questions asked and advice given change with progress in the quit attempt.
Intervention arm 2: 'Integrated' was tailored and interactive Internet intervention as adjunct to non‐internet‐based behavioural intervention. 'QuitCoach' and 'QuitonQ' were offered as a package, but in reality users could subsequently use either or both parts. The 2 programmes have complementary advice, with the brief snippets of advice in the text messages often summarising more detailed material in the tailored advice and supplementary materials. When integrated with 'QuitCoach', a few 'onQ' messages were based on responses to the 'QuitCoach' assessment Non‐internet‐based non‐active control arm 1: Participants were given brief information on web‐ and telephone‐based assistance available in Australia, www.quitnow.org.au and the Quitline number Non‐internet based active control arm 2: The 'onQ program' is based on the same cognitive–behavioural model as 'QuitCoach'. It provides a stream of SMS messages to the person that mix snippets of advice on strategy and things to do with motivational messages. The user can interact with it by reporting changes (e.g. a quit attempt) so that appropriate stage‐specific messages are sent, and once quit can also call up messages in crisis situations. The frequency of messages changes, with peaks on entry, around any actual quit attempt, and around any reported relapse crisis. |
|
| Outcomes | Outcome data were collected at 1 and 7 months. Outcomes were non‐bioverified 6‐month sustained abstinence at 7‐month follow‐up, 7‐day PPA, and proportion of quit attempts by 1 month | |
| Notes | At 1 month, 1 case was excluded from the outcome analyses due to hospitalisations (condition not identified). At 7 months, 2 participants were reported to have died ('onQ' and 'Integrated conditions'). 'QuitCoach' delivery costs were "negligible", and less than AUD 20 per user for onQ. A third (33.7%) of the sample used stop‐smoking medication, with no differences between groups. JB reported that he was employed part‐time during the conduct of this study through the University of Freiburg, Germany, on a project funded by a Pfizer Global Health Partnership. The 'Choice' arm was not included in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using a random‐number generator embedded in the baseline survey |
| Allocation concealment (selection bias) | Low risk | Allocation was computer‐based |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up for each treatment group: 'QuitCoach only' 13%; 'Integrated' 18%; Control 16%; 'Quit onQ' 10%; 'Choice' 16% |
Borland 2015.
| Methods | Randomised controlled trial Location: Australia Funding: National Health and Medical Research Council (grant number 1009767) Recruitment: Participants were recruited between May 2012 and July 2013 during visits to the 'QuitCoach' website. Participants were entered into a monthly draw to win a AUD 100 gift voucher. Participants were recruited between May 2012 and July 2013 |
|
| Participants | Participants (n = 2565) ('Rapid Implementation' n = 1601; 'Structured Planning' n = 964) were from the general population. Participants were excluded if they were using medication for a mental health condition, had already quit for at least 4 days, if they had never been a daily smoker or began smoking less than weekly more than a week ago, or were not interested in quitting. Participants were 66.1% female, and mean age was 37.6 (SD = 11.3) years. 36.5% of participants had secondary or lower education, 35.6% had some tertiary, 37.9% completed tertiary | |
| Interventions | All arms, 'QuitCoach + Rapid Implementation', 'QuitCoach', 'QuitCoach + Structured planning' were tailored and interactive. 'QuitCoach' is a web‐based automated tailored advice programmr that assesses a smoker’s situation by a 10‐minute online assessment and provides a tailored advice letter based on their answers. The programme allows smokers to quit to their own schedule, and it recommends a range of planning activities and has resources available to facilitate planning. 'QuitCoach + Rapid Implementation' included participants who had not committed to a quit date within the next 2 days. 'QuitCoach + Structured Planning' included provision of encouragement and tools for structured planning |
|
| Outcomes | Outcome data were collected at 2 weeks, 1 and 6 months. Outcomes were non‐bioverified 6 months sustained abstinence, and use of stop‐smoking medication. | |
| Notes | Authors reported that they had no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using hidden binary number generators in the QuitCoach assessment |
| Allocation concealment (selection bias) | Low risk | Allocation by a computer programme |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate: Overall 36.3% |
Brendryen 2008a.
| Methods | Randomised controlled trial Location: Norway. Funding: Co‐operation and co‐funding among the University of Oslo, Happy Ending AS, and the Norwegian Research Council Recruitment: by Internet advertisements. |
|
| Participants | 290 (144 intervention, 146 control), at least 18 years old, currently smoking 5+ CPD, willing to quit without using NRT, having daily access to the Internet and email, owning a mobile phone (a Norwegian‐registered phone number and postal address); av. age 39.5 years; 50% female; 49% intervention vs 52% control had a college degree | |
| Interventions | Intervention: Happy Ending (HE), intensive 1‐year smoking cessation programme delivered by the Internet and cell phone, consisting of more than 400 contacts by email, web pages, IVR, and SMS technology. Includes a craving helpline and a relapse prevention system, providing just‐in‐time therapy. All components fully automated Control: 44‐page self‐help booklet issued by the Norwegian Directorate for Health and Social Affairs. Contains general cessation information, quit calendar, 10‐day quit log, phone number of the national quitline, and links to relevant and open online tobacco cessation resources |
|
| Outcomes | Long‐term abstinence: prolonged abstinence at 12 months (i.e. repeated PPA at 1‐, 3‐, 6‐ and 12‐month assessments). No biochemical validation Short‐term abstinence: prolonged abstinence at 3 months (i.e. repeated PPA at 1‐ and 3‐month assessments) Other reported abstinence outcomes: PPA at 1, 3, 6, 12 months Other reported outcomes: Participant exposure (frequency and duration of each participant's visits to the web‐based programme), pharmacotherapy use, programme usability | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random digit. Stratified block randomisation applied to ensure equal numbers of both men and women in each group |
| Allocation concealment (selection bias) | Low risk | Centralised system |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 57 participants discontinued intervention at follow‐ups, none discontinued in control group. Cumulative dropout at 12months, 26 in intervention, 38 in control groups. All randomised participants were included in ITT analysis |
Brendryen 2008b.
| Methods | Randomised controlled trial Location: Norway. Funding: University of Oslo, Happy Ending AS and the Norwegian Research Council. Pfizer Norway provided a free supply of NRT Recruitment: by Internet advertisements |
|
| Participants | 396 (197 intervention, 199 control) aged 18 years or older, currently smoking more then 10 CPD, access to the Internet, email and cell phone on a daily basis, willing to quit smoking. Av. age 36 years; 50.8% female intervention vs 49.8% control; 42.1% intervention group vs 39.7% control with college degree; av. CPD 18 | |
| Interventions | All participants offered free NRT Intervention: Happy Ending intervention (HE) ‐ fully automated and digitally‐delivered smoking cessation intervention. The programme lasted 54 weeks and consisted of more then 400 contacts by email, web pages, IVR and SMS technology Control: received a self‐help booklet | |
| Outcomes | Long‐term abstinence: prolonged abstinence at 12 months (i.e. repeated PPA at 1‐, 3‐, 6‐ and 12‐month assessments). No biochemical validation Short‐term abstinence: PPA at 3 months Other reported abstinence outcomes: PPA at 1, 6, 12months Other reported outcomes: Programme use, NRT adherence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random digit |
| Allocation concealment (selection bias) | Low risk | Centralised system |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 post‐randomisation exclusions due to erroneous allocation. Response rate generally high across experimental condition and time (95.9% intervention vs 91.5% control at 12‐month assessment). All randomised participants were included in ITT analysis |
Bricker 2013.
| Methods | Pilot randomised controlled trial Location: USA Funding: This study was funded by the Fred Hutchinson Cancer Research Center. Dr. Bricker’s writing of this article was partly supported by a grant from the National Cancer Institute (R01CA166646 01A1). Dr. Heffner’s work on the project was supported by a grant from the National Institute on Drug Abuse (K23DA026517) Recruitment: Participants were recruited by radio and television, web‐based media, social networking sites, paid Internet advertisements, and emails to relevant professional organisations and employers. Participants received USD 10 compensation for completing study assessments at follow‐up. Participants were recruited over a 10‐week period starting June 15, 2010 |
|
| Participants | Participants (n = 222) ('ACT Webquit.org' n = 111; 'smokefree.gov' n = 111) were aged 18 or older, smoked at least 5 CPD for at least the past 12 months, wanted to quit within the next 30 days, were willing to be randomly assigned, US resident, had weekly access to a high‐speed Internet connection, willing and able to read in English, were not participating in any other smoking cessation interventions, and had never used the smokefree.gov website. No overall baseline characteristic were reported, but the trial arms were balanced on all recorded variables | |
| Interventions | 'ACT Webquit.org' was a tailored and interactive Internet intervention, targeting Acceptance and Commitment Therapy's (ACT) core process of values guiding quitting and containing videos of former smokers describing how quitting smoking changed their lives. Helped users apply their core values guiding quitting toward a personalised quit plan, and targeted processes of acceptance, being present, cognitive defusion, and awareness of the difference between one’s self and one’s thoughts. 'smokefree.gov' is a non‐tailored and non‐interactive Internet intervention which involves advice on planning a quit attempt, skills training, advice on pharmacotherapy, and social support for quitting |
|
| Outcomes | Outcome data were collected at 3 months. Outcomes were non‐bioverified 30‐day PPA, and the Avoidance and Inflexibility Scale | |
| Notes | Dr. Heffner has in the past served as a consultant for Pfizer. None of the other authors had competing interests to disclose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified blocked randomisation (with random block sizes), stratifying on gender and current depression |
| Allocation concealment (selection bias) | Low risk | “Randomised study arm assignments were computer generated and concealed from participants after study eligibility was determined”, “Neither research staff nor study participants had access to upcoming randomised study arm assignments” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 44% intervention, 45% control |
Brown 2014.
| Methods | Randomised controlled trial Location: United Kingdom Funding: This study was funded by a grant from the National Prevention Research Initiative (reference G0802035). Other support from Alzheimer’s Research Trust; Alzheimer’s Society, Biotechnology and Biological Sciences Research Council, British Heart Foundation, Cancer Research UK, Chief Scientist Office, Scottish Government Health Directorate, Department of Health, Diabetes UK, Economic and Social Research Council, Engineering and Physical Sciences Research Council, Health & Social Care Research & Development Office for Northern Ireland, Medical Research Council, The Stroke Association, Welsh Assembly Government Recruitment: Participants were recruited by the English Department of Health website called SmokeFree. All participants received a GBP 20 gift voucher. The study was conducted between Dec 6, 2011, and Oct 11, 2013. |
|
| Participants | Participants (n = 4613) ('StopAdvisor' n = 2321; control n = 2292) were high‐ and low‐socioeconomic status subpopulations. Inclusion criteria were aged 18 years and older, daily smoker, willing to make a serious quit attempt, and use a stop‐smoking website that sends email reminders, and agreed to study procedures. No overall study data reported | |
| Interventions | 'StopAdvisor' was a tailored and interactive Internet intervention, including advice on setting a quit date, use of smoking cessation medicines, reasons for quitting, making behavioural changes to minimise urge to smoke, developing specific coping strategies. Participants had access to an interactive calendar, frequently‐asked questions, a 'your progress' section, audio and video, and the 'StopAdvisor' Facebook page The control condition was a non‐tailored and non‐interactive Internet intervention, which was a 1‐page static website that presented brief and standard advice focusing on setting a quit date, use of smoking cessation medication Participants in both arms were encouraged to use medication, and use the NHS Stop Smoking Services |
|
| Outcomes | Outcome data were collected at 2, 4, 6, and 7 months. Outcomes were Russell Standard bio‐verified 6‐month sustained abstinence, website use, self‐reported abstinence, quit attempt, and website satisfaction | |
| Notes | All authors reported receiving grants from National Prevention Research Initiative during the study. JB reported grants from Pfizer, outside of the submitted work. LS reported personal fees from Pharmaceutical companies that make smoking cessation products, outside of the submitted work. RW reported receiving grants and personal fees from companies that develop and manufacture smoking cessation drugs, outside of the submitted work, and has had a patent issued for the “Nicotine Cannon” (novel nicotine delivery device) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was completely automated with no experimenter involvement by use of an unseen random‐number function embedded in the website code |
| Allocation concealment (selection bias) | Low risk | Randomisation was completely automated with no experimenter involvement by use of an unseen random‐number function embedded in the website code |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 29% StopAdvisor, 27% control |
Burford 2013.
| Methods | Randomised controlled trial Location: Perth, Australia. Funding: No information provided Recruitment: Participants were recruited from 8 metropolitan community pharmacies around Perth city centre, Western Australia, when presenting to collect prescribed medications or over‐the‐counter medications. No incentive was offered for participation. The study was conducted between January 2010 and December 2010. |
|
| Participants | Participants (n = 160) (Intervention n = 80; control n = 80) were aged between 18 and 30 years, smokers (defined as smoking 1+ CPD by self‐report); able to give consent; available for follow‐up at 6 months; no beards, moustaches, or non‐removable facial accessories; no body dysmorphia; and not using NRT or taking oral drugs for nicotine dependence. No overall baseline characteristics provided, and trial arms were balanced on all recorded characteristics | |
| Interventions | The face simulation software intervention was a tailored and interactive Internet‐based intervention as an adjunct to behavioural intervention, and was delivered over 1 brief session. In the intervention arm an Internet‐based 3‐dimensional age progression software package created a stream of aged images of faces from a standard digital photograph; the resulting aged image was adjusted to compare how the participant aged as a smoker versus as a non‐smoker. Participants also received standard 2‐minute smoking cessation advice from the pharmacist. The control arm was a brief face‐to‐face non‐internet‐based, non‐active control arm in which participants received standard 2‐minute smoking cessation advice from the pharmacist |
|
| Outcomes | Outcome data were collected at 1, 3, and 6 months. Outcomes were: bioverified (48 hours of follow‐up survey) PPA, quit attempts, transtheoretical stages of change, nicotine dependence, cost effectiveness of the intervention, and viability of delivering the intervention in a community pharmacy | |
| Notes | Cost of implementing the intervention was AUD 463, or the equivalent of AUD 5.79 per participant. The incremental cost‐effectiveness ratio was AUD 46 per additional quitter, or the equivalent of AUD 74 per additional lifetime quitter. Authors reported no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were recruited and assigned by the researcher to the different arms of the study on alternate weeks |
| Allocation concealment (selection bias) | High risk | Participants were recruited and assigned by the researcher to the different arms of the study on alternate weeks |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 26% intervention; 21% control |
Calhoun 2016.
| Methods | Randomised controlled trial Location: Durham, North Carolina, USA Funding: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Health Services Research and Development (IIR‐08‐032) Recruitment: Participants were recruited from the Durham VA Medical Center, and were compensated USD 25 per completed assessment, and received up to USD 50 for return of saliva samples. Study dates not reported. |
|
| Participants | Participants (n = 413) ('QuitNet' n = 206; Usual care n = 207) were Military Veterans, and inclusion criteria were current smoker, enrolled at the VA for primary care, and willing to make a quit attempt in the next 30 days. 16% were female, and mean age was 42.9 (SD = 13.9). 51% were white, 39% African‐American/black, 4% Hispanic/Latino. 24% had high school education or less, 76% had more than high school education. Mean number of CPD was 15.2 (SD = 8.7) | |
| Interventions | The Internet‐based intervention was tailored and interactive. Participants were provided a membership the full version of 'QuitNet®'. The website provides access to cessation support that is personalised based on each user's readiness to quit, access to online smoking cessation counsellors, and interactive features offering assistance in selecting a quit date and choosing medications, social features (i.e. forums, buddies, chat rooms), and email support. Participants were also offered NRT at baseline. The 'Specialty Care' arm was a non‐internet‐based active control arm in which participants were offered group and telephone counselling based on the 'QuitSmart™ Program', with smoking cessation medication offered as standard |
|
| Outcomes | Outcome data were collected at 3 and 12 months. Outcomes were bioverified 7‐day PPA, reach, and cost effectiveness | |
| Notes | The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough details provided |
| Allocation concealment (selection bias) | Low risk | Study staff members were blinded to the randomisation block size |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: Internet intervention 24%, specialty care 27% |
Cameron 2015.
| Methods | A repeat randomised controlled trial (based on Epton 2014) Location: Sheffield, UK Funding: UK National Prevention Research Initiative (NPRI) Phase 4 (grant number: MR/J0004501/1) Recruitment: Participants were invited by email upon university registration. Participants were entered into a GBP 100 prize draw as an incentive for completing each questionnaire. Participants completing all 3 questionnaires received a GBP 10 gift voucher and were entered into a draw for an iPad Mini. The study was conducted between September 2013 and March 2014. |
|
| Participants | Participants (n = 2621) (Intervention n = 1346; control n = 1275) were university students. Inclusion criteria were: incoming undergraduates at the University of Sheffield. There were no exclusion criteria. No overall study baseline characteristics were reported | |
| Interventions | The intervention was a non‐tailored and interactive Internet intervention. Participants assigned to the intervention condition completed a profile page that contained the self‐affirmation manipulation. They were directed to complete 4 modules about health‐related behaviour, content contained theory‐based messages and planning exercises. Once modules were completed participants were granted access to the full website with further health messages and links on each of the 4 targeted health behaviours The control condition was an email reminder to complete a questionnaire. |
|
| Outcomes | Outcome data were collected at 1 and 6 months. Outcomes were bioverified sustained cessation, fruit and vegetable intake, physical activity, alcohol consumption. Secondary outcomes: health behaviours at 1‐month follow‐up, social cognitive variables, health status, recreational drug use, BMI, health services usage, and biochemical measures | |
| Notes | The authors declared no competing interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated using the random function on LifeGuide |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated using the random function on LifeGuide |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: Intervention 62%, control 54% |
Choi 2014.
| Methods | Cluster‐randomised controlled trial Location: Michigan, USA Funding: Michigan foundation (n011646‐1465rfp) and the national institutes of health (5r21ca152247‐02). Recruitment: Participants were recruited during a regularly scheduled safety training session, where the study nurse described the study to potential participants. Participants who completed the baseline survey received USD 15, USD 15 for the 30‐day survey, and USD 20 for the 6‐month survey and cotinine test. The study was conducted between 2010 and 2012. |
|
| Participants | Participants (n = 145) ('Tobacco Tactics' n = 67; '1‐800‐quit‐now' n = 59) operating engineers aged 18 years or older, current smokers, and interested in participating in a cessation programme. Exclusion criteria: Operating engineers who were non‐English speaking, or pregnant. Participants were 20.7% female (n = 30), and mean age was 42.0 years (SD = 9.5). 86.2% were white (n = 125), 13.8% (n = 20) were non‐white. 61.1% (n = 88) completed high school or less than high school, 38.9% (n = 56) completed more than high school. Average/median CPD was 20.9 (SD = 9.9), and 86.9% (n = 126) reported a previous quit attempt. The proportion of participants thinking about quitting in the next 30 days was higher in the 1‐800‐quit‐now (n = 44, 56.4%) group compared to website arm (n = 32, 47.8%) (P = 0.042) | |
| Interventions | The 'Tobacco Tactics' website was a tailored and interactive Internet intervention as an adjunct to telephone‐based behavioural support, with pharmacotherapy. Behavioural and pharmacotherapy support were offered at 2, 7, 14, 21 and 30 days after the training, website access was ongoing during the study period. The website contains humorous graphics tailored to operating engineers, offered tailored cessation feedback, and follow‐up nurse counselling was offered by telephone or email or both, and/or online community. The content included interactive cognitive behavioural therapy exercises including a self‐assessment of tobacco habit, assessment of nicotine dependence, calculation of money savings, tips for preparing to quit, a change plan work sheet, and strategies for coping with relapses. Interactive components included mechanisms for users to assess their smoking habits, set a quit date, and monitor weekly progress; a nurse monitored the e‐community as a group moderator 3 times per week, answered questions, and stimulated group discussion. On each log‐off, participants answered questions about their tobacco habits which produced a graphic displaying their progress over time. Participants were offered over‐the‐counter nicotine patches, gum, lozenges, or a combination for highly‐dependent smokers. The nurse made follow‐up telephone and/or email counselling contacts at 2, 7, 14, 21 and 30 days after the training to reinforce website visits, promote skill building, and monitor pharmacologic treatment. '1‐800‐quit‐now' was a non‐internet‐based active control arm delivered at 2, 7, 14, 21, and 30 days after training, and smoking cessation medication was also offered to participants. In the '1‐800‐quit‐now' arm participants were encouraged by the study nurse to call and were given time to do so at their safety training class. The first time participants called the quit line, they received a personal coach who assisted them in setting a quit date and making an individualised quit plan, followed by up to 5 telephone coaching sessions around the caller’s quit date and free NRT (patches or gum), which were all equivalent to the tobacco tactics intervention. Those who had failed on NRT in the past discussed were offered bupropion or varenicline |
|
| Outcomes | Outcome data were collected at 1 and 6 months. Primary outcome was bioverified 7‐day PPA. Secondary outcomes were self‐reported quit rates; cotinine levels; number of quit attempts; nicotine dependence; CPD; smoking self‐efficacy; contacts with interventions; medications used; helpfulness of the interventions; and willingness to recommend the interventions to others | |
| Notes | The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 31% website‐tobacco tactics, 24% 1‐800‐quit‐now |
Clark 2004.
| Methods | Randomised controlled trial Location: USA Funding: Grant from the National Cancer Institute Recruitment: current smokers undergoing low‐dose fast spiral chest CT (SCTS) for lung cancer. Participants were recruited at the first annual follow‐up visit |
|
| Participants | 171 current cigarette smokers (85 intervention group, 86 control group), had access to a computer with Internet service. Average age 57.4 years, 50% female, 60% of participants were smoking < 20 CPD | |
| Interventions | Intervention: Hand‐out with a list of 10 Internet sites related to stopping smoking and a brief description of each site Control: received a copy of a publication of the National Cancer Institute |
|
| Outcomes | Long‐term abstinence: 7‐day PPA at 12‐months Short‐term abstinence: 7‐day PPA at 30 days Validation: CO measurement at 12‐month follow‐up Other reported outcomes: readiness to quit if not stopped, other tobacco use, number of quit attempts in previous year, other smokers in household, utilisation of intervention materials at 30‐day follow‐up | |
| Notes | Not included in any analyses, as intervention extremely low intensity and similar to control arms of other trials (self help). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given in the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts, as all study participants attended their annual review which corresponded with the 1‐year follow‐up assessment |
Cobb 2016.
| Methods | Randomised controlled fractional factorial trial Location: USA Funding: The National Cancer Institute of the National Institutes of Health (R01CA155369) Recruitment: Participants were recruited by Facebook advertising, and earned media. A sub‐sample (10%) of seed participants who completed a brief web‐based survey at baseline and 30 days after enrolment were reimbursed USD 20 for each completed survey. The study was conducted between December 2012 and October 2013. |
|
| Participants | Participants (n = 9042, n by intervention arm was not provided) were from the general population, and were a US resident, current smoker, aged 18 years or older, had an active English‐language Facebook account and email address, accepted Facebook permissions for application installation. Exclusion criteria: Participants with a Facebook friend that had already installed the application. Participants were 70% female (n = 6329), and were aged 43.9 years (SD = 14.1). Ethnicity among a subsample of survey participants (n = 857): 11% (n = 94) were non‐white, 4% (n = 34) were Hispanic. Education among a subsample of survey participants (n = 857), 58% (n = 497) had at least some college education. Cells were balanced on all recorded characteristics. | |
| Interventions | The Facebook intervention was a tailored and interactive Internet intervention, no details about intervention duration were provided. The Facebook intervention is based on the “5As” model (Ask, Advise, Assess, Assist, and Arrange). Intervention participants were asked if they smoke and were advised to quit, participant's readiness to quit was assessed and a “Quit Date Wizard” was provided to assist in planning a quit attempt and setting a quit date. If the participant set a quit date, the application displayed a countdown to that date or an estimate of savings since that date. Users who did not set a quit date in their first visit may set one at any time. Daily check‐ins provided tailored, personalised information and support and assessed smoking status. 'Facebook intervention with alerting' was a tailored Internet intervention with additional online alerts to remind users of the application. Participants received the Facebook application as described, and proactive Facebook application alerts reminding them to check in to the application. At each check participants confirmed their quit date or updated their smoking status. Smokers who had not set a quit date received various daily check‐ins that included prompts to set a quit date, as well as evidence‐based content incorporating the “5 Rs” (Relevance, Risk, Rewards, Roadblocks, and Repetition). |
|
| Outcomes | Outcome data were collected at 30 days. Outcomes were non‐bioverified 7‐day PPA, and diffusion through Facebook | |
| Notes | ClinicalTrials.gov Identifier: NCT01746472. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Seed users were randomised using "an adaptive biased‐coin strategy” by the programme |
| Allocation concealment (selection bias) | Low risk | The trial was conducted entirely within Facebook with all recruitment, screening, enrolment and randomisation automated by clinical trials management software |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
Dezee 2013.
| Methods | Randomised controlled trial Location: USA Funding: "no external funding". Recruitment: Participants were recruited through advertisements in a military medical centre and surrounding clinics in the southwestern United States. Participants were not compensated. Study enrolment was conducted between February 20, 2007 and May 27, 2008. |
|
| Participants | Participants (n = 217) (In‐person Counseling n = 44; Internet Counseling n = 173 were military personnel, or family members of military personnel. Inclusion criteria were aged 18 years or older, entitled to care within the US military medical system, smoked at least 10 CPD, used email regularly, planned to be geographically stable for 4 months, and planned on quitting in the next 30 days. No overall baseline characteristics were provided and there were no differences between arms for baseline characteristics. | |
| Interventions | The Internet counselling intervention was both tailored and interactive. Participants using the 'GetQuit' web‐based counselling programme received a daily email and invitation to complete an activity. The activities included a detailed smoking history, motivations to quit smoking, quit date advice, smoking triggers and alternatives, support systems, coping strategies, avoiding weight gain, and medication education. The control group received in‐person counselling of 1½‐hour group classes conducted once weekly for four weeks. The classes emphasised motivational intervention activities, practical counselling (problem‐solving), social support, healthy eating, stress management, and education about medications and approaches to quitting Participants in both arms were prescribed a standard dose of varenicline. |
|
| Outcomes | Outcome data were collected at 12 weeks. Outcomes were bioverified 7‐day PPA, nicotine dependence, depression, and anxiety. | |
| Notes | The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated using a random‐number table |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned using a sealed, opaque envelope |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rate: Overall 57% |
Dickinson 2013.
| Methods | Randomised controlled trial Location: Colorado, USA. Funding: The Robert Wood Johnson Foundation provided the funding for this project through its Prescription for Health initiative Recruitment: Participants were recruited from GPs practices. No study dates reported. |
|
| Participants | Participants (n = 169) (Basic Website n = 88; Enhanced Website n = 81) were from the general population. Inclusion criteria were aged between 18 and 65 years, had been seen in the practice within the last 18 months, and could read and write English or Spanish. Participants were: 79.7% female (n = 135), mean age was 43 years (range 18 ‐ 79 years). Some college or college graduate 88.2% (n = 149), Non‐Hispanic white 85.8%. 8.3% were current smokers. There were more smokers in the enhanced intervention group (P = 0.0118), and the participants in the enhanced group indicated that they had poorer physical health based on a higher number of unhealthy days during the previous month (P = 0.0472). | |
| Interventions | The basic site was a non‐tailored and non‐interactive Internet intervention that included educational content for healthy eating, activity level, alcohol intake, cigarette smoking and depression. Materials were designed to assist participants in behaviour change. The site also regularly updated information about behaviour change and an educational section about how they could better communicate with clinicians about behaviour change and related issues. The enhanced site was a tailored and interactive Internet intervention that included all the elements of the basic site plus an extensive section about action plans, where participants were prompted to develop an individualised action plan for changing behaviour, or a plan to monitor depression symptoms. Participants who developed an action plan were prompted to discuss this with their GP. The enhanced site also included an online forum where participants could post issues and discuss them with other participants working on similar behavioural changes, and an “Ask the Expert” section, where questions for the clinical team could be posted. Participants were encouraged to return to the site periodically. |
|
| Outcomes | Outcome data were collected at 3 and 6 months. Outcome data were health behaviour change around diet, physical activity, alcohol consumption, and self reported smoking status. Smoking outcome data were not reported, and data for smokers only were not available from study authors. | |
| Notes | No conflicts of interest were declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: 76% basic website, 67% enhanced website |
Elfeddali 2012.
| Methods | Randomised controlled trial Location: Netherlands Funding: Dutch Organization for Health Research and Innovation Recruitment: Flyers, ads in local newspapers, online ads on the websites of national health funds, a national news page, and the Dutch Foundation for a Smoke‐free Future |
|
| Participants | 2031 daily smokers aged 18 ‐ 65 years, willing to set a quit date within 1 month, motivated to quit smoking. Average age 42, 62.3% female, ethnicity not reported, average CPD was 20, 92.9% had previously attempted to quit at some point. Education: 10.2% low; 55.6% medium; 34.2% high | |
| Interventions | Intervention 1 ('AP'): Tailored feedback at baseline, plus invitations to do 6 preparatory and coping planning assignments, all online (the first 3 prior to quit date and the final 3 assignments after quit date). Based on I‐Change model Intervention 2 ('AP+'): as per AP but 11 assignments after quit attempt (14 total) Control: usual care. |
|
| Outcomes | Long‐term outcome: continuous abstinence at 12 months Secondary outcomes: programme evaluation, dose response Methods of assessing outcome: Primarily self‐report. Cotinine validation used in subsample (n = 70) Biochemical verification changed outcomes for 2 participants who were self‐reported non‐smokers |
|
| Notes | Numbers used in analysis come from sample 1 as reported in paper (ITT). Respondents who completed all parts of their assigned programme (including those in the control group) were eligible to win 1 of 20 prizes of EUR 250. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computerised allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High losses to follow‐up, although similar percentage followed up in each group: 202 control (31.8%), 190 AP (27.2%), 174 AP+ (25.0%) |
Emmons 2013.
| Methods | Randomised controlled trial Location: USA and Canada Funding: National Cancer Institute (5R01CA106914‐5 and 1K05 CA124415) Recruitment: Participants were recruited from cancer treatment centres, and the study was advertised on websites designed for and about childhood and young adult cancer survivors and survivorship. Baseline data collection began on December 2005 and follow‐up data collection ended in October 2009. |
|
| Participants | Participants (n = 374) (Web n = 201; Control n = 128) were childhood cancer survivors who smoked. Inclusion criteria were diagnosis of cancer before age 35, currently aged 18 ‐ 55 years, completed cancer treatment for ≥ 2 years, mentally able to provide informed consent, reachable by telephone, able to speak English, and a current smoker (i.e. defined as smoking within the previous 30 days). Participants were: 48.7% female, mean age was 32 (SD = 7.94) years; 86.4% were white; 36.1% had a high school education or less, 29.7% had at least a college degree. On average participants smoked 10 CPD | |
| Interventions | The web arm was a tailored and interactive Internet intervention. The web content was tailored based on participants' motivation to quit smoking; participants could re‐assess their motivation to quit at any time and the website content would change based on their responses. Participants had access to the website for 6 months regardless of their log‐in status. The control arm was a non‐internet‐based non‐active control, in which participants received a letter from the site oncologist encouraging smoking cessation, and a self‐help manual about how to quit smoking. In both arms, free nicotine patches or bupropion were offered to all participants, and spouses/significant others who wanted to quit |
|
| Outcomes | Outcome data were collected at 15 months. Outcomes were non‐bioverified 30‐day PPA, Internet access and utilisation, nicotine dependence, quit attempts, use of pharmacotherapy, motivation to quit smoking, self‐efficacy, cancer‐related distress, perceived control, perceived vulnerability, depression, contact with the healthcare system, and intervention use | |
| Notes | The authors declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not enough details provided about sequence generation. “The random allocation sequence was generated by the study biostatistician.” |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether future allocation was concealed from the survey team “The random allocation sequence was generated by the study biostatistician. Randomization was done by the survey team and supervised by the biostatistician, following completion of the baseline survey.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: web arm 43%, control arm 12% |
Epton 2014.
| Methods | Randomised controlled trial Location: Sheffield, UK Funding: The study was funded by the UK National Prevention Research Initiative (NPRI) Phase 4 (grant number: MR/J0004501/1) Recruitment: Participants were invited by email upon university registration. Participants were paid GBP 10 for completing all 3 questionnaires and were entered into a GBP 100 prize draw for each questionnaire they completed. The study was conducted from September 2012 to March 2013. |
|
| Participants | Participants (n = 1445) (Intervention n = 736; Control n = 709) were university students. Inclusion criteria were incoming undergraduate at the University of Sheffield. No overall participant baseline characteristics were reported. There were no differences between participants in the intervention and control arms in baseline measures of the 4 health behaviours. Gender and age did, however, differ between the 2 arms, with more women and younger participants in the intervention arm than in the control arm | |
| Interventions | The intervention was a tailored and interactive Internet‐based intervention. Participants assigned to the intervention arm were directed to the U@Uni website and asked to complete a profile page that contained the self‐affirmation manipulation. After completing their profile, participants were asked to sign in to the website and view the online resources, which included theory‐based messages relevant to the targeted health behaviours and a planner that contained instructions to form implementation intentions. Participants were able to access information that was of interest to them. Participants could download a smartphone app which was available throughout the year Measurement‐only control |
|
| Outcomes | Outcome data were collected at 1 and 6 months. Outcomes were bioverified sustained cessation, portions of fruit and vegetables, physical activity alcohol consumption, health status, recreational drug use, BMI, Health Service usage, academic performance, social cognitive variable, and engagement with the digital intervention | |
| Notes | The authors declare that they have no competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated using SurveyGizmo |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated using SurveyGizmo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: Intervention 40%, control 34% |
Etter 2005.
| Methods | Randomised controlled trial Location: Switzerland Funding: Health Department of the Canton of Geneva, Swiss Cancer League, Swiss Federal Office of Public Health, Novartis. The Health on the Net (HON) Foundation, provided an informatics engineer who developed the software that produced the counselling letters and who managed the data collection and storage. Recruitment: visitors to Stop‐tabac.ch, a French‐language website. Enrolment of participants took place between April 2003 and July 2004 |
|
| Participants | 11,969 visitors to the website (Intervention n = 5966; Control n = 6003), including current and ex‐smokers. Average age 34 years; 61% female; 19.5 CPD | |
| Interventions | Compares 2 Internet‐based interventions. Intervention: The original online programme was a tailored, interactive smoking cessation programme. It was based on psychological and addiction theory, and preliminary research conducted in the same population. The tailoring questionnaire assessed demographic characteristics, smoking status, stage of change, level of tobacco dependence, attitudes toward smoking, self‐efficacy, use of self‐change strategies and coping methods, and intention to use NRT. After answering the 62‐item questionnaire, participants received a personal counselling letter of 6 to 9 pages (3000 ‐ 4000 words) illustrated with cartoons and graphs that were also tailored to each participant's answers. The counselling letter consisted of about 20 paragraph of text, chosen by the computer from a library of 350 paragraphs according to pre‐established decision rules. Control: modified tailored programme was shorter, simplified version designed for NRT users. The modified programme used a shorter questionnaire (38 questions) that included ad hoc questions instead of validated multi‐item scales. The counselling letter was of similar length (3000 ‐ 4000 words), but contained more information on NRT and nicotine dependence and less information on health risks and coping strategies |
|
| Outcomes | Short‐term abstinence: self‐reported 7‐day PPA at 11 weeks post‐randomisation | |
| Notes | Short‐term outcomes only, so not included in comparisons. Differential drop‐out did not lead to substantial difference in relative effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation. |
| Allocation concealment (selection bias) | Low risk | Alternate questionnaires used by each separate person signing up to the website. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 50% lost to follow‐up. Follow‐up survey completed by 2,341 (39.2%) of those on original programme vs 1,896 (31.6%) of those on modified (p < 0.001). All randomised participants were included in ITT analysis. |
Fraser 2014.
| Methods | Evaulation study of interventions Location: USA Funding: Matthews Media Group, and ARRA funding to the National Cancer Institute. Additional funding was provided by the National Cancer Institute (5K05CA139871) Recruitment: Participants were recruited through the smokefree.gov home page. Participants were reimbursed for completing assessments: USD 10 for enrolment, USD 20 at 1 and 3 months, and USD 45 at 7 months. No study dates reported. |
|
| Participants | Participants (n = 1034) (participants had one or more components turned on: Quitline counselling n = 453; NRT n = 518; Messaging n = 523; Website n = 509; Brochures n = 517) were from the general population. Inclusion criteria were aged 17 years or older, daily smoker of at least 5 CPD, interested in quitting smoking within the next 30 days but not actively engaged in quitting, had phone and home Internet access, had an email address, no prior use of the smokefree.gov website, suitability for NRT (e.g. no allergies to NRT, not pregnant), willingness to perform study procedures and have use of smokefree.gov website tracked. Participants were: 68% female (n = 703), mean age was 39.3 years (SD = 12.3); 84.4% (n = 877) were white; 6.6% (n = 68) African‐American; 3.9% (n = 40) had below high school education; 20.4% (n = 211) high school only; 56.1% (n = 580) high school/college degree; 19.7% (n = 204) college graduate. For smokers, the mean CPD was 19.3 (SD = 8.9) | |
| Interventions | The study has 5 intervention components that were either 'turned on or off' for each participant: The National Cancer Institute’s website (smokefree.gov vs a 'lite' website), telephone quitline counselling (vs none), a smoking cessation brochure (vs a 'lite' brochure), motivational email messages (vs none), and mini‐lozenge NRT (vs none). This therefore resulted in 32 different combinations of intervention components | |
| Outcomes | Outcome data were collected at 1, 3 and 7 months. Outcomes were non‐bioverified 7‐day PPA, tobacco dependence, Wisconsin Inventory of Smoking Dependence Motives, Internet experience and resources, smoking history information, social support, alcohol use and problems, relapse proneness, and withdrawal symptoms, ratings of access to quitting resources, treatment satisfaction, and affect | |
| Notes | David Fraser, Kate Kobinsky, Stevens Smith, Jason Kramer, Wendy Theobald, and Timothy Baker declared that they have no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition: overall 20% |
Frederix 2015.
| Methods | Randomised controlled trial Location: Belgium Funding: Flanders Care (Grant number DEM2012‐02‐03) and from the Research Foundation Flanders (FWO; Grant number: 1128915N) Recruitment: Participants were recruited from Cardiology Departments of 3 Belgian hospitals. Participants were recruited offline at the hospitals’ rehabilitation centres during face‐to‐face information sessions. No incentive was offered for participation. the study was conducted from February 2013 to August 2014. |
|
| Participants | Included participants (n = 140) (Intervention group n = 70; Control group n = 70) entered cardiac rehabilitation for coronary heart disease and were treated with a percutaneous coronary intervention or with coronary artery bypass grafting, or congestive heart failure with reduced ejection fraction, had a computer at home with Internet access. Exclusion criteria: coronary heart disease class IV, symptomatic and/or exercise‐induced cardiac arrhythmia within the previous 6 months, physical disability related to musculoskeletal or neurological problems, or severe cognitive impairment. There were an equal number of smokers in each arm (n = 18 per arm). No other overall baseline characteristics provided | |
| Interventions | Center‐Based Cardiac Rehabilitation Program was a tailored and interactive Internet intervention delivered alongside a non‐internet‐based behavioural intervention and was delivered over 49 sessions over 24 weeks. Intervention‐group participants received a 24‐week, Internet‐based programme in addition to the conventional centre‐based cardiac rehabilitation. The telerehabilitation programme started at week 6 of the 12‐week centre‐based cardiac rehabilitation, allowing the intervention‐group participants to become familiarised with the telerehabilitation’s motion sensor and associated password‐protected web service during the 6‐week overlap period. The programme focused on multiple cardiac rehabilitation core components and used both physical activity telemonitoring and dietary/smoking cessation/physical activity telecoaching strategies. “For the telemonitoring part, intervention group patients were prescribed patient‐specific exercise training protocols based on achieved peak aerobic capacity during initial maximal cardiopulmonary exercise testing and calculated body mass index. Intervention group patients were instructed to continuously wear the accelerometer and to regularly transmit their registered activity data to the telerehabilitation centre’s local server. They were instructed to transmit their physical activity data at least once weekly. These data enabled a semiautomatic telecoaching system to provide the patients with feedback by email and SMS text messaging (once weekly), encouraging them to gradually achieve predefined exercise training goals. ” “In addition, patients received emails and/or SMS text messages (once weekly) with tailored dietary and smoking cessation recommendations... The smoking cessation telecoaching program included information on major risks associated with smoking, the health benefits of smoking cessation, and nicotine replacement therapy. It provided smokers with encouraging messages toward smoking cessation." The control group was a centre‐based rehabilitation programme which was a non‐internet‐based active control arm, which included 45 pluridisciplinary rehabilitation sessions and at least 2 exercise training sessions a week delivered over 24 weeks. The group had at least 1 consultation with the dietician who provided the participants with general guidelines on healthy diet, and at least 1 consultations with a psychologist who aimed to improve the participant’s self‐efficacy to change prior unhealthy lifestyle behaviour to a more healthy lifestyle behaviour, and assessed the participant's mood related to their cardiac event |
|
| Outcomes | Outcome data were collected at 24 weeks. Outcomes were: Peak oxygen consumption (VO2 peak), Daily physical activity, cardiovascular risk factor control (body weight, blood pressure, blood lipid profile, blood glucose level and HbA1c), HeartQol quality of life, IPAQ physical activity, EQ‐5D score, days lost due to cardiovascular rehospitalisation, days lost due to hospitalisations for any reason, time to first cardiovascular rehospitalisation, time to first hospitalisations for any reason. Smoking status was not recorded as an outcome, and data were unavailable from the authors. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by a central computerised randomisation system using block randomisation |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by a central computerised randomisation system using block randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 10% overall |
Graham 2011.
| Methods | Randomised controlled trial Location: USA Funding: National Cancer Institute Recruitment: Internet search engine – people searching for quit smoking variations who then clicked on link to cessation website being evaluated. March 2005 to May 2007 |
|
| Participants | 2005 US residents, 18 years or older currently smoking 5 or more CPD, no prior use of QuitNet website Av. age 35.9, 51.1% female, 86.5% white, 47.2% had college 1 to 3 years, 30.6% had 4 or more years of college, average CPD 20, 3.3 had quit attempts in past year |
|
| Interventions | 'Enhanced Internet' (EI; n = 651): free 6‐month access to QuitNet.com – interactive, commercial cessation website. Provides: advice to quit; assistance in setting quit date; assessment of motivation, smoking history, demographics, and nicotine dependence; individually‐tailored information based on assessment; problem‐solving and skill‐training content; tailored assistance for using pharmacotherapies; social support (large online social network) 'Enhanced Internet + phone' (EI+P; n = 675) : As per EI, plus proactive phone counselling through National Jewish Health (non‐profit academic medical centre). Counsellors part of larger quitline operation. Participants offered 5 calls in 'relapse sensitive' schedule, intensive support in first 30 days after quit attempt. Counsellors prompted and reinforced use of 'QuitNet' and could see participant's use of the site (not included in this review). Control: 'Basic Internet' (BI; n = 679): 6‐month free access to static information‐only version of content on 'QuitNet'. No interactive or individually‐tailored features, no social network |
|
| Outcomes | Short‐ and long‐term abstinence: self‐reported 30‐day single PPA at each follow‐up (3, 6, 12 and 18 months after randomisation) Other reported outcomes: self‐reported 30‐day multiple PPA at each follow‐up (3, 6, 12 and 18months after randomisation), Fagerström Test for Nicotine Dependence, confidence in quitting, perceived stress, network diversity and number of network members |
|
| Notes | Participants were offered a USD 25 incentive for the completion of each survey and a USD 20 bonus for completing all 4 surveys. Participants unreachable by telephone were offered USD 15 for completing the survey by the Internet. 'EI+P' arm not included in any analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐numbers table and stratified by sex and baseline motivation to quit |
| Allocation concealment (selection bias) | Unclear risk | Method is unclear from the study report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number completed study: 1348 (BI 68.6% (466), EI 69% (449), EI+P 67.1% (453)). All randomised participants were included in ITT analysis |
Harrington 2016.
| Methods | Randomised controlled trial Location: USA Funding: National Institute of Drug Abuse (U01DA031515) Recruitment: Participants were recruited from University of Alabama hospital, and received a USD 25 cheque for completing follow‐up surveys. The study was conducted between July 12, 2011, and May 22, 2013. |
|
| Participants | Participants (n = 1448) (Intervention n = 748; Control n = 740) were hospitalised patients, 48% (n = 714) were female, and aged 41.6 (13.1) years. 35.6% (n = 529) were non‐white, and 50.5% (n = 752) had a high school diploma or less. On average smoked 14.1 (SD = 9.9) CPD in the last 30 days. The intervention arm included more men, and participants smoked fewer cigarettes per day | |
| Interventions | Intervention: Web‐based smoking cessation programme that includes a 'transition coach' to hospitalised patients who assisted them in quitting as they were discharged from the hospital. Intervention arm participants had access to a tailored web‐based intervention that included e‐messages and activities tailored to their recent hospital stay. Control: Standard smoking cessation information provided to all hospitalised patients as part of discharge package |
|
| Outcomes | Self‐reported bioverified abstinence at 6 months, a subgroup of participants were tested for biochemical validation. Other outcomes collected were non‐quitters’ smoking intensity during the previous 30 days, staff costs, staff time, materials used, staff training and visit time, participant engagement | |
| Notes | Study authors reported no financial conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An assignment list developed by the study statistician with SAS PROC PLAN |
| Allocation concealment (selection bias) | Low risk | An assignment list developed by the study statistician with SAS PROC PLAN within blocks of 4 for each patient care unit. Access to the randomised list was limited to the study co‐ordinator who provided assignment upon each enrolment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: intervention 19%, control 15% |
Haug 2011.
| Methods | Quasi‐randomised controlled trial Location: Germany (3 inpatient rehabilitation centres) Funding: German Association for the Promotion of Research in Rehabilitation Recruitment: all consecutively‐admitted people at rehab centres screened by doctors or nursing staff regarding inclusion criteria.The intervention website was announced as a new and free service to aid in smoking cessation in the local and national media |
|
| Participants | 477 participants (242 intervention, 235 control) who have smoked at least 1 CPD, or abstinent for maximum 6 months and before that smoked at least 1 CPD; used Internet and email at least every 2 weeks. Average age was 46.5 years, 52% female, ethnicity was not reported, 83.5% had at least 10 years of school education (55.8% 10 years, 27.7% more than 10 years), average CPD was 14.1, 32.7% had quit attempt in previous year | |
| Interventions | Intervention: Internet‐based programme for exclusive use by registered patients of participating rehab hospitals. Access for 6 months. Consists of 3 modules: individual advice provided by computer expert system, information website, and message board. Up to 7 individual counselling sessions by 'expert system' – 1 at hospital, up to 6 after discharge (1 a month). Asked to answer questions based on stage of change, and then online and email system generated feedback letter. Depending on stage of change, letters link to specific sections of website. Control: Usual care. |
|
| Outcomes | Long‐term abstinence: 7‐day PPA at 6‐month follow‐up Other reported outcomes: 4‐week PPA at 6‐month follow‐up, stage of change, nicotine dependence, smoking cessation self‐efficacy, programme use |
|
| Notes | Information on random sequence generation and allocation concealment provided by correspondence with author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by week of attendance. Weeks were allocated to condition based on a randomisation list. Random permuted blocks of 4 weeks used to ensure that the number of intervention weeks and control weeks were similar |
| Allocation concealment (selection bias) | High risk | Quasi‐randomised trial; recruiting staff were aware of the condition to which participants would be allocated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up assessments in 214 participants (88%) of the intervention group and 217 participants (92%) of control group |
Herbec 2014.
| Methods | Randomised controlled trial Location: United Kingdom Funding: AH was funded by British Heart Foundation PhD Studentship. The study was funded by a grant from the National Prevention Research Initiative (G0802035). The Funding Partners relevant to this award: Alzheimer’s Research Trust, Alzheimer’s Society, Biotechnology and Biological Sciences Research Council, British Heart Foundation, Cancer Research UK, Chief Scientist Office, Scottish Government Health Directorate, Department of Health, Diabetes UK, Economic and Social Research Council, Engineering and Physical Sciences Research Council, Health & Social Care Research & Development Office for Northern Ireland, Medical Research Council, The Stroke Association, Welsh Assembly Government. Recruitment: Participants were recruited between March 2012 and October 2013 through an online advertisement on NHS Smokefree website, discussion forums, and websites for UK pregnant women. Participants were not compensated for participation in the study. Study recruitment was conducted between March 2012 and October 2013. |
|
| Participants | Participants (n = 200) ('MumsQuit' n = 99; Control n = 101) were pregnant women, and inclusion criteria were: had Internet access, female, pregnant, aged 18 years of older, UK‐based, daily smoker, willing to make a serious quit attempt, agreed to intervention and trial procedures. Mean age was 27.8 (SD = 5.9) years. 92.5% (n = 185) were white. 4.0% (n = 8) were in full‐time education, 59.5% (n = 119) had post‐16 educational qualifications. On average participants smoked 14.7 (SD = 6.6) CPD, and 41.5% had made a quit attempt in previous year. There were no differences between arms for baseline characteristics | |
| Interventions | The intervention arm 'MumsQuit' had access to a tailored and interactive website ('MumsQuit'). The website offered an interactive, personalised, and structured quit plan that emulates the support from an expert smoking cessation advisor from NHS Stop Smoking Services. The intervention delivered 33 evidence‐ or theory‐based behaviour change techniques and provided up to 4 weeks of pre‐quit date support and up to 4 weeks of post‐quit date support, with email reminders sent to notify users when new intervention sessions are being released. The control arm involved a 1‐page static, non‐personalised website that provided brief standard advice for users |
|
| Outcomes | Outcome data were collected at 8 weeks. Outcomes were non‐bioverified 4‐week continuous abstinence, website usage (number of log‐ins, number of pages viewed, and time spent browsing the website) | |
| Notes | JB has received unrestricted research funding from Pfizer. RW undertakes research and consultancy and receives fees for speaking from companies that develop and manufacture smoking‐cessation medications (Pfizer, J&J, McNeil, GSK, Nabi, Novartis, and Sanofi‐Aventis). He also undertakes training for smoking cessation advisors and has a share of a patent for a novel nicotine delivery device. All other authors reported no conflicts of interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by a computer, with allocation concealment and locking of emails |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by a computer, with allocation concealment and locking of emails |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 36.4% MumsQuit, 30.7% control |
Houston 2015.
| Methods | Cluster‐randomised controlled trial Location: USA Funding: National Cancer Institute at the National Institutes of Health (R01‐CA‐129091) and the National Center for Advancing Translational Sciences of the National Institutes of Health [Award Number UL1TR000161]. Dr. Houston directs the eHealth Quality Enhancement Research Initiative (grant number eHQ‐10‐190) and receives support from this national Veterans’ Affairs implementation centre, and Dr. Sadasivam’s effort was also supported by a National Cancer Institute Career Development Award (K07CA172677). Recruitment: Clinical practices were the clusters (n = 174). Participants were recruited by practices. The study began in June 2011, no end date was stated. |
|
| Participants | Participants (n = 900) (Control n = 299; Messaging n = 164; Personalised n = 437) were smokers aged 18 years or older. 63% were female, 17% were aged 19 ‐ 34 years, 50% aged 35 ‐ 55, 25% aged 55 ‐ 64, 8% aged 65+ . 85% were white ethnicity, 10% black or African‐American, 5% of other ethnicity. 8% had less than high school education, 30% were a high school graduate, 43% had some college, 19% were a college graduate or had more education. 27% smoked between 0 ‐ 10 CPD, 51% smoked 11 ‐ 20, and 22% smoked 20+ CPD. 53% of participants had made a previous quit attempt. There were no differences between arms for baseline characteristics | |
| Interventions | There were 3 arms, all tailored and interactive Internet interventions, with the addition of or without motivational messaging. The control group (n = 147) was allocated to Decide2Quit.org a smoking cessation website which included motivational information tailored to readiness to quit (not thinking of quitting, thinking of quitting, preparing to quit) and interactive risk, decisional balance, and cessation barrier calculators and games linking the chemicals in smoking with their other uses (e.g. formaldehyde is used in both cigarettes and in embalming). The website also included resources about smoking, seeking social support, and talking to your doctor about quitting. The 'Messaging Group' (n = 164) intervention arm were allocated to Decide2Quit.org, and also received brief motivational email messages that were tailored to an individual smoker’s readiness to quit (not ready to quit, thinking about quitting, preparing to quit, actively quitting), and included messages written by smokers for other smokers. The 'Personalised Group' (n = 437) intervention arm were allocated to Decide2Quit.org, received the same tailored motivational emails as in the 'Messaging Group', and in addition they had access to personal online support from trained tobacco treatment specialists, and a link to an online support group (BecomeAnEx.org). |
|
| Outcomes | Outcome data were collected at 6 months. Outcome was non‐bioverified 7‐day PPA. | |
| Notes | The authors declared no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was computer‐generated using a randomisation table with blocks of 10. Participants were randomised by a computer |
| Allocation concealment (selection bias) | Low risk | The random sequence was computer‐generated using a randomisation table with blocks of 10. Participants were randomised by a computer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition rate: 48%. Attrition rates by arm: 51% control, 38% messaging, 50% personalised |
| Other bias | High risk | Practices implementing the paper or e‐referral implementation strategies chose which smokers to refer. During training, the practices were encouraged to refer smokers regardless of whether they were ready to quit smoking. All smokers in these practices were eligible for referral. Additionally, Groups were not evenly balanced. N randomised: Personalised group = 437; The messaging group = 164; The control = 147 |
Humfleet 2013.
| Methods | Randomised controlled trial Location: USA Funding: National Institute on Drug Abuse and California Tobacco‐Related Disease Research Program Recruitment: Patients at 3 HIV+ clinics. The study was conducted between June 2006 to February 2010. |
|
| Participants | 209 (Website intervention n = 58; Individual counselling n = 69; Control n = 82) HIV+ smokers 18 years or older, smoke most days of the month (69 individual counselling, 57 computer‐based, 82 self‐help only), av. age 45, 15% female, av. CPD 20, av. past quit attempts 4. Ethnicity: 27% African‐American, 53% white, 2% American‐Indian, 18% multiple/other. Education: 21% less than high school, 45% high school/GED, 15% associates degree/vocational, 16% BA, 4% graduate degree |
|
| Interventions | Intervention 1. Website intervention: Orientation meeting of 45 ‐ 60 minutes – how to use website etc., no extra cessation guidance. Treatment components structured into 'steps' corresponding with counselling intervention sessions. 5 steps estimated to take 30 ‐ 45 minutes to complete (self‐assessment and homework assignments). Could access website for 12 months, no schedule suggested for website visits. Intervention 2. Individual counselling: 6 sessions of in‐person, individual counselling based on cognitive behavioural treatment model, targeted to the needs of HIV+ smokers. Personal Quit Plan. Sessions held during weeks 1, 2, 3, 5, 8 and 12, 40 ‐ 60 minutes long. Control: Self‐help manual. All participants had access to 10 weeks of NRT |
|
| Outcomes | Short‐ and long‐term abstinence: self‐reported 7‐day PPA at 3, 6, 9, 12 months following start of treatment Other outcomes: sustained abstinence at each follow‐up assessment and PPA verified by CO level |
|
| Notes | Paper reports percentage abstinent only; unclear if percentage is of completers or all participants enrolled. Calculated n quit assuming percentage based on completers only; as more participants dropped out of intervention than control group this was a conservative assumption to make and could underestimate the treatment effect. A sensitivity analysis using completers as the denominator increased the size of the effect (at 12 months, Internet vs control, RR 1.33, 95% CI 0.71 to 2.46). Sustained abstinence results were not provided and hence those included are for 7‐day PPA. Authors chose not to supplement CO measurement | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised, stratified based on CPD, depression status, and gender |
| Allocation concealment (selection bias) | High risk | All treatments were provided at the clinical sites, recruiting personnel knew participants Significant differences between treatment groups were found on 3 variables. Smokers in the self‐help condition were older and more likely to have a history of major depressive episode than participants in the other 2 conditions. Smokers in the CBI condition were more likely to have met criteria for bipolar disorder than the other 2 conditions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retention rates were 89% at 12 weeks, 84% at 24 weeks, 82% at 36 weeks, and 81% at 52 weeks |
Japuntich 2006.
| Methods | Randomised controlled trial Location: 2 research centres in Wisconsin, USA Funding: National Cancer Institute grant Recruitment: from October 2001 to July 2002 by billboards, bus interior posters, flyers, television advertisements and press releases. Recruitment materials did not state that the study tested an experimental computer programme. Interested individuals called a central telephone number |
|
| Participants | 284 (140 intervention, 144 control), ≥ 18 years old, smoking ≥ 10 CPD, with traditional telephone line, literate in English. Av. age 41 years; 54.9% female; 79.1% white; average CPD 21.6. Exclusion criteria: current depression, current use of psychiatric medication, medical conditions contra‐indicating bupropion SR use, current use of a smoking cessation product or treatment, being pregnant or likely to become pregnant during the treatment phase of the study | |
| Interventions | Intervention: 9 weeks of twice‐daily bupropion SR (150 mg), 3 brief counselling sessions and 5 follow‐up visits plus 12 weeks access to 'Comprehensive Health Enhancement Support System for Smoking Cessation and Relapse Prevention' (CHESS SCRP) website (plus study computer and dial‐up connection). The 'CHESS SCRP' is a web‐based guided universe of information, emotional support and problem‐solving assistance in a password‐protected environment. The 'CHESS SCRP' website was organised into 4 sections. The first section provided information about quitting smoking. The second section was a support centre that provided a variety of chat programmes as well as a cognitive behavioural therapy intervention for negative emotions. The third section was an information repository that allowed participants to save 'CHESS SCRP' documents in an easy‐to‐find folder ('my folder'). The final section allowed participants to search for information within 'CHESS SCRP', provided a list of recommended websites and offered tips on evaluating websites participants may have found on their own Control group: As intervention but no access to 'CHESS' |
|
| Outcomes | Long‐term abstinence: 7‐day PPA 6 months after quit date Short‐term abstinence: 7‐day PPA 3 months after quit date Validation: CO ≤ 10 ppm Other reported outcomes: Number of times participants used CHESS SCRP website | |
| Notes | 1‐year follow‐up results not reported in paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, method not described |
| Allocation concealment (selection bias) | Unclear risk | No details given in the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 63 participants withdrew from the study between randomisation and the 1‐year follow‐up (31 from intervention, 32 from control); 57 were lost to follow‐up (27 from intervention group, 30 from control). Dropouts were considered smokers and all randomised participants were included in ITT analysis |
Mananes 2014.
| Methods | Randomised controlled trial Location: Spain Funding: Spanish Ministry of Science and Technology (grant ID#SEJ2004‐03392/PSI) Recruitment: Not clear The intervention program was conducted between October 2009 and May 2010. |
|
| Participants | Participants (n = 23,213) (Interactive program n = 11588; Non‐interactive program n = 11625) were aged 18 years or older, wanted to quit smoking within 30 days, smoked at least 2 CPD, had Internet access and an email address, and accepted treatment conditions. Exclusion criteria were use of other smoking treatment. Participants were: 50.1% (n = 11,620) female, mean age was 39.5 (SD = 10.3). 93.57% (n = 21,721) were Spanish, 1.48% (n = 336) were other European Union, 4.98% (n = 1156) non‐European Union; 13.20% (n = 3064) had only primary school education, 21.55% (n = 5002) only high school education, 19.22% (n = 4461) had professional training, 46.03% (n = 10,686) had University education. Average/median CPD was 19.3 (SD = 10.3) | |
| Interventions | The content of the 'UNED' web‐based smoking cessation programme followed the Clinical Guidelines for the Treatment of Smoking and was based on cognitive behavioural therapy methods. Modules included education about the quit process, nicotine fading, self‐monitoring, self‐control, relapse prevention, coping skills, and lifestyle change. In the interactive format, modules incorporated an evaluation form, to ensure that users had received the contents gradually and that they had completed all modules. The non‐interactive version provided identical content as the interactive version through a static PDF file. |
|
| Outcomes | Outcome data were collected at 90 days. Outcomes were non‐bioverified PPA, dropout rates, module completion, user satisfaction, follow‐up response rate, and self‐reported smoking abstinence | |
| Notes | The authors reported no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The programme randomly assigned the users to either interactive or non‐interactive versions |
| Allocation concealment (selection bias) | Low risk | The programme randomly assigned the users to either interactive or non‐interactive versions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: intervention 97%, control 94% |
Mason 2012.
| Methods | Randomised controlled trial Location: UK Funding: Cancer Research UK Recruitment: visitors to 'QUIT' website, November 2008 to May 2010 |
|
| Participants | 1483 visitors to 'QUIT' website (746 intervention, 737 control). Author also recruited recent ex‐smokers ‐ excluded from this analysis, but including them would be 877 intervention, 737 control. Average age 38, 64% female, average CPD 18, 9% no educational qualifications, 48% quit attempt in previous 3 months Ethnicity: 94% white, 1% black, 4% Asian, 2% other | |
| Interventions | Intervention: Tailored advice report at baseline plus invitation 1 month later to complete second online assessment and receive tailored progress report. Reports delivered on‐screen and sent to email, presented immediately after completing questionnaire. Message content based on social cognitive theory and perspectives on change model. Tailored based on age, sex, previous quit attempts, reasons for quitting, dependence, motivation/determination to quit, proposed quit date, and other variables Control: 1 standardised advice report at baseline, not tailored, contained 'best advice for most smokers' |
|
| Outcomes | Long‐term abstinence: 3‐month prolonged abstinence at 6 months, self‐reported by phone or online Secondary outcomes: 1‐month prolonged abstinence, 7‐day and 24‐hour PPA, all at 6 months |
|
| Notes | We calculated n abstinent from percentages given Only current smokers included in analysis (excludes recent ex‐smokers ‐ including them would not change overall results) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Low risk | Allocation performed automatically by web server. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 50% lost to follow‐up overall. 40% intervention, 42% control followed up at 6 months No significant differences between intervention and control group. Predictors of attrition same in both arms |
Mavrot 2016.
| Methods | Randomised controlled trial Location: Switzerland Funding: Tobacco Control Fund of the Swiss Federal Office of Public Health (Grant Number 10.003634) Recruitment: Participants were recruited between March 2012 and March 2013 during visits to Stop‐Tabac. No incentive was offered for participation |
|
| Participants | Participants were current or ex‐smokers (n = 1120) (Intervention n = 580); Control n = 580), aged 18 years or older, valid email and postal addresses, phone number, provided informed consent. Participants were 65.7% (n = 736) female, and mean age was 36.5 years. 94.6% (n = 1060) were current smokers and 5.4% (n = 60) were former smokers. Trial arms were balanced on all recorded characteristics | |
| Interventions | Intervention: 'The coach' programme was a tailored and interactive Internet intervention, delivered during the 6‐month period after enrolment. The intervention included access to the Stop‐Tabac website which involved a series of automatic, personalised feedback reports based on the participant’s answers to a tailoring questionnaire. Participants received a progress report for each of the 3 answered questionnaires, and a personal web page with progress graphs which displayed the participant's change over time in tobacco dependence, withdrawal symptoms, motivation and self‐efficacy. Participants also received automatic, individually‐tailored, proactive email messages that took into account each participant’s smoking status, quit date (past or future) and level of dependence Control: The Stop‐Tabac website was a non‐tailored, non‐interactive Internet intervention in which participants had access to the website from enrolment. The website was based on the transtheoretical model of behaviour change, theories of relapse prevention, and tobacco dependence |
|
| Outcomes | Outcome data were collected at 3 and 6 months. Outcomes were: non‐bioverified 1‐month PPA, level of addiction, attitudes toward smoking, motivation to quit, withdrawal symptoms, use of self‐change strategies and self‐efficacy, use of smoking cessation aids, frequency of use of the Coach intervention | |
| Notes | The authors reported no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A list of random numbers was used to assign participants |
| Allocation concealment (selection bias) | Low risk | Participants were automatically assigned by a computer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: 64% intervention, 60% control |
McClure 2014.
| Methods | Randomised factorial trial. Location: USA. Funding: National Cancer Institute (R01 CA138598 to JM, principle investigator). Recruitment: Smokers were identified from automated health plan records, and then they were mailed a study invitation letter. Interested smokers were directed to the study web site, and were screened for eligibility online. Participants received $20 after completing the baseline survey and $10 for completing each follow‐up survey. To encourage participation at one year follow‐up, five participants were randomly chosen from among the 12‐month respondents to receive a $100 gift card. Data were collected between May 2010 and November 2012. |
|
| Participants | Participants (n = 1,865) were from the general population, and were aged 18 years or older, a Group Health member, smoked lifetime 100 cigarettes, smoked in the last seven days, averaged at least five cigarettes/ day, not using stop smoking treatment, had access to the Internet, willing to check their e‐mail at least once a week, fluent English reading and writing, no visual impairments preventing computer use, comfortable using a computer and the Internet. Participants were 63.2% Female (n = 1,178), mean age 44.2 (SD = 14.7) years. 82.3% (n = 1,534) were white non‐Hispanic. 28.1% (n = 524) had high school or less, 50.6% (n = 944) had some college, 21.2% (n = 396) had a college degree or higher. On average participants smoked 15.4 (SD = 7.4) cigarettes per day. | |
| Interventions | This study tested 16 variations of the Internet intervention. The 'Q2' intervention was organised into three content areas, based on different stages of readiness to quit: those not ready to quit, those ready to quit, and those already quit. Content contained motivational or action oriented information for quitting smoking tailored to each person’s interest in quitting smoking, gender, smoking history, self‐efficacy, and other baseline characteristics. Each participants intervention was similar, but varied based on the randomly assigned experimental factor levels: Message Tone, Navigation autonomy, Proactive Emails, Testimonials. | |
| Outcomes | Outcome data were collected at 2‐, 6‐ and 12‐months. Outcomes were non‐bioverified 30 day point‐prevalent abstinence, non‐bioverified 7 day point prevalence abstinence, use of either pharmacotherapy or phone counselling program, self‐reported utilization of any treatment. | |
| Notes | 26% (n = 478) of participants utilised the provided adjunct treatment (pharmacotherapy or counselling) at one year follow‐up. The authors reported no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate: 32% overall |
McClure 2016.
| Methods | Randomised controlled trial Location: Washington, USA Funding: Group Health Research Institute and the National Institute for Drug Abuse (R34DA034612) Recruitment: Participants from a primary care practice were identified by automated health plan records and mailed a study invitation. Participants received USD 20 for completing each follow‐up survey. Study recruitment began in 2014 and was completed in 2015. |
|
| Participants | Participants (n = 66) (Intervention n = 33; Control n = 33) were aged 18 ‐ 65 years, no plans to disenroll from Group Health over the next 6 months, smoked at least 10 CPD, fluent English, willing to use varenicline, ready to quit smoking in the next month, had a smartphone which they used at least once a week, willing to receive emails or text messages, eligible to receive varenicline as a covered insurance benefit. Exclusions: hearing, comprehension, or visual limitations that precluded study participation, used non‐cigarette forms of tobacco or nicotine, using other stop‐smoking treatments, unwilling to use contraceptives while taking varenicline, medical or psychiatric exclusion for varenicline use. Participants were 56% female (n = 37), and mean age was 49.5 (SD = 8.7) years. 92% (n = 61) were white. 27% (n = 18) had a college degree or higher. Participants smoked on average 18 CPD (SD = 7.2), and 42% (n = 28) had prior use of varenicline. Trial arms were balanced on all recorded characteristics | |
| Interventions | 'MyMAP Experimental Intervention' was a non‐tailored and non‐interactive Internet intervention; participant also received a 12‐week course of varenicline. The intervention was delivered up to 5 months. Participants in the intervention arm received the same self‐help Quit Guide as the control group. The intervention included 2 interactive features: (1) on‐demand adaptively‐tailored advice for managing nicotine withdrawal symptoms and (2) a secure messaging system. Participants could access the adaptive advice any time by completing a brief check‐in survey to report current symptoms and side effects, and then they received a personalised report with advice and motivational encouragement tailored to each person’s current level of motivation for quitting and confidence in quitting. Participants were also periodically prompted by text or email to complete a check‐in survey The 'MyMAP Control Intervention' was a non‐tailored and non‐interactive Internet‐based intervention, delivered up to 5 months. Participants in this arm also received a 12‐week course of varenicline. In the 'MyMAP Control Intervention' arm participants received an 'mHealth'‐delivered self‐help Quit Guide which included psycho‐educational content for quitting smoking, with content standardised and not tailored, and was designed to lead smokers through a 5‐step guide for how to quit smoking which was grounded in cognitive behavioural therapy |
|
| Outcomes | Outcome data were collected at 2 weeks, and 3 and 5 months. Outcomes were: non‐bioverified 7‐day PPA, programme use and satisfaction, varenicline use, number of log‐in visits, duration of time spent viewing intervention content, Quit Guide content viewed, number of secure messages sent, use of the check‐in surveys and adaptively‐tailored advice | |
| Notes | The authors reported no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 27% experimental arm; 39% control arm |
McDonnell 2011.
| Methods | Randomised controlled trial Location: USA Funding: Centers for Disease Control Recruitment: Korean‐language graphic ads for 3 Korean newspapers online, sponsored links based on search terms entered into Yahoo or Google, flyers, word of mouth, press conference, email campaign and local television campaign. Recruiting from September 2005 – April 2008 |
|
| Participants | 1409 Korean‐Americans, age 18 or older (702 intervention group, 707 control group) who had smoked at least 1 CPD for past 7 days, were current US residents and had valid email address. Average age 35 years, 12% female, 63% had at least a college degree, average CPD 14, past quit attempts were not specified | |
| Interventions | Intervention: access to website with cognitive behavioural self‐help programme based on stages of change, translated into Korean and adapted for Korean‐Americans. 6 sections: assess readiness to quit, discuss withdrawal, evaluate smoking patterns, provide opportunity to make public pledge to quit, discuss pharmacotherapies, and chart daily smoking. Also addressed relapse. Sections completed sequentially – participants could not advance without completing exercises in given section. Not tailored to individual responses Control: booklet of same content |
|
| Outcomes | Long‐term abstinence: self‐reported 30‐day abstinence at 50 weeks and 7‐day PPA at 50 weeks. Other outcomes assessed: completion of the Internet programme (data about programme activity captured directly by the web software) |
|
| Notes | Incentive of up to USD 100 for completion of 11 surveys was given to participants in order to provide adequate compensation and motivation. Additional information on intervention provided by correspondence with author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Computer‐allocated (centrally) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of 1409 randomised, 1112 participants were analysed (562 intervention, 550 control) ‐ 297 were excluded post‐randomisation because they did not meet inclusion criteria, enrolled more than once, or technological issue – email did not go out for 92, and 47 had data overwritten. At the end 587 participants completed study (48.4% of intervention, 57.3% of control). All randomised participants were included in ITT analysis |
McKay 2008.
| Methods | Randomised controlled trial Location: Web Study name: Smokers' Health Improvement Project (SHIP) Funding: The study was supported by grant from the National Cancer Institute Recruitment: An Internet‐based recruitment campaign was designed and executed. The campaign involved ad placement on Google and Yahoo search engines (keywords 'quit smoking' and 'stop smoking') and links to their relevant affiliated sites. Clicking those ads enabled users to (1) visit recruitment site (study description, inclusion/exclusion criteria), (2) submit answers to screening items, (3) provide their informed consent, and (4) complete the baseline assessment |
|
| Participants | 2318 current smokers (1159 intervention, 1159 control), ≥ 18 years, interested in quitting within next 30 days, willing to engage in moderate physical activity, had access to the Internet and gave written informed consent. 70.5% female; 30 ‐ 50 years old; 86.6% white; 40.7% of participants had some college education; 27.5% had college degree; smoked 20 to 40 CPD | |
| Interventions | Intervention: 'QSN' condition incorporated a hybrid information architecture in which first‐time users were directed through a series of tailored web pages (tunnel design) in order to introduce them to the key concepts and strategies of a behavioural programme for quitting smoking. Once they emerged from the tunnel, users were able to choose their own path to access a broad array (using a matrix design) of additional content on quitting and maintaining non‐smoking. Components of the smoking cessation intervention used in the study are based on Social Cognitive Theory. These components are designed to encourage tobacco abstinence with strategies that address each participant's behaviour, cognition, and environment Control: 'Active Lives' control condition accessed a web‐based programme designed to encourage them to engage in a personalised fitness programme that would help them quit smoking. The programme guided each participant through a multi‐step plan that included a motivational component (exploration of the benefits of physical activity and a clarification of personal goals and barriers), a behavioural action plan with extensive tracking features (e.g. weekly activity schedules personalised to each participant's schedule and types of activities), additional online resources (articles and tips sheets), and access to a web forum for peer support |
|
| Outcomes | Long‐term abstinence: self‐reported 7‐day PPA at 3 and 6 months post‐enrolment. In addition, repeated PPA at both the 3‐ and 6‐month assessments Other reported outcomes: exposure (frequency and duration of each participant's visits to the web‐based programme), physical activity, pharmacotherapy use, programme usability |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation |
| Allocation concealment (selection bias) | Low risk | Not described in the paper, but recruitment automated so risk of bias likely to be low |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27% of participants provided both 3‐ and 6‐month assessment data, no significant difference between groups. All randomised participants were included in ITT analysis |
Mehring 2014.
| Methods | Cluster‐randomised controlled trial Location: Germany Funding: HausMed eHealth Services GmbH Recruitment: Participants were recruited by GPs. Participants in the intervention group received free access to the smoking cessation programme (worth EUR 79). Participants in the control group received EUR 10 to attend final follow‐up. The study was conducted between May 19, 2011, and April 1, 2013. |
|
| Participants | Participants (n = 168) (Intervention group n =86; Control group n = 82) were from the general population. Inclusion criteria were aged 18 years or older, had Internet access. Exclusion criteria were insufficient German language skills, psychiatric disorder, or post‐traumatic stress disorder. There were no overall baseline characteristics available. The intervention and control group were similar in gender, age, weight, CPD, and number of years with nicotine consumption. However, participants in the control group were significantly taller. There was no significant group difference found for the use of NRT or for the intake of varenicline | |
| Interventions | Intervention: The coaching programme included 12 modules. Each module lasted 1 week and contained tasks which were supported by corresponding daily SMS reminders. The reminder included information about motivation, and encouraged daily performance of the task. The coaching programme offered printed material (i.e.. emergency plan, relaxation exercises, questionnaires, information, self‐agreements, etc.), and included interactive features, video clips, and quizzes. Each week participants provided feedback about their motivation and whether or not they completed tasks. Participants could also access an online forum for queries. Participants online activity was monitored online by a GP, and participants received 3 phone calls from a GP or a nurse to offer motivation and support Control: Usual smoking cessation treatment as provided by GP |
|
| Outcomes | Outcome data were collected at 12 weeks. Outcomes were bioverified continuous/sustained cessation, bioverified PPA, self‐reported smoking status, number of NRTs, weight in kilograms, CPD, physical activity (range from 0 ‐ 4), and breathing difficulties (range from 0 ‐ 4) | |
| Notes | The authors reported no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated busing the programme Research Randomiser |
| Allocation concealment (selection bias) | Low risk | “Randomization was concealed by using sequentially numbered, opaque, sealed envelopes held by the study coordinator” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: intervention 41%, control 15% |
Moskowitz 2016.
| Methods | Randomised controlled trial Location: USA Funding: Centers for Disease Control and Prevention (Cooperative Agreement #U48‐DP001908) Recruitment: Participants were recruited between July 2012 and September 2013 by Google AdWords (adwords.google.com). All advertising was in Korean. In the high‐reinforcement condition, participants were offered USD 2.00 for completion of each of 5 interim surveys, USD 25.00 for completion of the follow‐up survey, and USD 25.00 for completion of the programme. In the low‐reinforcement condition, the incentive was USD 25.00 for final survey completion. Participants were recruited between July 2012 and September 2013. |
|
| Participants | Included participants (n = 403) (High reinforcement n = 199; Low reinforcement n = 204) were of self‐identified Korean ethnicity, age 18 years or older, daily smoker (i.e. smoked at least 1 CPD during the previous 7 days), current US resident, valid email address, and regular Internet access. Participants were 14% female, mean age was 40.7 (SD = 10.6) years. 98% were born in Korea, 15% had high school education or less, 23% had some technical school/college, and 62% were college graduates. Participants smoked on average 13.1 CPD (SD = 6.8) | |
| Interventions | 'QiW programme with low reinforcement' was a non‐tailored and non‐interactive Internet intervention including 6 modules delivered during the study period. The intervention was "a cognitive‐behavioural, self‐help program based on the stages of change described in Prochaska's Transtheoretical Model". Modules led participants through the stages of quitting, and addressed relapse and withdrawal symptoms. The intervention also included short introductory videos using computer animations that were available in English and Korean. The 'QiW programme with high reinforcement' was a non‐tailored and non‐interactive Internet intervention delivered as an adjunct to non‐internet‐based behavioural intervention, and included 6 modules delivered during the study period. The intervention was the same as in the low‐reinforcement group, but included an online interim surveys with financial incentives for these assessments and also for programme completion, and participants received reminders about the incentive for programme completion with a monthly reminder to complete the interim survey |
|
| Outcomes | Outcome data were collected at 26 weeks. Outcomes were 30‐day PPA and no information was provided about bioverification; 7‐day PPA was also measured, and programme completion | |
| Notes | The authors reported no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by the online survey software |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by the online survey software |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition rate: 50%. Attrition rates by arm: 47% QiW program + low reinforcement; 54% QiW program + high reinforcement |
Muñoz 2006 Study 3.
| Methods | Randomised controlled trial Location: 74 countries Funding: Grants from the Tobacco‐Related Disease Research Program and from the University of California Committee on Latino Research to the UCSF/SFGH Latino Mental Health Research Program Recruitment: by press releases and standard links from search engines The study was conducted in English |
|
| Participants | 280 English‐speaking participants (139 intervention and 141 control), 18 years of age, smoking 5+ CPD, using email at least once weekly, and planning to quit within the next month; average age 38.4 years, 67.9% female, 76.3% white, 20.3 average CPD. Education: high school or less: 35.4%, some college: 29.3%, college grad: 25.4%, graduate degree: 10.0% | |
| Interventions | Compares variants of an Internet‐based intervention. Intervention: The smoking cessation intervention (Guía) was the Guía para dejear de fumar (brochure in Spanish, translated into English) and adapted as a web‐based brochure for this study. In addition to Guía, individually‐timed educational messages ('ITEMs') were used. These were emails inviting participants back to the site at specific times. The messages included encouraging comments and links to relevant sections of the assigned intervention, such as planning for the quit date, the early quit period, how to stay quit, and relapses if any. The component tested in the trial was an 8‐lesson social‐learning‐oriented mood management ('MM') course designed to improve quit rates. The course included instructions on how to use the materials; self‐monitoring screens to record cigarettes smoked mood and anxiety levels, pleasant activities, helpful and harmful thoughts, and contacts with helpful people; and relaxation instructions. Lessons were made available 1 a week to simulate how such lessons would be delivered in a traditional smoking cessation group Control: 'Guía' and 'ITEMs' alone |
|
| Outcomes | Long‐term abstinence: self‐reported 7‐day PPA at 12 months after entry Short‐term abstinence: self‐reported 7‐day PPA at 3 months Abstinence also assessed at 1 and 6 months Other reported outcomes: abstinence rates by history of major depression | |
| Notes | High level of incentives were used to encourage adherence | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | Results of the baseline questionnaires were used to automatically implement stratified randomisation by gender and major depressive episode status |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 50% lost to follow‐up. Follow‐up data were provided by 35.4%, and 34.6% of those completing baseline questionnaires and randomised at the 3‐ and 12‐month follow‐ups respectively. All randomised participants were included in ITT analysis |
Muñoz 2006 Study 4.
| Methods | Randomised controlled trial Location: 74 countries Funding: Grants from the Tobacco‐Related Disease Research Program and from the University of California Committee on Latino Research to the UCSF/SFGH Latino Mental Health Research Program. Recruitment: by press releases and standard links from search engines The study was conducted in Spanish |
|
| Participants | 288 Spanish‐speaking participants (142 intervention vs 146 control), 18 years of age, smoking 5+ CPD, using email at least once weekly, and planning to quit within the next month; average age 35 years, 41.3% female, 62% white, 22.8 average CPD. Education: high school or less: 22.6%, some college: 24.0%, college grade: 39.2%, graduate degree: 14.2% | |
| Interventions | Compares variants of an Internet‐based intervention Intervention: Same as Muñoz 2006 Study 3; 'Guía' + 'ITEMs' + 'MM' Control: 'Guía' + 'ITEMs' | |
| Outcomes | Long‐term abstinence: self‐reported 7‐day PPA at 12 months after entry Short‐term abstinence: self‐reported 7‐day PPA at 3 months Abstinence also assessed at 1 and 6 months Other reported outcomes: abstinence rates by history of major depression | |
| Notes | High level of incentives were used to encourage adherence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation. |
| Allocation concealment (selection bias) | Low risk | Results of the baseline questionnaires were used to automatically implement stratified randomisation by gender and major depressive episode status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition: 38.5% |
Muñoz 2009.
| Methods | Randomised controlled trial Location: 68 countries Funding: Tobacco‐Related Disease Research Program, and infrastructure grant from the University of California Committee on Latino Research. Tobacco Research Network programme, National Cancer Institute, National Institute on Drug Abuse, National Institutes of Health Recruitment: Participants were recruited using Google Ad Words campaigns targeted at users worldwide Smokers came to the study site by search engines, links from other websites, media stories, or word of mouth |
|
| Participants | 1000 participants aged 18 years or older, smoking 5+ CPD, intending to quit in the next month and using email at least once weekly. They were assigned to 4 conditions : 1 (n = 247); 2 (n = 251); 3 (n = 251); 4 (n = 251); average age 37.9 years, 45% female, 53% Hispanic/Latino, 19.8 average CPD. Education: Some college 39.5%, college graduate 28.7%, graduate degree 14.7% | |
| Interventions | Compares cumulative variants of an Internet‐based intervention Condition 1 ('Guía' alone): the online static Guía as used in Muñoz 2006 studies, a cigarette counter, and an online journal to record experiences while quitting. The Guía covered reasons to quit, cessation strategies, relapse prevention and management, pharmacological aids, and how to help a smoker quit Condition 2 ('Guía' + 'ITEMs'): As 1. plus Individually Timed Educational Messages ('ITEMs'); automated emails with links to sections of the Guía keyed to quit date Condition 3 ('Guía' + 'ITEMs' + 'MM'): As 2. plus 8‐lesson cognitive‐behavioural mood management course as used in Muñoz 2006 Condition 4 ('Guía' + 'ITEMs' + 'MM' + 'VG'): As 3. plus 'virtual group' asynchronous bulletin board for mutual support and suggestions |
|
| Outcomes | Long‐term abstinence: self‐reported 7‐day PPA at 12 months after entry Short‐term abstinence: self‐reported 7‐day PPA at 3 months Abstinence also assessed at 1 and 6 months Secondary outcome: satisfaction with website Other reported outcomes: website use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based |
| Allocation concealment (selection bias) | Low risk | Stratified randomisation using an automated algorithm programmed into the website |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 50% lost across follow‐ups. 90% responded to at least 1 follow‐up, 14% to 1, 18% to 2, 20% to 3 and 38% to all 4. No differences between number of assessments were found between language groups. No significant difference in number of completed assessments were found based on treatment condition, sex or major depressive episode history. All randomised participants included in ITT analysis |
Oenema 2008.
| Methods | Randomised controlled trial Location: Netherlands Funding: Netherlands Heart Foundation Recruitment: Members of an online research panel |
|
| Participants | 692 participants who were self‐reported smokers or recent ex‐smokers (within last 2 years) at baseline, from bigger sample of 2159 adults 30 years or older with Internet skills and sufficient understanding of Dutch language. Participant demographics not reported for smokers alone, within larger sample: mean age 43.6, 46% female, 96% native Dutch, 41% high education, 33% medium education, 27% low education level. CPD not reported | |
| Interventions | Intervention: Website with tailored information on saturated fat intake, physical activity, and smoking cessation. Smoking cessation module consisted of 2 parts and was based on Social Cognitive Stages Model. First part designed to enhance motivation to quit, feedback provided on outcomes of quitting. Second part designed to increase self‐efficacy, including individualised advice on NRT and selected skills for coping with high‐risk situations, which were assessed to be relevant to individual. Feedback provided on current smoking status and progression on psychological factors Control: Usual care (offered access to website after study end) |
|
| Outcomes | No long‐term abstinence measure Short‐term abstinence: assessed at 1 month, definition of abstinence not clear Self‐report only Other reported outcomes: Stage of change, diet and physical activity measures, website engagement (across whole sample) |
|
| Notes | All results reported in this review are for the subgroup of participants who were self‐reported smokers or recent ex‐smokers (within 2 years) at baseline Number quit not reported, calculated using complete‐case smokers multiplied by percentages given; then used ITT denominators in analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation concealed until exposure to the intervention, done centrally by general online research agency, researchers blind to study condition throughout |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76% smokers in intervention followed up, 83% smokers in control |
Patten 2006.
| Methods | Randomised controlled trial Location: Rochester, Minnesota; Madison, Wisconsin; Hartford, Connecticut ‐ USA. Funding: Supported by a grant from the National Cancer Institute Recruitment: television commercials, radio and newspaper announcements, and flyers displayed in the schools and clinics at each site |
|
| Participants | 139 adolescents aged 11 ‐ 18 years (69 intervention condition vs 70 control), smoked a total of 10+ cigarettes during the previous 30 days, willing and able to complete treatment and assessment visits, provided written informed consent; average age 16 years, 50% female, 90% white, av 10 CPD | |
| Interventions | Intervention (in this review):'Stomp Out Smokes' (SOS): Internet‐based intervention. SOS participants were provided access to SOS and the Internet for 24 weeks and except for the assessment visits, study staff did not have any personal contact with participants. General content of the SOS site was consistent with the clinical practice guidelines on effective tobacco use intervention but tailored to adolescents, and updated every 6 months as needed. Reading level for content was at the 6th grade. The web architecture and design of the SOS site was also consistent with the National Cancer Institute web usability guidelines Control (in this review): 'Brief office intervention' (BOI): Adolescents receiving the BOI met with a research counsellor for 4 consecutive weekly individual sessions. Duration of session 1 was projected to be 30 ‐ 40 minutes, while the remaining 3 sessions were about 10 ‐ 20 minutes each. Adolescents were given a specific homework exercise at the end of each session which focused on preparing to stop smoking or practising at least 1 of the techniques discussed in the session |
|
| Outcomes | Long‐term abstinence: 30‐day PPA at 9 months
Short‐term abstinence: 30‐day PPA at 3 months
Abstinence also assessed at 2 and 6 months
Validation: CO ≤ 8 ppm at each follow‐up Other reported outcomes: CPD and days smoked at 6 months, treatment compliance, concomitant behavioural and pharmacological treatment |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | No details were given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage attending assessment vi it in the intervention and control conditions respectively was 42% and 53% at 9 months. All randomised participants included in ITT analysis |
Pechmann 2016.
| Methods | Randomised controlled trial Location: USA Funding: NIH R34 Innovation grant DA030538 Recruitment: Participants were recruited by Google search engine and an advertisement on Google AdWords. No incentive was offered for participation |
|
| Participants | Participants (n = 160) ('Tweet2Quit' n = 80; Control n = 80) were from the general population and were resident in continental USA, English‐speaking, aged 18 – 59 years, smoked 100+ cigarettes in lifetime, currently smoking ≥ 5 CPD, intention to quit smoking in the next month, active email account, mobile phone with Internet access and unlimited texting, weekly texting, and daily Facebook use. Exclusion criteria: health contraindications to nicotine patch use, actively taking medication for depression, anxiety or quitting smoking, illicit hard drug use in the past 4 weeks, daily marijuana use, residence with another participant, failure to provide contact or collateral information, or failure to respond to a confirmatory text message, or both. Participants were 73.7% female (n = 118), and mean age 35.7 (SD = 9.9) years. 88.7% (n = 142) were white non‐Hispanic, 6.9% (n = 11) were African‐American, 4.4% (n = 7) were Hispanic. 31.2% (n = 50) had a college degree or higher, 40.0% (n = 64) had some college, 28.8% (n = 46) had a high school degree or less. Participants smoked on average 18 CPD (SD = 8.2) | |
| Interventions | 'Tweet2Quit' was a tailored and interactive Internet intervention + NRT, and was delivered over 100 days. Participants in the intervention arm also received the control intervention. 'Tweet2Quit' participants received daily discussion‐topic automated messages and daily engagement autofeedback. Participants received individualised automated feedback on their prior 24‐hour‐tweeting. Tweeters were praised (e.g. ‘Great job staying connected with your quit smoking group. Your tweets make a difference!’), while nontweeters were encouraged (e.g., ‘Missed hearing from you yesterday! Share how you are doing with your group’). In addition to the Internet intervention participants received by a 56‐day supply of nicotine patches titrated to their baseline smoking level (starting with 14 mg patches if < 10 CPD and 21 mg patches if > 10 CPD) The control group was a non‐tailored and non‐interactive Internet intervention (smokefree.gov) + NRT. Participants received a 56‐day supply of nicotine patches titrated their baseline smoking level (starting with 14 mg patches if < 10 CPD and 21 mg patches if > 10 CPD). At trial start date, an automated email encouraged participants to select a quit date and to start using the patches on that date. Participants were encouraged to access smokefree.gov, the National Cancer Institute’s quit‐smoking website. Automated emails sent smokefree.gov module links as follows: Prepare to Quit, Quitting, Help line/live chat, Staying Quit, Help line/live chat |
|
| Outcomes | Outcome data were collected at 7, 30 and 60 days. Outcomes were non‐bioverified sustained abstinence, nonbioverified 7‐day PPA, number of days used nicotine patches, number of visits to smokefree.gov, tweet volume (i.e. number of tweets each participant sent), days of tweeting (i.e. number of days a participant sent at least 1 tweet), tweeting duration | |
| Notes | JJP is serving as an expert witness in litigation against tobacco companies, and has consulted for Pfizer which makes cessation medications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned using a computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Participants were assigned using a computer‐generated randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: Tweet2Quit 19%, control 13% |
Rabius 2008.
| Methods | Randomised controlled trial Location: web‐based Funding: American Cancer Society Recruitment: Through Internet. The link placed on ACS website led smokers to the QuitLink study website, where they could answer eligibility questions, provide informed consent, and complete the baseline survey |
|
| Participants | 6451 English‐speaking daily smokers residing in the USA who provided informed consent and completed the baseline survey, randomised to 6 sites: Control Site (n = 1047), Site 1 (n = 1052), Site 2 (n = 1103), Site 3 (n = 1042), Site 4 (n = 1101), Site 5 (n = 1106). Average age 41 years, 70% female, 87% white intervention vs 74% control, had some college education 75% intervention vs 59% control, average CPD 21, 6.3 past quit attempts | |
| Interventions | Comparison between different Internet sites Intervention: received emailed access to 1 of 5 tailored interactive sites provided by co‐operating research partners (SmokeClinic, CAMH, V‐CC, ORCAS, QuitNet, and ProChange) Control: received access to a targeted, minimally‐interactive ACS site with text, photographs, and graphics providing stage‐based quitting advice and peer modelling |
|
| Outcomes | Long‐term abstinence: self‐reported 30‐day PPA, 13 months after randomisation Short‐term abstinence (Pike 2007): self‐reported 7‐day PPA at 3 months Other assessed outcomes: Use of the different interactive sites (reported in Pike 2007). Link between quitting success and number of visits to interactive sites. Effect modification by indicator of depression at baseline | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation |
| Allocation concealment (selection bias) | Low risk | No details given in the paper, but recruitment automated so risk of bias likely to be low |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 50% lost to follow‐up. 38% provided information on their smoking status 13 months after randomisation. All randomised participants were included in ITT analysis |
Schulz 2014.
| Methods | Randomised controlled trial Location: Netherlands Funding: By ZonMw, the Netherlands Organization for Health Research and Development (grant number: 120610012) Recruitment: Participants were recruited by 4 Health Authorities within the provinces of North‐Brabant and Zeeland. The study was conducted between 2009 and 2012. |
|
| Participants | Participants (n = 5390) (Sequential n = 1736; Simultaneous n = 1638) were from the general population. Inclusion criteria were aged between 18 and 65 years, had a computer with Internet access, basic Internet literacy, and a valid email address. Participants were 47.4% (n = 2394) female, mean age was 44.2 (SD = 12.7) years; Education low 10.4% (n = 515), medium 47.1% (n = 2334), high 42.6% (n = 2112). 34.2% of participants were current smokers. The whole cohort smoked on average 2.3 (SD = 6.5) CPD | |
| Interventions | All groups received a tailored and non‐interactive online intervention which involved a health risk appraisal (HRA) regarding physical activity, fruit and vegetable consumption, alcohol and cigarette consumption. Questionnaires were used to measure the psychosocial concepts of the I‐Change model. Participants were invited to change unhealthy behaviours and received feedback on all behaviours. Control condition received a minimal intervention. |
|
| Outcomes | Outcome data were collected at 12 and 24 months. Outcomes were physical activity, vegetable consumption, fruit consumption, alcohol intake | |
| Notes | Hein de Vries was scientific director of Vision2Health, a company that licenses evidence‐based, innovative, computer‐tailored health communication tools. No other authors reported any conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using computer software |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted using computer software |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates: Sequential arm 80%, simultaneous arm 80%, control arm 75% |
Shuter 2014.
| Methods | Randomised controlled trial Location: New York, USA Funding: Grants R21CA163100‐01 and P30CA051008 from the National Institutes of Health/ National Cancer Institute. Clinical Core of the Center for AIDS Research at the Albert Einstein College of Medicine and Montefiore Medical Center funded by the National Institutes of Health (NIH AI‐51519). Recruitment: Participants were recruited from Montefiore Medical Center’s Center for Positive Living Participants received travel vouchers, and a USD 30 incentive for each study visit. The study was conducted between March 2012 and April 2013. |
|
| Participants | Participants (n = 138) (Intervention n = 69; control n = 69) were persons living with HIV. Inclusion criteria were had a membership of the Center for Positive Living Clinic, confirmed HIV infection, used cigarettes, pipes, or cigars, and interested in quitting in the next 6 months. No overall baseline characteristics provided and there were no differences in baseline characteristics between arms | |
| Interventions | Intervention: 'Positively Smoke Free on the Web' was a non‐tailored and interactive Internet‐based intervention, plus NRT. The website aimed to educate, motivate, and increase self‐efficacy to quit. Participants were send sent email and text reminders to access the website. Control: The usual‐care arm was a non‐internet‐based active control arm in which participants were offered brief advice to quit, a self‐help brochure. Participants in both arms were offered a 3‐month supply of nicotine patches |
|
| Outcomes | Outcome data were collected at 6 weeks, and 3 months. Outcomes were bioverified 7‐day PPA, adherence, engagement, satisfaction, study contamination, nicotine addiction, motivation to quit, self‐efficacy, decisional balance, social support, loneliness, anxiety, depression, and perceived stress | |
| Notes | No details were provided about conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by study staff using a random‐number table and an even/odd allocation strategy |
| Allocation concealment (selection bias) | Low risk | Participants were randomised by study staff using a random‐number table and an even/odd allocation strategy. Given the use of a random‐number table prior to allocation, it would have been difficult to predict treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate: Overall 2.9% |
Simmons 2011.
| Methods | 4‐arm randomised controlled trial Location: Florida, USA Funding: James and Esther King Biomedical Research Program, Florida Department of Health Recruitment: Participants were recruited using a campus‐wide questionnaire at the University of South Florida |
|
| Participants | Participants (n = 341) (Didactic intervention n = 85; 'Web‐Smoke' n = 85; Group intervention n = 86; 'Web‐nutrition' intervention n = 85) were college student smokers, English‐speaking, 18 – 24 years of age, and smoked 5+ cigarettes a week. Participants were 44.1% female, and mean age was 20.54 (SD = 2.0) years. 81.3% were white, 11.8% Hispanic. 70.9 % were daily smokers, mean CPD was 46.4 (SD = 40.6) | |
| Interventions | 1. Web‐delivered experiential tailored and interactive Internet intervention to increase motivation to quit smoking and reducing smoking, using cognitive dissonance theory as a model 2. In‐person, group‐based, experiential smoking intervention 3. Web‐based traditional didactic smoking intervention 4. Web‐based experiential nutrition intervention |
|
| Outcomes | Intention to quit smoking and smoking status at 1 and 6 months following the intervention. Outcomes included 30‐day PPA, 7‐day PPA, motivation to quit, dissonance thermometer, risk perception questionnaire, smoking consequences questionnaire; decisional balance questionnaire, test of smoking knowledge, and comparable diet/nutrition measures. Self‐reported abstinence was biochemically verified using breath CO testing. Participants with CO < 10 ppm were classified as abstinent | |
| Notes | Web nutrition arm not included in meta‐analysis as it had no smoking content | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by an online random‐number generator |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned by an online random‐number generator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 9% web‐based experiential smoking (Web‐Smoke); 11% web‐based experiential nutrition; 4% web‐based didactic smoking; 7% group‐based experiential smoking |
Skov‐Ettrup 2016.
| Methods | Randomised controlled trial Location: Denmark Funding: The study was funded by the Danish Cancer Society Recruitment: Participants were recruited from the Danish Health Examination Survey (2007 – 2008) and the Danish Health and Morbidity Survey (2010). No incentive was offered for participation. Participants were enrolled from August to October 2011. Follow‐up was completed in January 2013. |
|
| Participants | Participants (n = 1810) (Proactive telephone counselling n = 452; Reactive telephone counselling n = 453; Internet‐based program n = 453; Booklet n = 452) were self‐reported daily smokers, with a Danish address in 2011, valid email address and mobile phone number. Participants were aged 41 ‐ 62 years. No other overall baseline characteristics were reported. | |
| Interventions | Intervention: 'e‐quit' was a tailored and interactive Internet intervention, with optional text message support, accessed freely online for the duration of the study. Upon signing up to the intervention webpage all participants received a tailored feedback letter based upon their level of dependence, and users were encouraged to select a quit date within the next 3 months. The website included personalised feedback according to quit date and overview of programme components, a daily video of a person at the same stage of the smoking cessation process, exercises for increasing motivation and identifying coping strategies, tailored feedback based on level of dependence (pharmacotherapy was encouraged for those with high nicotine dependence), blog option, action planning tool, urgent assistance for cravings and information about smoking and health emails and text messages from e‐quit were optional. Proactive telephone counselling was a non‐internet‐based, active control arm, including 5 sessions delivered over 8 weeks. The intervention was based on 5 themes from the Transtheoretical Model of behaviour change: clarification (smoking history and readiness), preparation (strengthening of motivation and planning coping strategies), action (maintaining participant engagement during the first days as smokefree), action/maintenance (maintaining engagement and recognition of success), and future (maintenance and the future as non‐smoker). Participants were encouraged to set a quit date, and counsellors assessed nicotine dependence, informed about the pros and cons of using pharmacotherapy accordingly. Control: Reactive telephone counselling was a non‐internet‐based non‐active control arm in which interested participants received 1 session that lasted for approximately 13 – 15 minutes, no information about how many sessions were provided. Participants were informed that they could receive free telephone counselling at the Danish national quitline; callers who were ready to quit were encouraged to set a quit date and information about pharmacotherapy was provided if relevant |
|
| Outcomes | Outcome data were collected at 1, 6 and 12 months. Outcomes were non‐bioverified prolonged abstinence, and 30‐day PPA | |
| Notes | No conflicts of interest were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation was conducted by applying a fixed sequence of 4 numbers repeatedly |
| Allocation concealment (selection bias) | High risk | Allocation was conducted by applying a fixed sequence of 4 numbers repeatedly. The person performing the allocation was blinded to names and ID numbers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 21% e‐quit; 21% proactive telephone counselling; 15% self‐help booklet |
Smit 2016.
| Methods | Randomised controlled trial Location: Netherlands Funding: Dutch Cancer Society (UM 2007‐3834) Recruitment: Participants were recruited from general practices, and were offered a EUR 10 gift voucher after the completion of all questionnaires. Participants were recruited between May 2009 to June 2010. |
|
| Participants | Participants (n = 414) ('Multiple Computer Tailoring + Counselling' n = 163; 'Multiple Computer Tailoring' n = 132; Usual Care n = 119) were current smokers, motivated to quit within 6 months, aged 18 years or older, sufficiently proficient in Dutch, access to the Internet. 59.9% were female, mean age was 48.0 (SD = 11.9) years. High level of education 22.7%, medium level of education 45.2%, low level of education 32.1%. Median number of quit attempts was 3 (interquartile range: 2 ‐ 4). No differences between groups' baseline characteristics | |
| Interventions | Intervention 1: 'Multiple Computer Tailoring' employed tailored feedback messages was an interactive and tailored Internet intervention. Smokers of > 10 CPD were advised to discuss smoking cessation medication options with their GP. Feedback messages were sent regularly during the intervention period Intervention 2: 'Multiple Computer Tailoring + Counselling' was an interactive and tailored Internet intervention plus behavioural support, in which participants received the tailored feedback letter that 'Multiple Computer Tailoring' group received, and at 6 weeks the letter was replaced by a counselling meeting with a nurse; nurses followed up participants by telephone at 6 months providing additional support Control: 'Usual care' was a non‐internet‐based non‐active control arm. Participants received smoking cessation guidance according to Dutch standard practice |
|
| Outcomes | Outcome data were collected at 6 and 12 months. Bioverified prolonged abstinence, quit attempts, tobacco consumption, intention to quit smoking or to maintain non‐smoking or both, attitude, self‐efficacy and social influence | |
| Notes | Hein de Vries is scientific director of Vision2Health, a company that licenses evidence‐based innovative computer‐tailored health communication tools | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted using computer software |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted using computer software |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 43% MTC, 43% MC, 46% UC |
Stanczyk 2014.
| Methods | Randomised controlled trial Location: Netherlands Funding: ZonMw, the Netherlands Organisation for Health Research and Development (grant number: 20011007) Recruitment: Participants were recruited by: 1) GP referral of smoking patients to the intervention website; 2) local newspapers, newspaper websites, and Dutch health fund websites; 3) international online social networking websites (e.g. Hyves and Facebook). Participants had the chance to win EUR 100 if they completed all the assessments. Participants were recruited from December 2010 to June 2012. |
|
| Participants | Participants (n = 2551) (Video computer tailoring n = 670; Text computer tailoring n = 708; Control n = 721) were smokers who were motivated to quit within the next 6 months, 18 years or older, and had Internet access. 60.9% were female (n = 1278), and mean age was 45.7 (SD = 12.8) years. 95.2% were of Dutch nationality (n = 1995), and education levels were: low 33.6% (n = 705), medium 37.3% (n = 782 ), and high 29.2% (n = 612), and smoked an average of 18.8 CPD (SD = 8.6). There were significant differences between trial arms in readiness to quit, preparatory planning and coping planning. Participants in the 2 experimental conditions were more likely to have made preparatory and coping plans. Control group were more ready to quit at final follow‐up | |
| Interventions | The interventions were both tailored and interactive Internet interventions (i.e. outcome data were combined across these interventions). Text‐ and video‐based web interventions were delivered over 4‐months, depending on motivation to quit ‐ 6 sessions were delivered over 8 weeks if motivated to quit and 8 ‐ 9 sessions over 3 ‐ 4 months if not motivated to quit. The content of the intervention was exactly the same in the text‐ and video‐based interventions Participants received multiple sessions of computer‐tailored advice, either text‐based or as a video message. Feedback was tailored to their smoking behaviour, attitude (pros and cons of smoking and quitting), perceived social influence (modelling and support), perceived self‐efficacy, and how to prepare to quit. Based on the participants “readiness to quit smoking” within the following month, they were allocated to received personalised feedback during subsequent multiple computer‐tailored sessions and received further advice on planning a quit attempt. Participants who were not ready to quit within 1 month received further advice on how to increase motivation Control: The control group was a non‐tailored and non‐interactive internet‐based intervention. The control group received 1 session of generic short text advice |
|
| Outcomes | Outcomes were prolonged abstinence data collected at 6 months, with no information provided about bioverification status. Secondary outcomes: 7‐day PPA was self‐assessed abstinence from smoking during the past 7 days. Programme appreciation was assessed by measuring “Attention to the tailored advice”, comprehensibility, adaptation, appreciation, and processing | |
| Notes | Hein de Vries is scientific director of Vision2‐Health, a company that licenses evidence‐based computer‐tailored health communication tools | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised into 1 of the 3 conditions by the website |
| Allocation concealment (selection bias) | Low risk | Participants were randomised into 1 of the 3 conditions by the website |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 33% combined intervention group; 27% in control group |
Stoddard 2008.
| Methods | Randomised controlled trial Location: web‐based Funding: supported by the Tobacco Control Research Branch of the National Cancer Institute. The project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health Recruitment: federal employees and contractors were invited by email. Emails contained information about a service for smokers interested in quitting, along with an embedded link redirecting interested participants to a site used to screen for eligibility |
|
| Participants | 1375 participants over 18 years of age (691 intervention vs 684 control) who were ready to quit in the next 30 days or who had begun an initiation attempt within 5 days before enrolment; average age 43.6 years, 54% female, non‐Hispanic white (69.1%), 16.9% non‐Hispanic black and 7.0% Hispanic, 49.2% had some college education, average CPD 18.3 | |
| Interventions | Compares variants of an Internet‐based intervention Intervention: website that included asynchronous bulletin board (BB condition). Beside basic content which was the same for both conditions, BB condition offered a forum where participants could respond to some seeded categories posted on the board or start their own message Control: publicly available smokefree.gov, designated as usual care (UC condition). The basic content was: 1) online quit guide and 5 unique self‐help materials targeted to specific populations; 2) links for reaching a counsellor for one‐on‐one help either by telephone or instant messaging; 3) an interactive list of clinical trials still recruiting smokers who wished to quit; 4) an interactive smoker's risk tool showing changes in risk of death due to smoking based on the smoker's history and time of quitting; and 5) a series of empirically‐based statements about positive health changes that commonly follow cessation |
|
| Outcomes | Short‐term abstinence: self‐reported 7‐day PPA at 3 months after enrolling in the study Other reported outcomes: time spent on the website, use of pages, cessation aids used in the past and during the study period | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation that selected from ID numbers generated with returned baseline questionnaires |
| Allocation concealment (selection bias) | Low risk | Centralised system |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Over 50% lost to follow‐up. 39.7% returned a follow‐up questionnaire after 3 months. All randomised participants were included in ITT analysis |
Strecher 2005.
| Methods | Randomised controlled trial Location: www in England and Republic of Ireland Funding: supported by GlaxoSmithKline Recruitment: smokers in the United Kingdom and Republic of Ireland who purchased NiQuitin CQ 21 mg patch and connected to a website to enrol for free behavioural support materials |
|
| Participants | 3971 participants 18 years of age or older (1991 intervention vs 1980 control), smoking > 10 CPD, but had a TQD that was within 7 days from the enrolment date and had purchased NiQuitin CQ21 mg; average age 36.9 years, 56.5% females, average CPD 23.5 | |
| Interventions | Compares variants of an Internet‐based intervention to support NRT‐assisted quit attempts Intervention: web‐based tailored behavioural smoking cessation materials (CQ PLAN). Information collected in the enrolment questionnaire was used to tailor CQ PLAN materials. Programme materials consisted of an initial web‐based cessation guide, 3 sequential tailored newsletters delivered by email over a 10‐week period. The content of the programme was based on cognitive‐behavioural methods of smoking cessation and relapse prevention. In addition, participants were allowed to identify a supportive person that would receive an email message with tailored advice for supporting the participant Control: web‐based non‐tailored materials (control condition). Cognitive behavioural concepts and instruction on product were similar to those addressed in the CQ PLAN. The differences were that control group did not receive: tailored materials, the 3 follow‐up newsletters and the opportunity to identify the supportive person |
|
| Outcomes | Primary outcome: Self‐reported continuous abstinence for 28 days (6‐week follow‐up) or 10 weeks (12‐week follow‐up) Secondary outcomes: Participant satisfaction |
|
| Notes | No long‐term follow‐up so not included in any comparisons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to assess risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 53.3% responded to the 6‐week and 43.2% responded to the 12‐week follow‐up survey. All randomised participants were included in ITT |
Strecher 2008.
| Methods | Randomised controlled trial Location: 2 HMOs: in Washington State and Michigan, USA Funding: National Cancer Institute grants. NRT was provided by GlaxoSmithKline Recruitment: participants were recruited from the memberships of 2 HMOs participating in the National Cancer Institute's Research Network: Group Health in Washington State and Henry Ford Health System in Michigan |
|
| Participants | 1866 participants aged 21 ‐ 70, currently smoking at least 10 CPD, seriously considering quitting in the next 30 days, were randomised to 1 of the 16 study arms. One of the inclusion criteria was that participants were not currently enrolled in another formal smoking cessation programme or were not currently using pharmacotherapy for smoking cessation and had no medical contraindications for NRT; average age 46.3 years, 59.5% female, 78.9% white, > High School 63.8%, average CPD 21.8 | |
| Interventions | Compares variants of an Internet‐based intervention to support NRT‐assisted quit attempts Intervention: A web‐based smoking cessation programme plus nicotine patch. 5 components of the intervention were randomised using a factorial design. Intervention group was assigned to high‐depth tailored success story, outcome expectation, and efficacy expectation messages; high personalised source; and multiple exposure to the intervention components Control: Participants in this group were assigned to low‐depth tailored success story, outcome expectation, and efficacy expectation messages; low personalised source; and single exposure to the intervention components |
|
| Outcomes | Primary outcome: self‐reported 7‐day PPA at the 6‐month post‐quit date follow‐up Secondary outcomes: programme and NRT use |
|
| Notes | Not included in comparisons as used fractional factorial design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised ‐ Stratified random allocation within HMO site immediately after assessment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 76% responded to the 6‐month follow‐up. All randomised participants were included in ITT |
Swan 2010.
| Methods | Randomised controlled trial Location: Internet, Group Health (nonprofit healthcare organisation serving Washington and Idaho), USA Funding: National Cancer Institute. Varenicline and nominal support for recruitment from Pfizer Recruitment: Group Health members recruited through health plan magazine advertisements, employee mailings, physician referrals and Free&Clear Quit for Life programme |
|
| Participants | 1202 health‐plan members aged ≥ 18 years (web = 401, PTC = 402, PTC‐Web = 399); smoked ≥ 10 CPD over past year and ≥ 5 CPD within past week; dependable phone and Internet access, comfortable using Internet; eligible for smoking cessation services, medically appropriate for varenicline use; average age 47.3 years, 66.9% female, 89.7% white, average CPD 21.8, quit attempts past year 48.3%, longest previous quit > 6 months 36.7% | |
| Interventions | All participants received a 12‐week supply of varenicline, written information about medication use, 5 ‐ 10 minutes orientation call, printed Quit Guides, access to toll‐free phone line for reactive support. Intervention 1: Up to 5 proactive telephone‐based calls from a Free & Clear tobacco treatment counsellor (PTC) Intervention 2: Interactive online programme, tools modified from PTC, tailored to stage in quit process, including discussion forums (Web) Intervention 3: PTC‐Web; combination of 1 and 2; counsellor had access to data entered online. (Does not contribute to this review) |
|
| Outcomes | Primary outcome: 30‐day PPA at 6 months Other reported outcomes: 7‐day PPA at 6 months, 7‐ and 30‐day PPA at 3 months, use by treatment group (number of contacts, contact duration in minutes), medication use (number of days varenicline taken, number of pills taken) | |
| Notes | Trial is registered at Clinicaltrials.gov (NCT00301145) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation using an automated algorithm |
| Allocation concealment (selection bias) | Low risk | Allocation at end of intake survey, algorithm built into study database |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76.3% reached for 3‐month interview, 74.2% reached for 6‐month interview. No differences between the 3 treatment groups at either time point. All randomised participants were included in ITT |
Swartz 2006.
| Methods | Randomised controlled trial Location: Internet Funding: National Cancer Institute Grant Recruitment: through large worksites. Promotional materials (e.g. posters and brochures) with smoking cessation messages and the website address (www.Quitcigs.org) were displayed in the worksites. Some organisations also placed a link to the Quitcigs website on their intranet websites or sent broadcast emails or electronic newsletters to employees promoting the research study |
|
| Participants | 351 participants (171 intervention, 180 control),18 years or older, currently smoking cigarettes on a daily basis, considering quitting smoking in next 30 days, and being able to access the website; 51.9% female, 82.1% white, 68% smoke up to 20 CPD; majority aged 26 ‐ 39 (38.2%) or 40 ‐ 55 years (48.4%) | |
| Interventions | Intervention: Consisted of a video‐based Internet site that presented current strategies for smoking cessation and motivational materials tailored to the users' race/ethnicity, sex and age. The programme contained approximately 20 hours of video material, although individuals saw only a fraction of that amount. The video segments presented 3 types of characters: a physician who presented a brief message on health importance of stopping smoking and information regarding pharmacological aids; an ex‐smoker‐guide matched to the user by sex and race/ethnicity; and many testimonials from ex‐smokers The entire intervention was provided by the website server programme Control: Received nothing for 90 days and were then allowed access to the programme |
|
| Outcomes | Short‐term abstinence: self‐reported 7‐day PPA at 90‐day assessment Other reported outcomes: programme use | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using computer algorithm |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 90‐day follow‐up 197 participants returned to complete the assessment, 87 (50.9%) of treatment participants, 110 (61.1%) of control. All randomised participants were included in ITT analysis |
Te Poel 2009.
| Methods | Randomised controlled trial Location: Netherlands Funding: The Netherlands Organization for Health Research and Development. The Netherlands Foundation for a Smoke‐Free Future Recruitment: advertisements in local newspapers, banners on websites, flyers and posters and by a random selection of smokers' email addresses purchased from a customer information management company |
|
| Participants | 458 participants (224 intervention, 234 control), 18 years or older, smoker of cigarettes or loose‐cut tobacco or both, intending to quit within 1 year; average age 46.1 years, 56.1% female, 15.7% had no or little vocational training, 48.7 had advanced vocational training, 31.7% had college/university training. Participants in the intervention group smoked on average significantly more tobacco products a day at baseline (mean = 22) compared with control (mean = 20) | |
| Interventions | Intervention: 7 ‐ 9‐page computer‐tailored email letter generated from responses to an online questionnaire Control: 7‐page generic, non‐tailored email letter, after completing same questionnaire Emails addressed motivational (attitudes, social influences, self‐efficacy) and post‐motivational (skills, action planning) determinants |
|
| Outcomes | Primary outcome: 7‐day PPA at 6 months Other reported outcomes: 24‐hour PPA at 6 months, programme evaluation | |
| Notes | Participants were offered EUR 7.50 to fill out all questionnaires. Not included in any comparisons as not clinically similar to other comparisons: compared 2 emails, no website component. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment |
| Allocation concealment (selection bias) | Low risk | Computerised, low risk of selection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 58.5% lost to follow‐up from intervention group, 56.4% lost from control. All randomised participants included in ITT analyses |
Voncken‐Brewster 2015.
| Methods | Randomised controlled trial Location: Netherlands Funding: ZonMw, the Netherlands Organization for Health Research and Development Recruitment: Participants were invited from 5 general practices by mail, and from a Dutch online panel Participants received EUR 2.55 per completed questionnaire. Study was conducted between May 2012 and July 2013. |
|
| Participants | Participants (n = 1325) (Intervention n = 662; Control n = 663) had a diagnosis of COPD, were at moderate or high risk for COPD, aged 40 – 70 years, fluent in Dutch, had access to the Internet, and had basic computer skills. Participants were 52% (n = 680) female, and were aged 57.6 (SD = 7.2) years. 29.5% (n = 386) attended primary school/basic vocational school only, 32.7% (n = 427) had a high school degree, 37.8% (n = 494) had a higher professional degree/university degree. 34.2% (n = 447) were current smokers. 8.8% had previously attempted to quit, and on average smoked 19.3 (SD = 12.1) CPD. The control group had a higher unemployment rate at 51.6%, compared to 45.9% in the intervention arm | |
| Interventions | Intervention: The 'Masteryourbreath' intervention was a tailored and interactive Internet intervention delivered over 6 months. In the lifestyle intervention arm participants received usual care or use other resources to help them manage their disease or improve their lifestyle. Participants received computer‐tailored feedback for lifestyle changes and application included 2 behaviour‐change modules, smoking cessation and physical activity. Each module had 6 components: health‐risk appraisal, motivational beliefs, social influence, goal‐setting and action plans, self‐efficacy, and feedback to maintain the healthy behaviour. Participants could switch behaviour‐change modules and choose to enter 1 or more intervention components according to their preference. The intervention was tailored to participants’ characteristics and behaviour and participants’ previous responses were also incorporated in the feedback so they could track their own behaviour change and goal attainment over the intervention period Control: The control group was a non‐internet‐based, non‐active control arm in which participants received usual care to help them manage their disease or improve their lifestyle |
|
| Outcomes | Outcome data were collected at 6 months. Outcomes were non‐bioverified continued abstinence, physical activity, health status, intention to change behaviour, number of quit attempts during the past 6 months, 24‐hour PPA, tobacco consumption, prolonged abstinence, 7‐day PPA | |
| Notes | The intervention application was used by 36% (n = 237) participants of the experimental group. 21.2% (n = 51) of smokers, and 1.7% (n = 7) non‐smokers completed at least 1 intervention component Hein de Vries is scientific director of Vision2Health, a company that licenses evidence‐based innovative computer‐tailored health‐communication tools The other authors declare that they had no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a permuted block design with a random block size varying from 4 to 20 |
| Allocation concealment (selection bias) | Low risk | A researcher not involved in data collection or analysis randomised participants using computer software |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 16% in treatment arm, 23% in control arm |
Wangberg 2011.
| Methods | Randomised controlled trial Location: Norway Funding: Norweigan Foundation for Health and Rehabiliatation, Norwegian Directorate of Health Recruitment: August 2006 to December 2007 by local and national media |
|
| Participants | 2298 current smokers (1171 intervention group, 1127 control group after), aged 16 years or older, registered at the website between August 2006 and December 2007. Average age 37 years, 72% female, ethnicity not reported, 17.1% had 17 or more years of education, average CPD 16.2, past quit attempts not reported | |
| Interventions | Intervention: 12‐month Internet‐based intervention for smoking cessation plus tailored messages sent based on questionnaires to personal web page and by email. Internet‐based intervention contains static information on dangers of smoking, general advice on cessation, and information about website, plus interactive tests for nicotine addiction, type of smoker, and motivation level, plus social support by discussion forum, guest book and personal diary. Receive up to 150 tailored messages over 12 months – first 14 days before, and last 1 ‐months after the quit date. Frequency first daily, then dropped off after quit date, using personalisation, adaption and feedback‐type tailoring Control: same Internet‐based intervention, but no messages – only emails containing notifications and reminders for follow‐up questionnaires |
|
| Outcomes | Long‐term abstinence: self‐reported 7‐day PPA at 6 and 12 months Short‐term abstinence: self‐reported 7‐day PPA at 1 and 3 months |
|
| Notes | For purposes of this report, 847 participants enrolled in time for 12‐month follow‐up (419 intervention, 428 control); 1798 included in 3‐month analysis (902 intervention, 896 control) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation by computer |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High losses to follow‐up (> 70%). 116 of 419 (response rate = 27.7%) in the intervention group and 128 of 428 (response rate = 29.9%) were followed up at 12 months Participants lost to follow‐up were counted as smokers in ITT analysis |
Wittekind 2015.
| Methods | Randomised controlled trial Location: Germany Funding: No funding information was provided Recrtuiment: Participants were recruited on smoking‐related Internet forums. No study dates were reported. |
|
| Participants | Participants (n = 257) (Standard approach‐avoidance task; n = 87; Modified approach‐avoidance task; n = 85; Control n = 85) were from the general population and were excluded if they had not smoked within the last month, or if they "did not answer the survey honestly". No other criteria were applied. Participant baseline characteristics were not reported by trial arm. Groups did not differ in any demographic or smoking‐related variable | |
| Interventions | Participants were randomly allocated to a standard or modified version of the 'Approach Avoidance Task (AAT)', or a wait list control. In both versions, participants were instructed to respond to the format of pictures which corresponded to smoking‐related or neutral items, by pushing or pulling a joystick independent of the content of the pictures. In the modified version, participants were shown their reaction time after each trial. | |
| Outcomes | Outcome data were collected at 4 weeks. Outcomes were non‐bioverified PPA, commitment to quitting smoking scale, Fagerström Test for Nicotine Dependence, and obsessive‐compulsive smoking scale | |
| Notes | 52.6% (n = 38) of participants were satisfied with the standard 'AAT' programme, and 42.4% (n = 33) with the modified 'AAT' programme. The authors reported no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates: 37% standard 'AAT', 46% modified 'AAT', 35% control |
Woodruff 2007.
| Methods | Cluster‐randomised controlled trial Location: 14 high school sites in San Diego County, CA USA Funding: Grant from California's Tobacco‐related Disease Research Program Recruitment: classroom presentations, lunch‐hour sign‐up tables, flyers, posters, school newspaper ads and articles, school‐wide announcements, and school liaison referrals. At the suggestion of school personnel, the recruitment approach and materials were different for intervention and control schools |
|
| Participants | 136 adolescent smokers (at least 1 cigarette smoked in last 30 days) from 14 high schools (77 intervention, 59 control, mean of 11 participants per intervention school, 8.4 per control school); average age 16 years, 46% female, 51% Hispanic, 28% white non‐Hispanic | |
| Interventions | Intervention: Internet‐based, virtual reality world combined with motivational interviewing conducted in real time by a smoking cessation counsellor (7 x 45‐minute virtual world sessions over a 7‐week period, and complete the 4 online surveys) Control: measurement‐only control condition (4 online surveys) |
|
| Outcomes | Long‐term abstinence: self‐reported 7‐day PPA at 12 months Short‐term abstinence: self‐reported 7‐day PPA at 3 months Secondary outcomes: satisfaction with the programme (5‐item questionnaire; ease of use, liking the programme, usefulness for "helping you quit" and for "helping other teen smokers quit") Other reported outcomes: programme use | |
| Notes | Participants were offered USD 50 to complete 4 online surveys over a 15‐month period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster‐randomised by school; method not described |
| Allocation concealment (selection bias) | High risk | Students recruited after schools randomised, with different recruitment methods. The 2 conditions did not differ significantly on demographic data, although a significantly greater proportion of intervention participants were alternative/continuation high‐school students. The groups differed significantly on several baseline smoking variables |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was 25% post‐intervention, 21% for the 3‐month follow‐up survey, and 27% at 12 months. Survey non‐response was higher among intervention participants than among controls (33% vs 15%). All randomised participants included in ITT analysis |
Yang 2016.
| Methods | Randomised controlled trial Location: China Funding: No details were reported Recrtuitment: Not clear. Participants were recruited between October 2014 to 2015. |
|
| Participants | Participants were 146 men with COPD ('Drug plus Wechat +NRT group' n = 42; usual care group + NRT n = 40; Control n = 38). Participants must have smoked for > 2 years, having smoked ≥ 5 CPD, upon diagnosis nicotine dependence score is ≥ 3. Must not have attempted quitting smoking, must be in stable medical conditions, willing to quit smoking, accepting medication or intervention for nicotine dependence. The patients were diagnosed having FEV1/FVC < 70% and after excluding possibilities of chronic cough, sputum and breathing difficulties | |
| Interventions | Intervention 1: 'Drug plus Wechat +NRT group' was a tailored and interactive Internet intervention, in which participants has access to a chat‐based smoking cessation support group. A doctor was also included in the support group. Every week information on smoking cessation was provided. If the participant had any they could use WeChat to communicate with the doctor and the Wechat support group. Participants also received NRT Intervention 2: 'The usual care group + NRT' received counselling and information on methods to quit and NRT Control: The 'usual care' group received counselling and information on methods to quit and no NRT |
|
| Outcomes | Outcome data were collected at 6 months. Smoking status was measured using non‐biovalidated 7‐day PPA, and sustained abstinence at 3 and 6 months | |
| Notes | No information about trial conflicts of interest was provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition rate: 26/146 participants were lost to follow‐up |
Zullig 2014.
| Methods | Randomised controlled trial Location: USA Fudning: No information reported Recruitment: Participants were sent a recruitment letter 2 weeks prior to a primary care clinic visit. A research staff member contacted participants by telephone, and arranged to meet in person to discuss the study. No study dates reported. |
|
| Participants | Participants (n = 96) (Intervention n = 47; Usual care n = 49) were patients of a primary care clinic for at least 1 year with 1 or more visits in the previous year, diagnosed with CVD or a CVD‐risk equivalent (e.g. diabetes), and had at least 1 modifiable outcome (e.g. hypertension or smoking). Exclusion criteria: metastatic cancer, dementia, psychosis or end‐stage renal disease, lacked Internet access, received nursing services, unable to read English, participating in another CVD study or a household member was a participant, received or were a candidate for a heart transplant, were hospitalised for cardiac‐related illnesses in the previous 3 months, or arm circumference exceeded 50 cm. Participants were 66.6% (n = 64) female, and were aged 63.1 (SD = 12.2) years. 65% (n = 62) were white, 32% (n = 33) were African‐America, 1% (n = 1) were of other ethnicity. 94% (n = 90) completed > 12 years of school. 17% (n = 16) of participants were current smokers. Trial arms were balanced on all recorded characteristics | |
| Interventions | Intervention: The intervention was a tailored and non‐interactive Internet intervention delivered over 3 months. The intervention comprised a web‐based Framingham risk calculator, in which participants adjusted their own risk scores and indicated areas they were willing to modify. Tailored educational information was provided, based on participants’ readiness to change. Each time the participants logged online they selected 2 behavioural/lifestyle modules. Follow‐up log‐ons were used to reinforce the previous interaction and maintain or revise health behaviour goals. The modules covered: diet, exercise, smoking, alcohol, patient‐provider relationships and medication management. Each module asked the participant about their current beliefs and health practices. Based on the participant’s responses to a series of questions, there was tailored feedback to reinforce behaviour change. Participants were also given information on CVD medication management and side effects Control: The control group was a non‐internet‐based, non‐active control arm in which participants received usual care, which was printed educational cardiovascular disease information and additional information at "their providers’ discretion." Participants were able to request intervention materials at the end of the study. All material provided was at a 6th‐grade reading level |
|
| Outcomes | Outcome data were collected at 3 months. Outcomes were non‐bioverified PPA, blood pressure, BMI, CVD risk, medication non‐adherence | |
| Notes | No information was provided about conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | After providing consent, participants were block‐randomised to the 3‐month intervention or to usual care |
| Allocation concealment (selection bias) | Low risk | Randomisation assignments were placed in sealed, consecutively‐numbered envelopes. The staff involved in the randomisation were blinded to the block size |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
av: average (mean) BMI: body mass index CO: carbon monoxide COPD: chronic obstructive pulmonary disease CPD: cigarettes per day IVR: interactive voice response NRT: nicotine replacement therapy PPA: point prevalence abstinence RCT: randomised controlled trial SMS: short message service TQD: target quit date
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abroms 2008 | Intervention used email for delivering counselling, but no Internet component |
| An 2007 | The Internet was used to quickly identify and enrol large numbers of college smokers in an online smoking cessation intervention, not as an intervention. There was no comparison group |
| Applegate 2007 | This study presents data that examined the feasibility of implementing a web and SMS text messaging programme to dose quitters properly and remind them to take medication at regular intervals |
| Baskerville 2015 | Mobile App |
| Bowen 2012 | Main aim of study is smoking prevention |
| Bravin 2015 | Not a randomised controlled trial |
| Buller 2008 | In this study the Internet was used as a tool for prevention of smoking, not as an intervention for smoking cessation |
| Buller 2014a | Mobile phone app |
| Buller 2014b | Mobile phone app |
| Calabro 2011 | Effect of Internet intervention confounded with in‐person counselling |
| Chen 2006 | This study does not have smoking cessation as an outcome. |
| Chew 2005 | This article describes the background, implementation, and evaluation of an Internet‐based health promotion network in the Czech Republic |
| Christoff 2015 | Offline intervention |
| Cobb 2006 | Not an RCT. The primary goal of this study was to characterise individuals who search for smoking cessation information on the Internet to determine appropriate triage and treatment strategies. The secondary goal was to estimate the incidence of searches for cessation information using a publicly‐available search engine |
| Cobb 2005 | Not an RCT; uncontrolled evaluation of 'QuitNet' with a 25.6% response rate |
| Dallery 2013 | Video‐based intervention |
| Danaher 2006 | This paper describes information architecture designs when creating effective web‐based interventions |
| Danaher 2011 | Study of intervention for smokeless tobacco cessation, not smoking cessation |
| Etter 2006a | This is a literature review and an Internet survey in 1506 current and former evaluation smokers. |
| Etter 2009a | Internet was not intervention, both groups had access to website but intervention was NRT |
| Etter 2009b | Very short follow‐up (48 hours after baseline) |
| Feil 2003 | Subsample of 370 participants followed for 3 months with no comparison group |
| Gala 2008 | Pilot study among college baseball players (smokeless tobacco users) with a small sample size and short follow‐up period (1 month) |
| Gillaspy 2010 | Outcome was stage of change at 1‐month follow‐up |
| Haug 2014 | Text messaging intervention |
| Houston 2005 | Randomised trial with 250 participants allocated to 2 different websites. Reported as an abstract, no further details available |
| Houston 2008a | Internet intervention targeted dentists, not smokers. No smoking cessation outcomes |
| Houston 2008b | Not an RCT. Pre‐post study evaluating a change in website content to change user behaviour |
| Houston 2013 | Not a smoking cessation intervention |
| Jacobs 2011 | Internet intervention confounded with in‐person counselling |
| Koo 2003 | The paper describes characteristics of websites for smoking cessation |
| Koo 2005 | The study evaluates strategies for recruiting teenagers for the evaluation of a smoking‐cessation website through the Internet |
| Lenert 2004 | Not an RCT. Compared variants of a cessation intervention with consecutive series of participants. See Muñoz 2006 Study 3, Muñoz 2006 Study 4 and Muñoz 2009 for trials of same intervention. |
| Linke 2012 | Tests the efficacy of exercise rather than an Internet‐based programme |
| Mermelstein 2006 | Previously included but excluded at 2013 update. Internet intervention confounded by additional phone counselling received by intervention arm |
| Muramoto 2007 | Trial compares the efficacy of in‐person training vs web‐based training vs a usual‐practice comparison group to teach non‐medical "health influencers" tobacco cessation skills |
| Mussulman 2014 | Video messaging intervention. |
| Muñoz 2012a | Not an RCT. Evaluates a website previously used in an RCT, modified so that users can choose which option they would like |
| Muñoz 2016 | Not randomised |
| Naughton 2014 | Text messaging intervention |
| NCT00865553 | Not a smoking cessation intervention |
| NCT01980017 | Not internet‐based intervention |
| NCT02046408 | Not an internet‐based intervention |
| NCT02103829 | Study was terminated according to clinicaltrials.gove (21‐4‐2017) |
| Norman 2004 | Study evaluates a strategy for online study of recruitment and retention, the influence of incentives on follow‐up response, and the impact of the Quit Smoking Network site on smoking behaviour |
| Norman 2008 | Classroom‐based smoking cessation and prevention intervention for adolescents. Did not assess smoking cessation as an outcome, only lowered smoking status |
| Ota 2005 | Cross‐sectional survey, no control group. |
| Pederson 2005 | Describes strategies for assisting patients in quitting smoking |
| Pisinger 2010 | Very low usage of the programme |
| Prochaska 2001 | Computer‐based intervention, but does not use Internet |
| Prochaska 2008 | Unable to confirm denominators for reported cessation rates which exclude losses to follow‐up. Study includes 136 smokers assigned to 3 conditions. Compared online tailored support to motivational interviewing as an adjunct to a health risk assessment |
| Prokhorov 2008 | Evaluates a computer‐assisted, counsellor‐delivered smoking cessation programme |
| Ray 2014 | All participants received intervention. Participants were randomised to 1 of 2 different referral methods |
| Reitzel 2011 | Evaluates hand‐held computer‐delivered intervention |
| Rowan 2007 | This study examined the relations between neighbourhood social context and smoking‐related factors among African‐Americans. A culturally‐tailored cessation treatment was delivered by palmtop computer |
| Schneider 1990 | Early Internet intervention, not considered comparable with other included interventions |
| Selby 2004 | Not an RCT |
| Severson 2008 | RCT of intervention for users of smokeless tobacco |
| Shegog 2005 | Pilot study evaluating the use of a web‐based tobacco prevention programme to change intentions of middle school children to smoke tobacco. Cross‐sectional survey with no control group |
| Skov‐Ettrup 2014 | Text messaging intervention |
| Stoddard 2005 | Feasibility study. No control group. |
| Stoops 2009 | All participants used web‐based components in the same way. The study differentiated between the incentive schedules used |
| Thieleke 2005 | Small sample size, no control group |
| Toll 2007 | Not internet‐based intervention |
| Velicer 2006 | Computer‐based intervention, but no Internet |
| Vilaplana 2014 | Text messaging intervention |
| Walters 2006 | A review of studies of computer‐ and Internet‐based interventions for smoking behaviour, published between 1995 and August 2004 |
| Wetter 2006 | This paper describes 3 projects ‐ computer‐delivered treatments for smoking cessation |
| Woolf 2006 | Nine‐month pre‐post comparison with non‐randomized control practices, 6 family practices (4 intervention, 2 control). Authors tested whether participants are more likely to pursue healthy behaviours (e.g. physical activity, smoking cessation) if referred to a tailored website that provides valuable information for behaviour change |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Buller 2014.
| Trial name or title | |
| Methods | Randomised controlled trial conducted in the continental USA. Participants were recruited by an online health risk assessment, and posters, cards with study URL and handouts by state tobacco control programmes, employers, and unions, online advertisements on Google Adwords, and screening by 4 state telephone quit lines |
| Participants | Participants were young adults aged 18 ‐ 30, and smoked at least 1 cigarette in the past 30 days, fluent in English, and a resident of the continental USA. Participants were 64% female, and the mean age was 25 years. 84% were non‐Hispanic white, 33.8% had a high school degree only, 34.2% had some college, and 13.2% had a college or postgraduate degree, 11.5% did not complete high school and 7.3% had a trade, technical or vocational education. On average participants smoked 18.1 CPD |
| Interventions | Intervention arm: 'Real e Quit website' was an Internet intervention (tailored and interactive) + NRT. Smokers could request a free 2‐week course of nicotine patches. Quit Coach provided tailored advice delivered in text format, and smokers could also read supplemental documents on issues such as benefits of quitting, strategies for stopping, using NRT, getting through early days of a quit, coping with nicotine withdrawal, and implementing a smoke‐free home. Quit Coach also contained testimonial videos of young adult smokers who had quit, provided e‐cards smokers could send to show support for quitting, and a blog by a smoking cessation counsellor. Participants in the control arm were referred to a telephone quit line service which was a non‐Internet‐based active control arm + NRT. The quit line used standard counselling protocols. Proactive calls were placed by counsellors to smokers, and smokers were offered up to 5 counselling sessions. During the initial sessions, a quit date was set, support provided, and information given on the correct use of medications. Follow‐up sessions were used to identify difficult situations and problem‐solving strategies to develop coping mechanisms during and after the quit process. The second control arm was The National Cancer Institute's self‐help cessation booklet which was a non‐active control arm + NRT. Smokers could request a free 2‐week course of nicotine patches, and the National Cancer Institute's self‐help cessation booklet was available for download in PDF |
| Outcomes | Outcome data were collected at 12 and 26 weeks. Outcomes were non‐bioverified 30‐day continuous abstinence, use of nicotine replacement therapy, predictors of use of nicotine replacement therapy, CPD, smokeless tobacco use, quit attempts since joining the study, self‐efficacy staying quit, use of help in quitting (telephone, Internet, reading a self‐help booklet, at a clinic or group, hypnosis or just tried on their own). Participants still smoking at follow‐up were asked the likelihood of quitting in the next 3 months and whether they had set a quit date; participants not smoking, were asked how long ago they had quit, 7‐day smoking prevalence, and likelihood that they might smoke again. |
| Starting date | |
| Contact information | |
| Notes | Smokers followed up tended to be older, better educated, employed, spent more time using the Internet, smoked on fewer days, were less addicted, had greater readiness to quit, and had made more previous quit attempts. David Buller was employed by Klein Buendel, Inc., a for‐profit health communication research firm and Klein Buendel, Inc. is owned by Dr. Buller's spouse. Erwin P. Bettinghaus is employed by Klein Buendel, Inc., a for‐profit health communication research firm, and is a member of the Board of Directors for Klein Buendel, Inc. Gary Cutter participated on data and safety monitoring committees for the following organizations focusing on medical research: Apotek, Biogen‐Idec,Cleveland Clinic,Glaxo Smith Klein pharmaceuticals, Gilead Pharmaceuticals, Modigenetech/Prolor, Merck/Ono Pharmaceuticals, Merck, Neuren, Revalesio, Sanofi‐Aventis, Teva, Vivus, National Heart, Lung, and Blood Institute (Protocol Review Committee), National Institute on Neurological Disorders and Stroke, National Multiple Sclerosis Society, and National Institute on Child Health and Development (OPRU oversight committee). He has consulted, received speaking fees, or served on advisory boards for the following organizations: Alexion, Allozyne, Bayer, Celgene, Coronado Biosciences, Consortium of MS Centers (grant), Diogenix, Klein‐Buendel Incorporated, Medimmune, Novartis, Nuron Biotech, Receptos, Spiniflex Pharmaceuticals, Teva pharmaceuticals. He is employed by the University of Alabama at Birmingham and President of Pythagoras. Inc. a private consulting. All other authors report no conflicts of interest. This project was supported by a grant from the National Cancer Institute (CA107444). |
Díaz‐Gete 2012.
| Trial name or title | Effectiveness of an email tracking Intervention among the continued abstinence of tobacco consumption |
| Methods | Randomised controlled trial |
| Participants | Smokers, aged 18 years or older, frequent user of email account |
| Interventions | Participants in the intervention arm received an email‐based intervention, compared to a brief advice control group |
| Outcomes | Primary outcome: Smoking status and maintenance of smoking cessation Secondary outcomes: PPA, self‐reported tobacco consumption, self‐reported smoking reduction, stage of change in Prochaska cycle, used time by professionals to achieve participants stop smoking, used time by participants, cost to get smoking help in primary care service, cost of helping people to leave smoking in regular conditions |
| Starting date | The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years. (No Study results posted, only linked publication is published protocol noted in references) |
| Contact information | Laura Díaz‐Gete, Institute Català de la Salut |
| Notes |
Henderson 2012.
| Trial name or title | Lakota Oyate Wicozani Pi Kte (LOWPK) trial |
| Methods | 2‐arm randomised controlled trial |
| Participants | 180 remote reservation‐dwelling adult American‐Indian men and women with type 2 diabetes who are at high risk for CVD |
| Interventions | A web‐based diabetes and nutritional intervention to reduce risk factors related to cardiovascular disease |
| Outcomes | The primary outcome variable is change in glycosylated haemoglobin level after an average 18‐month follow‐up period. Secondary outcome variables include changes in low‐density lipoprotein cholesterol, systolic blood pressure, body mass index, and smoking status, as well as an evaluation of intervention cost effectiveness |
| Starting date | 2009 |
| Contact information | |
| Notes | Multifactorial intervention; may not meet inclusion criterion for this review |
Humfleet 2007.
| Trial name or title | Lesbian, gay, bisexual, and transgender (LGBT) Internet‐based smoking treatment ‐ 1 |
| Methods | 2‐arm randomised controlled trial |
| Participants | 600 LGBT smokers |
| Interventions | 1) a self‐help intervention tailored to LGBT smokers plus social support plus email‐based counselling 2) a standard self‐help condition alone, similar to other general smoking cessation treatments |
| Outcomes | Smoking status will be determined at 1, 3, 6, and 12 months following the start of treatment |
| Starting date | September 2002. This study has been completed. (No study results posted and no linked publications) |
| Contact information | Gary Humfleet, ghumfleet@lppi.ucsf.edu. University of California, San Francisco |
| Notes | NCT00111501, NIDA sponsored |
Humfleet 2008.
| Trial name or title | Reaching and treating lesbian, gay, bisexual, and transgender (LGBT) cigarette smokers ‐ 2 |
| Methods | 4‐arm randomised controlled trial. |
| Participants | Lesbian, gay, bisexual and transgender (LGBT) smokers |
| Interventions | 1) a mail‐based self‐help (MSH) treatment; 2) MSH plus an Internet‐based smoking treatment (IST); 3) MSH plus telephone counselling (TC); 4) MSH plus IST plus TC |
| Outcomes | Smoking status will be determined at 3, 6, and 12 months following the start of treatment |
| Starting date | February 2008. This study has been completed. (No study results posted and no linked publications) |
| Contact information | Gary Humfleet, ghumfleet@lppi.ucsf.edu. University of California, San Francisco |
| Notes | NCT00634218 |
Kramer 2009.
| Trial name or title | Effectiveness of a web‐based self‐help smoking cessation intervention: protocol of a randomised controlled trial |
| Methods | Study design: randomised controlled trial Recruitment: Participants were recruited over a 1‐year period using advertisements in daily and weekly national or regional newspapers or on the Internet. Enrolment took place by a website |
| Participants | Inclusion criteria: Adults aged18 and older who were currently smoking cigarettes or rolling tobacco, were willing to quit smoking within 3 months and have Internet access Exclusion criteria: smokers who were already preparing to stop smoking with the support of a coach, a course of pharmacotherapy, or if they were already enrolled in another smoking cessation study |
| Interventions | Intervention: web‐based interactive self‐help intervention (Stop SIte) Control: access to the Dutch online self‐help guide developed by STIVORO |
| Outcomes | Primary outcome measure: prolonged abstinence in the past 3 months Secondary outcomes: PPA, number of cigarettes smoked, and incidence of quit attempts at follow‐up assessments Methods of assessing outcome: self‐reported smoking abstinence Methods of follow‐up for non‐respondents: ITT analysis Timing of outcome assessment: 3 and 12 months after 1‐month grace period from starting the intervention after baseline |
| Starting date | Trial status completed, only linked publication is published protocol noted in references |
| Contact information | jkramer@trimbos.nl |
| Notes |
NCT01103427.
| Trial name or title | Mobile text messaging as an adjunct function to an Internet‐based smoking cessation intervention implemented in the general population and in a health care setting |
| Methods | Randomised controlled trial |
| Participants | Adult smokers |
| Interventions | Participants in the intervention arm received Internet‐based coaching and SMS‐based coaching |
| Outcomes | Primary outcome: smoking cessation at 12 months |
| Starting date | May 2010. This study has been completed. (No study results posted and no linked publications) |
| Contact information | Inger T Gram, University of Tromso |
| Notes |
NCT01457469.
| Trial name or title | Enhanced quitline intervention in smoking cessation for patients with non‐metastatic lung cancer |
| Methods | Randomised controlled trial |
| Participants | Diagnosed with stage I ‐ IIIa/b non‐small cell lung cancer, or limited stage small cell lung cancer, smoked cigarettes in the past 7 days, willing to consider quitting smoking. Exclusion Criteria: Patients with drug and alcohol abuse. |
| Interventions | Participants in the intervention arm 'enhanced quitline' received a personalised letter and a smoking cessation booklet, plus an 8‐week supply of nicotine patches, and a 30‐ to 45‐minute counselling session focusing on the benefits of quitting smoking for cancer patients and addressing cancer‐specific concerns about smoking cessation. Participants also undergo a quitline‐based smoking cessation intervention comprising 5 individual 25‐ to 30‐minute telephone counselling sessions, and unlimited inbound phone‐based access to Quit Coaches over 8 to 11 weeks, mailed written materials, and an interactive online programme. Patients in the control arm received a personalised letter from their physician with advice to quit smoking and a copy of the National Cancer Institute's 'Cleaning the Air' smoking cessation booklet |
| Outcomes | Primary outcomes: Participation of people with lung cancer in the outpatient oncology setting, accrual of people with lung cancer in the outpatient oncology setting, participant retention, participant acceptance of the enhanced quitline‐based smoking cessation intervention, protocol fidelity Secondary outcomes: Abstinence, quality of life, stress, and depressive symptoms |
| Starting date | October 2011. Study completed. No published or unpublished reports located |
| Contact information | Kathryn Weaver, Comprehensive Cancer Center of Wake Forest University |
| Notes |
NCT01544153.
| Trial name or title | Improving adherence to web‐based cessation programs: a social network approach |
| Methods | Randomised controlled trial, 2 x 2 design |
| Participants | Aged 18 years and older, current smoker, registered user on BecomeAnEX.org. Exclusion criteria: pregnant or breastfeeding, cardiovascular conditions, current use of any stop‐smoking medication |
| Interventions | Comparison of an interactive, evidence‐based smoking cessation website alone and in conjunction with 1) a theory‐driven, social network protocol designed to integrate participants into the online community, and 2) a 4‐week supply of free NRT |
| Outcomes | Primary outcome: Self‐reported 30‐day PPA Secondary outcomes: Self‐reported 30‐day PPA |
| Starting date | February 2012. This study is ongoing, but not recruiting participants. |
| Contact information | Amanda Graham, Truth Initiative |
| Notes |
NCT01692730.
| Trial name or title | Web‐assisted tobacco intervention with community colleges |
| Methods | Randomised controlled trial |
| Participants | Aged 18 or older, smokes at least 1 CPD on average, attends community college |
| Interventions | Participants in the intervention arm received 'Enhanced Web Assisted Intervention' which was an enhanced and highly interactive website for cessation ‐ with current Public Health Service Guideline information and effective smoking cessation strategies, and novel interactive and social network features, including a variety of better‐practice features recommended by recent literature, and technologically advanced proactive features (i.e. emails, SMS texting, and social networking). Control arm received a basic web‐assisted intervention with current Public Health Service Guideline information and effective smoking cessation strategies, with minimal interactive web‐based features |
| Outcomes | Primary outcome: Biochemically‐verified abstinence Secondary outcome: Stages of change |
| Starting date | October 2012. This study has been completed. (No study results posted and no linked publications) |
| Contact information | Scott McIntosh, University of Rochester, USA |
| Notes |
NCT01812278.
| Trial name or title | Randomised trial of web‐delivered acceptance therapy for smoking cessation (WebQuit) |
| Methods | Randomised controlled trial |
| Participants | Aged 18 years or older, smokes at least 5 cigarettes daily for at least past 12 months, wants to quit in next 30 days, willing to be randomly assigned to either group, resides in USA, has at least weekly access to a high‐speed Internet connection and email, willing and able to read in English, not participating in other smoking cessation interventions (including our other intervention studies), has never used the mokefree.gov website, willing to complete all 3 follow‐up surveys, provide email, phone, and mailing address, provide contact information for 2w collaterals (e.g. relatives) |
| Interventions | Intervention: Acceptance & Commitment Therapy (ACT) website Control: Cognitive Behavioural Therapy (CBT) (website) |
| Outcomes | 30‐day PPA, 7‐day, 24‐hour, and 30‐day PPA quit rates |
| Starting date | March 2014. This study is ongoing, but not recruiting participants. |
| Contact information | Fred Hutchinson, Cancer Research Center |
| Notes |
NCT02021175.
| Trial name or title | Adaptation and development of a web and cell phone quit smoking treatment for Korean youth |
| Methods | Randomised controlled trial |
| Participants | Inclusion criteria: Self‐identify as Korean or Korean‐American; smoked at least 5 CPD for the past 6 months; interested in smoking cessation, aged between 14 ‐ 19 years; willing to provide information that can assist in locating the individual for follow‐up visits; living in Los Angeles County; has a phone capable of receiving SMS text messages; has a computer or other regular access to engage programme components; willing and able to provide consent if older than 18; willing and able to provide assent if under 18 and has a parent or legal guardian willing and able to provide consent; at least 6th‐grade English reading level due to requirements of assessment procedures |
| Interventions | Behavioural: Tailored CBME Therapy via Technology: 6 weeks of tailored interactive cognitive‐behavioural motivational enhancement therapy delivered through Internet and cell phones; Other: Standard care: referral to currently‐available resources for 6 weeks of a standard smoking cessation approach |
| Outcomes | 7‐day PPA, bioverified by urinary cotinine and CO will be assessed. Variables that mediate outcomes include measures of demographics, withdrawal symptoms, psychiatric and substance use status, impulsivity, health‐related quality of life, and neighbourhood status and acculturation |
| Starting date | June 2016. This study is not yet open for participant recruitment. |
| Contact information | Steve Shoptaw, University of California, Los Angeles |
| Notes |
NCT02050308.
| Trial name or title | Web‐based smoking cessation program for tribal college students |
| Methods | Randomised controlled trial |
| Participants | Adults enrolled at Salish Kootenai College, valid telephone number and email address, willing to participate in all study components, willing to be followed‐up for 6 months, who self‐identify as American‐Indian or Alaska Native, current smoker |
| Interventions | Internet‐All Nations Breath of Life is a culturally‐tailored Internet‐based intervention that will cover topics relevant to quitting smoking, American‐Indian culture, and health. Participants will have a choice of varenicline, bupropion, NRT or no pharmacotherapy |
| Outcomes | Primary outcome: 7‐day PPA, biochemically (salivary cotinine) verified PPA, cigarettes smoked, number of quit attempts, adherence to programme participation |
| Starting date | May 2015. This study is currently recruiting participants. |
| Contact information | Joseph A Pacheco, University of Kansas |
| Notes |
NCT02072772.
| Trial name or title | A trial of positively smoke‐free group therapy for HIV‐infected smokers |
| Methods | Randomised controlled trial |
| Participants | HIV‐infection, smoker, receives care at Montefiore Medical Center or Georgetown University, motivated to quit, willing to attend 8 x 90‐minute group sessions |
| Interventions | Positively Smoke Free group treatment will involve group sessions led by a professional and a "peer" HIV‐infected ex‐smoker with tobacco treatment training. Standard Care will involve advice to quit, and a self‐help brochure. All participants will be offered a 3‐month supply of nicotine patches |
| Outcomes | 6‐month abstinence from cigarettes, biochemically‐confirmed 7‐day PPA, cost per incremental quit |
| Starting date | May 2014. This study is not yet open for participant recruitment. |
| Contact information | Jonathan Shuter, Montefiore Medical Center |
| Notes |
NCT02099097.
| Trial name or title | Quit IT: Preliminary testing of a web‐based, 3D coping skills game to increase quitting self‐efficacy for maintaining smoking abstinence following hospitalisation |
| Methods | Randomised controlled trial |
| Participants | Aged 18 years or older, English‐speaking, cancer (solid tumour) diagnosis, or mass suspicious of cancer within past 6 months based on clinical judgement; cancer treatment to include hospitalisations for surgical treatment for at least 2 days at Memorial Sloan Kettering Cancer Center, referred to Tobacco Cessation Program, self‐reported cigarette use within the past 30 days, sufficient sensory acuity, and manual dexterity to use a computer game, can be reached by telephone |
| Interventions | Participants in the intervention arm received a web‐based video game “Smoking Cues Coping Skills Game”. Control arm received standard care |
| Outcomes | Primary outcome: Quitting smoking self‐efficacy Secondary outcome: Efficacy of the intervention for smoking abstinence and relapse prevention, smoking relapse following hospitalisations, biochemically‐verified 7‐day PPA |
| Starting date | March 2014. This study is ongoing, but not recruiting participants. |
| Contact information | Jamie Ostroff, Memorial Sloan Kettering Cancer Center, USA |
| Notes |
NCT02207036.
| Trial name or title | Social media Intervention for young adult smokers |
| Methods | Randomised controlled trial |
| Participants | Aged 18 ‐ 25 years old, English literate, access to Smartphone or computer with camera, Facebook users "most" (≥ 4) days a week, smoked ≥ 100 cigarettes in lifetime, current smoker of at least 1 CPD on 3 or more days of the week |
| Interventions | Participants in the intervention arm received “Tobacco Status Project“ which was a Facebook‐based intervention, versus control arm Smokefree.gov website |
| Outcomes | Primary outcome: Biochemically‐verified 7‐day PPA Secondary outcomes: Reduction of cigarette consumption, tobacco quit attempt, readiness to quit tobacco, abstinence goal, engagement in intervention |
| Starting date | October 2014. This study has been completed. (No study results posted and no linked publications) |
| Contact information | Daniel Ramo, University of California, USA |
| Notes |
NCT02329249.
| Trial name or title | Pharmacological aids for interactive smoking cessation (NRT 2) |
| Methods | Randomised controlled trial |
| Participants | Large worksites with Internet connection, thinking of quitting smoking, aged 18 years or older |
| Interventions | Behavioural intervention 'Smokefree Partners: 21 Days to Freedom' was a smoking cessation website programme with live personal coach. Wait‐list control |
| Outcomes | Primary outcome: Smoking cessation Secondary outcomes: Intentions to quit smoking, intentions to quit and remain smoke‐free, self‐efficacy for quitting, intentions to use a pharmacological smoking cessation aid |
| Starting date | December 2014. This study has been completed. (No study results posted and no linked publications) |
| Contact information | Susan Schroeder, Oregon Center for Applied Science |
| Notes |
NCT02378766.
| Trial name or title | “TAVIE en santé” or “Evaluation of Web‐based Interventions to Support People Living With HIV in the Adoption of Health Behaviours (LHIVEHEALTHY)” |
| Methods | Randomised controlled trial |
| Participants | Persons living with HIV, aged 18 years or older, able to read and understand French or English, have Internet access |
| Interventions | 'TAVIE en santé ' is a web‐based tailored tri‐component intervention addressing smoking cessation (SC), physical activity (PA) and healthy eating. Control group will receive a list of predetermined websites. |
| Outcomes | 7‐day PPA, physical activity, diet, intention to change health‐related behaviour, perceived control, attitude about health‐related behaviour |
| Starting date | December 2015. This study is currently recruiting participants. |
| Contact information | José Côté, University of Montreal |
| Notes |
NCT02585206.
| Trial name or title | Optimizing text messaging to improve adherence to web‐based cessation treatment |
| Methods | Randomised controlled trial |
| Participants | Adult smokers (every day/some days) who register on BecomeAnEX.org and enrol in the text message programme |
| Interventions | 'WEB' participants will have access to an evidence‐based cessation programme that educates smokers and provides the tools necessary to enhance self‐efficacy for quitting. 'WEB+TXT' participants will have access to the programme, and a text intervention |
| Outcomes | 30‐day PPA, motivation to quit, quit attempts, 7‐day PPA, continuous abstinence, intervention satisfaction |
| Starting date | July 2017. This study is not yet open for participant recruitment. |
| Contact information | Ryan Desrosiers, The Truth Initiative |
| Notes | Inclusion of Phase II (web intervention vs web and text intervention randomised controlled trial) but not Phase I (text intervention development/optimisation) |
NCT02602730.
| Trial name or title | Internet‐based non‐smoking program for postpartum women |
| Methods | Randomised controlled trial |
| Participants | For pregnant participants, aged 18 years or older, between 8 and 32 weeks pregnant, current smoker trying to quit, or tried to quit smoking within last 2 months, able to speak and read English, access to high‐speed or DSL Internet and email. For general population participants: male or female not pregnant, aged 18 years or older, current smoker trying to quit, or tried to quit smoking within last 2 months, able to speak and read English, access to high‐speed or DSL Internet and email |
| Interventions | Behavioural Internet‐based “Break the Chain” programme included digital coaching messages sent during the participant's quit attempt and as needed in response to participant questions or comments. The intervention was compared to a group receiving a PDF booklet “Cleaning the Air” which was emailed to participants |
| Outcomes | Primary outcome: Number of cigarettes smoked in the last 7 days at 10 months Secondary Outcomes: Knowledge of impact of smoking cessation. Pregnant smokers only: post‐natal impact, infant impact, infant health risk, infant health issues related to smoking, self‐efficacy to quit smoking, attitudes about smoking cessation, behavioural intentions about smoking cessation, programme satisfaction, user rating of system usability |
| Starting date | February 2012. This study has been completed. (No study results posted and no linked publications) |
| Contact information | Susan W Schroeder, Oregon Center for Applied Science |
| Notes |
Redfern 2014.
| Trial name or title | Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) study |
| Methods | Randomised controlled trial |
| Participants | Aged 18 years or older, access to the Internet by mobile phone, tablet or computer, at moderate‐to‐high risk of a CVD event |
| Interventions | The intervention group will participate in the CONNECT programme which is an e‐health strategy for cardiovascular risk management which includes access to interactive smart phone and Internet platforms. The control group will continue to participate in usual health care |
| Outcomes | Bioverified 7‐day PPA |
| Starting date | October 2014. Study active, but not recruiting |
| Contact information | Prof Julie Redfern, The George Institute for Global Health |
| Notes |
Westmaas 2013.
| Trial name or title | Tailored emails as a stand‐alone strategy for smoking cessation |
| Methods | 3‐arm randomised controlled trial. |
| Participants | 355 smokers recruited through American Cancer Society's website |
| Interventions | 1. Up to 30 tailored emails 2. 3 or 4 tailored emails 3. 1 non‐tailored email with links to web‐based smoking cessation resources |
| Outcomes | Smoking behaviour at 1, 3 and 6 months from baseline |
| Starting date | Not reported |
| Contact information | Lee Westmaas, lee.westmaas@cancer.org |
| Notes |
Wiers 2015.
| Trial name or title | |
| Methods | Randomised controlled trial conducted in Netherlands. Limited information available (abstract only) |
| Participants | Smokers who wanted to quit |
| Interventions | Attentional retraining compared to continued assessment control |
| Outcomes | Smoking cessation |
| Starting date | |
| Contact information | |
| Notes | The authors declare no possible conflict of interest. This study was made possible by a grant from the Dutch Medical Research Foundation (ZONMW) |
CPD: cigarettes per day CVD: cardiovascular disease PPA: point prevalence abstinence SMS: short message service
Differences between protocol and review
We used sensitivity analysis to investigate the impact of using data from complete cases (i.e. including only participants who were followed up) as compared to our ITT analysis (i.e. assuming those who dropped out or who were lost to follow‐up were smokers).
Contributions of authors
Gemma Taylor and Michael Dalili contributed equally on study selection, meta‐analysis, narrative analysis, and led on write‐up of the review. Gemma Taylor, Michael Dalili, and Monika Semwall equally contributed to data extraction and decisions about inclusion of studies. Lindsay Stead assisted with methodological support and conducted the searches. Josip Car conceived the idea for this review. All authors contributed to the design, and editing of the manuscript. All authors reviewed the final version of the manuscript.
Sources of support
Internal sources
Department of Primary Care and Social Medicine, Imperial College London, UK.
Ministry of Science, Education and Sport, Croatia.
-
Department of Primary Care Health Sciences, Oxford University, UK.
Editorial base for the Cochrane Tobacco Addiction Group
-
National Institute for Health Research School for Primary Care Research, UK.
Support for the Department of Primary Health Care, Oxford University
Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore eLearning grant, Singapore.
External sources
NHS Connecting for Health Evaluation Programme (NHS CFHEP 001), UK.
-
NHS Research and Development Programme, UK.
Infrastructure funding for the Cochrane Tobacco Addiction Group
Declarations of interest
Gemma MJ Taylor: None known. Michael Dalili: None known. Monika Semwal: None known. Marta Civljak: None known. Lindsay F Stead: None known. Aziz Sheikh: None known. Josip Car: None known.
Edited (no change to conclusions)
References
References to studies included in this review
An 2008 {published data only}
- An LC, Klatt C, Perry CL, Lein EB, Hennrikus DJ, Pallonen UE, et al. The RealU online cessation intervention for college smokers: a randomized controlled trial. Preventive Medicine 2008;47(2):194‐9. [DOI] [PubMed] [Google Scholar]
An 2013 {published data only}
- An LC, Demers MR, Kirch MA, Considine‐Dunn S, Nair V, Dasgupta K, et al. A randomized trial of an avatar‐hosted multiple behavior change intervention for young adult smokers. Journal of the National Cancer Institute 2013;2013(47):209‐15. [DOI: 10.1093/jncimonographs/lgt021] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bannink 2014 {published data only}
- Bannink R, Broeren S, Joosten‐van Zwanenburg E, As E, Looij‐Jansen P, Raat H. Effectiveness of a web‐based tailored intervention (E‐health4Uth) and consultation to promote adolescents' health: randomized controlled trial. Journal of Medical Internet Research 2014;16(5):e143. [Nederlands Trial Register: NTR 3596] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannink R, Broeren S, Joosten‐van Zwanenburg E, As E, Looij‐Jansen P, Raat H. Use and appreciation of a web‐based, tailored intervention (E‐health4Uth) combined with counseling to promote adolescents’ health in preventive youth health care: survey and log‐file analysis. JMIR Research Protocols 2014;3(1):e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berg 2014 {published data only}
- Berg CJ, Stratton E, Sokol M, Santamaria A, Bryant L, Rodriguez R. Novel incentives and messaging in an online college smoking intervention. American Journal of Health Behavior 2014;38(5):668‐80. [DOI: 10.5993/AJHB.38.5.4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bolman 2015 {published data only}
- Bolman C, Eggers SM, Osch L, Poel F, Candel M, Vries H. Is Action Planning Helpful for Smoking Cessation? Assessing the Effects of Action Planning in a Web‐Based Computer‐Tailored Intervention. Substance Use & Misuse 2015;50(10):1249‐1260. [DOI: 10.3109/10826084.2014.977397] [DOI] [PubMed] [Google Scholar]
Borland 2013 {published data only}
- Balmford J, Borland R, Benda P. Population‐level effects of automated smoking cessation help programs: a randomized controlled trial. Addiction 2013;108(3):618‐628. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Balmford J, Borland R, Benda P, Howard S. Factors associated with use of automated smoking cessation interventions: findings from the eQuit study. Health Education Research 2013;28(2):288‐99. [DOI: 10.1093/her/cys104] [DOI] [PubMed] [Google Scholar]
Borland 2015 {published data only}
- Borland R, Balmford J, Swift E. Effects of encouraging rapid implementation and/or structured planning of quit attempts on smoking cessation outcomes: a randomized controlled trial. Annals of Behavioral Medicine 2015;49(5):732‐742. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Borland R, Balmford J, Swift E. Effects of timing of initiation and planning on smoking cessation outcomes: study protocol for a randomised controlled trial. BMC Public Health 2013;13(235):1‐9. [DOI: 10.1186/1471-2458-13-235] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brendryen 2008a {published data only}
- Brendryen H, Drozd F, Kraft P. A digital smoking cessation program delivered through internet and cell phone without nicotine replacement (Happy Ending): randomized controlled trial. Journal of Medical Internet Research 2008;10(5):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brendryen 2008b {published data only}
- Brendryen H, Kraft P. Happy Ending: a randomized controlled trial of a digital multi‐media smoking cessation intervention. Addiction 2008;103(3):478‐84. [DOI] [PubMed] [Google Scholar]
Bricker 2013 {published data only}
- Bricker J, Wyszynski C, Comstock B, Heffner J. Does increased acceptance of urges boost quit rates of web‐delivered cessation interventions? Results from a randomized controlled trial. Society for Research on Nicotine and Tobacco 19th Annual Meeting March 13‐16 Boston MA. 2013:52.
- Bricker J, Wyszynski C, Comstock B, Heffner JL. Pilot randomized controlled trial of web‐based acceptance and commitment therapy for smoking cessation. Nicotine & Tobacco Research 2013;15(10):1756‐64. [DOI: 10.1093/ntr/ntt056] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker JB, Comstock BA, Wyszynski CM. Is it feasible to deliver acceptance and commitment therapy for smoking cessation as a web‐based intervention?. Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13‐16, 2012, Houston, Texas. 2012:93.
- Jones HA, Heffner JL, Mercer L, Wyszynski CM, Vilardaga R, Bricker JB. Web‐based acceptance and commitment therapy smoking cessation treatment for smokers with depressive symptoms. Journal of Dual Diagnosis 2015;11(1):56‐62. [DOI: 10.1080/15504263.2014.992588] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2014 {published data only}
- Brown J, Michie S, Geraghty AW, Yardley L, Gardner B, Shahab L, et al. Internet‐based intervention for smoking cessation (StopAdvisor) in people with low and high socioeconomic status: a randomised controlled trial.. Lancet Respiratory Medicine 2014;2(12):997‐1006. [DOI: 10.1016/S2213-2600(14)70195-X] [DOI] [PubMed] [Google Scholar]
- Brown J, Michie S, West R. Interpreting internet‐based trials: StopAdvisor for smoking cessation. Lancet Respiratory Medicine 2015;3(3):e5‐6. [DOI] [PubMed] [Google Scholar]
- Brown J, Michie S, West R. Interpreting internet‐based trials: StopAdvisor for smoking cessation‐‐authors' reply. Lancet Respiratory Medicine 2015;3(3):e6‐7. [DOI] [PubMed] [Google Scholar]
- Michie S, Brown J, Geraghty AW, Miller S, Yardley L, Gardner B, et al. A randomised controlled trial of a theory‐based interactive internet‐based smoking cessation intervention ('StopAdvisor'): study protocol. Journal of Smoking Cessation 2013;8(2):63‐70. [Google Scholar]
Burford 2013 {published data only}
- Burford O, Jiwa M, Carter O, Parsons R, Hendrie D. Internet‐based photoaging within Australian pharmacies to promote smoking cessation: randomized controlled trial. Journal of Medical Internet Research 2013;15(3):e64. [DOI: 10.2196/jmir.2337] [DOI] [PMC free article] [PubMed] [Google Scholar]
Calhoun 2016 {published data only}
- Calhoun PS, Datta S, Olsen M, Smith VA, Moore SD, Hair LP, et al. Comparative effectiveness of an Internet‐based smoking cessation intervention versus clinic‐based specialty care for veterans. Journal of Substance Abuse Treatment 2016;69:19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 2015 {published data only}
- Cameron D, Epton T, Norman P, Sheeran P, Harris PR, Webb TL, et al. A theory‐based online health behaviour intervention for new university students (U@Uni:LifeGuide): results from a repeat randomized controlled trial. Trials 2015;16(1):1‐15. [DOI: 10.1186/s13063-015-1092-4; ISRCTN: ISRCTN07407344] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2014 {published data only}
- Choi SH, Waltje AH, Ronis DL, Noonan D, Hong O, Richardson C, et al. Randomized controlled trial of the Tobacco Tactics website versus 1‐800‐QUIT‐NOW telephone line among Operating Engineers. Tobacco Induced Diseases 2014;12(Suppl 1):A13. [DOI: 10.1186/1617-9625-12-S1-A13] [DOI] [Google Scholar]
- Choi SH, Waltje AH, Ronis DL, Noonan D, Hong O, Richardson C, et al. Web‐enhanced tobacco tactics with telephone support versus 1‐800‐QUIT‐NOW telephone line intervention for operating engineers: randomized controlled trial. Journal of Medical Internet Research 2014;16(11):e255. [DOI: 10.2196/jmir.3375] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SA, Ronis DL, Richardson C, Waltje AH, Ewing LA, Noonan D, et al. Protocol of a randomized controlled trial of the Tobacco Tactics website for operating engineers. BMC Public Health 2012;12:335. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2004 {published data only}
- Clark MM, Cox LS, Jett JR, Patten CA, Schroeder DR, Nirelli LM, et al. Effectiveness of smoking cessation self‐help materials in a lung cancer screening population. Lung Cancer 2004;44(1):13‐21. [DOI] [PubMed] [Google Scholar]
Cobb 2016 {published data only}
- Cobb NK, Jacobs MA, Saul J, Wileyto EP, Graham AL. Diffusion of an evidence‐based smoking cessation intervention through Facebook: a randomised controlled trial study protocol. BMJ Open 2014;4(1):e004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb NK, Jacobs MA, Wileyto P, Valente T, Graham AL. Diffusion of an evidence‐based smoking cessation intervention through Facebook: a randomized controlled trial. BMJ Open 2016;106(6):1130‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dezee 2013 {published data only}
- Dezee KJ, Wink JS, Cowan CM. Internet versus in‐person counseling for patients taking varenicline for smoking cessation. Military Medicine 2013;178(4):401‐5. [DOI: 10.7205/MILMED-D-12-00272] [DOI] [PubMed] [Google Scholar]
Dickinson 2013 {published data only}
- Dickinson WP, Glasgow RE, Fisher L, Dickinson LM, Christensen SM, Estabrooks PA, et al. Use of a website to accomplish health behavior change: if you build it, will they come? And will it work if they do?. Journal of the American Board of Family Medicine 2013;26(2):168‐76. [DOI: 10.3122/jabfm.2013.02.110344] [DOI] [PubMed] [Google Scholar]
Elfeddali 2012 {published data only}
- Elfeddali I, Bolman C, Candel MJ, Wiers RW, Vries H. Preventing smoking relapse via web‐based computer‐tailored feedback: a randomized controlled trial. Journal of Medical Internet Research 2012; Vol. 14, issue 4:e109. [] [DOI] [PMC free article] [PubMed]
- Elfeddali I, Bolman C, Vries H. SQ4U a computer tailored smoking relapse prevention program incorporating planning strategy assignments and multiple feedback time points after the quit‐attempt: development and design protocol. Contemporary Clinical Trials 2012; Vol. 33, issue 1:151‐8. [; 6824] [DOI] [PubMed]
Emmons 2013 {published data only}
- Moor JS, Puleo E, Ford JS, Greenberg M, Hodgson DC, Tyc VL, et al. Disseminating a smoking cessation intervention to childhood and young adult cancer survivors: baseline characteristics and study design of the Partnership for Health‐2 study. BMC Cancer 2011;11(165):1‐9. [; DOI: 10.1186/1471-2407-11-165] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons KM, Puleo E, Sprunck‐Harrild K, Ford J, Ostroff JS, Hodgson D, et al. Partnership for health‐2, a web‐based versus print smoking cessation intervention for childhood and young adult cancer survivors: randomized comparative effectiveness study. Journal of Medical Internet Research 2013;15(11):e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler RH, Puleo E, Sprunck‐Harrild K, Emmons KM. Internet use among childhood and young adult cancer survivors who smoke: implications for cessation interventions. Cancer Causes and Control 2012;23(4):647‐52. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Epton 2014 {published data only}
- Epton T, Norman P, Dadzie AS, Harris PR, Webb TL, Sheeran P, et al. A theory‐based online health behaviour intervention for new university students (U@Uni): results from a randomised controlled trial. BMC Public Health 2014;14:563. [DOI: 10.1186/1471-2458-14-563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2005 {published data only}
- Etter JF. Comparing the efficacy of two internet‐based, computer‐tailored smoking cessation programs: a randomized trial. Journal of Medical Internet Research 2005;7(1):e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fraser 2014 {published data only}
- Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population‐based interventions for smoking cessation: a MOST trial. Translational Behavioral Medicine 2014;4(4):382‐90. [DOI: 10.1007/s13142-014-0278-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frederix 2015 {published data only}
- Frederix I, Hansen D, Coninx K, Vandervoort P, Vandijck D, Hens N, et al. Medium‐term effectiveness of a comprehensive internet‐based and patient‐specific telerehabilitation program with text messaging support for cardiac patients: randomized controlled trial. Journal of Medical Internet Research 2015;17(7):e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2011 {published data only}
- Cobb CO, Graham AL. Use of non‐assigned interventions in a randomized trial of internet and telephone treatment for smoking cessation. Nicotine & Tobacco Research 2014;16(10):1289‐97. [DOI: 10.1093/ntr/ntu066] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb CO, Niaura RS, Donaldson EA, Graham AL. Quit now? Quit soon? Quit when you're ready? Insights about target quit dates for smoking cessation from an online quit date tool. Journal of Medical Internet Research 2014;16(2):e55. [DOI: 10.2196/jmir.3086] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A, Papandonatos GD, Cobb CO, Niaura RS, Abrams DB, Tinkelman DG. Internet and telephone treatment for smoking cessation: mediators and moderators of short‐term abstinence. Nicotine & Tobacco Research 2015;17(3):299‐308. [DOI: 10.1093/ntr/ntu144] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Bock BC, Cobb NK, Niaura R, Abrams DB. Characteristics of smokers reached and recruited to an internet smoking cessation trial: a case of denominators. Nicotine & Tobacco Research 2006;8 Suppl 1:S43‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Chang Y, Fang Y, Cobb CO, Tinkelman DS, Niaura RS, et al. Cost‐effectiveness of internet and telephone treatment for smoking cessation: an economic evaluation of The iQUITT Study. Tobacco Control 2013;22(6):e11. [DOI: 10.1136/tobaccocontrol-2012-050465] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Cobb NK, Papandonatos GD, Moreno JL, Kang H, Tinkelman DG, et al. A randomized trial of internet and telephone treatment for smoking cessation.[Erratum appears in Archives of Internal Medicine 2011 Mar 14;171(5):395]. Archives of Internal Medicine 2011; Vol. 171, issue 1:46‐53. [] [DOI] [PMC free article] [PubMed]
- Graham AL, Papandonatos GD. Reliability of internet‐ versus telephone‐administered questionnaires in a diverse sample of smokers. Journal of Medical Internet Research 2008;10(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Papandonatos GD, Bock BC, Cobb NK, Baskin‐Sommers A, Niaura R, et al. Internet‐ vs. telephone‐administered questionnaires in a randomized trial of smoking cessation. Nicotine & Tobacco Research 2006;8 Suppl 1:S49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Papandonatos GD, Kang H, Moreno JL, Abrams DB. Development and validation of the online social support for smokers scale. Journal of Medical Internet Research 2011; Vol. 13, issue 3:427‐39. [] [DOI] [PMC free article] [PubMed]
- Graham AL, Papandontos GD, Erar B, Stanton CA. Use of an online smoking cessation community promotes abstinence: Results of propensity score weighting. Health Psychology 2015;34:1286‐95. [DOI: 10.1037/hea0000278] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA. Integrating comprehensive tobacco treatment into the evolving US health care system: it's time to act: comment on "A randomized trial of Internet and telephone treatment for smoking cessation". Archives of Internal Medicine 2011; Vol. 171, issue 1:53‐5. [] [DOI] [PubMed]
Harrington 2016 {published data only}
- Harrington KF, Kim YI, Chen M, Ramachandran R, Pisu M, Sadasivam RS, et al. Web‐based intervention for transitioning smokers from inpatient to outpatient care: an RCT. American Journal of Preventive Medicine 2016;51(4):620‐9. [DOI] [PubMed] [Google Scholar]
- Harrington KF, McDougal JA, Pisu M, Zhang B, Sadasivam RS, Houston TK, et al. Web‐based smoking cessation intervention that transitions from inpatient to outpatient: Study protocol for a randomized controlled trial. Trials 2012;13:123. [; DOI: 10.1186/1745-6215-13-123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haug 2011 {published data only}
Herbec 2014 {published data only}
- Herbec A, Brown J, Tombor I, Michie S, West R. Pilot randomized controlled trial of an internet‐based smoking cessation intervention for pregnant smokers ('MumsQuit'). Drug and Alcohol Dependence 2014;140:130‐6. [DOI: 10.1016/j.drugalcdep.2014.04.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Houston 2015 {published data only}
- Houston T, Rajani S, Jeroan A, Arlene A, Midge R, English T, et al. Evaluating the QUIT‐PRIMO clinical practice ePortal to increase smoker engagement with online cessation interventions: a national hybrid type 2 implementation study. Implementation Science 2015;10(1):154. [DOI: 10.1186/s13012-015-0336-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston T, Sadasivam R, Ford D, Richman J, Ray M, Allison J. The QUIT‐PRIMO provider‐patient Internet‐delivered smoking cessation referral intervention: a cluster‐randomized comparative effectiveness trial: study protocol. Implementation Science 2010;5(87):1‐9. [DOI: 10.1186/1748-5908-5-87] [DOI] [PMC free article] [PubMed] [Google Scholar]
Humfleet 2013 {published data only}
- Humfleet G, Hall SM. Using telephone‐based and internet‐based smoking treatments with LGBT smokers: preliminary findings (PA7‐3). Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13‐16 Houston Texas. 2012:26. []
- Humfleet GL, Delucchi K, Kelley K, Hall SM, Dilley J, Harrison G. Characteristics of HIV‐positive cigarette smokers: a sample of smokers facing multiple challenges. AIDS Education & Prevention 2009;21(3 Suppl):54‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humfleet GL, Dilley J, Hall SM, Delucchi KL. Smoking cessation in HIV+ clinical care settings [SYM 10D]. Society for Research on Nicotine & Tobacco 17th Annual Meeting, February 16‐19, Toronto. 2011:13‐4. []
- Humfleet GL, Hall SM, Delucchi KL, Dilley JW. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine & Tobacco Research 2013;15(8):1436‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Japuntich 2006 {published data only}
- Japuntich SJ, Zehner ME, Smith SS, Jorenby DE, Valdez JA, Fiore MC, et al. Smoking cessation via the internet: a randomized clinical trial of an internet intervention as adjuvant treatment in a smoking cessation intervention. Nicotine & Tobacco Research 2006;8 Suppl 1:S59‐67. [DOI] [PubMed] [Google Scholar]
Mananes 2014 {published data only}
- Mananes G, Vallejo MA. Usage and effectiveness of a fully automated, open‐access, Spanish web‐based smoking cessation program: randomized controlled trial. Journal of Medical Internet Research 2014;16(4):e111. [DOI: 10.2196/jmir.3091] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2012 {published data only}
Mavrot 2016 {published data only}
- Mavrot C, Stucki I, Sager F, Etter JF. Efficacy of an internet‐based, individually tailored smoking cessation program: A randomized trial. Journal of Telemedicine and Telecare 2016;23(5):521‐8. [DOI: 10.1177/1357633X16655476] [DOI] [PubMed] [Google Scholar]
McClure 2014 {published data only}
- McClure JB, Derry H, Riggs KR, Westbrook EW, John JS, Shortreed SM, et al. Questions About Quitting (Q2): design and methods of a multiphase optimization strategy (MOST) randomized screening experiment for an online, motivational smoking cessation intervention. Contemporary Clinical Trials 2012;33(5):1094‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Derry H, Riggs KR, Westbrook EW, Johna J, Shortreed SM, et al. Corrigendum to “Questions About Quitting (Q2): Design and Methods of a Multiphase Optimization Strategy (MOST) Randomized Screening Experiment for an Online, Motivational Smoking Cessation Intervention” [Contemp Clin Trials 33/5 (2012) 1094–1102]. Contemporary Clinical Trials 2013;33(1):179‐80. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Peterson D, Derry H, Riggs KR, Saint‐Johnson BS, Nair V, et al. Exploring the "active ingredients" of an online smoking intervention: a randomized factorial trial. Nicotine & Tobacco Research 2014;16(8):1129‐39. [DOI: 10.1093/ntr/ntu057] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure, JB, Shortreed SM, Bogart A, Derry H, Riggs K, John J, et al. The effect of program design on engagement with an internet‐based smoking intervention: randomized factorial trial. Journal of Medical Internet Research 2013;15(3):E69. [DOI: 10.2196/jmir.2508] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortreed SM, Bogart A, McClure JB, Derry H. Using multiple imputations to accommodate time‐outs in online interventions. Journal of Medical Internet Research 2013;15(11):e252.1‐8. [DOI: 10.2196/jmir.2781] [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2016 {published data only}
- McClure JB, Anderson ML, Bradley K, An LC, Catz SL. Evaluating an adaptive and interactive mHealth smoking cessation and medication adherence program: a randomized pilot feasibility study. JMIR mHealth and uHealth 2016;4(3):e94. [DOI: 10.2196/mhealth.6002] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonnell 2011 {published data only}
- McDonnell DD, Kazinets G, Lee HJ, Moskowitz JM. An internet‐based smoking cessation program for Korean Americans: results from a randomized controlled trial. Nicotine & Tobacco Research 2011; Vol. 13, issue 5:336‐43. [] [DOI] [PubMed]
- McDonnell DD, Lee HJ, Kazinets G, Moskowitz JM. Online recruitment of targeted populations: lessons learned from a smoking cessation study among Korean Americans. Social Marketing Quarterly 2010; Vol. 16, issue 3:2‐22. []
McKay 2008 {published data only}
- Danaher BG, Hart LG, McKay HG, Severson HH. Measuring participant rurality in web‐based interventions. BMC Public Health 2007;7:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaher BG, Lichtenstein E, McKay HG, Seeley JR. Use of non‐assigned smoking cessation programs among participants of a web‐based randomized controlled trial. Journal of Medical Internet Research 2009;11(2):e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay HG, Danaher BG, Seeley JR, Lichtenstein E, Gau JM. Comparing two web‐based smoking cessation programs: randomized controlled trial. Journal of Medical Internet Research 2008;10(5):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehring 2014 {published data only}
- Mehring M, Haag M, Linde K, Wagenpfeil S, Schneider A. Effects of a guided web‐based smoking cessation program with telephone counseling: a cluster randomized controlled trial. Journal of Medical Internet Research 2014;16(9):e218. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moskowitz 2016 {published data only}
- Moskowitz JM, McDonnell DD, Kazinets G, Lee H‐J. Online smoking cessation program for Korean Americans: randomized trial to test effects of incentives for program completion and interim surveys. Preventive Medicine 2016;86:70‐6. [DOI] [PubMed] [Google Scholar]
Muñoz 2006 Study 3 {published data only}
- Lenert L, Muñoz RF, Stoddard J, Delucchi K, Bansod A, Skoczen S, et al. Design and pilot evaluation of an internet smoking cessation program. Journal of the American Medical Informatics Association 2003;10(1):16‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz RF, Lenert LL, Delucchi K, Stoddard J, Perez JE, Penilla C, et al. Toward evidence‐based internet interventions: a Spanish/English web site for international smoking cessation trials. Nicotine & Tobacco Research 2006;8(1):77‐87. [DOI] [PubMed] [Google Scholar]
Muñoz 2006 Study 4 {published data only}
- Muñoz RF, Lenert LL, Delucchi K, Stoddard J, Perez JE, Penilla C, et al. Toward evidence‐based internet interventions: a Spanish/English web site for international smoking cessation trials. Nicotine & Tobacco Research 2006;8(1):77‐87. [DOI] [PubMed] [Google Scholar]
Muñoz 2009 {published data only}
- Muñoz RF, Barrera AZ, Delucchi K, Penilla C, Torres LD, Perez‐Stable EJ. International Spanish/English internet smoking cessation trial yields 20% abstinence rates at 1 year. Nicotine & Tobacco Research 2009;11(9):1025‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres LD, Barrera AZ, Delucchi K, Penilla C, Perez‐Stable EJ, Muñoz RF. Quitting smoking does not increase the risk of major depressive episodes among users of internet smoking cessation interventions. Psychological Medicine 2010; Vol. 40, issue 3:441‐9. [] [DOI] [PMC free article] [PubMed]
Oenema 2008 {published data only}
- Oenema A, Brug J, Dijkstra A, De Weerdt I, De Vries H. Efficacy and use of an internet‐delivered computer‐tailored lifestyle intervention, targeting saturated fat intake, physical activity and smoking cessation: a randomized controlled trial. Annals of Behavioural Medicine 2008;35(2):125‐35. [DOI] [PubMed] [Google Scholar]
Patten 2006 {published data only}
- Patten CA, Croghan IT, Meis TM, Decker PA, Pingree S, Colligan RC, et al. Randomized clinical trial of an internet‐based versus brief office intervention for adolescent smoking cessation. Patient Education and Counseling 2006;64(1‐3):249‐58. [DOI] [PubMed] [Google Scholar]
- Patten CA, Rock E, Meis TM, Decker PA, Colligan RC, Pingree S, et al. Frequency and type of use of a home‐based, internet intervention for adolescent smoking cessation. Journal of Adolescent Health 2007;41(5):427‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pechmann 2016 {published data only}
- Pechmann C, Delucchi K, Lakon C, Prochaska JJ. Randomised controlled trial evaluation of Tweet2Quit: a social network quit‐smoking intervention. BMJ Tobacco Control 2017;26:188‐194. [DOI: 10.1136/tobaccocontrol-2015-052768] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechmann C, Pan L, Delucchi K, Lakon CM, Prochaska JJ. Development of a Twitter‐based intervention for smoking cessation that encourages high‐quality social media interactions via automessages. Journal of Medical Internet Research 2015;17(2):e(50). [DOI: 10.2196/jmir.3772] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Pechmann C, Lakon C, Delucchi K. Randomized controlled trial of tweet2quit for smoking cessation. Society for Research on Nicotine and Tobacco 21st Annual Meeting Philadelphia. February 25‐28, 2015; Vol. 82:PA21‐2.
Rabius 2008 {published data only}
- Pike KJ, Rabius V, McAlister A, Geiger A. American Cancer Society's QuitLink: randomized trial of internet assistance. Nicotine & Tobacco Research 2007;9(3):415‐20. [DOI] [PubMed] [Google Scholar]
- Rabius V, Pike J, Geiger A, Hunter J, McAlister A. The American Cancer Society's Quitlink: A randomized clinical trial evaluating internet‐based smoking cessation programs and medication use (RPOS 3‐62). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
- Rabius V, Pike KJ, Wiatrek D, McAlister AL. Comparing internet assistance for smoking cessation: 13‐month follow‐up of a six‐arm randomized controlled trial. Journal of Medical Internet Research 2008;10(5):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman DF, Westmaas JL, Goldband S, Rabius V, Katkin ES, Pike KJ, et al. Randomized controlled trial of an interactive internet smoking cessation program with long‐term follow‐up. Annals of Behavioral Medicine 2010; Vol. 39, issue 1:48‐60. [] [DOI] [PubMed]
Schulz 2014 {published data only}
- Schulz DN, Kremers SPJ, Vandelanotte C, Adrichem MJG, Schneide F, Candel MJM, et al. Economic evaluation of a web‐based tailored lifestyle intervention for adults: findings regarding cost‐effectiveness and cost‐utility from a randomized controlled trial. Journal of Medical Internet Research 2014;16(3):e91. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz DN, Kremers SPJ, Vandelanotte C, Adrichem MJG, Schneide F, Candel MJM, et al. Effects of a web‐based tailored multiple‐lifestyle intervention for adults: a two‐year randomized controlled trial comparing sequential and simultaneous delivery modes. Journal of Medical Internet Research 2014;16(1):e26.1‐18. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shuter 2014 {published data only}
- Shuter J, Morales DA, Considine‐Dunn SE, An LC, Stanton CA. Feasibility and preliminary efficacy of a web‐based smoking cessation intervention for HIV‐infected smokers: a randomized controlled trial. Journal of Acquired Immune Deficiency Syndromes 2014;67(1):59‐66. [DOI: 10.1097/QAI.0000000000000226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 2011 {published data only}
- Simmons VN, Heckman B, Fink AC, Patel R, Bello L, Brandon TH. Efficacy of an experiential, web‐based smoking intervention for college smokers [PA2‐2]. Society for Research on Nicotine and Tobacco 17th Annual Meeting, February 16‐19, Toronto. 2011. []
- Simmons VN, Heckman BW, Fink AC, Small BJ, Brandon TH. Efficacy of an experiential, dissonance‐based smoking intervention for college students delivered via the internet. Journal of Consulting and Clinical Psychology 2013;81(5):810‐20. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skov‐Ettrup 2016 {published data only}
- Skov‐Ettrup LS, Dalum P, Bech M, Tolstrup JS. The effectiveness of telephone counselling and internet‐ and text‐message‐based support for smoking cessation: results from a randomized controlled trial. Addiction 2016;111(7):1257‐66. [DOI] [PubMed] [Google Scholar]
- Skov‐Ettrup LS, Dalum P, Ekholm O, Tolstrup JS. Reach and uptake of Internet‐ and phone‐based smoking cessation interventions: results from a randomized controlled trial. Preventive Medicine 2014;62:38‐43. [doi: 10.1016/j.ypmed.2014.01.020] [DOI] [PubMed] [Google Scholar]
- Skov‐Ettrup LS, Dalum P, Tolstrup JS. Internet‐based intervention and telephone counselling for smoking cessation: Results from a 4‐arm randomised controlled trial. European Journal of Epidemiology 2013;28(1 Supp 1):S48‐S49. [Google Scholar]
Smit 2016 {published data only}
- Smit ES, Vries H, Hoving C. Effectiveness of a web‐based multiple tailored smoking cessation program: a randomized controlled trial among Dutch adult smokers. Journal of Medical Internet Research 2012;14(3):e82. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit ES, Vries H, Hoving C. Results of the PAS study: a randomized controlled trial evaluating the effectiveness of a web‐based multiple tailored smoking cessation program combined with tailored counseling by practice nurses. Health Communication 2016;31(9):1165‐73. [DOI: 10.1016/j.cct.2010.03.001; Dutch Trial Register: NTR1351] [DOI] [PubMed] [Google Scholar]
- Smit ES, Vries H, Hoving C. The PAS study: a randomized controlled trial evaluating the effectiveness of a web‐based multiple tailored smoking cessation programme and tailored counselling by practice nurses. Contemporary Clinical Trials 2010;31(3):251‐8. [] [DOI] [PubMed] [Google Scholar]
- Smit ES, Evers SM, Vries H, Hoving C. Cost‐effectiveness and cost‐utility of Internet‐based computer tailoring for smoking cessation. Journal of Medical Internet Research 2013;15(3):55‐70. [DOI: 10.2196/jmir.2059] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit ES, Hoving C, Cox VC, Vries H. Influence of recruitment strategy on the reach and effect of a web‐based multiple tailored smoking cessation intervention among Dutch adult smokers. Health Education Research 2012; Vol. 27, issue 2:191‐9. [] [DOI] [PubMed]
Stanczyk 2014 {published data only}
- Stanczyk NE, Bolman C, Muris JW, Vries H. Study protocol of a Dutch smoking cessation e‐health program. BMC Public Health 2011; Vol. 11:847. [] [DOI] [PMC free article] [PubMed]
- Stanczyk NE, Bolman C, Smit ES, Candel MJ, Muris JW, Vries H. How to encourage smokers to participate in web‐based computer‐tailored smoking cessation programs: a comparison of different recruitment strategies. Health Education Research 2014;29(1):23‐40. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Stanczyk NE, Crutzen R, Bolman C, Muris J, Vries H. An economic evaluation of a video‐ and text‐based computer‐tailored intervention for smoking cessation: a cost‐effectiveness and cost‐utility analysis of a randomized controlled trial. PloS One 2014;9(10):e110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk NE, Crutzen R, Bolman C, Muris J, Vries H. Comparison of text and video computer‐tailored interventions for smoking cessation: randomized controlled trial. Journal of Medical Internet Research 2014;16(3):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanczyk NE, Crutzen R, Bolman C, Muris J, Vries H. Influence of delivery strategy on message‐processing mechanisms and future adherence to a Dutch computer‐tailored smoking cessation intervention. Journal of Medical Internet Research 2013;15(2):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoddard 2008 {published data only}
- Stoddard J, Delucchi K, Muñoz R, Collins N, Stable EP, Augustson E, et al. Smoking cessation research via the internet: a feasibility study. Journal of Health Communication 2005;10(1):27‐41. [DOI] [PubMed] [Google Scholar]
- Stoddard JL, Augustson EM, Moser RP. Effect of adding a virtual community (bulletin board) to smokefree.gov: randomized controlled trial. Journal of Medical Internet Research 2008;10(5):e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strecher 2005 {published data only}
- Strecher VJ, Shiffman S, West R. Moderators and mediators of a web‐based computer‐tailored smoking cessation program among nicotine patch users. Nicotine & Tobacco Research 2006;8 Suppl 1:S95‐101. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, Shiffman S, West R. Randomized controlled trial of a web‐based computer‐tailored smoking cessation program as a supplement to nicotine patch therapy. Addiction 2005;100(5):682‐8. [DOI] [PubMed] [Google Scholar]
Strecher 2008 {published data only}
- McClure JB, Greene SM, Wiese C, Johnson KE, Alexander G, Strecher V. Interest in an online smoking cessation program and effective recruitment strategies: results from Project Quit. Journal of Medical Internet Research 2006;8(3):e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecher VJ, McClure JB, Alexander GL, Chakraborty B, Nair VN, Konkel JM, et al. Web‐based smoking‐cessation programs: results of a randomized trial. American Journal of Preventive Medicine 2008;34(5):373‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swan 2010 {published data only}
- Javitz HS, Zbikowski SM, Deprey M, McAfee TA, McClure JB, Richards J, et al. Cost‐effectiveness of varenicline and three different behavioral treatment formats for smoking cessation. Translational Behavioral Medicine 2011;1(1):182‐90. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure JB, Jack L, Deprey M, Catz S, McAfee T, Zbikowski S, et al. Canary in a coal mine? Interest in bupropion SR use among smokers in the COMPASS trial. Nicotine & Tobacco Research 2008;10(12):1815‐6. [DOI] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Jack L, Catz SL, Zbikowski SM, McAfee TA, et al. Mood, side‐effects and smoking outcomes among persons with and without probable lifetime depression taking varenicline. Journal of General Internal Medicine 2009;24(5):563‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine 2010;38(5):482‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbikowski SM, Jack LM, McClure JB, Deprey M, Javitz HS, Mcafee TA, et al. Utilization of services in a randomized trial testing phone‐ and web‐based interventions for smoking cessation. Nicotine & Tobacco Research 2011;13(5):319‐27. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swartz 2006 {published data only}
- Swartz LHG, Noell JW, Schroeder SW, Ary DV. A randomised control study of a fully automated internet based smoking cessation programme. Tobacco Control 2006;15(1):7‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Te Poel 2009 {published data only}
- Poel F, Bolman C, Reubsaet A, Vries H. Efficacy of single computer‐tailored e‐mail for smoking cessation: results after 6 months. Health Education Research 2009;24(6):930‐40. [DOI] [PubMed] [Google Scholar]
Voncken‐Brewster 2015 {published data only}
- Voncken‐Brewster V, Tange H, Vries H, Nagykaldi Z, Winkens B, Weijden T. A randomised controlled trial testing a web‐based, computer‐tailored self‐management intervention for people with or at risk for chronic obstructive pulmonary disease: a study protocol. BMC Public Health 2013;13(1):1. [DOI: 10.1186/1471-2458-13-557] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voncken‐Brewster V, Tange H, Vries H, Nagykaldi Z, Winkens B, Weijden T. A randomized controlled trial evaluating the effectiveness of a web‐based, computer‐tailored self‐management intervention for people with or at risk for COPD. International Journal of Chronic Obstructive Pulmonary Disease 2015;10:1061‐73. [DOI: 10.2147/COPD.S81295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wangberg 2011 {published data only}
- Wangberg SC, Nilsen O, Antypas K, Gram IT. Effect of tailoring in an internet‐based intervention for smoking cessation: randomized controlled trial. Journal of Medical Internet Research. Canada, 2011; Vol. 13, issue 4:e121. [] [DOI] [PMC free article] [PubMed]
Wittekind 2015 {published data only}
- Wittekind CE, Feist A, Schneider BC, Moritz S, Fritzsche A. The approach‐avoidance task as an online intervention in cigarette smoking: A pilot study. Journal of Behavior Therapy and Experimental Psychiatry 2015;46:115‐20. [DOI] [PubMed] [Google Scholar]
Woodruff 2007 {published data only}
- Woodruff SI, Conway TL, Edwards CC. Sociodemographic and smoking‐related psychosocial predictors of smoking behavior change among high school smokers. Addictive Behaviors 2008;33(2):354‐8. [DOI] [PubMed] [Google Scholar]
- Woodruff SI, Conway TL, Edwards CC, Elliott SP, Crittenden J. Evaluation of an Internet virtual world chat room for adolescent smoking cessation. Addictive Behaviors 2007;32(9):1769‐86. [DOI] [PubMed] [Google Scholar]
Yang 2016 {published data only}
- Yang DX, Gu CJ, Ni L, Li N, Li QY, Zhou JP. Assessment of efficacy of medication combined with WeChat platform for quitting smoking in patients with chronic obstructive pulmonary disease.. Journal of Shanghai Jiaotong University (Medical Science) 2016;36(3):385‐9. [Google Scholar]
Zullig 2014 {published and unpublished data}
- Zullig LL, Sanders LL, Shaw RJ, McCant F, Danus S, Bosworth HB. A randomised controlled trial of providing personalised cardiovascular risk information to modify health behaviour. Journal of Telemedicine and Telecare 2014;20(3):147‐52. [DOI: 10.1177/1357633X14528446] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abroms 2008 {published data only}
- Abroms LC, Gill J, Windsor R, Simons‐Morton B. A process evaluation of e‐mail counselling for smoking cessation in college students: feasibility, acceptability and cost. Journal of Smoking Cessation 2009;4:26‐33. [Google Scholar]
- Abroms LC, Windsor R, Simons‐Morton B. Getting young adults to quit smoking: a formative evaluation of the X‐Pack Program. Nicotine & Tobacco Research 2008;10(1):27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
An 2007 {published data only}
- An LC, Hennrikus DJ, Perry CL, Lein EB, Klatt C, Farley DM, et al. Feasibility of internet health screening to recruit college students to an online smoking cessation intervention. Nicotine & Tobacco Research 2007;9 Suppl 1:S11‐8. [DOI] [PubMed] [Google Scholar]
Applegate 2007 {published data only}
- Applegate BW, Raymond C, Collado‐Rodriguez A, Riley WT, Schneider NG. Improving adherence to nicotine gum by SMS text messaging: a pilot study (RPOS3‐57). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007.
Baskerville 2015 {published data only}
- Baskerville NB, Struik LL, Hammond D, Guindon GE, Norman CD, Whittaker R, et al. Effect of a mobile phone intervention on quitting smoking in a young adult population of smokers: randomized controlled trial study protocol. JMIR Research Protocols 2015;4(1):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2012 {published data only}
- Bowen DJ, Henderson PN, Harvill J, Buchwald D. Short‐term effects of a smoking prevention website in American Indian youth. Journal of Medical Internet Research 2012; Vol. 14, issue 3:e81. [] [DOI] [PMC free article] [PubMed]
Bravin 2015 {published data only}
- Bravin JI, Bunge EL, Evare B, Wickham RE, Perez‐Stable EJ, Muñoz RF. Socioeconomic predictors of smoking cessation in a worldwide online smoking cessation trial.. Internet Interventions 2015;2(4):410‐8. [Google Scholar]
Buller 2008 {published data only}
- Buller DB, Young WF, Fisher KH, Maloy JA. The effect of endorsement by local opinion leaders and testimonials from teachers on the dissemination of a web‐based smoking prevention program. Health Education Research 2007;22(5):609‐18. [DOI] [PubMed] [Google Scholar]
Buller 2014a {published data only}
- Buller DB, Borland R, Woodall WG, Hall JR, Hines JM, Burris‐Woodall P, et al. Randomized trials on consider this, a tailored, internet‐delivered smoking prevention program for adolescents. Health Education and Behavior 2008;35(2):260‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buller 2014b {published data only}
- Buller DB, Borland Rk Bettinghaus EP, Shane JH, Zimmerman DE. Randomized trial of a smartphone mobile application compared to text messaging to support smoking cessation. Telemedicine Journal and E‐health 2014;20(3):206‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calabro 2011 {published data only}
- Calabro KS, Marani S, Yost T, Segura J, Mullin‐Jones M, Nelson S, et al. Project SUCCESS: Results from a randomized controlled trial. ISRN Public Health 2012;2012:Article ID 913713. [Google Scholar]
- Prokhorov AV, Marani S, Yost T, Luca M, Mullen M. Project SUCCESS: students using computerized coaching to end smoking successfully [PA2‐3]. Society for Research on Nicotine and Tobacco 17th Annual Meeting, February 16‐19, Toronto. 2011:18. []
Chen 2006 {published data only}
- Chen HH, Yeh ML. Developing and evaluating a smoking cessation program combined with an internet‐assisted instruction program for adolescents with smoking. Patient Education and Counseling 2006;61(3):411‐8. [DOI] [PubMed] [Google Scholar]
Chew 2005 {published data only}
- Chew F, Palmer S. Establishing an internet‐based tobacco‐control network for Czech health professionals. Health Promotion Practice 2005;6(1):109‐16. [DOI] [PubMed] [Google Scholar]
Christoff 2015 {published data only}
- Christoff AO, Boerngen‐Lacerda R. Reducing substance involvement in college students: a three‐arm parallel‐group randomized controlled trial of a computer‐based intervention. Addictive Behaviors 2015;45:164‐171. [DOI] [PubMed] [Google Scholar]
Cobb 2005 {published data only}
- Cobb NK, Graham AL, Bock BC, Papandonatos G, Abrams DB. Initial evaluation of a real‐world internet smoking cessation system. Nicotine & Tobacco Research 2005;7(2):207‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cobb 2006 {published data only}
- Cobb NK, Graham AL. Characterizing Internet searchers of smoking cessation information. Journal of Medical Internet Research 2006;8(3):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dallery 2013 {published data only}
- Dallery J, Raiff BR, Grabinski MJ. Internet‐based contingency management to promote smoking cessation: a randomized controlled study. Journal of Applied Behavior Analysis 2013;46(4):750‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danaher 2006 {published data only}
- Danaher BG, McKay HG, Seeley JR. The information architecture of behavior change websites. Journal of Medical Internet Research 2005;7(2):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danaher 2011 {published data only}
- Danaher BG, Severson HH, Zhu S‐H, Lichtenstein E, Andrews JA, Yearick C. Evaluating the relative efficacy of web‐based intervention and helpline in smokeless tobacco cessation: the CHEWFREE II RCT [SM 12C]. Society for Research on Nicotine & Tobacco 17th Annual Meeting, February 16‐19, Toronto. 2011:15. [; 9400123000006079]
Etter 2006a {published data only}
- Etter JF. Internet‐based smoking cessation programs. International Journal of Medical Informatics 2006;75(1):110‐6. [DOI] [PubMed] [Google Scholar]
Etter 2009a {published data only}
- Etter JF, Huguelet P, Perneger TV, Cornuz J. Nicotine gum treatment before smoking cessation: a randomized trial. Archives of Internal Medicine 2009;169(11):1028‐34. [DOI] [PubMed] [Google Scholar]
Etter 2009b {published data only}
- Etter JF. Comparing computer‐tailored, internet based smoking cessation counseling reports with generic, untailored reports: a randomized trial. Journal of Health Communication 2009;14(7):646‐57. [DOI] [PubMed] [Google Scholar]
Feil 2003 {published data only}
- Feil EG, Noell J, Lichtenstein E, Boles SM, McKay HG. Evaluation of an internet‐based smoking cessation program: lessons learned from a pilot study. Nicotine & Tobacco Research 2003;5(2):189‐94. [DOI] [PubMed] [Google Scholar]
Gala 2008 {published data only}
- Gala S, Dobbs S, Murray J, Pesek F, Kavanagh C, Ellison J, et al. Internet‐based spit tobacco (ST) cessation study. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March; Prague, Czech Republic. 2005.
- Gala S, Pesek F, Murray J, Kavanagh C, Graham S, Walsh M. Design and pilot evaluation of an internet spit tobacco cessation program. Journal of Dental Hygiene 2008;82(1):11. [PubMed] [Google Scholar]
Gillaspy 2010 {published data only}
- Gillaspy S, Mignogna M, Mignogna J, Zaitshik J, Bright B, Leffingwell T, et al. Development & testing of an interactive web‐based program to facilitate readiness & motivation for smoking cessation. Journal of Investigative Medicine 2010;58(3):440. [] [Google Scholar]
Haug 2014 {published data only}
- Haug S, Castro RP, Filler A, Kowatsch T, Fleisch E, Schaub MP. Efficacy of an Internet and SMS‐based integrated smoking cessation and alcohol intervention for smoking cessation in young people: study protocol of a two‐arm cluster randomised controlled trial. BMC Public Health 2014;14:1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Houston 2005 {published data only}
- Houston T, Ford DE. Smoking cessation websites as an approach to increasing physician visits for smoking cessation. Journal of General Internal Medicine 2005;20(Suppl 1):190. [Google Scholar]
Houston 2008a {published data only}
- Houston TK, Richman JS, Ray MN, Allison JJ, Gilbert GH, Shewchuk RM, et al. Internet delivered support for tobacco control in dental practice: randomized controlled trial. Journal of Medical Internet Research 2008;10(5):e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Houston 2008b {published data only}
- Houston TK, Ford DE. A tailored Internet‐delivered intervention for smoking cessation designed to encourage social support and treatment seeking: usability testing and user tracing. Informatics for Health and Social Care 2008;33(1):5‐19. [DOI] [PubMed] [Google Scholar]
Houston 2013 {published data only}
- Houston TK, Delaughter KL, Ray MN, Gilbert GH, Allison JJ, Kiefe CI, et al. Cluster‐randomized trial of a web‐assisted tobacco quality improvement intervention of subsequent patient tobacco product use: a National Dental PBRN study. BMC Oral Health 2013;13:13. [DOI: 10.1186/1472-6832-13-13] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2011 {published data only}
- Claes N, Jacobs N, Clays E, Schrooten W. Effectiveness of cardiovascular prevention programs in primary care (PreCardio): A randomised clinical trial. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(1 Suppl 1):S102. [] [Google Scholar]
- Jacobs N, Clays E, Bacquer D, Backer G, Dendale P, Thijs H, et al. Effect of a tailored behavior change program on a composite lifestyle change score: a randomized controlled trial. Health Education Research 2011; Vol. 26, issue 5:886‐95. [] [DOI] [PubMed]
- Jacobs N, Bourdeaudhuij I, Thijs H, Dendale P, Claes N. Effect of a cardiovascular prevention program on health behavior and BMI in highly educated adults: a randomized controlled trial. Patient Education and Counseling 2011; Vol. 85, issue 1:122‐6. [] [DOI] [PubMed]
- Jacobs N, Drost R, Ament A, Evers S, Claes N. Willingness to pay for a cardiovascular prevention program in highly educated adults: a randomized controlled trial. International Journal of Technology Assessment in Health Care. United States: Cambridge University Press (40 West 20th Street, New York NY 10011‐4211, United States), 2011; Vol. 27, issue 4:283‐9. [] [DOI] [PubMed]
Koo 2003 {published data only}
- Koo M, Skinner H. Improving web searches: case study of quit‐smoking web sites for teenagers. Journal of Medical Internet Research 2003;5(4):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koo 2005 {published data only}
- Koo M, Skinner H. Challenges of internet recruitment: a case study with disappointing results. Journal of Medical Internet Research 2005;7(1):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lenert 2004 {published data only}
- Lenert L, Muñoz RF, Perez JE, Bansod A. Automated e‐mail messaging as a tool for improving quit rates in an internet smoking cessation intervention. Journal of the American Medical Informatics Association 2004;11(4):235‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linke 2012 {published data only}
- Linke SE. Intermittent exercise in response to nicotine cravings in the context of an internet‐based smoking cessation program. Dissertation Abstracts International 2012;72(11‐B):7054. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke SE, Rutledge T, Myers MG. Intermittent exercise in response to cigarette cravings in the context of an internet‐based smoking cessation program. Mental Health and Physical Activity 2012;5(1):85‐92. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mermelstein 2006 {published data only}
- Mermelstein R, Turner L. Web‐based support as an adjunct to group‐based smoking cessation for adolescents. Nicotine & Tobacco Research 2006;8 Suppl 1:S69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muñoz 2012a {published data only}
- Muñoz RF, Aguilera A, Schueller SM, Leykin Y, Perez‐Stable EJ. From online randomized controlled trials to participant preference studies: morphing the San Francisco Stop Smoking site into a worldwide smoking cessation resource. Journal of Medical Internet Research 2012;14(3):e64. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muñoz 2016 {published data only}
- Muñoz RF, Bunge E, Chen K, Schueller S, Bravin JI, Shaughnessy EA, et al. Massive open online interventions: a novel model for delivering behavioral‐health services worldwide. Clinical Psychological Science 2016;4(2):194‐205. [Google Scholar]
Muramoto 2007 {published data only}
- Muramoto M, Campbell J, Connolly T, Aickin M. Project Reach: training community‐based "health influencers" to help tobacco users quit (POS2‐122). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007.
- Muramoto M, Connolly T, Matthews E, Yuan NP, Mack J. Development of a client‐centered tobacco brief intervention training for health influencers. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March; Prague, Czech Republic. 2005.
Mussulman 2014 {published data only}
- Mussulman L, Ellerbeck EF, Cupertino AP, Preacher KJ, Spaulding R, Catley D, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. Journal of Medical Internet Research 2015;17(5):e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussulman L, Ellerbeck EF, Cupertino AP, Preacher KJ, Spaulding R, Catley D, et al. Design and participant characteristics of a randomized‐controlled trial of telemedicine for smoking cessation among rural smokers. Contemporary Clinical Trials 2014;38(2):173‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naughton 2014 {published data only}
- Naughton F, Jamison J, Boase S, Sloan M, Gilbert H, Prevost AT, et al. Randomized controlled trial to assess the short‐term effectiveness of tailored web‐ and text‐based facilitation of smoking cessation in primary care. Addiction 2014;109(7):1184‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton F, Jamison J, Boase S, Sloan M, Gilbert H, Prevost AT, et al. Study protocol for iQuit in Practice: a randomised controlled trial to assess the feasibility, acceptability and effectiveness of tailored web‐ and text‐based facilitation of smoking cessation in primary care. BMC Public Health 2013;13:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00865553 {unpublished data only}
- NCT00865553. Computer‐assisted stop‐smoking program in helping doctors counsel patients who smoke cigarettes. clinicaltrials.gov/ct2/show/record/NCT00865553 (first received 9 March 2009).
NCT01980017 {published data only}
- NCT01980017. Evaluation of the Ottawa Model for Smoking Cessation in Diabetes Education Programs (OMSC in DEP). clinicaltrials.gov/ct2/show/NCT01980017 (first received 1 November 2013).
NCT02046408 {published data only}
- NCT02046408. Computer‐facilitated 5A's for smoking cessation in primary care. clinicaltrials.gov/ct2/show/NCT02046408 (first received 23 January 2014). [clinicaltrials.gov: NCT02046408]
NCT02103829 {unpublished data only}
- NCT02103829. "Quit Smoking on Your Own" Brief Online Study (TC‐PAU‐1). clinicaltrials.gov/ct2/show/NCT02103829 (first received 1 April 2014).
Norman 2004 {published data only}
- Norman C. CATCH‐IT report: evaluation of an internet‐based smoking cessation program: lessons learned from a pilot study. Journal of Medical Internet Research 2004;6(4):8‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Norman 2008 {published data only}
- Norman CD, Maley O, Li X, Skinner HA. Using the internet to assist smoking prevention and cessation in schools: a randomized, controlled trial. Health Psychology 2008;27(6):799‐810. [DOI] [PubMed] [Google Scholar]
- Skinner H, Maley O, Norman C. Website intervention for youth smoking prevention: findings from school‐based randomized controlled trial. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
Ota 2005 {published data only}
- Ota A, Takahashi Y. Factors associated with successful smoking cessation among participants in a smoking cessation program involving use of the internet, e‐mails, and mailing‐list. Nippon Koshu Eisei Zasshi [Japanese Journal of Public Health] 2005;52(11):999‐1005. [PubMed] [Google Scholar]
Pederson 2005 {published data only}
- Pederson LL, Blumenthal D. Smoking cessation: what works in primary care settings. Ethnicity and Disease 2005;15(2 Suppl 2):S10‐3. [PubMed] [Google Scholar]
Pisinger 2010 {published data only}
- Pisinger C, Jorgensen MM, Moller NE, Dossing M, Jorgensen T. A cluster randomized trial in general practice with referral to a group‐based or an internet‐based smoking cessation programme. Journal of Public Health 2010;32(1):62‐70. [DOI] [PubMed] [Google Scholar]
Prochaska 2001 {published data only}
- Prochaska JO, Velicer WF, Fava JL, Ruggiero L, Laforge RG, Rossi JS, et al. Counselor and stimulus control enhancements of a stage‐matched expert system intervention for smokers in a managed care setting. Preventive Medicine 2001;32(1):23‐32. [DOI] [PubMed] [Google Scholar]
Prochaska 2008 {published data only (unpublished sought but not used)}
- Prochaska JO, Butterworth S, Redding CA, Burden V, Perrin N, Leo M, et al. Initial efficacy of MI, TTM tailoring and HRI's with multiple behaviors for employee health promotion. Preventive Medicine 2008;46(3):226‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prokhorov 2008 {published data only}
- Prokhorov AV, Yost T, Mullin‐Jones M, Moor C, Ford KH, Marani S, et al. "Look At Your Health": outcomes associated with a computer‐assisted smoking cessation counseling intervention for community college students. Addictive Behaviors 2008;33(6):757‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 2014 {published data only}
- Coley HL, Sadasivam RS, Williams JH, Volkman JE, Schoenberger YM, Kohler CL, et al. Crowdsourced peer‐ versus expert‐written smoking‐cessation messages. American Journal of Preventive Medicine 2013;45(5):543‐50. [DOI: 10.1016/j.amepre.2013.07.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray MN. Predictors of smoking cessation among dental patients participating in a web‐assisted tobacco intervention: A national dental practice‐based research network study. Dissertation Abstracts International 2014;76(5‐B‐E):1‐119. [Google Scholar]
- Ray MN, Funkhouser E, Williams JH, Sadasivam RS, Gilbert GH, Coley HL, et al. Smoking‐cessation e‐referrals: a national dental practice‐based research network randomized controlled trial. American Journal of Preventive Medicine 2014;46(2):158‐65. [DOI: 10.1016/j.amepre.2013.10.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitzel 2011 {published data only}
- Reitzel LR, McClure JB, Cofta‐Woerpel L, Mazas CA, Cao Y, Cinciripini PM, et al. The efficacy of computer‐delivered treatment for smoking cessation. Cancer Epidemiology, Biomarkers and Prevention 2011;20(7):1555‐7. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowan 2007 {published data only}
- Rowan PJ, Cofta‐Woerpel L, Mazas CA, Cinciripini PM, Jones LA, Li Y, et al. Neighborhood social context independently predicts tobacco dependence among African American smokers (POS2‐135). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007.
Schneider 1990 {published data only}
- Schneider SJ, Walter R, O’Donnell R. Computerized communication as a medium for behavioral smoking cessation treatment: controlled evaluation. Computers in Human Behavior 1990;6:141‐51. [Google Scholar]
Selby 2004 {published data only}
- Selby P, McIntosh S, Bock B, Graham A, McDonald S. Web‐assisted tobacco interventions (WATI): potential, promotion and pitfalls (SYM6). Society for Research on Nicotine and Tobacco 10th Annual Meeting February 18‐21, Phoenix, Arizona. 2004.
Severson 2008 {published data only}
- Danaher BG, Smolkowski K, Seeley JR, Severson HH. Mediators of a successful web‐based smokeless tobacco cessation program. Addiction 2008;103(10):1706‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JS, Akers L, Severson HH, Danaher BG, Boles SM. Successful participant recruitment strategies for an online smokeless tobacco cessation program. Nicotine & Tobacco Research 2006;8 Suppl 1:S35‐41. [DOI] [PubMed] [Google Scholar]
- Gordon JS, Severson HH, Akers L, Boles SM. Chewfree.com: development, recruitment, and user engagement. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
- Severson HH, Gordon J, Danaher BG, Boles SM, Akers L. Outcome of a web delivered cessation program for smokeless tobacco users (POS1‐118). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007.
- Severson HH, Gordon JS, Akers L, Boles SM. User characteristics for smokeless tobacco cessation on the internet. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
- Severson HH, Gordon JS, Boles SM, Danaher BG, Akers L. Chewfree.com: Results of a web‐delivered smokeless tobacco cessation program (POS1‐71). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
- Severson HH, Gordon JS, Danaher BG, Akers L. ChewFree.com: evaluation of a web‐based cessation program for smokeless tobacco users. Nicotine & Tobacco Research 2008;10(2):381‐91. [DOI] [PubMed] [Google Scholar]
Shegog 2005 {published data only}
- Shegog R, McAlister AL, Hu S, Ford KC, Meshack AF, Peters RJ. Use of interactive health communication to affect smoking intentions in middle school students: a pilot test of the "Headbutt" risk assessment program. American Journal of Health Promotion 2005;19(5):334‐8. [DOI] [PubMed] [Google Scholar]
Skov‐Ettrup 2014 {published data only}
- Skov‐Ettrup LS, Ringgaard LW, Dalum P, Flensborg‐Madsen T, Thygesen LC, Tolstrup JS. Comparing tailored and untailored text messages for smoking cessation: a randomized controlled trial among adolescent and young adult smokers. Health Education Research 2014;29(2):195‐205. [DOI] [PubMed] [Google Scholar]
Stoddard 2005 {published data only}
- Stoddard J, Delucchi K, Muñoz R, Collins N, Stable EP, Augustson E, et al. Smoking cessation research via the internet: a feasibility study. Journal of Health Communication 2005;10(1):27‐41. [DOI] [PubMed] [Google Scholar]
Stoops 2009 {published data only}
- Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, et al. An internet‐based abstinence reinforcement smoking cessation intervention in rural smokers. Drug and Alcohol Dependence 2009;105(1‐2):56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thieleke 2005 {published data only}
- Thieleke J, McMahon J, Meyer G, Yun KA. An evaluation of the Freedom From Smoking Online cessation program among Wisconsin residents. Wisconsin Medical Journal 2005;104(4):41‐4. [PubMed] [Google Scholar]
Toll 2007 {published data only}
- Toll BA, O'Malley SS, Katulak NA, Wu R, Dubin JA, Latimer A, et al. Comparing gain‐ and loss‐framed messages for smoking cessation with sustained‐release bupropion: a randomized controlled trial. Psychology of Addictive Behaviors 2007;21(4):534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Velicer 2006 {published data only}
- Velicer WF, Friedman RH, Fava JL, Gulliver SB, Keller S, Sun X, et al. Evaluating nicotine replacement therapy and stage‐based therapies in a population‐based effectiveness trial. Journal of Consulting and Clinical Psychology 2006;74(6):1162‐72. [DOI] [PubMed] [Google Scholar]
Vilaplana 2014 {published data only}
- Vilaplana J, Solsona F, Abella F, Cuadrado J, Alves R, Mateo J. S‐PC: an e‐treatment application for management of smoke‐quitting patients. Computer Methods and Programs in Biomedicine 2014;115(1):33‐45. [DOI] [PubMed] [Google Scholar]
Walters 2006 {published data only}
- Walters ST, Wright JA, Shegog R. A review of computer and internet‐based interventions for smoking behavior. Addictive Behaviors 2006;31(2):264‐77. [DOI] [PubMed] [Google Scholar]
Wetter 2006 {published data only}
- Wetter D, Cinciripini PM. Palmtop computer‐delivered treatments for smoking cessation (SYM4C). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
Woolf 2006 {published data only}
- Woolf SH, Krist AH, Johnson RE, Wilson DB, Rothemich SF, Norman GJ, et al. A practice‐sponsored web site to help patients pursue healthy behaviors: an ACORN study. Annals of Family Medicine 2006;4(2):148‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Buller 2014 {published data only (unpublished sought but not used)}
- Buller D, Borland R, Severson H, Bettinghaus E, Haperin A, Tinkelman D, et al. Results of a randomized trial comparing an online smoking cessation program to a self‐help booklet and telephone quit line among young adults (POS2‐17). Society for Research on Nicotine and Tobacco 18th Annual Meeting March 13‐16, Houston, Texas. 2012.
- Buller DB, Halperin A, Severson HH, Borland R, Slater MD, Bettinghaus EP, et al. Effect of nicotine replacement therapy on quitting by young adults in a trial comparing cessation services. Journal of Public Health Management and Practice 2014;20(2):E7‐E15. [DOI: 10.1097/PHH.0b013e3182a0b8c7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Díaz‐Gete 2012 {published and unpublished data}
- Díaz‐Gete L, Puigdomènech E, Briones EM, Fàbregas‐Escurriola M, Fernandez S, Val JL, et al. Effectiveness of an intensive e‐mail based intervention in smoking cessation (TABATIC study): study protocol for a randomized controlled trial.. BMC Public Health 2013;18(13):364. [DOI: 10.1186/1471-2458-13-364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2012 {published data only}
- Henderson JA, Chubak J, O'Connell J, Ramos MC, Jensen J, Jobe JB, et al. Design of a randomized controlled trial of a web‐based intervention to reduce cardiovascular disease risk factors among remote reservation‐dwelling American Indian adults with type 2 diabetes. Journal of Primary Prevention 2012;33(4):209‐22. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Humfleet 2007 {published data only}
- Humfleet G, Hall S, Muñoz R, Greenwood G. Reaching and treating LGBT smokers: does the internet work? (POS1‐117). Society for Research on Nicotine and Tobacco 13th Annual Meeting February 21‐24, Austin, Texas. 2007.
- Humfleet GL, Greenwood G, Hall S, Muñoz R, Taylor A. Characteristics of lesbian, gay, bisexual, and transgender (LGBT) smokers seeking internet‐based treatment (POS1‐53). Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
- Humfleet GL, Greenwood G, Hall SM, Muñoz R, Hunt C, Taylor A. Internet‐based smoking treatment for lesbian, gay, bisexual and transgender (LGBT) cigarette smokers. Society for Research on Nicotine and Tobacco 11th Annual Meeting, 20‐23 March 2005; Prague, Czech Republic. 2005.
Humfleet 2008 {published data only}
- NCT00634218. Reaching and treating lesbian, gay, bisexual, and transgender (LGBT) cigarette smokers. clinicaltrials.gov/ct2/show/NCT00634218 (first received 4 March 2008).
Kramer 2009 {published data only}
- Kramer JJ, Willemsen MC, Conijn B, Emst AJ, Brunsting S, Riper H. Effectiveness of a web‐based self‐help smoking cessation intervention: protocol of a randomised controlled trial. BMC Public Health 2009;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01103427 {unpublished data only}
- Mobile text messaging as an adjunct function to an Internet‐based smoking cessation intervention implemented in the general population and in a health care setting. Ongoing study May 2010. This study has been completed. (No study results posted and no linked publications).
NCT01457469 {unpublished data only}
- NCT01457469. Enhanced quitline intervention in smoking cessation for patients with non‐metastatic lung cancer. clinicaltrials.gov/ct2/show/NCT01457469 (first received 21 October 2011).
NCT01544153 {unpublished data only}
- Cha S, Erar B, Niaura RS, Graham AL. Baseline Characteristics and Generalizability of Participants in an Internet Smoking Cessation Randomized Trial. Annals of Behavioral Medicine 2016;50(5):751–761. [DOI: 10.1007/s12160-016-9804-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Cha S, Cobb NK, Fang Y, Niaura RS, Mushro A. Impact of seasonality on recruitment, retention, adherence, and outcomes in a web‐based smoking cessation intervention: randomized controlled trial. Journal of Medical Internet Research 2013;15(11):e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Cha S, Fang Y, Cobb NK, Papandonatos GD, Niaura RS, et al. Improving adherence to web‐based smoking cessation programs: a randomized controlled trial. Society for Research on Nicotine and Tobacco 19th Annual Meeting March 13‐16 Boston MA. 2013:11.
- Graham AL, Cha S, Papandonatos GD, Cobb NK, Mushro A, Fang Y, Niaura RS, Abrams DB. Improving adherence to web‐based cessation programs: a randomized controlled trial study protocol. Trials 2013;14(48):1‐15. [DOI: 10.1186/1745-6215-14-48] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01692730 {unpublished data only}
- NCT01692730. Web assisted tobacco intervention with community colleges (WATI). clinicaltrials.gov/ct2/show/NCT01692730 (first received 17 September 2012).
NCT01812278 {published data only}
- NCT01812278. Randomized trial of web‐delivered acceptance therapy for smoking cessation (WebQuit). clinicaltrials.gov/ct2/show/NCT01812278 (first received 14 March 2013).
NCT02021175 {published data only}
- NCT02021175. Korean youth smoking cessation study. clinicaltrials.gov/ct2/show/NCT02021175 (first received 6 February 2013).
NCT02050308 {published data only}
- NCT02050308. Web‐based smoking cessation program for tribal college students. clinicaltrials.gov/ct2/show/NCT02050308 (first received 28 January 2014).
NCT02072772 {published data only}
- NCT02072772. A trial of positively smoke free group therapy for HIV‐infected smokers. clinicaltrials.gov/ct2/show/NCT02072772 (first received 25 February 2014).
NCT02099097 {unpublished data only}
- NCT02099097. Quit IT: Preliminary Testing of a Web‐based, 3D Coping Skills Game to Increase Quitting Self‐Efficacy for Maintaining Smoking Abstinence Following Hospitalization. clinicaltrials.gov/ct2/show/NCT02099097 (first received 25 March 2014).
NCT02207036 {unpublished data only}
- Ramo DE, Thrul J, Delucchi KL, Ling PM, Hall SM, Prochaska JJ. The Tobacco Status Project (TSP): Study protocol for a randomized controlled trial of a Facebook smoking cessation intervention for young adults. BMC Public Health 2015;15:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02329249 {unpublished data only}
- NCT02329249. Pharmacological aids for interactive smoking cessation (NRT_2). clinicaltrials.gov/ct2/show/NCT02329249 (first received 29 December 2014).
NCT02378766 {published data only}
- Cote J, Cossette S, Ramirez‐Garcia P, Pokomandy A, Worthington C, Gagnon MP, et al. Evaluation of a web‐based tailored intervention (TAVIE en santé) to support people living with HIV in the adoption of health promoting behaviours: an online randomized controlled trial protocol. BMC Public Health 2015;15:1042. [DOI: 10.1186/s12889-015-2310-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02378766. Evaluation of web‐based interventions to support people living with HIV in the adoption of health behaviors (LHIVEHEALTHY). clinicaltrials.gov/ct2/show/NCT02050308 (first received 17 February 2015).
NCT02585206 {published data only}
- Graham AL, Jacobs MA, Cohn AM, Cha S, Abroms LC, Papandonatos GD, Whittaker R. Optimising text messaging to improve adherence to web‐based smoking cessation treatment: a randomised control trial protocol. BMJ Open 2016;6(3):e010687. [DOI: 10.1136/bmjopen-2015-010687] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02602730 {unpublished data only}
- NCT02602730. Internet‐based nonsmoking program for postpartum women (PostPartum_2). clinicaltrials.gov/ct2/show/NCT02602730 (first received 2 January 2015).
Redfern 2014 {published data only}
- Redfern J, Usherwood T, Harris MF, Rodgers A, Hayman N, Panaretto K, et al. A randomised controlled trial of a consumer‐focused e‐health strategy for cardiovascular risk management in primary care: the Consumer Navigation of Electronic Cardiovascular Tools (CONNECT) study protocol. BMJ Open 2014;4(2):e004523. [DOI: 10.1136/bmjopen-2013-004523] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westmaas 2013 {unpublished data only}
- Westmaas JL, Abroms L, Bontemps‐Jones J, Mellerson J. Tailored emails as a stand‐alone strategy for smoking cessation: Evidence from a randomized controlled trial. Society for Research on Nicotine and Tobacco 19th Annual Meeting March 13‐16 Boston MA. 2013:174.
Wiers 2015 {published data only (unpublished sought but not used)}
- Wiers R, Elfeddali I, Pronk TW, Bolman C, Vries H. Online attentional re‐training for smoking. Drug and Alcohol Dependence 2015;146:e22. [DOI: 10.1016/j.drugalcdep.2014.09.741] [DOI] [Google Scholar]
Additional references
Benowitz 2010
- Benowitz NL. Nicotine Addiction. New England Journal of Medicine 2010;362(24):2295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blankers 2016
- Blankers M, Smit ES, Pol P, Vries H, Hoving C, Laar M. The missing= smoking assumption: a fallacy in internet‐based smoking cessation trials?. Nicotine & Tobacco Research 2016;18(1):25‐33. [DOI] [PubMed] [Google Scholar]
Bock 2004
- Bock B, Graham A, Sciamanna C, Krishanamoorthy J, Whiteley J, Carmona‐Barros R, et al. Smoking cessation treatment on the internet: content, quality, and usability. Nicotine & Tobacco Research 2004;6(2):207‐19. [DOI] [PubMed] [Google Scholar]
Brown 2013
Chen 2012
- Chen YF, Madan J, Welton N, Yahaya I, Aveyard P, Bauld L et al. Effectiveness and cost‐effectiveness of computer and other electronic aids for smoking cessation: a systematic review and network meta‐analysis. Health Technology Assessment 2012;16(38):1‐205. [DOI] [PubMed] [Google Scholar]
Coleman 2004
- Coleman T. Cessation interventions in routine health care. BMJ 2004;328(7440):631‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2006b
- Etter JF. A list of the most popular cessation web sites and a comparison of their quality. Nicotine & Tobacco Research 2006;8 Suppl 1:S27‐34. [DOI] [PubMed] [Google Scholar]
Eysenbach 2002
- Eysenbach G. Issues in evaluating health websites in an internet‐based controlled trial. Journal of Medical Internet Research 2002;4(3):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eysenbach 2007
- Eysenbach G. Poverty, human development and the role of e Health. Journal of Medical Internet Research 2007;9(4):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiore 2008
- Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service, May 2008. [Google Scholar]
Graham 2007
- Graham AL, Cobb NK, Raymond L, Sill S, Young J. Effectiveness of an internet‐based worksite smoking cessation intervention at 12 months. Journal of Occupational and Environmental Medicine 2007;49(8):821‐8. [DOI] [PubMed] [Google Scholar]
Graham 2016
- Graham AL, Carpenter KM, Cha S, Cole S, Jacobs MA, Raskob M, et al. Systematic review and meta‐analysis of Internet interventions for smoking cessation among adults. Substance Abuse and Rehabilitation 2016;7:55‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartman‐Boyce 2014
- Hartman‐Boyce J, Lancaster T, Stead LF. Print‐based self‐help interventions for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001118.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;18(343):d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hou 2014
- Hou SI, Charlery SA, Roberson K. Systematic literature review of Internet interventions across health behaviours. Health Psychology and Behavioral Medicine 2014;2(1):455‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
ICT 2016
- Information & Communication Technologies. ICT Facts and Figures 2016. ICT Statistics Online (www.itu.int/en/ITU‐D/Statistics/Pages/facts/default.aspx) 2016 (accessed 21st August 2017).
Kontos 2007
- Kontos EZ, Bennett GG, Viswanath K. Barriers and facilitators to home computer and internet use among urban novice computer users of low socioeconomic position. Journal of Medical Internet Research 2007;9(4):e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lader 2009
- Lader D. Opinions survey report no. 40: Smoking‐related behaviour and attitudes, 2008/09. Office for National Statistics 2009.
Lancaster 2017
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001292.pub3] [DOI] [PubMed] [Google Scholar]
Mathieu 2013
- Mathieu E, McGeechan K, Barratt A, Herbert R. Internet‐based randomized controlled trials: a systematic review. Journal of the Americal Medical Informatics Association 2013;20(3):568‐76. [DOI: 10.1136/amiajnl-2012-001175] [DOI] [PMC free article] [PubMed] [Google Scholar]
MMWR 2011
- Centers for Disease Control. Quitting smoking among adults ‐‐‐ United States, 2001‐‐2010. Morbidity and Mortality Weekly Report 2011;60(44):1513‐9. [PubMed] [Google Scholar]
Murray 2009
- Murray E, Khadjesari Z, White IR, Kalaitzaki E, Godfrey C, McCambridge J, et al. Methodological challenges in online trials. Journal of Medical Internet Research 2009;11(2):e9. [DOI: 10.2196/jmir.1052] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muñoz 2006
- Muñoz RF, Lenert LL, Delucchi K, Stoddard J, Perez JE, Penilla C, et al. Toward evidence‐based internet interventions: A Spanish/English web site for international smoking cessation trials. Nicotine & Tobacco Research 2006;8(1):77‐87. [DOI] [PubMed] [Google Scholar]
Muñoz 2012b
- Muñoz RF, Aguilera A, Schueller SM, Leykin Y, Pérez‐Stable EJ. From online randomized controlled trials to participant preference studies: morphing the San Francisco StopSmoking site into a worldwide smoking cessation resource. Journal of Medical Internet Research 2012;14(3):e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myung 2009
- Myung SK, McDonell DD, Kazinets G, Seo HG, Moskowitz JM. Effects of web‐and computer‐based smoking cessation programs. Archives of Internal Medicine 2009;169(10):929‐37. [DOI] [PubMed] [Google Scholar]
ONS 2016
- Office for National Statistics. Internet users in the UK: 2016. www.ons.gov.uk/businessindustryandtrade/itandinternetindustry/bulletins/internetusers/2016 2016.
Pike 2007
- Pike KJ, Rabius V, McAlister A, Geiger A. American Cancer Society's QuitLink: randomized trial of internet assistance. Nicotine & Tobacco Research 2007;9(3):415‐20. [DOI] [PubMed] [Google Scholar]
SCTH 1998
- Scientific Committee on Tobacco and Health and HMSO. Report of the Scientific Committee on Tobacco and Health. London: Her Majesty's Stationery Office, 1998. [Google Scholar]
Shahab 2009
- Shahab L, McEwen A. Online support for smoking cessation: a systematic review of the literature. Addiction 2009;104(11):1792‐804. [DOI] [PubMed] [Google Scholar]
SRNT 2002
- SRNT Subcommittee on Biochemical Verification. Outcome criteria in smoking cessation trials:proposal for a common standard: Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research 2002;4:149‐59. [Google Scholar]
Stead 2013a
- Stead LF, Buitrago D, Preciado N, Sanchez G, Hartmann‐Boyce J, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD000165.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stead 2013b
- Stead LF, Hartmann‐Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD002850.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2017
- Stead LF, Carroll AJ, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD001007.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strecher 2006
- Strecher VJ, Shiffman S, West R. Moderators and mediators of a web‐based computer‐tailored smoking cessation program among nicotine patch users. Nicotine & Tobacco Research 2006;8 Suppl 1:S95‐101. [DOI] [PubMed] [Google Scholar]
Surgeon General 2006
- U.S. Department of Health and Human Services. The health consequences of involuntary exposure to tobacco smoke: a report of the Surgeon General. www.surgeongeneral.gov/library/reports/secondhandsmoke/fullreport.pdf. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2006 (accessed 21 August 2017).
Surgeon General 2014
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. www.surgeongeneral.gov/library/reports/50‐years‐of‐progress/exec‐summary.pdf. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014 (accessed 21 August 2017).
Tzelepis 2017
- Tzelepis F, Paul CL, Williams CM, Gilligan C, Regan T, Daly J, et al. Real‐time video counselling for smoking cessation. Cochrane Database of Systematic Reviews 2017, Issue 5. [DOI: 10.1002/14651858.CD012659] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verheijden 2007
- Verheijden MW, Jans MP, Hildebrandt VH, Hopman‐Rock M. Rates and determinants of repeated participation in a web‐based behaviour change program for healthy body weight and healthy life style. Journal of Medical Internet Research 2007;9(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittaker 2016
- Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile phone‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD006611.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization. Building Blocks for Tobacco Control ‐ A Handbook. www.who.int/tobacco/resources/publications/tobaccocontrol_handbook/en/. Serial, 2004 (accessed 21 August 2017).
WHO 2017
- World Health Organization Tobacco Free Initiative. Tobacco Facts. http://www.who.int/mediacentre/factsheets/fs339/en/ 2017 (accessed 31 Aug 2017).
References to other published versions of this review
Civljak 2010
- Civljak M, Sheikh A, Stead LF, Car J. Internet‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007078.pub3] [DOI] [PubMed] [Google Scholar]
Civljak 2013
- Civljak M, Stead L, Hartmann‐Boyce J, Sheikh A, Car J. Internet‐based interventions for smoking cessation. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD007078.pub4] [DOI] [PubMed] [Google Scholar]


