Abstract
The retrosplenial cortex (RSC) is positioned at the interface between cortical sensory regions and the hippocampal/parahippocampal memory system. As such, it has been theorized that RSC may have a fundamental role in linking sensory stimuli together in the service of forming complex representations. To test this, three experiments were carried out to determine the effects of RSC damage or temporary inactivation on learning and performance of a negative patterning discrimination. In this procedure, two conditioned stimuli are reinforced when they are presented individually (i.e., stimulus elements) but are non-reinforced when they are presented simultaneously as a compound stimulus. Normal rats successfully discriminate between the two types of trials as evidenced by more responding to the elements compared to the compound stimulus. This is thought to reflect the formation of a configural representation of the compound stimulus; that is, the two cues are linked together in such a fashion that the compound stimulus is a wholly different, unique stimulus. Permanent lesions of RSC made prior to training (Experiment 1) had no effect on learning the discrimination. However, lesions (Experiment 2) or temporary chemogenetic inactivation (Experiment 3) of RSC made after training impaired subsequent performance of the discrimination. We argue that this pattern of results indicates that RSC may normally be involved in forming the configural representations manifested in negative patterning, but that absent the RSC, other brain systems or structures can compensate sufficiently to result in normal behavior.
1. Introduction
The retrosplenial cortex (RSC) is positioned at the interface between cortical sensory regions and structures that compose the hippocampal/parahippocampal memory system (Burwell & Amaral, 1998; Todd et al., 2016; Sugar et al., 2011; van Groen & Wyss, 1992, 2003; Vogt & Miller, 1983). As such, one theory of RSC function posits that the RSC is involved in forming associations among sensory cues, providing the basis for complex representations utilized by areas such as hippocampus (Bucci & Robinson, 2014; Kobayashi & Amaral, 2007; Wolbers & Buchel, 2005). This idea is supported by findings that RSC lesions or inactivations impair spatial memory (Harker & Wishaw, 2002; Vann & Aggleton, 2002; Keene & Bucci, 2009; van Groen et al., 2004; Cain et al., 2006) and contextual fear memory (Keene & Bucci, 2008a, 2008b; Todd et al., 2016; Corcoran et al., 2011; Kwapis et al., 2015). Contexts are typically defined as the constellation of background sensory stimuli (e.g., olfactory, visual, tactile) that compose the training environment (Bouton, 2010), and one theory of contextual learning (“configural” theory) holds that the various sensory stimuli are linked together to form a cohesive, gestalt representation of the context (Fanselow, 2000; Rudy, 2009; Rudy et al., 2004). Thus, the observation of reduced contextual fear memory after manipulations of RSC could be interpreted as evidence that RSC contributes to linking sensory cues, particularly in the service of forming a unique configural representation of the environment.
In most fear conditioning procedures, however, the sensory features that compose the context are always present (i.e., static, background stimuli). Thus, it is difficult to be certain that fear conditioning to the context necessarily involves an association between shock and a configural representation of the sensory stimuli. Instead, context fear conditioning could also be supported by the formation of an association between an individual stimulus present in the chamber and shock (Rudy, 2009), simply because the animal happens to be attending to that stimulus at the time shock is delivered. A variant of fear conditioning that has been used to circumvent this issue makes use of the “immediate-shock deficit.” That is, when rats are shocked immediately upon introduction to a novel training chamber (instead of being allowed to explore the environment for several minutes before the first shock), they exhibit little or no fear memory upon re-exposure to the context (Fanselow, 1986). The common explanation for this effect is that there is not enough time to link together the sensory features that compose the context, which prevents the formation of a context-shock association. Therefore, rats exhibit a deficit in context fear retrieval upon reintroduction to the training context. However, the immediate-shock deficit can be overcome if rats receive prior exposure to the context before fear conditioning. In other words, if rats are exposed to the training chamber (with no shocks delivered) prior to conditioning with the immediate shock, contextual fear memory is robust, a finding known as the “context pre-exposure facilitation effect” (CPFE; Fanselow, 1986, 1990; Kiernan and Westbrook, 1993; Rudy & O’Reilly, 1999). The idea is that pre-exposure to the chamber allows rats the opportunity to link together the sensory features of the environment into a cohesive whole, or configural representation, which can then become associated with shock during training. Prior studies in our lab have shown that lesions of RSC carried out after training impair CPFE (Todd et al., 2017), further supporting the idea that RSC may be involved in configural learning and memory.
The present series of three experiments sought to determine whether the involvement of RSC in configural learning and memory also includes non-contextual forms of configural learning, specifically, learning that involves linking discrete cues (e.g., punctate auditory and visual cues) with an outcome, rather than linking together static background cues. In addition, we tested whether the involvement of RSC in configural learning extends to conditioning that uses an appetitive unconditioned stimulus (US) rather than an aversive US. To these ends, we tested the effects of RSC manipulations using a behavioral procedure known as negative patterning (Woodbury, 1943; Redhead & Pearce, 1995; Bussey et al, 2000). In this procedure, two different conditioned stimuli (e.g., a light and a tone) are reinforced with food when they are presented individually (stimulus elements, E) but are not non-reinforced they are presented simultaneously as a single compound stimulus (C). Normal rats successfully discriminate between the two types of trials as evidenced by more responding during E trials than C trials. According to elemental theories of learning (e.g., Rescorla & Wagner, 1972), which posit that the individual elements in the compound each form as association with the US, rats should exhibit summation on C trials: that is, responding to C should be higher than to E. However, over the course of training, normal rats actually come to exhibit lower levels of conditioned responding during C trials. Hence, it is thought that rats represent C as a wholly different, unique stimulus, consistent with configural theories of learning (Redhead & Pearce, 1995, Pearce, 1987). Indeed, negative patterning is often considered to be a rigorous test of configural learning (Bouton, 2016). Experiments 1 and 2 tested the effects of permanent lesions of RSC carried out either before (Experiment 1) or after (Experiment 2) negative patterning training. Finding an effect of only the post-training lesion, Experiment 3 then used chemogenetics to more selectively and transiently silence RSC neurons after conditioning.
2. EXPERIMENT 1
2.1. Materials and Methods
2.1.1. Subjects
Sixteen experimentally naïve male Long Evans rats (~250g) were obtained from Harlan (Envigo) Laboratories (Indianapolis, IN). Upon arrival to the colony, rats were pair-housed and allowed to acclimate to the vivarium for at least three days with food (Purina standard rat chow; Nestle Purina, St. Louis, MO) and water were available ad libitum. After the acclimation period surgery was conducted as described below. Rats were housed individually after surgery and for the remainder of the experiment. During the recovery period (7 – 9 days), food was available ad libitum and rats were allowed to return to pre-surgical weight before being food restricted to gradually reduce their weight to 85% of free-feed body weight in preparation for behavioral training. Rats were weighed and handled daily and maintained at 85% weight by supplementing the food delivered during the behavioral procedures with additional chow. Throughout the experiment, rats were maintained on a 14:10 light-dark cycle and were monitored and cared for in compliance with the association for Assessment and Accreditation of Laboratory Animal care guidelines and the Dartmouth College Institutional Animal Care and Use Committee. All testing occurred during the light cycle.
2.1.2. Surgery
Half of the rats (n = 8) received bilateral electrolytic RSC lesions and the other half received sham lesions. Rats were anesthetized with isoflurane gas (1.5 – 3% in oxygen) and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The skin was retracted and a craniotomy was performed. For the RSC lesion group, skull penetrating burr holes were drilled above the intended lesion site using the coordinates shown in Table 1. An epoxy-coated electrode with a 1 mm exposed tip was lowered into each hole and current (2 mA) was applied for 15 s at each site. Sham-lesion rats (n = 8) underwent the craniotomy and received non-penetrating burr holes to avoid damaging the underlying cortex.
Table 1:
Stereotaxic coordinates for Experiments 1–3
| Anterior-Posterior | Medial-Lateral | Dorsal-Ventral | |
|---|---|---|---|
| Expts. 1 & 2 | −2.0 | ±0.3 | −2.0 & −2.7 |
| −3.5 | ±0.4 | −2.0 & −2.7 | |
| −5.0 | ±0.4 & ± 1.0 | −2.0 & −2.7 | |
| −6.5 | ±0.8 & ± 1.5 | −2.0 & −2.8 | |
| −8.0 | ±1.6 & ± 2.4 | −2.5 & −3.1 | |
| −9.0 | ±3.4 | −4.0 | |
| Expt. 3 | −2 | ±0.3 | −2.6 |
| −3.5 | ±0.3 | −2.4 | |
| −5.0 | ±0.3 | −2.6 | |
| −6.5 | ±1.0 | −2.4 | |
| −8.0 | ±1.5 | −2.5 |
All anterior-posterior and medial-lateral measurements are derived from bregma and midline respectfully. Experiment 1 & 2 dorsal-ventral measurements were derived from skull surface. Experiment 3 dorsal-vental measurements were derived from the cortical surface.. All measurements are in mm.
2.1.3. Behavioral Apparatus
Eight conditioning chambers (Med Associates, Inc., St. Albans, VT, ENV-007; 24 cm W × 30.5 cm L × 29 cm H) were used in the experiment, each housed in an individual sound attenuating chamber (Med Associates, ENV-017M; 66 cm W × 56 cm H × 56 cm D). A 2.8 W bulb (with a red cover) mounted to the ceiling of the sound attenuating chamber provided low level background illumination for video recording. The conditioning chambers had acrylic sidewalls and ceilings and the front and rear walls were made of brushed aluminum. The floors consisted of evenly spaced (1.5 cm) stainless steel rods (5 mm diameter). Background noise (~68 db) and airflow was created by an exhaust fan mounted to the sound attenuating chamber. A food cup was recessed in the center of the front wall of each chamber, and two retractable levers (Med Associates, ENV-112CM) were positioned to the left and right of the food cup (levers remained retracted for the duration of the experiment and were not used). Four panel lights (Med Associates, ENV-221M) were mounted in each chamber: a light was located above each lever and the food cup (~10.8 cm above the grid floor) and an additional light was positioned 16 cm directly above the food cup. Only the light located 10.8 cm above the food cup was used as the visual stimulus (V) in the experiment. When activated, the light flashed at a frequency of 2 Hz for 10 sec. A speaker (Med Associates, ENV-224AM) was mounted 20 cm above and to the right of food magazine and was the source of the auditory stimulus (A; a 74dB, 10Hz clicker). Two 45mg pellets (BioServ, Flemington, NJ) served as the US and were delivered into the food cup immediately after the presentation of A or V alone. An infrared photobeam transmitter/receiver was located at the front of the food cup to monitor food cup entries.
2.1.4. Behavioral Procedures
All rats first received a single 30-min magazine training session during which food pellets were delivered on a random interval schedule that resulted in 1 pellet delivery every 30 seconds on average. The purpose of this session was to orient the rat to the food cup and familiarize it to the pellets. The next day negative patterning training began (one 70-min session per day) and continued for 28 consecutive days. During each session there were three types of trials: Each individual stimulus (A, V) was presented four times and co-terminated with delivery of the US. The compound stimulus (AV) was presented 24 times during each session and was not reinforced (Redhead & Pearce, 1995; Bussey et al, 2000). Thus, there were a total of 32 trials per session. The stimuli were presented for 10 seconds on each trial and the intertrial interval (ITI) was variable and averaged 120 sec. The order of the trials was quasi-random such that no stimulus was presented more than twice in successive order and the order was counterbalanced across days.
2.1.5. Data Analysis
The amount of time spent with the snout inside the food cup during presentation of the conditioned stimuli (CSs) served as the primary measure of conditioned responding. As described in the Results (section 2.2.2), there were no significant differences in responding during presentation of the two elements (A, V) in either group of rats. Thus, responding during A-alone and V-alone trials was averaged together for all subsequent analyses.
The primary analysis of responding during presentation of the CSs consisted of a repeated-measures analysis of variance (ANOVA) with Group (sham, lesion) as the between-subjects variable, and Session (in 2-session blocks, 1–14) and Trial-type (element, compound) as the within-subjects variables. In addition, responding during the 10-sec period before stimulus onset (i.e., “pre-CS” behavior) was analyzed to test for any lesion-induced changes in baseline activity. Analyses were carried out using R statistical software and the alpha level was set at 0.05.
2.1.6. Lesion Verification and Analysis
After behavioral training was complete, rats were deeply anesthetized with sodium pentobarbital and transcardially perfused with 0.9% saline (~250 ml) followed by 10% buffered formalin (~300 ml). Brains were removed and remained in formalin for 24 hours followed by 72 hrs in 30% sucrose. A freezing microtome was used to collect 60 μm coronal brain sections throughout the RSC (and just anterior and just posterior to RSC), which were kept at 4° in phosphate buffer until being mounted the next day onto gelatin-coated glass slides. The sections (~27/rat) were then thionin-stained and viewed under a compound microscope (Axioskop I, Zeiss, Inc., Thornwood NY) and analyzed using StereoInvestigator software (Microbrightfield, Inc., Williston, VT). Two analyses were conducted: 1) Sections at six rostro-caudal levels (AP −1.8, −3.0, −4.2, −5.4, −6.6, & −7.8 mm from bregma) were used to obtain areas measurements of RSC damage (defined as missing tissue, gliosis, cortical thinning) and the average percentage of RSC area damage was calculated; 2) All ~27 sections collected for each rat were observed under the microscope and noted as having RSC damage or not. This was used to calculate the number of sections across the rostro-caudal extent of RSC that were damaged. Lesion tracings for each rat were made by hand using the Paxinos & Watson (2009) rat brain atlas as a guide and processed using ImageJ.
2.2. Results
2.2.1. Histology
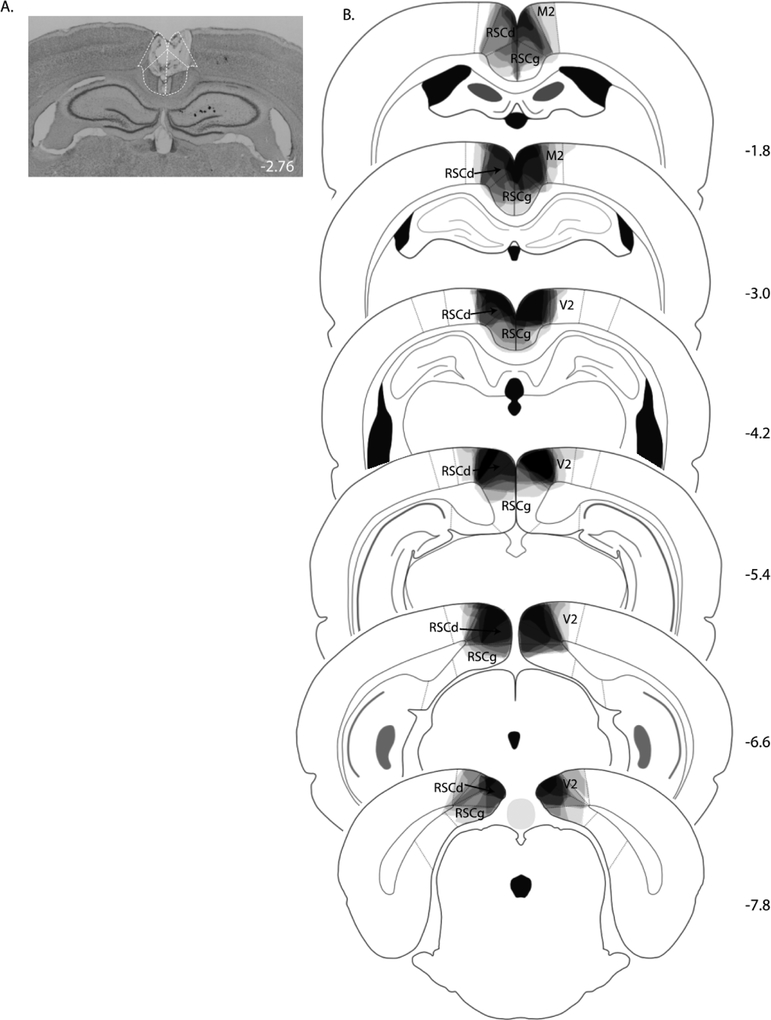
All rats in the lesion group exhibited bilateral damage of RSC. A photomicrograph of a representative RSC lesion is shown in Figure 1A. On average, the area of RSC that was damaged on each coronal section that was analyzed was 63 ± 6%. Damage to the RSC was present on 99 ± 1% of the sections collected for each rat (~27 sections/rat), indicating that damage extended throughout the rostro-caudal extent of the RSC (Figure 1B). Minor damage to the adjacent secondary visual cortex was observed in four of the eight lesioned rats, on 1 – 2 sections per rat. In two of those four cases, minor damage to the cingulate cortex was also observed on one section/rat. Three rats also exhibited slight damage to the cingulum bundle on one section analyzed from each rat.
Figure 1.
(A) Photomicrograph of a representative RSC lesion in Experiment 1. (B) Drawings of lesions at six levels along the rostro-caudal extent of the RSC. At each level, lesion drawings were stacked onto a single image. The darkness of an area indicates the number of lesion cases that include that area. M2 = secondary motor cortex; RSCd = restrosplenial dysgranular; RSCg = retrosplenial granula; V2 = secondary visual cortex.
2.2.2. Behavior
There were no significant differences in conditioned responding (time in food cup) during presentation of the stimulus elements (A, V) in either group of rats over the course of training (ps > 0.1). In addition, responding during each element was significantly greater than during the compound stimulus (ps < 0.05). Accordingly, responding during A-alone and V-alone trials was averaged together.
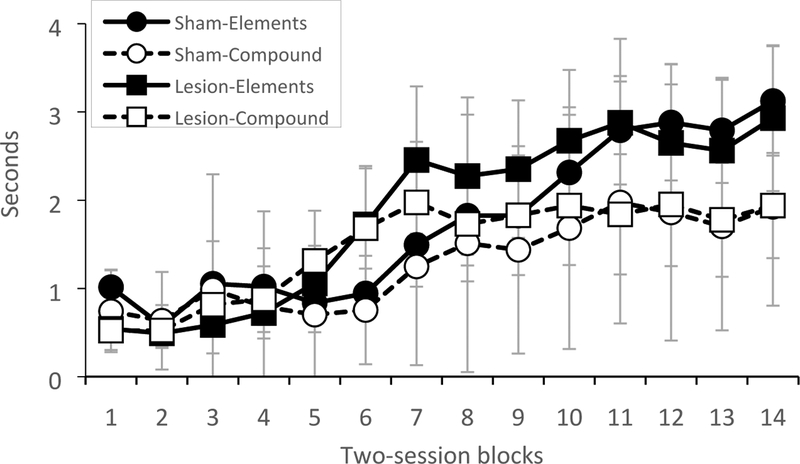
The amount of time sham-lesioned and RSC-lesioned rats spent with the snout inside the food cup during presentation of the elements and the compound stimulus over two-session blocks of training is shown in Figure 2. An ANOVA revealed significant main effects of Session [F (13, 182) = 10.9, p < 0.0001] and of Trial-type [F (1, 14) = 25.2, p < 0.0002], as well as a significant Session X Trial-type interaction [F (13,182) = 9.8, p < 0.0001]. Hence, while responding increased over the course of training, responding to the elements was greater than responding to the compound stimulus, indicating that rats learned the negative patterning discrimination. As shown in Figure 2, rats with RSC lesions exhibited comparable levels of responding to the sham-lesioned rats. Consistent with this, there was no main effect of Group [F (1, 14) = 0.06, p > 0.8] nor any significant interactions between Group and Session [F (13, 182) = 0.8, p > 0.6], Group and Trial-type [F (1, 14) = 0.3, p > 0.6], or Group, Session, and Trial-type [F (13, 182) = 0.8, p > 0.6]. In addition, there were no lesion effects on food cup behavior during the 10-sec period before stimulus onset (pre-CS period; ps > 0.6), indicating that RSC damage did not impact baseline levels of responding.
Figure 2.
Conditioned responding during presentation of the stimulus elements and the compound stimulus in sham-lesioned rats and RSC-lesioned rats in Experiment 1.
2.3. Discussion
The results of Experiment 1 indicate that electrolytic lesions of RSC prior to training do not impact the ability of rats to learn a negative patterning discrimination. Similarly, Nelson and colleagues (2018) recently demonstrated that neurotoxic lesions RSC made prior to training do not have an effect on an operant negative patterning discrimination. To the extent that negative patterning represents a rigorous test of configural learning, the consistency of these findings across both operant (Nelson et al., 2018) and classical conditioning procedures (present experiment) suggests that an intact RSC is not necessary for linking individual stimuli to form a cohesive, unique stimulus (i.e., the AV compound stimulus). The conclusion is strengthened by the fact that null results were obtained with two different lesions methods: electrolytic lesions (present study), which often produce more damage within RSC and may damage fibers of passage, and neurotoxic lesions (Nelson et al., 2018), which spare fibers of passage. In other words, even when a more extreme lesion method was used, which could also potentially disconnect RSC from other areas, there was still no effect on negative patterning.
Nonetheless, it remains possible that RSC could normally be involved in learning a negative patterning discrimination, but that absent RSC, other brain systems can compensate and result in intact performance. A similar argument has made in the case of hippocampal involvement in contextual fear memory (Fanselow, 2010) and supported by the consistent finding that hippocampal damage that occurs prior to training has little or no effect on contextual fear memory. However, damage that occurs soon after training produces a dramatic impairment in expressing contextual fear memory. Similarly, lesions of RSC made prior to training do not impact the context pre-exposure facilitation effect, but damage to RSC after training greatly reduces the effect and results in poor fear memory (Todd et al., 2017). Thus, at least under these conditions, another brain system or learning strategy may be able to compensate for the loss of RSC. Based on these findings, Experiment 2 tested the effects of RSC lesions that were carried out after rats learned the negative patterning discrimination. We hypothesized that if RSC is normally involved in learning the discrimination, damage after training would impair subsequent performance.
3. EXPERIMENT 2
3.1. Material and Methods
3.1.1. Subjects
Sixteen experimentally naïve male Long Evans rats (~250g) were obtained from Harlan (Envigo) Laboratories. All rats were allowed to acclimate to the vivarium for seven days while pair-housed with food and water were available ad libitum. Following the acclimation period, rats were separated into individual cages and body weights were reduced to 85% of free-feed weight in preparation for behavioral training. Rats were otherwise maintained as in Experiment 1.
3.1.2. Behavioral Procedure and Surgery
First, rats were trained in the same behavioral apparatus and negative patterning procedures used in Experiment 1 (“Training” phase of the experiment). After 28 daily training sessions, rats were divided into two groups and then underwent surgery (n = 8 RSC lesions, n = 8 sham lesions) as in Experiment 1. Half of the rats in each group received surgery the day after the final Training session; the other half received surgery the following day.
After the recovery period (8 – 9 days), all rats underwent a single Test session (30 min) consisting of four non-reinforced presentations of each stimulus (A, V, and AV). In order to isolate the effects of RSC damage on memory, we chose to test the stimulus elements under extinction conditions in order to minimize the opportunity for additional conditioning. The stimuli were presented on a variable ITI (average of 120 sec) and the order of the stimulus presentation was quasi-random (no stimulus occurred more than twice in a row) and counterbalanced across groups (half of the rats in each group received an element stimulus first, the other half received the compound stimulus first).
The final phase of the procedures began the next day and consisted of six daily Savings test sessions. During the Savings sessions, the original negative patterning procedure resumed (i.e., 4 reinforced trials of each individual stimulus (A, V) and 24 non-reinforced presentations of the compound stimulus, AV) and continued for six daily sessions. As during the initial Training phase, the order of the trials was quasi-random such that no stimulus was presented more than twice in successive order and the order was counterbalanced across days. The Saving sessions were included because, as described in section 3.2.2.2, we found that control rats failed to respond differentially to the elements versus the compound stimulus during the Test session. The Saving sessions were thus used to detect the originally-acquired associative strength to the elements and compound (Landeira et al., 2006), which may have been obscured following surgery and the relatively long recovery period. Indeed, savings tests can be especially sensitive at detecting differences in conditioning (Holland & Ross, 1983).
3.1.3. Data Analysis
A repeated-measures ANOVA was used to test for any pre-existing differences between rats that would be assigned to the sham-lesion or RSC-lesion groups. Thus, for the Training data, we conducted an ANOVA with Group (sham, lesion) as the between-subjects variable, and Session (2-session blocks, 1–14) and Trial-type (element, compound) as the within-subjects variables. Data collected during the Test session was analyzed using a repeated measures ANOVA with Group (sham, lesion) as the between-subjects variable and Trial type (element, compound) as the within-subjects variable. Responding was averaged during the 10-sec period before stimulus onset (pre-CS behavior) and analyzed to test for any lesion-induced changes in baseline activity during the Test session. For the Savings sessions, a repeated measures ANOVA was conducted with Group (sham, lesion) as the between-subjects variable, and Trial-type (element, compound), and Session (1–6) as the within-subjects variables. Significant interactions were decomposed with ANOVAs and pair-wise t-tests as appropriate.
3.1.4. Lesion Verification and Analysis
After the final Savings session, rats were euthanized and brain tissue was processed and analyzed as described in Experiment 1.
3.2. Results
3.2.1. Histology
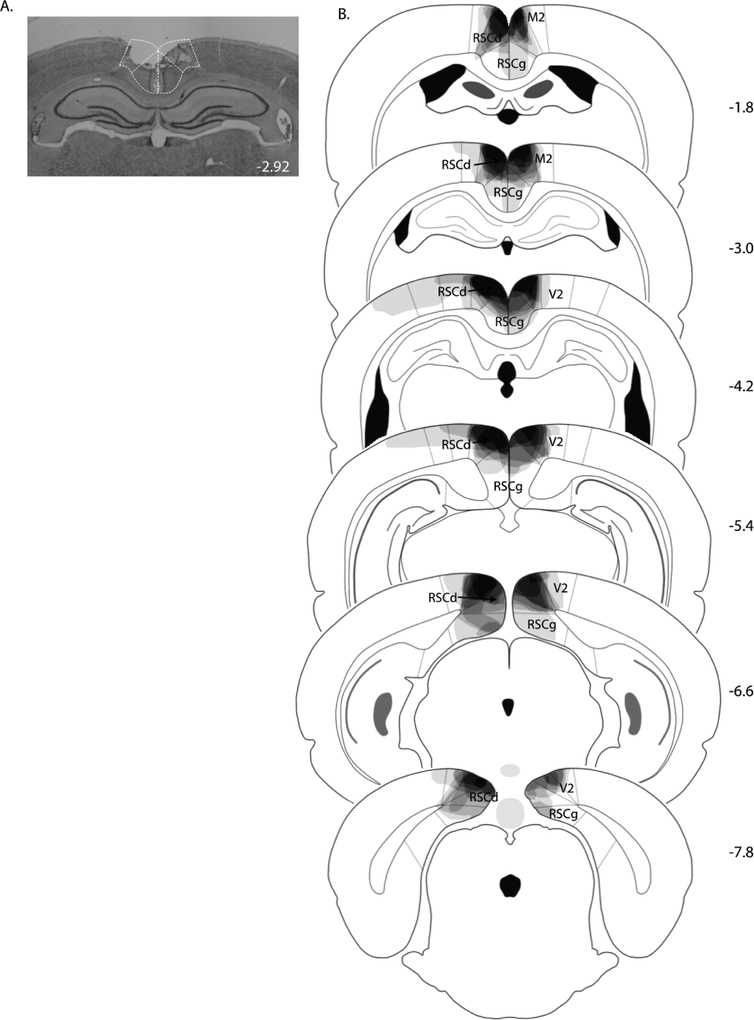
As in Experiment 1, each rat in the lesion group exhibited bilateral damage of RSC. A photomicrograph of a representative RSC lesion is shown in Figure 3A. On average, the area of RSC that was damaged on each coronal section that was analyzed was 49 ± 4%. Damage to the RSC was present on 99 ± 1% of the sections collected for each rat (~26 sections), extending throughout the entire rostro-caudal extent of the RSC (Figure 3B). In six of eight lesioned rats, minor damage to adjacent secondary visual cortex was observed in two sections on average, and in five of those cases, minor damage to the cingulate cortex was also observed on one section/rat. Five lesions rats also exhibited slight damage to the cingulum bundle on 1 – 2 sections per rat.
Figure 3.
(A) Photomicrograph of a representative RSC lesion in Experiment 2. (B) Drawings of lesions at six levels along the rostro-caudal extent of the RSC. At each level, lesion drawings were stacked onto a single image. The darkness of an area indicates the number of lesion cases that include that area. Abbreviations as in Figure 1.
3.2.2. Behavior
There were no significant differences in conditioned responding during presentation of the two elements at any point during the behavioral procedures (ps > 0.8). In addition, responding during each element was significantly greater than during the compound stimulus (ps < 0.001); hence, responding during A-alone and V-alone trials was averaged together.
3.2.2.1. Training
There were no pre-existing Group differences across the Training phase for rats that would receive sham-lesions or RSC-lesions after Training. This was supported by the results of an ANOVA that indicated that there was no main effect of Group [F (1, 14) = 0.02, p > 0.9], nor any significant Group interactions [Group X Trial type, F (1, 14) = 0.9, p > 0.3; Group X Session, F (13, 182) = 0.5, p > 0.9; Group X Trial-type X Session, F (13, 182) = 0.9, p > 0.5]. The only significant effects were a main effect of Trial-type [F (1, 14) = 22.0, p < 0.0004], a main effect of Session [F (13, 182) = 8.4, p < 0.0001], and a significant Trial-type X Session interaction [F (13, 182) = 21.4, p < 0.0001], indicating that all rats learned the discrimination before surgery.
3.2.2.2. Test session and Savings sessions
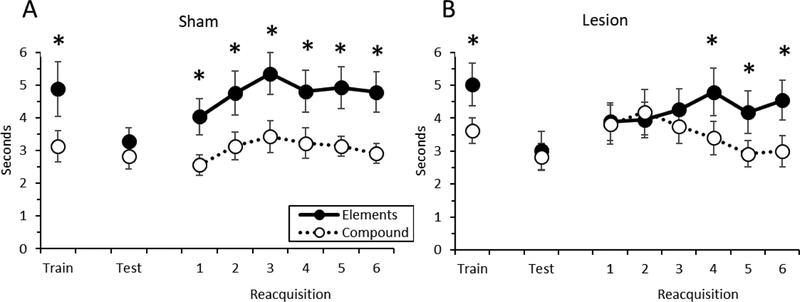
The primary data from Experiment 2 are shown in Figure 4. During the post-surgery Test session, neither group of rats exhibited differential responding during presentation of the non-reinforced elements and the non-reinforced compound stimulus (Figure 4). That is, neither the sham-lesioned rats nor the RSC-lesioned discriminated between the two trial types. This impression was supported by a repeated measures ANOVA, in which there was no significant main effects of Group [F (1, 14) = 0.06, p > 0.8] or Trial-type [F (1, 14) = 0.8, p > 0.4], and no significant Group X Trial-type interaction [F (1, 14) = 0.1, p > 0.7]. There were no lesion effects on food cup behavior during the 10-sec period before stimulus onset (ps > 0.1), indicating that RSC damage did not impact baseline levels of responding.
Figure 4.
Conditioned responding during presentation of the stimulus elements and the compound stimulus during the last day of Training, the Test session, and the Savings Sessions in sham-lesioned rats (A) and RSC-lesioned rats (B) in Experiment 2. * = p < 0.05.
During the Savings sessions, sham-lesioned rats immediately discriminated between the elements and the compound stimulus as soon as the original negative pattern procedures resumed (i.e., Savings session 1), reflecting the originally-acquired associative strength to the stimuli (Landeira et al., 2006). In contrast, RSC-lesioned rats needed several training sessions until they discriminated between the trial types. This was supported by the results of a repeated measures ANOVA, which revealed no main effect of Group, but a significant Group X Trial-type interaction [F (1, 14) = 6.9, p < 0.02] and a significant Group X Trial-type X Session interaction [F (5, 70) = 3.1, p < 0.02]. Subsequent decomposition of the three-way interaction revealed significant Group X Trial-type interactions during Session 1 [F (1, 14) = 11.3, p< 0.005], Session 2 [F (1, 14) = 20.8, p < 0.0005], and Session 3 [F (1, 14) = 9.6, p < 0.008]. In each case, sham-lesioned rats responded more during the element trials than the compound trials (ps < 0.002), but RSC-lesioned rats did not (ps > 0.2). For Sessions 4–6 there was a main effect of Trial-type (ps < 0.0001) and no significant Group X Trial-type interactions (ps > 0.4), indicating that by Session 4, RSC-lesioned rats again discriminated between the stimulus elements and compound.
3.3. Discussion
The results of Experiment 2 indicate that damage to RSC after rats have learned a negative patterning discrimination impairs subsequent expression of that learning. This is consistent with the notion that in intact rats, RSC may normally contribute to configural learning as it manifests in negative pattering. However, the finding that neither group of rats responded differentially to the elements versus the compound stimulus during the Test session was surprising, and suggests that factors other than RSC damage may have affected performance. For example, there was a significant gap (10 days) between the final day of Training and the Test day to allow for surgery and recovery that may have adversely affected performance. To address this, Experiment 3 used a chemogenetic approach (Designer Receptors Exclusively Activated by Designer Drugs; DREADDs) in which a virus containing the DNA for a synthetic inhibitory G-protein coupled receptor (Urban & Roth, 2015) was infused throughout RSC before any behavioral training (Robinson et al., 2014). The receptor is expressed but is otherwise inactive until the activating drug (clozapine-N-oxide, CNO) is administered systematically. In this way, we could train the rats in Experiment 3 without CNO on board (i.e., with RSC functioning normally), and then the next day, carry out the Test session with RSC silenced. The chemogenetic approach also addressed the possibility that damage to fibers of passage could have caused the effects in Experiment 2.
4. EXPERIMENT 3
4.1. Materials and Methods
4.1.1. Subjects
Twenty-four experimentally naïve male Long Evans rats (~250g) were obtained from Harlan Laboratories (Envigo). Rats were acclimated to the colony room and maintained as described in Experiment 1, except that the surgery recovery period was extended to ~2 weeks in order to allow time for full expression of the DREADDs receptor by the time of the Test phase (Smith et al., 2016; Armbruster et al., 2007).
4.1.2. Surgery
Rats were prepared for surgery as described in Experiment 1. Skull-penetrating burr holes were drilled above each intended infusion site at the coordinates described in Table 1. At each site, a 28-g Hamilton syringe was used to infuse 0.8μl (0.2 μl/min) one of three different DREADDs viruses (Addgene, Inc., Watertown, MA). Eighteen rats received infusions of pAAV8-hSyn-hM4Di-mCherry (abbreviated “Gi”), which contained the DNA for the hM4Di inhibitory receptor. Six rats received infusions of a control virus, pAAV8-hSyn-EGFP (abbreviated “GFP”), which was otherwise identical to the Gi virus but lacked the DNA for the inhibitory receptor (the control virus also contained a different fluorescent reporter, i.e., GFP vs. mCherry). The syringe remained in place for one minute before injection and two minutes after injection to reduce spread away from the target area. After recovery from surgery rats were food restricted to 85% of their free-food weight and maintained accordingly for the duration of the experiment.
4.1.3. Behavioral Procedures
Rats were trained in the same behavioral apparatus and behavioral procedures described in Experiment 2 (except that Savings sessions were not included).
4.1.4. Drug Preparation and Administration
CNO solution was freshly prepared immediately before the test session. CNO (NIMH Chemical Repository) was weighed and dissolved into dimethyl sulfoxide (1% DMSO) followed by 0.9% sterile saline to obtain a final concentration of 2 mg/ml. CNO (4mg/kg) or vehicle solution was administered intraperitoneally (i.p.) 30 min. before the Test session.
4.1.5. Data Analysis
The behavioral data were analyzed as in Experiment 2.
4.1.6. Verification of Virus Expression and Analysis
After behavioral training was complete, rats were euthanized and brain sections collected as described in Experiment 1. A compound fluorescent microscope (Axioskop I) was used to visualize the fluorescent reporters, mCherry and GFP. The percentage of sections exhibiting virus expression in RSC was determined for each rat. In addition, the level of viral expression was assessed by rating the extent of expression on a 0 – 5 scale (0 = no expression) as in our prior studies (Robinson et al., 2014, Todd et al., 2016). Virus expression in adjacent structures was also noted.
4.2. Results
4.2.1. Histology
As described below in section 4.2.2., rats that received the Gi virus infusions were subdivided into two groups after the Training phase. One group was designated to receive CNO during the subsequent Test session (Group Gi:CNO, n = 12) and the other group was designated to receive the vehicle solution (Group Gi:Veh, n = 6). Accordingly, we report the Gi expression for each of the two groups separately.
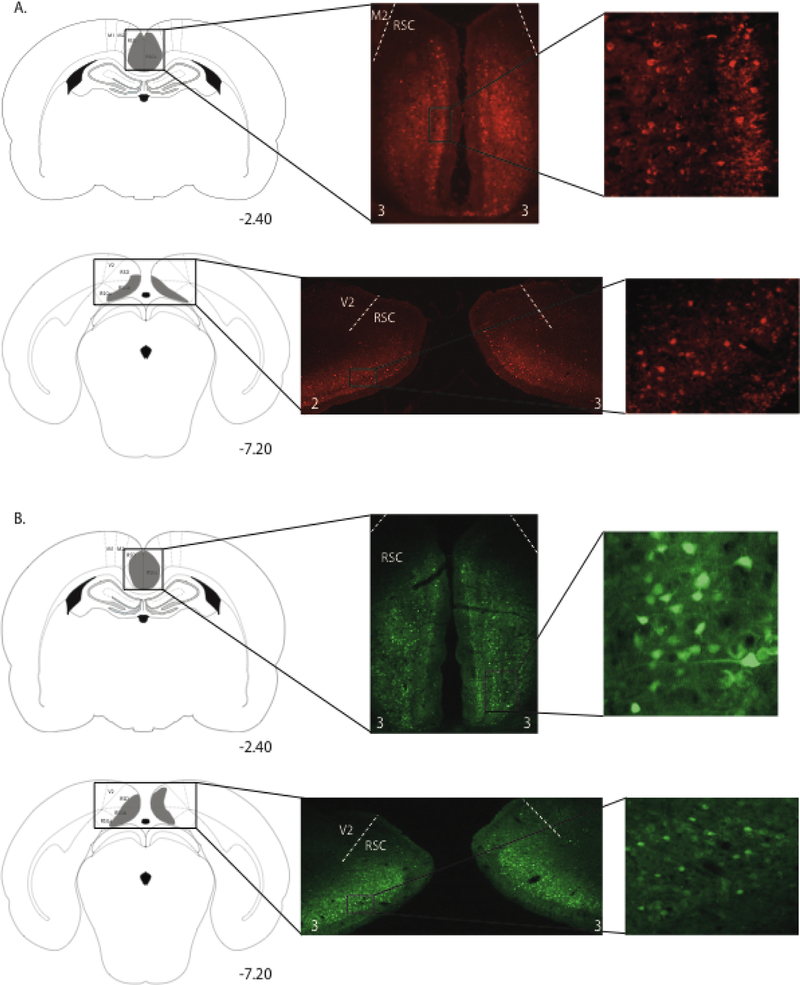
Virus expression is illustrated in Figure 5. One rat from Gi:CNO group and one rat from the Gi:vehicle group were removed from the study because expression was only unilateral in RSC. In the remaining rats in each of the Gi groups, expression of the mCherry reporter was bilateral and visible throughout the rostro-caudal extent of RSC as shown in Figure 5A. For Group Gi:CNO, the average percentage of RSC-containing sections (~27 per rat) with virus expression was 98 ± 1% and average virus expression rating was 1.9 ± 0.1. For Group Gi:vehicle, the average percentage of RSC-containing sections with virus expression was 99 ± 1% and average virus expression rating was 2.0 ± 0.3. In both Gi groups, expression was limited entirely to the RSC. For the rats that received the GFP virus (Group GFP:CNO), the average percentage of RSC-containing sections with virus expression was 99 ± 1% and average virus expression rating was 2.2 ± 0.3 (Figure 5B).
Figure 5.
Virus expression in anterior and posterior portions of the RSC in a rat from group Gi:CNO (A) and a rat from group GFP (B) in Experiment 3. Schematic diagrams in the left column depict the extent of virus expression within the RSC, with the numbers below each section indicating the A/P position in mm relative to bregma based on Paxinos and Watson (2009). Low magnification (middle column) and high magnification (right column) images show virus-expressing cells. The small white numbers at the bottom of each photomicrograph in the middle column indicate the expression rating for that section.
4.2.2. Behavior
During the Training phase, rats responded more during presentation of A than V (p < 0.004; mean for A = 3.3 sec, mean for B = 2.6 sec). Nevertheless, responding during each element was significantly greater than during the compound stimulus (ps < 0.002). Thus, responding during A-alone and V-alone trials was averaged together as in Experiments 1 and 2.
One rat in the Gi:CNO group and one rat in the Gi:vehicle group were excluded from the behavioral analyses because of low levels of responding during Training (> 3 standard deviations from the group means). Two rats in Group GFP:CNO exhibited responding during the compound stimulus that was at least as high as during the elements during the Training phase (i.e., failed to discriminate between the trial types). These rats were excluded because we were interested in understanding how inactivation impacted performance of the discrimination, thus it was critically important that rats first learned the discrimination. An analysis of levels of responding exhibited by the remaining rats in the two control groups (Gi:vehicle and GFP:CNO) using an independent measures t-test revealed no significant differences (ps > 0.2), indicating that CNO itself did not affect behavior. Thus, rats in those groups were combined into a single Control group (n = 8). The final sample size for Group Gi:CNO was n = 10.
4.2.2.1. Training phase
There were no pre-existing Group differences across the Training phase. This was supported by the results of an ANOVA that indicated that there was no main effect of Group [F (1, 21) = 0.1, p > 0.7], nor any significant Group interactions [Group X Trial type, F (1, 21) = 0.9, p > 0.4; Group X Session, F (14, 294) = 1.0, p > 0.5; Group X Trial-type X Session, F (14, 294) = 0.5, p > 0.9]. The only significant effects were a main effect of Trial-type [F (1, 21) = 35, p < 0.0001], a main effect of Session [F (14, 294) = 15, p < 0.0001], and a significant Trial-type X Session interaction [F (14, 294) = 14.2, p < 0.0001], indicating all rats learned the discrimination before being treated with CNO.
4.2.2.2. Test session
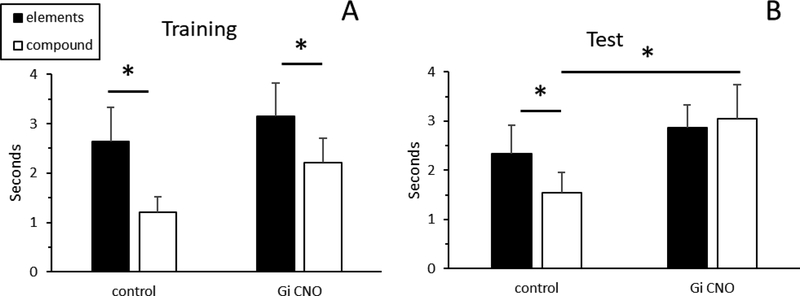
Prior to the Test session, rats received an injection of either CNO (Groups Gi:CNO and GFP:CNO) or vehicle solution (Group Gi:vehicle). Data obtained during the Test session is illustrated in Figure 6. A repeated measures ANOVA did not reveal any main effects of Group (p > 0.2) or Trial-type (p > 0.2), but did reveal a significant Group X Trial-type interaction [F (1, 16) = 5.5, p < 0.03]. Decomposition of the interaction showed that rats in the Control group exhibited more responding during presentation of the stimulus elements than the compound stimulus [t (7) = 3.9, p < 0.01], indicative of successful discrimination between the two trial types. However, rats in Group Gi:CNO did not discriminate between the stimulus elements and the compound stimulus [t (9) = 0.6, p > 0.6], instead exhibiting comparably high levels of responding on both types of trials. In addition, while there was no significant difference between the two groups during presentation of the stimulus elements [t (16) = 0.7, p > 0.5], responding to the compound stimulus was significantly higher in Group Gi:CNO compared to the Control group [t (16) = 1.8, p < 0.05]. There were no group differences in food cup behavior during the 10-sec period before stimulus onset (pre-CS period; ps > 0.1), indicating that RSC damage did not impact baseline levels of responding.
Figure 6.
Conditioned responding during presentation of the stimulus elements and the compound stimulus during the last day of Training (A) and during the Test session (B) in Experiment 3. * = p < 0.05.
4.3. Discussion
The results of Experiment 3 replicate the findings in Experiment 2 in that a manipulation of RSC after negative patterning training impaired subsequent performance of the discrimination. In the present experiment, a selective chemogenetic approach allowed for the transient silencing RSC neurons only during the Test session, which was carried out immediately after the training phase. We found that control rats continued to discriminate between the stimulus elements and the compound during the Test session, while silencing RSC activity resulted in high levels of responding to both the stimulus elements and the compound stimulus. This finding also suggests that the impairments observed in Experiment 2 cannot be attributed to damage of fibers of passage coursing through RSC.
5. GENERAL DISCUSSION
Three experiments tested the effects of RSC damage or inactivation on the ability of rats to learn or perform a negative patterning discrimination. In Experiment 1, RSC lesions carried out prior to training did not affect the ability of rats to learn to discriminate between reinforced presentations of an auditory (A) or visual (V) cue presented alone, and non-reinforced presentations of the simultaneous compound stimulus, AV. That is, both sham-lesioned rats and RSC-lesioned rats exhibited comparably high levels of conditioned responding during presentation of the stimulus elements and comparably low levels of responding during the compound stimulus. Similarly, Nelson and colleagues (2018) recently showed that pre-training lesions of RSC do not affect learning a negative patterning discrimination using a different behavioral procedure and lesion method. In their study, Nelson et al. (2018) trained rats in an operant version of the negative patterning procedure and separated training into distinct phases. That is, rats first learned to press a lever to obtain a food pellet when either of the auditory or visual cues were presented alone. Then, in the second phase of training, the cues were presented together and non-reinforced (these trials were intermixed with continued reinforcement of the stimulus elements). Pre-training neurotoxic lesions of RSC did not affect the ability to rats to discriminate between the elements and the compound stimulus. Thus, regardless of whether rats were trained using a Pavlovian negative pattering procedure after electrolytic RSC lesions (Experiment 1) or an operant procedure following neurotoxic RSC lesions (Nelson et al., 2018), there was no effect on learning the discrimination. Although in principal, non-configural strategies could be used to solve the negative patterning discrimination, other studies using procedures similar to ours have not found evidence for this (e.g., Bussey et al, 2000). Indeed, Nelson et al. (2018) demonstrated that rats with pre-training RSC lesions acquire the discrimination using a configural strategy.
The similar finding using both lesion methods is also noteworthy because it has been suggested that electrolytic lesions of RSC could produce behavioral impairments due to damage to fibers of passage, rather than damage to RSC neurons per se (Nelson et al., 2018). Thus, at least in the case of negative patterning, even potential damage to fibers of passage through RSC does not affect learning. This has additional relevance when compared to the pattern of effects of different lesion methods used to damage hippocampus prior to training in a negative pattering procedure. Specifically, it has been argued that early demonstrations of impairments in negative patterning following hippocampal lesions were due to the use of a more aggressive lesion approach (Rudy & Sutherland 1989), while more selective lesions of hippocampus were without effect (Davidson et al., 1993). In contrast, neither selective lesions of RSC neurons nor lesions that may produce more extensive damage of RSC affect learning the negative patterning discrimination.
In Experiment 2, rats were first trained until stable discrimination was exhibited and then received either sham-lesions or RSC lesions (i.e., a post-training lesion approach). When the negative patterning procedures were resumed, sham-lesioned rats immediately responded more during presentation of the elements compared to the compound stimulus. However, RSC-lesioned rats exhibited high levels of responding to both the elements and the compound stimulus. With several additional daily training sessions, RSC-lesioned rats were again able to perform the discrimination. In Experiment 3, as in Experiment 2, rats were trained until discrimination was stable before any manipulations took place. When RSC was temporarily silenced the next day, rats no longer discriminated between the elements and the compound stimulus, while control rats continued to respond more to the elements than the compound stimulus.
In light of the results of Experiments 2 and 3, a parsimonious account for the spared behavior in Experiment 1 and in the Nelson et al. (2018) study is that other brain regions/systems (e.g., hippocampus; Rudy & Sutherland 1989) can support negative patterning learning absent the RSC. One possibility is that regions such as hippocampus and RSC normally contribute to learning a negative patterning discrimination and forming configural representations, but that absent one or the other, there is enough learning supported by the remaining region to result in normal behavior. Indeed, the results of Experiments 2 and 3 suggest that that RSC may normally have a role in learning a negative patterning discrimination. That is, in intact rats, the RSC is functioning and involved in learning to discriminate between the reinforced elements and the non-reinforced compound stimulus. Hence, if RSC is subsequently taken off line, rats are impaired in performing the discrimination. Further, the finding in Experiment 2 that RSC-lesioned rats only needed four training sessions to exhibit significant discrimination after the lesion is suggestive of significant savings, which is perhaps supported by another region. In future studies, it would therefore be interesting to test the effects of having neither region available during learning (i.e., combined lesions of hippocampus and RSC). Similarly, the two regions could be selectively disconnected using chemogenetic approaches to test whether communication between them is essential for negative patterning.
An alternative account of the present findings is that RSC damage impairs any type of discrimination, not just those involving configural representations. Indeed, we have previously shown that rats with pre-training RSC lesions were slower than controls to learn a discrimination between one visual cue that was paired with foot shock, and another visual cue that was not reinforced (Todd et al. 2016). Similarly, Gabriel et al. (1987) found that aspirative / electrolytic lesions of RSC impacted discrimination by increasing responding to a CS-. We have also shown that pre-training RSC lesions impact Pavlovian feature negative discriminations (Keene & Bucci, 2008; Robinson et al., 2011). On the other hand, several studies have failed to find effects of RSC lesions on discrimination learning. For example, lesions of RSC had no effect on learning a complex appetitive discrimination using two visual cues (Nelson et al., 2014), nor did the affect early phases of learning in a conditional visual discrimination procedure (Bussey et al., 1996, 1997). Moreover, neither Nelson et al. (2018, Experiment 1) nor Experiment 1 in the present study found effects of pre-training RSC lesions on negative patterning. Thus, it seems unlikely that RSC damage has a general effect on learning discriminations. That said, it is important to note that all of the aforementioned studies involved pre-training lesions. Other than the studies in Experiments 2 and 3 here, few studies, to our knowledge, have examined the effects of post-training RSC lesions on previously learned discriminations. Thus, future research is needed to fully address whether post-training manipulations of RSC affect the ability to perform previously-learned discriminations.
We and others have posited that the RSC may have a fundamental role in combining the sensory features of a stimulus or a context, perhaps providing the basis for formation of complex representations (Bucci & Robinson, 2014; Kobayashi & Amaral, 2007; Wolbers & Buchel, 2005). Consistent with this idea, manipulations of RSC have repeatedly been shown to impair contextual fear conditioning (Keene & Bucci, 2008a, 2008b; Todd et al., 2016; Corcoran et al., 2011; Kwapis et al., 2015) and spatial learning (Harker & Wishaw, 2002; Vann & Aggleton, 2002; Keene & Bucci, 2009; van Groen et al., 2004; Cain et al., 2006). In addition, lesions of RSC abolish context pre-exposure facilitation effect (Todd et al., 2017). Perhaps most notably, RSC lesions or inactivation have also been shown to impair sensory preconditioning (Robinson et al., 2011; 2014), a procedure that provides a rigorous test of sensory integrationby requiring the formation an association between two neutral sensory cues (Brogden, 1939; Bouton, 2016). Inactivation of RSC specifically at the point in training when a tone and light are initially paired in the absence of reinforcement subsequently reduces conditioned responding to the tone after the light had been reinforced (Robinson et al., 2014). Nonetheless, the role of RSC in sensory integration has been called into question recently by the finding that RSC lesions made prior to training had no effect on the ability of rats to learn an operant negative patterning discrimination (Nelson et al., 2018). Instead, Nelson et al. (2018) have suggested that the contributions of RSC to learning and memory become apparent when there is a mismatch between prior learning and current contingencies (Nelson et al., 2018). While that may be true, the present findings indicate that RSC does indeed contribute to negative patterning; behavioral impairments are observed when RSC lesions or inactivation occurs following training. To the extent that our procedure involves the linking of various sensory stimuli into a unique configural representation, the present findings are consistent with the notion RSC may have a fundamental role in combining the sensory features of a stimulus or a context.
Pretraining lesions of retrosplenial cortex do not impair learning a negative pattering discrimination.
However, lesions or temporary silencing of retrosplenial cortex impairs the expression negative patterning.
This pattern of findings suggests that retrosplenial cortex is normally involved in forming configural representations.
8. ACKNOWLEDGEMENTS
This work was supported by National Science Foundation Grant IOS1353137 (DJB), NIH Grants T32 DA037202 (DIF) and K01 MH116158 (TPT), and a Neukom Institute for Computional Science CompX Faculty Grant (DJB). The authors thank Dr. Emily Cooper for valuable discussions that led to the present study and Ryan Monasch for assistance in carrying out Experiment 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. REFERENCES
- Armbruster BN, Li X, Pausch MH, Hertzile S, Roth BL. (2007) Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci 104: 5163–5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden WJ. (1939) Sensory preconditioning. J Exp Psychol. 25:323–332. [Google Scholar]
- Bouton ME (2010). The multiple forms of “context” in associative learning theory In Mesquita B, Barrett L.Feldman, & Smith ER(Eds.), The mind in context (pp. 233–258). New York, NY: Guilford Press. [Google Scholar]
- Bouton ME (2016) Learning and Behavior: A Contemporary Synthesis. Sinauer Associates. [Google Scholar]
- Bucci DJ, Robinson S. (2014) Toward a conceptualization of retrohippocampal contributions to learning and memory. Neurobiol Learn Mem 116: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. (1998) Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Journal of Comparative Neurology. 398:1–27. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Everitt BJ, Robbins TW, (1996). Dissociable effects of anterior and posterior cingulate cortex lesions on the acquisition of a conditional visual discrimination: facilitation of early learning vs. impairment of late learning. Behav. Brain Res. 82, 45–56. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Everitt BJ, Robbins TW, (1997). Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav. Neurosci 111, 908–919. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Dias R, Redhead ES, Pearce JM, Muir JL, Aggleton JP. (2000) Intact negative patterning in rats with fornix or combined perirhinal and postrhinal cortex lesions. Exp Brain Res. 134(4):506–19. [DOI] [PubMed] [Google Scholar]
- Cain DP, Humpartzoomian R, Boon F. (2006) Retrosplenial cortex lesions impair water maze strategies learning or spatial place learning depending on prior experience of the rat. Behavioural Brain Research. 170:316–325 [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzmán YF, Gao C, Jovasevic V, Radulovic J. (2011) NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci 31: 11655–11659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, McKernan MG, Jarrard LE. (1993) Hippocampal lesions do not impair negative patterning: a challenge to configural association theory. Behav Neurosci. 107(2):227–34. [DOI] [PubMed] [Google Scholar]
- Fanselow MS (1986). Associative vs. topographical accounts of the immediate shock deficitin rats: Implications for the response selection rules governing species specific defensive reactions. Learning and Motivation, 17, 16–39. [Google Scholar]
- Fanselow MS (1990). Factors governing one-trial contextual conditioning. Animal Learning & Behavior, 18, 264–270. [Google Scholar]
- Fanselow MS (2000). Contextual fear, gestalt memories, and the hippocampus. Behavioural Brain Research, 110, 73–81. [DOI] [PubMed] [Google Scholar]
- Gabriel M, Sparenborg SP, Stolar N, (1987). Hippocampal control of cingulate cortical and anterior thalamic information processing during learning in rabbits. Exp. Brain Res. 67, 131–152. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. (2002) Impaired spatial performance in rats with retrosplenial lesions: importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci. 22:1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. (2009) Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiol Learn Mem 91: 408–414 [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. (2008a) Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci 122: 89–97. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. (2008b) Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci 122: 1070–1077. [DOI] [PubMed] [Google Scholar]
- Kiernan MJ, & Westbrook RF (1993). Effects of exposure to a to-be-shocked environment upon the rat’s freezing response: Evidence for facilitation, latent inhibition, and perceptual learning. The Quarterly Journal of Experimental Psychology, 46, 271–288. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. (2007) Macaque monkey retrosplenial cortex: III. Cortical efferents. J Comp Neurol. 502:810–833. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Helmstetter FJ. (2015) The retrosplenial cortex is involved in the formation of memory for context and trace fear conditioning. Neurobiol Learn Mem 123: 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landeira-Fernandez J1, DeCola JP, Kim JJ, Fanselow MS. (2006) Immediate shock deficit in fear conditioning: effects of shock manipulations. Behav Neurosci. 120(4):873–9. [DOI] [PubMed] [Google Scholar]
- Nelson AJD, Hindley EL, Haddon JE, Vann SD, Aggleton JP, (2014). A novel role for the rat retrosplenial cortex in cognitive control. Learn. Mem 21, 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJD, Hindley EL, Vann SD, and Aggleton JP (2018). When is the rat retrosplenial cortex required for stimulus integration? Behav. Neurosci 132, 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. (2009) The rat brain in stereotaxic coordinates: compact sixth edition. Elsevier, London. [Google Scholar]
- Pearce JM. (1987) A model for stimulus generalization in Pavlovian conditioning. Psychol Rev. 94(1):61–73 [PubMed] [Google Scholar]
- Redhead ES, and Pearce JM (1995). Stimulus salience and negative patterning. Q. J. Exp. Psychol. B 48, 67–83. [PubMed] [Google Scholar]
- Rescorla RA, & Wagner AR (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement In Black AH & Prokasy WF (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). New York, NY: Appleton-Century-Crofts. [Google Scholar]
- Robinson S, Keene CS, Iaccarino HF, Duan D, Bucci DJ. (2011) Involvement of retrosplenial cortex in forming associations between multiple sensory stimuli. Behav Neurosci 125: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ. (2014) Chemogenetic silencing of neurons in the retrosplenial cortex disrupts sensory preconditioning. J Neurosci 34: 10982–10988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RT and Holland PC (1983). Savings test for associations between neutral stimuli. Animal Learning and Behavior, 11, 83–90. [Google Scholar]
- Rudy JW (2009). Context representations, context functions, and the parahippocampal-hippocampal system. Learning & Memory, 16, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, & Matus-Amat P (2004). Understanding contextual fear conditioning: Insights from a two-process model. Neuroscience and Biobehavioral Reviews, 28, 675–685 [DOI] [PubMed] [Google Scholar]
- Rudy JW, & O’Reilly RC (1999). Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience, 113, 867–880. [DOI] [PubMed] [Google Scholar]
- Rudy JW, and Sutherland RJ (1989). The hippocampal formation is necessary for rats to learn and remember configural discriminations. Behav. Brain Res. 34, 97–109. [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV. (2016) DREADDS: Use and application in behavioral neuroscience. Behav Neurosci. 130(2):137–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar J, Witter MP, van Strien NM, Cappaert NL. (2011) The retrosplenial cortex: intrinsic connectivity and connections with the (para)hippocampal region in the rat. An interactive connectome. Front Neuroinform 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Mehlman ML, Keene CS, DeAngeli NE, & Bucci DJ (2016). Retrosplenial cortex is required for the retrieval of remote memory for auditory cues. Learning & Memory, 23, 278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, DeAngeli NE, Jiang MY, Bucci DJ. (2017) Retrograde amnesia of contextual fear conditioning: Evidence for retrosplenial cortex involvement in configural processing. Behav Neurosci. 131(1):46–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Roth BL. (2015) DREADDs (Designer Receptors Exclusively Activated by Designer Drugs): chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol 55: 399–417 [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. (1992) Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol 315: 200–216. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM. (2003) Connections of the restrosplenial granular b cortex in the rat. J Comp Neurol 463: 249–263. [DOI] [PubMed] [Google Scholar]
- van Groen T, Kadish I, and Wyss JM (2004). Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav. Brain Res. 154, 483–491. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. (2002) Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci. 116:85–94. [PubMed] [Google Scholar]
- Vogt BA, Miller MW. (1983) Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol 216: 192–210. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Buchel C. (2005) Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. Journal of Neuroscience. 25:3333–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury CB (1943). The learning of stimulus patterns by dogs. J. Comp. Psychol 35, 29–40. [Google Scholar]