Abstract
Brain metastases encompass nearly 80% of all intracranial tumors. A late stage diagnosis confers a poor prognosis, with patients typically surviving less than two years. Poor survival can be equated to limited effective treatment modalities. One reason for the failure rates is the presence of the blood-brain (BBB) and blood-tumor barriers (BTB) that limit the access of potentially effective chemotherapeutics to metastatic lesions. Strategies to overcome these barriers include new small molecule entities capable of crossing into the brain parenchyma, novel formulations of existing chemotherapies, and disruptive techniques. Herein, we review BBB physiology and BTB pathophysiology. Additionally, we review the limitations of routinely practiced therapies and three current methods being explored for blood-brain/blood-tumor barrier disruption for improved delivery of chemotherapy to brain tumors.
Keywords: Blood-brain barrier, Blood-tumor barrier, permeability, disruption
Brain Metastases and Treatment Failure
Brain metastasis is an overwhelming morbidity of late stage cancer progression. Central nervous system (CNS) metastases occur in approximately 10% of all cancer types [1]. Recent increases in brain metastases are thought to be caused by improved control of systemic disease and increasingly sensitive imaging modalities [2]. Patients with CNS disease typically succumb within two years of diagnosis [3-5]. Therapies for brain lesions are mostly palliative, and rarely ever curative. These therapies include bulk surgical resection of the tumor(s), radiation therapy (either whole-brain and/or stereotactic), and/or systemic chemotherapy [6]. The blood-brain barrier (BBB), the brain’s innate defense system against blood delivered harmful substances, prevents delivery of most all efficacious systemic chemotherapies into brain tissue [7]. To improve efficacy of chemotherapeutics and small molecules, a way to bypass the BBB is necessary. In this article we discuss the utility and potential mechanisms of three widely explored BBB disruption techniques aimed at improving chemotherapeutic distribution to brain lesions. Understanding these techniques may result in the ability to progress treatment and affect survival of patients with this grim diagnosis.
The Blood-brain Barrier
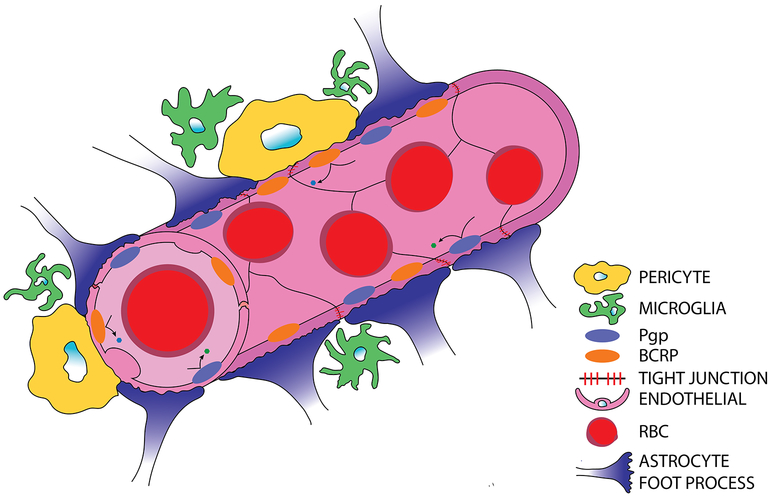
The BBB’s unique properties occur as a result of specific interactions between endothelial cells, pericytes, astrocytes, microglia and neurons, and their molecular components as seen in Figs. 1, Key Fig. 3a [8]. Proper regulation and function of the BBB is dependent on uninhibited interaction and communication between these cells.
Figure 1. Normal blood-brain barrier anatomy and physiology.
Brain capillary endothelial cells are tightly held to one another through continuous tight junction proteins and express P-glycoprotein (P-gp) and Breast Cancer Resistance Protein (BCRP) efflux transporters. Astrocytic end-feet processes further seal and support BBB integrity. Pericytes further regulate cerebral blood flow and BBB permeability. Microglia, the brain’s resident immune cells, can influence BBB permeability through inflammatory cascades and serve as the innate response to pathogens within the brain.
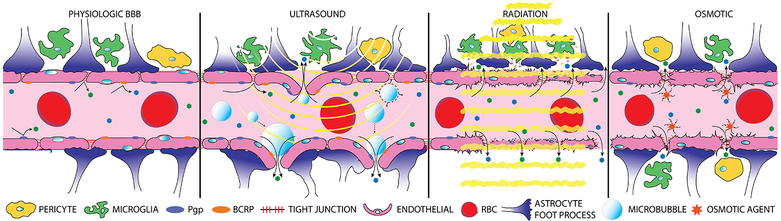
Figure 3, Key Figure. Blood-brain barrier disruption techniques.
Normal, undisrupted BBB with non-fenestrated endothelial cells sealed by tight junction proteins, further supported by astrocytic end-feet, pericytes, and microglia (a). Focused ultrasound (yellow curves) in combination with intravenously injected disrupts the BBB through cavitation and acoustic forces, ultimately leading to decreased molecular expression of tight junction proteins and an inflammatory response (b). Radiation therapy (yellow lines) disrupts the BBB through mechanisms of endothelial cell death and a neuro-inflammatory response from astrocytes and microglial cells (c). Hyperosmotic solutions are able to induce contraction and shrinkage of endothelial cells through a calcium dependent mechanism prompting widening of tight junctions (d).
Endothelial Cells
Brain microvascular endothelial cells (EC) form the foundation layer of the BBB and are crucial to the maintenance of its integrity. The ECs of the BBB are polarized in structure, as their luminal and abluminal surfaces have diverse biochemical and functional features; e.g. increased luminal γ-glutamyl transpeptidase expression [9]. The specialized BBB ECs have a high degree of expression of various transporters, including P-glycoprotein (P-gp, ABCB1), breast cancer resistance protein (BCRP, ABCG2), multi drug resistance protein, and various nutrient transporters [9]. These transporters move nutrients into the brain and efflux waste and other molecules out of the brain. Efflux pump expression is a major obstacle in overcoming drug delivery to the brain.
One of the most crucial features of ECs is their expression of tight junctions (TJ), which stabilizes the integrity of the BBB. The expression of TJ’s is induced by pericytes and results in a non-fenestrated vasculature preventing any unwanted “leaking” of luminal contents into the parenchyma of the brain. The TJ proteins are comprised of various transmembrane proteins including claudins, occludins, junctional adhesion molecules and accessory proteins, such ZO-1 and ZO-2 [10]. Another important trafficking molecule in normal BBB anatomy is major facilitator superfamily domain 2a (Mfsd2a). This protein is important for development of a functional BBB and is required for movement of docosahexaenoic acid into brain tissues [11, 12]. Importance of this transporter in BBB integrity and functionality is demonstrated by mice with genetically removed Mfsd2a that have decreased docosahexaenoic acid transport and increased disruption of the vascular barrier in brain [13].
Pericytes
Pericytes share the basement membrane with ECs and attach to them by ‘peg-socket’ junctions within the cerebral vasculature [14, 15]. During developmental stages and adult life, pericytes are recruited to EC of the BBB through several signaling methods, primarily the platelet derived growth factor-β pathway [14].
The presence of pericytes is critical for proper BBB function and development. These accessory cells directly influence permeability of the BBB by inducing EC TJ formation [16]. Next, pericytes regulate cerebral blood flow and waste clearance, disruption of which is associated with multiple brain pathologies, such as Alzheimer’s [15, 16]. Pericytes are shown to polarize astrocytic end-foot processes surrounding the BBB, and further are shown to regulate EC gene expression, increasing their viability through the Bcl2l2 pathway [17, 18].
Astrocytes
Astrocytic end-feet processes surround the BBB almost entirely. Their end-feet connect to the basement membrane through junctional molecules, including dystroglycan as well as channels like aquaporin 4, a molecule shown to maintain water homeostasis in the brain [10, 19]. Astrocytes play several roles in the regulation of the BBB. They assist in regulation of cerebral blood flow through Ca2+ signaling following neuronal perturbation [20]. Further, astrocytes are responsible for maintenance and formation of EC TJ. Sonic hedgehog, ang-1, and transforming growth factor signaling pathways influence this maintenance [10, 21]. Lastly, astrocytes directly impact vascular growth and proliferation through ang-1 and vascular endothelial growth factor (VEGF) secretion [10, 22, 23].
Microglia
Microglia are the resident immune cells of the brain. These cells play a role in both pro- and anti-inflammatory responses. Depending on their pro-inflammatory (M1) or anti-inflammatory (M2) phenotypes, they control inflammation through release of various molecular cytokines. Microglia are involved in angiogenesis, especially near EC tip cells, suggesting their influence in cerebral vascular development [24, 25]. However their role in maintenance of the integrity of a healthy BBB is still unknown.
The Blood-tumor Barrier
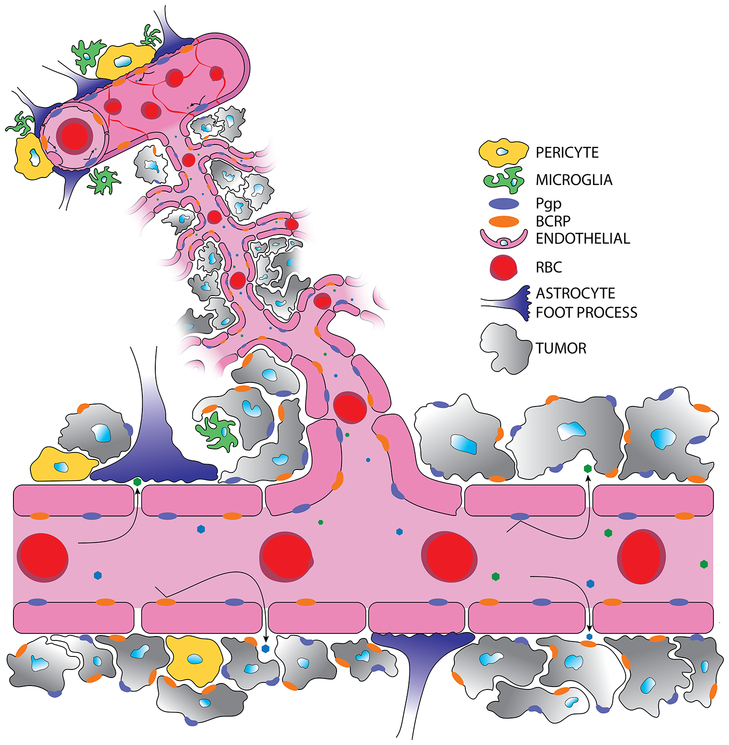
Of the primary cancers that migrate to the brain, lung, breast, melanoma, and renal cancers comprise the majority of metastatic brain tumors affecting ~50%, ~15%, ~10%, and ~5% of patients respectively [2]. Brain metastasis occurs when a circulating tumor cell, of a primary systemic tumor (i.e., breast, lung, melanoma, renal), detaches from the initial tumor mass and arrests in the brain microvascular capillary network, extravasates through the vessel wall into the perivascular space, and survives and proliferates into a new lesion [26, 27]. From initial metastatic colonization, the newly “seeded” brain metastatic tumor cells co-opt the brain vasculature eliciting neo-angiogenesis and microenvironment remodeling to promote tumor growth and further invasion. The newly formed neurovascular-tumor unit is termed the blood-tumor barrier (BTB, Fig. 2) and has differential properties concerning therapy pharmacokinetics and action in comparison to the intact BBB.
Figure 2. The blood-tumor barrier has altered anatomy and physiology.
Cancer cells coopt the cerebral vasculature and induce neo-angiogenesis resulting in fenestrated endothelia lacking tight junctional expression. Fenestrated, mal-formed vasculature allows for heterogeneous uptake of drug solutes. Cancer cells have increased expression of the P-gp and BCRP efflux transporters. At the BTB, less astrocytic end-foot processes and pericytes exist contributing to altered BTB integrity.
The BTB is inherently “leaky”, lacking tight junctions and astrocytic-endothelial contacts resulting in significant heterogeneous permeability from lesion to lesion within the brain [28, 29]. As lesions continue to outgrow their oxygen supply, angiogenesis occurs driven largely by VEGF. These new vessels are inherently leaky compared to the BBB phenotype. Dynamic angiogenesis during metastatic progression is different among brain lesions, which is thought to contribute to the heterogeneity in tumor permeability to chemotherapy. Additional contributions to increased permeability of the BTB include the lack of physiological TJ protein expression causing fenestra and discontinuous endothelia [28, 30]. Inconsistencies of junctional protein expression can allow for the passive permeability of cytotoxic therapies into tumor tissue. Interestingly mfsd2a is down-regulated at the BTB and promotes brain metastatic outgrowth due to lack of astrocytes promoting endothelial expression of mfsd2a, further contributing to BBB leakage in brain tumors [31].
Efflux mediated by P-gp (ABCB1) and BCRP (ABCG2) at the BBB limits distribution to normal brain of most chemotherapeutic agents. In the BTB setting, P-gp and BCRP have been found to be increased at the luminal membrane, as well as in the plasma membrane of tumor cells [32-34]. In preclinical mouse models, Elmquist and colleagues have demonstrated the active efflux of a host of agents used to treat melanoma and lung cancer brain metastases [32, 35, 36].
Other cellular and molecular properties of the BTB are prompted by astrocytes, pericytes and microglia. Astrocytes function to support and protect neuronal cells from damage and apoptosis through secretion of inflammatory cytokines such as TNFα, IL1, and IL6. Release of these cytokines encourages tumor proliferation and survival.[37] Additionally, astrocytes release exosomes containing miRNA-19a, which serves to induce loss of PTEN and promote further outgrowth and invasion of tumor cells within the brain [37, 38]. Microglia in the brain tumor microenvironment are known to secrete multiple growth factors and cytokines, such as TGFβ, TNFα, IL1, IL6, VEGF, EGF, and many metalloproteinases [39]. The molecular entities secreted by microglia promote tumor proliferation and invasion, as well as support angiogenesis [39]. Microglia cell populations also support colonization through the Wnt pathway, an effect attenuated with addition of Wnt inhibitors [40]. Pericyte subpopulations are known to contribute to BBB integrity and therefore permeability. Desmin+ pericytes are found in high concentrations in brain metastases and their presence is associated with high permeability [41].
Taken together, the distinct physical and molecular impedance the BTB plays in cancer treatment may seem insurmountable. In fact, the BTB, even in the presence of heterogeneous disruption, limits drug accumulation to the degree that there is limited apoptosis and cytotoxicity in nearly 90% of metastatic lesions in experiments utilizing preclinical models of breast cancer brain metastasis [42-47]. Inability of drugs to distribute to brain tumor tissues has led to the progression of techniques aimed at disrupting the BBB.
BBB/BTB Disruption for Increased Therapeutic Potential.
Disruptive CNS barrier techniques have increasingly become a research focus. Three highly investigated areas include the use of focused transcranial ultrasound (t-FUS) coupled with intravenously delivered microbubbles, hyperosmotic agents, and to a lesser degree radiation therapy that elicits transient changes in BBB permeability. Each of these applied to the treatment of metastatic brain lesions may lead to increased drug distribution and improve efficacy of many approved therapeutics. A list of ongoing or completed clinical trials utilizing disruption techniques can be found in Table 1.
Table 1.
BBB/BTB disruption techniques in ongoing or completed clinical trials.
| Title | Trial number | Mode of disruption | Type of disease |
|---|---|---|---|
| Super-selective Intra-arterial Repeated Infusion of Cetuximab for the Treatment of Newly Diagnosed Glioblastoma | [v] | Intra-arterial Mannitol | Glioblastoma Brain Neoplasm, Malignant EGFR Gene Overexpression GBM |
| Blood Brain Barrier Disruption (BBBD) Using MRgFUS in the Treatment of Her2-positive Breast Cancer Brain Metastases (BBBD) | [vi] | ExAblate Model 4000 Type-2 | Her-2 positive Breast Cancer, Brain Metastases |
| ExAblate Blood Brain Barrier Disruption (BBBD) for Planned Surgery in Glioblastoma | [vii] | ExAblate 4000 - Type 2 | GBM |
| Assessment of Safety and Feasibility of ExAblate Blood-Brain Barrier (BBB) Disruption for Treatment of Glioma | [ii] | ExAblate Neuro Model 4000 Type 2.0 | Glioblastoma |
| Blood-Brain Barrier Disruption Using Transcranial MRI-Guided Focused Ultrasound | [viii] | Transcranial ExABlate | Brain Tumor |
| ExAblate Blood-Brain Barrier Disruption for Glioblastoma in Patients Undergoing Standard Chemotherapy | [ix] | ExAblate 4000 type 2.0 | Glioblastoma Multiforme |
| Safety of BBB Disruption Using NaviFUS System in Recurrent Glioblastoma Multiforme (GBM) Patients | [x] | Neuronavigation-guided focus ultrasound system (NaviFUS) | GBM, Neoplasm, glioma |
| Safety of BBB Opening With the SonoCloud (SONOCLOUD) | [iv] | SonoCloud | Glioblastoma, Glioma, Brain Tumor |
| MRI Study of Changes in Blood-Brain/Tumor-Barrier Permeability in Patients With Brain Metastases During and After Radiotherapy | [xi] | SRS, Fractinated WBRT, Fractionated SRS | Brain Metastases (Breast, Lung, Melanoma, etc.) |
Focused Transcranial Ultrasound
Transient focused transcranial ultrasound (t-FUS) with concurrent administered intravenous microbubbles has been investigated as it can increase barrier permeability and improve distribution of CNS targeted therapeutics. Preliminary studies on mechanisms of BBB disruption indicate that the minimally invasive low intensity t-FUS coupled with the acoustic cavitation produced by the microbubbles cause molecular changes in tight junctions through decreased expression of claudin-5, ZO-1 and occludin, which enable the paracellular transport of genomic and chemical therapeutics as well as initiate inflammatory responses associated with damage-associated molecular patterns (Fig. 3b) [48, 49]. Combined with the higher hydraulic conductivity of interstitial fluid to the solid tumors, these changes have been used not only for higher tumor targeted delivery of many small molecule therapeutics but also for genes and immune cells [50-52].
Ultrasound influences the rate and extent of microbubble cavitation through its physicochemical properties that may lead to the production of stable or inertial cavitations. Under the influence of the FUS, microbubbles can undergo harmonic or non-harmonic oscillations which are responsible for the transient tight junction disruption; or undergo expansion and eventual collapse which can result in supplemental leakage or permanent damage [53, 54]. The amplitude and frequency of the ultrasound govern the mechanical index of the microbubbles and lead to enhanced disruption by specialized mechanisms including the push-pull action mediated broadening of ECs, high shear stress through micro-stream production, acoustic radiation, and pressure gradient mediated microbubble displacement [53, 55]. However, when microbubbles undergo unstable expansions and collapse it can lead to high EC lining pressure which may cause fragmentation of microbubbles resulting in mircro-jets and shock-waves. Additionally, microbubbles may also undergo free radical formation depending on microbubble lipid content and the degree of cell membrane permeabilization [56]. Altering the parameters of microbubbles enables their use as drug delivery devices as shown by a recent study that used a novel nitrogen based folate conjugated microbubble system encapsulated with methotrexate to increase its site-specific delivery and thus drug efficacy using high intensity focused ultrasound [57].
A recent study investigated the BBB/BTB penetration and cellular uptake of small (Doxorubicin) and large (ado-trastuzumab emtansine) molecules for an orthotopic brain metastasis of HER2 positive breast cancer model [50]. The study demonstrated that the small hydrophobic molecule showed a much higher (7-fold) concentration in the extravascular compartment along with high tumor penetration when FUS was used as opposed to control. In contrast, despite showing a 2-fold increase in the extravasation and slightly higher tumor penetration, the long (4-6d) drug circulation and transient effect of ultrasound diminished the overall effect when compared to control on day 5. Another study investigated the antitumor efficacy of polymeric polysorbate 80 modified paclitaxel nanoparticles and found an increase in the median survival of U87-Luc glioma-bearing mice to 37 days when to the control’s 26 days [58]. They demonstrated that the ultrasound mediated reduction in P-gp expression and tight junction disruption as well as apolipoprotein mediated endocytosis was responsible for the enhanced permeation of the nanoparticles. These pre-clinical studies in animal models have shown high efficacy leading to multiple trials to test the use of ultrasound in drug delivery for neurological diseases including Alzheimer’s [i], Parkinson’s Disease [ii] with dementia and multiple gliomas [iii].
Despite promising results, there are challenges such as high inertial cavitations of the microbubbles that cause vascular and tissue damage, reliance on expensive techniques like contrast magnetic resonance imaging to detect disruption, and lack of normalized experimental conditions. A study to reduce the inertial cavitation and provide an alternate treatment modality used closed loop cavitation mechanism to accurately provide 274.3 kHz of ultrasound; increased both survival and tumor regression by increased doxorubicin delivery in glioma bearing rats [59]. An alternate semiautomatic approach to deliver the ultrasound used unfocused ultrasound devices implanted in patients with glioblastoma. The study correlated local acoustic brain pressures with signal enhancement of greater than 10 percent observed through ultrasound which was more in gray matter [iv].
Radiation Therapy
The effects of radiation therapy on the BBB have been studied since the early 1980s [60]. However, the precedent of radiation therapy with subsequently timed chemotherapy was first suggested in 2002 by van Vulpen et. al [61]. The dose dependent response and time course of disruption of the BBB following radiation therapy is highly debated with the existence of contradictory reports. The pathophysiological changes following BBB disruption induced by radiation have been segregated into two main categories, acute and late phases [62-64]. Acute effects are thought to occur within the first 24 hours following cranial irradiation and, late effects are those described thereafter [65].
Mechanisms of radiation induced permeability (Fig. 3c) during the early stages after therapy include EC death and an increase in neuro-inflammation. Microvascular cell density and tight junction protein, ZO-1, expression was shown to decrease from 1 to 180 days following a single 10Gy whole brain radiotherapy dose [66]. A similar study reported EC density decreases at a single 10Gy dose are greatest at 10 days following radiation therapy [67]. Another study indicating the death of ECs as an early event following cranial radiation observed an increase in apoptotic ECs peaking at 12 hours after radiotherapy at doses ranging from 5Gy to 100Gy [68]. From these data, it appears evident that changes at the endothelial level occur, but the exact timing and mechanism are not clear.
The neuro-inflammatory response following radiation insult is characterized by activation of astrocytes, microglia, ECs, and their inflammatory mediators. Astrocytic and microglia activation following cranial exposure to radiation have been indicated as early as 4 hours and as late as 6 months following radiation treatments demonstrated by increased GFAP and CD11b staining [69, 70]. While these indicators of cellular activation are present, a number of cytokines and adhesion molecules are also variably increased following radiotherapy. In studies by Hong et al. and Kyrkanides et al. at four hours post radiation treatment, increases in CNS levels of TNFα, IL-1 β, and IL-6 were shown [71, 72]. In a similar study, Ruimeng et al. demonstrated the capacity for radiation therapy, at a dose of 50Gy, to increase immune cell activation and a panel of cytokines, including TNF-α and IL-6, at 12-weeks post treatment [73]. These research data suggest a critical role of the neuro-inflammatory response to radiation.
Taken together, the physiological responses to radiation alter the BBB/BTB in a manner which increases permeability. Data on the time course of increased permeability have been reported, but are variable among studies. Wilson et al. reported significantly altered permeability at 24 and 48 hours following cranial irradiation with a single dose of 20Gy, which could be rescued with anti TNF-α treatment [63]. Confirming this, a study of the rat BBB saw significant increases in permeability peaking at 24 hours posttherapy at a single dose of 20Gy to 4.4-, 10-, 38.2-, and 70-kDa FITC-dextran molecules [74]. Interestingly in Yuan et al.’s study, the time dependent increase in BBB permeability correlated well with an increase number of rolling leukocytes at the BBB, suggesting an increase in ICAM-1, a molecule expressed on the luminal surface of the BBB to aid in leukocyte trafficking to the brain parychema during an immune response [74]. Another study confirming early BBB disruption as soon as 24 hours following irradiation with single doses of 20 and 40Gy [75]. Each of these studies used a different means of irradiation, resulting in a specific dose rate for each respective study. This may provide information regarding the effect of dose rate on permeability related outcomes.
Another factor potentially contributing to permeability of the BBB/BTB may be fractionation schemes. Using daily doses of 4Gy for 3 consecutive days, Crowe et al. demonstrated enhanced permeability of irradiated tumors at 24 hours post-treatment compared to their contralateral sham treated counterparts when analyzed using DCE MRI [76]. Fractionation may elicit potentially altered permeability outcomes. Additionally, the particular mode of irradiation may play a role in pathophysiologic response to irradiation as well. When comparing broad beam radiation to micro-beam radiation therapy, Bouchet et all showed higher permeability increases in tumors treated with microbeam radiation therapy compared to those treated with conventional broadbeam radiotherapy at all time points, with a maximum at 7 days following radiation treatment [77]. Of note, there was increased permeability in lesions treated with BBRT compared to non-treated regions [77].
These studies all provide insight as to when the permeability changes may occur following radiation treatment. Contrary to this work another study by Murrell et al. noted that a dose of 20Gy in 2 fractions was not able to increase tumor permeability in a preclinical model of breast cancer brain metastasis [63]. Their work was subjected to only two time points however, one week and 11 days post radiation treatments. It is important to note that both authors may be suggesting the correct response. BBB/BTB opening following radiation therapy treatment may be transient or biphasic in nature, with points of high and low permeability in different phases, similar to that of stroke pathology [78].
Clinically there is evidence of breakdown of the BBB and BTB after radiotherapy as well. In a study of 30 patients receiving WBRT or SRS, with 64 analyzed metastatic lesions, radiotherapeutic treatments improved the permeability of initial low leaky tumors at 2 weeks and 1 month post therapy [79]. However, there was little or decreased permeability in initially very leaky metastases [79]. Zeng and colleagues also showed that in NSCLC patients treated with WBRT and concurrent gefitinib therapy, increased drug penetration was observed in accordance with escalation of radiation dose [80]. Lim et al. saw increased gadolinium deposition in peri-tumoral areas in 44 glioblastoma patients, but no change in untreated areas, indicating BBB/BTB disruption following radiation therapy [81]. These date provide evidence for increased permeability following radiation, but none give information elucidating the time course or magnitude of increased permeability.
Hyperosmotic Agents
Pre-clinical and clinical strategies have targeted the transient loosening or disruption of the BBB to increase permeability of therapeutics by techniques such as ultrasound, radiation or hyperosmotic agents like mannitol. One of the earliest techniques to disrupt the BBB using hyperosmotic agents was described by Neuwelt et al; wherein hyperosmotic mannitol administered via an intra-carotid injection was used to reversibly disrupt the BBB in canines [82]. The work demonstrated that when methotrexate was administered after the hyperosmotic agent, the drug levels were significantly higher (nearly 5-9 times as compared to control) in the ipsilateral cerebral hemisphere and contralateral hemisphere [82].
Although subsequent studies have failed to identify a singular mechanism underlying the mannitol mediated disruption, multiple distinct phenomena have been proposed. The most widely accepted theory of BBB opening is dehydration of the ECs followed by vasodilation induced shrinkage or contraction of the cells due to altered intracellular calcium levels (Fig. 3d) [83]. The resulting tension along with the calcium dependent actin and cadherin interaction leads to the widening of the tight junctions by increased bulk flow and solute diffusion. Other factors like nitric oxide, inflammatory mediators, bradykinin and mannitol induced tyrosine phosphorylation of Axl and beta-catenin have been implicated to augment the BBB disruption; however the exact mechanism is still not understood [83, 84].
Despite facing early challenges like potential neurotoxicity, osmotic disruption has been successfully used in pre-clinical models for improving drug therapy. Pharmacological agents such as oligonucleotides that have poor brain delivery have improved distribution by hyperosmotic mannitol mediated BBB disruption [85]. The study further demonstrated a high dissemination of the oligonucleotide in the ipsilateral brain regions including the striatum, somatosensory cortex and thalamus upon co-administration of 25% mannitol and the oligonucleotide which was modified with a hydrophobic moiety. In addition, the striatum, thalamus, motor cortex, hippocampus and somatosensory cortex showed Huntington gene mRNA silencing even a week after the initial therapy administration.
Concluding Remarks
Disruption of the BBB/BTB by ultrasound, radiation or hyperosmotic agents appears to be a promising aid to the delivery of chemotherapy for brain metastases. Studies using these disruptive techniques have shown to have an auxiliary impact on the brain distribution of traditional therapy. However many questions still remain unanswered like the length and extent of its effect, translation to the clinic, cost to benefit and many more (see outstanding questions). Still, these disruptive techniques in combination with chemotherapy offer a unique system to combat the otherwise poor prognosis of brain metastases.
To what extent can barrier efflux transporters be implicated in the failure of drug therapy?
Do different tumor types alter the blood-brain barrier integrity in discrete ways?
Are inflammatory mediators involved in propagating ultrasound and radiation mediated blood-brain barrier disruption?
Do barrier disruptive techniques have long term detrimental effects?
Can targeting the molecular structures in the blood-brain and or blood-tumor barrier reduce the effects of therapy failure due to tumor heterogeneity?
Can disruptive techniques like ultrasound and radiation affect tumor microenvironment and improve health outcomes?
Can inertial cavitations be completely eliminated in t-FUS to limit permanent damage?
How long does the blood-brain and or blood-tumor barrier remain functionally open after disruption?
Can the effects of osmotic disruption be controlled?
Will the effects of blood-brain and or blood-tumor barrier disruption be clinically relevant?
Can radiation and ultrasound be used to eradicate tumor masses directly without affecting healthy cells?
How long after blood-brain and or blood-tumor barrier disruption is the window of maximum permeability?
Can blood-brain and or blood-tumor barrier disruption with co-administration of drug therapy be made more cost effective?
Does the vascular and structural heterogeneity in the cranium attenuate effects of blood-brain and or blood-tumor barrier disruption?
How can blood-brain and or blood-tumor barrier disruptive techniques be modified for increased drug delivery to the other organs including the liver or kidney?
Highlights.
The blood-brain and blood-tumor barriers are responsible for therapy failure in many patients having brain metastases.
The blood-brain and blood-tumor barriers have specialized structures and efflux transporters which limit the brain permeation of majority of the drugs that are administered intravenously.
The neurovascular unit has been implicated in certain inflammatory responses that can aggravate the tumor invasion; however their exact role has not been elucidated.
The use of blood-brain and blood-tumor barrier disruptive mechanisms like radiation, ultrasound or osmotic agents; may transiently increase permeability which may improve therapeutic distribution to tumor.
Acknowledgements
This work was supported by the generous grant support from the National Cancer Institue (R01CA166067-05) and the National Institute of General Medical Sciences (P20GM121322-01A1).
Glossary
- Active Transport
The movement of molecules into the cell across the cellular membrane assisted by enzymes.
- Blood-Brain Barrier
The physicochemical barrier existing at the interface between the systemic circulation (blood) and brain limiting the passive and active transport of small molecules, proteins, toxins, and other potentially pathogenic entities into the brain.
- Blood-Brain Barrier Disruption
A physical opening, transient or persistent, of the BBB or BTB through a variety of mechanisms with the intent of increasing distribution of therapeutics into brain tissues.
- Blood-Tumor Barrier
Similar to the BBB in healthy individuals, the BTB is the interface between the blood and metastatic or primary tumor cells. This barrier is inherently “leaky” due to lack of tight junctions and neo-angiogenesis induced by the tumor.
- Brain Metastases
Tumors formed in the brain by cancer cells that have detached and migrated from a primary tumor site.
- Central Nervous System
Comprised of the brain and spinal cord, this complex of nerves controls the activities of the body.
- Endothelial Cells
Cells that line the interior (luminal) surfaces of blood and lymphatic vessels.
- Focused Transcranial Ultrasound
The use of low frequency ultrasonic waves, penetrating through the cranium to target particular sites within the brain.
- Glioblastoma
Also known as glioblastoma multiforme. A form of primary CNS tumor arising from one of the glial cell types.
- Neo-angiogenesis
The growth of new blood vessels.
- Passive Diffusion
The movement of molecules across a membrane or between cells without the need for energy. Molecules down a concentration gradient, from a high concentration to a lower concentration.
- Radiation Therapy
The use of X-rays, or similar forms of radiation, in the treatment of cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shen CJ et al. (2016) Controversies in the Therapy of Brain Metastases: Shifting Paradigms in an Era of Effective Systemic Therapy and Longer-Term Survivorship. Curr Treat Options Oncol 17 (9), 46. [DOI] [PubMed] [Google Scholar]
- 2.Nayak L et al. (2012) Epidemiology of brain metastases. Curr Oncol Rep 14 (1), 48–54. [DOI] [PubMed] [Google Scholar]
- 3.Witzel I et al. (2016) Breast cancer brain metastases: biology and new clinical perspectives. Breast Cancer Res 18 (1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yousefi M et al. (2017) Lung cancer-associated brain metastasis: Molecular mechanisms and therapeutic options. Cell Oncol (Dordr) 40 (5), 419–441. [DOI] [PubMed] [Google Scholar]
- 5.Glitza Oliva I et al. (2017) Melanoma Brain Metastases: Current Areas of Investigation and Future Directions. Cancer J 23 (1), 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matzenauer M et al. (2016) Treatment of brain metastases. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 160 (4), 484–490. [DOI] [PubMed] [Google Scholar]
- 7.Abbott NJ (2013) Blood-brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36 (3), 437–49. [DOI] [PubMed] [Google Scholar]
- 8.Chow BW and Gu C (2015) The molecular constituents of the blood-brain barrier. Trends Neurosci 38 (10), 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betz AL et al. (1980) Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res 192 (1), 17–28. [DOI] [PubMed] [Google Scholar]
- 10.Michinaga S and Koyama Y (2019) Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int J Mol Sci 20 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen LN et al. (2014) Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509 (7501), 503–6. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Zvi A et al. (2014) Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature 509 (7501), 507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreone BJ et al. (2017) Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 94 (3), 581–594 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rustenhoven J et al. (2017) Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol Sci 38 (3), 291–304. [DOI] [PubMed] [Google Scholar]
- 15.Zhao Z et al. (2015) Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163 (5), 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daneman R et al. (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468 (7323), 562–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto J et al. (2014) Tumor necrosis factor-alpha-stimulated brain pericytes possess a unique cytokine and chemokine release profile and enhance microglial activation. Neurosci Lett 578, 133–8. [DOI] [PubMed] [Google Scholar]
- 18.Armulik A et al. (2010) Pericytes regulate the blood-brain barrier. Nature 468 (7323), 557–61. [DOI] [PubMed] [Google Scholar]
- 19.Amtul Z and Hepburn JD (2014) Protein markers of cerebrovascular disruption of neurovascular unit: immunohistochemical and imaging approaches. Rev Neurosci 25 (4), 481–507. [DOI] [PubMed] [Google Scholar]
- 20.Manninen T et al. (2018) Computational Models for Calcium-Mediated Astrocyte Functions. Front Comput Neurosci 12, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y et al. (2014) Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One 9 (10), e110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Z et al. (2014) Ectopic expression of human angiopoietin-1 promotes functional recovery and neurogenesis after focal cerebral ischemia. Neuroscience 267, 135–46. [DOI] [PubMed] [Google Scholar]
- 23.Venkat P et al. (2018) Angiopoietin-1 Mimetic Peptide Promotes Neuroprotection after Stroke in Type 1 Diabetic Rats. Cell Transplant 27 (12), 1744–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki A (2017) Microglia and brain macrophages: An update. Neuropathology 37 (5), 452–464. [DOI] [PubMed] [Google Scholar]
- 25.Lee E and Chung WS (2019) Glial Control of Synapse Number in Healthy and Diseased Brain. Front Cell Neurosci 13, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramakrishna R and Rostomily R (2013) Seed, soil, and beyond: The basic biology of brain metastasis. Surg Neurol Int 4 (Suppl 4), S256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paget S (1989) The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev 8 (2), 98–101. [PubMed] [Google Scholar]
- 28.Lockman PR et al. (2010) Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res 16 (23), 5664–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah N et al. (2018) Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol Res 132, 47–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendricks BK et al. (2015) Novel delivery methods bypassing the blood-brain and blood-tumor barriers. Neurosurg Focus 38 (3), E10. [DOI] [PubMed] [Google Scholar]
- 31.Tiwary S et al. (2018) Metastatic Brain Tumors Disrupt the Blood-Brain Barrier and Alter Lipid Metabolism by Inhibiting Expression of the Endothelial Cell Fatty Acid Transporter Mfsd2a. Sci Rep 8 (1), 8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gampa G et al. (2017) Drug delivery to melanoma brain metastases: Can current challenges lead to new opportunities? Pharmacol Res 123, 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aryal M et al. (2017) Effects on P-Glycoprotein Expression after Blood-Brain Barrier Disruption Using Focused Ultrasound and Microbubbles. PLoS One 12 (1), e0166061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adkins CE et al. (2013) P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Front Pharmacol 4, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gampa G et al. (2019) Brain Distribution and Active Efflux of Three panRAF Inhibitors: Considerations in the Treatment of Melanoma Brain Metastases. J Pharmacol Exp Ther 368 (3), 446–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim M et al. (2019) Brain distribution of a panel of EGFR inhibitors using cassette-dosing in wild-type and Abcb1/Abcg2 deficient mice. Drug Metab Dispos. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wasilewski D et al. (2017) Reactive Astrocytes in Brain Metastasis. Front Oncol 7, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L et al. (2015) Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527 (7576), 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu SY and Watabe K (2017) The roles of microglia/macrophages in tumor progression of brain cancer and metastatic disease. Front Biosci (Landmark Ed) 22, 1805–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pukrop T et al. (2010) Microglia promote colonization of brain tissue by breast cancer cells in a Wnt-dependent way. Glia 58 (12), 1477–89. [DOI] [PubMed] [Google Scholar]
- 41.Lyle LT et al. (2016) Alterations in Pericyte Subpopulations Are Associated with Elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin Cancer Res 22 (21), 5287–5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adkins CE et al. (2016) Characterization of passive permeability at the blood-tumor barrier in five preclinical models of brain metastases of breast cancer. Clin Exp Metastasis 33 (4), 373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohn KA et al. (2016) Semi-automated rapid quantification of brain vessel density utilizing fluorescent microscopy. J Neurosci Methods 270, 124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bohn KA et al. (2017) Inhibition of VEGF and Angiopoietin-2 to Reduce Brain Metastases of Breast Cancer Burden. Front Pharmacol 8, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittapalli RK et al. (2017) Quantitative Fluorescence Microscopy Measures Vascular Pore Size in Primary and Metastatic Brain Tumors. Cancer Res 77 (2), 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittapalli RK et al. (2013) Quantitative fluorescence microscopy provides high resolution imaging of passive diffusion and P-gp mediated efflux at the in vivo blood-brain barrier. J Neurosci Methods 219 (1), 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terrell-Hall TB et al. (2017) Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 8 (48), 83734–83744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao B et al. (2018) Blood-brain barrier disruption induced by diagnostic ultrasound combined with microbubbles in mice. Oncotarget 9 (4), 4897–4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kovacs ZI et al. (2017) Disrupting the blood-brain barrier by focused ultrasound induces sterile inflammation. Proc Natl Acad Sci U S A 114 (1), E75–E84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arvanitis CD et al. (2018) Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption. Proc Natl Acad Sci U S A 115 (37), E8717–E8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mead BP et al. (2016) Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J Control Release 223, 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curley CT et al. (2017) Focused Ultrasound Immunotherapy for Central Nervous System Pathologies: Challenges and Opportunities. Theranostics 7 (15), 3608–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dasgupta A et al. (2016) Ultrasound-mediated drug delivery to the brain: principles, progress and prospects. Drug Discov Today Technol 20, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin Q et al. (2016) Inertial cavitation initiated by polytetrafluoroethylene nanoparticles under pulsed ultrasound stimulation. Ultrason Sonochem 32, 1–7. [DOI] [PubMed] [Google Scholar]
- 55.Chen KT et al. (2019) Theranostic Strategy of Focused Ultrasound Induced Blood-Brain Barrier Opening for CNS Disease Treatment. Front Pharmacol 10, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jia C et al. (2018) Generation of Reactive Oxygen Species in Heterogeneously Sonoporated Cells by Microbubbles with Single-Pulse Ultrasound. Ultrasound Med Biol 44 (5), 1074–1085. [DOI] [PubMed] [Google Scholar]
- 57.Lee S et al. (2018) Ultrasound-mediated drug delivery by gas bubbles generated from a chemical reaction. J Drug Target 26 (2), 172–181. [DOI] [PubMed] [Google Scholar]
- 58.Li Y et al. (2018) Mechanisms of enhanced antiglioma efficacy of polysorbate 80-modified paclitaxel-loaded PLGA nanoparticles by focused ultrasound. J Cell Mol Med 22 (9), 4171–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun T et al. (2017) Closed-loop control of targeted ultrasound drug delivery across the blood-brain/tumor barriers in a rat glioma model. Proc Natl Acad Sci U S A 114 (48), E10281–E10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lundqvist H et al. (1982) Permeability of the blood-brain barrier in the rat after local proton irradiation. Acta Radiol Oncol 21 (4), 267–71. [DOI] [PubMed] [Google Scholar]
- 61.van Vulpen M et al. (2002) Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? (Review). Oncol Rep 9 (4), 683–8. [PubMed] [Google Scholar]
- 62.Khatri A et al. (2011) Effect of radiation on the penetration of irinotecan in rat cerebrospinal fluid. Cancer Chemother Pharmacol 68 (3), 721–31. [DOI] [PubMed] [Google Scholar]
- 63.Wilson CM et al. (2009) Radiation-induced astrogliosis and blood-brain barrier damage can be abrogated using anti-TNF treatment. Int J Radiat Oncol Biol Phys 74 (3), 934–41. [DOI] [PubMed] [Google Scholar]
- 64.FitzGerald TJ et al. (2006) The effect of radiation therapy on normal tissue function. Hematol Oncol Clin North Am 20 (1), 141–63. [DOI] [PubMed] [Google Scholar]
- 65.Nordal RA and Wong CS (2005) Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol Biol Phys 62 (1), 279–87. [DOI] [PubMed] [Google Scholar]
- 66.Deng Z et al. (2017) Distinct Expression of Various Angiogenesis Factors in Mice Brain After Whole-Brain Irradiation by X-ray. Neurochem Res 42 (2), 625–633. [DOI] [PubMed] [Google Scholar]
- 67.Sandor N et al. (2014) Low dose cranial irradiation-induced cerebrovascular damage is reversible in mice. PLoS One 9 (11), e112397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pena LA et al. (2000) Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 60 (2), 321–7. [PubMed] [Google Scholar]
- 69.Mildenberger M et al. (1990) An animal model of prophylactic cranial irradiation: histologic effects at acute, early and delayed stages. Int J Radiat Oncol Biol Phys 18 (5), 1051–60. [DOI] [PubMed] [Google Scholar]
- 70.Kyrkanides S et al. (1999) TNF alpha and IL-1beta mediate intercellular adhesion molecule-1 induction via microglia-astrocyte interaction in CNS radiation injury. J Neuroimmunol 95 (1-2), 95–106. [DOI] [PubMed] [Google Scholar]
- 71.Hong JH et al. (1995) Induction of acute phase gene expression by brain irradiation. Int J Radiat Oncol Biol Phys 33 (3), 619–26. [DOI] [PubMed] [Google Scholar]
- 72.Kyrkanides S et al. (2002) Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res Mol Brain Res 104 (2), 159–69. [DOI] [PubMed] [Google Scholar]
- 73.Yang R et al. (2018) Inhibitors of HIF-1alpha and CXCR4 Mitigate the Development of Radiation Necrosis in Mouse Brain. Int J Radiat Oncol Biol Phys 100 (4), 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan H et al. (2003) Radiation-induced permeability and leukocyte adhesion in the rat blood-brain barrier: modulation with anti-ICAM-1 antibodies. Brain Res 969 (1-2), 59–69. [DOI] [PubMed] [Google Scholar]
- 75.Nakata H et al. (1995) Early blood-brain barrier disruption after high-dose single-fraction irradiation in rats. Acta Neurochir (Wien) 136 (1-2), 82–6; discussion 86-7. [DOI] [PubMed] [Google Scholar]
- 76.Crowe W et al. (2018) MRI Evaluation of the effects of Whole Brain Radiotherapy on Breast Cancer Brain Metastasis. Int J Radiat Biol, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bouchet A et al. (2017) Permeability of Brain Tumor Vessels Induced by Uniform or Spatially Microfractionated Synchrotron Radiation Therapies. Int J Radiat Oncol Biol Phys 98 (5), 1174–1182. [DOI] [PubMed] [Google Scholar]
- 78.Merali Z et al. (2017) Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One 12 (2), e0171558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teng F et al. (2017) Blood-tumor barrier opening changes in brain metastases from pre to one-month post radiation therapy. Radiother Oncol 125 (1), 89–93. [DOI] [PubMed] [Google Scholar]
- 80.Zeng YD et al. (2015) Blood-brain barrier permeability of gefitinib in patients with brain metastases from non-small-cell lung cancer before and during whole brain radiation therapy. Oncotarget 6 (10), 8366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim WH et al. (2018) Does radiation therapy increase gadolinium accumulation in the brain?: Quantitative analysis of T1 shortening using R1 relaxometry in glioblastoma multiforme patients. PLoS One 13 (2), e0192838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Neuwelt EA et al. (1979) Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest 64 (2), 684–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rapoport SI (2000) Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol 20 (2), 217–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilhelm I et al. (2008) Hyperosmotic stress induces Axl activation and cleavage in cerebral endothelial cells. J Neurochem 107 (1), 116–26. [DOI] [PubMed] [Google Scholar]
- 85.Godinho B et al. (2018) Transvascular Delivery of Hydrophobically Modified siRNAs: Gene Silencing in the Rat Brain upon Disruption of the Blood-Brain Barrier. Mol Ther 26 (11), 2580–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]