Abstract
A novel association can form between two memories even when the events to which they correspond are not physically present. For example, once an integrated memory has formed that binds the (when, where, and what) components of an event together, this memory can be triggered by one of its components, and updated with coincident information in the environment. The neural basis of this form of retrieval-mediated learning is unknown. Here, we show, for the first time, that NMDA receptors in the rat hippocampus are required for retrieval-mediated learning involving episodes, but not for the expression of such learning or for retrieval-mediated learning involving simple associations between the components of episodes. These findings provide a novel insight into learning processes that serve the desirable function of integrating stored information with new information, but whose operation might also provide a substrate for some of the cognitive symptoms of schizophrenia and Alzheimer's disease.
Introduction
When two novel events occur in close succession in the world, an association can develop between their memories that allows future encounters with one to retrieve the memory of the other. However, new learning can also take place when a memory, itself revived by association, coincides with a different event in the world (Holland, 1981; Hall, 1996). This form of retrieval-mediated learning enables stored memories to be updated with new information in the environment and provides a foundation for the otherwise puzzling observation that retrieved memories are susceptible to interference: the cost of allowing associatively retrieved memories to be updated is their susceptibility to interference (see also Sara and Deweer, 1982; Sara et al., 1980; Nader et al., 2000; Nader and Einarsson, 2010). There is an additional cost of such updating: the content of the resulting memories can be arbitrarily related to real-world relationships; and this process of updating could provide a substrate for the “loosening of associations” and their oft-bizarre nature evident in patients with schizophrenia (Bleuler, 1911) and various forms of dementia (e.g., Alzheimer's disease) (Doughty et al., 2010). The synaptic processes that underlie retrieval-mediated learning are unknown. Here, we investigated the involvement of NMDA receptor-dependent synaptic plasticity in the rat hippocampus, a brain region that functions abnormally in both people with schizophrenia (Harrison, 1999; Shenton et al., 2001) and those with Alzheimer's disease (Ball et al., 1985), in retrieval-mediated learning.
Extant research implicates the hippocampus in forming memories for patterns of stimulation (Kim and Fanselow, 1992; Ergorul and Eichenbaum, 2004; Ryan et al., 2010), and in the retrieval of memories with episodic content [i.e., what happened where and when (see also Fortin et al., 2004; Iordanova et al., 2009)]. Retrieval-mediated learning, however, requires not only the retrieval of stored memories, but also the updating of these memories with new information. We therefore investigated the contribution of synaptic processes in the hippocampus to retrieval-mediated learning. Two kinds of retrieval-mediated learning in rats were examined: one involved the retrieved memories for configurations of when and where a specific auditory stimulus (what) was presented (henceforth an episode) (Baddeley et al., 2002); and the other involved the memories for the components of such an episode (when or where an auditory stimulus was presented) (see Fig. 1). Briefly, after the memory formation stage, presenting the auditory stimulus was used to trigger the target memories at the same time that a novel event (a mildly aversive footshock) was delivered during the retrieval-mediated learning stage. The final, expression test assessed whether the stored memories had been updated with the new information about shock, by presenting rats with the when and where configurations (or their separate components) that should have been evoked and associated with shock during the retrieval-mediated learning stage. We examined the effects of blocking (1) synaptic transmission in the hippocampus (with muscimol) (cf. Iordanova et al., 2009), and (2) NMDA receptor-dependent synaptic plasticity in the same structure [with AP5 (d-2-amino-5-phosphonovaleric acid)] (Morris et al., 1990), on retrieval-mediated learning involving episodes or their components.
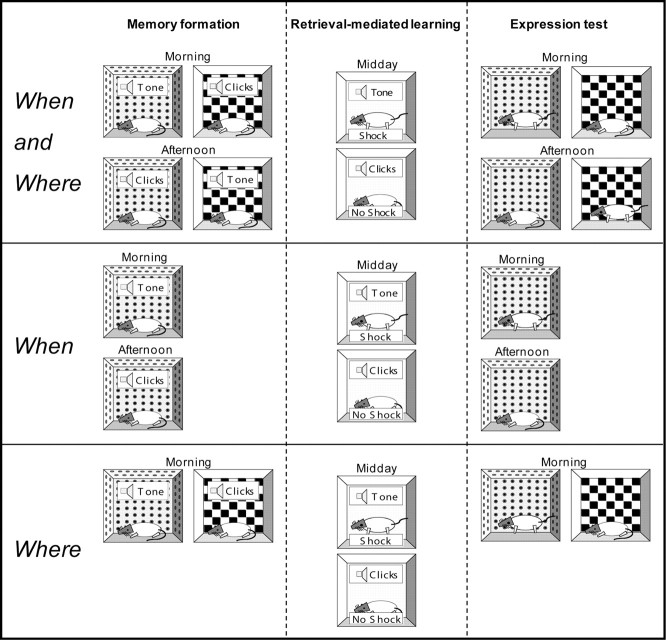
Figure 1.
A schematic for the behavioral procedures used in experiments 1 and 2. In experiment 1, rats received the three-stage when-where procedure outlined in the top row of the figure. During the memory formation stage, each rat received four patterns: morning + spotted + tone, morning + checked + clicks, afternoon + spotted + clicks, and afternoon + checked + tone. In the retrieval-mediated learning stage (days 5 and 6), rats received presentations of the tone which, in a third context at midday, were paired with shock. These trials should allow the evoked memories of the morning + spotted + tone and afternoon + checked + tone to become associated with shock. Presentations of clicks, and allied evoked memories, were paired with no shock. During the final expression test, the level of fear to the when-where configurations that had been paired with the tone and clicks during the memory formation stage was assessed. In the example shown here, control rats should be more likely to show fear to the when-where configurations that had been presented with the tone in the memory formation stage (i.e., morning + spotted and afternoon + checked) than to the remaining configurations. In experiment 2, rats received the three-stage when and where procedures outlined in the middle and lower rows, respectively. In the when and where subgroups, the memory formation stage involved presenting the tone or clicks with different times of day (when: morning or afternoon) or different contexts (where: spotted or checked). This retrieval-mediated learning stage should permit the time of day or context associated with the tone (or click) to become associated with shock (and no shock). During the expression test, the when or where components, which had been paired with the tone and clicks, were presented.
Materials and Methods
Subjects
Seventy-eight naive adult male hooded Lister rats (Rattus norvegicus; supplied by Harlan Olac Ltd.) were used in experiments 1a (n = 16), 1b (n = 24), 1c (n = 16), and 2 (n = 22). The rats were ∼6 months old immediately before surgery, and weighed between 292 and 349 g. The rats were housed in pairs in a temperature-controlled colony room illuminated between 8:00 A.M. and 8:00 P.M., with food and water available ad libitum in the home cages throughout the experiment. All procedures were conducted between 8:00 A.M. and 6:00 P.M. Animal husbandry and experimental procedures were conducted in accordance with the “principles of laboratory animal care” (Guide for the Care and Use of Laboratory Animals, NIH publication No. 85-23, revised 1985) and the UK Animals (Scientific Procedures) Act (1986).
Surgery and histology
Before behavioral testing, all rats were implanted with a double 26 gauge guide cannula (Plastics One). The ends of the guide cannulae were aimed at the dorsal hippocampus bilaterally by positioning them 2.0 mm below bregma through a hole drilled 3.0 mm posterior to and 1.5 mm lateral to bregma. Guide cannulae were fixed in position with dental cement and four screws, and anchored by Super Glue. Dummy cannulae were placed in these guides when the rats were not receiving microinjections. The microinjections were made using 33 gauge double microinjection cannulae, which were connected to a 1 μl glass syringe operated by an infusion pump (Harvard Apparatus), and were inserted into the guide cannulae. Microinjection cannulae projected a further 1 mm ventral to the tip of the guide cannulae. The amount of artificial CSF (aCSF), muscimol (1 μg/μl), and AP5 (6 μg/μl) infused into the dorsal hippocampus was 0.5 μl at the rate of 0.25 μl per minute, with a diffusion time of 1 min. Following surgery, the rats were allowed to recover until they reestablished their preoperative weights. The rats were handled and weighed daily during recovery. After behavioral testing, all rats were given a lethal overdose of sodium pentobarbitone (Euthatal); their brains were then extracted, postfixed for 24 h, and transferred to phosphate-buffered (0.1 m) 30.0% sucrose solution, in which they remained for a further 24 h. All brains were frozen in a −20°C cryostat and sectioned coronally. The 40 μm sections were collected on gelatin-coated slides, left to dry at room temperature for 24 h, and then stained with cresyl violet. The sections were examined under a microscope and the placements of dorsal hippocampal cannulae were verified with reference to the boundaries defined by Paxinos and Watson (1998).
Apparatus
The apparatus was the same as that described previously (Iordanova et al., 2009). Briefly, four chambers (23.0 × 24.5 × 21.0 cm, length × width × height; supplied by Campden Instruments Ltd.) were arranged in a 2 × 2 array for the memory formation and expression stages of all experiments. The walls and ceilings of the top pair of boxes in the array were covered with spotted laminated paper (black circles on a white background), whereas the walls and ceiling of the lower two chambers were covered with black and white checked laminated paper. A series of stainless steel rods served as the chamber floor. Each chamber was locally illuminated by a single 15 V, 24 W jewel light positioned in the center of the ceiling and received ambient illumination from the experimental room light. The chambers were placed in separate sound-attenuating cabinets, but the doors of the cabinets were left open to permit observation of each rat during the test phase through the transparent plastic front doors to the chambers. The behavior of the rats was recorded using a Panasonic VHS movie camera (model NV-M40).
Two additional chambers were used for the stage in which the retrieval-mediated learning occurred. These chambers were identical to those used during the other two stages with the exception that the walls were not decorated. These two chambers were placed below the set of four chambers, and were not locally illuminated by either a houselight or by the experimental room light. Also, these chambers were not housed in a sound-attenuating cabinet. A 0.5 s, 0.5 mA electric shock could be delivered to the grid floor of the chambers using a shock generator that was linked to a shock scrambler (Campden Instruments Ltd., models 521C and 521S, respectively). Each of the chambers was equipped with a speaker mounted above the ceiling that was used to deliver a 2 kHz tone and a 10 Hz click presented at an intensity of ∼78 dB (A; Brüel & Kjaer, type 2235).
Behavioral procedures
Experiments 1a, 1b, and 1c: Retrieval-mediated learning involving episodes.
Figure 1 (top row) provides a summary of the design used to assess retrieval-mediated learning involving when and where auditory stimuli were presented (Iordanova et al., 2009). On each of the memory formation days (days 1–4), rats were placed in two visual contexts (spotted and checked) in the morning and the same two visual contexts in the same sequence in the afternoon (spotted and then checked for half of the rats, and checked and then spotted for the remainder). In the morning, one auditory stimulus (e.g., a tone) was presented in one visual context (e.g., spotted), and the second auditory stimulus (e.g., a series of clicks) was presented in the other context (e.g., checked). In the afternoon, this arrangement was reversed (e.g., the clicks were presented in the spotted context and the tone in the checked context). The order in which the visual contexts were presented for the 4 d of exposure was the same for any given rat. Morning and afternoon sessions took place between 9:00 A.M. and 11:00 A.M., and 4:30 P.M. and 6:30 P.M., respectively; the interval between the morning and afternoon sessions within a day was ∼7 h. Each exposure to a visual context was 5 min, and there was an interval of 5 min between exposure to spotted and checked contexts during which the rats were placed in their holding cages and transferred back to the colony room. There were ten, 10 s presentations of each auditory stimulus in each of the four sessions. The interval between presentations was 20 s, and the first auditory stimulus was presented 20 s after the rat was placed in a given visual context. Assignment of visual context and auditory cue to the designated roles in the morning and afternoon was fully counterbalanced. After each session, the rats were placed back in their holding cages and taken back to the colony room where they remained between sessions.
During the critical retrieval-mediated learning stage (days 5 and 6), the rats received aversive conditioning at approximately midday (between 12:30 P.M. and 2:30 P.M.). On day 5, rats received two sessions. In the first session, they were placed in the undecorated chamber for 90 s and received three presentations of one of the auditory stimuli (e.g., the tone) that were each preceded by a 20 s interval. Each presentation of this stimulus terminated with the delivery of shock. The other session was identical with the exception that the remaining stimulus (e.g., clicks) was presented and no shocks were delivered. In between the two sessions, the rats were removed from the undecorated chamber and placed in their home cage in a dark room for 10 min. On day 5, for half of the rats the first session involved aversive conditioning and the second did not, and for the remainder, this arrangement was reversed. On day 6, the order in which the rats received the two types of sessions was reversed. The aim of experiment 1a was to determine whether the dorsal hippocampus is required during updating of past episodes with new information. To this end, rats received infusions of either aCSF (aCSF group; n = 8) or muscimol (muscimol group; n = 8) into the dorsal hippocampus immediately before each of the retrieval-mediated learning sessions on days 5 and 6. Muscimol is a potent agonist for GABAA receptors, and inhibits synaptic transmission within the area that it is infused. A previous experiment has shown that muscimol infusions into the dorsal hippocampus selectively disrupt the retrieval of configural memories for episodes when it is administered in the final, expression test (Iordanova et al., 2009). However, whether these infusions influence retrieval-mediated learning has not been examined. The aim of experiment 1b was to examine whether the retrieval-mediated learning involving episodes is impaired when NMDA receptor-dependent synaptic plasticity is disrupted. To do so, one group received aCSF infusions into the dorsal hippocampus immediately before the retrieval-mediated learning sessions (aCSF group; n = 8), while two other groups received AP5 infusions into the dorsal hippocampus either immediately before these sessions (AP5-before group; n = 8) or 30 min after the sessions (AP5-after group; n = 8). AP5 is a potent, selective NMDA antagonist; thus, an infusion of AP5 into the dorsal hippocampus serves to prevent synaptic plasticity by inhibiting activation of the NMDA receptors. Critically, an infusion of AP5 before retrieval-mediated learning should disrupt NMDA receptor-mediated synaptic plasticity during the process of updating past memories with new information. The additional group, which received an infusion of AP5 after retrieval-mediated learning, allowed us to determine whether NMDA receptors modulate this process of updating over a more extended period. The infusion of AP5 after training also provides a control for any possible permanent disruptive effects the drug may have on hippocampal function that are independent of modulating synaptic plasticity during the time period of interest. In experiment 1c, rats received no infusions before aversive conditioning on days 5 and 6 but, rather, received such infusions immediately before the final expression test (see below for details). If NMDA receptor-dependent synaptic plasticity is critical to retrieval-mediated learning, then infusing AP5 into the dorsal hippocampus immediately before the expression test should not influence test performance: retrieval-mediated learning would already have taken place, and such infusions should not influence the expression of retrieval-mediated learning because they should not block synaptic transmission (cf. Iordanova et al., 2009).
During the final expression test for retrieval-mediated learning (days 7 and 8), we assessed the tendency of rats to freeze in both contexts in the morning and afternoon. These sessions occurred at the same times of day as during the memory formation stage. Each test session was 3 min; rats were placed in their home cages and taken to the colony room in the 3 min interval between pairs of test trials, and in the interval between the morning and afternoon test sessions. On day 7, half of the rats received the test order spotted/checked in both the morning and afternoon test sessions, and the remainder received the test order checked/spotted in both sessions. For half of the rats in each of these subconditions, the first visual context that was presented in the morning was the one that should retrieve a memory of the auditory stimulus that had been paired with shock, and the second visual context should not, whereas in the afternoon, the opposite was the case. For the remaining rats, these arrangements were reversed. On day 8, the order of testing was reversed for each rat. In experiment 1c, half of the rats received aCSF infusions before the tests on day 7 and AP5 infusions before the tests on day 8, and the remainder received AP5 infusions before the tests on day 7 and aCSF infusions before the tests on day 8. Rats in experiments 1a and 1b received no infusions before expression tests on days 7 and 8. Finally, on day 9, the levels of freezing provoked by the tone and clicks were evaluated. This test was arranged in a manner identical to that of the aversive conditioning phase, but no shocks were delivered.
Experiment 2: retrieval-mediated learning involving the components of episodes.
The aim of experiment 2 was to assess whether any influence of our neural manipulations on retrieval-mediated learning was specific to retrieved memories for episodes involving the configuration of when and where something happened. Experiment 2 assessed retrieval-mediated learning involving the when or where components of episodes, using the stimuli and apparatus from experiments 1a, 1b, and 1c. For the when subgroup (n = 11; Fig. 1, middle row), in the memory formation stage, half of the rats received presentations of the tone in the morning and clicks in the afternoon sessions (days 1–4), and for the remaining rats, this arrangement was reversed. For half of the rats in the resulting subgroups, exposure and test sessions occurred in a spotted chamber, and for the rest, they occurred in a checked chamber. In the where subgroup (n = 11; Fig. 1, bottom row), rats were placed in one visual context (e.g., spotted), where they received one auditory stimulus (e.g., tone), and a second visual context (e.g., checked), where they received the other auditory stimulus (e.g., clicks; days 1–4). These sessions took place in the morning. The sequence in which the rats received the two visual contexts was arranged in the same way as in experiments 1a, 1b, and 1c.
In the retrieval-mediated learning stage (days 5 and 6), all rats received aversive conditioning trials in the undecorated chamber at midday, in a manner identical to that described for experiments 1a, 1b, and 1c: one of the auditory stimuli was paired with shock and the other was not. Immediately before these training sessions, on days 5 and 6, rats received an infusion of either aCSF (n = 8), muscimol (n = 7), or AP5 (n = 7) into the dorsal hippocampus.
Finally, all rats in the when subgroup received tests in which expression of what had been learnt in the previous stages was assessed over days 7–10. On each day, rats received a single test either in the morning or the afternoon. For half of the rats the sequence of tests was morning (day 7), afternoon (day 8), afternoon (day 9), morning (day 10), and for the remainder the sequence of tests across the 4 d was afternoon, morning, morning, afternoon. For rats in the where subgroup, the expression test sessions took place on days 7 and 8. These test sessions were arranged in the same fashion as in experiments 1a, 1b, and 1c, with the exception that rats only received a single pair of test sessions in the visual contexts in the morning (i.e., at the same time that they had been presented during memory formation).
Scoring and statistics
During the test, the behavior of the rats was video recorded and their freezing (or inactivity) was rated using a time-sampling procedure in which each rat was observed every 2 s and scored as either freezing/inactive or moving/active. Freezing was defined as the absence of all movements, except for those related to breathing; and ratings were made by an observer who was blind with respect to the treatment received by the rats (M.D.I.). A percentage freezing score was calculated for the proportion of the observations on which each rat froze during the tests. To reduce individual variability in levels of freezing, the percentage freezing scores were, in turn, converted into two ratio scores for rats in experiments 1a, 1b, and 1c: one for the morning test and one for the afternoon test. These morning and afternoon ratios were calculated in the same way: percentage freezing in the visual context that should be feared in the morning (e.g., spotted), because morning + spotted had previously accompanied the revalued auditory stimulus (Fig. 1, e.g., the tone), divided by the total percentage of freezing in the morning (in the spotted and checked contexts). Using this ratio, a score >0.50 in the morning indicates that the rat showed greater freezing to the configuration (e.g., morning + spotted) that should retrieve the revalued auditory stimulus (e.g., the tone) than to the configuration (e.g., morning + checked) that should retrieve the other auditory stimulus (e.g., the clicks). A score <0.50 in the afternoon indicates that the rat is showing more freezing to the configuration (e.g., checked + afternoon) that should retrieve the revalued auditory stimulus (e.g., the tone) than to the configuration (e.g., spotted + afternoon) that should retrieve the other auditory stimulus (e.g., the clicks).
In experiment 2, for the rats in the when condition, the ratios again took the form: freezing at the time of day that was previously paired with the now aversive auditory stimulus, divided by freezing at both times of day. A score >0.50 indicates that a rat is showing greater freezing at the time of day at which the now feared auditory stimulus (e.g., the tone in Fig. 1, middle row) had been presented during the first stage of training, than at the other time of day. The ratios for the where conditions in these experiments took the following form: freezing in the visual context that had signaled the auditory stimulus that was later paired with shock, divided by freezing during both visual contexts; a score >0.50 indicates that a rat is showing more freezing in the visual context that had signaled the now feared auditory stimulus (the tone in the example depicted in Fig. 1, bottom row) than in the other visual context.
Results
Retrieval-mediated learning involving episodes
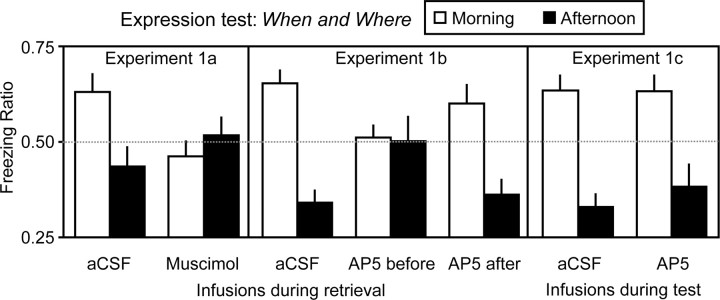
The behavioral results from the critical expression test in experiment 1 are summarized in Figure 2. Inspection of this figure reveals that when either muscimol (experiment 1a) or AP5 (experiment 1b) had been infused into the dorsal hippocampus (Fig. 3) immediately before retrieval-mediated learning involving entire episodes (when and where something happened), there was no evidence that such learning had taken place (Fig. 2, left and center). However, when AP5 was infused either after retrieval-mediated learning (experiment 1b) or immediately before the test (experiment 1c), there was evidence of such learning (Fig. 2, center and right). ANOVA revealed an interaction between the time of day at which testing occurred (morning or afternoon) and group (experiment 1a: F(1,14) = 17.61, p < 0.05; experiment 1b: F(2,21) = 8.55, p < 0.05); there was also a main effect of time of day in experiments 1a and 1b (smallest F(1,14) = 5.57, p < 0.05), but no main effect of group in either experiment (F values <1). Subsequent analysis confirmed that for the aCSF groups in experiments 1a and 1b, and the AP5-after condition in experiment 1b, the morning and afternoon ratios differed significantly from one another (minimum F(1,14) = 21.50, p < 0.05); and when the scores were pooled in such a way as to preserve the direction of the difference from chance (0.50) (Iordanova et al., 2009), the scores differed from 0.50 (minimum t(7) = 5.36, p < 0.05). The morning and afternoon scores for the corresponding muscimol and AP5-before groups in experiments 1a and 1b did not differ from one another (F values <1) or from chance (largest t(7) = 1.12, p > 0.05). The contrasting results from groups AP5 before and AP5 after reveals that the role of NMDA receptors in retrieval-mediated learning is temporally restricted. In a final test, in which the level of conditioned fear to the auditory stimuli was assessed, there was no interaction between the levels of fear to the stimuli directly paired with shock and no shock and group in either experiment 1a (aCSF: shock mean = 80.83%, no shock mean = 15.00%; muscimol: shock mean = 65.56, no shock mean = 18.89%; main effect of stimulus F(1,10) = 53.54, p < 0.05, no effect of group and no interaction, F values <1) or experiment 1b (aCSF: shock mean = 56.67, no shock mean = 10.83%; AP5 before: shock mean = 61.67, no shock mean = 4.17%; AP5 after: shock mean = 62.50%, no shock mean = 6.67%; main effect of stimulus F(1,21) = 95.39, p < 0.05, no effect of group and no interaction, F values <1). Unsurprisingly, conventional associative learning is not influenced by our neural manipulations of the hippocampus (Kim and Fanselow, 1992).
Figure 2.
Mean freezing ratios (+SEM) for experiment 1: retrieval-mediated learning involving episodes. Mean freezing ratios (+SEMs) in experiments 1a (left), experiment 1b (middle), and experiment 1c (right). Scores >0.50 in the morning, and scores <0.50 in the afternoon, indicate that retrieval-mediated learning has taken place and is evident at test.
Figure 3.
Histology for experiments 1 and 2. Position of the tips of the infusion cannulae, which were similarly placed in the dorsal hippocampus (specifically in CA1) in all experiments are shown (experiment 1a: closed square, aCSF; open squares, muscimol; experiment 1b: closed squares, aCSF; open squares, AP5 before; open triangles, AP5 after; experiment 1c: closed squares, aCSF on test 1; open squares, AP5 on test 1; experiment 2: closed squares,= aCSF; open squares, muscimol; open triangles, AP5).
In experiment 1c, infusing AP5 during the expression test had no effect on the ability of the when and where test patterns to access the memories that were formed during memory encoding, and were then updated during retrieval-mediated learning (Fig. 2, right). ANOVA revealed a main effect of time of day (F(1,15) = 41.48, p < 0.05), no effect of drug (aCSF or AP5), and no interaction (F values <1); and the morning and afternoon scores, pooled as above, differed from 0.50 (aCSF: t(15) = 4.66, p < 0.05; AP5: t(15) = 4.35, p < 0.05). This latter finding, together with the fact that blocking synaptic plasticity had a selective influence on retrieval-mediated learning involving episodes, but not the separate components of those episodes (see below), indicates that AP5 is not exerting its effect in experiment 1b through a state-dependent memory effect.
Retrieval-mediated learning involving the components of episodes
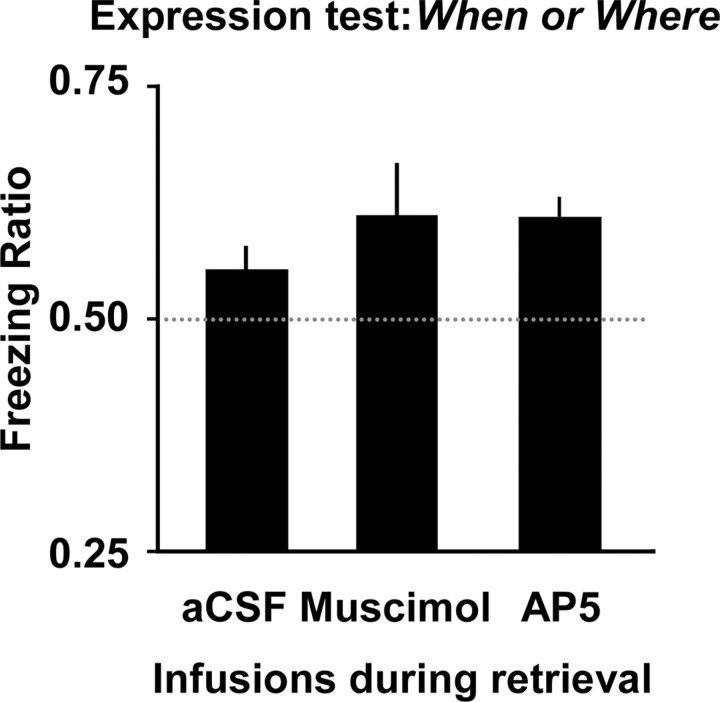
The results from the expression test in experiment 2 are shown in Figure 4. Inspection of this figure shows that the neural manipulations, which proved effective in disrupting retrieval-mediated learning in experiment 1 (i.e., infusions of muscimol and AP5 into the dorsal hippocampus during retrieval), were completely without effect on retrieval-mediated learning involving the components of episodes in experiment 2. Thus, there was no effect of infusing either muscimol or AP5, immediately before the retrieval-mediated learning sessions involving pairing one auditory stimulus with shock, on the later expression of fear to the time of day or context linked to that auditory stimulus. ANOVA revealed no effect of the nature of the component subgroup (when or where; F values <1), no effect of drug (aCSF versus either muscimol or AP5; largest F(1,16) = 1.38, p > 0.05), and no interaction between these factors (largest F(1,16) = 1.77, p > 0.05). In experiment 2, the ratios differed from chance (i.e., 0.50; when: t(10) = 3.16, p < 0.05; where: t(10) = 2.45, p < 0.05).
Figure 4.
Mean freezing ratios (+SEM) in experiment 2: retrieval-mediated learning involving the components of episodes. In this case, freezing ratios >0.50 indicate that retrieval-mediated learning has taken place and is evident at test.
Freezing levels
The overall levels of freezing during the tests, from which the ratios shown in Figure 2 were derived, were as follows. In experiment 1a, the level of freezing in group aCSF (mean = 23.4%) was higher than that exhibited by rats in group muscimol (mean = 16.2%; t(14) = 2.25, p < 0.05). In experiment 1b, an overall ANOVA revealed no differences between the groups (group aCSF mean = 17.7%, group AP5 before mean = 19.4%; group AP5 after mean = 18.44; F < 1). Similarly, in experiment 1c, the levels of freezing did not differ depending on whether rats were tested immediately following an infusion of aCSF or AP5 (aCSF mean = 11.5%; AP5 mean = 12.6%; t(15) = 0.695, p > 0.05). Finally, in experiment 2, an overall ANOVA revealed no differences in the levels of test freezing between rats infused with aCSF (mean = 38.2), muscimol (mean = 29.5), or AP5 (mean = 36.7; F < 1).
Discussion
Associative learning is often viewed as a simple process that allows relationships between stimuli in the world to be represented in the brain and thereby enable future adaptive behavior (Dickinson, 1980). The purview of associative processes is vastly extended once it is allowed that memories that have been retrieved by dint of the operation of associative processes can themselves enter into novel associations involving events in the world (Holland, 1981; Hall, 1996). Importantly, retrieval-mediated learning provides a fundamental mechanism for integrating established memories with new information. However, this form of learning also provides a basis for linking events that have no real-world relationship, and it could provide a substrate for distorted patterns of thinking and reasoning. It is, therefore, of both theoretical interest and potential therapeutic significance to understand the neural basis of retrieval-mediated learning. Here, we showed for the first time that retrieval-mediated learning involving the integrated memories for episodes requires NMDA receptor-dependent plasticity in the hippocampus. In contrast, retrieval-mediated learning involving the separate components of such episodes (i.e., when or where something happened) does not require hippocampal synaptic transmission or NMDA receptor-mediated plasticity. It is worth highlighting the fact that this dissociation clearly indicates that the involvement of NMDA receptor-dependent plasticity does not reflect a simple influence on conventional Pavlovian conditioning (involving the auditory stimuli): had this been the case, then our neural manipulations during retrieval-mediated learning should have exerted an equally disruptive effect on test performance across all of our behavioral assays. Moreover, direct assessment of fear conditioned to the auditory stimuli revealed no effects of our neural manipulations. Together, the results of experiments 1 and 2 have broad-ranging implications.
The general idea that retrieved memories become labile—subject to modification—is supported by studies examining the neurophysiology of memory permanence. For example, it has been argued that when previously acquired memories are retrieved, they undergo a process of reconsolidation that is underpinned by protein synthesis (Nader et al., 2000; Nader and Einarsson, 2010). The adaptive significance of such lability is that it could allow previously acquired memories to be updated with new information (see also Lee, 2008; Kuhl et al., 2010). The set of studies reported here indicates that NMDA receptor-dependent synaptic plasticity in the rat hippocampus mediates the updating of configural memories involving episodes with new information, but that this plasticity is not necessary for the updating of the components of such episodes.
The finding that the hippocampus plays a role in retrieval-mediated learning involving integrated memories for episodes (i.e., when and where something happened), but not the components of such memories (i.e., when or where something happened), is consistent with the view that this structure is involved in specific aspects of memory retrieval, more formally referred to as pattern completion (Rudy and O'Reilly, 1999). In both types of behavioral task, the presentation of an auditory cue provided the basis for a given training memory to be retrieved and linked to an aversive outcome. However, in only one case did the auditory stimulus retrieve the memory for a training pattern, whose separation from the memories of other overlapping training patterns requires configural processes: in the when and where procedure, the presentation of the tone was not linked to a specific time of day or context but, rather, to a combination or configuration of the two (Fig. 1, top row), whereas in the when or where procedures, the tone could simply retrieve a specific time of day or context in which they were embedded during training (Fig. 1, middle and bottom rows). The results of experiments 1 and 2 therefore provide support for the contentions that both configural and componential processes contribute to pattern memory, and that hippocampal NMDA receptors are required for updating the former but not the latter types of memory. However, this is not to imply that these two processes operate in parallel; they might interact (see below).
Hippocampal function is atypical in both schizophrenia (Harrison, 1999; Shenton et al., 2001) and Alzheimer's disease (Ball et al., 1985), and the cognitive symptoms of both include anomalous chains of associations. One potential basis for this particular cognitive symptom follows naturally from our findings. It is possible that retrieval-mediated learning about episodes or configurations (involving NMDA receptors in the hippocampus) ordinarily constrains retrieval-mediated learning about the components of such episodes (involving other neural mechanisms). Without this constraint, the extensive web of links involving the components of episodes would then form the basis for the anomalous chains of associations found in schizophrenia and Alzheimer's disease.
Footnotes
This research was supported by a grant from the Biotechnology and Biological Sciences Research Council (UK).
References
- Baddeley A, Conway MA, Aggleton JP. Episodic memory: new directions in research. Oxford: Oxford UP; 2002. [Google Scholar]
- Ball MJ, Hachinski V, Fox A, Kirshen AJ, Fisman M, Blume W, Kral VA, Fox H, Merskey H. A new definition of Alzheimer's disease: a hippocampal dementia. Lancet. 1985;325:14–16. doi: 10.1016/s0140-6736(85)90965-1. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox, or the group of schizophrenias (translated 1950 by Zinkin J) New York: International Universities; 1911. [Google Scholar]
- Dickinson A. Contemporary animal learning theory. Cambridge, UK: Cambridge UP; 1980. [Google Scholar]
- Doughty OJ, Lawrence VA, Al-Mousawi A, Ashaye K, Done DJ. Overinclusive thought and loosening of associations are not unique to schizophrenia and are produced in Alzheimer's dementia. Cogn Neuropsychiatry. 2010;14:149–164. doi: 10.1080/13546800902857918. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. Learning about associatively activated stimulus representations: implications for acquired equivalence and perceptual learning. Anim Learn Behav. 1996;24:233–255. [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Holland PC. Acquisition of representation-mediated conditioned food aversions. Learn Motiv. 1981;12:1–12. doi: 10.1016/j.lmot.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanova MD, Burnett DJ, Aggleton JP, Good M, Honey RC. The role of the hippocampus in mnemonic integration and retrieval: complementary evidence from lesion and inactivation studies. Eur J Neurosci. 2009;30:2177–2189. doi: 10.1111/j.1460-9568.2009.07010.x. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nat Neurosci. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- Morris RG, Davis S, Butcher SP. Hippocampal synaptic plasticity and NMDA receptors: a role in information storage? Philos Trans R Soc Lond B Biol Sci. 1990;329:187–204. doi: 10.1098/rstb.1990.0164. [DOI] [PubMed] [Google Scholar]
- Nader K, Einarsson EÖ. Memory reconsolidation: an update. Ann N Y Acad Sci. 2010;1191:27–41. doi: 10.1111/j.1749-6632.2010.05443.x. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. Sydney: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav Neurosci. 1999;113:867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Ryan L, Lin CY, Ketcham K, Nadel L. The role of medial temporal lobe in retrieving spatial and nonspatial relations from episodic and semantic memory. Hippocampus. 2010;20:11–18. doi: 10.1002/hipo.20607. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Deweer B. Memory retrieval enhanced by amphetamine after a long retention interval. Behav Neural Biol. 1982;36:146–160. doi: 10.1016/s0163-1047(82)90145-5. [DOI] [PubMed] [Google Scholar]
- Sara SJ, DeWeer B, Hars B. Reticular stimulation facilitates retrieval of a “forgotten” maze habit. Neurosci Lett. 1980;18:211–217. doi: 10.1016/0304-3940(80)90328-6. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Res. 2001;29:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]