Abstract
Background: Approximately one-half of cervical cancer cases in the United States occur in underscreened or never-screened women. We examined predictors to completing Papanicolaou (Pap) testing and whether a positive human papillomavirus (HPV) self-collection result affects Pap testing adherence among underscreened women.
Materials and Methods: Low-income women aged 30–65 years who reported no Pap testing in ≥4 years were recruited in North Carolina. Knowledge, attitudes, and barriers regarding cervical cancer and Pap testing were assessed by telephone questionnaires. We mailed self-collection kits for HPV testing and provided information regarding where to obtain affordable Pap testing. Participants received $45 for completing all activities. We used multivariable logistic regression to assess the predictors of longer reported time since last Pap (≥10 vs. 4–9 years) and of completion of Pap testing following study enrollment (follow-up Pap).
Results: Participants (n = 230) were primarily black (55%), uninsured (64%), and with ≤high school education (59%). Cost and finding an affordable clinic were the most commonly reported barriers to screening. White women and those with ≤high school education reported longer intervals since last Pap test. Half of the participants reported completing a follow-up Pap test (55%). Women with a positive HPV self-collection were five times more likely to report completing a follow-up Pap test than those with negative self-collection (odds ratio = 5.1, 95% confidence interval 1.4–25.7).
Conclusions: Improving awareness of resources for affordable screening could increase cervical cancer screening in underserved women. Home-based HPV self-collection represents an opportunity to re-engage infrequently screened women into preventive screening services.
Keywords: barriers, underscreened, human papillomavirus, cervical cancer screening, self-collection
Introduction
Widespread cervical cytology screening by Papanicolaou (Pap) testing has dramatically reduced cervical cancer mortality in the last half century in the United States.1 Despite the overall reduction in cervical cancer mortality due to national screening programs, racial and ethnic minorities and medically underserved women still face a higher risk of dying from cervical cancer in the United States.2
Cervical cancer incidence and mortality rates are higher in geographical areas with relatively higher poverty and lower educational level.3 In North Carolina, counties with lower economic prosperity have a higher incidence of and mortality from invasive cervical cancer.4 National surveillance data show that women with higher educational attainment are more likely to report having had a Pap test within the past 3 years.5 Other structural and intrapersonal factors are also barriers to cervical cancer screening, including time constraints, lack of insurance coverage, lack of health care access, difficulty with transportation, discomfort, cancer fatalism, and cultural concerns.6–13
Although the prevalence of insufficient cervical cancer screening varies worldwide, 81% of U.S. women in 2013 self-reported completing a Pap test in the preceding 3 years, whereas only 62% of uninsured women had been screened according to these national screening recommendations.5,14 In North Carolina, 60% of Medicaid-enrolled women aged 21–64 years were screening compliant,15 well below the Healthy People 2020 objective of 93%.16 While self-reported cervical cancer screening shows few differences between white and minority women,17 errors in overreporting of Pap testing are common among all women, particularly among minority women.18 which may account for much of the disparities in cervical cancer morbidity and mortality.2
Testing for infection with high-risk (oncogenic) human papillomavirus (HPV) is significantly more sensitive than Pap test alone for the detection of high-grade cervical precancer14 and has the potential to reduce the burden to patients and health care systems by allowing for a 5-year interval between screenings.14 However, in the United States, HPV testing is approved for clinical use only when conducted on cervical samples collected by a trained provider, which must be collected during pelvic examination, therefore presenting similar barriers as Pap testing.14 HPV testing on self-collected samples has been shown to be as sensitive as testing on provider-collected samples and may increase screening coverage among underscreened women.19
Few studies in the United States have assessed predictors of Pap test completion specifically among populations of low-income women who are overdue for cervical cancer screening.20–23
Data are currently needed to further identify barriers to, knowledge of, attitudes toward, and predictors of Pap testing among women with an infrequent history of Pap test screening, a population at elevated risk for developing invasive cervical cancer. Approximately half of cervical cancers diagnosed in the U.S. are in women never or infrequently screened,24 and the longer a woman goes without screening, the greater her risk for developing invasive cervical cancer.1
Therefore, we present data among low-income infrequently screened women in North Carolina to identify barriers, knowledge, attitudes, and predictors of cervical cancer screening among low-income women overdue for screening. Primary study outcomes were to assess the predictors of longer time since last Pap testing (≥10 vs. 4–9 years) and of completing in-clinic Pap testing after receiving HPV results from home-based self-collected samples.
Materials and Methods
Phase 1 of the My Body My Test study (MBMT-1) was conducted to determine the acceptability and feasibility of conducting mail-based self-collection for HPV testing for cervical cancer screening among infrequently screened women in North Carolina, as previously described.25 A total of 10 counties were included: Wake, Durham, Harnett, Guilford, Wayne, Cumberland, Robeson, Richmond, Hoke, and Scotland. Complete study methods have been described in detail elsewhere25 and are summarized below.
Study participants
Women were eligible to participate if they were between the ages of 30 and 65 years, reported not having had a Pap test in 4 years or more, were not pregnant, and were of low income (income at or below 200% of the poverty level). We conducted recruitment through a variety of outreach efforts, including referral from the United Way (Cary, NC) 2-1-1 social assistance hotline, newspaper advertisements, and posters and postcards placed in agencies and businesses serving low-income populations. At the end of noncrisis calls with female callers, United Way 2-1-1 staff gave brief information about the study, asked simple eligibility criteria questions, and ascertained interest in receiving a self-collection kit. If the caller met initial eligibility criteria and expressed interest, she was referred to the study hotline to receive more information about the MyBodyMyTest study hotline and complete full eligibility screening.
Procedures
Participants were mailed a kit to self-collect a cervicovaginal sample at home and mail it back for HPV DNA testing (“self-collection”). When HPV self-collection results were ready, agents at a call center run by the American Sexual Health Association (ASHA) contacted participants by phone to conduct a 20- to 25-minute “acceptability” questionnaire assessing factors including knowledge about HPV and cervical cancer, attitudes toward the self-collection and Pap testing, health history, and barriers to completing self-collection or Pap test. HPV self-collection results were verbally provided to participants near the end of this acceptability questionnaire. At the end of this phone call, participants were encouraged to complete an in-clinic Pap test and provided with information on local clinics providing free or low-cost Pap testing. After the participant self-reported completing an in-clinic Pap test by returning a postcard, or after 2 months without the participant reporting a Pap test, a “follow-up” questionnaire was administered by phone. Women who completed all study components received $45 in incentives: $30 for returning the self-collected sample and completing the acceptability questionnaire, $10 for completing an in-clinic Pap test, and $5 for completing the follow-up questionnaire.
The institutional review boards at the University of North Carolina at Chapel Hill and at East Carolina University approved the study.
Measures
The questionnaires contained ordinal, categorical, and Likert scale items. Time since last Pap test was based on participant responses to the acceptability questionnaire item, “When was your last Pap smear?” The term “Pap smear” was used in the questionnaire, given that pilot testing showed this wording to be more familiar to women than “Pap test.” Follow-up Pap test completion was determined by self-report during the acceptability or follow-up questionnaire or by returning a postcard reporting that the participant had completed a Pap test during the study.
Barriers to Pap testing were assessed by the open-ended question, “What are some reasons that you've not had a Pap smear recently?” with multiple responses permitted. Similar responses to this question were categorized for analysis. The acceptability questionnaire contained several closed-ended Likert scale questions eliciting the degree to which women perceived specific barriers, for example, “How hard do you think it would be to find a doctor or clinic that would give you a Pap smear?” For analysis, responses to these questions were dichotomized in the following manner: “not hard at all” versus “somewhat hard/very hard,” “strongly agree/somewhat agree” versus “somewhat disagree/strongly disagree,” and “very likely/somewhat likely” versus “very unlikely/somewhat unlikely.” Responses of “Do not know” and “Other” and missing data were excluded in calculation of p-values, although these counts and percentages are included in tables to aid interpretation.
Data analysis
Chi-square and Fisher's exact tests were used to assess differences in sociodemographic characteristics, barriers, and other factors of interest by time since last Pap test and by Pap completion. Odds ratios (ORs) and 95% confidence intervals (95% CI) were computed to identify predictors of (1) not having a Pap test in ≥10 years versus 4–9 years and (2) reporting completing a follow-up Pap test. Previous studies have defined “rarely screened” based on cutoffs of time since last Pap ranging from ≥5 to ≥9 years.26–28 We used a cutoff of ≥10 years to assess for predictors of notably longer intervals since last screening.
Both logistic models included participant characteristics listed in Table 1 (participant age, HPV self-collection result, race, and educational level), and barriers presented in Table 2 (locating a clinic to obtain a Pap test, locating an affordable clinic to obtain a Pap test, locating childcare, taking time off work, and miles to clinic). Items in Table 3 that may influence obtaining a Pap test were also included, for example, “In the past year, has a doctor said you should get a Pap smear?” and “How likely are you to get a Pap smear in the next 3 months?” Logistic regression models adjusted for potential confounding variables simultaneously. Insurance status was not included in final logistic regression analysis since more than 70% of women were uninsured or of unknown insurance status. No collinearity in the final model was detected.
Table 1.
Characteristics of Infrequently Screened Women in North Carolina by Years Since Last Pap Test and by Completion of Pap Test During Study
| Years since last Pap test, N = 162a | Completed Pap test during study, N = 145b | ||||||
|---|---|---|---|---|---|---|---|
| Overall | 4–9 Years, n (%) | ≥10 Years, n (%) | pc | Yes, n (%) | No, n (%) | pc | |
| N (%) | 230 | 132 (81) | 30 (19) | 80 (55) | 65 (45) | ||
| Age (years) | 0.72 | 0.33 | |||||
| 30–39 | 82 (36) | 42 (32) | 8 (27) | 23 (29) | 21 (32) | ||
| 40–49 | 83 (36) | 50 (38) | 14 (47) | 27 (34) | 27 (42) | ||
| 50–65 | 65 (28) | 40 (30) | 8 (27) | 30 (38) | 17 (26) | ||
| HPV self-test result | 0.54 | 0.01 | |||||
| Negative | 185 (86) | 109 (87) | 23 (82) | 61(77) | 58 (94) | ||
| Positive | 31 (14) | 16 (13) | 5 (18) | 18 (23) | 4 (6) | ||
| Not availabled | 14 | 7 | 2 | 1 | 3 | ||
| Race | 0.13 | 0.55 | |||||
| Non-Hispanic black | 127 (55) | 75 (57) | 13 (43) | 48 (60) | 33 (51) | ||
| Non-Hispanic white | 78 (34) | 45 (34) | 16 (53) | 25 (31) | 26 (40) | ||
| Othere | 22 (10) | 12 (9) | 1 (3) | 6 (8) | 5 (8) | ||
| Do not know/missing | 3 | 0 | 0 | 1 | 1 | ||
| Health insurance | 0.37 | 0.56 | |||||
| Medicaid | 53 (23) | 26 (20) | 8 (27) | 20 (25) | 15 (23) | ||
| Uninsured | 148 (64) | 93 (70) | 18 (60) | 49 (61) | 43 (66) | ||
| Other insurancef | 19 (8) | 11 (8) | 4 (13) | 9 (11) | 4 (6) | ||
| Missing | 10 | 2 | 0 | 2 | 3 | ||
| Educational attainment | 0.14 | 1.00 | |||||
| Some college or more | 81 (35) | 52 (39) | 7 (23) | 28 (37) | 23 (35) | ||
| GED/HS grad or less | 135 (59) | 79 (60) | 22 (73) | 47 (59) | 38 (58) | ||
| Refused/missing | 14 | 1 | 1 | 5 | 4 | ||
| Annual household income | 0.74 | 0.84 | |||||
| <$10,000 | 96 (42) | 53 (40) | 14 (47) | 37 (46) | 28 (43) | ||
| $10,000–$20,000 | 79 (34) | 46 (35) | 10 (33) | 24 (30) | 20 (31) | ||
| >$20,000 | 34 (15) | 26 (20) | 4 (13) | 13 (16) | 8 (12) | ||
| Missing | 21 | 7 | 2 | 6 | 9 | ||
| Urbanity | 1 | 0.42 | |||||
| Rural | 48 (21) | 28 (21) | 6 (20) | 15 (19) | 16 (25) | ||
| Urban | 182 (79) | 104 (79) | 24 (80) | 65 (81) | 49 (75) | ||
Percentages may not sum to 100% due to rounding.
Excludes 68 participants missing data on exact time since last Pap test.
Excludes 85 participants missing data on whether they completed a Pap test during the study period.
Calculated by Fisher's exact test. Excluded “Do not Know” in calculation of p-value.
HPV result not available because result was inconclusive (n = 4), sample leaked in transit (n = 5), participant did not return sample (n = 3), or participant did not return consent form for HPV testing (n = 2).
“Other” race includes Asian (n = 2), American Indian or Alaska Native (n = 6), Hispanic (n = 12), and multiple races (n = 2).
“Other” insurance includes Medicare, private or employer provided, and military insurance.
GED, General Educational Development test; HPV, human papillomavirus; HS, high school; Pap, Papanicolaou.
Table 2.
Barriers to Cervical Cancer Screening by Years Since Last Pap Test and Completion of Pap Test During Study
| Years since last Pap test (n = 162)a | Completed Pap test during study (n = 143)b | ||||||
|---|---|---|---|---|---|---|---|
| Overall, N (%) | 4–9 Years, n (%) | ≥10 Years, n (%) | pc | Yes, n (%) | No, n (%) | pc | |
| N | 224d | 132 (81) | 30 (19) | 79 (55)b | 64 (45) | ||
| What are some reasons that you've not had a Pap smear recently?e | |||||||
| Cost too much | 63 (28) | 44 (33) | 14 (47) | 0.05 | 19 (24) | 21 (33) | 0.73 |
| No health insurance | 17 (8) | 16 (12) | 1 (3) | 5 (6) | 6 (9) | ||
| Do not have time | 17 (8) | 14 (11) | 1 (3) | 5 (6) | 6 (9) | ||
| Low priority/did not perceive need/other health issues | 15 (7) | 13 (10) | 2 (7) | 4 (5) | 5 (8) | ||
| Afraid/nervous | 10 (4) | 8 (6) | 1 (3) | 4 (5) | 2 (3) | ||
| Have not been to doctor/no regular provider | 6 (3) | 4 (3) | 2 (7) | 3 (4) | 0 (0) | ||
| Limited transportation | 4 (2) | 1 (1) | 3 (10) | 1 (1) | 1 (2) | ||
| Do not know/not specified/missing | 92 | 32 | 6 | 38 | 23 | ||
| How hard do you think it would be to find a doctor or clinic that would give you a Pap smear? | |||||||
| Not hard at all | 154 (69) | 87 (66) | 17 (57) | 0.19 | 57 (72) | 39 (61) | 0.34 |
| Hardf | 60 (27) | 37 (28) | 13 (43) | 19 (24) | 20 (31) | ||
| Do not know | 10 (5) | 8 (6) | 0 (0) | 3 (4) | 5 (8) | ||
| How hard do you think it would be to find a doctor or clinic where you can afford a Pap smear? | |||||||
| Not hard at all | 95 (42) | 45 (34) | 11 (37) | 0.83 | 34 (43) | 26 (40) | 1.00 |
| Hardf | 115 (51) | 78 (59) | 17 (57) | 43 (54) | 31 (48) | ||
| Do not know | 14 (6) | 9 (7) | 2 (7) | 2 (3) | 7 (11) | ||
| How hard do you think it would be to find someone to watch your children so you could get a Pap smear? | |||||||
| Not hard at all | 97 (43) | 54 (41) | 9 (30) | 0.24 | 32 (41) | 28 (44) | 0.61 |
| No children at home | 91 (41) | 58 (44) | 13 (43) | 36 (46) | 24 (38) | ||
| Hardf | 34 (15) | 19 (14) | 8 (27) | 10 (13) | 11 (17) | ||
| Do not know | 2 (1) | 1 (1) | 0 (0) | 1 (1) | 1 (2) | ||
| How hard do you think it would be to take time off work to get a Pap smear? | |||||||
| Do not work | 52 (23) | 35 (27) | 8 (27) | 1.0 | 18 (23) | 14 (22) | 0.76 |
| Not hard at all | 118 (53) | 63 (48) | 14 (47) | 45 (57) | 32 (50) | ||
| Hardf | 49 (22) | 31 (24) | 7 (23) | 15 (19) | 15 (23) | ||
| Refused/do not know | 5 (2) | 3 (2) | 1 (3) | 1 (1) | 3 (5) | ||
| About how many miles do you think you need to travel to get a Pap smear? | |||||||
| <5 miles | 72 (32) | 39 (30) | 7 (23) | 0.22 | 23 (29) | 22 (34) | 0.61 |
| 5–10 miles | 88 (39) | 61 (46) | 10 (33) | 33 (42) | 28 (44) | ||
| >10 milesg | 57 (25) | 30 (23) | 11 (37) | 19 (24) | 11 (17) | ||
| Do not know | 7 (3) | 2 (2) | 2 (7) | 4 (5) | 3 (5) | ||
Percentages may not sum to 100% due to rounding.
Excludes 68 participants missing data on exact time since last Pap test.
Excludes 85 participants missing data on whether they completed a Pap test during the study period, and an additional 2 missing data for variables in the table.
Calculated by Fisher's exact test. Excluded “Do not Know” in calculation of p-value.
Excludes six participants missing data for variables in the table.
Multiple responses allowed.
“Somewhat hard” and “very hard” were combined.
“11 to 20 miles” and “more than 20 miles” were combined.
Table 3.
Knowledge, Beliefs, Behaviors, and Attitudes by Years Since Last Pap Test and Completion of Pap Test During Study
| Years since last Pap test (n = 162)a | Completed Pap test during study (n = 143)b | ||||||
|---|---|---|---|---|---|---|---|
| Overall, N (%) | 4–9 Years, n (%) | ≥10 Years, n (%) | pc | Yes, n (%) | No, n (%) | pc | |
| 224d | 132 (81) | 30 (19) | 79 (55) | 64 (45) | |||
| Do you think HPV can cause abnormal Pap smears? | |||||||
| Yes | 130 (58) | 80 (61) | 18 (60) | 1.0 | 46 (58) | 35 (55) | 1.0 |
| No | 32 (14) | 17 (13) | 3 (10) | 11 (14) | 9 (14) | ||
| Do not know/refusede | 62 (27) | 35 (27) | 9 (30) | 22 (28) | 20 (31) | ||
| In the past year, did you try to get a Pap smear, but were unable to get one? | |||||||
| Yes | 77 (34) | 52 (39) | 9 (30) | 0.41 | 26 (33) | 18 (28) | 0.59 |
| No | 145 (65) | 80 (61) | 21 (70) | 53 (67) | 45 (70) | ||
| Do not know | 2 (1) | 0 | 0 | 0 | 1 | ||
| In the past year, has a doctor said you should get a Pap smear? | |||||||
| Yes | 76 (34) | 44 (33) | 6 (20) | 0.19 | 27 (34) | 24 (38) | 0.60 |
| No | 144 (64) | 86 (65) | 23 (77) | 52 (66) | 37 (58) | ||
| Do not know | 4 | 2 | 1 | 0 | 3 | ||
| How likely are you to get a Pap smear in the next 3 months? | |||||||
| Unlikelyf | 61 (27) | 26 (20) | 11 (37) | 0.09 | 27 (34) | 13 (20) | 0.09 |
| Likely | 154 (69) | 99 (75) | 18 (60) | 50 (63) | 47 (73) | ||
| Do not know | 9 | 7 | 1 | 2 | 4 | ||
| If I got regular Pap smears, I would trust them to find cervical cancer when it is still treatable. | |||||||
| Agreeg | 212 (95) | 124 (94) | 28 (93) | 1.0 | 77 (97) | 60 (94) | 0.09 |
| Disagree | 6 (3) | 5 (4) | 1 (3) | 0 (0) | 3 (5) | ||
| Do not know | 6 | 3 | 1 | 2 | 1 | ||
| The North Carolina Breast and Cervical Cancer Control Program offers Pap smears. Did you hear about this program before this study? | |||||||
| Yes | 31 (14) | 16 (12) | 4 (13) | 0.77 | 13 (16) | 11 (17) | 1.00 |
| No/do not know | 191 (86) | 115 (87) | 26 (87) | 65 (82) | 53 (83) | ||
| Missing | 2 | 1 | 0 | 1 | 0 | ||
| Has a doctor or other medical professional ever told you that you have HPV, cervical cancer, or cervical disease? | |||||||
| Yes | 10 (4) | 7 (5) | 0 (0) | 0.35 | 3 (4) | 3 (5) | 1.00 |
| No | 211 (94) | 124 (94) | 30 (100) | 75 (95) | 59 (92) | ||
| Do not know/missing | 3 | 1 | 0 (0) | 1 | 2 | ||
| How worried are you about getting cervical cancer? | |||||||
| Not at all | 69 (31) | 38 (29) | 8 (27) | 0.92 | 20 (25) | 20 (31) | 0.28 |
| A little | 57 (25) | 31 (23) | 7 (23) | 23 (29) | 16 (25) | ||
| Moderately | 47 (21) | 34 (26) | 7 (23) | 12 (15) | 15 (23) | ||
| Very | 43 (19) | 23 (17) | 7 (23) | 21 (27) | 10 (16) | ||
| Do not know/missing | 8 | 6 | 1 | 3 | 3 | ||
Percentages may not sum to 100% due to rounding.
Excludes 68 participants missing data on exact time since last Pap test.
Excludes 85 participants missing data on whether they completed a Pap test during the study period, and an additional two missing data for variables in the table.
Calculated by Fisher's exact test. Excluded “Do not know” in calculation of p-value.
Excludes six participants missing data for variables in the table.
One respondent “refused” to reply.
“Very unlikely” and “somewhat unlikely” were combined. “Somewhat likely” and “very likely” were combined.
“Somewhat disagree” and “strongly disagree” were combined. “Strongly agree” and “somewhat agree” were combined.
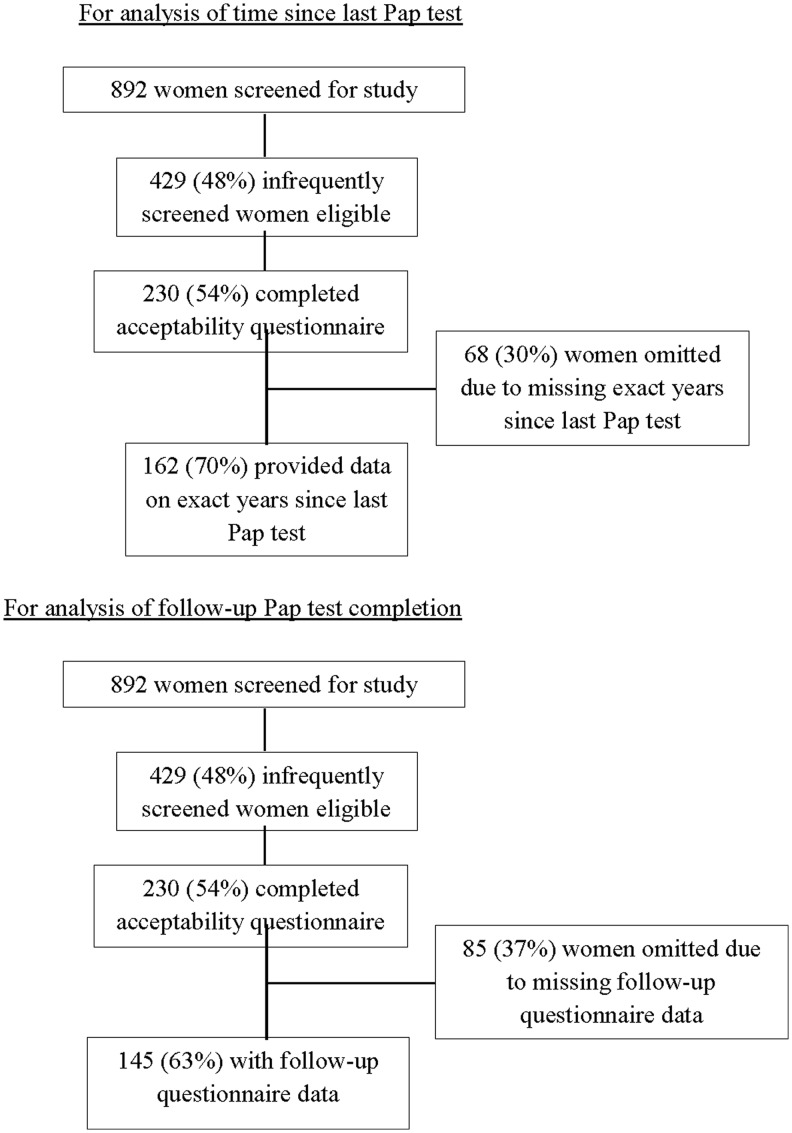
Among 429 women who met the eligibility criteria to participate, 227 women (53%) returned a self-collected sample and completed the acceptability questionnaire and 3 women (1%) completed the acceptability questionnaire without returning a self-collected sample. Women who returned a self-collected sample received their HPV results by phone during completion of the acceptability questionnaire. Of these 230 women, 68 women (30%) reported no cervical cancer screening in the last 4 years and were unable to report the exact time since prior screening; thus, a total of 162 women were included in the analysis of time since last Pap test (Fig. 1). The 68 women omitted from subsequent time since last Pap test analyses did not differ significantly from those who remained in the analysis with respect to demographic characteristics (data not shown). Of the 230 women who completed an acceptability questionnaire, an additional 6 women were excluded from analysis for Tables 2–4 due to missing data for the presented variables. Of the 230 women who completed an acceptability questionnaire, 145 started the follow-up questionnaire and are included in analysis of completion of follow-up Pap test (Fig. 1). Tests were two-tailed with statistical significance defined by p < 0.05 using SAS version 9.3.
FIG. 1.
Flow diagram of participants in study of infrequently screened women in North Carolina.
Results
Study participants
Eighty-one percent (132/162) of participants reported that their last Pap test was 4–9 years before, and 19% (30/162) reported completing their last Pap test ≥10 years before study participation (Table 1). Four women (2.5%) reported having never obtained a Pap test.
Most women were uninsured (64%), had a high school education or less (59%), and had a household income under ≤$20,000 annually (76%). Over half (55%) of the participants were non-Hispanic black women. Approximately 14% were self-collection positive for HPV infection. There were no significant differences in sociodemographic characteristics between women who reported 4–9 years versus ≥10 years since their last Pap test or between women who reported completing and reported not completing a follow-up Pap test (Table 1). Women who tested HPV self-collection positive were more likely to complete a follow-up Pap test (82%) than those who tested negative (51%), p = 0.01.
Attitudes and barriers to getting a Pap test
The most frequently reported barriers to Pap testing were cost of Pap testing (28%), lack of health insurance (8%), lack of time (8%), and low priority of getting screened (7%) (Table 2). Fear, lack of provider, and limited transportation were less commonly identified barriers to Pap testing. About one-quarter of the participants reported that locating a clinic to obtain a Pap test was somewhat hard or very hard (27%), yet over half reported that locating an affordable clinic was somewhat hard or very hard (51%) (Table 2). Approximately 22% of women reported that taking time off from work was somewhat or very hard (22%), whereas 53% responded that it was “not hard at all” and 23% did not work. A smaller proportion of women (15%) said that finding childcare was somewhat hard or very hard, 43% that it was “not hard at all,” and 41% of women had did not have children or their children were grown. Most women (71%) reported that they lived 10 miles or less from a clinic. Overall, no significant differences in barriers were found between women with a last reported Pap test of 4–9 years compared with ≥10 years.
Seventy-nine women (55%) reported completing a Pap test since enrolling in the study (Table 2). There were no statistically significant differences in barriers reported between women who completed a follow-up Pap test and those who did not.
Knowledge, beliefs, and behaviors regarding Pap testing
Most participating women were aware that HPV infection can cause abnormal Pap tests (58%), and almost all (95%) somewhat agreed or strongly agreed that they trusted Pap tests to find cervical cancer when it is still treatable (Table 3). Although participants reported a median of 3 visits to a health care provider in the past year (data not shown), of 164 who reported at least one visit to a provider, most (64%) reported not having received a physician recommendation for a Pap test during that time. Approximately one-third (34%) of women reported that they tried to get a Pap test in the past year but were unable to get one. Although 69% of women reported on the acceptability questionnaire being somewhat likely or very likely to get a Pap test in the next 3 months, there was no difference in reported Pap test intentions between women who did and did not report completing a follow-up Pap test (Table 3).
The North Carolina Breast and Cervical Cancer Control Program (NC-BCCCP) is a federally funded program that provides free or low-cost breast and cervical cancer screening and treatment services to uninsured women who earn at or below 250% of the poverty line. Only 22 (14%) of uninsured participants had heard of the program, although these women were likely to be eligible for this program (data not shown). There was little variation in reported attitudes toward and beliefs about Pap tests between women with a last Pap test 4–9 years versus ≥10 years before or between women who reported completing a Pap test while participating in the study compared with those who did not (Table 3).
Predictors of interval since last Pap test and of completion of follow-up Pap test
Table 4 presents the predictors of longer intervals since previous Pap test (≥10 years vs. 4–9 years) and of reporting completion of a follow-up Pap test. Significant predictors of ≥10 years since previous Pap test were being non-Hispanic white (OR = 4.8, 95% CI: 1.5–16.7 vs. non-Hispanic black) and having a high school education or less (OR = 3.6, 95% CI: 1.1–13.9 vs. some college). Other barriers may be associated with ≥10 years since last Pap test, although these estimates were relatively imprecise.
Table 4.
Predictors of Longer Interval Since Last Pap Test and Completing a Pap Test During the Study
| ≥10 years since last Pap testa | Completed Pap test during studyb | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| N = 162 | N = 143 | |
| Age (years) | ||
| ≥45 | Reference | Reference |
| <45 | 1.4 (0.4–4.7) | 0.5 (0.2–1.2) |
| HPV DNA self-test result | ||
| Negative | Reference | Reference |
| Positive | 1.5 (0.3–6.4) | 5.1 (1.4–25.7) |
| Race | ||
| Black | Reference | Reference |
| Non-Hispanic white | 4.8 (1.5–16.7) | 0.6 (0.2–1.6) |
| Other racec | 0.7 (0–6.3) | 1.6 (0.3–10) |
| Educational attainment | ||
| Some college | Reference | Reference |
| ≤GED/HS | 3.6 (1.1–13.9) | 1.3 (0.5–3.2) |
| In the past year, has a doctor recommended a Pap smear? | ||
| No | Reference | Reference |
| Yes | 0.4 (0.1–1.3) | 0.8 (0.3–2.0) |
| How likely are you to get a Pap smear in the next 3 months? | ||
| Unlikelyd | Reference | Reference |
| Likely | 0.4 (0.1–1.3) | 0.4 (0.2–1.2) |
| How hard do you think it would be to find a doctor or clinic that would give you a Pap smear? | ||
| Not harde | Reference | Reference |
| Hard to find a doctor | 1.7 (0.5–6.2) | 0.6 (0.2–1.9) |
| How hard do you think it would be to find a doctor or clinic where you can afford a Pap smear? | ||
| Not harde | Reference | Reference |
| Hard | 0.2 (0.1–0.9) | 0.6 (0.2–1.8) |
| How hard do you think it would be to find someone to watch your children so you could get a Pap smear? | ||
| Hard to find childcare | Reference | Reference |
| Not hard to find childcaree | 0.5 (0.1–2.6) | 0.5 (0.1–2.3) |
| Do not have children or children grown | 0.5 (0.1–2.8) | 0.9 (0.2–4.3) |
| How hard do you think it would be to take time off work to get a Pap smear? | ||
| Hard to take time off worke | Reference | Reference |
| Not hard | 1.9 (0.4–10.9) | 2.0 (0.6–6.6) |
| Do not work | 2.0 (0.4–11.9) | 1.2 (0.3–4.6) |
| About how many miles did you travel from home to work to get a Pap smear? | ||
| <5 miles | Reference | Reference |
| 5–10 miles | 0.5 (0.1–2.1) | 1.7 (0.6–4.6) |
| ≥11 milesf | 2.3 (0.6–9.5) | 3.6 (1.0–14.2) |
Logistic regression model adjusted for all variables simultaneously.
Referent group is women who obtained a Pap test 4–9 years ago, n = 132.
Referent group is women who did not complete a Pap test during the study, n = 64.
Other race includes Asian, American Indian or Alaska Native, Hispanic, and multiple races.
“Very unlikely” and “somewhat unlikely” were combined. “Somewhat likely” and “very likely” were combined.
“Somewhat hard” and “very hard” were combined.
“11 to 20 miles” and “more than 20 miles” were combined.
CI, confidence interval; OR, odds ratio.
Receiving a positive HPV self-collection result was a predictor of completing a follow-up Pap test (OR = 5.1, 95% CI: 1.4–25.7). Need to travel 11 miles or more to get a Pap test was positively associated with completing a follow-up Pap test, although significance was borderline (OR = 3.6, 95% CI: 1.0–14.2).
Discussion
Among 230 women in North Carolina with infrequent cervical cancer screening histories, cost and lack of health insurance were the most commonly reported barriers to cervical cancer screening. While most participants reported that it was not hard to locate a clinic to be screened for cervical cancer, over half reported that it was hard to find an affordable clinic. Most women were aware that HPV is a risk factor for cervical cancer and trusted Pap tests to catch cervical cancer while it can still be treated. White non-Hispanic women and those with a high school education or less were more likely to report a longer interval since their previous Pap test (≥10 vs. 4–9 years).
Our findings that women who received an HPV-positive self-collection result were more likely to report completing a Pap test than those with an HPV-negative result suggest that self-collection HPV-positive status motivated subsequent in-clinic screening. While our study was not designed to allow for detailed assessment of self-collection HPV results as a motivator for in-clinic screening, our findings are consistent with other studies that found relatively high clinic attendance among underscreened women with positive HPV results from self-collection.29,30 For example, studies in Europe found 87%–95% attendance at in-clinic follow-up among women with a positive HPV result from home self-collection.31,32 A randomized control trial with underscreened women in North Carolina is currently underway, which will evaluate completion of in-clinic screening among self-collection HPV-positive women in more detail (NCT02651883).
Low-income underscreened women face multiple barriers to completion of in-clinic cervical cancer screening. Our finding that women with lower educational levels were more likely to have a longer interval since their last Pap test is consistent with previous studies that found that relatively less educated women were less likely to obtain Pap testing compared with those with more education.22,33,34 In our study population, non-Hispanic white women were more likely to report longer intervals since their last Pap test compared with non-Hispanic black women. This finding is consistent with national survey data finding somewhat higher self-reported rates of screening in the previous 3 years among black women compared with white women.35 However, in contrast, one study that compared self-reported screening history with medical records found overreporting among all women, but particularly high overreporting among black and Hispanic women, with adjusted screening rates estimated as low as 58% among black women compared with 73% among white women.18 It is important to note that mortality from invasive cervical cancer is highest among black women, making them overall a higher risk group for adverse outcomes compared with white women.36
Our finding that the cost of screening was a major barrier is consistent with previous studies among primarily low-income white women in Appalachia (aged 40–64 years),8,22,23 women residing in public housing,37 and low-income black women in the American South,38,39 who commonly reported financial circumstances, including lack of health insurance, as a barrier to screening.
HPV self-collection alone, however, does not remove cost as a primary barrier to in-clinic follow-up screening. Cost and lack of insurance were the most commonly reported barriers to screening, and more than half of our study population was uninsured, calling attention to the need to make all steps of the care continuum—HPV self-collection, in-clinic screening, follow-up diagnostics, and treatment, when needed—affordable and accessible to all women. Given that 18% of HPV-positive women in our study reported not having completed follow-up Pap testing, additional efforts are needed to assist some high-risk women to overcome barriers.
Some of our findings were not consistent with other studies of barriers to cervical cancer screening. We found that difficulty taking time off from work was not a significant predictor of longer interval since last screening or of completing a follow-up Pap test, which may be because our medically underserved women faced relatively more barriers overall than the general female population, and one-quarter of our participants reported not working. Previously reported evidence as to whether employment status predicts cervical cancer screening adherence has been mixed, possibly due to differing definitions of employment status between studies.22,37 Attitudes such as fear, discomfort, or embarrassment were not commonly reported as barriers to screening among participants in our study, although such factors have been identified in other U.S. studies.7,39 Although lack of trust in screening has previously been identified as a barrier to cancer screening,40 most of our participants (95%) trusted screening to find cervical cancer when it is still treatable, which was similar in another survey of North Carolina women (92%).41
Our finding that women living or working 11 or more miles away from a clinic were more likely than those living or working fewer than 5 miles away to report completing follow-up screening is difficult to interpret. A possible explanation is that car ownership, a factor we did not measure, may be greater in women living farther from a clinic compared with those who live closer to a clinic to obtain screening. Measurement of time and cost to travel to a clinic may be a better measure of the barrier of transportation in future studies.42
Two of our findings were somewhat concerning with regard to missed opportunities to access existing resources. One noteworthy finding was that most participants reported not being aware of the NC-BCCCP screening program, although the majority would have been eligible to receive free or low-cost cervical cancer screening through this program. Interventions designed to increase awareness of and facilitate access to the NC-BCCCP program could improve screening coverage in this higher risk group. Second, the most common predictor to obtaining a Pap test is physician recommendation,38,39 but 64% of our participants who reported at least one visit to a health care provider in the past year also reported that they did not receive a physician recommendation for a Pap test during that time period.
The main strength of this study is the identification and recruitment of a population of low-income women overdue for cervical cancer screening residing in both urban and rural areas of the southeastern United States. We used multiple recruitment strategies to identify and recruit these hard-to-reach infrequently screened women from diverse settings. Comprehensive telephone questionnaires collected detailed information on attitudes and barriers from this high-risk medically underserved population. Furthermore, we provided participants with clinic information to obtain a low-cost Pap test and tracked completion of follow-up Pap testing.
In terms of study limitations, women self-selected for entry into the study by responding to recruitment advertising; therefore, this study population may have been more motivated to be screened for cervical cancer than the general population of North Carolina women overdue for cervical cancer screening. Conduct of enrollment and study activities by phone may have biased the sample toward women who owned phones, although some participants reported that they used the phone of a friend or relative. Determination of Pap test completion by self-report, rather than by medical record review, is a potential study limitation. Completion of a Pap test was incentivized with a $10 gift card, which may have led to potential overreporting of in-clinic Pap attendance. Loss to follow-up of some participants between enrollment and completion of the follow-up questionnaire further limited our ability to assess whether reported Pap completion rates were representative of the entire recruited sample. Our finding of few significant differences in reported barriers between the analyzed groups may be due to our inclusion of only infrequently screened women, as these women were more likely to experience barriers to preventive screening than the general population of women eligible to be screened.
Most rarely screened women surveyed had accurate knowledge about the effectiveness of cervical cancer screening, yet were largely unaware of where to obtain affordable screening. Thus, outreach efforts to this high-risk population, including basic education on the importance of cervical cancer screening and connection to affordable services, may have the potential to increase the uptake of screening. Improving awareness of the NC-BCCCP program, in particular, may increase the use of Pap test services among uninsured women. However, many women in our study did not complete Pap testing even after receiving information on affordable screening options, indicating that awareness alone is likely to be insufficient and additional support may be needed to address other barriers. The $10 incentive for completing a Pap test, although small, may have helped some women by supporting the cost of transportation or clinic fee.
Community health workers and lay navigation staff could also be instrumental in navigating and supporting never or infrequently screened women to access preventive screening services, a process that has proven successful to engage women in screening services in other settings.43 Women with less than a high school education emerged as a special population to target in future efforts to improve adherence to cervical cancer screening recommendations. Given that women with home-collection HPV-positive results were notably more likely to obtain in-clinic Pap testing, our results further support the use of home-based HPV self-collection as a means to engage infrequently or never-screened women into preventive screening services.
Acknowledgments
This research was supported by a grant from the Kate B. Reynolds Charitable Trust and the National Cancer Institute of the National Institutes of Health under award numbers 5U54CA156735 and NIH NCI R01 CA183891. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We appreciate contributions to study design, implementation, and recruitment from Dr. Allen Rinas, Dr. Belinda Yen-Lieberman, Dr. Jerome Belinson, Meredith Kamradt, Rachel Larsen, Kristen Ricchetti, and Kelly Murphy, as well as from Rovers Medical Devices for donation of the Viba sample collection brushes, and QIAGEN for donation of kits for Hybrid Capture 2 HPV testing.
Author Disclosure Statement
Dr. J.S.S. has received research grants, supply donations, and consultancies; served on paid advisory boards; and/or been a paid speaker for Arbor Vita, Becton Dickenson Corporation, Hologic, Rovers Medical Devices, and Trovagene in the past 5 years. Dr. A.R.R. has received research grants from Merck & Co., Inc. in the past 5 years. No competing financial interests exist for the remaining authors.
References
- 1. Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012;137:516–542 [DOI] [PubMed] [Google Scholar]
- 2. Horner MJ, Altekruse SF, Zou Z, Wideroff L, Katki HA, Stinchcomb DG. U.S. geographic distribution of prevaccine era cervical cancer screening, incidence, stage, and mortality. Cancer Epidemiol Biomarkers Prev 2011;20:591–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer 2004;101:1051–1057 [DOI] [PubMed] [Google Scholar]
- 4. Denslow SA, Knop G, Klaus C, Brewer NT, Rao C, Smith JS. Burden of invasive cervical cancer in North Carolina. Prev Med 2012;54:270–276 [DOI] [PubMed] [Google Scholar]
- 5. Sabatino SA, White MC, Thompson TD, Klabunde CN, Centers for Disease Control and Prevention (CDC). Cancer screening test use—United States, 2013. MMWR Morb Mortal Wkly Rep 2015;64:464–468 [PMC free article] [PubMed] [Google Scholar]
- 6. National Institutes of Health Consensus Development Conference. Statement on cervical cancer. Gynecol Oncol 1997;66:351–361 [DOI] [PubMed] [Google Scholar]
- 7. Ackerson K, Gretebeck K. Factors influencing cancer screening practices of underserved women. J Am Acad Nurse Pract 2007;19:591–601 [DOI] [PubMed] [Google Scholar]
- 8. Schoenberg NE, Studts CR, Hatcher-Keller J, Buelt E, Adams E. Patterns and determinants of breast and cervical cancer non-screening among Appalachian women. Women Health 2013;53:552–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Malley AS, Forrest CB, Mandelblatt J. Adherence of low-income women to cancer screening recommendations. J Gen Intern Med 2002;17:144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. del Carmen MG, Avila-Wallace M. Effect of health care disparities on screening. Clin Obstet Gynecol 2013;56:65–75 [DOI] [PubMed] [Google Scholar]
- 11. Behbakht K, Lynch A, Teal S, Degeest K, Massad S. Social and cultural barriers to Papanicolaou test screening in an urban population. Obstet Gynecol 2004;104:1355–1361 [DOI] [PubMed] [Google Scholar]
- 12. Leach CR, Schoenberg NE. The vicious cycle of inadequate early detection: A complementary study on barriers to cervical cancer screening among middle-aged and older women. Prev Chronic Dis 2007;4:A95. [PMC free article] [PubMed] [Google Scholar]
- 13. Scarinci IC, Garcia FA, Kobetz E, et al. Cervical cancer prevention: New tools and old barriers. Cancer 2010;116:2531–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moyer VA U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;156:880–891, W312 [DOI] [PubMed] [Google Scholar]
- 15. Moss JL, McCarthy SH, Gilkey MB, Brewer NT. Application of the carolina framework for cervical cancer prevention. Gynecol Oncol 2014;132 Suppl 1:S33–S40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion, Healthy People 2020. Available at: www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives Accessed February4, 2018 [PubMed]
- 17. White A, Thompson TD, White MC, et al. Cancer screening test use—United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: A meta-analysis. Cancer Epidemiol Biomarkers Prev 2008;17:748–757 [DOI] [PubMed] [Google Scholar]
- 19. Snijders PJ, Verhoef VM, Arbyn M, et al. High-risk HPV testing on self-sampled versus clinician-collected specimens: A review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer 2013;132:2223–2236 [DOI] [PubMed] [Google Scholar]
- 20. Castle PE, Rausa A, Walls T, et al. Comparative community outreach to increase cervical cancer screening in the Mississippi delta. Prev Med 2011;52:452–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kobetz E, Kish JK, Campos NG, et al. Burden of human papillomavirus among Haitian immigrants in Miami, Florida: Community-based participatory research in action. J Oncol 2012;2012:728397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hatcher J, Studts CR, Dignan MB, Turner LM, Schoenberg NE. Predictors of cervical cancer screening for rarely or never screened rural Appalachian women. J Health Care Poor Underserved 2011;22:176–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Collins T, Stradtman LR, Vanderpool RC, Neace DR, Cooper KD. A community-academic partnership to increase Pap testing in Appalachian Kentucky. Am J Prev Med 2015;49:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leyden WA, Manos MM, Geiger AM, et al. Cervical cancer in women with comprehensive health care access: Attributable factors in the screening process. J Natl Cancer Inst 2005;97:675–683 [DOI] [PubMed] [Google Scholar]
- 25. Smith JS, Des Marais AC, Deal AM, et al. Mailed human papillomavirus self-collection with Papanicolaou test referral for infrequently screened women in the United States. Sex Transm Dis 2018;45:42–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benard VB, Royalty J, Saraiya M, Rockwell T, Helsel W. The effectiveness of targeting never or rarely screened women in a national cervical cancer screening program for underserved women. Cancer Causes Control 2015;26:713–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Broberg G, Gyrd-Hansen D, Miao Jonasson J, et al. Increasing participation in cervical cancer screening: Offering a HPV self-test to long-term non-attendees as part of RACOMIP, a Swedish randomized controlled trial. Int J Cancer 2014;134:2223–2230 [DOI] [PubMed] [Google Scholar]
- 28. Darlin L, Borgfeldt C, Forslund O, et al. Comparison of use of vaginal HPV self-sampling and offering flexible appointments as strategies to reach long-term non-attending women in organized cervical screening. J Clin Virol 2013;58:155–160 [DOI] [PubMed] [Google Scholar]
- 29. Vanderpool RC, Jones MG, Stradtman LR, Smith JS, Crosby RA. Self-collecting a cervico-vaginal specimen for cervical cancer screening: An exploratory study of acceptability among medically underserved women in rural appalachia. Gynecol Oncol 2014;132 Suppl 1:S21–S25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gupta S, Palmer C, Bik EM, et al. Self-sampling for human papillomavirus testing: Increased cervical cancer screening participation and incorporation in international screening programs. Front Public Health 2018;6:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Virtanen A, Nieminen P, Luostarinen T, Anttila A. Self-sample HPV tests as an intervention for nonattendees of cervical cancer screening in finland: A randomized trial. Cancer Epidemiol Biomarkers Prev 2011;20:1960–1969 [DOI] [PubMed] [Google Scholar]
- 32. Sanner K, Wikstrom I, Strand A, Lindell M, Wilander E. Self-sampling of the vaginal fluid at home combined with high-risk HPV testing. Br J Cancer 2009;101:871–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Limmer K, LoBiondo-Wood G, Dains J. Predictors of cervical cancer screening adherence in the United States: A systematic review. J Adv Pract Oncol 2014;5:31–41 [PMC free article] [PubMed] [Google Scholar]
- 34. Damiani G, Basso D, Acampora A, et al. The impact of level of education on adherence to breast and cervical cancer screening: Evidence from a systematic review and meta-analysis. Prev Med 2015;81:281–289 [DOI] [PubMed] [Google Scholar]
- 35. King CJ, Chen J, Garza MA, Thomas SB. Breast and cervical screening by race/ethnicity: Comparative analyses before and during the great recession. Am J Prev Med 2014;46:359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention (CDC). Cervical cancer rates by race and ethnicity. Cervical cancer rates by race and ethnicity. Available at: https://www.cdc.gov/cancer/cervical/statistics/race.htm Accessed October7, 2018
- 37. Bazargan M, Bazargan SH, Farooq M, Baker RS. Correlates of cervical cancer screening among underserved Hispanic and African-American women. Prev Med 2004;39:465–473 [DOI] [PubMed] [Google Scholar]
- 38. Peterson NB, Murff HJ, Cui Y, Hargreaves M, Fowke JH. Papanicolaou testing among women in the southern United States. J Womens Health (Larchmt) 2008;17:939–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reiter PL, Linnan LA. Cancer screening behaviors of African American women enrolled in a community-based cancer prevention trial. J Womens Health (Larchmt) 2011;20:429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gauss JW, Mabiso A, Williams KP. Pap screening goals and perceptions of pain among Black, Latina, and Arab women: Steps toward breaking down psychological barriers. J Cancer Educ 2013;28:367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Salz T, Gottlieb SL, Smith JS, Brewer NT. The association between cervical abnormalities and attitudes toward cervical cancer prevention. J Womens Health (Larchmt) 2010;19:2011–2016 [DOI] [PubMed] [Google Scholar]
- 42. McDonald YJ, Goldberg DW, Scarinci IC, et al. Health service accessibility and risk in cervical cancer prevention: Comparing rural versus nonrural residence in New Mexico. J Rural Health 2017;33:382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thompson B, Carosso EA, Jhingan E, et al. Results of a randomized controlled trial to increase cervical cancer screening among rural Latinas. Cancer 2017;123:666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]