Abstract
Objective: Chronic wounds associated with diabetes are an important public health problem demanding new treatments to improve wound healing and decrease amputations. Monocytes/macrophages play a key role in sustained inflammation associated with impaired healing and local administration of peroxisome proliferator-activated receptor (PPAR)γ agonists may modulate macrophage, improving healing. In this study, we investigated the effects of GQ-11, a partial/dual PPARα/γ agonist, on macrophage function and wound healing in diabetes.
Approach: Wounds were surgically induced at the dorsum of C57BL/6J and BKS.Cg-Dock7m +/+ Leprdb/J (db/db) mice and treated with hydrogel (vehicle), pioglitazone or GQ-11, for 7 or 10 days, respectively. After treatment, wounds were analyzed histologically and by quantitative PCR (qPCR). In addition, bone marrow-derived macrophages (BMDM) were cultured from C57BL/6J mice and treated with vehicle, pioglitazone, or GQ-11, after challenge with lipopolysaccharide or interleukin-4 to be analyzed by qPCR and flow cytometry.
Results: GQ-11 treatment upregulated anti-inflammatory/pro-healing factors and downregulated pro-inflammatory factors both in wounds of db/db mice and in BMDM.
Innovation: Wounds of db/db mice treated with GQ-11 exhibited faster wound closure and re-epithelization, increased collagen deposition, and less Mac-3 staining compared with vehicle, providing a new approach to treatment of diabetic wound healing to prevent complications.
Conclusion: GQ-11 improves wound healing in db/db mice, regulating the expression of pro- and anti-inflammatory cytokines and wound growth factors, leading to increased re-epithelization and collagen deposition.
Keywords: wound closure, diabetes, biomarkers, cell biology

Dulcineia S.P. Abdalla, PhD

Timothy J. Koh, PhD
Introduction
Prevalence of diabetes among adults >18 years of age is 8.8% worldwide and is a major cause of blindness, kidney failure, heart attacks, stroke, and lower limb amputation.1 Chronic wounds associated with diabetes is an important health problem, affecting up to 25% of diabetic patients, and 14–24% of these wounds will lead to amputation.2
Hyperglycemia and hyperlipidemia likely contribute to many of the complications associated with diabetes,3,4 and may contribute to several characteristics of diabetic wounds: a persistent inflammatory response with prolonged monocytes/macrophages (Mo/Mp) accumulation, increased pro-inflammatory cytokine release, and impaired growth factor release as well as impaired angiogenesis and extracellular matrix.5,6
Mo/Mp have been established as key regulators of wound healing.7,8 In normal wound healing, these cells adopt a pro-inflammatory phenotype in the early stages of healing that is efficient for killing pathogens and clearing the wound of damaged tissue. During the later stages of healing, they assume a pro-healing phenotype associated with the release of anti-inflammatory cytokines and growth factors that promote angiogenesis, cell proliferation/differentiation, and collagen deposition as well as wound closure. Importantly, wound macrophages in diabetic mice exhibit an impaired phenotype transition resulting in a sustained pro-inflammatory phenotype, which may be an important contributor to the persistent inflammatory response in diabetic wounds. Associated with the impaired phenotype transition are increased levels of interleukin (IL)-1β and tumor necrosis factor (TNF)-α along with decreased levels of vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β, which likely contribute to impaired healing of wounds of diabetic mice.5,9,10
A number of factors have been postulated to induce the switch of macrophages from pro-inflammatory to noninflammatory and promote the resolution of inflammation.5,9,10
Among them, peroxisome proliferator-activated receptors (PPARs) may help to resolve inflammation by transrepression of pro-inflammatory genes and activation of anti-inflammatory genes expression in Mo/Mp and other cells.11 Importantly, local administration of PPARγ agonists to wounds of diabetic mice has been shown to promote a noninflammatory Mp phenotype and improve wound healing, suggesting a promising approach to downregulate inflammation and improve healing in chronic wounds.12
Clinical Problem Addressed
Common PPARγ agonists such as thiazolidinediones (TZDs) are used to treat insulin resistance associated with diabetes, and while they induce a pleiotropic anti-inflammatory effect, they have important adverse effects, including weight gain, bone loss, and cardiovascular events,13,14 encouraging the search for new TZDs with preserved efficacy and reduced side effects. GQ-11 is a new thiazolidine compound, exhibiting partial/dual PPARα/γ agonism with classical antidiabetic effects of TZDs, improves lipid profile and reduces chronic inflammation associated with obesity in mice with reduced side effects.15 Therefore, the aim of this study was to determine whether GQ-11 reduces inflammation and improves wound healing in diabetic mice in basic research, providing better understanding of underlying mechanisms, improving diabetic wound healing clinical trials and purposing a new approach to diabetic wound healing treatment to prevent its complications such as amputations.
Materials and Methods
GQ-11 synthesis
GQ-11 [(Z)-5-((1H-indol-3-yl)methylene)-3-(4-methylbenzyl)thiazolidine-2,4-dione] was synthesized as previously described,15 in the Laboratory of Drug Design and Synthesis of the Federal University of Pernambuco (Recife, Pernambuco, Brazil).
Animals and treatment
Male homozygous C57BL/6J and BKS.Cg-Dock7m +/+ Leprdb/J (db/db) mice were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in the animal facility at College of Medicine Research Building—University of Illinois at Chicago. The study protocols were approved by the Animal Care Committee of University of Illinois at Chicago (No. 15-180) and followed the rules of the Guide for the Care and the Use of Laboratory Animals, published by the U.S. National Institutes of Health (NIH).16 Mice were maintained in plastic cages at 22°C, 12 h light–dark cycle, and given free access to food and water during all the experiments. Sample size calculation was based on evidence from previous preclinical studies for testing TZDs antidiabetic effects.15,17,18
At 10 weeks of age, animals were anesthetized with isoflurane (3%), the skin shaved and cleaned with 70% alcohol, and four full thickness wounds were created on the dorsum using 8 mm biopsy punch. On day 3 postinjury, after initial inflammatory response was allowed to proceed normally, animals were randomly allocated into three treatment groups (n = 6/group): vehicle (F-127® Pluronic Gel, Sigma-Aldrich, Cat No. P2443, 25% in phosphate-buffered saline [PBS], 0.1% dimethyl sulfoxide [DMSO]), pioglitazone (Sigma-Aldrich, Cat No. E6910, powder, vehicle diluted, 2 mM), or GQ-11 (powder, vehicle diluted, 2 mM). For each group, 50 μL of drug or vehicle gel was applied topically with a pipette on the wound surface. For C57BL/6J mice, treatment was performed daily for 4 days and for db/db mice, treatment was performed daily for 7 days. To ensure the gel stayed on the wound and moist wound healing, Tegaderm® film was applied over the wounds and changed daily. As a control for gene expression evaluation, a separate group with three C57BL/6J mice was submitted to the same wounding procedures. These control mice were euthanized and wounds harvested for analysis on day 3 postsurgery.
For all experimental mice, wound closure was assessed by digital images taken daily at a distance of 10 cm from the lesions and a scale was placed in the field of view to calibrate the images. At the end of the treatment period (7 days for wild type, 10 days for db/db), animals were euthanized through cervical dislocation under isoflurane anesthesia (3%). Wounds were harvested and prepared for messenger RNA (mRNA) analysis or histological analysis.
Histology and hematoxylin-eosin/Trichrome staining
Samples used for histology were fixed in 4% paraformaldehyde (4°C) for 4 h and then immersed in 15% sucrose for 48 h and in 30% sucrose for 24 h (all at 4°C). After sucrose protection, wounds were embedded in Optimum Cutting Temperature medium (Thermo Fisher Scientific, Cat No. 2373571) on dental wax to prevent folding, and frozen in 2-methylbutane cooled with liquid nitrogen. Samples were stored at −80°C until sectioning.
Frozen wounds were sectioned in a cryostat (Leica Biosystems). Both chamber and specimen head temperatures were set at −32°C. Sections (10 μm) were collected on Superfrost Plus microscope slides (Thermo Fisher Scientific, Cat No. 4951PLUS4) and stored at −20°C. Each wound was sectioned from one edge through the center of the wound, and sections showing the largest wound diameter were deemed to represent the center of the wound and used for further analysis. Hematoxylin-eosin (HE) and Trichrome staining were performed according to our standard protocol.5,19
Immunohistochemistry
For Mac-3 staining, slides were defrosted for 30 min, washed in PBS for 5 min then immersed in citrate buffer (pH = 6), warmed in a microwave for 5 min, and cooled for 25 min at room temperature. Sections were then marked with PAP pen, washed with PBS (twice, 2 min each), glycine (three times, 2 min each), and PBS again (twice, 2 min each). Next, sections were incubated in hydrogen peroxide (0.3%, 5 min), washed with PBS (twice, 2 min each), and blocked with PBS-bovine serum albumin solution (0.2%, 30 min). After blocking, sections were incubated with primary antibody against Mac-3 (2 h, room temperature—Biolegend, Inc., Cat No. 108501, dilution 1:50), washed with PBS (three times, 2 min each), incubated with secondary antibody (30 min, at room temperature—Invitrogen, Cat No. 11481782, 1:200 dilution), and washed again with PBS (once, 30 min, room temperature). Then, sections were incubated with horseradish peroxidase (30 min, at room temperature), washed with PBS (once, 10 min, room temperature), and developed with DAB Kit (Vector Labs, Cat No. SK4100) for 3 min, under microscope observation. Sections were then washed with PBS for 2 min and counterstained with QS Hematoxylin (Vector Labs, Cat No. H3404,) for 5 s. A final wash in distilled water was then performed before mounting samples in Vectashield® (Vector Labs, Cat No. H1000).
Image analysis for wound measurements
Images of the wounds surface were analyzed using a Java-based image processing software (ImageJ, NIH). The percentage of wound closure was calculated (% wound closure = [100 × (wound size “postsurgery” – wound size “after treatment period”)]/wound size “postsurgery”). Histology images were acquired using a Nikon Instruments 80i microscope with a 2 × /0.06 objective and a DS-QI1 digital camera, and were analyzed using the NIS-Elements® Advanced (Nikon Corporation) software. Data were processed on Excel (Microsoft) and plotted on GraphPad Prism for graphs and statistics. For histology analysis, the percentage of re-epithelization [(distance traversed by epithelium over wound from wound edge/distance between wound edges) × 100] was calculated for two sections per wound and was averaged over sections to provide a representative value for each wound. Average granulation thickness was measured in the same sections by dividing the wound bed area by wound length. In the slides stained with Trichrome, the percentage of blue stain was measured in granulation tissue area using NIS-Elements Advanced (Nikon Corporation) software.
Cell culture
Bone marrow-derived macrophages (BMDM) were isolated and differentiated as previously described.20 In brief, femurs were harvested from C57Bl/6J male mice, bone marrow was flushed, and monocytes were seeded in 15 cm dishes and differentiated with macrophage colony-stimulating factor (10 ng/mL) added to Roswell Park Memorial Institute Medium high glucose (10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin), for 7 days at 37°C, 5% carbon dioxide.
After differentiation, 6 × 104 cells were seeded in 24-well plates and after allowing 12 h for attachment, cells were treated with vehicle (DMSO, Sigma-Aldrich, Cat No. 276855, 0.01%), 10 μM pioglitazone (Sigma-Aldrich, Cat No. E6910), or 10 μM GQ-11. After 24 h treatment, cells were challenged with 100 ng/mL lipopolysaccharide (LPS) (Sigma-Aldrich, Cat No. L2630) or 20 ng/mL IL-4 (Sigma-Aldrich, Cat No. I1020). Cells and medium were collected in the intervals of 3, 6, 12, and 24 h after challenge.
Cytokine assay
Levels of cytokines/chemokines secreted by BMDM were measured in cell culture medium by cytometric bead array (CBA) Mouse Inflammation Kit (BD Biosciences, Cat No.552364). Levels of IL-6, IL-10, monocyte chemoattractant protein-1 (MCP-1), and TNF-α were measured following the manufacturer instructions.
Quantitative PCR analysis
mRNA was isolated from wounds and from BMDM using TRIzol Reagent (Invitrogen, Cat No. 15596026). One microgram of RNA was reverse-transcribed into complementary DNA (cDNA) using the high-capacity cDNA SuperScript Vilo® Kit, according to the manufacturer's instructions (Thermo Fisher Scientific, Cat No. 11706050). Quantitative PCR (qPCR) was performed in an ABI 7500 Fast Real-Time PCR using SYBR green master mix (Thermo Fisher Scientific, Cat No. 4385610) and primers for Arg-Il-6, Il-10, Tgf-β, Tnf-α, Il-1β, Vegf, PPARγ, PPARα (primer sequences available in Supplementary Table S1). Expression levels of each target gene were normalized to Rpl-4 and mRNA relative expression as internal efficiency controls. The mRNA fold change was calculated using the 2(-Delta Delta C(t)) method,21 and values expressed as fold increases relative to the control group.
Statistical analyses
Statistical analyses were performed using GraphPad Prism software, version 5.0. One-way analysis of variance followed by Tukey's test was used to calculate statistical significance as appropriate. All data in this study are expressed as the mean ± standard deviation. Values of p < 0.05 were considered significant.
Results
GQ-11 decreases expression and release of pro-inflammatory cytokines and induces expression of anti-inflammatory cytokines and growth factors in BMDM
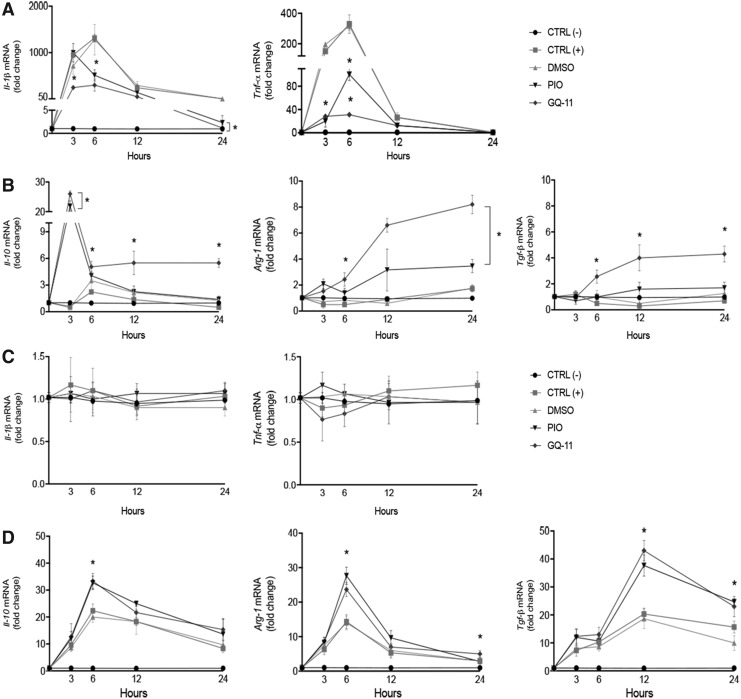
We first sought to determine whether pretreatment of cultured macrophages with pioglitazone or GQ-11 altered responses to either LPS or IL-4. When cells were challenged with LPS, pretreatment with pioglitazone and GQ-11 blunted induction of the pro-inflammatory cytokines Il-1β and Tnf-α (Fig. 1A). Importantly, both pioglitazone and GQ-11 upregulated the expression of Il-10, Arg-1 and Tgf-β in BMDM challenged by LPS, indicating that even in the presence of a strong inflammatory stimulus these drugs can maintain a macrophage anti-inflammatory phenotype (Fig. 1B). Accordingly, pretreatment with pioglitazone and GQ-11 did not alter the expression of pro-inflammatory cytokines when BMDM were challenged with IL-4 (Fig. 1C), but upregulated the expression of Il-10, Arg-1, and Tgf-β, compatible with the anti-inflammatory phenotype (Fig. 1D).
Figure 1.
GQ-11 increases anti-inflammatory cytokine expression and decreases pro-inflammatory cytokine expression in BMDM. (A) Fold change of Il-1β and Tnf-α after challenge with LPS. (B) Fold change of Il-10, Arg-1, and Tgf-b after LPS challenge. (C) Fold change of Il-1β and Tnf-α after IL-4 challenge. (D) Fold change of Il-10, Arg-1 and Tgf-b after IL-4 challenge. BMDM were pretreated for 24 h with vehicle (DMSO 0.01%), pioglitazone (PIO 10 μM), or GQ-11 (10 μM) and challenged with LPS (100 ng/mL) or IL-4 (20 ng/mL). mRNA expression of BMDM was quantified by qPCR. Negative controls [CTRL (−)] are represented by nontreated and nonchallenged cells. Positive controls [CTRL (+)] are represented by nontreated but challenged cells. Data are expressed as the mean ± SD of biological triplicates. Statistical analyses were performed using ANOVA/Tukey's multiple comparison tests. *p < 0.05. ANOVA, analysis of variance; BMDM, bone marrow-derived macrophages; DMSO, dimethyl sulfoxide; IL, interleukin; LPS, lipopolysaccharide; mRNA, messenger RNA; qPCR, quantitative PCR; SD, standard deviation; TGF, transforming growth factor; TNF, tumor necrosis factor.
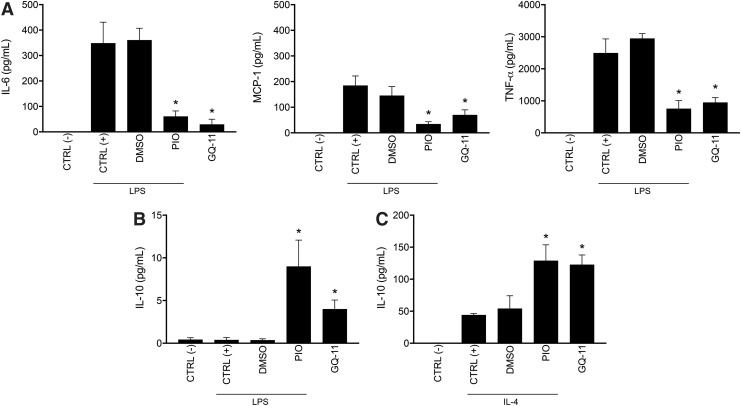
When measuring cytokine/chemokine protein levels in cell culture medium, pretreatment with pioglitazone or GQ-11 blunted the release of the pro-inflammatory cytokines IL-6, MCP-1, and TNF-α, after the challenge with LPS (Fig. 2A). Although pro-inflammatory cytokine release was below detectable levels in the medium of cells challenged with IL-4, pretreatment with pioglitazone or GQ-11 was able to increase IL-10 release, after challenge with either LPS or IL-4 (Fig. 2B, C).
Figure 2.
GQ-11 decreases pro-inflammatory cytokine release and increases anti-inflammatory cytokine release by BMDM. (A) Concentrations of IL-6, MCP-1, and TNF-α protein of BMDM supernatant. (B) IL-10 concentration from supernatant of BMDM pretreated for 24 h with vehicle (DMSO 0.01%), pioglitazone (PIO 10 μM), or GQ-11 (10 μM) and challenged with LPS (100 ng/mL). (C) IL-10 concentration in supernatant of BMDM pretreated for 24 h with vehicle (DMSO 0.01%), pioglitazone (PIO 10 μM), or GQ-11 (10 μM) and challenged with IL-4 (20 ng/mL). Total protein of BMDM supernatant was accessed by flow cytometry, with a CBA. Negative controls [CTRL (−)] are represented by nontreated and nonchallenged cells. Positive controls [CTRL (+)] are represented by nontreated but challenged cells. Data are expressed as the mean ± SD of biological triplicates. Statistical analyses were performed using ANOVA/Tukey's multiple comparison tests. *p < 0.05. CBA, cytometric bead array; MCP-1, monocyte chemoattractant protein-1.
GQ-11 increases expression of anti-inflammatory cytokines and growth factors in wounds of both nondiabetic and diabetic mice
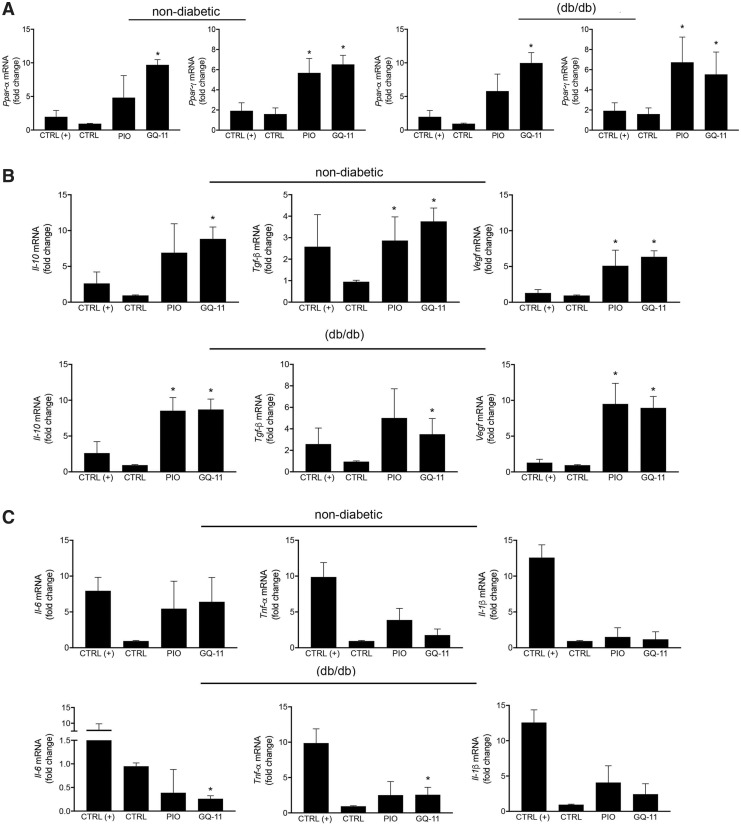
To determine whether GQ-11 can ameliorate inflammation in vivo, we applied GQ-11 topically to wounds in nondiabetic and diabetic mice. We first assessed the effect of GQ-11 on expression of PPARs in wound tissue, and found that GQ-11 treatment upregulated the expression of Ppar-α and Ppar-γ, and pioglitazone upregulated expression of Ppar-γ, in wounds of both nondiabetic and diabetic mice compared with vehicle control treatment (Fig. 3A), confirming targeting of the PPAR pathway by these drugs.
Figure 3.
GQ-11 increases the expression of anti-inflammatory cytokines and decreases the expression of pro-inflammatory cytokines in wounds of db/db mice. (A) Fold change of Ppar-α and Ppar-γ in wounds of nondiabetic and diabetic db/db mice. (B) Fold change of Il-10, Tgf-β, and Vegf in nondiabetic and db/db mice. (C) Fold change of Il-6, Tnf-α, and Il-1β in nondiabetic and db/db mice. mRNA expression was quantified by qPCR. Data are expressed as the mean ± SD of six mice per group. Statistical analyses were performed using ANOVA/Tukey's multiple comparison tests. *p < 0.05.
In addition, treatment with GQ-11 or pioglitazone upregulated the expression of anti-inflammatory cytokines and growth factors in wounds, including Il-10, Tgf-β, and Vegf in both nondiabetic and diabetic mice compared with vehicle controls (Fig. 3B), indicating that GQ-11 can induce anti-inflammatory cytokines and growth factors associated with tissue repair. Whereas there was no effect of either GQ-11 or pioglitazone treatment on expression of pro-inflammatory cytokines in wounds of nondiabetic mice compared with controls, GQ-11 downregulated Il-6 in wounds of diabetic mice compared with control or pioglitazone groups (Fig. 3C). The lack of effect of GQ-11 on Il-1β and Tnf-α expression may be due to the time point examined; the expression of these cytokines was significantly lower in all treatment groups at the time point examined compared with the positive controls collected on day 3 postinjury.
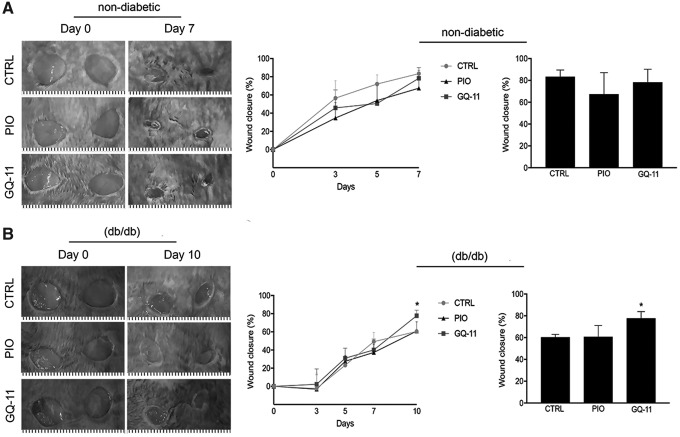
GQ-11 accelerates wound closure in diabetic mice
To determine whether GQ-11-induced alterations in gene expression were associated with improved wound healing, we first assessed the effects of GQ-11 on wound closure using external measurements. In nondiabetic mice, there was no effect of GQ-11 on wound closure (Fig. 4A). However, in diabetic db/db mice, we observed a substantial increase of about 30% in wound closure on day 10 postwounding in animals treated with GQ-11, compared with pioglitazone or control groups (Fig. 4B; Supplementary Table S2). Interestingly, the improvement was only apparent at the later stage of wound closure, indicating an effect during the resolution of inflammation.
Figure 4.
GQ-11 improves wound closure in db/db mice. (A) Representative lesions at scapula of nondiabetic mice (postsurgery and after 7 days), decrease of wound area along 7 days postsurgery and total wound closure. (B) Representative lesions at scapula of diabetic db/db mice (postsurgery and after 10 days), decrease of wound area along 10 days postsurgery and total wound closure. Mice were submitted to surgical procedure to induce scapula lesions and were treated at the 3rd day postsurgery with vehicle (CTRL—Pluronic Gel®), pioglitazone (PIO—2 mM), or GQ-11 (2 mM) for 4 days (nondiabetic mice) and 7 days (db/db mice). Positive controls [CTRL (+)] are represented by wounds of nondiabetic mice at the 3rd day postsurgery without treatment. Data are expressed as the mean ± SD of six mice per group. Statistical analyses were performed using ANOVA/Tukey's multiple comparison tests. *p < 0.05.
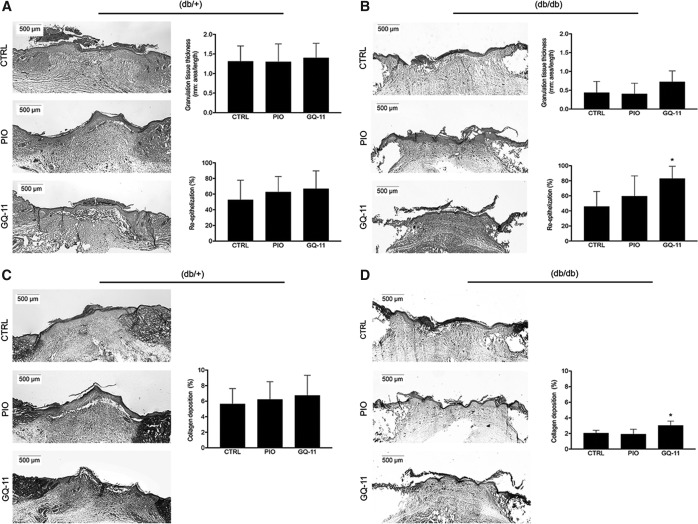
Wound healing was also assessed through histological analysis. HE staining of wound cryosections allowed us to measure granulation tissue thickness and re-epithelization. Again, no difference in either granulation tissue thickness or re-epithelization was observed between treatment groups in nondiabetic mice (Fig. 5A). In db/db mice, treatment with GQ-11 significantly increased re-epithelization to 83% compared with 45% for controls and 59% for pioglitazone treatment, consistent with the wound closure data. GQ-11 also induced a trend of an increase in granulation tissue thickness, but this did not reach statistical significance (Fig. 5B; Supplementary Table S2).
Figure 5.
GQ-11 increases re-epithelization and collagen deposition in wounds of db/db mice. (A) Representative sections of wounds edge of nondiabetic mice, HE stained, and the respective thickness measurement and re-epithelization calculation. (B) Representative sections of wounds edge of db/db mice, HE stained and the respective thickness measurement and re-epithelization calculation. (C) Representative sections of wounds edge of nondiabetic mice, Trichrome stained, and respective collagen deposition. (D) Representative sections of wounds edge of db/db mice, Trichrome stained, and respective collagen deposition. Mice were submitted to surgical procedure to induce scapula lesion and were treated at the 3rd day postsurgery with vehicle (CTRL—Pluronic Gel), pioglitazone (PIO—2 mM), or GQ-11 (2 mM) for 4 days (nondiabetic mice) and 7 days (db/db mice). Positive controls [CTRL (+)] are represented by wounds of db/+ mice at the 3rd day postsurgery without treatment. Histological sections were collected with frozen samples with cryostat. Magnification 2 × . Data are expressed as the mean ± SD of six mice per group. Statistical analyses were performed using ANOVA/Tukey's multiple comparison tests. *p < 0.05. HE, hematoxylin-eosin.
GQ-11 increases collagen deposition in diabetic mice
In addition to measuring re-epithelization in HE-stained cryosections, Trichrome staining allowed collagen deposition in wounds to be assessed in both nondiabetic and diabetic mice. Treatment of wounds in nondiabetic mice did not alter collagen deposition compared with controls (Fig. 5C). In contrast, GQ-11 treatment of wounds in diabetic mice increased collagen deposition by about 50%, compared with control and pioglitazone groups (Fig. 5D; Supplementary Table S2).
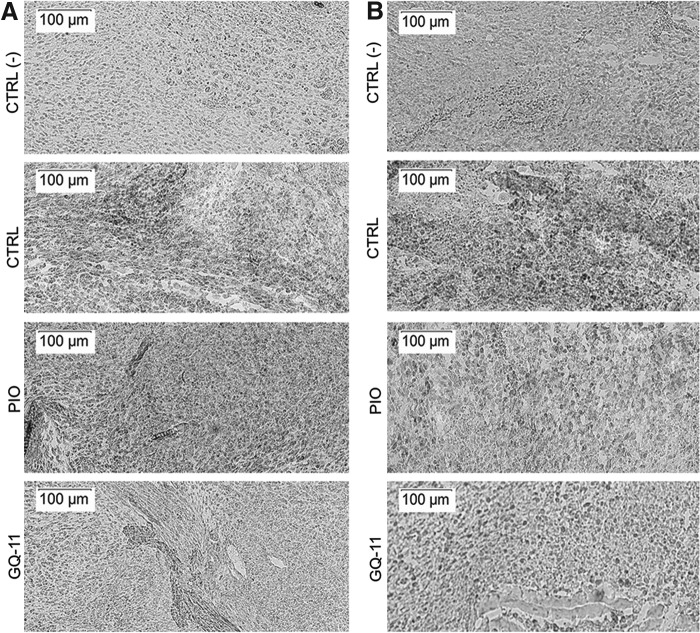
GQ-11 may decrease macrophage infiltration in wounds of db/db mice
Qualitatively, we observed that in wounds of both nondiabetic and diabetic mice, those treated with GQ-11 showed less macrophage infiltration compared with pioglitazone and vehicle in nondiabetic mice (Fig. 6A), and compared with vehicle in diabetic mice (Fig. 6B). These findings indicate that GQ-11 may induce resolution of inflammation in diabetic wounds.
Figure 6.
GQ-11 decreases macrophage infiltration in wounds of db/db mice. (A) Representative sections of Mac-3 staining in wounds of nondiabetic mice. (B) Representative sections of Mac-3 staining in wounds of db/db mice. Mice were submitted to surgical procedure to induce scapula lesion and were treated at the 3rd day postsurgery with vehicle (CTRL—Pluronic Gel), pioglitazone (PIO—2 mM), or GQ-11 (2 mM) for 4 days (nondiabetic mice) or 7 days (db/db mice). Positive controls [CTRL (+)] are represented by wounds of db/+ mice at the 3rd day postsurgery without treatment. Histological sections were collected with frozen samples with cryostat and staining was performed by immunohistochemistry with specific antibodies against Mac-3 (1:50). Magnification 10 ×.
Discussion
Our previous studies indicated beneficial properties of a novel PPAR agonist (GQ-11) that may represent a promising candidate for the therapy of diabetes complications.15 In this study, our major findings are that GQ-11 induces a pro-healing phenotype both in cultured macrophages and when applied topically to wounds of diabetic mice, which resulted in accelerated re-epithelization and collagen deposition.
GQ-11 is a dual, partial PPAR-γ/α agonist, and may influence wound healing through effects on inflammation. PPAR-γ/α can affect inflammation direct or indirectly.22 First, PPAR-γ might modulate the expression/activity of G protein-coupled receptors (GPCRs) and transcriptional regulation of GPCRs kinase-2 activity, inducing anti-inflammatory responses.23 Second, PPAR-α, whose activation stimulates the expression of genes encoding cytochrome P450 and β-oxidation enzymes, contributes to resolution of inflammation, through leukotriene B4 activity limitation.24 Importantly, the inhibition of PPAR-γ activity is related to sustained production of IL-1β, whereas activation of PPAR-γ induces a protective anti-inflammatory effect.25 Interestingly, it has been shown that the inflammation repression by PPAR-γ is dependent on PPAR-α, through IκBα induction, both in vitro and in vivo.26 Thus, a dual/partial PPAR-γ/α agonist may represent a promising approach to dampen inflammation and improve wound healing, particularly in the setting of diabetes. In this study, GQ-11 increased anti-inflammatory and pro-healing factors expression, including IL-10, Arg-1, Tgf-β, and Vegf, both in cultured macrophages and in vivo in wounds (Figs. 1–3). GQ-11 also downregulated pro-inflammatory cytokines Il-1β and Tnf-α expression in strongly pro-inflammatory cultured macrophages, but did not affect expression of these cytokines in less inflammatory macrophages and during later stages of wound healing. These findings corroborate previous studies reporting anti-inflammatory effects of PPAR-γ agonists.11,27,28
In the context of normal wound healing, pro-inflammatory cytokines likely play important roles in the early inflammatory stage of wound healing.29 We reported that loss of the NLRP3 inflammasome resulted in reduced IL-1β in wounds of nondiabetic mice and delayed healing.30 In vivo studies report that macrophages are the main producers of IL-1β in wounds, both in nondiabetic and diabetic animals, especially in the early inflammatory stage, when macrophages are polarized to a pro-inflammatory phenotype.9,27,31 This pro-inflammatory phenotype is also associated with macrophage phagocytosis of apoptotic cells,32 which in turn is thought to induce transition to a noninflammatory healing-associated phenotype.8,33 The transition from pro-inflammatory to noninflammatory macrophage phenotypes is also important for normal wound healing, where macrophages with the latter phenotype release growth factors important for the proliferative and remodeling stages of wound healing.34 One such growth factor, TGF-β binds to TGF-β receptor I and induces phosphorylation of Smad4 resulting in further downregulation of pro-inflammatory genes and induction of fibroblast migration and proliferation.35 In addition, the binding of Smad4 transcription factor induces the Il-10 gene promoter, stimulating further Il-10 expression in macrophages.36 Thus, induction of such a phenotype switch in wounds of diabetic mice by GQ-11 may help to restore normal wound healing.
In the diabetic environment, a number of factors may promote a chronic inflammatory response that impairs wound healing. The persistent hyperglycemia observed in diabetes causes a set of glycation reactions resulting in generation of advanced glycation end-products (AGEs).37 In turn, AGEs have direct and indirect actions in many cell types, including the induction of oxidative stress through the AGE Receptor (RAGE). Sustained activity of a reactive oxygen species-mediated pathway—induced either by RAGEs activation and/or impaired phagocytosis of apoptotic cells—may activate the NLRP3 inflammasome/IL-1β pathway inducing a pro-inflammatory positive feedback loop and contributing to the persistent inflammatory response related to impaired healing.9,10 In addition, the activation of many factors, such as RAGE and inflammation mediators as MCP-1, contributes to upregulation of nuclear factor-κB and its target genes, including TNF-α,38 which may also be involved in impaired wound healing. TNF-α can induce cell cycle arrest and apoptosis through forkhead box protein O1-dependent process39 resulting in increased expression of pro-inflammatory cytokines, setting up another inflammatory positive feedback loop that impairs healing. GQ-11 may help to break these pro-inflammatory positive feedback loops and induce resolution of inflammation in diabetic wounds. When compared with pioglitazone, the downregulation of Il-6 and Tnf-α observed in wounds treated with GQ-11 might also be related to decreased macrophage infiltration in the same groups, since the animals treated with pioglitazone did not show the same effect even upregulating Il-10 and Vegf.
Previous studies by Sakai et al.40 showed some different results when analyzing the effects of pioglitazone on wound healing. When comparing both studies, we can observe a different pattern in wound closure with greater re-epithelization on the first 7 days in Sakai et al.'s study,40 whereas our model showed greater re-epithelization from 7th to 10th days. This difference could be related to distinct drug solubilization and local delivery on wounds as different vehicles were used in both studies. For sure, new formulations to improve drug delivery and efficacy are necessary for the future development of a new wound healing therapeutic agent for clinical use, especially with new structures, such as GQ-11.
Innovation
Based on this, our findings suggest that wounds in diabetic mice treated with GQ-11 were in later stages of healing compared with controls, exhibiting greater re-epithelization and increased collagen deposition, likely transitioning into remodeling stage of tissue repair. The effects on healing can be attributed to the partial PPAR-γ/α agonism of GQ-11, which promoted cytokines modulation, leading to healing improvement. The search for a new PPAR agonist that shares classic TZDs therapeutic effects with less adverse effects is a key to a new approach in diabetic wound healing clinical trials to prevent its complications, such as amputations.
Key Findings
Greater re-epithelization when compared with pioglitazone and control groups.
Increased collagen deposition when compared with pioglitazone and control groups.
Inflammation modulation when compared with pioglitazone and control groups.
Supplementary Material
Acknowledgments and Funding Sources
This study was supported by São Paulo Research Foundation (FAPESP grant 2012/51316-5 to D.S.P.A.), the National Council for Scientific and Technological Development (National Institute of Science and Technology for Pharmaceutical Innovation [INCT_if/CNPq] grant 573663/2008-4 to I.R.P. and CNPq/MICCIN grant BFU2011-2476 to D.S.P.A.), and National Institutes of Health (NIH R01 GM092850 to T.J.K.). J.C.S. was supported by FAPESP fellowships (2016/00233-3 and 2016/16850-1).
Abbreviations and Acronyms
- AGEs
advanced glycation end-products
- ANOVA
analysis of variance
- RAGE
AGE receptor
- BMDM
bone marrow-derived macrophages
- CBA
cytometric bead array
- cDNA
complementary DNA
- DMSO
dimethyl sulfoxide
- GPCRs
G protein-coupled receptors
- HE
hematoxylin-eosin
- IL
interleukin
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- Mo/Mp
monocytes/macrophages
- mRNA
messenger RNA
- NIH
National Institutes of Health
- PBS
phosphate-buffered saline
- PPARs
peroxisome proliferator-activated receptors
- qPCR
quantitative PCR
- SD
standard deviation
- TGF
transforming growth factor
- TNF
tumor necrosis factor
- TZDs
thiazolidinediones
- VEGF
vascular endothelial growth factor
Author Disclosure and Ghostwriting
The authors state no competing financial interests. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Jacqueline Cavalcante Silva, the first author, is currently a PhD candidate in University of São Paulo (São Paulo, Brazil) at Biochemistry Laboratory, under the supervision of Dulcineia Saes Parra Abdalla. Marina Galdino da Rocha Pitta is currently leading Laboratory of Drug Design and Synthesis in Federal University of Pernambuco (Pernambuco, Brazil). Ivan da Rocha Pitta is currently a visitor professor in Core of Therapeutic Innovation in Federal University of Pernambuco (Pernambuco, Brazil). Timothy Jon Koh is currently a full professor in the Department of Kinesiology and Nutrition of University of Illinois at Chicago (Chicago, IL). Dulcineia Saes Parra Abdalla, the last author, is currently a full professor in the Faculty of Pharmaceutical Sciences of University of São Paulo (São Paulo, Brazil).
References
- 1. Ogurtsova K, Fernandes JDR, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diab Res Clin Pract 2017;28:40–50 [DOI] [PubMed] [Google Scholar]
- 2. ADA (American Diabetes Association). Diabetes mellitus and exercise. Diabetes Care 2017;40:S88–S98 [DOI] [PubMed] [Google Scholar]
- 3. Brownlee M. Glycation and diabetic complications. Diabetes 1994;43:836–841 [DOI] [PubMed] [Google Scholar]
- 4. Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation 1996;93:1905–1912 [DOI] [PubMed] [Google Scholar]
- 5. Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011;56:256–264 [DOI] [PubMed] [Google Scholar]
- 6. Goren I, Müller E, Schiefelbein D, et al. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J Invest Dermatol 2007;127:2259–2267 [DOI] [PubMed] [Google Scholar]
- 7. Koh TJ, DiPietro LA. Inflammation and wound healing: the role of macrophage. Expert Rev Mol Med 2011;11:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novak ML, Koh TJ. Phenotypic transitions of macrophages orchestrate tissue repair. Am J Pathol 2013;183:1352–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mirza R, Fang MM, Ennis WJ, Koh TJ. Blocking interleukin-1β induces a healing- associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013;62:2579–2587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mirza R, Fang MM, Weinheimmer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetes. Diabetes 2014;63:1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pascual G, Fong AL, Ogawa S, et al. A sumoylation-dependent pathway mediating transrepression of inflammatory response genes by PPARγ. Nature 2005;437:759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mirza RE, Fang MM, Novak ML, et al. Macrophage PPARγ and impaired wound healing in type 2 diabetes. J Pathol 2015;236:433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lebovitz HE. Differentiating members of the thiazolidinedione class: a focus on safety. Diabetes Metab Res Rev 2002;18:S23–S29 [DOI] [PubMed] [Google Scholar]
- 14. Patel RR. Thiazolidinediones and congestive heart failure: a judicious balance of risks and benefits. Cardiol Rev 2009;17:132–135 [DOI] [PubMed] [Google Scholar]
- 15. Silva JC, de Oliveira EM, Turato WM, et al. GQ-11: a new PPAR agonist improves obesity-induced metabolic alterations in LDLr −/− mice. Int J Obes (Lond) 2018;42:1062–1072 [DOI] [PubMed] [Google Scholar]
- 16. NIH (National Institute of Health), N 85-23: Committee for the Update of the Guide for the Care and Use of Laboratory Animal. Guide for Care and Use of Laboratory Animals, 8th ed. Washington, DC: The National Academies Press, 2011 [Google Scholar]
- 17. Silva JC, César FA, de Oliveira EM, et al. New PPARγ partial agonist improves obesity-induced metabolic alterations and atherosclerosis in LDLr −/− mice. Pharmacol Res 2016;104:49–60 [DOI] [PubMed] [Google Scholar]
- 18. Amato AA, Rajagopalan S, Lin JZ, et al. GQ-16, a novel peroxisome proliferator-activated receptor γ (PPARγ) ligand, promotes insulin sensitization without weight gain. J Biol Chem 2012;287:28169–28179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009;175:2454–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weischenfeldt J, Porse B. Bone marrow-derived macrophages (BMM): isolation and applications. CSH Protoc 2008;3:1–6 [DOI] [PubMed] [Google Scholar]
- 21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 22. Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab 2012;23:351–363 [DOI] [PubMed] [Google Scholar]
- 23. Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol 2009;49:123–150 [DOI] [PubMed] [Google Scholar]
- 24. Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature 1996;384:39–43 [DOI] [PubMed] [Google Scholar]
- 25. Vijay SK, Mishra M, Kumar H, Tripathi K. Effect of pioglitazone and rosiglitazone on mediators of endothelial dysfunction, markers of angiogenesis and inflammatory cytokines in type-2 diabetes. Acta Diabetol 2009;46:27–33 [DOI] [PubMed] [Google Scholar]
- 26. Orasanu G, Ziouzenkova O, Devchand PR, et al. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone represses inflammation in a peroxisome proliferator-activated receptor-alpha-dependent manner in vitro and in vivo in mice. J Am Coll Cardiol 2008;52:869–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mirza RE, Koh TJ. Contributions of cell subsets to cytokine production during normal and impaired wound healing. Cytokine 2015;71:409–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hou Y, Moreau F, Chadee K. PPARγ is an E3 ligase that induces the degradation of NFκB/p65. Nat Commun 2012;3:1300. [DOI] [PubMed] [Google Scholar]
- 29. Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010;184:3964–3977 [DOI] [PubMed] [Google Scholar]
- 30. Weinheimer-Haus EM, Mirza RE, Koh TJ. Nod-like receptor protein-3 inflammasome plays an important role during early stages of wound healing. PLoS One 2015;10:e0119106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–550 [DOI] [PubMed] [Google Scholar]
- 32. Fadok VA, McDonald PP, Bratton DL, Henson PM. Regulation of macrophage cytokine production by phagocytosis of apoptotic and post-apoptotic cells. Bioch Soc Transac 1998;26:653–656 [DOI] [PubMed] [Google Scholar]
- 33. Cvetanovic M, Ucker DS. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol 2004;172:880–889 [DOI] [PubMed] [Google Scholar]
- 34. Leibovich SJ, Ross R. The role of the macrophage in wound reapir. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975;78:71–100 [PMC free article] [PubMed] [Google Scholar]
- 35. Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-β1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF- β1-mediated fibrosis. JEM 2003;198:1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity 2008;28:468–476 [DOI] [PubMed] [Google Scholar]
- 37. Hori M, Yagi M, Nomoto K, et al. Experimental models for advanced glycation end-product formation using albumin, collagen, elastin, keratin and proteoglycan. Anti Aging Med 2012;9:125–134 [Google Scholar]
- 38. Xu F, Zhang C, Graves DT. Abnormal cell responses and role of TNF-α in impaired diabetic wound healing. Biomed Res Int 2013;2013:754802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang W, Liu E, Zhang J, et al. Activation of PPAR alpha by fenofibrate inhibits apoptosis in vascular advential fibroblasts partly through SIRT1-mediated deacetylation of FOXO1. Exp Cell Res 2015;338:54–63 [DOI] [PubMed] [Google Scholar]
- 40. Sakai S, Sato K, Tabata Y, Kishi K. Local release of Pioglitazone (a peroxisome proliferator-activated receptor γ agonist) accelerates proliferation and remodeling phases of wound healing. Wound Repair Regen 2015;24:57–64 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.