Abstract
Striatal medium spiny neurons (MSNs) are divided into two subpopulations exerting distinct effects on motor behavior. Transgenic mice carrying bacterial artificial chromosome (BAC) able to confer cell type-specific expression of enhanced green fluorescent protein (eGFP) for dopamine (DA) receptors have been developed to characterize differences between these subpopulations. Analysis of these mice, in contrast with original pioneering studies, showed that striatal long-term depression (LTD) was expressed in indirect but not in the direct pathway MSNs. To address this mismatch, we applied a new approach using combined BAC technology and receptor immunohistochemistry. We demonstrate that, in physiological conditions, DA-dependent LTD is expressed in both pathways showing that the lack of synaptic plasticity found in D1 eGFP mice is associated to behavioral deficits. Our findings suggest caution in the use of this tool and indicate that the “striatal segregation” hypothesis might not explain all synaptic dysfunctions in Parkinson's disease.
Introduction
Dopamine (DA), released from the neurons of the substantia nigra pars compacta into the striatum, exerts a critical role in the modulation of basal ganglia activity whose alteration is implicated in the pathophysiology of Parkinson's disease (PD) (Graybiel, 2005). The loss of DA in experimental PD causes the impairment of striatal synaptic long-term depression (LTD) in medium spiny neurons (MSNs), suggesting that a critical role of endogenous DA is to enable a form of plasticity representing the possible synaptic correlate of motor memory (Calabresi et al., 1992a,b, 1999, 2007a; Lovinger et al., 1993; Tang et al., 2001; Wang et al., 2006; Lovinger, 2010). These pioneering electrophysiological studies on striatal LTD found that this form of synaptic plasticity was virtually expressed in all striatal MSNs.
The classical model of basal ganglia functioning suggests that the ability of the striatum to select differential action of DA in the control of movement is due to the segregation of D1 and D2 DA receptors in two distinct groups of MSNs. According to this model, D1 receptor (D1R)-expressing MSNs of the direct pathway project to the substantia nigra pars reticulata, while D2 receptor (D2R)-expressing MSNs of the indirect pathway project to the medial globus pallidus (Gerfen et al., 1990). These two efferent pathways are believed to exert opposing effects on locomotor behavior (Gerfen, 1992). Direct confirmation of these hypotheses has been hindered by difficulty in selectively targeting direct and indirect pathway neurons with traditional techniques. Thus, bacterial artificial chromosome (BAC) transgenic mice conferring cell type-specific expression of enhanced green fluorescent protein (eGFP) in distinct neuronal subpopulations, including MSNs of the direct and indirect pathways, have been used in the last years (Kreitzer and Malenka, 2007; Ade et al., 2008; Shen et al., 2008; André et al., 2010). Utilizing BAC transgenic mice in which direct and indirect pathway neurons were labeled with GFP, Kreitzer and Malenka (2007) found that DA-dependent plasticity was selectively expressed in MSNs of the indirect pathway, while MSNs of the direct pathway did not express this form of synaptic plasticity. However, in a previous study from Wang et al. (2006), in which eGFP specifically targeted the neurons of the direct or of the indirect pathway, no substantial differences was found between the two neuronal populations.
These findings had obvious implications for understanding the normal functions of the basal ganglia and these tools were produced and promptly made available to address questions in a cell-specific manner.
Nevertheless, caution in the interpretation of data resulting from the use of eGFP transgenic mice is needed since it has been indeed recently reported that D2R-eGFP mice express an altered behavioral and physiological phenotype due to D2 receptor overexpression (Kramer et al., 2011).
Thus, in the present study, we have addressed the mismatch between the original pioneering studies on striatal LTD and the recent findings obtained using BAC transgenic mice. In particular, we have analyzed the expression of LTD in the two populations of MSNs identified by a new approach using the BAC technology combined with substance P (SP) (direct pathway) and adenosine A2A (indirect pathway) receptor immunohistochemistry (Rosin et al., 2003; Deng et al., 2006). Our findings support the original view that LTD is expressed in MSNs of both direct and indirect pathways and suggest caution in the use of BAC mice targeting DA receptors because genetic manipulation in these mice might result per se in behavioral and electrophysiological phenotypic abnormalities. These findings also indicate the “striatal segregation” hypothesis might not explain all synaptic dysfunctions observed in PD.
Materials and Methods
All the experiments were conducted in conformity with the European Communities Council Directive of November 1986 (86/609/ECC). Two- to 3-month-old male Wistar rats (Harlan) (n = 85), C57BL/6 mice (n = 44), and 6- to 12-week-old male C57BL/6J mice carrying BAC (n = 52) that express enhanced green fluorescent protein (BAC-eGFP) under the control of D1R promoter (drd1-eGFP) or D2R promoter (drd2-eGFP) were used for electrophysiological experiments. BAC-eGFP mice were originally generated by the GENSAT (Gene Expression Nervous System Atlas) program at the Rockefeller University (Gong et al., 2003) and were backcrossed into C57BL/6 mice. All experiments were performed in male hemizygous mice.
6-Hydroxydopamine lesion in the rats.
Procedures for obtaining rats with 6-hydroxydopamine (6-OHDA)-induced striatal DA denervation have been previously given in detail (Picconi et al., 2003, 2008). In brief, deeply anesthetized rats were unilaterally injected with 6-OHDA (12 μg/4 μl of saline containing 0.1% ascorbic acid) into the medial forebrain bundle (MFB) (Picconi et al., 2008). For rats receiving sham surgery, the scalp was incised and holes were drilled through the skull at sites corresponding to the lesion coordinates (Paxinos et al., 1985). In this case, rats underwent the injection of the vehicle alone. Following 15 days, rats were tested with 0.05 mg/kg subcutaneously administered apomorphine, and turns contralateral to the lesion were counted for 40 min. Rats with >200 contralateral turns were assigned to the group of the DA-denervated animals. Sham-operated animals did not show turning behavior. One and one-half months after the lesion, the rats were used for electrophysiological experiments. The severity of the lesion was confirmed afterward by striatal and nigral immunohistochemistry tyrosine hydroxylase (Picconi et al., 2003).
6-Hydroxydopamine lesion in the mice.
Mice were deeply anesthetized and mounted in a stereotaxic frame (Kopf Instruments) with a mouse adaptor. 6-OHDA-HCl (Sigma-Aldrich) was dissolved in 0.01% ascorbate-saline at the concentration of 3.0 μg/μl freebase 6-OHDA. Mice received an injection of 1 μl of 6-OHDA into the left MFB (Lundblad et al., 2004). To minimize nonspecific tissue damage, the injections were performed at a rate of 0.5 μl/min using a glass capillary with an outer diameter of ∼50 μm attached to a 10 μl Hamilton syringe. The injection cannula was left in place for additional 3 min before slowly retracting it. Sham lesions were performed by injection of 0.01% ascorbate saline in the MFB.
Preparation and maintenance of corticostriatal slice.
Briefly, corticostriatal coronal slices were cut from rats or mice brains (thickness, 240 μm) using a vibratome (Picconi et al., 2011; Tozzi et al., 2011). A single slice was transferred to a recording chamber and submerged in a continuously flowing Krebs' solution (room temperature; 2.5–3 ml/min) bubbled with a 95% O2–5% CO2 gas mixture. The composition of the solution was as follows (in mm): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 10 glucose, and 25 NaHCO3. Drugs were bath applied by switching the solution to one containing known concentrations of drugs. Total replacement of the medium in the chamber occurred within 1 min.
Electrophysiology.
Sharp electrodes for the intracellular recordings were filled with 2 m KCl (30–60 MΩ). Signals were recorded using an Axoclamp 2B amplifier (Molecular Devices), displayed on a separate oscilloscope, stored, and analyzed on a digital system (pClamp 9; Molecular Devices). Glutamatergic EPSPs and EPSCs were evoked every 10 s by means of a bipolar electrode connected to a stimulation unit (Grass-Telefactor). As conditioning high-frequency stimulation (HFS), we used three trains (3 s duration, 100 Hz frequency, at 20 s intervals). During tetanic stimulation, the intensity was increased to suprathreshold levels. For patch-clamp recordings, neurons were visualized using differential interference contrast and infrared (IR) microscopy (Nikon). MSNs from slices of mice expressing BAC-eGFP under the control of D1R promoter (D1 eGFP) or D2R promoter (D2 eGFP) were visualized with an IR- and fluorescence-equipped microscope (Nikon; Eclipse FN1). Whole-cell voltage-clamp (holding potential, −70 mV) recordings were performed with borosilicate glass pipettes (4–7 MΩ) filled with the following internal solution (in mm): 120 CsMeSO3, 15 CsCl, 8 NaCl, 0.2 EGTA, 10 HEPES, 2 Mg-ATP, 0.3 Na-GTP, 10 TEA (tetraethylammonium), 5 QX-314, adjusted to pH 7.2 with CsOH. In some experiments performed in current-clamp mode, the following solution (in mm) was adopted: 120 K-gluconate, 0.1 CaCl2, 2 MgCl2, 0.1 EGTA, 10 HEPES, 0.3 Na-GTP, and 2 Mg-ATP, adjusted to pH 7.3 with KOH. Signals were amplified with a MultiClamp 700B amplifier (Molecular Devices), recorded, and stored on PC using pClamp 10. Whole-cell access resistance was 10–30 MΩ. The stimulating electrode was located in the striatum to activate glutamatergic fibers. The recording electrodes were placed within the dorsolateral striatum. All the experiments were conducted in the continuous presence of 50 μm picrotoxin (Sigma-Aldrich). Input resistances and injected currents were monitored throughout the experiments. Variations of these parameters >20% led to the rejection of the experiment. Quantitative data on EPSP or EPSC modifications induced by HFS are expressed as a percentage of the controls, the latter representing the mean of responses recorded during a stable period (15–20 min) before the tetanus.
Tissue processing and double immunofluorescence.
The brain sections were postfixed overnight at +4°C with 4% paraformaldehyde in saline solution and then cryoprotected in phosphate buffer (PB) (0.1 m) with 0.02% sodium azide for 48 h at 4°C. The sections filled with biocytin (during electrophysiological recordings) were incubated with streptavidin-Cy3 (catalog #S6402; Sigma-Aldrich) diluted 1:600 in PB-TX-100 (0.3%) for 2 h at room temperature to verify the presence of cells filled with biocytin. Sections (from each group) were preincubated with a primary antibody goat anti-SP (Immunological Science) or rabbit polyclonal anti-A2A (Alexis) to label the medium spiny projection neurons that are component, respectively, of the “direct” or “indirect” basal ganglia pathway (Beckstead and Kersey, 1985; Svenningsson et al., 1999; Rosin et al., 2003; Rebola et al., 2005; Deng et al., 2006).
The primary antisera were used at a concentration of 1:400 for SP and 1:250 for A2A in 0.1 m PB containing 0.3% Triton X-100 and 0.02% sodium azide for 24 h at room temperature and 48 h at 4°C. Sections were then rinsed three times for 15 min at room temperature, and subsequently incubated with a mixture of anti-goat Cy2- and anti-rabbit Cy5-conjugated secondary antibody (Jackson ImmunoResearch) for 2 h at room temperature. All the secondary antibodies were used at 1:200 concentration. Tissue was mounted on gelatin-coated slides, coverslipped with Gel Mount, and all the images were acquired with a confocal laser-scanning microscope Zeiss LSM700 with 40× oil magnification. To acquire the inserts showing the branched dendrites, a 100× oil zoom 6 magnification was used.
Behavioral measurements, open field.
The apparatus consists of a circular open field arena (diameter, 60 cm) made in opaque black Plexiglas and surrounded by four black 20-cm-high walls. The animals were gently placed in the middle of the open field (OF) and were allowed to freely explore the area for 10 min. Behavioral measures included the following: total distance traveled, average velocity and rearing frequency (number of times the animals stood on their hindpaws), and duration. In addition to general activity levels, the amount of time spent along the periphery in the border areas, as opposed to the central areas, was used as an index of anxiety. Mice were considered in the central compartment only when all their four paws were within its border. The OF was washed with a 70% ethanol solution between each behavioral session to eliminate possible bias due to odors left by previous mice. All activity was recorded digitally and analyzed off-line by a computer-based video tracking system, EthoVision (Noldus).
Two-way active avoidance test.
Fifteen mice per group were used in the training experiments. Active avoidance (AA) task requires to learn that an explicit conditioned stimulus (CS) (light) precedes the delivery of a negative unconditioned stimulus (US) (footshock). The test has been performed in a shuttle box (40 × 10 × 15 cm). The used box is divided in two compartments by a partition with an opening at the floor level. The box has a transparent cover with a light bulb (10 W) attached above the compartment. The floor is made of a stainless-steel grid. Mice were subjected to one AA session (duration, 30 min; 60 avoidance trials) for 5 consecutive days. Each trial consisted of 22 s of darkness followed by a 4 s light signal (CS) presented in one compartment and subsequently 4 s of light associated to an electric footshock (0.2 mA, 25 s) in the same compartment (US). The number of conditioned responses (AA) (crossings occurring within 8 s of CS), the average escape latency to cross to the other side of the box after the beginning of each conditioned stimulus, and the number of intertrial crossings between the two box compartments were recorded automatically by the apparatus (Ugo Basile). Footshock sensitivity was evaluated by placing mice in the cage where the grid floor was connected to a shock producer. Mice were individually placed in the cage and their pain thresholds were evaluated by increasing current intensity from 0 to a maximum of 0.6 mA. The minimal intensity eliciting vocalization and jumping was retained as the score. Mice failing to squeak were given the maximum score of 0.6 mA. Two-way ANOVA was performed to compare means with one factor between groups (genotype) and one factor within groups (day).
Statistics.
Values given in the text and in the figures are mean ± SEM of changes in the respective cell populations. Paired Student's t test was used for the electrophysiological analysis of the pre- versus post-HFS protocol in the same cell population. Statistical comparisons of the electrophysiological experiments between different neuronal populations were analyzed using a two-way ANOVA. If the interactions were significant, the tests were followed by Bonferroni's test for post hoc comparison. The analyses were done using Prism 4.0 software (GraphPad Software).
For behavioral experiments, group data were calculated as mean ± SEM. Student's t test and two-way ANOVAs were used to analyze statistical differences between genotypes. Group means were compared with Bonferroni's post hoc tests. Statistically significant data were reported if p < 0.05.
Drugs.
Picrotoxin and l-sulpiride were from Tocris Bioscience. Apomorphine hydrochloride and 6-OHDA were from Sigma-Aldrich.
Results
LTD in striatal MSNs in physiological condition and after 6-OHDA lesion
LTD has been described as a persistent decrease in the amplitude of glutamatergic evoked events occurring at corticostriatal synapses following HFS protocol (Calabresi et al., 1992b; Lovinger et al., 1993).
In the present study, we show that, whatever the technique used for the electrophysiological recordings, sharp intracellular (Fig. 1A,B) and patch-clamp (Fig. 1C,D), or the configuration adopted, current (Fig. 1A,B)- and voltage-clamp mode (Fig. 1C,D), LTD was induced after the delivery of HFS, in almost the totality of healthy MSNs (n = 70 of 80 recorded cells), recorded from either rats or mice slices (n = 20 for each group, pre- vs post-HFS, p < 0.0001).
Figure 1.
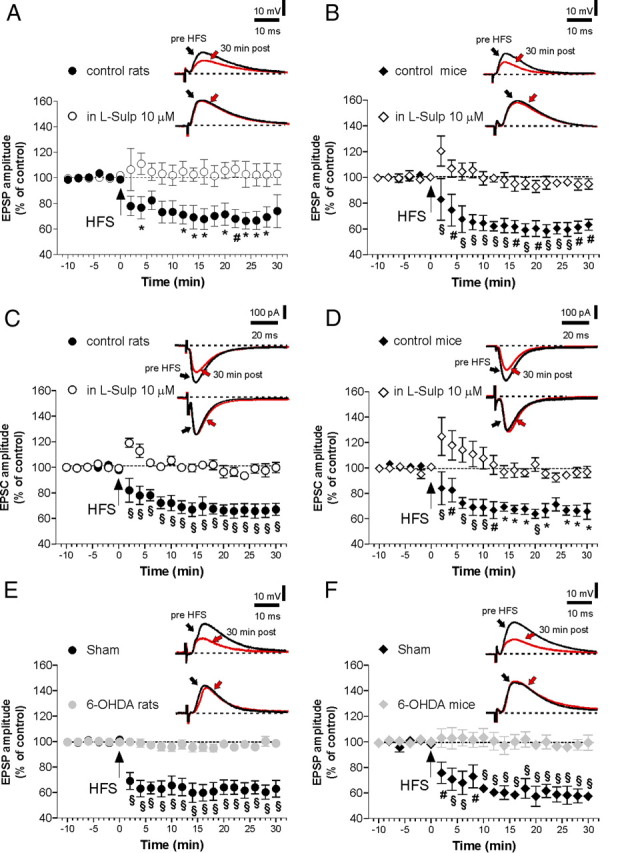
HFS elicits LTD in MSNs recorded from control rats and mice but not in 6-OHDA-lesioned animals. A, B, The graphs show the time course of LTD in MSNs recorded by patch-clamp/sharp electrode techniques in current-clamp mode, respectively, from control rats slices and control mice slices, before (n = 20 for each group) and after the application of the D2 receptor antagonist l-sulpiride (n = 10). Insets, n = 8 averaged traces of EPSPs before and 30 min after the delivery of HFS. Calibration bars: 10 mV, 10 ms. Stimulus artifacts have been truncated. C, D, EPSC amplitudes before and after LTD induction protocol in control condition and in the presence of l-sulpiride in patch-clamp recordings in voltage clamp, respectively, from MSNs in rats and mice (n = 10 for each condition). Insets, Waveforms are averages of n = 8 EPSCs from single experiments at the times indicated. Calibration bars: 100 pA, 20 ms. Stimulus artifacts have been truncated. Pre- versus post-HFS in l-sulpiride, p > 0.05; control versus plus l-sulpiride, *p < 0.05, #p < 0.01, §p < 0.001. E, F, EPSC plotted amplitude before and after HFS in 6-OHDA-lesioned and sham-operated animals, either in rats (E) (n = 10) or mice (F) (n = 10). Averaged EPSC traces are shown as insets, at the times indicated. Pre- versus post-HFS in 6-OHDA animals, p > 0.05; sham versus 6-OHDA rats, #p < 0.01, §p < 0.001. Error bars indicate SEM.
In agreement with previous reports (Calabresi et al., 1992b, 1999; Wang et al., 2006), LTD induction was fully prevented by the application of the D2R antagonist l-sulpiride (10 μm) in all experimental conditions (Fig. 1A–D; n = 10 for each condition, pre- vs post-HFS in l-sulpiride, p > 0.05; control vs plus l-sulpiride, *p < 0.05, #p < 0.01, §p < 0.001).
LTD is abolished in MSNs following striatal DA denervation, indicating that this form of synaptic plasticity is dependent on the endogenous DA (Calabresi et al., 1992a,b; Paillé et al., 2010). In line with these findings, we found that, in all the MSNs recorded from either mice or rats, in which the striatal DAergic fibers were lesioned by 6-OHDA, LTD was fully abolished (Fig. 1E,F). Conversely, this form of synaptic plasticity was normal in sham-operated animals (Fig. 1E,F; 6-OHDA rats, 6-OHDA mice, n = 10 for each condition; pre- vs post-HFS, p > 0.05; sham vs 6-OHDA rats, #p < 0.01, §p < 0.001).
LTD in the direct and indirect pathway striatal MSNs
To reveal possible heterogeneity in the determinants of LTD induction between direct and indirect pathway neurons following HFS, cells were backfilled with biocytin during recordings and immunofluorescence double labeling was performed a posteriori. Direct pathway MSNs were immunohistochemically identified as “substance P positive” (SP+) (Fig. 2A) (Beckstead and Kersey, 1985), whereas those from the indirect pathway were recognized as “A2A receptor positive” (A2A+) (Fig. 2A) (Svenningsson et al., 1999; Rebola et al., 2005). In Figure 2B, the time course of LTD in MSNs recorded from SP+ and A2A+ neurons in control rats is reported. Both the amplitude and the time course of LTD were similar in these cell types (n = 5, pre- vs post- HFS, p < 0.0001 in SP+; n = 5, pre- vs post-HFS, p < 0.0001 in A2A+). l-Sulpiride, an antagonist of the D2 receptor, fully prevented LTD induction in both neuronal populations (Fig. 2B; n = 6, pre- vs post-HFS, p > 0.05 in SP+ neurons; n = 6, pre- vs post-HFS, p > 0.05 in A2A+ neurons; SP+ vs plus l-sulpiride, *p < 0.05, #p < 0.01, §p < 0.001; A2A+ vs plus l-sulpiride, §p < 0.001).
Figure 2.
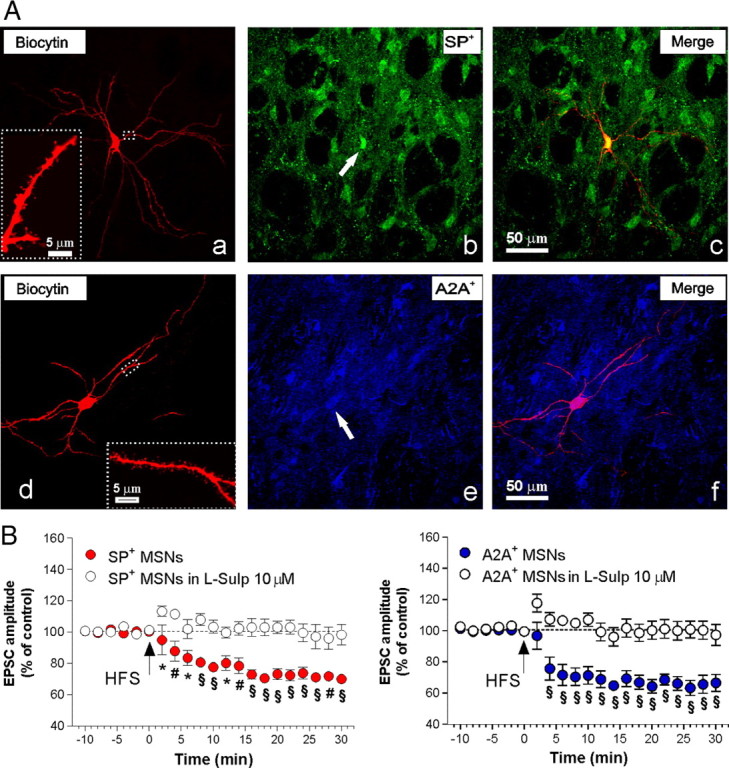
D2 receptor antagonism prevents synaptic plasticity in both the direct and indirect pathway neurons. A, Confocal laser-scanning microscopy images of double-labeled immunofluorescence for biocytin and SP (a–c) and biocytin and adenosine A2A receptor (A2A) (d–f). Biocytin immunolabeling is visualized in streptavidin-Cy3 fluorescence (a, d), while SP (b) and A2A (e) are, respectively, visualized in green-Cy2 or blue-Cy5 fluorescence. The merged image is shown in the two panels on the right (c, f). The white arrows show the colocalization of SP- or A2A-positive medium spiny projection neurons with the cell filled with biocytin. Scale bar, 50 μm. In a and d, higher magnification shows branched dendrites characteristic of intracellularly filled medium spiny neurons (MSNs). Scale bar, 5 μm. B, The left panel shows the time course of the changes in EPSC amplitude in SP+ MSNs after the delivery of HFS, in absence (n = 5; filled circles) or in presence of l-sulpiride (10 μm) (n = 6; open circles). In the right panel is reported the time course of EPSC from A2A+ MSNs after HFS in control condition (n = 5) or in the presence of l-sulpiride (n = 6). Pre- versus post-HFS in l-Sulp, p > 0.05 in SP+ neurons; pre- versus post-HFS in l-Sulp, p > 0.05 in A2A+ neurons; SP+ versus plus l-sulpiride, *p < 0.05, #p < 0.01, §p < 0.001; A2A+ versus plus l-sulpiride, §p < 0.001. Error bars indicate SEM.
Severe 6-OHDA denervation blocks LTD induction in both SP+ and A2A+ MSNs
One peculiar property of striatal synaptic plasticity induction is its reliance on DA (Calabresi et al., 1992b; Picconi et al., 2003, 2011). However, recent data on spike-timing-dependent synaptic plasticity have questioned this important concept and raised the possibility that synaptic plasticity is differentially impaired in the two pathways after nigrostriatal lesion (Shen et al., 2008).
Hence, to distinguish MSNs from direct and indirect pathways, we recorded all the neurons from 6-OHDA-lesioned rats, filling them with biocytin (Fig. 3Aa,d). In these experiments, HFS failed to induce significant changes of the glutamatergic transmission in all the denervated cells of both subtypes, while LTD was intact in slices obtained from sham-operated animals (Fig. 3B, left panel; n = 5 for each group; 6-OHDA SP+, pre- vs post-HFS, p > 0.05; sham SP+ vs 6-OHDA SP+, *p < 0.05, #p < 0.01) (Fig. 3C, left panel; n = 5 for each group; 6-OHDA A2A+ pre- vs post-HFS, p > 0.05; sham A2A+ vs 6-OHDA A2A+, §p < 0.001). These neurons were subsequently demonstrated, by means of double-labeled immunofluorescence for biocytin and SP (Fig. 3Aa–c) or biocytin and A2A (Fig. 3Ad–f), to belong either to the direct or the indirect pathway (n = 5 SP+; n = 5 A2A+).
Figure 3.
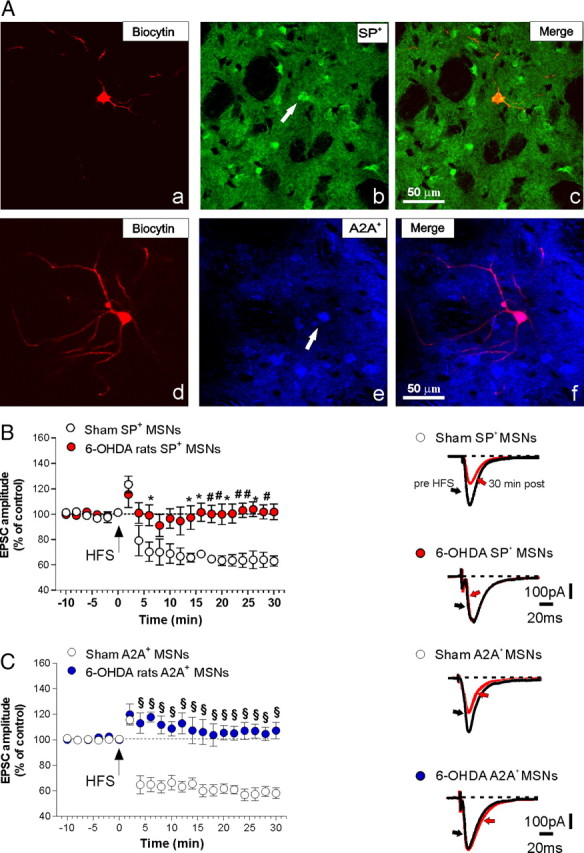
Striatal DA denervation blocks LTD induction in both SP+ and A2A+ medium spiny neurons (MSNs). A, Confocal laser-scanning microscopy images of double-labeled immunofluorescence showing biocytin and SP (a–c) and biocytin and adenosine A2A receptor (A2A) (d–f) from 6-OHDA-lesioned rats. A, Biocytin immunolabeling is visualized in streptavidin-Cy3 fluorescence (a, d), while SP (b) is revealed by green-Cy2 fluorescence and A2A (e) is visualized by blue-Cy5 fluorescence. The merged image is shown in the left panel (c, f). The white arrows show the colocalization of SP- or A2A-positive medium spiny projection neurons with the cell filled with biocytin. Scale bar, 50 μm. B, C, In the left panel is reported the time course of LTD in MSNs recorded from SP+, or A2A+, either from control rats or 6-OHDA-lesioned animals (n = 5 for each group). The right panels in B and C show averaged traces of the EPSC 10 min before and 30 min after the delivery of HFS. 6-OHDA SP+, pre- versus post-HFS, p > 0.05; sham SP+ versus 6-OHDA SP+, *p < 0.05, #p < 0.01. 6-OHDA A2A+ pre- versus post-HFS, p > 0.05; sham A2A+ versus 6-OHDA A2A+, §p < 0.001. Error bars indicate SEM. Calibration bars: 100 pA, 20 ms.
LTD in D1 eGFP and D2 eGFP mice
Studies using mice expressing eGFP targeting direct or indirect pathway MSNs have shown several differences between MSNs of the two categories (Day et al., 2006; Kreitzer and Malenka, 2007; Bertran-Gonzalez et al., 2008, 2009; Shen et al., 2008; Matamales et al., 2009; André et al., 2010; Kramer et al., 2011), also showing that striatal LTD is segregated within the indirect pathway neurons (Kreitzer and Malenka, 2007) and raising concerns on the conclusions of studies conducted before the introduction of this new tool. Moreover, findings obtained using eGFP mice appear also in contrast with the data resulting from the immunohistochemical and electrophysiological characterization of MSNs of the present study. To investigate the underlying reasons of these discordant results, we also analyzed LTD in eGFP D1 and D2 mice.
MSNs recorded in slices obtained from mice expressing eGFP under the control of D1R (D1 eGFP) or D2R promoter (D2 eGFP) were visualized with an infrared and fluorescence-equipped microscope. Only neurons displaying a marked fluorescence were approached for patch-clamp recordings and underwent subsequent electrophysiological characterization. As reported in Figure 4, A and B, no significant differences between the two pathway neurons were observed in response to positive and negative current pulses injected, although D2 cells were more prone to fire compared with D1 cells (n = 8 for each group) (Fig. 4A,B). Examination of action potentials evoked with depolarizing current pulses or with continuous current injection did not reveal significant differences in amplitude (88.54 ± 5.82 mV, D1 neurons, vs 89.0 ± 4.76 mV, D2 neurons), half-amplitude duration (0.8 ± 1 ms, D1 neurons, vs 0.9 ± 1 ms, D2 neurons), or afterhyperpolarization amplitudes (13.88 ± 4 mV, D1 neurons, vs 12.96 ± 3.5 mV, D2 neurons) between the two cell populations.
Figure 4.
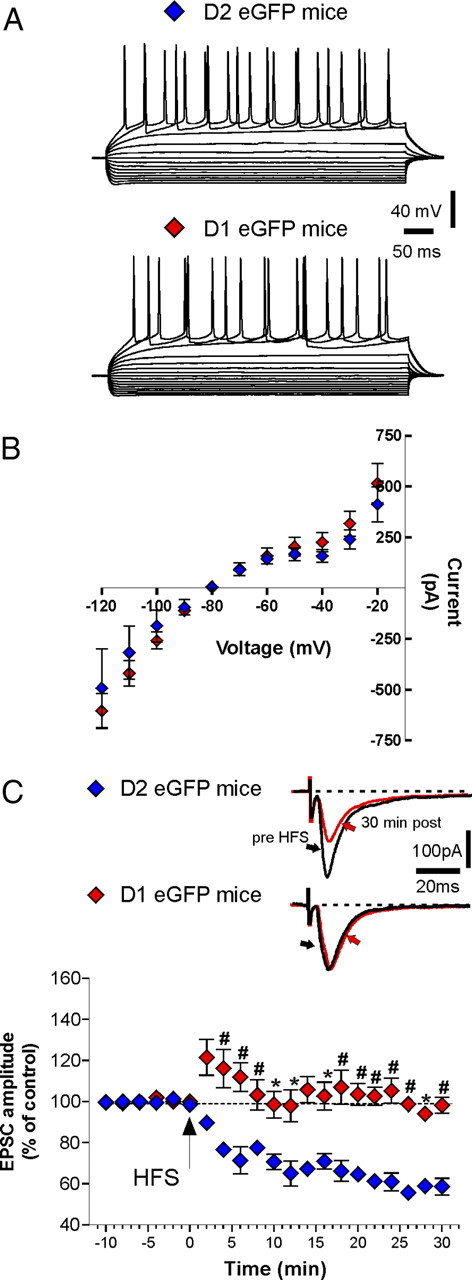
Electrophysiological characterization of D1 and D2 eGFP mice and lack of LTD in D1 eGFP mice. A, Examples of current-clamp recordings obtained from two MSNs in D1 eGFP and D2 eGFP mice, representing responses to a series of hyperpolarizing and depolarizing pulses of currents (from −400 pA up to +200 pA, delta between pulses 50 pA, 500 ms duration). B, The graph shows current–voltage plots generated by measuring the voltage change as a function of current intensity in MSNs recorded from direct and indirect pathway (n = 8 for each group). C, The graph shows LTD in D2 eGFP (blue diamonds; n = 12) mice but not in D1 eGFP (red diamonds; n = 13). Pre- versus post-HFS, p > 0.05; D2 eGFP MSNs versus D1 eGFP MSNs, *p < 0.05, #p < 0.01. Error bars indicate SEM. The insets show representative averaged traces of the EPSC evoked, respectively, 10 min before and 30 min after the delivery of HFS, respectively, in D2 (top inset) and D1 (bottom inset) mice. Calibration bars: 100 pA, 20 ms.
HFS induced a robust LTD in all the fluorescent medium spiny neurons recorded from D2 eGFP mice (Fig. 4C; n = 12; pre- vs post-HFS, p < 0.0001). Conversely, in line with other results previously reported on BAC transgenic mice labeled for direct and indirect pathways (Kreitzer and Malenka, 2007), the same protocol failed to induce LTD in all the fluorescent MSNs recorded from D1 eGFP mice (Fig. 4C; n = 13; pre vs post-HFS, p > 0.05; D2 eGFP MSNs vs D1 eGFP MSNs, *p < 0.05, #p < 0.01).
Combined immunohistochemical and electrophysiological analysis in D1 and D2 eGFP mice
From these latter results, one would conclude that LTD is a form of synaptic plasticity restricted only to the indirect pathway neurons. However, this result was in sharp contrast with the findings obtained with blind recordings and immunofluorescence a posteriori. Therefore, our working hypothesis was that the results obtained in D1 eGFP mice could be a consequence of the manipulation of D1R expression and signaling. For this reason, we recorded the nonfluorescent neurons either from D1 or D2 eGFP mice to study LTD avoiding possible alterations resulting from the eGFP expression. As shown in Figure 5A, the nonfluorescent neurons were recorded from D2 eGFP mice, and they were found to be SP+ (putative direct pathway neurons) (n = 6). Surprisingly, HFS was able to induce a robust and reliable LTD in these direct pathway neurons (pre- vs post-HFS, p < 0.0001). This result was unexpected according to the findings obtained from D1 cells expressing eGFP. In parallel, we recorded nonfluorescent neurons from D1 eGFP mice (A2A+) and also in this neuronal population, the tetanic stimulation induced LTD (n = 6; pre- vs post-HFS A2A+ neurons, p < 0.0001). Interestingly, in both groups (Fig. 5A,B), LTD induction was fully prevented by bath-application of l-sulpiride (n = 6 for each group; SP+ vs SP+ plus l-sulpiride or A2A+ vs A2A+ plus l-sulpiride, #p < 0.01, §p < 0.001).
Figure 5.

LTD is expressed in nonfluorescent MSNs recorded from either D1 eGFP or D2 eGFP mice. A, In the top panel are represented confocal laser-scanning microscopy images of double-labeled immunofluorescence for biocytin and SP in eGFP D2 mice (a–d). a, In the left panel is shown a nonfluorescent MSN in a D2 eGFP mice (white arrow). Biocytin immunolabeling is visualized in streptavidin-Cy3 fluorescence (b), while SP (c) is visualized in blue-Cy5 fluorescence. The merged image is shown in d. The white arrows show the colocalization of substance P-positive medium spiny projection neurons with the cell filled with biocytin. Scale bar, 50 μm. In the left bottom panel is represented the time course of LTD in nonfluorescent neurons recorded from eGFP D2 mice in the presence and absence of l-sulpiride (n = 6 for each group). The right panel shows the averaged traces of EPSCs recorded 10 min before and 30 min after the delivery of HFS protocol. Calibration bars: 100 pA, 20 ms. B, In the top panel are represented confocal laser-scanning microscopy images of double-labeled immunofluorescence for biocytin and SP in eGFP D2 mice (a–d). Biocytin immunoreactivity is revealed by streptavidin-Cy3 fluorescence (b), while A2A (c) is labeled by blue-Cy5 fluorescence. The merged image is shown in the right panel (d). The white arrows show the colocalization of substance P-positive medium spiny projection neurons with the cell filled with biocytin. Note in this field the cell filled with biocytin co-contains A2A, whereas is devoid of D1 receptor (white arrows). Scale bar, 50 μm. The bottom panel on the left shows the time course of LTD in nonfluorescent neurons recorded from D1 eGFP mice, identified as A2A-positive neurons, in the presence and absence of l-sulpiride (n = 6 for each group). SP+ versus SP+ plus l-sulpiride or A2A+ versus A2A+ plus l-sulpiride, #p < 0.01, §p < 0.001. Error bars indicate SEM. In the right panel are reported representative traces of the EPSCs evoked 10 min before and 30 min after the delivery of HFS. Calibration: 100 pA, 20 ms.
Locomotor activity in D1 eGFP mice
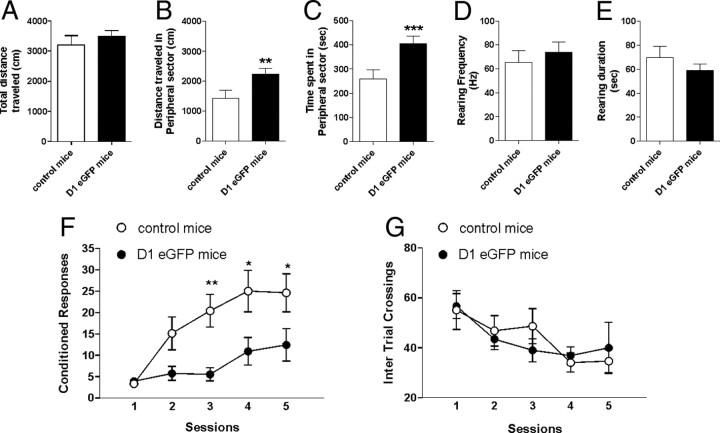
Since it has been hypothesized that DA-dependent striatal synaptic plasticity influences movements and cognition (Calabresi et al., 2006, 2007a,b), locomotor activity and cognitive performances were analyzed in D1 eGFP mice as well as in control animals. Spontaneous locomotor activity in a novel environment was evaluated by open field test. Exploratory behavior declined progressively over the 10 min observation interval following introduction of animals into the open field arena in both experimental groups (data not shown). In addition to general activity levels, which was comparable between the two groups in terms of total distance traveled (Fig. 6A, control mice vs D1 eGFP mice, respectively, n = 8 and n = 9; p > 0.05), the amount of time spent along the periphery in the border areas, as opposed to the central areas, was used as an index of anxiety (thigmotaxis) (Simon et al., 1994). Surprisingly, D1 eGFP mice appeared significantly more anxious in the novel environment compared with the control mice since they showed increased activity in the peripheral sector in terms of distance traveled (Fig. 6B; control mice vs D1 eGFP mice, **p < 0.01) and time spent (Fig. 6C; control mice vs D1 eGFP mice, ***p < 0.001). No significant difference in vertical activity (rearing) was observed between D1 eGFP and control mice (Fig. 6D,E; control mice vs D1 eGFP mice, p > 0.05).
Figure 6.
Behavioral alterations in D1 eGFP mice. A–E, Mice were exposed to the open field and their locomotor activity was monitored for 10 min (D1 eGFP, n = 9, black bar; control mice, n = 8, white bar). A, Total distance traveled in the open field test in D1 eGFP and control mice. B, C, Bar graphs showing, respectively, the distance traveled and the time spent by D1 eGFP and control mice in the peripheral area. Control mice versus D1 eGFP mice, **p < 0.01. Control mice versus D1 eGFP mice, ***p < 0.001. D, E, Vertical exploratory activity is indicated as rearing duration and rearing frequency in the two bar graphs. F, G, Striatal-dependent learning abilities of D1 eGFP mice and control mice were tested using a two-way AA test. F, The graph shows the mean number of avoidance responses per day ± SEM recorded for each group (n = 15). Two-way ANOVA revealed an effect of interaction session by genotype, indicating that performances were lower in D1 eGFP (filled circles) than in control mice (open circles). *p < 0.05; **p < 0.01. G, Mean number of crossings between the two compartments per day ± SEM are reported. Two-way ANOVA revealed no significant group main effect, indicating that locomotion did not significantly differ between genotypes.
Cognitive performances in D1 eGFP mice
Cognitive performance in D1 eGFP and control mice in the two-way AA test was examined. This paradigm requires the association of a cue (light or sound) with a footshock and the learning of a footshock avoidance strategy. The formation of this association in the avoidance behavior largely depends on the dorsolateral striatum (Fibiger and Mason, 1978; White and McDonald, 2002), and it involves a functional DA signaling (Koob et al., 1984; Da Cunha et al., 2002).
To analyze learning in the AA test, we considered two measurements: number of avoidance responses (conditioned responses) and the intertrial crossing. In this experiment, 15 mice for each genotype were subjected to daily sessions for 5 consecutive days. Performances increased in each group as training proceeded (significant effect of “session,” F(4,112) = 14.64; p < 0.001); however, a significant genotype–sessions interaction (F(4,112) = 3.88; p < 0.01) revealed a different trend in performance according to the group. In fact, as shown in Figure 6F, on the last day of training, D1 eGFP mice reached lower active avoidance scores than control mice (*p < 0.05; **p < 0.01). The same statistical analysis was performed on the number of intertrial crossings. The results did not reveal any significant effect of genotype or sessions for this variable, revealing that impairment is not secondary to motor deficits (Fig. 6G).
Discussion
Long-lasting, activity-dependent synaptic changes are thought to underlie the ability of the brain to translate experiences into memories and seem to represent the cellular model underlying learning and memory processes (Kandel, 2001). Alteration of brain plasticity may lead to the motor and cognitive disturbances observed in neurodegenerative diseases such as PD (Calabresi et al., 2007b; Picconi et al., 2008; Di Filippo et al., 2009; Bagetta et al., 2010). Therapeutic approaches targeting synaptic plasticity could prevent neuronal degeneration and restore altered motor and cognitive functions (Calabresi et al., 2006, 2010). LTD has been described at corticostriatal excitatory synapses and it might underlie motor-skill learning, cognitive performance, and reward mechanisms (Gerdeman et al., 2002; Calabresi et al., 2007a; Di Filippo et al., 2009). A unique feature of striatal LTD, among other forms of brain synaptic plasticity, is its dependence on endogenous DA and its loss in experimental models of PD (Calabresi et al., 1992b; Tang et al., 2001; Goldberg et al., 2005; Lovinger, 2010; Paillé et al., 2010). A common finding of these studies was the demonstration of a rather homogeneous expression of LTD in all MSNs recorded in physiological condition. In sharp contrast with these studies, the use of eGFP-expressing mice in the direct or indirect pathway has shown that LTD is segregated only in D2-positive neurons of the indirect pathway (Kreitzer and Malenka, 2007). Striatal MSNs, in fact, represent a heterogeneous population in terms of DA receptor expression, and the prevailing view is that D1 and D2 receptors are segregated into two subpopulations of projecting GABAergic spiny neurons, forming two large efferent streams that are thought to differ in their axonal targets (Gerfen, 1992). Since neuronal identification represents an important resource facilitating the physiological characterization of different cellular populations, D1 eGFP and D2 eGFP mice have provided a very efficient approach in the recognition of distinct neuronal populations within the striatum (Gong et al., 2003). Therefore, many researchers have adopted this method to identify and highlight the characteristics of the direct and indirect pathway neurons. Compelling evidence obtained by the use of this transgenic mouse model have demonstrated the existence of differences between striatonigral and striatopallidal MSNs, with respect to their intrinsic membrane properties and synaptic plasticity, in physiological and pathological conditions (Day et al., 2006; Kreitzer and Malenka, 2007; Cepeda et al., 2008; Shen et al., 2008; Bertran-Gonzalez et al., 2009).
However, a recent comprehensive report on homozygous D2 eGFP mice, based on behavioral, electrophysiological, and molecular characterization, showed that mice expressing eGFP through the BAC vector are not comparable with the wild-type littermates (Kramer et al., 2011). Interestingly, the present study provides a clear support to this observation, revealing that, in addition to D2 eGFP mice, also D1 eGFP mice seem to be dissimilar from control mice. In fact, here we report three main results further substantiating the hypothesis that BAC clone itself might determine severe modifications in neurons and circuit physiology.
The first surprising result is the lack of LTD in D1 fluorescent neurons from D1 eGFP mice. In fact, many experiments performed using blind intracellular sharp electrodes and patch-clamp recordings have never revealed a segregation in the LTD phenomenon. Noteworthy, in the first paper dealing with synaptic plasticity in D1 and D2-eGFP labeled neurons, the expression of LTD was homogeneously found (Wang et al., 2006). Moreover, even when electrophysiological recordings were coupled to immunohistochemical analysis a posteriori, striatonigral and striatopallidal neurons did not appear as two different populations in terms of LTD expression.
Indeed, in line with a previous study (Wang et al., 2006), we have recently shown that LTD is dependent upon CB1 and D2 receptor activation (Picconi et al., 2011), in both direct and indirect pathway neurons. Here, we further confirm this concept so that, in conclusion, the determinants of LTD in both subclasses of neurons appear the same, at least with this type of synaptic plasticity paradigm. Moreover, we show that, in both subpopulations of MSNs, LTD is dependent on D2 receptor activation in line with previous studies (Calabresi et al., 1992a; Tang et al., 2001; Wang et al., 2006).
Additionally, the relevance of the experimental paradigm inducing LTD should not be undervalued as demonstrated by the findings obtained in rats subjected to nigrostriatal lesion. In fact, with our conditioning protocol, the possibility to induce LTD was fully blocked in DA-denervated animals, in contrast with other results obtained with spike-timing-dependent plasticity (Shen et al., 2008). An additional explanation for the discrepancy between our results and other studies using spike-timing-dependent plasticity (Shen et al., 2008), might be that our experiments were performed 2 months after the lesion, when the degeneration of DAergic neurons reached a stable and severe degree, resembling the late parkinsonian state.
Moreover, the experimental procedure could explain the discrepancy between our results and those reported in the study by Wang et al. (2006), which found LTD in both D1 and D2 BAC mice. In fact, the authors used mice younger (P17–P25) than those analyzed in our study. Furthermore, a possible explanation for the mismatch observed could be related to the stimulating electrode positioning. Indeed, we have activated glutamatergic fibers directly in the striatum, thus leading to the recruitment of excitatory terminals arising from cortex and thalamus, while Wang et al. (2006) mainly activated corticostriatal inputs. It is also worth noting that the choice of this kind of stimulation could have modified the engagement of striatal interneurons that have been thoroughly demonstrated capable of modulating the induction of plasticity (Wang et al., 2006).
The second puzzling result is the expression of LTD in MSNs recorded from D2 eGFP mice that did not show fluorescence and were identified as SP positive (putative D1 neurons of the direct pathway). This finding raises the possibility that, in D1 fluorescent neurons, the lack of LTD might result from injured signaling downstream DA receptor.
The third result we report here regards the behavioral alterations found in D1 eGFP mice. In the open field test, an increase of thigmotaxis was observed in these mice, suggesting an increased anxiety (Simon et al., 1994). Interestingly, studies on animals with dorsomedial striatal lesion (Devan et al., 1999) suggest a central role for the striatum in the development of anxiety. Moreover, in a model of parkin mutant mouse, characterized by altered DAergic metabolism in the striatum, an increased thigmotactic behavior has been described (Zhu et al., 2007). D1 eGFP mice also show cognitive deficits. This interesting observation arises from the analysis of the active avoidance task, a paradigm depending on the DAergic striatal system (Darvas et al., 2011).
Together, these results may imply an abnormal DAergic signal within the striatum in D1 eGFP mice. Although we cannot conclude with certainty that the BAC vector is the cause of the hypothesized altered DAergic signaling in D1 eGFP mice, our results could give a clue to address this issue.
As suggested in a recent report (Kramer et al., 2011), several factors acting alone or in combination might be responsible for such impairment, such as, for instance, the insertion of the clone or the transgene presence in one or more copies. These events might lead to altered expression levels of the reporter gene or its expression pattern.
In conclusion, the findings of the present study have provided a hint that more caution should be taken working with eGFP mice in regard to their DAergic system, at least as they have been developed at the moment. In light of our results, we could state that, although defining the fingerprint of a recorded cell a posteriori may be considered a limitation, at least this approach preserves the physiology of neurons and the related circuits. Subsequent studies should bring further understanding about the specific mechanisms that dictate the complex alterations at the corticostriatal synapses in D1 and D2 eGFP mice.
The “segregation hypothesis” of striatal direct and indirect pathways has been applied to the interpretation of synaptic plasticity and basal ganglia circuit abnormalities observed in experimental models of PD. Our results demonstrating absence of physiological LTD expression in both the striatal pathways after DA depletion suggest caution in the use of this model to explain all basal ganglia physiological events in experimental PD.
Footnotes
This work was supported by the European Community FP7–Thematic Priority HEALTH Contract 222918 (REPLACES) (P.C.), by the Ministry of Health Grants Progetto Strategico 2007 (B.P., P.C.), Progetti Finalizzati 2006–2008 (B.P., P.C.), “Giovani Ricercatori 2008” (B.P.) and Cariplo Foundation contract number 0661-2010 (B.P.), and Prin 2008 (P.C.). We thank Prof. D. Centonze and A. Pisani for critically discussing this manuscript and Prof. P. Greengard who provided us with the eGFP mice.
The authors declare no competing financial interests.
References
- Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci. 2008;28:1185–1197. doi: 10.1523/JNEUROSCI.3908-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André VM, Cepeda C, Cummings DM, Jocoy EL, Fisher YE, William Yang X, Levine MS. Dopamine modulation of excitatory currents in the striatum is dictated by the expression of D1 or D2 receptors and modified by endocannabinoids. Eur J Neurosci. 2010;31:14–28. doi: 10.1111/j.1460-9568.2009.07047.x. [DOI] [PubMed] [Google Scholar]
- Bagetta V, Ghiglieri V, Sgobio C, Calabresi P, Picconi B. Synaptic dysfunction in Parkinson's disease. Biochem Soc Trans. 2010;38:493–497. doi: 10.1042/BST0380493. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Kersey KS. Immunohistochemical demonstration of differential substance P-, met-enkephalin-, and glutamic-acid-decarboxylase-containing cell body and axon distributions in the corpus striatum of the cat. J Comp Neurol. 1985;232:481–498. doi: 10.1002/cne.902320406. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Håkansson K, Borgkvist A, Irinopoulou T, Brami-Cherrier K, Usiello A, Greengard P, Hervé D, Girault JA, Valjent E, Fisone G. Histone H3 phosphorylation is under the opposite tonic control of dopamine D2 and adenosine A2A receptors in striatopallidal neurons. Neuropsychopharmacology. 2009;34:1710–1720. doi: 10.1038/npp.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Mercuri NB, Bernardi G. Coactivation of D1 and D2 dopamine receptors is required for long-term synaptic depression in the striatum. Neurosci Lett. 1992a;142:95–99. doi: 10.1016/0304-3940(92)90628-k. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Maj R, Pisani A, Mercuri NB, Bernardi G. Long-term synaptic depression in the striatum: physiological and pharmacological characterization. J Neurosci. 1992b;12:4224–4233. doi: 10.1523/JNEUROSCI.12-11-04224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Marfia GA, Bernardi G. Glutamate-triggered events inducing corticostriatal long-term depression. J Neurosci. 1999;19:6102–6110. doi: 10.1523/JNEUROSCI.19-14-06102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Parnetti L, Di Filippo M. A convergent model for cognitive dysfunctions in Parkinson's disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006;5:974–983. doi: 10.1016/S1474-4422(06)70600-7. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007a;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Galletti F, Saggese E, Ghiglieri V, Picconi B. Neuronal networks and synaptic plasticity in Parkinson's disease: beyond motor deficits. Parkinsonism Relat Disord. 2007b;13(Suppl 3):S259–S262. doi: 10.1016/S1353-8020(08)70013-0. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B. Levodopa-induced dyskinesias in patients with Parkinson's disease: filling the bench-to-bedside gap. Lancet Neurol. 2010;9:1106–1117. doi: 10.1016/S1474-4422(10)70218-0. [DOI] [PubMed] [Google Scholar]
- Cepeda C, André VM, Yamazaki I, Wu N, Kleiman-Weiner M, Levine MS. Differential electrophysiological properties of dopamine D1 and D2 receptor-containing striatal medium-sized spiny neurons. Eur J Neurosci. 2008;27:671–682. doi: 10.1111/j.1460-9568.2008.06038.x. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Angelucci ME, Canteras NS, Wonnacott S, Takahashi RN. The lesion of the rat substantia nigra pars compacta dopaminergic neurons as a model for Parkinson's disease memory disabilities. Cell Mol Neurobiol. 2002;22:227–237. doi: 10.1023/A:1020736131907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvas M, Fadok JP, Palmiter RD. Requirement of dopamine signaling in the amygdala and striatum for learning and maintenance of a conditioned avoidance response. Learn Mem. 2011;18:136–143. doi: 10.1101/lm.2041211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat. 2006;32:101–116. doi: 10.1016/j.jchemneu.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Di Filippo M, Picconi B, Tantucci M, Ghiglieri V, Bagetta V, Sgobio C, Tozzi A, Parnetti L, Calabresi P. Short-term and long-term plasticity at corticostriatal synapses: implications for learning and memory. Behav Brain Res. 2009;199:108–118. doi: 10.1016/j.bbr.2008.09.025. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Mason ST. The effects of dorsal bundle injections of 6-hydroxydopamine on avoidance responding in rats. Br J Pharmacol. 1978;64:601–605. doi: 10.1111/j.1476-5381.1978.tb17322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu Rev Neurosci. 1992;15:285–320. doi: 10.1146/annurev.ne.15.030192.001441. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, Vortherms TA, Kitada T, Costa C, Tong Y, Martella G, Tscherter A, Martins A, Bernardi G, Roth BL, Pothos EN, Calabresi P, Shen J. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia: learning new tricks and loving it. Curr Opin Neurobiol. 2005;15:638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Koob GF, Simon H, Herman JP, Le Moal M. Neuroleptic-like disruption of the conditioned avoidance response requires destruction of both the mesolimbic and nigrostriatal dopamine systems. Brain Res. 1984;303:319–329. doi: 10.1016/0006-8993(84)91218-6. [DOI] [PubMed] [Google Scholar]
- Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Tyler EC, Merritt A. Short- and long-term synaptic depression in rat neostriatum. J Neurophysiol. 1993;70:1937–1949. doi: 10.1152/jn.1993.70.5.1937. [DOI] [PubMed] [Google Scholar]
- Lundblad M, Picconi B, Lindgren H, Cenci MA. A model of l-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol Dis. 2004;16:110–123. doi: 10.1016/j.nbd.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Matamales M, Bertran-Gonzalez J, Salomon L, Degos B, Deniau JM, Valjent E, Hervé D, Girault JA. Striatal medium-sized spiny neurons: identification by nuclear staining and study of neuronal subpopulations in BAC transgenic mice. PLoS One. 2009;4:e4770. doi: 10.1371/journal.pone.0004770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillé V, Picconi B, Bagetta V, Ghiglieri V, Sgobio C, Di Filippo M, Viscomi MT, Giampà C, Fusco FR, Gardoni F, Bernardi G, Greengard P, Di Luca M, Calabresi P. Distinct levels of dopamine denervation differentially alter striatal synaptic plasticity and NMDA receptor subunit composition. J Neurosci. 2010;30:14182–14193. doi: 10.1523/JNEUROSCI.2149-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Picconi B, Centonze D, Håkansson K, Bernardi G, Greengard P, Fisone G, Cenci MA, Calabresi P. Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- Picconi B, Paillé V, Ghiglieri V, Bagetta V, Barone I, Lindgren HS, Bernardi G, Angela Cenci M, Calabresi P. l-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiol Dis. 2008;29:327–335. doi: 10.1016/j.nbd.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Picconi B, Bagetta V, Ghiglieri V, Paillè V, Di Filippo M, Pendolino V, Tozzi A, Giampà C, Fusco FR, Sgobio C, Calabresi P. Inhibition of phosphodiesterases rescues striatal long-term depression and reduces levodopa-induced dyskinesia. Brain. 2011;134:375–387. doi: 10.1093/brain/awq342. [DOI] [PubMed] [Google Scholar]
- Rebola N, Canas PM, Oliveira CR, Cunha RA. Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience. 2005;132:893–903. doi: 10.1016/j.neuroscience.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–S18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Tang K, Low MJ, Grandy DK, Lovinger DM. Dopamine-dependent synaptic plasticity in striatum during in vivo development. Proc Natl Acad Sci U S A. 2001;98:1255–1260. doi: 10.1073/pnas.031374698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, de Iure A, Di Filippo M, Tantucci M, Costa C, Borsini F, Ghiglieri V, Giampà C, Fusco FR, Picconi B, Calabresi P. The distinct role of medium spiny neurons and cholinergic interneurons in the D2/A2A receptor interaction in the striatum: implications for Parkinson's disease. J Neurosci. 2011;31:1850–1862. doi: 10.1523/JNEUROSCI.4082-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Kai L, Day M, Ronesi J, Yin HH, Ding J, Tkatch T, Lovinger DM, Surmeier DJ. Dopaminergic control of corticostriatal long-term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron. 2006;50:443–452. doi: 10.1016/j.neuron.2006.04.010. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Zhu XR, Maskri L, Herold C, Bader V, Stichel CC, Güntürkün O, Lübbert H. Non-motor behavioural impairments in parkin-deficient mice. Eur J Neurosci. 2007;26:1902–1911. doi: 10.1111/j.1460-9568.2007.05812.x. [DOI] [PubMed] [Google Scholar]