Abstract
New neurons are continuously generated in the adult mammalian olfactory bulb. The role of these newborn neurons in olfactory learning has been debated. Blocking the addition of neurons has been reported either to result in memory alteration or to have no effect at all (Imayoshi et al., 2008; Breton-Provencher et al., 2009; Lazarini et al., 2009; Sultan et al., 2010). These discrepancies may have arisen from differences in the behavioral paradigms used: operant procedures indicated that neurogenesis blockade had substantial effects on long-term memory (Lazarini et al., 2009; Sultan et al., 2010) whereas other methods had little effect (Imayoshi et al., 2008; Breton-Provencher et al., 2009). Surprisingly, while operant learning is known to modulate the survival of new neurons, the effect of non-operant learning on newborn cells is unknown. Here we use mice to show that compared with operant learning, non-operant learning does not affect cell survival, perhaps explaining the current controversy. In addition, we provide evidence that distinct neural substrates at least partly underlie these two forms of learning. We conclude that the involvement of newborn neurons in learning is subtly dependent on the nature of the behavioral task.
Introduction
The formation of new functional neurons in the olfactory bulb (OB) originates in the proliferation of stem cells in the subventricular zone which generate neuronal progenitors migrating along the rostral migratory stream to reach the OB. Once in the OB, adult-born cells differentiate into granule and periglomerular interneurons, and shape the output message of the OB (Lledo et al., 2006). Numerous studies have investigated the role of newborn neurons in olfactory learning and have yielded conflicting results (Imayoshi et al., 2008; Breton-Provencher et al., 2009; Lazarini et al., 2009; Sultan et al., 2010). Indeed, a deficit in long-term olfactory memory was reported after reducing bulbar neurogenesis by irradiation (Lazarini et al., 2009) or by using an antimitotic agent (Sultan et al., 2010), with no effect on learning performance. In sharp contrast, other studies using genetic ablation of neurogenesis (Imayoshi et al., 2008) or an antimitotic agent (Breton-Provencher et al., 2009) reported no deficit on long-term memory.
Because the age of the ablated newborn neurons was similar in the studies reporting an effect (Lazarini et al., 2009; Sultan et al., 2010) or no effect (Imayoshi et al., 2008; Breton-Provencher et al., 2009) on long-term memory, this parameter cannot account for the discrepancy. Furthermore, the technique used to block neurogenesis cannot explain the discrepancies. Indeed, two independent studies using irradiation or antimitotic drug infusion reported impairment in long-term olfactory memory while the two studies using antimitotic infusions yielded conflicting data. We thus made the hypothesis (Sultan et al., 2010) that discrepancies may arise from differences in the behavioral paradigms used. Indeed, the studies reporting no effect of neurogenesis blockade on long-term memory used an associative task in which animals made a non-operant association between an odor and a reward that were simultaneously present and accessible to the animals (Imayoshi et al., 2008; Breton-Provencher et al., 2009). Animal behavior during training did not determine whether it obtained the reward. In contrast, the studies reporting an effect of neurogenesis blockade on olfactory memory used a task with an operant component in which the animals used the odor cue to elicit specific behavior aimed at obtaining the reward (Lazarini et al., 2009; Sultan et al., 2010). Thus, as in the non-operant task, the animals made an odor-reward association but this association also reinforced an active, motivated behavior to obtain the reward. For simplicity, we will name this odor-cue associative task including an operant component the operant task. The different nature of the task may imply differential needs for adult-born neurons. Furthermore, while operant learning has been shown to modulate newborn cell survival, surprisingly, no such modulation in the non-operant paradigm has been studied.
To clarify this question, we studied the effect of operant or non-operant conditioning on the survival and functional involvement of bulbar newborn cells using bromodeoxyuridine (BrdU) labeling and expression of the immediate early gene Zif268 respectively. In addition, to documenting the neural substrate of the two paradigms, we investigated the engagement of the brain's olfactory structures in processing the learned odorant.
Materials and Methods
Experimental design
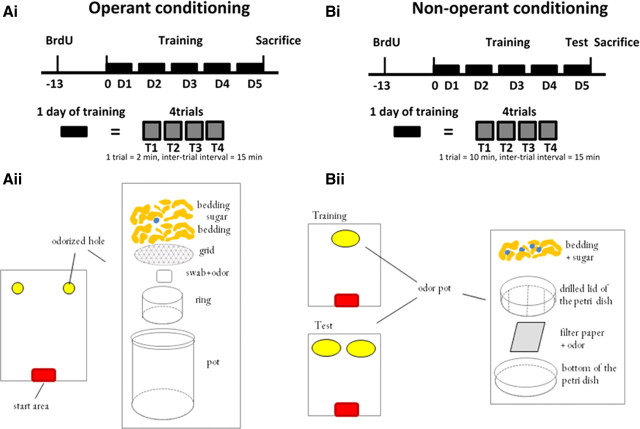
Non-operant conditioning was done following the experimental design described by Breton-Provencher et al. (2009) and Imayoshi et al. (2008). Operant conditioning was similar to our previously described protocol (Sultan et al., 2010) except for the odorants used. To measure newborn cell survival in the operant behavioral task, the DNA synthesis marker BrdU was administered 13 d before conditioning (Fig. 1Ai,Bi). This protocol allowed labeling of a cohort of newborn cells integrating the OB network at the beginning of training (Petreanu and Alvarez-Buylla, 2002), and thus in their critical period for learning-dependent survival (Mouret et al., 2008). The same timing of BrdU injections was applied in the non-operant behavioral task.
Figure 1.
Experimental design. A, Operant conditioning. Ai, Two groups of animals (conditioned and pseudo-conditioned; n = 10 per group) were trained for 5 d. BrdU was administered 13 d before training to label newborn cells arriving in the OB at the beginning of training. All mice were killed on day 5, 1 h after the last trial. Aii, Experimental apparatus used for operant conditioning. B, Non-operant conditioning. Bi, Two groups of animals (conditioned and pseudo-conditioned; n = 10 per group) were trained for 4 d and tested for learning on day 5, 1 h before they were killed. Again, BrdU was administered 13 d before training. Bii, Experimental apparatus used for non-operant conditioning.
Animals and BrdU injections
A total of 40 male C57BL/J mice, aged 8 weeks, were used. Mice were housed under a 12 h reverse light/dark cycle in an environmentally controlled room with ad libitum access to water. The animals were food-restricted 5 d before and during the olfactory learning (−20% daily food consummation, leading to a 15% reduction in body weight). Every effort was made to minimize both the number of animals used and their suffering during the experimental procedure in accordance with the European Community Council Directive of November 24, 1986 (86/609/EEC). Thirteen days before the beginning of the behavioral training, the mice received 3 injections of BrdU (50 mg/kg in saline; i.p., every 2 h).
Odorants
In the behavioral tasks, (+)-carvone (purity 96%, Aldrich Chemical Co.) and (−)-carvone (purity >99%, Merck-Schuchardt) were used. Odorants were diluted to a concentration of 15% in mineral oil, and 0.05 ml of this solution was used for each presentation.
Behavioral experiments
Operant olfactory learning
Mice were tested on a computer-assisted 2-hole board apparatus (Mandairon et al., 2006, 2009). A polypropylene swab, impregnated when needed with the odorant, was placed at the bottom of each hole (4.5 cm deep) and covered with bedding (Fig. 1Aii).
Shaping.
Before conditioning, all the mice, in the absence of the odorant, learned to retrieve a reward by digging through the bedding in a hole.
Conditioning.
A reward was systematically associated with (+)-carvone while (−)-carvone was nonreinforced (conditioned group, n = 10). Mice were trained during one session per day for 5 d. A daily session consisted in 4 trials (2 min per trial, 15 min intertrial interval). For each trial, the odorants were randomly placed in the 2 holes, to avoid spatial learning (Fig. 1Aii).
Pseudo-conditioning.
The reward was randomly associated with either of the 2 odorants (pseudo-conditioned group, n = 10).
Data analysis.
Learning was assessed through the percentage of correct choices on each training day. For each trial, a correct choice was recorded if the first nose-poke was into the (+)-carvone odorized hole. The mean percentage of correct choices was calculated for each day and averaged within groups. In the figure, the results are given as mean ± SEM. Between-group comparison was done using ANOVA for repeated measures followed by Bonferroni post hoc tests (Systat software). Statistical significance was set at p < 0.05.
Non-operant olfactory learning
The protocol used has previously been described by Schellinck et al. (2001; Imayoshi et al., 2008; Breton-Provencher et al., 2009). An odorized filter paper was placed at the bottom of a Petri dish; this was kept in place by the Petri dish cover in which 9 holes (1 mm in diameter) had been drilled to allow diffusion of the odor. Bedding was placed on top of this odor pot (1 cm high) and the reward (sugar pearls, EuroSugar, Paris, France) was mixed in with it (Fig. 1Bii).
Conditioning.
Mice were trained for 1 session per day over 4 d. For all mice, a daily session consisted in 4 randomized trials (10 min per trial, 15 min intertrial interval): (+)-carvone was presented in 2 of the trials and (−)-carvone in the other two (Fig. 1Bii). The reward was systematically associated with (+)-carvone and (−)-carvone was nonreinforced (conditioned group, n = 10).
Pseudo-conditioning.
The same sequence of trials was used except that the reward was randomly associated with (+)-carvone or (−)-carvone during a session (pseudo-conditioned group, n = 10).
Learning test.
Twenty-four hours after the last conditioning session (day 5), the 2 odors were presented simultaneously in two odor pots without any reward. The mice were allowed to dig in the bedding for 2 min (Fig. 1Bii).
Data analysis.
On test day, the time spent digging in each odor pot was recorded as an index of learning. Mean digging time was calculated and averaged within groups. In the figures, all results are given as mean ± SEM. Between-group comparisons were done using the unilateral t test. Statistical significance was set at p < 0.05.
Brain preparation
All the mice were killed on day 5 (1 h after the last behavioral test, Fig. 1). They were deeply anesthetized (Pentobarbital, 0.2 ml/30 g) and killed by intracardiac perfusion of 50 ml of fixative (4% paraformaldehyde in phosphate buffer, pH 7.4). Their brains were removed, postfixed, cryoprotected in sucrose (20%), frozen rapidly and then stored at −20°C before sectioning with a cryostat (Jung).
Newborn cell survival assessment
BrdU immunohistochemistry
As previously described (Sultan et al., 2010), 14-μm-thick sections of the OB were incubated overnight at 4°C in a mouse anti-BrdU primary antibody (1:100, Millipore Bioscience Research Reagents). BrdU was revealed using a horse biotinylated anti-mouse secondary antibody (1:200, Vector Laboratories) and an avidin-biotin-peroxidase complex (ABC Elite Kit, Vector Laboratories).
Quantification and mapping in the OB
BrdU-positive cells were counted using mapping software (Mercator Pro, Explora Nova) coupled to a Zeiss microscope. Cells were counted in the granule cell layer of the OB, which is the main target of neurogenesis and modulation by learning. All counts and mapping were done on five mice per group, without knowledge of the mouse's status. For each animal, cells were counted on 32 sections distributed along the rostrocaudal axis of the OB (intersection interval 70 μm). BrdU mean density was then calculated by dividing the number of positive cells by the surface of this region.
Newborn cell survival assessment in the dentate gyrus
Using the same method, BrdU-positive cells were counted on 7–10 sections (14 μm thick) of the dentate gyrus of the hippocampus distributed along the anteroposterior axis. Densities were compared using unilateral Student's t tests.
BrdU/zif 268 and BrdU/NeuN double labeling
Double labeling was performed using rabbit anti-Zif268 (1:500, Santa Cruz Biotechnology), mouse anti-NeuN (1:500, Millipore Bioscience Research Reagents) and rat anti-BrdU (1:100, Harlan Sera Laboratory) primary antibodies. The appropriate secondary antibodies were Alexa Fluor 546 for BrdU, Alexa Fluor 633 for NeuN, and streptavidin-Alexa Fluor 488 for Zif268. The labeled cells (mean 35 cells counted per animal, n = 5) were observed and analyzed by pseudo-confocal scanning microscopy using a Zeiss microscope equipped with an Apotome. For the operant and non-operant groups, conditioned and pseudo-conditioned animals were compared using a unilateral t test.
Zif268 expression analysis in the OB
In the sections of the OB treated for BrdU/Zif268 double labeling, Zif268-labeled cells were counted on a series of photographs randomly taken in the granule cell layer (∼20 per animal, n = 5). The Zif268-positive cell density was calculated and averaged within each experimental group. Between-group comparisons were performed by ANOVA followed by a post hoc Fisher test. The level of significance was set to 0.05.
Zif268 expression in orbital, infralimbic and prelimbic cortices
To assess the expression of Zif268 in response to the learned odorant, mice were killed on day 5 (1 h after the last behavioral test).
Immunohistochemistry
Brain sections were transferred to 10% normal goat serum (Sigma) with 2% BSA and 0.1% Triton X-100 for 1 h to block nonspecific binding and were then incubated overnight in a rabbit anti-Zif268 antibody (1:1000, Santa Cruz Biotechnology) at 25°C. Sections were then incubated in a biotinylated anti-rabbit secondary antibody (1:200, Vector Laboratories) for 2 h. The remaining treatments were similar to those for the BrdU labeling.
Quantification
Zif268 expression was assessed in the orbital, infralimbic and prelimbic cortices (n = 3 for each group). In the orbital cortex, 3–4 sections were counted (Mercator Pro, Explora Nova) between bregma 2.80 mm and bregma 2.10 mm (Franklin and Paxinos, 2008). A counting frame (200 × 300 μm) was positioned in the orbital cortex and the labeled cell density was calculated. Zif268-positive cells were also counted on a series of 4–5 sections between bregma 1.98 mm and 1.54 mm in the area of the prelimbic (counting frame 200 × 600 μm) or infralimbic (200 × 500 μm) cortex determined with regards to the shape of the adjacent corpus callosum (Franklin and Paxinos, 2008; Fig. 2C). For all structures, cell density was averaged within each experimental group. Between-group comparisons were performed by unilateral t test. The level of significance was set to 0.05.
Figure 2.
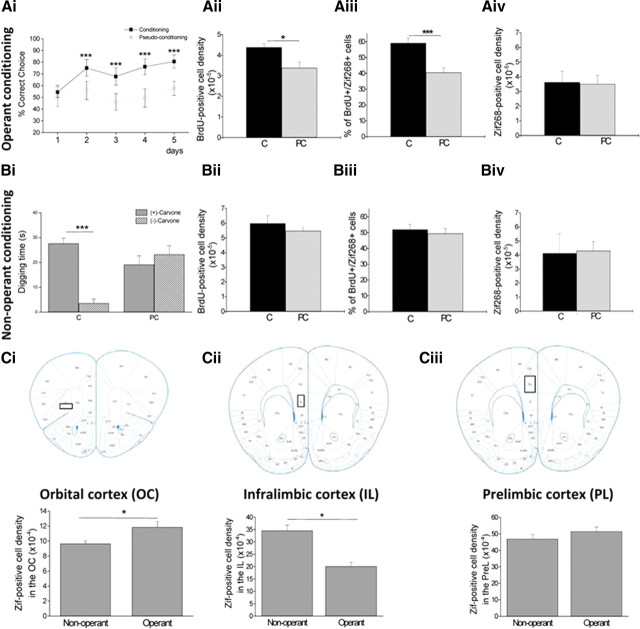
Only operant conditioning modulates newborn cell survival. A, Operant conditioning. Ai, Behavioral performances. Aii, BrdU-positive cell density in the granule cell layer is increased in the conditioned compared with the pseudo-conditioned group. Aiii, Counts of BrdU+/Zif268+ cells indicate that newborn cells are functionally more solicited in the conditioned than the pseudo-conditioned group. Aiv, The overall expression of Zif268 in the granule cell layer is similar between the conditioned and pseudo-conditioned groups. *p < 0.05, ***p < 0.005 for difference between conditioned and pseudo-conditioned. B, Non-operant conditioning. Bi, Behavioral performances. Bii, BrdU-positive cell density in the granule cell layer is not modulated by conditioning. Biii, The percentage of BrdU+/Zif268+ cells is similar between the conditioned and pseudo-conditioned groups. Biv, The overall expression of Zif268 in the granule cell layer is similar between the conditioned and pseudo-conditioned groups (***p < 0.005). C, Conditioned group; PC, pseudo-conditioned group. All cell densities are cell/μm2. Zif268-positive cell density in orbital cortex (Ci), infralimbic cortex (Cii), and prelimbic cortex (Ciii). *p < 0.05.
Results
To understand why neurogenesis blockade has different effects on operant versus non-operant olfactory learning, we investigated the impact of these two behavioral paradigms on newborn neuron survival.
The mice trained using operant conditioning learned the odor-reward association (Fig. 2Ai), as shown by the increase over time of the percentage of correct choices until it reaches 80% for the conditioned mice (day effect; F(4,70) = 21.108; p = 0.026), whereas it remained at chance level in the pseudo-conditioned animals (day effect; F(4,60) = 0.383; p = 0.820). This result was confirmed by a significant difference between the performance of the conditioned and pseudo-conditioned animals (group effect; F(1,26) = 14.787; p = 0.001).
With the operant learning, we found that BrdU-positive cell density was significantly higher in the conditioned compared with the pseudo-conditioned mice (p = 0.03; Fig. 2Aii). This finding confirmed results from previous studies (Alonso et al., 2006; Mouret et al., 2008; Sultan et al., 2010), and indicated an increased survival of adult-born cells within the granule cell layer mediated by operant learning. No difference in neural differentiation was found between the conditioned and pseudo-conditioned mice (p = 0.82); ∼92% of BrdU-positive cells were NeuN-positive in both groups. To analyze the functional implication of newborn neurons in response to the learned odorants, we looked at the BrdU/Zif268 colabeled cells in the granule cell layer. We found that the functional recruitment of newborn cells was greater in the operant conditioned group compared with the pseudo-conditioned animals (p = 0.003, Fig. 2Aiii). This significant difference between groups represents a true increase in the functional involvement of these newborn neurons since the total density of Zif268-positive cells was similar between the conditioned and pseudo-conditioned groups (p = 0.80; Fig. 2Aiv).
Together, these results show that operant learning increases the density of newborn cells in the granule cell layer, and increases the involvement of these cells in processing the learned odorants.
Mice conditioned using the non-operant task learned the odor-reward association as measured by their digging time during the test. The conditioned mice actually spent significantly more time digging in the pot with the reinforced odor (+)-carvone than in the nonreinforced odor (−)-carvone (p < 0.001). Moreover, mice from the pseudo-conditioned group spent an equal time digging in each odor pot (p = 0.42) (Fig. 2Bi). To assess whether non-operant learning affects newborn cell survival, BrdU-positive cell density was measured in the granule cell layer of the conditioned and pseudo-conditioned mice. No difference was found between the two groups (p = 0.19; Fig. 2Bii). Interestingly, this result indicates that non-operant learning does not affect BrdU-positive cell survival. Moreover, the proportion of BrdU-positive cells expressing Zif268 was found to be similar in both the conditioned and pseudo-conditioned groups (p = 0.19), suggesting no specific involvement of newborn cells in the task (Fig. 2Biii). In addition, no difference was found in the rate of neuronal cell differentiation (i.e., BrdU/NeuN colabeling) between the conditioned and pseudo-conditioned mice (p = 0.53), or total Zif268 expression (p = 0.79; Fig. 2Biv). Finally, we observed that the density of newborn cells in the non-operant task was higher than in the operant task for both the conditioned and pseudo-conditioned groups (p < 0.05). This result is in accordance with the longer exposure time to the odor in the non-operant group (40 min per day) versus the operant group (8 min per day) and suggests an effect of odor exposure on newborn cell survival, independent of associative learning.
It is worth noting that neurogenesis was not modified in any of the two behavioral tasks in the hippocampus (operant p = 0.45; non-operant p = 0.15 for conditioned versus pseudo-conditioned groups).
To summarize, while operant learning increased newborn neuron survival, non-operant learning apparently had no such effect.
To further document the potential difference of the nature of the operant versus non-operant task, we analyzed brain activation after the last behavioral session. We specifically focused on three structures: the orbital, prelimbic and infralimbic cortices which have been shown to be involved in olfactory learning and memory (Tronel and Sara, 2002; Roullet et al., 2004; Dardou et al., 2006, 2007; Chapuis et al., 2009). Using Zif268 expression as an indicator of activity, we found more positive cells in the orbital cortex after the operant compared with the non-operant task (unilateral t test, p < 0.05) (Fig. 2Ci). In contrast, fewer positive cells were observed in the infralimbic cortex after the operant compared with the non-operant task (unilateral t test, p < 0.05) (Fig. 2Cii) while no difference was found in the prelimbic cortex (Fig. 2Ciii).
Together, these results indicated that the two learning paradigms (non-operant versus operant) differentially modulated newborn neuron survival and that the learned odorants were differentially processed in olfactory brain structures depending on the behavioral paradigm used.
Discussion
We found that operant conditioning increased the number of newborn neurons in the OB, whereas non-operant conditioning did not.
The cohorts of newborn neurons labeled and tracked in both behavioral paradigms (operant versus non-operant) were aged between 13 and 18 d at the time of learning. At this age, newborn neurons are at their most critical period for integration into the bulbar network (Carleton et al., 2003; Kelsch et al., 2009) and during this period learning has been shown to increase their survival rate by a few days (Mouret et al., 2008). Our findings clearly show that non-operant learning had no influence on newborn neurons of this age. This is consistent with the absence of any effect of the neurogenesis blockade observed in the Breton-Provencher et al. study. Indeed, in their study, the timing of the antimitotic infusion prevented the arrival of newborn neurons of the same age as the ones tracked in the present study. Non-operant learning could theoretically influence the fate of older newborn neurons. However, this possibility is unlikely since the genetic long-term ablation of neurogenesis depriving the OB of neurons aged from a few days to several weeks did not affect non-operant learning or memory (Imayoshi et al., 2008). Together, these data suggest that non-operant learning is independent of neurogenesis regardless of the age of the newborn neurons.
The fact that non-operant learning did not affect neurogenesis could explain why a neurogenesis blockade did not induce long-term memory impairment in the studies using non-operant learning (Imayoshi et al., 2008; Breton-Provencher et al., 2009). Conversely the mice that learned the task through operant conditioning and showed an increase of neurogenesis could not remember the task when neurogenesis was blocked (Lazarini et al., 2009; Sultan et al., 2010). This conclusion is further supported by our own (Kermen et al., 2010; Sultan et al., 2010; Sultan et al., 2011) and others findings (Lazarini et al., 2009) showing that operant conditioning selects newborn neurons for survival and that these newborn neurons are used for long-term memory.
To further document the potential differences in the neuronal substrate underlying the two forms of learning, we looked at the activation of cerebral structures known to be involved in olfactory memory (Tronel and Sara, 2002; Roullet et al., 2004; Dardou et al., 2006, 2007; Chapuis et al., 2009). We found a differential activation between the two types of learning in the orbital cortex with a more pronounced engagement in the operant group. This finding is consistent with altered behavioral performance when lesions were made in the orbital cortex a very similar learning paradigm using auditory cues (Ostlund and Balleine, 2007). Both prelimbic and infralimbic areas are closely synaptically connected with the piriform cortex but their functional role essentially remains unknown. Interestingly, their connections with the piriform cortex and many other brain areas are quite distinct (Vertes, 2004) suggesting different functions. Our data further suggest that they are differentially engaged in processing the learned odorant, depending on the operant component of the learning paradigm. These data suggest that the olfactory information is differently processed depending on the form of learning. Thus, these two forms of learning although both associative, solicited different substrates including modulation of neurogenesis and activation of olfactory brain structures.
In the present study, the non-operant groups exhibited higher BrdU-positive cell density than did the operant groups. This increase in newborn cell survival was unrelated to learning since it was observed in both the conditioned and pseudo-conditioned animals. However, this suggests that the odor exposure time in the non-operant paradigm (40 min per day versus 8 min in the operant groups) may have been sufficient to improve the survival of newly formed cells within the OB similarly to that observed with odor enrichment (Rochefort et al., 2002; Moreno et al., 2009; Veyrac et al., 2009).
Our findings, beyond allowing the reconciliation of current literature on the role of newborn neurons in olfactory associative learning, points to the importance of the behavioral demands made on the animals. It seems that newborn neurons subserve specific roles in particular forms of learning and memory and that modulation of neurogenesis is therefore not a general requirement for olfactory learning. Operant versus non-operant learning could be an example of this task-dependent involvement of newborn neurons. Even more subtle differences should be taken into consideration. Fear conditioning is one such example, a particular form of associative learning consisting of an aversive association and involving a specific neural circuit (LeDoux, 2000) has been shown to be partially dependent on newborn cell survival (Valley et al., 2009). Thus, depending on the task performed by the animal, different plasticity mechanisms are solicited, leading to specific changes in the neural representation of the odor which may or may not depend on newborn neurons. Among these mechanisms the role of centrifugal inputs deserve particular attention since they are likely to be differentially involved depending on the behavioral task (Mandairon and Linster, 2009).
Footnotes
This work was supported by the CNRS and Claude Bernard-Lyon 1 University.
The authors declare no competing financial interests.
References
- Alonso M, Viollet C, Gabellec MM, Meas-Yedid V, Olivo-Marin JC, Lledo PM. Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci. 2006;26:10508–10513. doi: 10.1523/JNEUROSCI.2633-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Provencher V, Lemasson M, Peralta MR, 3rd, Saghatelyan A. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29:15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton A, Petreanu LT, Lansford R, Alvarez-Buylla A, Lledo PM. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6:507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- Chapuis J, Garcia S, Messaoudi B, Thevenet M, Ferreira G, Gervais R, Ravel N. The way an odor is experienced during aversive conditioning determines the extent of the network recruited during retrieval: a multisite electrophysiological study in rats. J Neurosci. 2009;29:10287–10298. doi: 10.1523/JNEUROSCI.0505-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardou D, Datiche F, Cattarelli M. Fos and Egr1 expression in the rat brain in response to olfactory cue after taste-potentiated odor aversion retrieval. Learn Mem. 2006;13:150–160. doi: 10.1101/lm.148706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardou D, Datiche F, Cattarelli M. Does taste or odor activate the same brain networks after retrieval of taste potentiated odor aversion? Neurobiol Learn Mem. 2007;88:186–197. doi: 10.1016/j.nlm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. The mouse brain in stereotaxic coordinates. Ed 3. 2008. Compact. [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Kelsch W, Lin CW, Mosley CP, Lois C. A critical period for activity-dependent synaptic development during olfactory bulb adult neurogenesis. J Neurosci. 2009;29:11852–11858. doi: 10.1523/JNEUROSCI.2406-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermen F, Sultan S, Sacquet J, Mandairon N, Didier A. Consolidation of an olfactory memory trace in the olfactory bulb is required for learning-induced survival of adult-born neurons and long-term memory. PLoS One. 2010;5:e12118. doi: 10.1371/journal.pone.0012118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Mouthon MA, Gheusi G, de Chaumont F, Olivo-Marin JC, Lamarque S, Abrous DN, Boussin FD, Lledo PM. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4:e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. J Neurophysiol. 2009;101:2204–2209. doi: 10.1152/jn.00076.2009. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Sacquet J, Garcia S, Ravel N, Jourdan F, Didier A. Neurogenic correlates of an olfactory discrimination task in the adult olfactory bulb. Eur J Neurosci. 2006;24:3578–3588. doi: 10.1111/j.1460-9568.2006.05235.x. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Sultan S, Rey N, Kermen F, Moreno M, Busto G, Farget V, Messaoudi B, Thevenet M, Didier A. A computer-assisted odorized hole-board for testing olfactory perception in mice. J Neurosci Methods. 2009;180:296–303. doi: 10.1016/j.jneumeth.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Moreno MM, Linster C, Escanilla O, Sacquet J, Didier A, Mandairon N. Olfactory perceptual learning requires adult neurogenesis. Proc Natl Acad Sci U S A. 2009;106:17980–17985. doi: 10.1073/pnas.0907063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouret A, Gheusi G, Gabellec MM, de Chaumont F, Olivo-Marin JC, Lledo PM. Learning and survival of newly generated neurons: when time matters. J Neurosci. 2008;28:11511–11516. doi: 10.1523/JNEUROSCI.2954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–2689. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet F, Datiche F, Liénard F, Cattarelli M. Cue valence representation studied by Fos immunocytochemistry after acquisition of a discrimination learning task. Brain Res Bull. 2004;64:31–38. doi: 10.1016/j.brainresbull.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Schellinck HM, Forestell CA, LoLordo VM. A simple and reliable test of olfactory learning and memory in mice. Chem Senses. 2001;26:663–672. doi: 10.1093/chemse/26.6.663. [DOI] [PubMed] [Google Scholar]
- Sultan S, Mandairon N, Kermen F, Garcia S, Sacquet J, Didier A. Learning-dependent neurogenesis in the olfactory bulb determines long-term olfactory memory. FASEB J. 2010;24:2355–2363. doi: 10.1096/fj.09-151456. [DOI] [PubMed] [Google Scholar]
- Sultan S, Lefort JM, Sacquet J, Mandairon N, Didier A. Acquisition of an olfactory associative task triggers a regionalized down-regulation of adult born neuron cell death. Front Neurosci. 2011;5:52. doi: 10.3389/fnins.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronel S, Sara SJ. Mapping of olfactory memory circuits: region-specific c-fos activation after odor-reward associative learning or after its retrieval. Learn Mem. 2002;9:105–111. doi: 10.1101/lm.47802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valley MT, Mullen TR, Schultz LC, Sagdullaev BT, Firestein S. Ablation of mouse adult neurogenesis alters olfactory bulb structure and olfactory fear conditioning. Front Neurosci. 2009;3:51. doi: 10.3389/neuro.22.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Sacquet J, Nguyen V, Marien M, Jourdan F, Didier A. Novelty determines the effects of olfactory enrichment on memory and neurogenesis through noradrenergic mechanisms. Neuropsychopharmacology. 2009;34:786–795. doi: 10.1038/npp.2008.191. [DOI] [PubMed] [Google Scholar]