Abstract
Aging affects all levels of neural processing, including changes of intracortical inhibition and cortical excitability. Paired-pulse stimulation, the application of two stimuli in close succession, is a useful tool to investigate cortical excitability in humans. The paired-pulse behavior is characterized by the second response being significantly suppressed at short stimulus onset asynchronies. While in rat somatosensory cortex, intracortical inhibition has been demonstrated to decline with increasing age, data from human motor cortex of elderly subjects are controversial and there are no data for the human somatosensory cortex (SI). Moreover, behavioral implications of age-related changes of cortical excitability remain elusive. We therefore assessed SI excitability by combining paired-pulse median nerve stimulation with recording somatosensory evoked potentials in 138 healthy subjects aged 17–86 years. We found that paired-pulse suppression was characterized by substantial interindividual variability, but declined significantly with age, confirming reduced intracortical inhibition in elderly subjects. To link the age-related increase of cortical excitability to perceptual changes, we measured tactile two-point discrimination in a subsample of 26 aged participants who showed either low or high paired-pulse suppression. We found that tactile performance was particularly impaired in subjects showing markedly enhanced cortical excitability. Our data demonstrate that paired-pulse suppression of human SI is significantly reduced in older adults, and that age-related enhancement of cortical excitability correlates with degradation of tactile perception. These findings indicate that cortical excitability constitutes an important mechanism that links age-related neurophysiological changes to behavioral alterations in humans.
Introduction
Aging induces major reorganization and remodeling at all levels of brain structure and function (Rowe and Kahn, 1987; Arnsten, 1998; Cabeza, 2002; Dinse, 2006). Numerous lines of evidence converge on the observation that during aging, intracortical inhibition is particularly affected and that much of age-related impairment of sensation and perception may result from this phenomenon. A study performed several years ago reported an enhanced electrical coupling in the hippocampus of aged rats, which has been assumed to contribute to an increase in cellular excitability with age (Barnes et al., 1987). More recently, evidence for a significant degradation of visual orientation and direction selectivity, together with enhanced spontaneous activity, was described in old macaque monkeys and cats (Schmolesky et al., 2000; Hua et al., 2006). The authors suggested that the decreased selectivity and increased excitability of cells in old animals might be attributable to an age-related degeneration of intracortical inhibition. For the somatosensory cortex, an expansion of the representations within primary somatosensory cortex (SI) in elderly subjects aged 60–85 years has been reported. In the same subjects, tactile spatial two-point discrimination performance showed a strong decline with age (Kalisch et al., 2009). Map expansion typically observed in young and adult subjects during learning is associated with a gain in performance. It has been suggested that age-related map changes are related to a reduction of intracortical inhibition developing with age, as a reduced level of inhibition would allow excitatory processes to spread. It is undisputed that receptive fields (RFs) are kept small through active inhibition (Hicks and Dykes, 1983). In a study analyzing receptive fields and point-spread functions in somatosensory cortex of aged rats, an enlargement of RF size and of the size of the cortical point-spread function has been described (Spengler et al., 1995; Godde et al., 2002), compatible with a general reduction of cortical inhibition.
Stimulation with pairs of stimuli in close succession (paired-pulse stimulation) has become a common tool to investigate paired-pulse suppression (PPS). PPS describes the phenomenon of cortical responses to the second stimulus becoming significantly reduced at short stimulus onset asynchronies (SOA). PPS is quantified in terms of the ratio of the amplitude of the second response divided by the first response amplitude. Accordingly, small amplitude ratios are associated with strong PPS, and large ratios are associated with reduced PPS. For the somatosensory system, paired-pulse stimulation in combination with somatosensory evoked potentials (SEP) recordings over primary somatosensory cortex has been used to investigate paired-pulse behavior and to obtain markers of cortical excitability (Schwartz and Shagass, 1964; Shagass and Schwartz, 1964; Ragert et al., 2004; Höffken et al., 2007).
For aged rats, significant reductions of PPS have been described for somatosensory cortex, corroborating that intracortical inhibition is reduced at higher ages (David-Jürgens and Dinse, 2010). So far, no data are available for human somatosensory system. For human primary motor cortex, controversial findings have been reported about age-related changes of PPS (Peinemann et al., 2001; Wassermann, 2002; Oliviero et al., 2006; Smith et al., 2009), and the behavioral implications of age-related changes of PPS remain elusive at present. Therefore, we tested the hypothesis of age-related reduction of intracortical inhibition in human somatosensory cortex in 138 individuals aged 17–86 years. We measured paired-pulse SEPs following median nerve stimulation at short SOA. In addition, to link age-related changes of cortical excitability to perceptual abilities, we measured tactile discrimination performance in a subsample of aged participants.
Materials and Methods
Subjects.
We tested 138 right-handed volunteers aged 17–86 years (91 female; mean age, 48 ± 21.5 years). In all subjects, handedness was determined using the Edinburgh Handedness Inventory (Oldfield, 1971). All subjects were neurologically healthy, as assessed by a neurologist. Individuals with diseases of the central or peripheral nervous system were excluded from the study. Eligibility criteria were lucidity, independence in activities of daily living, and absence of motor handicaps such as functional impairment because of arthritis or other causes of joint immobility. Single-pulse SEP latencies of the N20 component were used to ascertain that peripheral processing was intact. Typically, early symptoms of polyneuropathies of the peripheral nerves include lengthening of conduction, which can increase up to 30% (Yiannikas and Vucic, 2008). In our sample, no participants were included who had latencies longer than the in-house critical value of 22.9 ms. Furthermore, medication with central nervous effects in present or reported history was a criterion for exclusion. Medication intake that was permitted is documented in Table 1. The study was approved by the Ethics Committee of the Ruhr-University of Bochum and was performed in accordance to the Declaration of Helsinki. All subjects gave informed consent before participating.
Table 1.
Demographic and experimental data of young and elderly subjects
| Young (<46 years old) | Elderly (≥46 years old) | |
|---|---|---|
| N | 69 | 69 |
| Sex (F) | 48 | 42 |
| Age, years, mean ± SD (range) | 28 ± 7.3 (17–45) | 67.9 ± 8.5 (47–86) |
| Medication (N) | 37 | |
| Anticonvulsant | 0 | |
| Antidepressant | 0 | |
| Antihypertensive | 32 | |
| Beta-blocker | 12 | |
| ASA | 11 | |
| NSAID | 3 | |
| Antacids | 4 | |
| Steroids | 3 | |
| Statins | 11 | |
| Thyroxine | 8 | |
| Tamsulosin (α1a-selective alpha blocker) | 4 | |
| Others (Mictonorm, Norfloxacin, Phenprocoumon, Quinine Sulphate) | 4 | |
| Stimulation intensity (mean ± SEM) | 2.28 ± 0.81** | 3.89 ± 0.25 |
| Single-pulse A1 (μV; mean ± SEM) | 4.62 ± 0.35 | 4.55 ± 0.39 |
| Paired-pulse A1 (μV; mean ± SEM) | 4.02 ± 0.27 | 4.32 ± 0.38 |
| Paired-pulse A2 (μV; mean ± SEM) | 1.77 ± 0.14** | 2.72 ± 0.33 |
| Ratio A2/A1 (mean ± SEM) | 0.45 ± 0.03*** | 0.64 ± 0.05 |
| Two-point discrimination thresholds (mm; mean ± SEM) | 3.33 ± 0.14 | |
| For elderly subjects with ratios <1 (N = 13) | 3.07 ± 0.18 | |
| For elderly subjects with ratios >1 (N = 13) | 3.58 ± 0.18 |
F, Female; ASA, acetylsalicylic acid; NSAID, non-steroidal anti-inflammatory drugs.
** p < 0.01;
*** p < 0.001.
Paired-pulse evoked somatosensory potentials.
To assess possible age-related changes of excitability of somatosensory cortex, we applied a paired-pulse protocol consisting of paired electrical stimulation of the median nerve with an SOA of 30 ms while recording SEPs. Nerve stimulation was performed using a block electrode placed on the wrist of the right hand. Pulse duration was 0.2 ms and the repetition rate of the paired stimuli was 3 Hz. Subjects had to report a prickling sensation in the thumb, index, and middle fingers of the stimulated hand to verify correct positioning of the stimulating block electrode. As a rule, stimulation intensity was individually adjusted to the twofold of individual sensory thresholds (Table 1). Median nerve stimulation at individually adjusted intensity induced a small muscular twitch in the thenar muscles. During the recording session, participants were seated on a comfortable chair and were instructed to relax but stay awake with closed eyes. In addition, single-pulse stimulation was performed using the same parameters as for the paired-pulse stimulation (pulse duration, 0.2 ms; repetitive rate, 3 Hz). SEPs were recorded using an electrode over the left SI, 2 cm posterior to C3 (CP3) according to the international 10–20 system (American Electroencephalographic Society, 1994). A reference electrode was placed over midfront (FZ) position. SEPs were recorded in epochs from 20 ms before to 200 ms after stimulus onset with a 32-channel amplifier (bandpass filter, 100–2000 Hz; Brain AMP MR; Brain Products) and stored for off-line analysis. For each single- and paired-pulse stimulation, 800 stimulus-related epochs were recorded. Offline, SEP raw data were segmented and baseline corrected, movement and muscle artifacts (amplitudes > 100 μV) were rejected, and averaging was performed. Peak-to-peak amplitudes of the cortical N20–P25 SEP components were analyzed, which are assumed to be generated in SI (Lueders et al., 1983; Allison et al., 1989; Namiki et al., 1996). Paired-pulse suppression was expressed as a ratio (A2/A1) of the amplitudes of the second (A2) and the first (A1) N20–P25 peak (Fig. 1). Amplitude ratios <1 describe a PPS while ratios >1 indicate paired-pulse facilitation. The influence of age on SEP paired-pulse ratio (A2/A1) was assessed by calculating Pearson's correlation coefficient. In a post hoc approach, SEP data were split into two groups using the median age of all participants (46 years; N = 69 per group). Independent two-sample t test was performed to assess differences between amplitude ratios of subjects <46 years and subjects ≥46 years.
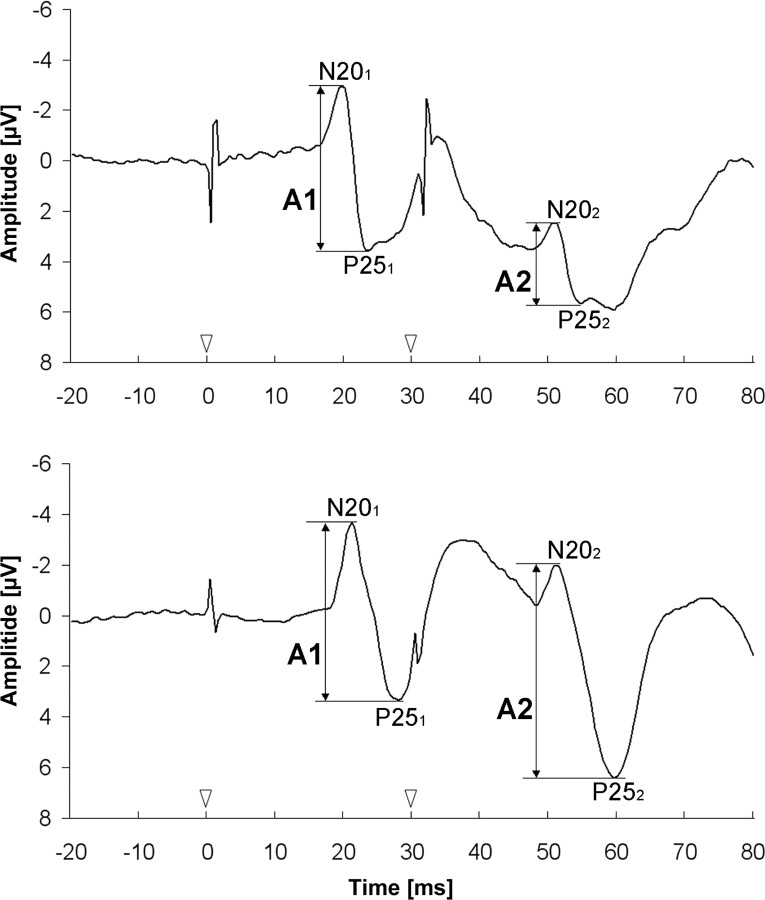
Figure 1.
Cortical responses to paired-pulse stimulation at an SOA of 30 ms of one young (top) and one elderly (bottom) subject. Onset of stimulation is marked by white arrowheads. The N20–P25 amplitudes of the first (A1) and second (A2) response are marked by black arrows. Amplitude ratio = A2/A1. Note the small paired-pulse suppression in the example of the elderly subject (bottom).
Tactile discrimination performance.
In addition, a subpopulation of 26 subjects (age, 57–81 years; mean age, 70.2 ± 6.2 years) was tested in a simultaneous spatial two-point discrimination task using the method of constant stimuli (Godde et al., 1996, 2000; Pleger et al., 2001; Dinse et al., 2003). Acuity thresholds obtained by gratings or by two-point measurements are largely equivalent (Dinse et al., 2006), although thresholds obtained by gratings are slightly lower in general. For two-point discrimination testing, seven pairs of pins with different spacings were mounted on a rotatable disc (diameter, 200 mm) that allowed switching rapidly between pairs. We used two different test devices, depending on the age of the subjects and their discrimination skills. With one device, we presented 1.0, 1.4, 1.8, 2.2, 2.6, 3.2, and 4.0 mm spacing between pins. With the other device, we presented 1.5, 2.3, 3.2, 3.9, 4.7, 5.6, and 7.0 mm spacing. In both cases, zero distance was tested with a single pin. To achieve a standardized type of stimulation, the arm and fingers of the subjects were fixed on a plate that could be moved up and down by the examiner. The disc was installed in front of the plate. The down-movement was stopped at a fixed position above the pins. The index finger of the right hand was placed above a small hole through which the finger touched the tips of the pins at approximately the same indentations in each trial (Godde et al., 2000; Pleger et al., 2001; Dinse et al., 2003). To obtain a stable baseline discrimination performance, each distance was tested eight times in randomized order in each of three separate sessions, resulting in a total of 192 trials. The subjects had to decide immediately whether they had the sensation of one or two tips. The summed responses were plotted against distance as a psychometric function for absolute threshold, fitted by a binary logistic regression. Threshold was taken from the fit at the distance for which 50% correct responses were reached. In the subgroup of elderly subjects (>55 years old), a correlation between paired-pulse SEP ratios (A2/A1) and two-point discrimination thresholds was analyzed using Pearson's correlation coefficient. All statistical analyses were performed using the SPSS 17.0 software package (version 17.0.0).
Results
Paired-pulse SEPs
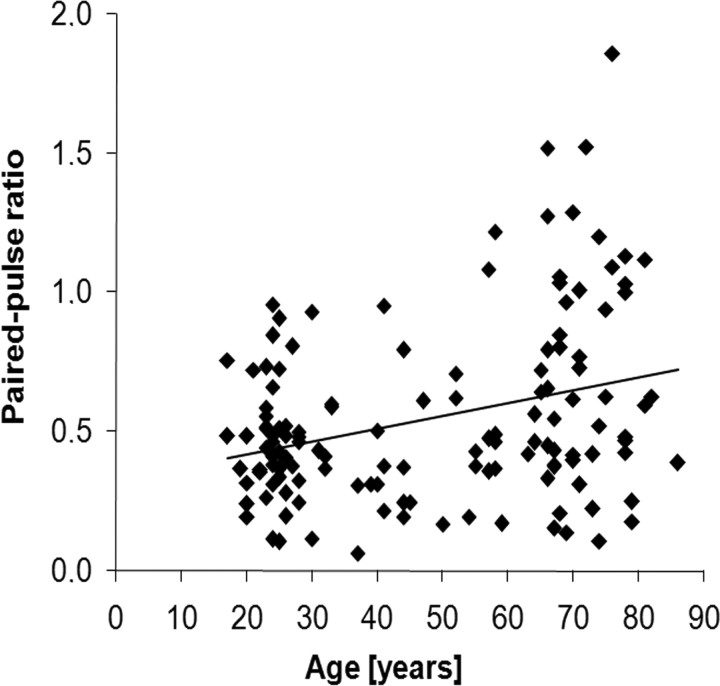
We measured SEP following right median nerve stimulation in 138 participants aged 17–86 years by analyzing the cortical N20-P25 component. We found increasing paired-pulse amplitude ratios (A2/A1) with increasing age (Pearson's correlation coefficient r = 0.307, p < 0.001; Fig. 2). Generally, we observed a substantial interindividual scatter of amplitude ratios. However, while none of the subjects <55 years old showed amplitude ratios exceeding 1, 25% of the subjects >55 years old did. For post hoc analysis, we split the data at the median age of our subjects, which was 46 years. Post hoc unpaired t test revealed significant (p < 0.001) increases in mean amplitude ratios in individuals aged 46 years or older (0.64 ± 0.05, mean ± SEM) compared with subjects younger than 46 years (0.45 ± 0.03). The N20-P25 peak-to-peak amplitudes of the first (A1) paired-pulse SEP were comparable to single-pulse N20-P25 peak-to-peak amplitudes (Pearson's correlation coefficient r = 0.863, p < 0.001), but neither of them were correlated with the age of the subjects (Pearson's correlation: single-pulse A1, r = −0.046, p = 0.608; paired-pulse A1, r = 0.052, p = 0.546). Additionally, we found no significant difference in paired-pulse A1 between elderly (46 years or older; 4.32 ± 0.38, mean ± SEM) and adult (<46 years; 4.02 ± 0.27) subjects. In contrast, we found a positive correlation between age and the second amplitude of the paired-pulse SEP (A2; Pearson's correlation coefficient, r = 0.233, p = 0.006). An unpaired t test revealed significantly (p = 0.008) higher A2 amplitudes in elderly subjects (46 years or older; 2.72 ± 0.33, mean ± SEM) compared with adult subjects (under 47 years; 1.77 ± 0.14).
Figure 2.
Individual SEP amplitude ratios (A2/A1) plotted against the age of the subjects. Paired-pulse ratios increase with increasing age. Pearson's correlation, r = 0.307, p < 0.001.
Stimulation thresholds did not correlate with paired-pulse ratios or any N20–P25 amplitude.
The significant increase of the paired-pulse ratio indicates enhanced cortical excitability and is consistent with the assumption of a general reduction of intracortical inhibition with increasing age.
Relation of age-related decline of tactile discrimination performance with cortical excitability
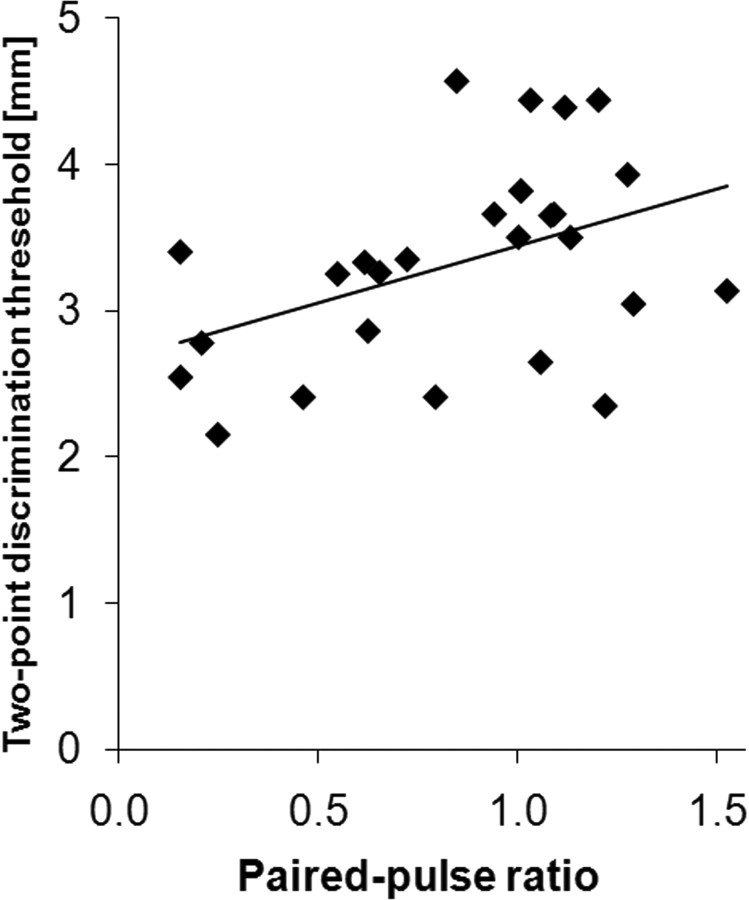
To obtain information about potential perceptual consequences of enhanced cortical excitability during aging, we measured tactile discrimination thresholds in a subset of 26 elderly participants, who showed either particularly low or high paired-pulse ratios. The mean (±SEM) tactile two-point discrimination thresholds were 3.33 ± 0.14 mm, confirming previous reports of a severe age-related decline in tactile discrimination abilities. There was a positive linear correlation between paired-pulse amplitude ratios (A2/A1) and two-point discrimination thresholds (Pearson's correlation coefficient, r = 0.428, p = 0.029; Fig. 3). For subjects with amplitude ratios <1, we found mean (±SEM) discrimination thresholds of 3.07 ± 0.18 mm, while subjects with amplitude ratios >1 showed discrimination thresholds of 3.58 ± 0.18 mm. Thus, tactile discrimination thresholds showed significantly higher impairment in subjects characterized by particularly high paired-pulse ratios indicative of decreased intracortical inhibition. Within the tested group of individuals, there was no correlation between age and two-point discrimination performance. There was also a lack of correlation between paired-pulse ratio and tactile discrimination thresholds for a cohort of young subjects aged 19–30 years (n = 40, data not shown).
Figure 3.
Individual two-point discrimination thresholds plotted against paired-pulse amplitude ratios (A2/A1). Discrimination thresholds increase with increasing paired-pulse ratios. Pearson's correlation, r = 0.428, p = 0.029.
Discussion
We used a paired-pulse protocol of electrical median nerve stimulation in 138 participants aged 17–86 years to measure age-related changes of intracortical excitability in human primary somatosensory cortex. PPS describes the phenomenon of neuronal responses to the second stimulus being significantly reduced at short SOA. We found that in elderly individuals, paired-pulse suppression was significantly reduced. By combining assessment of tactile discrimination performance in a subpopulation of elderly subjects, we also demonstrated that particularly high levels of cortical excitability were associated with severe impairment of tactile discrimination performance.
We use the term “paired-pulse suppression” to refer to a reduction in the neuronal response to the second of a pair of two successive stimuli, a phenomenon often referred to as “forward suppression”. We use “inhibition” to refer to one possible candidate for this suppression, namely GABAergic intracortical inhibition arising from postsynaptic activation of GABAA or GABAB receptor gated channels. “Synaptic depression” refers to a reduction in synaptic drive, including mechanisms such as postsynaptic receptor desensitization, presynaptic depletion of releasable vesicles, and other presynaptic mechanisms depressing vesicle release (Bellingham and Walmsley, 1999).
Typically, even in young subjects, measurements of paired-pulse suppression reveal substantial interindividual variability. Therefore, whether and how aging affects paired-pulse behavior remains largely elusive. The available data from human motor cortex of elderly subjects are controversial. According to Peinemann et al. (2001), the motor-evoked potential magnitude of intracortical PPS elicited by transcranial magnetic stimulation (TMS) was significantly smaller in elderly than in young adults. In contrast, a study using a different stimulation device found no evidence for age-related changes of intracortical PPS (Oliviero et al., 2006). Another TMS study in young and elderly subjects reached a similar conclusion, reporting a large variability of paired-pulse behavior on the one hand and no influence of age on paired-pulse behavior on the other hand (Wassermann, 2002). However, recently a significant age-related reduction of paired-pulse suppression has been reported for somatosensory cortex of aged rats, based on a large number of neuron recordings (David-Jürgens and Dinse, 2010). So far, no data for human somatosensory system are available. By investigating a sample of 138 participants, we were able to demonstrate—despite substantial interindividual variability—that paired-pulse suppression in human SI is in fact reduced, which is in line with the notion of a general age-related decline of inhibition (Barnes et al., 1987; Schmolesky et al., 2000; Peinemann et al., 2001; Hua et al., 2006; David-Jürgens and Dinse, 2010; Schmidt et al., 2010). As documented in Table 1, elderly participants had higher sensory thresholds than young subjects. It is, however, unlikely that differences in stimulation intensities might have affected paired-pulse suppression. First, we did not find a correlation between stimulation intensity and PPS. Second, we have recently systematically investigated the effect of stimulation intensities on PPS (our unpublished data), which revealed no effect except for extreme intensity values very close to sensory threshold, or at maximally tolerable intensities; both conditions are unlikely to hold for our present cohort of subjects.
While our data provide evidence that aging affects paired-pulse behavior at a systemic level and that this effect is mediated by a decrease in the second amplitude, they cannot provide information about possible underlying mechanisms. Despite substantial experimental and theoretical work, the mechanisms mediating paired-pulse behavior are not fully understood, not even in young animals. There is agreement that presynaptic mechanisms play a crucial role (Hashimoto and Kano, 1998; David-Jürgens and Dinse, 2010). Wehr and Zador (2005) reported that in rat auditory cortex, GABA receptor-mediated inhibition does not play a major role in forward suppression for interstimulus intervals (ISIs) beyond 100 ms. For longer ISIs, synaptic depression is assumed to be responsible for the observed PPS (Wehr and Zador, 2005). In the visual cortex, suppression is also more consistent with thalamocortical synaptic depression than with inhibition (Carandini et al., 2002; Freeman et al., 2002). In addition, there is evidence that GABAB receptors seem to be involved in regulation of PPS (Porter and Nieves, 2004). Recently, an altered composition of GABAA receptors, especially a reduction of subunit α 5, has been reported in aged animals (Schmidt et al., 2010). In addition to the contribution of GABAergic mechanisms, there is evidence for the involvement of glutamatergic transmission in the paired-pulse phenomenon (Takahashi et al., 1996; von Gersdorff et al., 1997). Because of differences in the PPS between cortical and thalamic cells, it has been argued that inheritance of thalamic response properties is unlikely to account for long-lasting forward suppression (Wehr and Zador, 2005). For human subjects, based on multichannel SEP recordings after paired median nerve stimulation, it has been shown that paired-pulse suppression is generated at least rostral to the brainstem nuclei (Höffken et al., 2010).
In many studies, assessment of paired-pulse suppression is used as a marker of altered cortical excitability, without linking changes of excitability to behavior (Ragert et al., 2004). However, there is evidence that reduced inhibition developing at high age is associated with decrease of function. For example, a recent ultrastructural study revealed a significant age-related decline in the numerical density of presumptive inhibitory synapses of sensorimotor cortex (Poe et al., 2001), demonstrating a deficit in the intrinsic inhibitory circuitry of the aging neocortex. This observation is in agreement with recent reports where comparison of stimulus selectivity of cells in V1 in young and old macaque monkeys revealed a significant degradation of orientation and direction selectivity in old animals (Schmolesky et al., 2000). In contrast, application of GABA and its agonist muscimol on V1 cells in senescent monkeys resulted in an improvement of visual cortical function (Leventhal et al., 2003). We therefore hypothesized that elevated cortical excitability in elderly subjects leading to a lack of paired-pulse suppression might be associated with particularly impaired tactile perceptual abilities. In fact, in our study, elderly participants characterized by amplitude ratios >1 revealed the largest discrimination impairment, while elderly subjects characterized by intermediate amplitude ratios displayed moderate age-related decline. These observations corroborate that in somatosensory system, age-related loss of inhibition is associated with significant age-related degradation of tactile perception, offering the possibility to use the assessment of paired-pulse suppression in elderly individuals as an indicator of the intactness of tactile performance or vice versa. Conceivably, many factors contribute to differences of performance within a subpopulation of elderly individuals. According to our data, alterations in paired-pulse behavior, i.e., cortical excitability, appears to contribute. In addition, other factors, such as changes in skin properties, changes in daily use and behavior, and changes in CNS processing, influence discrimination performance. Therefore, our results cannot provide insight into possible cause and consequence. On the one hand, older subjects may have less somatosensory input due to peripheral or central degenerational processes, which is then compensated for by the somatosensory cortex with less inhibitory effects on cortical processing. On the other hand, reduced cortical inhibition may directly interact with the processing of somatosensory input, leading to lower discrimination performances.
In our study, none of the young subjects revealed amplitude ratios >1. In contrast, ratios <1 were found in both young and elderly individuals, although tactile discrimination differed substantially between young and elderly subjects (Stevens, 1992; Woodward, 1993; Wohlert, 1996; Dinse et al., 2006; Kalisch et al., 2009). Accordingly, the close association between paired-pulse suppression and discrimination performance discussed above appears limited to elderly subjects. In a sample of young adults (N = 40; 19–30 years of age), we found no correlation between paired-pulse ratios and discrimination thresholds (Pearson's correlation coefficient, r = −0.022, p = 0.892; our unpublished data). In young subjects, reduction of paired-pulse suppression is linked to a gain of behavioral and perceptual performance (Muellbacher et al., 2001; Ziemann et al., 2001; Ragert et al., 2003, 2004, 2008; Neary et al., 2005; Höffken et al., 2007). To account for the dual effects of enhanced excitability, being linked to impaired behavior, such as during aging, or to enhanced behavior, such as during learning, one has to consider that many different mechanisms may lead to paired-pulse facilitation as observed at a phenomenological level using EEG recordings. Accordingly, the mechanisms mediating reduced paired-pulse suppression during aging must be fundamentally different from those mediating learning. A similar observation has been made recently for changes in cortical maps. Using electric source localization, Kalisch et al. (2009) demonstrated that the distance between the dipoles of the index and the little fingers increased in elderly subjects, indicative of map expansion, which was paralleled by a decline in tactile acuity. Because cortical map expansion in young subjects is typically associated with skill and performance acquisition (Elbert et al., 1995; Pleger et al., 2001), it has been concluded that map expansion observed during aging must represent a specific form of map alteration associated with aging processes, which differs qualitatively from learning-related reorganization occurring in young and adult subjects.
To provide a model-based explanation for these observations, we recently used a mean-field approach to model cortical population activation to understand both the process of aging and the process of tactile discrimination performance (Wilimzig et al., 2006). In this model, the activation after stimulation depends not only on the distance between inputs on the skin, but also on interactions within the cortex, which were depicted by Mexican-hat interaction characterized by local excitation and a broader range inhibition (Wilson and Cowan, 1973; Amari, 1977). Aging is modeled by a broadening of the inhibitory kernel, while learning is modeled by a reduction of the amplitude of the inhibitory interaction. In the “young” model, both the excitatory and the inhibitory interaction components are sharp. leading to focused representations. The “aging” model has a broader excitatory and a broader and weaker inhibitory component consistent with broader distributed representations and higher excitability. According to our simulations, the fine structure of excitatory and inhibitory interaction is differentially affected by aging and learning processes (Wilimzig et al., 2006). It is therefore reasonable to assume that intracortical interaction is a key factor explaining the size of cortical representation, the amount of cortical excitability, as well as the outcome of perceptual tasks.
Combined, our data showed that paired-pulse suppression in human SI is significantly reduced in older adults, and that a pronounced age-related enhancement of cortical excitability correlates with degradation of tactile perception. These findings indicate that cortical excitability constitutes one important mechanism that links age-related neurophysiological changes to behavioral alterations in humans. Accordingly, modulation of cortical excitability might be an interesting means to interfere with age-related changes of behavior and perception.
Footnotes
This study was supported by the Deutsche Forschungsgemeinschaft, project numbers TE 315/4-1 and Di 334/19-1. We thank Heike März for data collection. We are indebted to the subjects who participated in the study for their consent and cooperation. This study was performed as a part of the Collaborative Research Center 874 (research area A1 and A5) and was supported by the Deutsche Forschungsgemeinschaft DFG.
The authors report no financial conflicts of interest.
References
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J Neurophysiol. 1989;62:694–710. doi: 10.1152/jn.1989.62.3.694. [DOI] [PubMed] [Google Scholar]
- Amari S. Dynamics of pattern formation in lateral-inhibition type neural fields. Biol Cybern. 1977;27:77–87. doi: 10.1007/BF00337259. [DOI] [PubMed] [Google Scholar]
- American Electroencephalographic Society. Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–113. [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, McNaughton BL. Increased electrotonic coupling in aged rat hippocampus: a possible mechanism for cellular excitability changes. J Comp Neurol. 1987;259:549–558. doi: 10.1002/cne.902590405. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. doi: 10.1016/s0896-6273(00)80762-x. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ, Senn W. A synaptic explanation of suppression in visual cortex. J Neurosci. 2002;22:10053–10065. doi: 10.1523/JNEUROSCI.22-22-10053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David-Jürgens M, Dinse HR. Effects of aging on paired-pulse behavior of rat somatosensory cortical neurons. Cereb Cortex. 2010;20:1208–1216. doi: 10.1093/cercor/bhp185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinse HR. Cortical reorganization in the aging brain. Prog Brain Res. 2006;157:57–80. doi: 10.1016/s0079-6123(06)57005-0. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. GABAergic mechanisms gate tactile discrimination learning. Neuroreport. 2003;14:1747–1751. doi: 10.1097/00001756-200309150-00018. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Kleibel N, Kalisch T, Ragert P, Wilimzig C, Tegenthoff M. Tactile coactivation resets age-related decline of human tactile discrimination. Ann Neurol. 2006;60:88–94. doi: 10.1002/ana.20862. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- Freeman TC, Durand S, Kiper DC, Carandini M. Suppression without inhibition in visual cortex. Neuron. 2002;35:759–771. doi: 10.1016/s0896-6273(02)00819-x. [DOI] [PubMed] [Google Scholar]
- Godde B, Spengler F, Dinse HR. Associative pairing of tactile stimulation induces somatosensory cortical reorganization in rats and humans. Neuroreport. 1996;8:281–285. doi: 10.1097/00001756-199612200-00056. [DOI] [PubMed] [Google Scholar]
- Godde B, Stauffenberg B, Spengler F, Dinse HR. Tactile coactivation-induced changes in spatial discrimination performance. J Neurosci. 2000;20:1597–1604. doi: 10.1523/JNEUROSCI.20-04-01597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godde B, Berkefeld T, David-Jürgens M, Dinse HR. Age-related changes in primary somatosensory cortex of rats: evidence for parallel degenerative and plastic-adaptive processes. Neurosci Biobehav Rev. 2002;26:743–752. doi: 10.1016/s0149-7634(02)00061-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Kano M. Presynaptic origin of paired-pulse depression at climbing fibre-Purkinje cell synapses in the rat cerebellum. J Physiol. 1998;506:391–405. doi: 10.1111/j.1469-7793.1998.391bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks TP, Dykes RW. Receptive field size for certain neurons in primary somatosensory cortex is determined by GABA-mediated intracortical inhibition. Brain Res. 1983;274:160–164. doi: 10.1016/0006-8993(83)90533-4. [DOI] [PubMed] [Google Scholar]
- Höffken O, Veit M, Knossalla F, Lissek S, Bliem B, Ragert P, Dinse HR, Tegenthoff M. Sustained increase of somatosensory cortex excitability by tactile coactivation studied by paired median nerve stimulation in humans correlates with perceptual gain. J Physiol. 2007;584:463–471. doi: 10.1113/jphysiol.2007.140079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffken O, Lenz M, Tegenthoff M, Schwenkreis P. Multichannel SEP-recording after paired median nerve stimulation suggests origin of paired-pulse inhibition rostral of the brainstem. Neurosci Lett. 2010;468:308–311. doi: 10.1016/j.neulet.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiol Aging. 2006;27:155–162. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Ragert P, Schwenkreis P, Dinse HR, Tegenthoff M. Impaired tactile acuity in old age is accompanied by enlarged hand representations in somatosensory cortex. Cereb Cortex. 2009;19:1530–1538. doi: 10.1093/cercor/bhn190. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300:812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Lueders H, Lesser RP, Hahn J, Dinner DS, Klem G. Cortical somatosensory evoked potentials in response to hand stimulation. J Neurosurg. 1983;58:885–894. doi: 10.3171/jns.1983.58.6.0885. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M. Role of the human motor cortex in rapid motor learning. Exp Brain Res. 2001;136:431–438. doi: 10.1007/s002210000614. [DOI] [PubMed] [Google Scholar]
- Namiki J, Takase M, Ohira T, Goto K, Ishikawa M, Ajimi Y, Toya S. The neural origin generating early cortical components of SEP: topographical analysis using temporal-second-order-differentiation of cortical SEPs. Brain Topogr. 1996;8:229–232. doi: 10.1007/BF01184774. [DOI] [PubMed] [Google Scholar]
- Neary K, Anand S, Hotson JR. Perceptual learning of line orientation modifies the effects of transcranial magnetic stimulation of visual cortex. Exp Brain Res. 2005;162:23–34. doi: 10.1007/s00221-004-2117-5. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55:74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Conrad B, Siebner HR. Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett. 2001;313:33–36. doi: 10.1016/s0304-3940(01)02239-x. [DOI] [PubMed] [Google Scholar]
- Pleger B, Dinse HR, Ragert P, Schwenkreis P, Malin JP, Tegenthoff M. Shifts in cortical representations predict human discrimination improvement. Proc Natl Acad Sci U S A. 2001;98:12255–12260. doi: 10.1073/pnas.191176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe BH, Linville C, Brunso-Bechtold J. Age-related decline of presumptive inhibitory synapses in the sensorimotor cortex as revealed by the physical disector. J Comp Neurol. 2001;439:65–72. doi: 10.1002/cne.1335. [DOI] [PubMed] [Google Scholar]
- Porter JT, Nieves D. Presynaptic GABAB receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J Neurophysiol. 2004;92:2762–2770. doi: 10.1152/jn.00196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Dinse HR, Pleger B, Wilimzig C, Frombach E, Schwenkreis P, Tegenthoff M. Combination of 5 Hz repetitive transcranial magnetic stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. Neurosci Lett. 2003;348:105–108. doi: 10.1016/s0304-3940(03)00745-6. [DOI] [PubMed] [Google Scholar]
- Ragert P, Becker M, Tegenthoff M, Pleger B, Dinse HR. Sustained increase of somatosensory cortex excitability by 5 Hz repetitive transcranial magnetic stimulation studied by paired median nerve stimulation in humans. Neurosci Lett. 2004;356:91–94. doi: 10.1016/j.neulet.2003.11.034. [DOI] [PubMed] [Google Scholar]
- Ragert P, Franzkowiak S, Schwenkreis P, Tegenthoff M, Dinse HR. Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. Exp Brain Res. 2008;184:1–11. doi: 10.1007/s00221-007-1073-2. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Redecker C, Bruehl C, Witte OW. Age-related decline of functional inhibition in rat cortex. Neurobiol Aging. 2010;31:504–511. doi: 10.1016/j.neurobiolaging.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci. 2000;3:384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Shagass C. Recovery functions of human somatosensory and visual evoked potentials. Ann N Y Acad Sci. 1964;112:510–525. doi: 10.1111/j.1749-6632.1964.tb26765.x. [DOI] [PubMed] [Google Scholar]
- Shagass C, Schwartz M. Recovery functions of somatosensory peripheral nerve and cerebral evoked responses in man. Electroencephalogr Clin Neurophysiol. 1964;17:126–135. doi: 10.1016/0013-4694(64)90144-0. [DOI] [PubMed] [Google Scholar]
- Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res. 2009;198:489–500. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- Spengler F, Godde B, Dinse HR. Effects of ageing on topographic organization of somatosensory cortex. Neuroreport. 1995;6:469–473. doi: 10.1097/00001756-199502000-00016. [DOI] [PubMed] [Google Scholar]
- Stevens JC. Aging and spatial acuity of touch. J Gerontol. 1992;47:P35–P40. doi: 10.1093/geronj/47.1.p35. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Forsythe ID, Tsujimoto T, Barnes-Davies M, Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Schneggenburger R, Weis S, Neher E. Presynaptic depression at a calyx synapse: the small contribution of metabotropic glutamate receptors. J Neurosci. 1997;17:8137–8146. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Wilimzig C, Pleger B, Ragert P, Kalisch T, Tegenthoff M, Dinse HR. Differential effects of aging and learning processes on cortical interaction. Soc Neurosci Abstr. 2006;32:53.6. [Google Scholar]
- Wilson HR, Cowan JD. A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik. 1973;13:55–80. doi: 10.1007/BF00288786. [DOI] [PubMed] [Google Scholar]
- Wohlert AB. Tactile perception of spatial stimuli on the lip surface by young and older adults. J Speech Hear Res. 1996;39:1191–1198. doi: 10.1044/jshr.3906.1191. [DOI] [PubMed] [Google Scholar]
- Woodward KL. The relationship between skin compliance, age, gender, and tactile discriminative thresholds in humans. Somatosens Mot Res. 1993;10:63–67. doi: 10.3109/08990229309028824. [DOI] [PubMed] [Google Scholar]
- Yiannikas C, Vucic S. Utility of somatosensory evoked potentials in chronic acquired demyelinating neuropathy. Muscle Nerve. 2008;38:1447–1454. doi: 10.1002/mus.21078. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]