Abstract
Cognitive flexibility is known to depend on the striatum. However, the striatum does not act in isolation to bias cognitive flexibility. In particular, cognitive flexibility also implicates the frontal cortex. Here we tested the hypothesis that the human frontal cortex controls cognitive flexibility by regulating striatal function via topographically specific frontostriatal connections. To this end, we exploited a repetitive transcranial magnetic stimulation (TMS) protocol over frontal cortex that is known to increase dopamine release in the striatum. This intervention was combined with functional magnetic resonance imaging to determine the functional and topographic specificity of its consequences at the whole brain level. Participants were scanned both before and after off-line TMS while performing a cognitive switching task that is known to depend on a specific striatal substructure, the putamen. Frontal stimulation perturbed task-specific functional signals in the putamen, while reducing fronto-striatal functional connectivity. There were no such effects of TMS over the medial parietal cortex. These data strengthen the hypothesis that cognitive flexibility involves topographic frontal control of striatal function.
Introduction
The human striatum is increasingly recognized to be important for higher cognitive functions, in particular “cognitive flexibility,” the ability to update behavioral goals in response to changing contextual demands (Cools et al., 2004, 2006). However, the striatum does not function alone; it interacts with the frontal cortex. This is consistent with the fact that these two regions are strongly interconnected via functionally and anatomically relatively segregated topographic loops (Alexander et al., 1986). Here we aimed to assess whether cognitive flexibility, and associated striatal functional signals, are controlled by the frontal cortex.
To this end, we used an off-line repetitive transcranial magnetic stimulation (TMS) protocol known to increase dopamine release in the striatum. Using [11C]raclopride positron emission tomography (PET), Strafella et al. (2001, 2003) showed that cortical stimulation altered striatal dopamine release in a manner restricted by cortico-striatal circuit structure. Stimulation over primary motor cortex increased dopamine release in anatomically connected regions of the putamen (Strafella et al., 2003), while dorsolateral prefrontal cortex stimulation increased dopamine release focally in the caudate nucleus (Strafella et al., 2001). This TMS-induced dopamine release was observed while subjects were at rest, in the absence of any psychological task.
The functional importance of striatal dopamine for cognitive flexibility is supported by psychopharmacological and functional magnetic resonance imaging (fMRI) studies, which have revealed that cognitive switching and associated striatal activity (Rogers et al., 2000; Leber et al., 2008) are sensitive to dopaminergic drug administration (Mehta et al., 2004; Cools et al., 2007) and polymorphisms in dopamine genes (Aarts et al., 2010; Stelzel et al., 2010). Furthermore, dopaminergic manipulations modulate functional connectivity between the striatum and frontal cortex (Nagano-Saito et al., 2008; Wallace et al., 2011).
Previous work suggests that the putamen is critical for cognitive switching between concrete stimulus exemplars, but not between abstract rules that have no direct instantiation in the motor or sensory domain. When healthy volunteers switched between concrete stimuli, but not abstract rules, fMRI signal in the putamen was increased (Cools et al., 2004). Further, patients with focal putamen lesions were selectively impaired during stimulus switching but not rule switching (Cools et al., 2006).
Here we aimed to test the hypothesis that cognitive flexibility involves topographic frontal control of striatal function. If the frontal cortex has a causal role in cognitive flexibility by controlling striatal function, then the functional impact of frontal stimulation should be particularly pronounced when subjects are in a cognitive state that depends critically on putamen signaling (O'Shea et al., 2007a).
Subjects performed a cognitive switching task during fMRI (Cools et al., 2004), both before and after TMS. We predicted that TMS over primary motor cortex, but not medial parietal cortex, would alter functional signal in the putamen, specifically, when subjects switched between stimuli (but not between abstract rules). If the expected change in putamen functional signal is indeed a direct consequence of motor cortex stimulation, then this should be reflected in a TMS-induced change in task-specific functional connectivity between these regions.
Materials and Methods
Subjects.
Twenty-nine right-handed healthy volunteers participated in this study. Data from one subject were excluded because the scan had to be aborted during the critical period immediately after the TMS. Fourteen subjects received TMS over the left primary motor cortex (M1) (9 female; mean age 24.4, SD 3.1) and 14 subjects received TMS over the medial parietal cortex (POz; 60% of the vertex-inion distance) (8 female; mean age 23.1, SD 3.0). One subject in the control group mistakenly received TMS over PPOz (30% of the vertex-inion distance). Analyses performed with and without this subject yielded the same results.
The study was approved by the Central Oxford Research Ethics Committee (07/Q1606/1) and was conducted in accordance with the Declaration of Helsinki. Exclusion criteria were personal or family history of neurological or psychiatric disorder, cardiovascular disease, regular use of medication or recreational drugs, heavy smoking, claustrophobia, or metal parts in the body. All subjects gave written informed consent and were compensated for their participation.
Procedure.
Subjects were invited to spend on average 4 h at the University of Oxford Centre for Clinical Magnetic Resonance Research at the John Radcliffe Hospital. After extensive practice on the experimental paradigm they underwent two fMRI scans, one pre-TMS scan and one post-TMS scan, in counterbalanced order (M1 group: 7 subjects received TMS first; control group: 7 subjects received TMS first) (Fig. 1A). The average delay between the last TMS pulse and the first experimental trial of the post-TMS fMRI scan was 3 min 44 s for the M1 group (SEM 8.5 s; range 3 min 14 s to 5 min) and 3 min 38 s for the control group (SEM 7.6 s; range 2 min 29 s to 4 min 20 s). For subjects who received TMS first, the minimum (washout) delay between the end of TMS and the start of the second (baseline, so-called “pre-TMS” scan) fMRI scan was 70 min. During both fMRI scans, subjects performed four runs of the behavioral paradigm (described below), which lasted ∼30 min. For one subject in the control group only two runs were obtained during the pre-TMS session, so data analysis was performed on these two sessions.
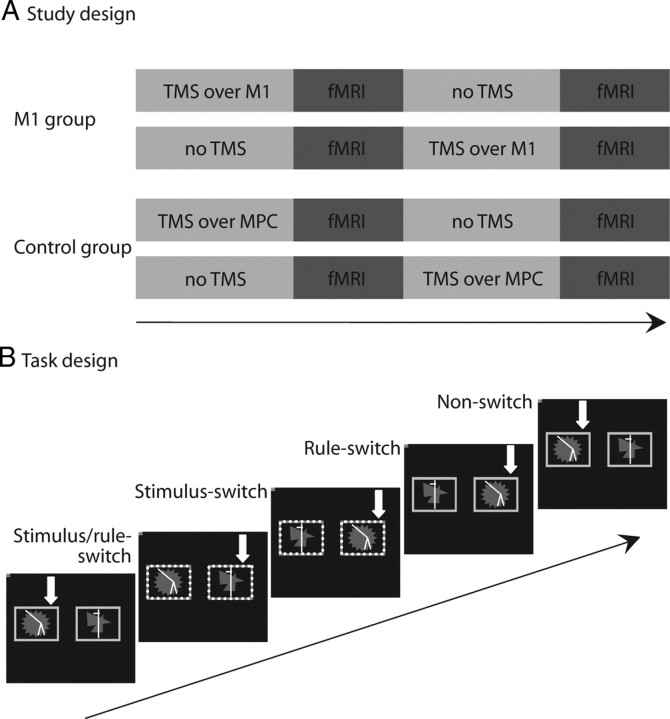
Figure 1.
A, We used a between-subjects design such that one group of subjects (n = 14) received TMS over the primary motor cortex, and one group of subjects (n = 14) received TMS over the medial parietal cortex. Subjects were scanned before and after TMS. Importantly, the order of the pre- and post-TMS scans was counterbalanced within groups. B, On each trial the same two abstract patterns were presented within a pair of colored stimulus cue windows. The yellow (solid) stimulus windows cued participants to choose the same pattern as on the previous trial (match rule), while the blue (dotted) stimulus windows cued participants to respond to the other pattern (non-match rule). This design allowed us to separate four trial types: (1) trials on which both the task rule and the target stimulus were the same as on the previous trial (non-switch trials), (2) trials on which the task rule remained the same but the target stimulus switched (stimulus-switch trials), (3) trials on which the target stimulus remained the same but the task rule was different from the previous trial (rule-switch trials), and (4) trials on which both the task rule and the target stimulus were different from the previous trial (stimulus/rule-switch trials). The white arrows indicate the correct response (not shown to subjects).
Behavioral paradigm.
On each trial, the same two abstract colored patterns were presented simultaneously (left–right location randomized) (Fig. 1B), and subjects were required to choose one of the two patterns on each trial. Responses were made according to one of two response rules using the index and middle finger of the right hand on a button box. The patterns were presented within and at the same time as either blue or yellow stimulus windows. If the windows were yellow, subjects were required to choose the same stimulus as on the previous trial (i.e., the target stimulus remained the same). If the windows were blue, subjects were required to choose the pattern that they did not choose on the previous trial (i.e., they switched responding from target stimulus A to target stimulus B). The design allowed us to separate four trial types: (1) non-switch trials on which both the task rule and the target stimulus were the same as on the previous trial (i.e., yellow trials after yellow trials), (2) stimulus-switch trials on which the task rule remained the same but the target stimulus switched (i.e., blue trials after blue trials), (3) rule-switch trials on which the target stimulus remained the same but the task rule switched (i.e., yellow trials after blue trials), and (4) stimulus/rule-switch trials on which both the task rule and the target stimulus were different from the previous trial (i.e., blue trials after yellow trials) (Fig. 1B). Each subject performed four runs of 114 trials (6.3 min per run), and stimuli were presented in a pseudorandom fixed order so that (1) rule switching was unpredictable (the probability of a rule switch was 0.5 on each trial), (2) the number of stimulus-repetition and stimulus-switching trials was matched within each block, and (3) response repetition was approximately matched across the four trial types. Stimuli and cue windows were presented for 2000 ms or until a response was made. If a response was not made within 2000 ms, a “too late” message was presented. Feedback, consisting of a green smiley face for correct responses or a red sad face for incorrect responses, was presented immediately after the response. The feedback faces were presented centrally between the two stimuli for 500 ms, during which the stimuli also remained on the screen. After feedback, the stimuli were removed, and the face was replaced by a fixation cross for a variable interval so that the overall interstimulus interval was 3.32 ms, enabling desynchronization from the repetition time (of 1600 ms) and sufficient sampling across the hemodynamic response function.
Upon arrival each subject performed four practice blocks to ensure subjects understood the task and to minimize test–retest effects during the two following experimental sessions. The task was programmed in Microsoft Visual Basic 6.0, and stimuli were presented using a beamer and projected onto a mirror in the MR scanner.
TMS.
This was delivered via a biphasic Magstim SuperRapid machine (Magstim Company) through a 70 mm figure-of-eight coil held tangential to the skull and fixed in position using a mechanical arm. Stimulation intensity was determined for each individual with reference to the hand motor “hotspot,” the optimal scalp position overlying the left primary motor cortex (M1) at which the lowest intensity single-pulse TMS evoked a just noticeable twitch from the relaxed first dorsal inter-osseous muscle of the right hand. Stimulation was applied at 90% of the resting motor threshold, and defined as the lowest TMS intensity to elicit motor-evoked potentials of ∼ 50 μV amplitude on 5 of 10 consecutive trials. The resting motor threshold was measured for each individual on a different day before the fMRI session. In the same session we confirmed for each individual that a train of the repetitive stimulation protocol at this subthreshold intensity did not elicit motor-evoked potentials. Mean stimulation intensities were 48.3% (SD ± 6.6) of maximum stimulator output for our cortical area of interest, M1, and 50% (SD ± 11.4) for the control region, medial parietal cortex.
Medial parietal cortex (60% of the vertex-inion distance, area POz according to the International 10–20 electrode system) was chosen as a control region. In common with other TMS/fMRI studies (O'Shea et al., 2007a), we selected a cortical region that is not a critical node in the functional network controlling the function of interest, cognitive flexibility. Hence, the data for this region control for any general non-specific effects of repetitive brain stimulation and for non-specific connectional spread of stimulation from cortex to striatum. Over M1, the TMS coil handle was oriented posterior-anterior at ∼45° from the mid-sagittal axis, inducing latero-medial current flow in the brain. Over medial parietal cortex, the coil was oriented perpendicular to the floor.
Motor-evoked potentials were recorded using silver chloride surface electrodes in a tendon-belly montage. Electromyographic responses were sampled, amplified, and filtered using a CED 1902 amplifier, a CED 1401 analog-to-digital converter, and a Pentium 4 computer running Signal (version 2.14) software (Cambridge Electronic Design). The sampling rate was 5 kHz and signals were notch filtered at 50 Hz and bandpass filtered between 10 and 1000 Hz.
The repetitive TMS protocol was identical to that previously shown to induce focal dopamine release in the striatum (Strafella et al., 2001, 2003, 2005). Three blocks of TMS were delivered 10 min apart. Each block consisted of fifteen 10-pulse trains of 1 s duration (i.e., 10 Hz) with an intertrain interval of 10 s. Stimulation was performed in the MRI control room, immediately adjacent to the scanner room.
Image acquisition.
Whole-brain imaging was performed on a 3 T MR scanner (Magnetom Trio TIM; Siemens Medical Systems). Four runs of 250 T2*-weighted echo-planar images were obtained using a gradient-echo echo-planar scanning sequence (25 axial-oblique slices, repetition time = 1600 ms, echo time = 28 ms, slice thickness = 4 mm, interslice gap = 1 mm, descending slice acquisition, field-of-view = 224 mm, flip angle = 80°). Visual stimuli were projected on a screen and were viewed through a mirror attached to the head coil. In addition, a high-resolution T1-weighted MP-RAGE anatomical scan was obtained from each subject (192 sagittal slices, repetition time = 2300 ms, echo time = 3.03 ms, voxel size = 1.0 × 1.0 × 1.0 mm, field-of-view = 256 mm).
Image analysis.
Univariate data analysis was performed using SPM5 (Statistical Parametric Mapping; Wellcome Trust Centre for Cognitive NeuroImaging, London). The first 12 functional scans of each dataset were discarded to avoid T1 equilibrium effects. The first task trial began immediately after that. Anatomical images were spatially coregistered to the mean of the functional images and normalized using a unified segmentation approach. Preprocessing procedures for functional images included within-subject realignment, spike removal, spatial normalization using the same transformation matrix as estimated from the anatomical images, and spatial smoothing using a Gaussian kernel of 10 mm full-width at half-maximum.
In a general linear model (GLM) we included four regressors of interest: (1) non-switch trials, (2) stimulus-switch trials, (3) rule-switch trials, and (4) combined stimulus/rule-switch trials. The first trial in each block, error trials (including omissions and premature responses), and trials immediately after such error trials were not included in the model. The six realignment parameters were modeled as regressors of no interest. All paradigm-related regressors were modeled as delta functions at the onset of the stimulus (which co-occurred with the onset of the cue) and were convolved with a canonical hemodynamic response function including time derivatives. Time series were high-pass filtered (128 s). The parameter estimate, derived from the mean least-squares fit of the model to the data, reflects the strength of covariance between the data and the canonical response function for a given condition.
We predicted that TMS would modulate blood oxygenation level-dependent (BOLD) signal on trials requiring switching between stimuli. Hence, we defined a “stimulus switching” contrast that was used for all analyses. This stimulus switching condition was defined as the contrast between stimulus-switch and stimulus/rule-switch trials versus rule-switch and non-switch trials. Contrast images for “stimulus switching” were calculated separately at the subject level for each fMRI session (pre- and post-TMS). Next, these contrast images were tested in a random effects second-level factorial design with the factors TMS Time (pre-TMS vs post-TMS) and TMS Site (M1 vs medial parietal cortex). This allowed us to assess all of the following within a single statistical model: (1) main effect of Task (“stimulus switching network”), (2) Task × TMS Site × TMS Time interaction, and (3) Task × TMS Time interactions separately for each of the two TMS sites.
The main effect of Task, collapsed across TMS Site and TMS Time conditions, was tested and displayed at a threshold of p < 0.05 familywise error (FWE) corrected for the whole brain (pFWE).
We predicted that M1 TMS would modulate activity in the putamen in a task-specific manner. Since stimulation was delivered over the left M1, we expected the effect to be particularly strong in the left putamen. To investigate this hypothesis, we generated a functionally defined putamen volume of interest (VOI), based on an a priori expected pattern of putamen activity in the main task condition (BOLD increase during stimulus switching; data collapsed across subjects, TMS Site, and TMS Time). The VOI was centered on an activation cluster in the left anterior putamen [MNI coordinates: peak, (−20, 6, 2); cluster size, 412 voxels]. VOI definition and data extraction were done using MarsBaR (Brett et al., 2002).
TMS effects in the putamen were assessed at the cluster level, corrected for multiple comparisons in our small search volume (VOI) [i.e. small volume corrected (svc)] (psvc < 0.05). The height threshold at the voxel level was set at p < 0.001 uncorrected for multiple comparisons.
In additional analyses we assessed TMS effects in all task-activated regions using MarsBaR; p values were divided by the number of regions tested to correct for multiple comparisons.
Figures were displayed using MRIcroN (Rorden et al., 2007). Statistical parametric maps were superimposed on a skull-stripped template in standard MNI space.
Psychophysiological interaction analysis.
Functional connectivity was assessed using psychophysiological interaction (PPI) analysis (Friston et al., 1997). PPI works under the assumption that the degree to which the BOLD signal in one area can predict the BOLD signal in another corresponds to the degree of influence that the first region has on the second region. In order words, it tests whether region A shows higher or lower connectivity with region B, during condition C, compared with condition D. The PPI analysis was used to test whether this relationship was changed by TMS. Time series were extracted for each individual participant from a seed voxel in the left putamen that showed significant BOLD signal for the stimulus switching contrast (i.e., main effect of “Task” at p < 0.05 FWE corrected for the whole brain). Because the exact locations of activation maxima varied across subjects, we localized the peak voxel in the putamen for each individual according to the constraints that it (1) exceeded a threshold of p < 0.05 (uncorrected) for the specified contrast and (2) was within 10 mm of the group maximum [MNI coordinates (−20, 6, 2)] for the stimulus switching contrast. For datasets in which no significant voxels were found using these constraints (4 of the 14 pre-TMS sessions and 6 of the 14 post-TMS sessions), the threshold was lowered to p < 0.5 (uncorrected). Once the peak voxel was located for each individual subject, time series data were averaged across a 3 mm spherical VOI centered on that voxel. More specifically, regional time series were summarized by computing the first eigenvector across all suprathreshold voxels (p < 0.05 or p < 0.5 uncorrected) within 3 mm of this peak voxel. The deconvolved time series were then multiplied by a vector coding for the experimental condition of interest (stimulus switching) to obtain the PPI regressor.
On the subject level, we included the PPI regressor in a GLM, together with regressors modeling the experimental conditions and the extracted time series. This allowed us to assess functional connectivity between the seed and all other voxels in the brain over and above shared functional activation and task-independent correlations in BOLD signal between the seed and other regions. These regressors were convolved with a canonical hemodynamic response function and high-pass filtered (128 s). In addition, the six realignment parameters were modeled. The PPI analysis was performed separately for each fMRI session (pre- and post-TMS). The PPI maps from the pre- and post-TMS sessions were brought to the second level in a paired sample t test. We predicted that TMS would change functional connectivity between the putamen and the stimulated left M1. For the M1 hand area, we defined a 6 mm spherical VOI around the MNI coordinates (−32, −21, 69) based on our previous work (O'Shea et al., 2007b).
TMS effects were assessed at the cluster level, corrected for multiple comparisons in our small search volume (VOI) (psvc < 0.05) or the whole brain (p < 0.05). The height threshold at the voxel level was set at p < 0.005 uncorrected for multiple comparisons. Note that although this voxel threshold is quite liberal, statistical inferences were done after correction for multiple comparisons in our volume of interest.
Behavioral analysis.
The first trial in each block, incorrect trials, trials on which subjects did not respond within the maximum of 2000 ms (omissions), premature responses (<300 ms), and trials after errors and omissions (to avoid potential bias across trial types in the reaction time data owing to potentially differential rates of “post-error slowing”) (Rabbitt, 1966) were excluded from reaction time analyses. All 28 subjects performed well on the task, and individual percentage errors and omissions did not differ between the two experimental groups (M1, mean 8.2%; medial parietal cortex, mean 7.7%). Data were analyzed using repeated-measures ANOVA (SPSS) with Greenhouse-Geisser correction where appropriate. In line with the fMRI analyses, we performed repeated-measures ANOVA with one between-subject factor (TMS Site: M1 vs medial parietal cortex) and two within-subject factors (Task: stimulus-switch and stimulus/rule-switch trials vs rule-switch and non-switch trials; TMS: pre-TMS vs post-TMS) and tested for a three-way interaction.
Results
Main effect of stimulus switching
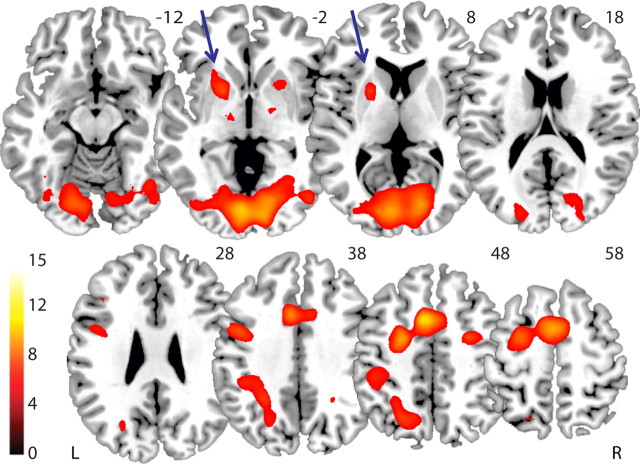
Based on previous work (Cools et al., 2004, 2006), we focused hypothesis-driven analyses on trials that required switching between stimuli (i.e., stimulus-switch and stimulus/rule-switch trials vs rule-switch and non-switch trials). First, to identify the network activated by stimulus switching, we assessed the main effect across the whole group (data pooled across pre- and post-TMS conditions and TMS site). Consistent with our prior study (Cools et al., 2004), BOLD signal was increased in the anterior putamen, when subjects switched between stimuli. Regions that showed a similar increase during stimulus switching included the supplementary motor area, inferior frontal cortex, thalamus, inferior parietal cortex, and visual regions (Fig. 2; Table 1).
Figure 2.
Task network for stimulus switching. Main effect on BOLD signal during trial types that required switching between stimuli (stimulus-switch and stimulus/rule-switch) relative to trial types that did not require switching between stimuli (rule-switch and non-switch), with data pooled across TMS Time (pre-TMS vs post-TMS) and TMS Site (M1 vs medial parietal cortex). A cluster in the left putamen was defined as a volume of interest (indicated by arrows). Bar indicates t values, and figure is thresholded for a t value of 5.22, corresponding to a p value of 0.05 FWE corrected for multiple comparisons.
Table 1.
Clusters showing a main effect of stimulus switching at 0.05 FWE corrected
| Region | Cluster size | Local maximum |
Cluster statistics T value | M1 TMS effect p value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Putamen left | 412 | −20 | 6 | 2 | 7.49 | 0.004 |
| Putamen right | 97 | 22 | 14 | −6 | 6.09 | 0.009 |
| Premotor left/ACC | 2802 | −8 | 12 | 46 | 11.31 | n.s. |
| Occipital cortex | 6400 | −6 | −84 | 2 | 11.00 | 0.026 |
| Premotor right | 173 | 26 | −2 | 48 | 7.35 | n.s. |
| Parietal cortex left | 1383 | −28 | −50 | 42 | 7.05 | n.s. |
| Thalamus right | 51 | 12 | −4 | −8 | 6.09 | n.s. |
| Thalamus left | 22 | −16 | −16 | 0 | 5.85 | n.s. |
| Parietal cortex right | 16 | 26 | −50 | 36 | 5.68 | n.s. |
| Occipital cortex left | 19 | −38 | −58 | −14 | 5.67 | n.s. |
| Inferior frontal cortex left | 7 | −40 | 26 | 24 | 5.56 | n.s. |
| Occipital cortex right | 2 | 22 | −86 | 22 | 5.30 | n.s. |
| Thalamus/pallidum right | 1 | 14 | 4 | −10 | 5.26 | n.s. |
The last column shows effects of M1 TMS, as revealed by a supplementary VOI analysis for each of these clusters. The p values for these supplementary analyses are corrected for multiple comparisons.
Effect of TMS on task-specific BOLD signal in the putamen
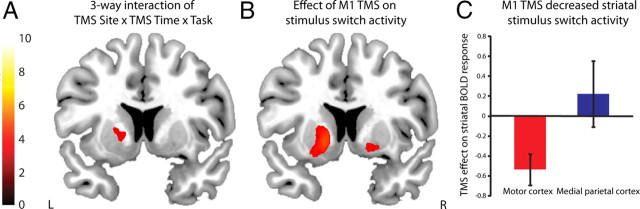
To test the hypothesis that TMS over the left M1, but not the medial parietal cortex, would modulate BOLD signal in the left putamen, we focused analyses on the cluster in the left putamen that showed a main effect of stimulus switching (Fig. 2). In this region, we found, as predicted, a significant three-way interaction between Task (stimulus-switch and stimulus/rule-switch vs rule-switch and non-switch trials), TMS Time (pre- vs post-TMS), and TMS Site (M1 vs medial parietal cortex) [cluster 1: peak voxel, (−18, 8, 2), F = 14.09, psvc = 0.020; cluster 2: peak voxel, (−16, 8, −4), F = 12.53, psvc = 0.034] (Fig. 3A). This confirmed that TMS induced a significantly different effect on functional activity in the anterior putamen depending on where the stimulation was applied. To further explore this interaction, we assessed Task × TMS Time interactions separately in each TMS Site group. As predicted, we found that M1 TMS significantly changed putamen BOLD signal [cluster 1: peak voxel, (−16, 8, −6), F = 28.14, psvc < 0.0005; cluster 2: peak voxel, (−20, 0, 10), F = 12.72, psvc = 0.032; cluster 3: peak voxel, (−24, 2, 10), F = 12.17, psvc = 0.038], but TMS at the control site did not (Fig. 3B,C).
Figure 3.
Stimulation induced a task-specific reduction in striatal activity during stimulus switching. A, The statistical parametric maps were masked by the main effect of stimulus switching (thresholded at p < 0.001 uncorrected). The 3-way interaction of TMS Site (M1 vs medial parietal cortex) × TMS Time (pre-TMS vs post-TMS) × Task revealed a significant effect in the putamen. TMS had a different effect on BOLD signal in the putamen during stimulus switching depending on whether stimulation was applied to M1 or medial parietal cortex. B, This interaction was driven by a significant effect of M1 TMS, and no significant effect of TMS over the medial parietal cortex (not displayed). Bar indicates t values and figures are thresholded for a t value of 3.25 corresponding to a p value of 0.001 uncorrected for multiple comparisons. C, Bar graphs (representing parameter estimates extracted from the peak voxel of the 3-way interaction [MNI coordinates: (−18, 8, 2)] (cluster shown in A)) showing that TMS over M1, but not the medial parietal cortex, decreased BOLD signal in the anterior putamen during stimulus switching.
Effect of TMS on task-specific BOLD signal in other regions
To test the regional selectivity of the M1 TMS effect we assessed TMS Time × Task interactions in all clusters that showed a main effect of Task (Fig. 2; Table 1). After correction for multiple comparisons, we found an effect of TMS in three regions. One of these regions was the left putamen, as described above. In addition we found an effect in the right putamen and in an occipital cluster (Table 1).
TMS reduced task-specific connectivity between the motor cortex and the anterior putamen
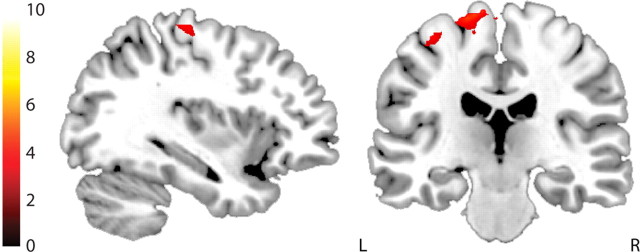
Next we assessed whether the effect of M1 TMS on switch-related BOLD signal in the anterior putamen was accompanied by changes in functional connectivity between M1 and the anterior putamen. Functional connectivity was assessed via PPI analyses, contrasting pre-TMS versus post-TMS data from the left putamen seed region with the stimulus switching contrast as the task regressor. Random-effects analysis with multiple comparison correction (SVC in the a priori defined M1; whole-brain correction elsewhere) revealed that M1 TMS reduced switch-related connectivity between the left putamen and left M1, adjacent to the motor hand knob, which was the target for TMS (Yousry et al., 1997) [peak voxel, (−32, −26, 66), t = 3.20, psvc = 0.031] (Fig. 4). No other effects were found at the whole-brain level. The exact same analysis was performed on the data from the control group who received TMS over the medial parietal cortex. No effects were found.
Figure 4.
TMS reduced switch-related functional connectivity between the motor cortex and putamen. TMS over left M1 reduced functional connectivity between the left putamen and left M1. Bar indicates t values and figure is thresholded for a t value of 3.01 corresponding to a p value of 0.005 uncorrected for multiple comparisons.
Hence, TMS over M1 reduced switch-related functional connectivity between the putamen and M1.
Effect of TMS on behavior
The overall pattern of task performance replicated our previous studies (Cools et al., 2004, 2006), so it is not reported in detail here. Just as for fMRI signal, we expected that TMS would alter task performance and predicted a three-way interaction of Task × TMS Site × TMS Time on error rates or reaction times. There was no effect of TMS on reaction time. In the error rate data, the three-way interaction did not reach significance (p = 0.1). Rather, analyses revealed a generalized reduction in performance accuracy specifically after M1 stimulation, regardless of trial type. Error rate analyses showed a significant interaction between TMS Site and TMS Time (F(1,26) = 6.84, p = 0.015). Separate analyses by group revealed that accuracy changed only after M1 stimulation (effect of TMS Time: F(1,13) = 8.76, p = 0.011). After M1 TMS, there was a generalized increase in error rates that was not specific to any trial type (no Task × TMS Time interaction: F(1,13) = 2.89, p = 0.1). There was no main effect or interaction when TMS was applied over the medial parietal cortex. We also tested for a significant correlation between the behavioral and neural effects of TMS, but there was no relationship that survived correction for multiple comparisons.
Discussion
This study confirmed the hypothesis that striatal functional signals associated with cognitive switching are under topographic frontal cortical control. Frontal but not medial parietal cortex TMS attenuated cognitive switch-related signal selectively in the putamen. The effect was anatomically constrained: a focal reduction in putamen activity was accompanied by weakened cortico-striatal functional connectivity. Hence, the induced signal changes were specific to the anatomical loop connecting the cortical stimulation target and its topographically connected striatal substructure. In addition, the TMS effect was functionally specific, expressed only on trials that are known to depend critically on the putamen. TMS suppressed BOLD signal only on trials that required subjects to switch between concrete stimuli, with no effect on trials where subjects had to switch between abstract rules. Hence, frontal interference disrupted fronto-striatal connectivity in a functionally and topographically specific way.
These findings extend the work of Strafella et al. (2001, 2003) in an important way. Those authors observed that frontal stimulation induced focal striatal dopamine release. However, the functional consequences were not addressed. Here, for the first time, we demonstrate the functional consequences of this intervention by using a task designed to specifically assay putamen-dependent cognitive functioning. Frontal TMS perturbed putamen signal selectively on trials in which subjects were required to switch between concrete stimuli. This task has been previously shown to activate the putamen (Cools et al., 2004) and to be impaired by focal putamen lesions (Cools et al., 2004, 2006). As predicted, stimulation had no effect on brain activity during trials in which subjects had to switch between abstract rules, a task that is not striatum-dependent. Hence, the effect of TMS varied as a function of participants' cognitive state, arising only on trials that imposed a cognitive demand for which the putamen is functionally specialized. Hence, the stimulation effects were not a simple consequence of passive connectional spread; rather, their trial-by-trial expression was cognitive state dependent.
The TMS effects were also anatomically specific to the stimulation site: no such effects were observed when stimulation was applied over the medial parietal cortex. Hence, the results cannot be explained by some general, non-specific effect of cortical stimulation. The region in the putamen that was modulated by TMS is remarkably close to the region found in the previous neurochemical study (Strafella et al., 2003), and is known to receive anatomical projections from the hand area of M1 (Takada et al., 1998). To test whether the changes in putamen BOLD signal were indeed a direct effect of M1 stimulation, we performed functional connectivity analysis. In support of this hypothesis, stimulation weakened functional interaction between the anterior putamen and the stimulated M1 in a task-specific manner.
The topographic specificity of the TMS-induced functional changes concurs with and extends the focal neurochemical findings of Strafella et al. (2001, 2003). In those studies, focal increases in striatal dopamine varied by cortical stimulation site: M1 TMS selectively affected the putamen, while dorsolateral prefrontal cortex TMS affected the caudate nucleus. Hence, dopamine release was altered specifically in the subregion of the striatum known to receive most of its projections from the frontal area that was stimulated. The topographical specificity of those neurochemical data, combined with the present pattern of functional activation changes, indicate that the connectional spread of stimulation was constrained by the known anatomical pattern of relatively segregated fronto-striatal-thalamic loops (Alexander et al., 1986; Kelly and Strick, 2004).
To determine the regional specificity of the observed TMS effects, supplementary analysis was conducted on functionally defined VOIs derived from the main task contrast. In addition to the predicted effect in the left putamen, M1 TMS also modulated BOLD signal significantly in the right putamen and the occipital cortex (Table 1). Such distributed effects of cortical stimulation are a typical finding in the TMS-fMRI literature (e.g., O'Shea et al., 2007a; Sack et al., 2007; Ruff et al., 2008). Indeed, given that TMS attenuated functional signal in the putamen, a structure known to have a key role in stimulus switching, it would be surprising if there were no accompanying changes in the activity of functionally interconnected regions within the stimulus switching network (Fig. 2). The right putamen effect likely reflects interhemispheric connections, either at the level of the putamen or the motor cortex (Künzle, 1975). The effect on occipital cortex might reflect indirect downstream consequences of the perturbation of striatal BOLD signal. Consistent with this, in a previous study we showed that activity in the basal ganglia can influence visual processing by modulating fronto-posterior connections (van Schouwenburg et al., 2010). Importantly, there were no observed effects in somatosensory regions, such as posterior thalamus or primary somatosensory cortex, ruling out a role for somatosensory feedback in driving the observed results.
In a previous [11C]raclopride PET study, continuous theta burst stimulation (cTBS) to left dorsolateral prefrontal cortex was shown to reduce striatal dopamine release and impair performance on a Wisconsin Card Sort task involving set shifting between higher order abstract rules (Ko et al., 2008). Notably, unlike the effects of the current TMS protocol, the impact of cTBS in that study was neither topographically specific nor restricted to a specific subregion of the striatum. Reductions in dopamine release were observed in both the caudate nucleus and putamen. In addition, that study did not include a task control, so the functional specificity of the effects could not be determined. The difference in topographical specificity between the results of Ko et al. (2008) and the present findings might reflect differences in the TMS protocol (cTBS vs 10 Hz TMS), imaging duration (60 min acquisition time for PET), or cognitive state. In any case, the present study is the first to demonstrate the feasibility of using cortical TMS to modulate subcortical function in a topographically specific manner. The question of whether the current TMS protocol applied to dorsolateral prefrontal cortex would selectively perturb rule-switching but not stimulus-switching functions should be addressed in future work.
The exact same TMS protocol used in the current study was previously shown to increase dopamine release in the putamen (Strafella et al., 2001, 2003, 2005). The idea that the present functional effects of TMS may be dopamine dependent concurs with evidence that cognitive switching is sensitive to dopaminergic drug manipulations and polymorphisms in dopamine genes (Cools et al., 2001, 2003; Mehta et al., 2004; Aarts et al., 2010; Stelzel et al., 2010). Hence, we hypothesize that the observed TMS effects on functional striatal signal are caused by modulation of striatal dopamine transmission. This remains to be tested, however, since the dopamine findings were observed at rest, in the absence of any psychological task, while the present functional results were shown to be cognitive state dependent. This causal dopamine hypothesis could be directly tested in future experiments by assessing whether the TMS-induced functional effects are blocked following pretreatment with sulpiride, a dopamine receptor antagonist that blocks striatal dopamine transmission (van Holstein et al., 2011). The finding that M1 TMS decreased BOLD signal in the putamen might at first seem surprising, given that this protocol is known to increase dopamine release, at least when subjects are at rest. However, it is established that there is an optimal level of dopamine transmission for cognitive function, with either too much or too little dopamine impairing cognitive performance (Arnsten, 1998; Cools and Robbins, 2004; Cools and D'Esposito, 2011). Accordingly, one might speculate that the observed decrease in putamen signal reflects a detrimental “overdose”-like effect caused by a TMS-induced abnormal increase in dopamine release. Contrary to predictions, however, this task-specific perturbation of functional signals was not accompanied by a task-specific behavioral interference effect. Rather, TMS degraded accuracy across all trial types. This may reflect an impact of TMS on the directly stimulated motor and adjacent premotor cortices, which play important roles in arbitrary stimulus-response selection and execution (O'Shea et al., 2007a,b).
The topographic specificity of the current results suggests that it is possible to manipulate cognitive functions associated within distinct cortico-striatal circuits by means of noninvasive transcranial stimulation. The present study targeted the putamen-motor cortical loop and provides the first proof-of-principle demonstration. It is an empirical question whether other loops can be modulated as effectively. If so, then this approach could have interesting therapeutic potential. For example, in Parkinson's disease, where patients suffer motor and cognitive deficits caused by dopamine loss in the basal ganglia, dopamine agonists can restore motor and some forms of cognitive control. However, these drugs are systemic and lack specificity, such that improvements in some functions are accompanied by impairment of other functions associated with other cortico-striatal loops that are overdosed by dopaminergic drugs (Cools, 2006). Hence, a noninvasive intervention, such as the current TMS protocol, is of in-principle theoretical interest, since it demonstrates the feasibility of intervening selectively to alter functioning within a specific cortico-striatal circuit without unwanted side effects in adjacent loops.
In summary, the present study confirmed the hypothesis that striatal functional signals associated with cognitive flexibility are under topographic frontal cortical control. Cortical TMS can be used to manipulate subcortical cognitive functions in a functionally and topographically specific manner.
Footnotes
R.C. was supported by a Royal Society University Research Fellowship and is currently supported by a VIDI grant from the Innovational Research Incentives Scheme of the Netherlands Organisation for Scientific Research (NWO), and a Human Frontiers Science Program research grant (to Kae Nakamura, R.C., and Nathaniel Daw). J.O'S. is supported by a Royal Society Dorothy Hodgkin Research Fellowship and Research Grant. R.B.M. and M.F.S.R. are supported by the Medical Research Council UK (G0802146).
References
- Aarts E, Roelofs A, Franke B, Rijpkema M, Fernández G, Helmich RC, Cools R. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35:1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox [abstract]. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6; Sendai, Japan. 2002. [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-u-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Robbins TW. Chemistry of the adaptive mind. Philos Transact A Math Phys Eng Sci. 2004;362:2871–2888. doi: 10.1098/rsta.2004.1468. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson's disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Robbins TW. Differential responses in human striatum and prefrontal cortex to changes in object and rule relevance. J Neurosci. 2004;24:1129–1135. doi: 10.1523/JNEUROSCI.4312-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Ivry RB, D'Esposito M. The human striatum is necessary for responding to changes in stimulus relevance. J Cogn Neurosci. 2006;18:1973–1983. doi: 10.1162/jocn.2006.18.12.1973. [DOI] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–459. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Ko JH, Monchi O, Ptito A, Bloomfield P, Houle S, Strafella AP. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task: a TMS-[(11)C]raclopride PET study. Eur J Neurosci. 2008;28:2147–2155. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88:195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Leber AB, Turk-Browne NB, Chun MM. Neural predictors of moment-to-moment fluctuations in cognitive flexibility. Proc Natl Acad Sci U S A. 2008;105:13592–13597. doi: 10.1073/pnas.0805423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology (Berl) 2004;176:331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28:3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea J, Johansen-Berg H, Trief D, Göbel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007a;54:479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Sebastian C, Boorman ED, Johansen-Berg H, Rushworth MF. Functional specificity of human premotor-motor cortical interactions during action selection. Eur J Neurosci. 2007b;26:2085–2095. doi: 10.1111/j.1460-9568.2007.05795.x. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–272. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Andrews TC, Grasby PM, Brooks DJ, Robbins TW. Contrasting cortical and subcortical activations produced by attentional-set shifting and reversal learning in humans. J Cogn Neurosci. 2000;12:142–162. doi: 10.1162/089892900561931. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Bestmann S, Blankenburg F, Bjoertomt O, Josephs O, Weiskopf N, Deichmann R, Driver J. Distinct causal influences of parietal versus frontal areas on human visual cortex: evidence from concurrent TMS–fMRI. Cereb Cortex. 2008;18:817–827. doi: 10.1093/cercor/bhm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DE, Dechent P, Goebel R, Baudewig J. Imaging the brain activity changes underlying impaired visuospatial judgments: simultaneous fMRI, TMS, and behavioral studies. Cereb Cortex. 2007;17:2841–2852. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci. 2010;30:14205–14212. doi: 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Fraraccio M, Dagher A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain. 2003;126:2609–2615. doi: 10.1093/brain/awg268. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Ko JH, Grant J, Fraraccio M, Monchi O. Corticostriatal functional interactions in Parkinson's disease: a rTMS/[C-11]raclopride PET study. Eur J Neurosci. 2005;22:2946–2952. doi: 10.1111/j.1460-9568.2005.04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Tokuno H, Nambu A, Inase M. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res. 1998;120:114–128. doi: 10.1007/s002210050384. [DOI] [PubMed] [Google Scholar]
- van Holstein M, Aarts E, van der Schaaf ME, Geurts DE, Verkes RJ, Franke B, van Schouwenburg MR, Cools R. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology (Berl) 2011;218:567–578. doi: 10.1007/s00213-011-2340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg MR, den Ouden HE, Cools R. The human basal ganglia modulate frontal-posterior connectivity during attention shifting. J Neurosci. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vytlacil JJ, Nomura EM, Gibbs SE, D'Esposito M. The dopamine agonist bromocriptine differentially affects fronto-striatal functional connectivity during working memory. Front Hum Neurosci. 2011;5:32. doi: 10.3389/fnhum.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120(Pt. 1):141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]