Abstract
Peripheral nerve hyperexcitability (PNH) is one of the distal peripheral neuropathy phenotypes often present in patients affected by type 2 diabetes mellitus (T2DM). Through in vivo and ex vivo electrophysiological recordings in db/db mice, a model of T2DM, we observed that, in addition to reduced nerve conduction velocity, db/db mice also develop PNH. By using pharmacological inhibitors, we demonstrated that the PNH is mediated by the decreased activity of Kv1-channels. In agreement with these data, we observed that the diabetic condition led to a reduced presence of the Kv1.2-subunits in juxtaparanodal regions of peripheral nerves in db/db mice and in nerve biopsies from T2DM patients. Together, these observations indicate that the T2DM condition leads to potassium channel-mediated PNH, thus identifying them as a potential drug target to treat some of the DPN related symptoms.
Introduction
Diabetic peripheral neuropathy (DPN) is characterized by either “positive” (paresthesia, dysesthesia, allodynia, cramps, fasciculations) and/or “negative” (hypesthesia, anesthesia, tiredness, muscle weakness) symptoms (Quasthoff, 1998). Previous studies suggested that changes in neuronal ion channel expression and function may contribute to DPN symptoms (Quasthoff, 1998; Misawa et al., 2005, 2009). In myelinated axons, ion channels are localized at specific spatially restricted domains (Salzer, 2003). Sodium channels (Nav), predominantly containing the α-subunit Nav1.6, are clustered at the node of Ranvier and play a critical role in the conduction of the action potential. The juxtaparanodal region is enriched in voltage-gated Shaker-like potassium channels (Kv1) that are responsible for the fast potassium conductance in axons. In addition, nodal Kv7 channels were recently shown to mediate the slow axonal potassium conductance (Schwarz et al., 2006). While previous clinical electrophysiological studies suggested that peripheral nerve hyperexcitability (PNH), which is part of the distal peripheral neuropathy phenotype present in type 2 diabetes mellitus (T2DM), is a consequence of alterations in voltage-gated channels (Misawa et al., 2005, 2009), more direct evidence is lacking.
Approximately 90% of all diabetic patients suffer from T2DM (Nolan et al., 2011). We therefore decided to get more insight into the DPN associated with this form of diabetes by studying a rodent model of T2DM, the db/db mice (Hummel et al., 1966). Our characterization of db/db animals by electrophysiological recordings revealed the presence of PNH as part of their DPN phenotype. We observed that the altered Kv1-channel function contributes to the PNH phenotype in db/db animals and that these functional changes are paralleled by altered distribution of the juxtaparanodal Kv1.2-subunit in peripheral nerves of db/db mice and in nerve biopsies from T2DM patients indicating the clinical relevance of our observations.
Materials and Methods
Animals.
Db/db breeding pairs were obtained from Janvier, France [B6.BKS(D)-Leprdb/J; Stock Number: 000697] and the generated animals were genotyped as previously described (http://jaxmice.jax.org/strain/000642.html). All animals were housed in a controlled environment with a 12 h light/12 h dark cycle and free access to water and standard laboratory diet. Experiments were performed in accordance with the legal requirements of the University of Lausanne and the Canton of Vaud.
Only male mice were used in this study. Tail vein blood glucose was determined with a glucometer Ascencia Contour (Bayer). Plasma insulin levels were measured by using the Rat/Mouse Insulin ELISA Kit from Millipore (catalog #EZRMI-13K) according to the manufacturer's protocol.
Human biopsies.
All donors gave a written consent for the biopsy. Biopsies of the peroneal nerve and adjacent muscle were performed under local anesthesia, fixed in 3.6% glutaraldehyde and embedded in paraffin for routine analysis, or were embedded in plastic for semithin and ultrathin sectioning, analyzed by light and electron microscopy respectively. Paraffin sections were stained with hematoxylin-eosin and Masson's trichrome. Semithin sections were stained with thionine blue.
Electrophysiology.
Nerve conduction velocity recordings and ex vivo compound action potential (CAP) recordings have been performed as previously described (Cartoni et al., 2010; de Preux Charles et al., 2010). For pharmacological analysis, the isolated nerves were exposed to the drugs between 30 min and 1 h until the effects seemed stable. Tetrodotoxin (TTX) was purchased from Enzo Life Sciences, tetraethylammonium (TEA) and 4-aminopyridine (4-AP) from Sigma, and flupirtine from Tocris Bioscience. All other chemicals were purchased from VWR.
Immunohistochemistry.
Mouse tissues were processed as described previously (Arnaud et al., 2009). Twenty-micrometer-thick sciatic nerve sections were prepared and fixed with Zamboni's fixative for 15 min at room temperature (RT). For immunostainings, the following primary antibodies were used: Kv1.2 (1:200; NeuroMab, K14/16), pan-Nav (1:100; Sigma, SP19 S6936), Kvβ2 (1:100, Alomone, APC-117), Kv1.1 (1:100; Alomone, APC-009) and MBP (1:100, Millipore Bioscience Research Reagents, MAB386) with the appropriate fluorescent secondary antibodies (Alexa Fluor 594 or 488 conjugated anti-rabbit, anti-mouse or anti-rat at a dilution of 1:200; Invitrogen). Nile Red staining on teased fibers was performed as previously described (Arnaud et al., 2009).
Quantitative PCR.
RNA extraction, reverse transcription and qPCR conditions have been performed as previously described (Arnaud et al., 2009). The primers used were as follows: forward 5′-CTGGTACCCATCTGCAAG-3′, reverse 5′-GTGTGCTCTAGGACTGGATG-3′ for Kv1.2; forward 5′-AAGGACGGGAAACGCGAGGG-3′, reverse 5′-ATCGATGGACGCTGGCGGG-3′ for Kv1.1; forward 5′-AGACAGGCTCCCCCGGGATG-3′, reverse 5′-CATGGCCCGCACGGTCTCTTC-3′ for Kvβ2; forward 5′-ACACTAGTGGAAGAGCTGGA-3′, reverse 5′-ACGATCAGGTTCACAATCTC-3′ for Nav1.6; forward 5′-TTCACAAGTCTTCTAAGGACTCCTCG-3′, reverse 5′-GCACTGGCGTCTGCCG-3′ for MPZ and forward 5′-TTGCTCTTCGTCTCCACCATC-3′, reverse 5′-TCGTGTGTCCATTGCCCAC-3′ for PMP22. Results were normalized by using the reference gene β-actin (forward primer: 5′-GCCCTGAGGCTCTTTTCCAG-3′; reverse primer: 5′-TGCCACAGGATTCCATACCC-3′).
Western blot analysis.
Sciatic nerve endoneurium and dorsal root ganglia (DRG) isolated from mice at 23 weeks of age from at least three animals per genotype were pooled and lysed in ice-cold lysis buffer (20 mm Na2H2PO4, 250 mm NaCl, 1% Triton X-100, SDS 0.1%) supplemented with Complete protease inhibitors (Roche). After spin down for 15 min at 4°C, proteins were extracted from the supernatant. Protein levels were quantified using the Bio-Rad protein assay with BSA as a standard. Equal amounts of protein extracts were boiled for 5 min at 95°C and were then resolved by 12% SDS-PAGE and electro-transferred onto a polyvinylidene difluoride membrane (GE Healthcare). Membrane was blocked in Tris-buffered saline containing 0.1% Tween (TBS-T) supplemented with 5% milk powder for 1 h at RT, and subsequently incubated overnight at 4°C in the same buffer supplemented with antibodies against Kv1.2 (1:500; NeuroMab, K14/16), and standardized using β-Actin (1:2000; Sigma, A5316) or α/β-Tubulin (1:1000, Cell Signaling Technology, #2148). After washing in TBS-T, blots were exposed to the appropriate horseradish peroxidase-conjugated secondary antibodies (Dako) in the blocking buffer for 1 h at RT. Finally, the blots were developed using ECL reagents (Pierce) and Kodak Scientific Imaging Films (Kodak).
Morphometric analysis.
Morphometric analysis, including the g-ratio calculation, was performed as previously described (Arnaud et al., 2009).
Statistics.
All data are presented as mean ± SEM. P values have been calculated with the Student's t test.
Results
Our characterization of db/db mice revealed an increase in body weight and blood glucose levels at 6 weeks of age. To study the consequences of T2DM on peripheral nerve function, we exposed the db/db mice to hyperglycemia for 16 weeks (this, based on the estimated lifespan, represents ∼10 years in humans). Therefore, we performed all physiological analysis using 23-week-old mice. At this age, db/db mice were severely obese and hyperglycemic. The initial insulin resistance was followed by a β-cell dysfunction leading to a decline in the plasma insulin levels in db/db mice. Nerve conduction measurements on the sciatic nerve, containing both motor and sensory fibers, revealed a significantly reduced motor nerve conduction velocity (from 40.15 ± 3.49 m/s−1 in wild-type (WT) to 30.02 ± 4.46 m/s−1 in db/db mice, p < 0.05) and we also detected a reduction in the tail sensory nerve conduction velocity (27.32 ± 2.48 m/s−1 in WT; 18.62 ± 2.44 m/s−1 in db/db mice, p < 0.05). Interestingly, the stimulus threshold to initiate a first response during the motor nerve conduction studies was significantly lower in db/db mice compared with WT mice (p < 0.0003), indicating hyperexcitability of db/db nerves (Fig. 1a). However, the area and amplitude of the compound muscle action potential were not altered in db/db mice compared with WT mice at 23 weeks of age (data not shown).
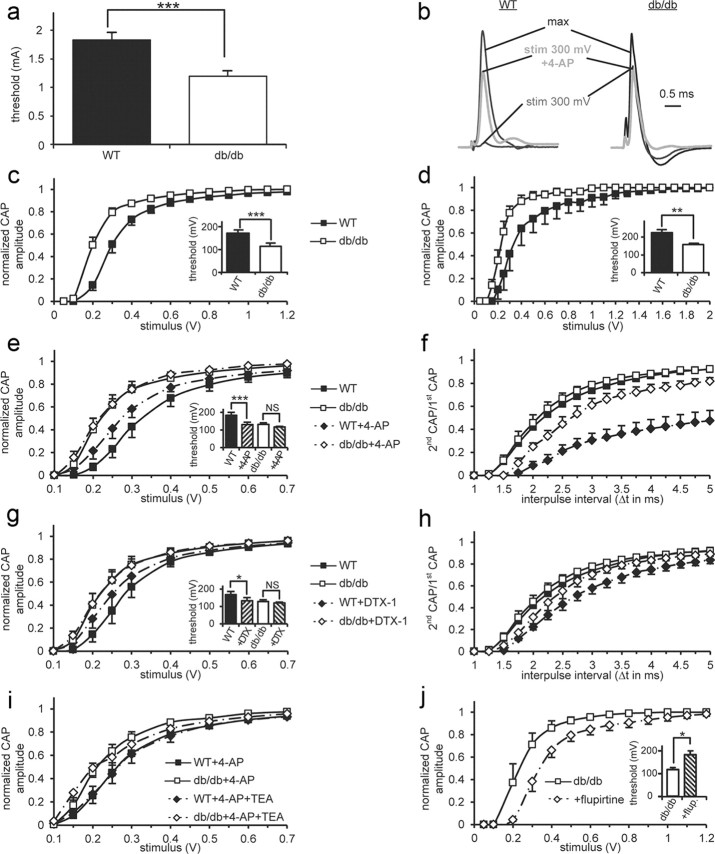
Figure 1.
Hyperexcitability of db/db peripheral nerves is mediated by decreased Kv1-channel function. a, In vivo measurements of the threshold stimulus to induce a first compound muscle action potential on sciatic nerves. The threshold is significantly decreased in db/db (n = 20) compared with WT (n = 20) mice. b–j, Data obtained by performing ex vivo recordings of compound action potentials (CAPs) on isolated mouse sciatic nerves. All except d are related to Aαβ CAPs. b, Typical traces of CAPs of WT and db/db nerves at different stimulus strengths (300 mV or the one to get the maximal amplitude) in presence or absence of 4-AP. The amplitudes of the traces are normalized to get the same maximum amplitude in WT and db/db nerves. c, Aαβ CAP amplitude of WT (n = 11) and db/db (n = 12) nerves normalized by the maximal amplitude as a function of the stimulus strength. We observed a leftward shift of the db/db curve corresponding to peripheral nerve hyperexcitability (PNH). Inset: a significantly reduced threshold in db/db nerves confirms PNH. d, Aδ CAP amplitude/stimulus strength curves. As for larger Aαβ fibers, thinly myelinated Aδ sensory fibers are more excitable in db/db (n = 5) compared with WT (n = 7) nerves. Inset: the threshold to generate an Aδ CAP is also significantly decreased in db/db nerves. e, 4-AP (500 μm), a blocker of Kv1-channels, induces hyperexcitability in WT (n = 6) but not in db/db (n = 6) nerves, suggesting decreased function of these juxtaparanodal potassium channels. f, Effect of 4-AP on Aαβ CAP refractory period (RP). The RP is represented as the ratio of the second CAP on the first CAP amplitude (second CAP/first CAP) as a function of the interpulse interval (Δt). 4-AP increases RP significantly more in WT (n = 6) than in db/db (n = 6) nerves confirming a decrease in Kv1-channel function. g, DTX-1, a toxin specifically blocking Kv1-channels, has a similar effect than 4-AP on db/db and WT nerve excitability (n = 6). h, Effect of DTX-1 on RP. As for 4-AP, WT RP (n = 6) is more increased by DTX-1 than db/db RP (n = 6). i, Effect of coexposure of TEA (10 mm) with 4-AP (500 μm) on nerve excitability. TEA, which blocks nodal Kv7-channels, does not induce any further increase in excitability in WT (n = 5) or in db/db (n = 4) nerves. Therefore, a change in Kv7-channels is unlikely to be responsible for PNH in db/db nerves. j, Flupirtine (10 μm), an activator of Kv7-channels, significantly decreases db/db nerve excitability (n = 5) thus reversing PNH as shown by the curves. Inset: threshold increases back to WT values. Error bars indicate SEM. *p < 0.05; **p < 0.01; ***p < 0.001. Legends for c–d, e–f, and g–h are shown between them.
We extended our above mentioned in vivo electrophysiological characterization by ex vivo CAP recordings (Stys et al., 1991) on sciatic nerves of 23-week-old mice. The normalized Aαβ CAP amplitude/stimulus strength curves confirmed the presence of an increased excitability in db/db nerves (Fig. 1b,c). The threshold (Fig. 1c, see inset) and the stimulus to get 50% of the maximal CAP amplitude (stim1/2) were significantly reduced (p < 0.001). We observed similar results with thinly myelinated Aδ sensory fibers (Fig. 1d).
A decrease of fast Kv1-currents in several disease situations was previously described to induce PNH (Arimura et al., 2002; Tomlinson et al., 2010; Shibuya et al., 2011). Therefore, we evaluated the contribution of Kv1-channels to PNH in T2DM by exposing isolated sciatic nerves to 4-AP (500 μm) and DTX-1 (100 nm), which were previously used to block these channels in axons (Röper and Schwarz, 1989; Vabnick et al., 1999). As expected, in WT nerves, Kv1-channel block with 4-AP (Fig. 1b,e) induced hyperexcitability. The threshold (Fig. 1e, see inset) and stim1/2 were significantly reduced (p < 0.001). Interestingly, db/db nerve excitability was only barely affected by 4-AP (Fig. 1b,e). Moreover, we observed a similar lack of 4-AP effect on db/db refractory period (RP; Fig. 1f). DTX-1 changed nerve excitability and RP of WT and db/db nerves in a similar way but was less potent than 4-AP (Fig. 1g,h), probably as a consequence of its previously described more limited diffusion to juxtaparanodal channels (Devaux and Gow, 2008). Together these results suggest a decreased function of Kv1-channels in db/db mice that could be responsible for the observed PNH.
We evaluated the possibility that other ion channels might be implicated in PNH. Previous studies suggested that PNH in diabetic patients could be a consequence of increased persistent nodal Nav-currents (Misawa et al., 2009). As these currents are mainly mediated by NaV1.9 TTX-resistant (TTX-R) Nav-channels (Matsutomi et al., 2006), we tested the sensitivity of CAPs to TTX. The residual CAP amplitude was similar in WT and db/db nerves after exposure to 100 nm TTX (respectively 38 ± 2% and 37 ± 6%) and 1 μm TTX (<2% for both tested genotypes). As mutations affecting nodal Kv7.2 lead to PNH (Taylor et al., 1992; Maljevic et al., 2008), we studied a potential role of these channels in the observed PNH. We evaluated whether TEA (10 mm), which blocks slow potassium currents in axons (Röper and Schwarz, 1989), would in presence of 4-AP induce a further increase in the excitability of WT nerves reaching similar values as observed in db/db nerves. Excitability was not significantly affected by coexposure of 4-AP and TEA either in WT or in db/db nerves (Fig. 1i). Therefore, Kv7- and TTX-R Nav-channels are unlikely key players in the PNH observed in db/db mice.
We tested whether the PNH could be reversed by modulation of potentially preserved ion channels present in db/db nerves. Flupirtine (10 μm), an analgesic activating Kv7-channels, suppressed PNH in db/db nerves as revealed by the increased threshold and stim1/2 (p < 0.05, Fig. 1j).
Altered channel localization may explain the decreased function of Kv1-channels and the consequent PNH phenotype observed in 23-week-old db/db mice (Devaux, 2010; Shibuya et al., 2011). Therefore, we characterized the Kv1-channel distribution along peripheral axons. Teased fibers of sciatic nerves from 5- and 23-week-old WT mice coimmunostained for Kv1.2 and pan-Nav showed typical accumulation of nodal Nav-subunits and specific localization of juxtaparanodal Kv1.2-subunits (Fig. 2a). The same expression pattern was detected in db/db teased fibers at 5 weeks of age before the onset of T2DM and DPN (Fig. 2a). Interestingly, we observed a strong reduction of juxtaparanodal Kv1.2-subunits in db/db teased fibers at 23 weeks of age, whereas Nav-clusters were well preserved (Fig. 2a). Quantification revealed that 80% of all analyzed nodes present a reduced or absent Kv1.2 signal in db/db mice (Fig. 2a). Moreover, internodal localization of Kv1.2 was also affected in db/db fibers (Fig. 2b). However, other Kv1-channel subunits, Kv1.1 and Kvβ2, were not affected in db/db mice compared with WT mice and were normally distributed even at juxtaparanodes with reduced or absent Kv1.2 (Fig. 2c).
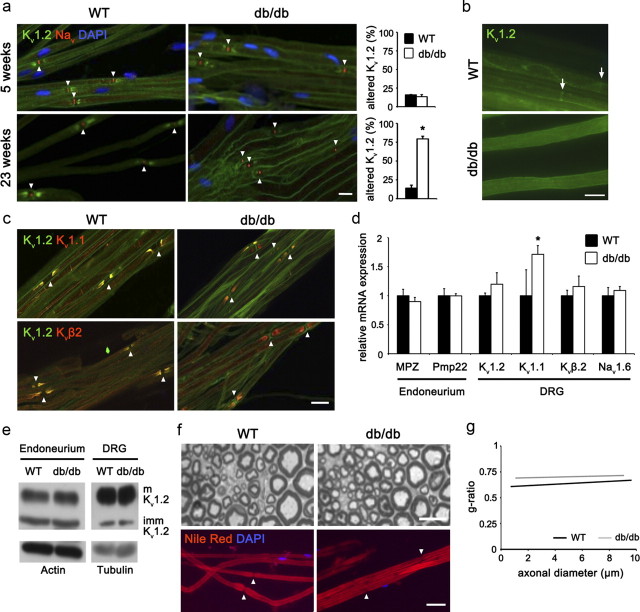
Figure 2.
Kv1.2 distribution is altered in db/db mice. a, At 5 weeks of age, before the onset of T2DM and DPN in db/db mice, nodal Nav-channels and juxtaparanodal Kv1.2-subunits are correctly clustered in teased fibers from both WT (n = 2) and db/db mice (n = 3). At 23 weeks of age, WT mice (n = 6) show the typical expression pattern of Kv1.2 and Nav but in db/db teased fibers (n = 6) the Kv1.2 expression was significantly reduced. However, the nodal Nav-clusters remained unaffected. Bar graphs on the right illustrate the percentage of juxtaparanodes with reduced or absent Kv1.2 signal of 100 counted nodes. Arrowheads present in a, c, f mark the localization of nodal regions. b, Internodal Kv1.2 localization is altered in 23-week-old db/db mice compared with age-matched WT mice. Schmidt-Lanterman incisures apposing localization is indicated by arrows. c, At 23 weeks of age, all juxtaparanodal regions show colocalization of Kv1.2 and Kv1.1 or Kvβ2 respectively in WT mice (n = 3). While also in db/db mice (n = 3) Kv1.1 or Kvβ2 are appropriately positioned in all juxtaparanodal regions, Kv1.2 is lost in some of the regions (red staining). d, qPCR data showing that, except for a mild increase of Kv1.1, all ion channel subunits are normally expressed in db/db compared with WT DRGs (n = 3). The peripheral myelin genes MPZ and Pmp22 show no difference in their expression level in db/db and WT sciatic nerve endoneurium. e, Kv1.2 protein expression of both mature and immature isoforms is not reduced in db/db DRGs and sciatic nerve endoneurium compared with WT levels (n = 3). f, Toluidine blue-stained semithin cross sections and Nile red-stained teased fibers of WT (n = 3) and db/db (n = 3) sciatic nerves show that 16 weeks of hyperglycemia has no detectable effect on the axonal and myelin structures. g, The g-ratio corroborates this result (n = 3). Scale bars in a, c, f: 20 μm; in b: 8 μm. Error bars indicate SD; *p < 0.05.
Defects in multiple molecular mechanisms can lead to the observed altered distribution of Kv1.2-channels and we evaluated some of them. We tested for potential changes in expression of ion channel subunits in DRG and sciatic nerve endoneurium of 23-week-old mice since it was previously shown that the alteration in the distribution of Kv1.2 can be due to their decreased expression in the neurons (Shibuya et al., 2011). In DRG, we observed no differences in the mRNA expression levels of Kv1.2, Kvβ2 and Nav1.6 (the major subunit of nodal Nav-channels) while Kv1.1 showed a slight upregulation (Fig. 2d). Moreover, the protein expression of the mature (glycosylated) and immature isoforms of Kv1.2 in DRG and in sciatic nerve endoneurium was similar between db/db and WT mice (Fig. 2e) indicating that Kv1.2 is normally expressed and transported to the axon. Demyelination is also known to affect juxtaparanodal Kv1-channel clustering (Arroyo et al., 2004). We analyzed the myelin morphology of db/db mice at 23 weeks of age. Semithin cross sections (Fig. 2f) and g-ratio [ratio between axonal area and total (axonal + myelin) area] measurements (Fig. 2g) as well as qPCR analysis (Fig. 2d) for two important peripheral myelin genes (MPZ and Pmp22) failed to reveal any demyelination in db/db mice. Moreover, no segmental demyelination was observed by Nile Red stainings on teased fibers of db/db mice compared with WT mice (Fig. 2f). Together these observations indicate that, most probably, the Kv1.2-channel anchoring into the axolemma may be affected under diabetic condition.
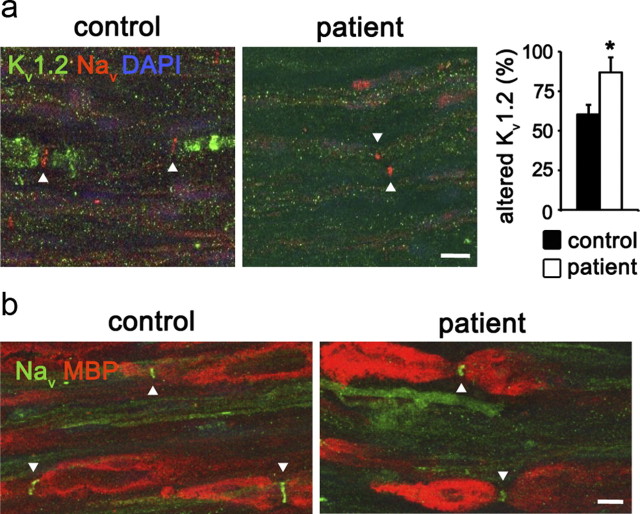
To assess the clinical relevance of our data, we evaluated the channel distribution in nerve biopsies from T2DM patients with a predominantly axonal form of DPN (Table 1 and Fig. 3a). Coimmunostainings of Kv1.2 and pan-Nav on longitudinal sections of the peroneal nerve, a major branch of the mixed sciatic nerve, of control subjects revealed the typical accumulation of nodal Nav-channels and juxtaparanodal Kv1-channels. Similar to the phenotype of db/db mice, we observed a significant reduction of juxtaparanodal Kv1.2-subunits in all analyzed T2DM patients (Fig. 3a) while nodal Nav-clusters were well preserved. Eighty-seven percent of the counted nodes had an impaired or absent Kv1.2 signal in T2DM patients (Fig. 3a). Although all T2DM patients presented a loss of myelinated fibers (Table 1), none of them showed obvious signs of segmental demyelination (Fig. 3b).
Table 1.
Clinical profile of diabetic and control subjects
| Patient | Sex | Age (y) | Age of onset of T2DM (y) | Neuropathy | Symptoms | Nerve biopsy |
|---|---|---|---|---|---|---|
| 08N01013 | M | 52 | Unaffected by T2DM | — | Neurogenic muscular atrophy, sensory defects until knee, squeezed lateral cutaneous nerve from thigh to spinal cord | Normal |
| 09N01460 | F | 63 | Unaffected by T2DM | — | Chronic neurogenic muscular atrophy, walking/balance problems since 2 years, progressive, sensory defects | Normal |
| 10N00090 | M | 51 | Unaffected by T2DM | Impairment of L5-S1 (bilateral) | Muscular atrophy, disease of vertebral discs, pain, motor and sensory defects | Normal, rare regeneration |
| 08N00894 | M | 57 | 54 | Axonal | Fast progressive motor defects, sensory defects in lower limbs, amyotrophy | Loss of medium/small size myelinated fibers as well as unmyelinated fibers, WD, infiltration of immune cells |
| 09N01529 | F | 72 | 58 | Axonal | Progressive motor/sensory defects since 4 years, hypoesthesia, reduced balance/vibration sense | Loss of medium/small size myelinated fibers as well as unmyelinated fibers, signs of WD and microangiopathy |
| 10N00300 | M | 41 | n.d. | Axonal and demyelinating | Paresthesia of lower limbs since 6 months, sensory defects of all four limbs | Moderate loss of large myelinated fibers, WD and edema, inflammatory processes, diabetic microangiopathy |
WD, Wallerian degeneration; n.d., not determined; —, not detected.
Figure 3.
Altered Kv1.2 distribution in peripheral nerves of diabetic patients. a, Longitudinal sections of peroneal nerve biopsies from T2DM (n = 3) and control subjects (n = 3) were immunostained for Nav and Kv1.2. In control subjects, Nav-channels accumulate at the node of Ranvier and Kv1.2-channels at the juxtaparanode. Interestingly, as observed in db/db mice, T2DM patients show a striking loss of juxtaparanodal Kv1.2-channels. The bar graph on the right represents the percentage of juxtaparanodal regions with reduced or abnormal Kv1.2 distribution (100 nodes per controls and subjects were counted). b, Immunostaining for MBP and Nav on human biopsies did not reveal any presence of segmental demyelination in diabetic subjects. Arrowheads mark the localization of nodal regions. Scale bars: 10 μm. Error bars indicate SD; *p < 0.05.
Discussion
Similar to previously published data (Hummel et al., 1966; Herberg and Coleman, 1977; Robertson and Sima, 1980) we observed a relatively early onset of T2DM in db/db mice. This diabetic phenotype was progressive and accompanied by DPN. Importantly, we have identified, for the first time, the presence of PNH in db/db mice thus validating it as a model to characterize this part of the DPN phenotype. Similar to the situation in diabetic patients where PNH is thought to be responsible of the positive symptoms (Quasthoff, 1998; Misawa et al., 2005, 2009), it could explain allodynia in db/db mice (Cheng et al., 2009).
Using ex vivo CAP recordings, we have clearly demonstrated that a reduced activity of Kv1-channels was involved in the PNH phenotype of db/db mice. A role of other channels (e.g., Kv7.2 or NaV-channels) is unlikely critical but cannot be completely ruled out. Kv1-channels are clustered at the juxtaparanodal regions and along the internodes of myelinated peripheral axons (Salzer, 2003). It was previously observed that paranodal/juxtaparanodal potassium binding sites are reduced in nerves from diabetic patients (Seneviratne and Weerasuriya, 1974). We have specifically shown that the Kv1.2 distribution is altered in both db/db mice and in T2DM nerve biopsies thus providing, to our knowledge, the first direct demonstration of such an alteration in axonal ion channels in diabetic patients. Importantly, previous clinical studies already demonstrated that alterations in Kv1-channel function may lead to PNH or myokymia in different pathological situations (Arimura et al., 2002; Tomlinson et al., 2010; Shibuya et al., 2011).
We excluded a reduction of Kv1.2 expression in the soma of neurons as the cause of the decreased Kv1.2 signal in axons. Furthermore, we did not observe any accumulation of Kv1.2 subunits in DRG of db/db mice and its expression (as detected by Western blot) was normal in sciatic nerve endoneurium suggesting that axonal targeting of Kv1.2 is not responsible for the phenotype. We also clearly excluded a demyelinating phenotype in db/db and human nerves as being the cause of the altered Kv1.2 distribution. Interestingly, it was previously observed that the absence of Caspr2 or TAG-1, two adhesion molecules responsible for the juxtaparanodal targeting of Kv1.2, lead to redistribution of Kv1.2-channels to the internodal regions (Poliak et al., 2003). However, in db/db mice even the internodal Kv1.2 localization is affected suggesting that the diabetic condition leads to a diffuse Kv1.2 distribution along the axolemma. As opposed to most ion channel subunits which possess intrinsic targeting motifs, both the membrane targeting and TAG1-mediated clustering of Kv1.2 is mainly dependent on its phosphorylation status (Gu and Gu, 2011) which might be affected under diabetic condition. Further experiments are therefore needed to clarify the mechanisms underlying the observed Kv1.2 mislocalization.
Our data indicate at least two different approaches how to improve DPN condition (particularly PNH) for which there are no effective therapies. First, one may try to enhance the targeting of Kv1.2 to its juxtaparanodal and internodal locations thus improving the hypersensitivity phenotype. Alternatively, our expression and pharmacology data generated in db/db mice showed that there are multiple ion channel-types (including Nav1.6, Kv1.1, Kv7.2 and Kv7.3) that are well preserved in the diabetic peripheral nerve. Both the inhibition of Nav-channels and the activation of preserved Kv-channels might alleviate the PNH phenotype. We validated the clinical potential of the second approach by suppressing PNH in db/db nerves with the Kv7-activator flupirtine. Interestingly, flupirtine, which is an analgesic, has been shown previously to suppress PNH resulting from exposure of rat and human peripheral nerves to oxaliplatin (Sittl et al., 2010).
In conclusion, we demonstrated that Kv1-channels, particularly Kv1.2, play a critical role in the PNH phenotype of db/db mice and corroborated these data with the characterization of biopsies from T2DM patients. We anticipate that these results may have direct clinical implications for the development of new treatments against T2DM-related PNH.
Footnotes
This work was supported by a grant from the Swiss National Science Foundation to R.C. (Grant 31003A_135735/1). We thank the Cellular Imaging Facility of the University of Lausanne for help with the confocal microscopy and Dr. Jérome Devaux for helpful discussions.
References
- Arimura K, Sonoda Y, Watanabe O, Nagado T, Kurono A, Tomimitsu H, Otsuka R, Kameyama M, Osame M. Isaacs' syndrome as a potassium channelopathy of the nerve. Muscle Nerve. 2002;(Suppl 11):S55–S58. doi: 10.1002/mus.10148. [DOI] [PubMed] [Google Scholar]
- Arnaud E, Zenker J, de Preux Charles AS, Stendel C, Roos A, Médard JJ, Tricaud N, Kleine H, Luscher B, Weis J, Suter U, Senderek J, Chrast R. SH3TC2/KIAA1985 protein is required for proper myelination and the integrity of the node of Ranvier in the peripheral nervous system. Proc Natl Acad Sci U S A. 2009;106:17528–17533. doi: 10.1073/pnas.0905523106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo EJ, Sirkowski EE, Chitale R, Scherer SS. Acute demyelination disrupts the molecular organization of peripheral nervous system nodes. J Comp Neurol. 2004;479:424–434. doi: 10.1002/cne.20321. [DOI] [PubMed] [Google Scholar]
- Cartoni R, Arnaud E, Médard JJ, Poirot O, Courvoisier DS, Chrast R, Martinou JC. Expression of mitofusin 2(R94Q) in a transgenic mouse leads to Charcot-Marie-Tooth neuropathy type 2A. Brain. 2010;133:1460–1469. doi: 10.1093/brain/awq082. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Dauch JR, Hayes JM, Hong Y, Feldman EL. Nerve growth factor mediates mechanical allodynia in a mouse model of type 2 diabetes. J Neuropathol Exp Neurol. 2009;68:1229–1243. doi: 10.1097/NEN.0b013e3181bef710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Preux Charles AS, Verdier V, Zenker J, Peter B, Médard JJ, Kuntzer T, Beckmann JS, Bergmann S, Chrast R. Global transcriptional programs in peripheral nerve endoneurium and DRG are resistant to the onset of type 1 diabetic neuropathy in Ins2 mice. PLoS One. 2010;5:e10832. doi: 10.1371/journal.pone.0010832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux J, Gow A. Tight junctions potentiate the insulative properties of small CNS myelinated axons. J Cell Biol. 2008;183:909–921. doi: 10.1083/jcb.200808034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ. The C-terminal domain of ssIV-spectrin is crucial for KCNQ2 aggregation and excitability at nodes of Ranvier. J Physiol. 2010;588:4719–4730. doi: 10.1113/jphysiol.2010.196022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Gu Y. Clustering and activity tuning of Kv1 channels in myelinated hippocampal axons. J Biol Chem. 2011;286:25835–25847. doi: 10.1074/jbc.M111.219113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg L, Coleman DL. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism. 1977;26:59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153:1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- Maljevic S, Wuttke TV, Lerche H. Nervous system KV7 disorders: breakdown of a subthreshold brake. J Physiol. 2008;586:1791–1801. doi: 10.1113/jphysiol.2008.150656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsutomi T, Nakamoto C, Zheng T, Kakimura J, Ogata N. Multiple types of Na(+) currents mediate action potential electrogenesis in small neurons of mouse dorsal root ganglia. Pflugers Arch. 2006;453:83–96. doi: 10.1007/s00424-006-0104-3. [DOI] [PubMed] [Google Scholar]
- Misawa S, Kuwabara S, Kanai K, Tamura N, Hiraga A, Nakata M, Ogawara K, Hattori T. Axonal potassium conductance and glycemic control in human diabetic nerves. Clin Neurophysiol. 2005;116:1181–1187. doi: 10.1016/j.clinph.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Misawa S, Sakurai K, Shibuya K, Isose S, Kanai K, Ogino J, Ishikawa K, Kuwabara S. Neuropathic pain is associated with increased nodal persistent Na(+) currents in human diabetic neuropathy. J Peripher Nerv Syst. 2009;14:279–284. doi: 10.1111/j.1529-8027.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quasthoff S. The role of axonal ion conductances in diabetic neuropathy: a review. Muscle Nerve. 1998;21:1246–1255. doi: 10.1002/(sici)1097-4598(199810)21:10<1246::aid-mus2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Robertson DM, Sima AA. Diabetic neuropathy in the mutant mouse [C57BL/ks(db/db)]: a morphometric study. Diabetes. 1980;29:60–67. doi: 10.2337/diab.29.1.60. [DOI] [PubMed] [Google Scholar]
- Röper J, Schwarz JR. Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibres. J Physiol. 1989;416:93–110. doi: 10.1113/jphysiol.1989.sp017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573:17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne KN, Weerasuriya A. Nodal gap substance in diabetic nerve. J Neurol Neurosurg Psychiatry. 1974;37:502–513. doi: 10.1136/jnnp.37.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Misawa S, Arai K, Nakata M, Kanai K, Yoshiyama Y, Ito K, Isose S, Noto Y, Nasu S, Sekiguchi Y, Fujimaki Y, Ohmori S, Kitamura H, Sato Y, Kuwabara S. Markedly reduced axonal potassium channel expression in human sporadic amyotrophic lateral sclerosis: An immunohistochemical study. Exp Neurol. 2011;232:149–153. doi: 10.1016/j.expneurol.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Sittl R, Carr RW, Fleckenstein J, Grafe P. Enhancement of axonal potassium conductance reduces nerve hyperexcitability in an in vitro model of oxaliplatin-induced acute neuropathy. Neurotoxicology. 2010;31:694–700. doi: 10.1016/j.neuro.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Stys PK, Ransom BR, Waxman SG. Compound action potential of nerve recorded by suction electrode: a theoretical and experimental analysis. Brain Res. 1991;546:18–32. doi: 10.1016/0006-8993(91)91154-s. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Burke D, Heywood J. Physiological evidence for a slow K+ conductance in human cutaneous afferents. J Physiol. 1992;453:575–589. doi: 10.1113/jphysiol.1992.sp019245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson SE, Tan SV, Kullmann DM, Griggs RC, Burke D, Hanna MG, Bostock H. Nerve excitability studies characterize Kv1.1 fast potassium channel dysfunction in patients with episodic ataxia type 1. Brain. 2010;133:3530–3540. doi: 10.1093/brain/awq318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabnick I, Trimmer JS, Schwarz TL, Levinson SR, Risal D, Shrager P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J Neurosci. 1999;19:747–758. doi: 10.1523/JNEUROSCI.19-02-00747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]