ABSTRACT
The primary site for KRAS signaling is the inner leaflet of the plasma membrane (PM). We previously reported that oxanthroquinone G01 (G01) inhibited KRAS PM localization and blocked KRAS signaling. In this study, we identified acylpeptide hydrolase (APEH) as a molecular target of G01. APEH formed a stable complex with biotinylated G01, and the enzymatic activity of APEH was inhibited by G01. APEH knockdown caused profound mislocalization of KRAS and reduced clustering of KRAS that remained PM localized. APEH knockdown also disrupted the PM localization of phosphatidylserine (PtdSer), a lipid critical for KRAS PM binding and clustering. The mislocalization of KRAS was fully rescued by ectopic expression of APEH in knockdown cells. APEH knockdown disrupted the endocytic recycling of epidermal growth factor receptor and transferrin receptor, suggesting that abrogation of recycling endosome function was mechanistically linked to the loss of KRAS and PtdSer from the PM. APEH knockdown abrogated RAS–RAF–MAPK signaling in cells expressing the constitutively active (oncogenic) mutant of KRAS (KRASG12V), and selectively inhibited the proliferation of KRAS-transformed pancreatic cancer cells. Taken together, these results identify APEH as a novel drug target for a potential anti-KRAS therapeutic.
KEY WORDS: KRAS, Acylpeptide hydrolase, Recycling endosome, Oxanthroquinone, Plasma membrane, Sphingomyelin metabolism
Summary: APEH is a novel regulator of the plasma membrane localization and function of KRAS. Therefore, APEH is a potential drug target for KRAS-dependent human cancers.
INTRODUCTION
RAS proteins are small GTPases that oscillate between active GTP-bound and inactive GDP-bound states in growth factor signaling pathways to regulate cell growth, proliferation, and differentiation (Hancock, 2003). Four RAS isoforms, KRAS4A and KRAS4B (both encoded by KRAS), as well as HRAS and NRAS, are ubiquitously expressed in human cells. These RAS isoforms contain a near identical G-domain that interacts with a common set of guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) that regulate RAS-GTP levels, as well as a common set of effector proteins (Prior and Hancock, 2001, 2012; Zhou and Hancock, 2015).
To recruit downstream effectors and activate signaling pathways, RAS must localize primarily to the inner leaflet of the plasma membrane (PM) (Hancock, 2003). Each RAS isoform is targeted to the PM by a C-terminal anchor comprising a C-terminal cysteine farnesyl carboxyl-methyl ester, common to all isoforms, acting in concert with a distinct second signal (Hancock et al., 1989). In the case of NRAS and HRAS, this signal is palmitoylation of one or two cysteine residues adjacent to the farnesylated cysteine (Hancock et al., 1989; Laude and Prior, 2008). For KRAS4B (hereafter referred to as KRAS), the signal is a polybasic domain, (Hancock et al., 1990) whereas in KRAS4A there is a combination of a polybasic domain and palmitoylation (Tsai et al., 2015). These different C-terminal anchors allow different biological activities for each RAS isoform (Prior and Hancock, 2001, 2012; Zhou and Hancock, 2015). RAS genes are the most frequently mutated genes in human cancers (Cox and Der, 2010; Cox et al., 2014; Prior and Hancock, 2012); with the mutation locking RAS proteins into a constitutively active GTP-bound state. KRAS mutations are the most prevalent, accounting for ∼85% of all RAS mutations reported (Prior et al., 2012).
Although the RAS C-terminal membrane anchor is required and sufficient for PM binding, spatial organizing systems are also required to actively return RAS to the PM following endocytosis. Thus, HRAS and NRAS are depalmitoylated by a thioesterase and released into the cytosol after endocytosis, and depalmitoylated HRAS and NRAS are transported to the endoplasmic reticulum and Golgi complex for re-palmitoylation, allowing forward transport back to the PM via the exocytic pathway and recycling endosome (RE) (Dekker et al., 2010; Goodwin et al., 2005; Misaki et al., 2010; Rocks et al., 2010, 2005). KRAS dissociates from endocytic vesicles and binds to the cytosolic solubilization factor PDEδ, which prevents interaction with bulk endomembrane. KRAS is released from PDEδ through the action of ARL2 in the vicinity of the RE to allow selective binding to RAB11-positive RE vesicles for transportation back to the PM (Schmick et al., 2015, 2014).
Blocking the localization and spatial organization of RAS on the PM inhibits the biological function of RAS, because RAS must assemble into nanoclusters on the PM for recruiting and activating downstream effectors (Hancock, 2003; Zhou and Hancock, 2015). Farnesyltransferase inhibitors (FTIs) are effective at inhibiting cancers with HRAS mutations, but do not block the PM localization of KRAS and NRAS, because the CAAX box of these RAS proteins can be alternately geranylgeranylated and localize normally to the PM in the presence of FTIs (Cox et al., 2014; Rowinsky, 2006; Sebti and Der, 2003). By contrast, inhibiting components of the RAS spatial organizing systems effectively reduces KRAS PM localization. For example, knockdown of RAB11 or inhibition of PDEδ redistributes KRAS from the PM, by interfering with the endocytic trafficking of KRAS (Schmick et al., 2014; Zimmermann et al., 2013).
The KRAS membrane anchor binds with high affinity to phosphatidylserine (PtdSer), a major component phospholipid of the inner leaflet of the PM (Zhou and Hancock, 2015; Zhou et al., 2017). Reducing the PtdSer content of the PM therefore impairs KRAS PM binding and abrogates KRAS signal output (Zhou and Hancock, 2015; Zhou et al., 2017). Multiple classes of inhibitors of sphingomyelin (SM) metabolism reduce PM PtdSer content and therefore mislocalize KRAS from the PM (Cho et al., 2012, 2016; Maekawa et al., 2016; van der Hoeven et al., 2018). We recently reported that the oxanthroquinone G01, a representative derivative of a novel class of Streptomyces polyketide, oxanthroquinones, caused the mislocalization KRAS by a mechanism that involved perturbation of both RE function and SM metabolism (Tan et al., 2018). In this current study, we report the identification of a molecular target of G01 and reveal a novel biological function of this protein in regulating KRAS PM localization.
RESULTS
G01 binds to and inhibits the enzyme acylpeptide hydrolase
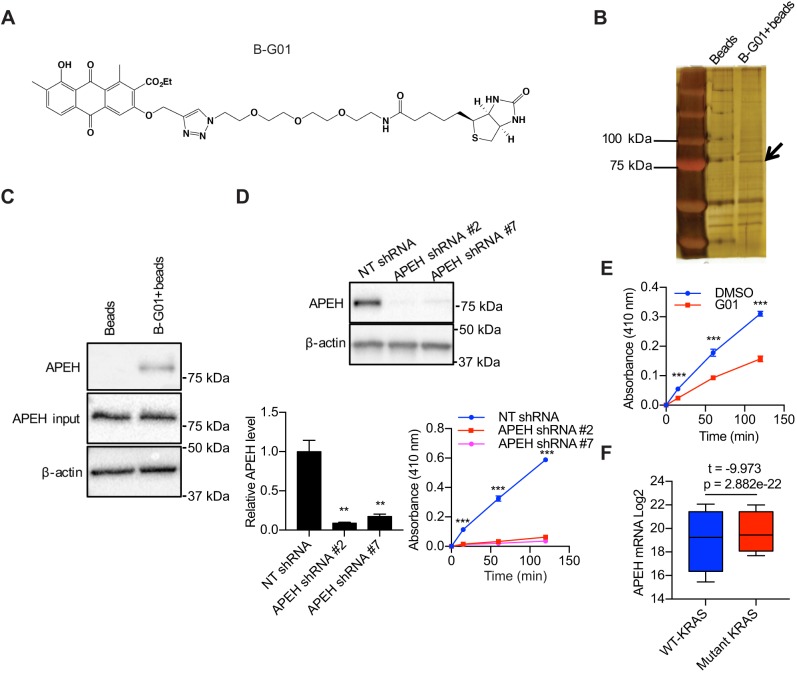
To determine the protein target of G01, a compound named as B-G01 was synthesized, whose structure consists of a biotin and G01 moiety (Fig. 1A). We incubated CaCO2 cell lysate with vehicle (DMSO) or B-G01. B-G01, along with its bound proteins, was pulled down by streptavidin-conjugated beads and analyzed by means of silver staining. A band of ∼75 kDa was detected in the sample incubated with B-G01 but not in the control sample incubated with streptavidin-conjugated beads alone (Fig. 1B). The silver-stained band was excised and identified by mass spectrometry as acylpeptide hydrolase (APEH). Protein identity was then confirmed by immunoblotting (Fig. 1C). APEH, is a prolyl-oligopeptidase that is responsible for catalyzing the removal of Nα-acylated amino acids from peptides and has a general role in protein degradation (Gonen et al., 1994; Hershko et al., 1984; Palmieri et al., 2011; Zeng et al., 2017). APEH is also capable of breaking down oxidized and glycated proteins and has been alternately named oxidized protein hydrolase (Fujino et al., 2000a,b). To biochemically verify this result, we measured the enzymatic activity of APEH by analyzing the release of p-nitroaniline (pNA) from a synthetic peptide, acetyl-alanine-p-nitroaniline (ac-Ala-pNA). Lysates from MDCK cells in which APEH expression was reduced by ∼90% using two different shRNAs exhibited minimal APEH activity compared to control lysates (Fig. 1D). APEH enzymatic activity was also significantly reduced in the G01-treated cell lysate (Fig. 1E). Together, these data are consistent with APEH being the molecular target of G01. Although none of the previously identified functions of APEH seemed related to the PM localization of KRAS, bioinformatics analysis showed that APEH is more highly expressed in human cancers with mutant KRAS than those with wild-type KRAS, which is consistent with a possible role of APEH in KRAS oncogenesis (Fig. 1F). We therefore examined the cell biology of APEH in the context of KRAS in more detail. Further characterization of the mechanism of action of G01 on APEH enzymatic activity was precluded by the lack of commercially available, biochemically active recombinant APEH.
Fig. 1.
APEH is bound and inhibited by G01. (A) Structure of B-G01. (B) Beads collected from cell lysates incubated with or without B-G01 were analyzed by silver staining. The band that was analyzed by mass spectrometry is indicated (arrow). (C) Beads collected from 200 μg cell lysates incubated with or without B-G01 were immunoblotted for APEH. Controls including 20 μg whole-cell lysate (input) were immunoblotted for APEH and β-actin. Representative blots were shown. (D) Lysates of MDCK cells transfected with NT shRNA or APEH shRNAs were blotted for APEH. The level of APEH was normalized to the level of β-actin. Representative western blots are shown. **P<0.01 between APEH knockdown and control APEH levels (one-way ANOVA). Lysates were incubated with 1 mM ac-Ala-pNA for 2 h at 37°C. Absorbance at 410 nm was measured at intervals. ***P<0.001 (one-way ANOVA). Results are mean±s.e.m. (n=3). (E) MDCK cell lysates were incubated with vehicle (DMSO) or 50 μM G01 for 1 h at room temperature and 1 mM ac-Ala-pNA was added. Absorbance at 410 nm was measured following warming to 37°C. Results are mean±s.e.m. (n=3). ***P<0.001 between DMSO- and G01-treated background-subtracted absorbance (Student's t-tests). (F) Box plots indicating quartiles of APEH expression level in patient tissues (n=20,163) of 33 types of cancers with or without KRAS mutants were generated using the UCSC Xena Browser. Whiskers represent upper and lower quartiles. The statistical significance was analyzed with Welch's t-test.
APEH knockdown or inhibitors abolish the localization and nanoclustering of KRAS on the PM
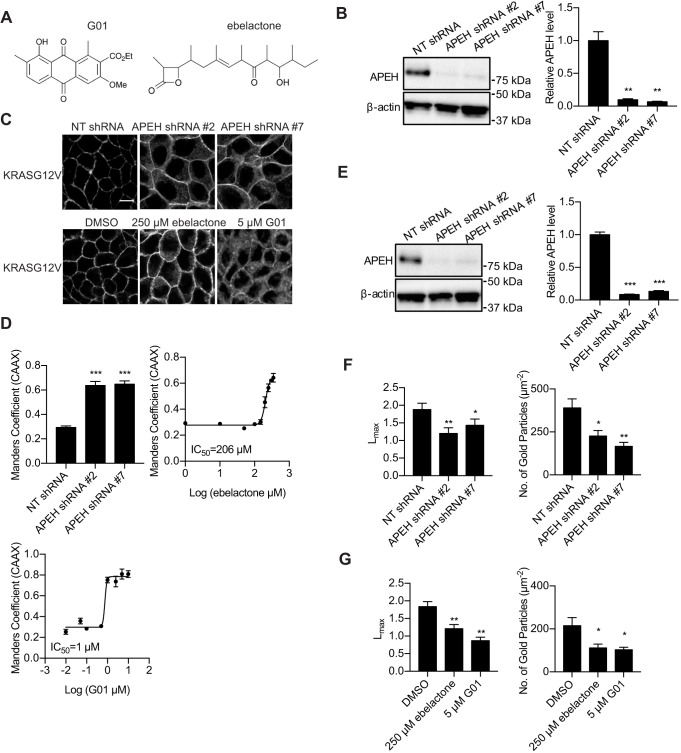
To determine whether downregulation of APEH inhibits PM localization of the constitutively active (oncogenic) mutant of KRAS (KRASG12V), we transfected non-targeting (NT) shRNA or knocked down APEH with APEH shRNAs in MDCK cells stably expressing mGFP-tagged KRASG12V and mCherry-tagged CAAX, an endomembrane marker (Fig. 2B). MDCK cells are well-suited to confocal imaging because the restricted basolateral expression of KRAS in these polarized cells (Fig. S1) facilitates accurate quantification of PM localization. Confocal imaging revealed mislocalization of KRASG12V from the PM in cells transfected with APEH shRNAs, whereas KRASG12V was predominantly localized on the PM in cells transfected with NT shRNA (Fig. 2C). The mislocalization of mGFP–KRASG12V from the PM was quantified by determining Manders’ coefficients (Cho et al., 2012), which evaluate the extent of colocalization between mGFP–KRASG12V and mCherry–CAAX. We observed a significant increase in the Manders’ coefficient in APEH-knockdown cells, indicating a significant mislocalization of KRASG12V from the PM (Fig. 2D). To verify that APEH KD left the KRAS membrane anchor intact, we fractionated KRASG12V expressing APEH-knockdown cells into P100 (membrane) and S100 (cytosolic) fractions. Fig. S2 shows that that ∼80% of KRASG12V was found in the P100 fraction of both control and APEH-knockdown cells. Thus the mislocalization observed in confocal imaging (Fig. 2D) reflects KRAS localization to endomembrane and not the cytosol, as occurs when post-translational processing is abrogated (Hancock et al., 1990). G01 also causes the mislocalization other RAS isoforms (Tan et al., 2018). Consistent with this, we observed a significant mislocalization of HRASG12V and NRASG12V from the PM in APEH-knockdown cells (Fig. S3).
Fig. 2.
APEH inhibition or knockdown inhibits KRASG12V PM localization and nanoclustering. (A) Structure of G01 and ebelactone. (B) MDCK cells stably expressing mGFP–KRASG12V and mCherry–CAAX transfected with NT shRNA or APEH shRNAs were lysed and blotted for APEH. The level of APEH was normalized to the level of β-actin. Representative western blots are shown. Results are mean±s.e.m. (n=3). **P<0.01 between APEH-knockdown and control APEH levels (one-way ANOVA). (C) Cells treated as in B together with MDCK cells stably expressing mGFP–KRASG12V and mCherry–CAAX and treated with ebelactone or G01 for 48 h were imaged in a confocal microscope. Representative images are shown. Scale bar: 10 μm. (D) Upper left, the extent of KRAS mislocalization was quantified by determining the Manders’ coefficients, which evaluate the extent of colocalization of mCherry–CAAX and mGFP–KRASG12V. Results are mean±s.e.m. (n=3). ***P<0.001 between APEH-knockdown and control Manders’ coefficients (one-way ANOVA). Upper right and bottom left, estimated IC50 values for ebelactone or G01 were obtained from the respective Manders’ coefficient (mean±s.e.m.; n=3) dose–response plots. (E) MDCK cells stably expressing mGFP–KRASG12V transfected with NT shRNA or APEH shRNAs were lysed and blotted for APEH. The level of APEH was normalized to the level of β-actin. Representative western blots are shown. Results are mean±s.e.m. (n=3). ***P<0.001 between APEH-knockdown and control APEH levels (one-way ANOVA). (F) Basal PM sheets were generated from cells from E and imaged by EM after incubation with anti-GFP antibody conjugated to 4.5 nm gold particles. Left, the extent of clustering was analyzed with Ripley's K-function expressed as L(r)−r functions, and normalized on the 99% confidence interval (c.i.). Lmax, which is the maximum value of L(r)−r, serves as the summary statistic. Values of Lmax above the c.i. indicate clustering, with the extent of clustering given by the value of Lmax (mean±s.e.m.; n≥12). *P<0.05; **P<0.01 comparing the pattern for control cells with that for APEH knockdown cells (bootstrap tests). Right, gold labeling density on the PM sheets is shown as mean±s.e.m. (n≥12). *P<0.05; **P<0.01 for the difference in gold labeling density (one-way ANOVA). (G) Lmax and gold labeling density calculated as in F of basal PM sheets generated from cells treated with vehicle (DMSO) or ebelactone or G01 for 48 h.
Previous work has identified ebelactone A (hereafter ebelactone) (Fig. 2A) as a commercially available APEH inhibitor (Palmieri et al., 2011; Sandomenico et al., 2012). We therefore treated MDCK cells stably expressing mGFP–KRASG12V and mCherry–CAAX with ebelactone. Similar to the observation in APEH-knockdown cells, mGFP–KRASG12V localization to the PM was inhibited by ebelactone (Fig. 2C) with an IC50 of 206 µM (Fig. 2D), which is similar to the concentration at which ebelactone inhibited APEH and its downstream proteasomal function in previous studies (Palmieri et al., 2011).
RAS nanoclusters are sites of effector recruitment and are critical for the signaling downstream of RAS (Hancock, 2003; Zhou and Hancock, 2015). We therefore determined whether the nanoclustering of KRAS is disrupted by inhibitors or knockdown of APEH. Intact basal PM sheets from MDCK cells expressing mGFP–KRASG12V transfected with NT shRNA or APEH shRNAs (Fig. 2E) were labeled with gold-conjugated anti-GFP antibodies and imaged by electron microscopy (EM). Spatial mapping showed significant decreases in the peak values of the L(r)–r clustering statistic Lmax (Plowman et al., 2005), indicating a decrease of nanoclustered KRASG12V on the PM in APEH-knockdown cells (Fig. 2F). There was also a significant reduction in anti-GFP immunogold labeling of KRASG12V in APEH-knockdown cells, again showing that APEH knockdown depleted KRASG12V from the inner leaflet of the PM (Fig. 2F), in concordance with the results obtained by confocal microscopy (Fig. 2C). Similar effects were observed in ebelactone- or G01-treated cells (Fig. 2G).
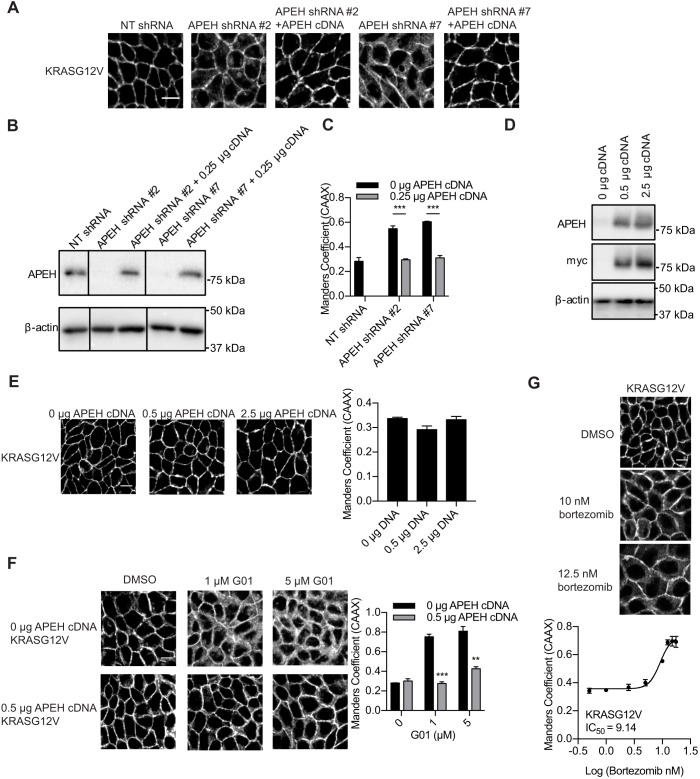
Next, we confirmed that reduced expression of APEH is causative of KRAS mislocalization. MDCK cells stably expressing mGFP–KRASG12V and mCherry–CAAX with or without APEH knockdown were transfected with APEH cDNA, and imaged with confocal microscopy. mGFP-KRASG12V was localized predominantly on the PM in the cells transfected with NT shRNA and mislocalized from the PM in cells transfected with APEH shRNAs (Fig. 3A–C). The PM localization of mGFP–KRASG12V was rescued by the restoration of ectopic APEH expression using exogenous cDNA (Fig. 3A–C). These observations confirmed that the KRAS mislocalization was induced by the knockdown of APEH and not by other off-target effects. Similarly, overexpression of APEH in MDCK cells stably expressing mGFP–KRASG12V and mCherry–CAAX did not affect KRAS cellular localization (Fig. 3D,E), but overexpression of APEH was able to restore PM localization of KRAS in cells treated with 1 or 5 µM G01 (Fig. 3F). Previous studies suggested that APEH regulates the chymotrypsin-like activity of proteasome (Bergamo et al., 2016; Palmieri et al., 2011; Palumbo et al., 2016). Therefore, we tested whether proteasome inhibitors can also mislocalize KRAS from the PM. MDCK cells stably expressing mGFP–KRASG12V and mCherry–CAAX were treated with bortezomib and imaged. Similar to the observation in APEH-knockdown cells, mGFP–KRASG12V localization to the PM was inhibited by bortezomib with an IC50 of ∼10 nM, which is a concentration that effectively blocks proteasomal function (Kelley et al., 2004; Liu et al., 2009; Yu et al., 2003) (Fig. 3G).
Fig. 3.
Expression of exogenous APEH rescues the KRASG12V mislocalization induced by APEH knockdown or G01 treatment. (A) Cells as in Fig. 2B were transfected with 0.25 μg APEH–Myc cDNA and imaged in a confocal microscope. Representative images are shown. (B) Parallel cultures of the cells as in A were analyzed by immunoblotting. Representative blots are shown. (C) The extent of KRAS mislocalization was quantified by determining the Manders’ coefficients. ***P<0.001 (Student's t-test). (D) MDCK cells stably expressing mGFP–KRASG12V and mCherry–CAAX were transfected with 0.5 or 2.5 μg APEH–Myc cDNA and lysed for immunoblotting. Representative blots are shown. (E) Parallel cultures of the cells as in D were imaged in a confocal microscope and representative images are shown. The extent of KRAS mislocalization was quantified by determining the Manders’ coefficients. There was no significant difference between APEH-overexpressing and control cells (one-way ANOVA). (F) Cells as in D were seeded on 12-well plates and treated with vehicle (DMSO) or G01 for 48 h. Representative images are shown. The extent of KRAS mislocalization was quantified by determining the Manders’ coefficients. **P<0.01; ***P<0.001 compared with cells without APEH overexpression (one-way ANOVA). (G) MDCK cells stably expressing mGFP–KRASG12V and mCherry–CAAX were treated with vehicle (DMSO) or bortezomib for 48 h. Cells were imaged in a confocal microscope. Representative images are shown. The extent of KRAS mislocalization was quantified determining the Manders’ coefficients. Estimated IC50 values were obtained from the respective Manders’ coefficients dose–response plots. All graphs show mean±s.e.m. (n=3). Scale bars: 10 μm.
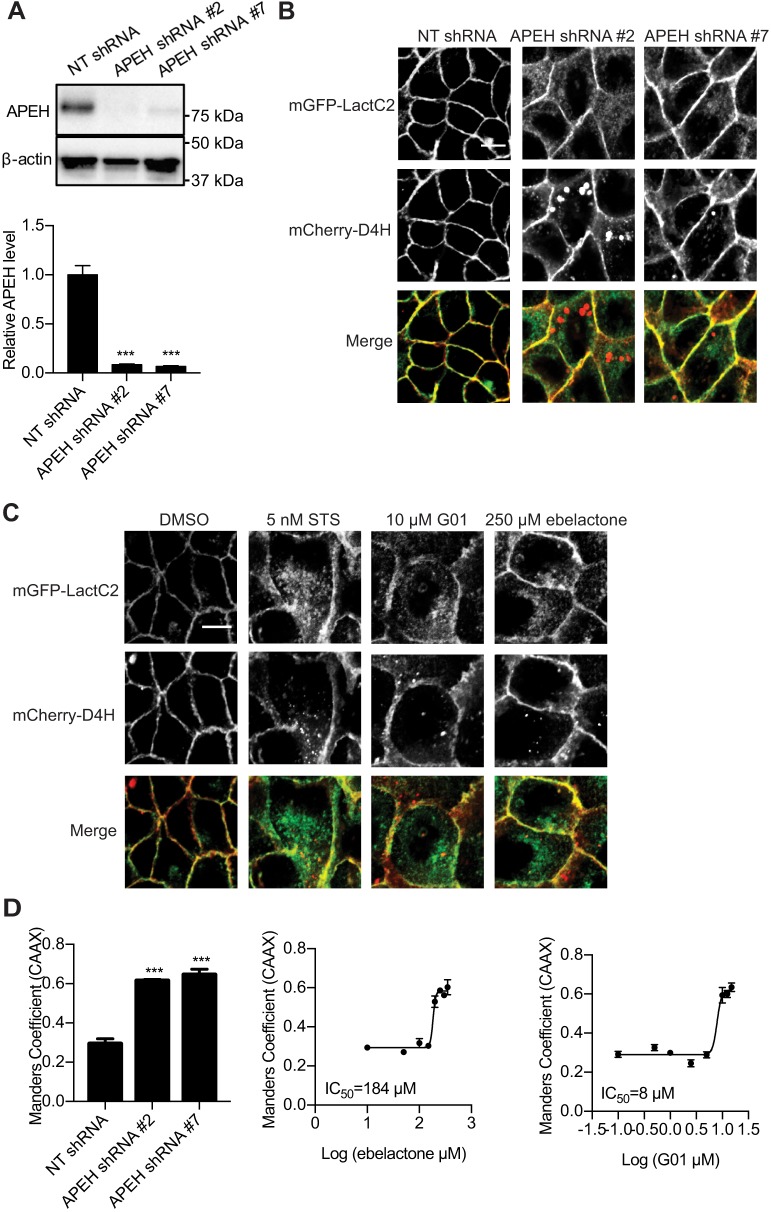
APEH knockdown or inhibitors mislocalize PtdSer and cholesterol from the PM
To explore potential molecular mechanisms for how APEH maintains KRAS PM localization and organization we investigated whether APEH regulates the PM localization of PtdSer and cholesterol, which are in turn critically important for the PM localization and clustering of KRAS and HRAS (Zhou and Hancock, 2015). APEH was knocked down in MDCK cells stably expressing mGFP–LactC2 and mCherry–D4H, which are probes for PtdSer and cholesterol, respectively (Maxwell et al., 2018; Yeung et al., 2008) (Fig. 4A). The localization of mGFP–LactC2 and mCherry–D4H was imaged in a confocal microscope. In control cells, the majority of mGFP–LactC2 and mCherry–D4H was localized on the PM. However, in APEH-knockdown cells, mGFP–LactC2 and mCherry–D4H were redistributed to endomembrane, suggesting a disruption of PtdSer and cholesterol localization (Fig. 4B). We next tested whether APEH inhibitors have similar effect on the localization of PtdSer and cholesterol. MDCK cells stably expressing mGFP–LactC2 and mCherry–D4H were treated with ebelactone or G01 for 48 h and imaged. mGFP–LactC2 and mCherry–D4H were mislocalized in both G01- and ebelactone-treated cells (Fig. 4C). The extent of PtdSer mislocalization was quantified by calculation of the Manders’ coefficients in cells co-expressing mGFP–LactC2 and mCherry–CAAX. When APEH was knocked down or inhibited, a significant increase in the Manders’ coefficients was observed (Fig. 4D). Further quantification showed that G01 and ebelactone led to the mislocalization of LactC2 with IC50 values of 8 µM and 184 µM, respectively (Fig. 4D).
Fig. 4.
PtdSer and cholesterol are mislocalized upon APEH knockdown or inhibition. (A) MDCK cells stably expressing mGFP–LactC2 and mCherry–D4H were transfected with NT shRNA or APEH shRNAs and lysed for quantitative immunoblotting. The level of APEH was normalized to the level of β-actin. Representative western blots are shown. Results are mean±s.e.m. (n=3). ***P<0.001 between APEH-knockdown and control cells (one-way ANOVA). (B) Parallel cultures of cells as in A were imaged in a confocal microscope. Representative images are shown. (C) MDCK cells stably expressing mGFP–LactC2 and mCherry–D4H were treated with vehicle (DMSO) or staurosporine (STS) or ebelactone or G01 for 48 h and imaged in a confocal microscope. Representative images are shown. (D) Left, MDCK cells stably expressing mGFP–LactC2 and mCherry–CAAX were transfected with NT shRNA or APEH shRNAs and imaged in a confocal microscope. The extent of LactC2 mislocalization was quantified by determining the Manders’ coefficients. ***P<0.001 (one-way ANOVA). Middle and right, MDCK cells stably expressing mGFP–LactC2 and mCherry–CAAX were treated with G01 or ebelactone for 48 h and imaged in a confocal microscope. The extent of LactC2 mislocalization was quantified by determining the Manders’ coefficients. Estimated IC50 values for ebelactone or G01 were obtained from the respective Manders’ coefficient dose–response plots. Results are mean±s.e.m. (n=3). Scale bars: 10 μm.
APEH knockdown or inhibitors disrupt the endocytic recycling of EGFR and transferrin receptor
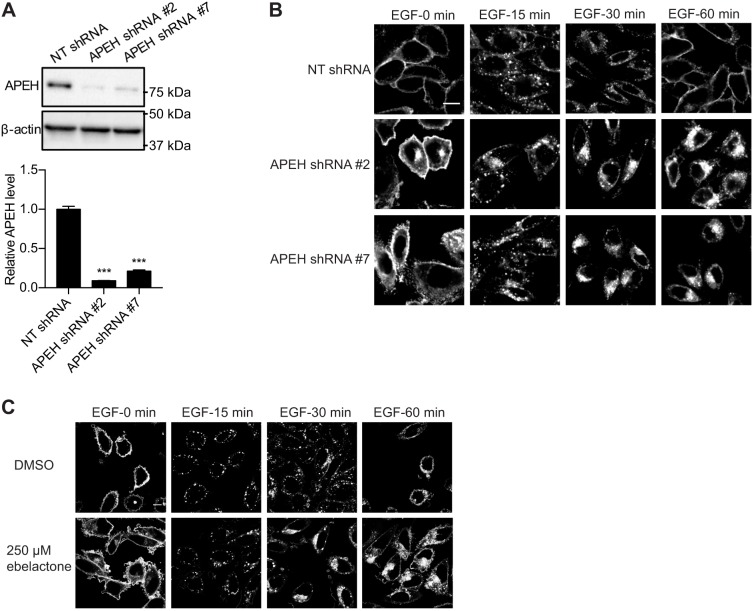
Normal functioning of the RE is required to maintain KRAS on the PM (Misaki et al., 2010; Schmick et al., 2014). We reported previously that G01 treatment disrupts the endocytic recycling of the EGFR and transferrin receptor (TfR) (Ghosh and Maxfield, 1995; Rodman and Wandinger-Ness, 2000; Roepstorff et al., 2009; Tan et al., 2018). Therefore, we investigated whether knockdown of APEH had a similar effect. To carry out live-cell imaging of EGFR trafficking, knockdown of APEH was performed in CHO cells stably expressing mGFP–EGFR (Fig. 5A). This is a useful cell system because CHO cells do not express any endogenous EGFR and were shown previously to be highly sensitive to G01 treatment (Tan et al., 2018). Cells were serum starved, incubated with EGF on ice, and then imaged in a confocal microscope at intervals following warming to 37°C. In control cells, mGFP–EGFR was predominantly localized to the PM at 0 min, to endosomal vesicles at 15−30 min and restored to the PM by 60 min (Fig. 5B). In APEH-knockdown cells, mGFP–EGFR was already partially mislocalized from the PM at 0 min and thereafter progressively accumulated in the perinuclear region (Fig. 5B). Similar results were observed in ebelactone-treated cells (Fig. 5C), suggesting that, in both cases, exit of the EGFR from the RE was inhibited. Neither ebelactone treatment nor APEH knockdown affected the EGFR expression level (Fig. S4).
Fig. 5.
The endocytic recycling of EGFR is disrupted by APEH knockdown or inhibition. (A) CHO cells stably expressing mGFP–EGFR were transfected with NT shRNA or APEH shRNAs and lysed for quantitative immunoblotting. The level of APEH was normalized to the level of β-actin. Results are mean±s.e.m. (n=3). Representative western blots are shown. ***P<0.001 between APEH-knockdown and control APEH levels (one-way ANOVA). (B) Cells as in A were serum-starved for 2 h and incubated with 50 ng/ml EGF on ice for 20 min. Excess EGF was washed away and cells were incubated with fresh warm medium. Cells were fixed at different time points and imaged in a confocal microscope. Representative images are shown. (C) CHO cells stably expressing mGFP–EGFR were treated with vehicle (DMSO) or ebelactone. Cells were serum-starved for 2 h and incubated with 50 ng/ml EGF on ice for 20 min. Excess EGF was washed away and cells were incubated with fresh warm medium. Cells were fixed at different time points and imaged in a confocal microscope. Representative images are shown. Scale bars: 10 μm.
Next, A431 cells with or without APEH knockdown (Fig. S5A) were incubated with Alexa Fluor 555-conjugated transferrin (Tf–555) on ice. Excess Tf–555 was washed away and cells were immediately imaged in a confocal microscope. Surface fluorescence intensity was lower in cells with APEH knockdown, suggesting a lower level of TfR on the PM (Fig. S5B). Cells were then imaged at intervals following warming to 37°C. In control cells, Tf–555 was internalized and predominantly returned to the PM by 60 min (Fig. S5C). However, Tf–555 was internalized but not returned to the PM in APEH-knockdown cells (Fig. S5C). The relative level of Tf–555 on the PM was quantified by determining the Manders’ coefficients after co-staining the PM of cells with Alexa Fluor 488 conjugated to wheat germ agglutinin (WGA–488), an impermeable PM marker that binds N-acetylglucosamine and N-acetylneuraminic acid (Fig. S5D). In similar experiments, ebelactone treatment also reduced the level of TfR on the PM and blocked the return of Tf–555 to the PM after internalization (Fig. S5E–G). By contrast there was no significant difference in the kinetics of Alexa Fluor 647-conjugated cholera toxin b subunit (CTB–647) internalization in A431 cells after APEH knockdown or ebelactone treatment (Fig. S6). Thus, APEH knockdown does not affect the internalization of ganglioside GM1, which is independent of RE function.
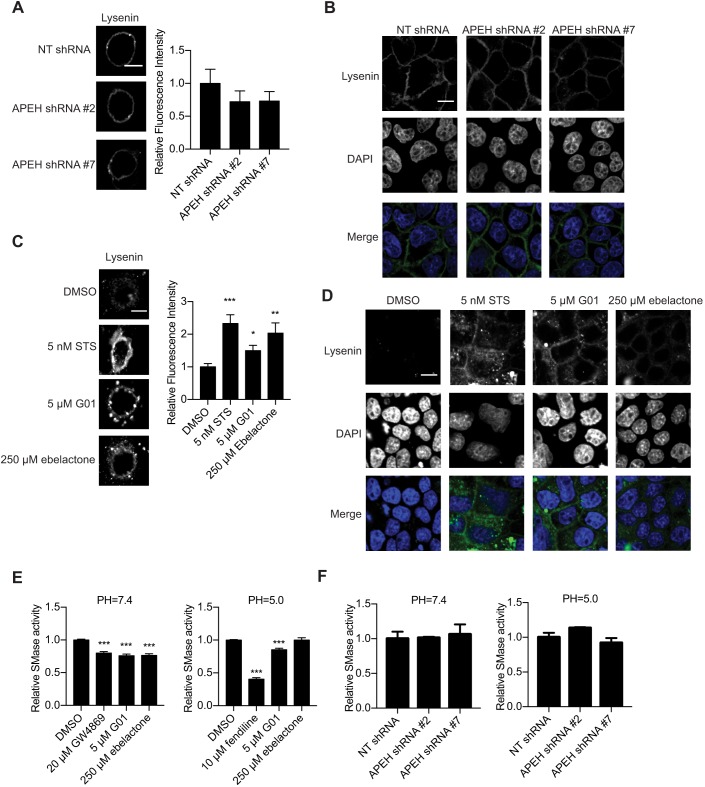
Knockdown of APEH does not affect SM metabolism
Previously, we reported that G01 treatment enhanced cellular SM content (Tan et al., 2018). We therefore investigated whether APEH knockdown had a similar effect. Wild-type MDCK cells were transfected with NT shRNA or APEH shRNAs, and stained with GFP–lysenin, a probe that binds specifically to SM. Cells were then imaged in a confocal microscope with fixed imaging parameters to compare fluorescence intensity. No changes of fluorescence intensity or GFP–lysenin localization were observed on the exofacial PM (Fig. 6A) or intracellular membranes (Fig. 6B) in APEH-knockdown cells, indicating APEH does not regulate cellular SM levels. An increase in SM levels was, however, observed in cells treated with ebelactone and again in cells treated with G01 (Fig. 6C,D). We therefore examined whether G01 and ebelactone additionally affected the activity of sphingomyelinases (SMases). G01 reduced SMase activity in both neutral and acidic pH environments, suggesting an inhibitory activity towards acid SMase (ASMase) and neutral SMase (NSMase) (Fig. 6E). Ebelactone preferentially inhibited SMase activity in a neutral pH environment, suggesting that it has NSMase inhibitory activity (Fig. 6E). As for cells transfected with NT or APEH shRNAs, no significant change in SMase activity was observed (Fig. 6F). Therefore, these observations indicate that G01 or ebelactone likely increases SM levels by inhibiting SMases in an APEH-independent manner.
Fig. 6.
APEH knockdown does not alter cellular SM levels. (A) Cells as in Fig. 1D were stained with GFP–lysenin and imaged in a confocal microscope with fixed imaging parameters to compare fluorescence intensities. Representative images are shown. The GFP fluorescence intensity was quantified using ImageJ and normalized to the mean of those in NT shRNA group (set at one). Results are mean±s.e.m. (n≥12). There was no significant difference in the relative fluorescence intensity (one-way ANOVA). (B) Cells as in Fig. 1D were stained with GFP–lysenin and DAPI following by cell permeabilization. Cells were imaged in a confocal microscope with fixed imaging parameters for comparison of fluorescent intensities. Representative images are shown. (C) MDCK cells were treated with vehicle (DMSO), staurosporine (STS), ebelactone or G01 for 48 h, and imaged and analyzed as in A. *P<0.05; **P<0.01; ***P<0.001 compared with DMSO (one-way ANOVA). (D) MDCK cells were treated with vehicle (DMSO), STS, ebelactone or G01 for 48 h and permeabilized. Cells were stained with GFP–lysenin and DAPI and were imaged as in B. Representative images are shown. (E) MDCK cells were treated with vehicle (DMSO), GW4869, G01 or ebelactone for 48 h and lysed. Cell lysates were analyzed for neutral and acid SMase activity. ***P<0.001 (one-way ANOVA). (F) Cells as in Fig. 1D were lysed and analyzed for neutral and acid SMase activity. There was no significant difference between the control and knockdown cells (one-way ANOVA). Graphs in E and F show mean±s.e.m. (n=3). Scale bars: 10 μm.
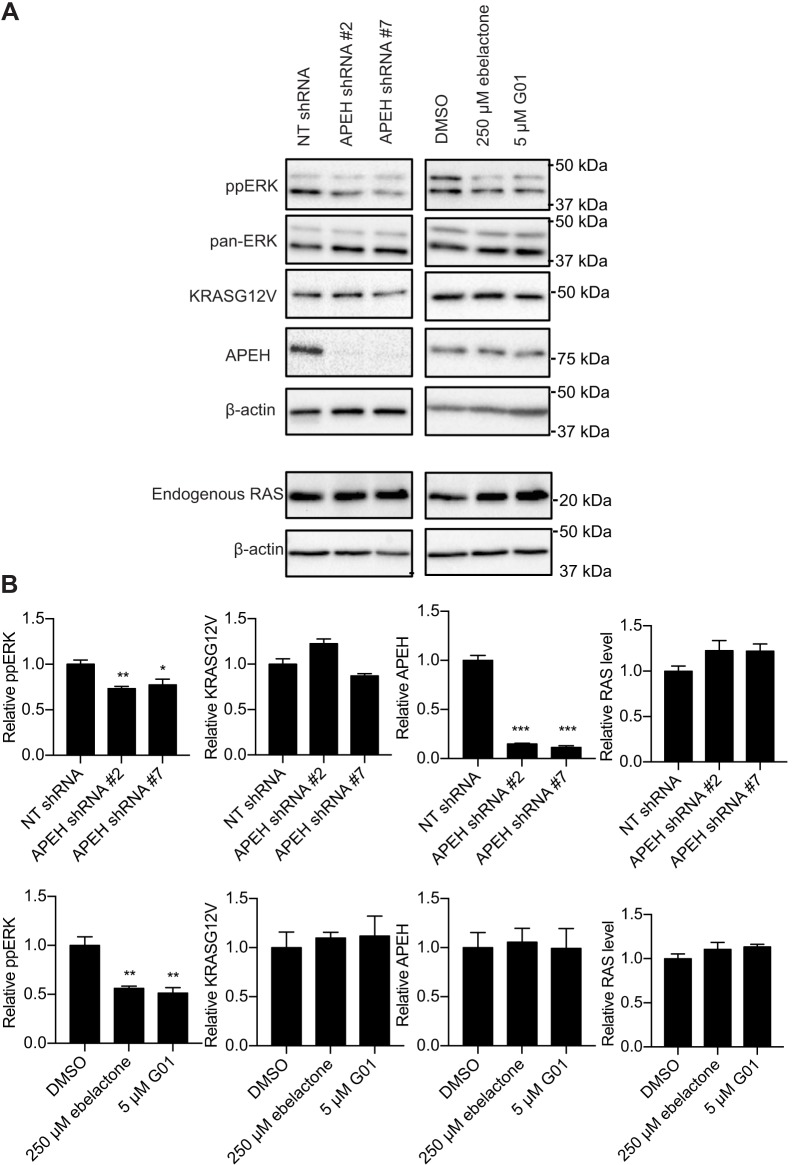
APEH knockdown or inhibitors abrogate MAPK signaling downstream of oncogenic KRAS
Since APEH knockdown disrupted KRASG12V PM localization and nanocluster formation, which are essential for the downstream signal transmission (Hancock, 2003; Zhou and Hancock, 2015), we determined whether MAPK signaling was also inhibited. In MDCK cells expressing KRASG12V the phospho-Erk1/2 (ppERK) levels were significantly lower in cells with APEH knockdown, indicating suppression of MAPK signaling (Fig. 7A,B). In these cells KRASG12V and endogenous RAS expression was unaffected by APEH knockdown (Fig. 7A,B). Identical observations were made in cells treated with ebelactone or G01 (Fig. 7A,B). Of note, G01 and ebelactone treatment did not alter cellular APEH expression levels (Fig. 7A,B).
Fig. 7.
MAPK signaling is reduced by APEH knockdown or inhibition. (A) MDCK cells expressing KRASG12V were treated with vehicle (DMSO) or ebelactone or G01 for 48 h. These cells, together with the cells as in Fig. 2E, were lysed for quantitative immunoblotting. Representative blots are shown. (B) Levels of ppERK were normalized to the total level of ERK. Levels of KRASG12V, APEH, and endogenous RAS were normalized to the total level of β-actin. Results are mean±s.e.m. (n=3). *P<0.05; **P<0.01; ***P<0.001 between drug-treated or shRNA-transfected and control protein levels (one-way ANOVA).
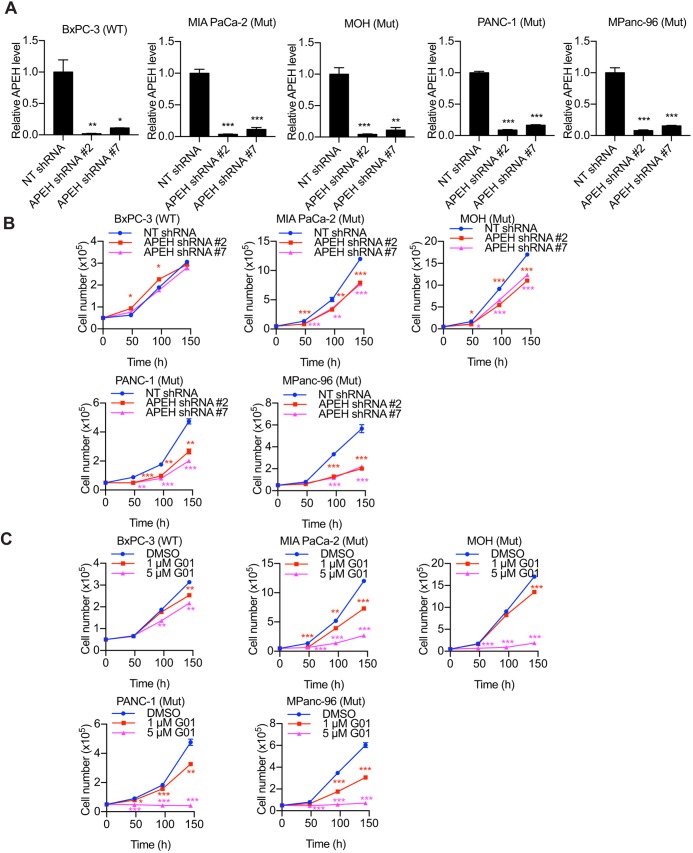
APEH knockdown or G01 inhibits the proliferation of KRAS-transformed pancreatic cancer cells
Finally, we tested whether the knockdown or inhibition of APEH hinders the proliferation of cancer cells that are dependent on oncogenic KRAS. We knocked down APEH in BxPC3, MiaPaCa-2, MOH, PANC-1 and Mpanc-96 cells (Fig. 8A). Cells were then seeded and cultured for 6 days and counted. In parallel experiments, parental cells were treated daily with G01. We found that APEH knockdown more potently inhibited the proliferation of KRAS-dependent cancer cells (Fig. 8B). Similarly, G01 dose-dependently inhibited the proliferation of the same KRAS-dependent cancer cells (Fig. 8C). The data also show that the suppression of cancer cell proliferation was greater with G01 treatment than APEH knockdown.
Fig. 8.
The proliferation of KRAS-dependent pancreatic cancer cells is inhibited by APEH knockdown or inhibition. BxPC-3, MiaPaCa-2, MOH, PANC-1 and MPanc-96 cells were transfected with NT shRNA or APEH shRNAs and lysed for quantitative immunoblotting. The level of APEH was normalized to the level of β-actin. *P<0.05; **P<0.01; ***P<0.001 between APEH-knockdown and control APEH cells (one-way ANOVA). (B) Cells as in A were grown in 12-well plates for 6 days. Cells were counted every 48 h. *P<0.05; **P<0.01; ***P<0.001 (one-way ANOVA). (C) BxPC-3, MiaPaCa-2, MOH, PANC-1 and MPanc-96 cells were treated with vehicle (DMSO) or daily doses of two concentrations of G01 in 12-well plates for 6 days. Cells were counted every 48 h. *P<0.05; **P<0.01; ***P<0.001 (one-way ANOVA). All graphs show mean±s.e.m. (n=3).
DISCUSSION
In this study, we show that the molecular target of G01, a novel KRAS inhibitor, is the enzyme APEH. G01 bound to APEH with sufficient avidity to allow affinity purification from cell lysates, and G01 inhibited the enzymatic activity of APEH in biochemical assays. Consistent with the biological effect of G01 (Tan et al., 2018), shRNA-mediated knockdown of APEH expression resulted in KRAS mislocalization from the PM, which was rescued by the restoration of ectopic APEH expression. Together, these observations reveal that APEH is a novel regulator of KRAS PM localization. Two major cellular processes are required for the fidelity of KRAS PM targeting. First, the maintenance of a high concentration of PtdSer on the inner leaflet of the PM, which is required because the KRAS membrane anchor exhibits exquisite binding specificity for this phospholipid (Zhou and Hancock, 2015; Zhou et al., 2017). Second, a functioning spatial organization system, which in the case of KRAS requires the RE operating in concert with the chaperone PDEδ and its regulator ARL2 (Cho et al., 2016; Misaki et al., 2010; Schmick et al., 2014). We show here that both of these components are compromised when APEH expression is suppressed. PM inner leaflet PtdSer is reduced, resulting in redistribution of the PtdSer probe LactC2 to endomembrane. In addition, there is a malfunction at the RE as evidenced by aberrant endosomal recycling of the EGFR and TfR. It seems likely that the failure to maintain PM PtdSer content in APEH-knockdown cells is at least in part also due to aberrant RE function, since lipids as well as proteins must be appropriately sorted and recycled after endocytosis. Therefore, the mechanism of action of APEH knockdown for KRAS mislocalization involves disruption of RE function, which is consistent with previous observations in G01-treated cells, where it was shown that impairment of the KRAS spatial organizing system and reduced PtdSer content of PM are causative of KRAS mislocalization. These results were recapitulated by a previously described, albeit much less potent, APEH inhibitor, ebelactone (Palmieri et al., 2011; Sandomenico et al., 2012), which we show here also led to the mislocalization of KRAS and reduced the PtdSer content of the inner PM. Consistent with the effects on KRAS PM localization, APEH knockdown blocked MAPK signaling downstream of the oncogenic mutant KRASG12V and inhibited the proliferation of KRAS-dependent pancreatic cancer cells. By contrast APEH knockdown had minimal effect on the proliferation of pancreatic cancer cells that expressed wild-type KRAS.
Of note, APEH knockdown was less efficacious at inhibiting pancreatic cancer cell growth than treatment with G01. Moreover, whereas G01 elevates cellular SM levels (Tan et al., 2018), knockdown of APEH had no such effect, suggesting that G01 may have other cellular targets in addition to APEH. Consistent with this interpretation of the results, biochemical assays indeed showed that G01 can inhibit both ASMase and NSMase, whereas the activity of these enzymes was unaffected in APEH-knockdown cells. Interestingly, ebelactone also elevated cellular SM levels and inhibited NSMase. Previous work has shown that inhibition of SMases is of itself sufficient to impair KRAS PM localization (Cho et al., 2016; Maekawa et al., 2016; van der Hoeven et al., 2018); it therefore seems likely that simultaneous perturbation of SM metabolism and RE function via APEH inhibition results in the increased potency of G01 for inhibition of KRAS-dependent pancreatic cancer cell proliferation.
α-Acetylation of the N-terminal amino acid of proteins (Nα acetylation) is a highly prevalent post-translational modification in eukaryotic cells (Zhang et al., 2011). The function of Nα acetylation is not fully understood, but there are examples where the modification can regulate protein stability, enzymatic activity and protein–protein interactions. Nα acetylation is irreversible, unlike amino acid side chain acetylation. In eukaryotic cells APEH, a ubiquitously expressed cytosolic enzyme, catalyzes removal of the Nα-acetylated amino acid from peptides and therefore regulates the overall cellular levels of Nα acetylated proteins (Palmieri et al., 2011). APEH is also able to hydrolyze oxidized or glycated proteins and has been alternatively named as oxidized protein hydrolase in older literature (Fujino et al., 2000a,b). Recent studies have shown that knockdown or inhibition of APEH blocks the chymotrypsin-like activity of the proteasome (Bergamo et al., 2016; Palmieri et al., 2011; Palumbo et al., 2016). Thus, APEH has been advocated to operate as a regulator of proteasome that degrades of poly-ubiquitylated and/or oxidized proteins. Separately, APEH has also been identified as a component of an enzyme complex that repairs DNA single-strand breaks and, consistent with this, has been shown to translocate into the cell nucleus in response to DNA damage (Zeng et al., 2017).
Linking these disparate activities of APEH to the perturbation of RE function and consequent mislocalization of KRAS that we have described is not immediately straightforward. Taken together, the previous studies broadly suggest that APEH is a regulator of cellular protein quality control, given its peptidase activity and role in proteasomal regulation. We therefore speculate that aberrant protein quality control causes damage to the endolysosome, which is in turn critical for the recycling of RAS and PM lipids (Cho et al., 2016; van der Hoeven et al., 2018). This may be a consequence of oxidative stress induced by accumulation of oxidatively damaged proteins (Shimizu et al., 2003, 2004), which in turn causes oxidative damage to the endolysosome (Aits and Jäättelä, 2013; Berndtsson et al., 2009; Yeung et al., 2006) and/or dysregulation of specific proteins involved in regulation of RE function. Identifying the precise molecular target(s) may prove difficult, but in support of the general mechanism that aberrant protein quality control is involved, we show that treatment of cells with the proteasome inhibitor bortezomib phenocopies the effect of G01 on KRAS mislocalization.
Finally, it is interesting to note that APEH is expressed at higher levels in cancers expressing oncogenic mutant KRAS than in cancers expressing wild-type KRAS. This correlation, when taken in the context of our observations that APEH activity is required to maintain KRAS and PtdSer on the inner leaflet of the PM, highlights the potential utility of APEH inhibitors, and perhaps proteasomal inhibitors, in the treatment of KRAS-dependent pancreatic cancer.
MATERIALS AND METHODS
Materials
Oxanthroquinone G01 (G01) was synthesized as described previously (Salim et al., 2014). All reagents for the synthesis of G01 and B-G01 (a G01 analog incorporating a PEG linker and biotin moiety) were used as purchased from Sigma-Aldrich. Spectroscopic characterization of G01 and B-G01 was carried out on a Bruker 600 MHz NMR spectrometer, and a Bruker micrOTOF mass spectrometer. Staurosporine (STS) was obtained from BioAustralis (Australia). Ebelactone A (ebelactone; ALX-270-013-M001) was purchased from Enzo Biochem (Farmingdale, NY). Fendiline was synthesized at Translational Chemistry Core Facility at the MD Anderson Cancer Center (Houston, TX). GW4869 (D1692) was purchased from Sigma-Aldrich. All compounds were dissolved in dimethyl sulfoxide (DMSO). Cell culture media and fetal bovine serum (FBS) were purchased from HyClone (Chicago, IL) unless otherwise described. MISSION Non-Target (NT) shRNA (SHC216) and APEH shRNAs (SHCLNG-NM_001640) were purchased from Sigma-Aldrich (St. Louis, MO). The sequences of APEH shRNA #2 and APEH shRNA #7 are: 5′-CCGGCGAGTCCTTCTTTCAGACCAACTCGAGTTGGTCTGAAAGAAGGACTCGTTTTTG-3′ and 5′-CCGGCCTGTATTATGTGGACCTCATCTCGAGATGAGGTCCACATAATACAGGTTTTTG-3′, respectively. APEH cDNA in pcDNA3.1+C-MYC plasmid (NM_001640.3) was purchased from GenScript (Piscataway, NJ). Rabbit anti-phospho-p44/42 mitogen-activated protein kinase (MAPK) (ppERK1/2; Thr202/Tyr204) (9101, 1:1000), rabbit anti-ERK1/2 (4695, 1:1000) antibodies were purchased from Cell Signaling Technology (Beverly, MA). Mouse anti-β-actin (A1978, 1:5000) antibody was purchased from Sigma-Aldrich. Mouse anti-pan-RAS (610001, 1:500) antibody was purchased from BD Biosciences (Franklin Lakes, NJ). Rabbit anti-mGFP antibody for immunogold labeling was generated as previously described and used at 1:30 (Prior et al., 2003b). Rabbit anti-acylpeptide hydrolase (APEH) antibody (PA5-43137, 1:000), Pierce Streptavidin resins (20359), transferrin from human serum, Alexa Fluor 555 conjugate (T35352), Cholera toxin subunit B (Recombinant), Alexa Fluor 647 conjugate (C34778), wheat germ agglutinin, Alexa Fluor 488 conjugate (W11261), and Pierce silver stain kit (24612) were purchased from Thermo Fisher Scientific (Waltham, MA).
Synthesis of B-G01
B-G01 was prepared from G01 via the corresponding 3-O-propargyl ether. A mixture of G01 (50 mg; 0.15 mmol) and potassium carbonate (41 mg; 0.30 mmol) in acetone (25 ml) was treated with propargyl bromide (107 mg; 0.90 mmol) at ambient temperature, in five portions at an interval of 3 h, and the mixture stirred at room temperature for 18 h. The reaction mixture was then filtered, acidified to pH 4 with 0.1 M HCl and extracted with diethyl ether (3×30 ml). The combined organic layers were concentrated in vacuo, and the residue purified using reverse phase flash column chromatography [Biotage SNAP KP-C18-HS (12 g) column, 30 min gradient elution at 15 ml/min from 10% acetonitrile (MeCN) in H2O, to 100% MeCN with a constant 0.01% trifluoroacetic acid (TFA) modifier] to yield the 3-O-propargyl ether of G01 (50.5 mg; 89%). A solution of the 3-O-propargyl ether of G01 (4 mg, 0.011 mmol), biotin-PEG3-azide (5 mg, 0.011 mmol), CuSO4 (10 mol %) and sodium ascorbate (20 mol %) in water (2 ml) was stirred at room temperature for 12 h. The resulting precipitate was extracted with ethyl acetate (3×15 ml) and subjected to reversed phase chromatography (Biotage SNAP KP-C18-HS (12 g) column, 30 min gradient elution at 15 ml/min from 10% MeCN in H2O to 100% MeCN with a constant 0.01% TFA as modifier) to yield B-G01 (5.4 mg, 60%, HRMS-ESI, m/z 845.3263 [M+Na]+, calculated for C40H50N6O11Na, 845.3263).
Cell lines
BxPC3, MiaPaCa-2, MOH and MPanc-96 cell lines were provided by Craig Logsdon at MD Anderson Cancer Center, Houston, TX. Other cell lines were purchased from the American Type Culture Collection (ATCC). The Madin–Darby canine kidney (MDCK), MPanc-96, PANC-1 and A431 cell lines were maintained in Dulbecco's modified Eagle's medium (GIBCO) supplemented with 2 mM L-glutamine and 10% fetal bovine serum (FBS). Chinese hamster ovary (CHO; ATCC) cells were maintained in Ham's F-12K medium supplemented with 10% FBS 2 mM L-glutamine. BxPC3 and MOH cells were grown in RPMI-1640 supplemented with 10% FBS. MiaPaCa-2 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 2.5% horse serum. CaCO-2 cells were maintained in Eagle's minimum essential medium supplemented with 20% FBS.
Pulldown assay
CaCO-2 cell lysates were incubated with or without B-G01 for 1 h at room temperature. The cell lysates were then incubated with a 20 μl 50% streptavidin bead suspension. The beads were collected and analyzed by SDS-PAGE and silver staining. The bands of interest were analyzed by mass spectrometry in Clinical and Translational Proteomics Service Center for Precision Biomedicine, Institute of Molecular Medicine, Houston, TX. The potential protein targets shown in the report were confirmed with immunoblotting followed by another identical pulldown assay.
APEH enzyme assay
The enzyme substrate acetyl-alanine-p-nitroaniline (Ac-Ala-pNA) was synthesized by Bachem. MDCK cells were seeded and were lysed. Samples each containing 250 μg proteins were incubated with DMSO or G01 for 1 h at room temperature. The samples were then heated at 37°C for 2 min before 1 mM ac-Ala-pNA was added. Absorbance at 410 nm were measured at 15 min, 60 min and 120 min following incubation at 37°C. The background-subtracted absorbance was used for evaluating significance of differences using two-tailed Student's t-tests or one-way ANOVA.
Bioinformatic analysis using UCSC Xena browser
The APEH mRNA expression levels in patients of 33 types of cancer with or without KRAS mutation were obtained and analyzed by using the UCSC Xena brower (https://xenabrowser.net/).
Electron microscopy and spatial analysis
MDCK cells expressing mGFP–KRASG12V were seeded on fibronectin-coated gold EM grids (IGG200, Ted Pella Inc). A PBS-soaked Whatman filter paper was put onto the cells for 5 min for facilitation of the removal of apical PM. By applying brief pressure and then removing the PBS-soaked filter paper, the cytosolic surface of the adherent basal PM was exposed because the apical PM was removed. The cytosolic leaflet of the basal PM was washed, fixed with paraformaldehyde and glutaraldehyde, and labeled with anti-GFP antibody conjugated to 4.5-nm gold particles. The basal PM sheets were imaged via a JEOL 1400 transmission electron microscope at 100,000× magnification. Using ImageJ, 1 µm2 areas of the PM sheet were identified and the (x,y) coordinates of the gold particles were determined. Univariate K-functions were calculated and standardized on the 99% confidence interval (c.i.) (Hancock and Prior, 2005; Prior et al., 2003a; Ripley, 1977). Bootstrap tests were used to examine differences between replicated point patterns were constructed, and statistical significance was evaluated against 1000 bootstrap samples (Diggle et al., 2000).
Confocal microscopy
Cells were fixed with 4% paraformaldehyde for 30 min and treated with 50 mM NH4Cl for 10 min. Cells were observed and imaged with a Nikon A1R confocal microscope.
Western blotting
Cells were washed with cold PBS twice and lysed in buffer containing 50 mM Tris-HCl pH 7.5, 75 mM NaCl, 25 mM NaF, 5 mM MgCl2, 5 mM EGTA, 1 mM dithiothreitol, 100 μM NaVO4 and 1% NP-40 plus protease inhibitors. SDS-PAGE and immunoblotting were performed with 15 μg total proteins in each sample. The signal was detected by enhanced chemiluminescence (SuperSignal; Pierce) with ChemiDoc MP System (Bio-Rad). The intensity of bands were quantified using ImageJ (1.47v).
Lysenin staining
A maltose binding protein (MBP)–GFP–lysenin fragment (amino acid residues 161 to 297) was purified as previously described (Maekawa et al., 2016). MDCK cells were fixed with 4% paraformaldehyde for 30 min. Cells were treated with or without 0.05% saponin, before being incubated with 60 μg/ml MBP–GFP–lysenin. Cells that were permeabilized were stained with 4′,6-diamidino-2-phenylindole (DAPI) for indication of cell nucleus. Cells were imaged in a confocal microscopy with fixed parameters for comparison of fluorescence intensities.
Sphingomyelinase activity assay
The SMase activity assay was undertaken with an Amplex Red SMase Assay Kit (A12220; Invitrogen; Carlsbad, CA) according to the manufacturer's instructions. Briefly for the NSMase assay, 10 μg of whole-cell lysates (at 1 μg/μl concentration) prepared in lysis buffer B without DTT was mixed with 40 μl of 1× reaction buffer (0.5 M Tris-HCl pH 7.4, 50 mM MgCl2), and loaded on a well of black 96-well plate. 50 μl of Amplex Red reaction mixture (100 μM Amplex Red reagent, 2 U/ml horseradish peroxidase, 0.2 U/ml choline oxidase, 8 U/ml alkaline phosphatase, and 0.5 mM sphingomyelin in 1× reaction buffer) was added to each well, and the plate was incubated in dark at 37°C for 60 min. The fluorescence was measured using BioTek Synergy H1 microplate reader (excitation λ=540 nm, emission λ=590 nm). 0.1 U/ml SMase and 1× reaction buffer were used as a positive and a negative control, respectively. For the ASMase assay, 10 μg of whole-cell lysates (at 1 μg/μl concentration) prepared in lysis buffer B without DTT was mixed with 40 μl of low-pH buffer (50 mM sodium acetate, pH 5.0), and loaded on a well of black 96-well plate. 5 µl of 5 mM sphingomyelin was added to each well, and the plate was incubated in dark in 37°C for 1 h. After the incubation, the pH was raised to 7.4 by adding 50 µl of Amplex Red reaction mixture [100 µM Amplex Red reagent, 2 U/ml horseradish peroxidase, 0.2 U/ml choline oxidase, 8 U/ml alkaline phosphatase in high-pH buffer (100 mM Tris-HCl, pH 8.0)]. The plate was further incubated in the dark in 37°C for 60 min, and the fluorescence was measured using BioTek Synergy H1 microplate reader (excitation λ=540 nm, emission λ=590 nm). 0.1 U/ml SMase and low-pH buffer were used as a positive and negative control, respectively.
Cell proliferation assay
Cells were seeded in 12-well plates and treated with or without chemical compounds when attached to the bottom of the plates. Cells were counted every 48 h.
Statistical analysis
Microsoft Excel 2016 and Prism v7.0a were used for one-way ANOVA and two-tailed Student's t-tests.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: L.T., P.N., R.J.C., J.F.H.; Methodology: L.T., K.-J.C., W.E.K., C.M.G., Y.Z., P.N., R.J.C., J.F.H.; Software: L.T., K.-J.C., W.E.K., C.M.G., Y.Z., P.N., R.J.C., J.F.H.; Validation: L.T., K.-J.C., W.E.K., C.M.G., Y.Z., P.N., R.J.C., J.F.H.; Formal analysis: L.T., K.-J.C., W.E.K., C.M.G., Y.Z., P.N., R.J.C., J.F.H.; Investigation: L.T., K.-J.C., W.E.K., C.M.G., Y.Z., P.N., R.J.C., J.F.H.; Resources: L.T., K.-J.C., C.M.G., Y.Z., P.N., R.J.C., J.F.H.; Data curation: L.T., K.-J.C., Y.Z., J.F.H.; Writing - original draft: L.T., J.F.H.; Writing - review & editing: L.T., K.-J.C., W.E.K., C.M.G., P.N., R.J.C., J.F.H.; Visualization: L.T., Y.Z., P.N., R.J.C., J.F.H.; Supervision: L.T., K.-J.C., Y.Z., R.J.C., J.F.H.; Project administration: L.T., J.F.H.; Funding acquisition: J.F.H.
Funding
This work was supported by grants from the Cancer Prevention and Research Institute of Texas (CPRIT; RP170233 to J.F.H.) and the National Cancer Institute (R00CA188593 to K.-J.C.). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.232132.supplemental
References
- Aits S. and Jäättelä M. (2013). Lysosomal Cell Death at a Glance. J. Cell. Sci. 126, 1905-1912. 10.1242/jcs.091181 [DOI] [PubMed] [Google Scholar]
- Bergamo P., Palmieri G., Cocca E., Ferrandino I., Gogliettino M., Monaco A., Maurano F. and Rossi M. (2016). Adaptive response activated by dietary cis9, trans11 conjugated linoleic acid prevents distinct signs of gliadin-induced enteropathy in mice. Eur. J. Nutr. 55, 729-740. 10.1007/s00394-015-0893-2 [DOI] [PubMed] [Google Scholar]
- Berndtsson M., Beaujouin M., Rickardson L., Havelka A. M., Larsson R., Westman J., Liaudet-Coopman E. and Linder S. (2009). Induction of the lysosomal apoptosis pathway by inhibitors of the ubiquitin-proteasome system. Int. J. Cancer 124, 1463-1469. 10.1002/ijc.24004 [DOI] [PubMed] [Google Scholar]
- Cho K.-J., Park J.-H., Piggott A. M., Salim A. A., Gorfe A. A., Parton R. G., Capon R. J., Lacey E. and Hancock J. F. (2012). Staurosporines disrupt phosphatidylserine trafficking and mislocalize Ras proteins. J. Biol. Chem. 287, 43573-43584. 10.1074/jbc.M112.424457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K.-J., van der Hoeven D., Zhou Y., Maekawa M., Ma X., Chen W., Fairn G. D. and Hancock J. F. (2016). Inhibition of acid sphingomyelinase depletes cellular phosphatidylserine and mislocalizes K-Ras from the plasma membrane. Mol. Cell. Biol. 36, 363-374. 10.1128/MCB.00719-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A. D. and Der C. J. (2010). Ras history: the saga continues. Small GTPases 1, 2-27. 10.4161/sgtp.1.1.12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A. D., Fesik S. W., Kimmelman A. C., Luo J. and Der C. J. (2014). Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Discov. 13, 828 10.1038/nrd4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker F. J., Rocks O., Vartak N., Menninger S., Hedberg C., Balamurugan R., Wetzel S., Renner S., Gerauer M., Schölermann B. et al. (2010). Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat. Chem. Biol. 6, 449 10.1038/nchembio.362 [DOI] [PubMed] [Google Scholar]
- Diggle P. J., Mateu J. and Clough H. E. (2000). A comparison between parametric and non-parametric approaches to the analysis of replicated spatial point patterns. Adv. Appl. Probab. 32, 331-343. 10.1239/aap/1013540166 [DOI] [Google Scholar]
- Fujino T., Ando K., Beppu M. and Kikugawa K. (2000a). Enzymatic removal of oxidized protein aggregates from erythrocyte membranes. J. Biochem. 127, 1081-1086. 10.1093/oxfordjournals.jbchem.a022701 [DOI] [PubMed] [Google Scholar]
- Fujino T., Watanabe K., Beppu M., Kikugawa K. and Yasuda H. (2000b). Identification of oxidized protein hydrolase of human erythrocytes as acylpeptide hydrolase1. Biochim. Biophys. Acta 1478, 102-112. 10.1016/S0167-4838(00)00004-2 [DOI] [PubMed] [Google Scholar]
- Ghosh R. N. and Maxfield F. R. (1995). Evidence for nonvectorial, retrograde transferrin trafficking in the early endosomes of HEp2 cells. J. Cell Biol. 128, 549-561. 10.1083/jcb.128.4.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonen H., Smith C. E., Siegel N. R., Kahana C., Merrick W. C., Chakraburtty K., Schwartz A. L. and Ciechanover A. (1994). Protein synthesis elongation factor EF-1 alpha is essential for ubiquitin-dependent degradation of certain N alpha-acetylated proteins and may be substituted for by the bacterial elongation factor EF-Tu. Proc. Natl. Acad. Sci. USA 91, 7648-7652. 10.1073/pnas.91.16.7648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Drake K. R., Rogers C., Wright L., Lippincott-Schwartz J., Philips M. R. and Kenworthy A. K. (2005). Depalmitoylated Ras traffics to and from the Golgi complex via a nonvesicular pathway. J. Cell Biol. 170, 261-272. 10.1083/jcb.200502063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F. (2003). Ras proteins: different signals from different locations. Nat. Rev. Mol. Cell Biol. 4, 373 10.1038/nrm1105 [DOI] [PubMed] [Google Scholar]
- Hancock J. F. and Prior I. A. (2005). Electron microscopic imaging of Ras signaling domains. Methods 37, 165-172. 10.1016/j.ymeth.2005.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock J. F., Magee A. I., Childs J. E. and Marshall C. J. (1989). All ras proteins are polyisoprenylated but only some are palmitoylated. Cell 57, 1167-1177. 10.1016/0092-8674(89)90054-8 [DOI] [PubMed] [Google Scholar]
- Hancock J. F., Paterson H. and Marshall C. J. (1990). A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63, 133-139. 10.1016/0092-8674(90)90294-O [DOI] [PubMed] [Google Scholar]
- Hershko A., Heller H., Eytan E., Kaklij G. and Rose I. A. (1984). Role of the alpha-amino group of protein in ubiquitin-mediated protein breakdown. Proc. Natl. Acad. Sci. USA 81, 7021-7025. 10.1073/pnas.81.22.7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley T. W., Alkan S., Srkalovic G. and Hsi E. D. (2004). Treatment of human chronic lymphocytic leukemia cells with the proteasome inhibitor bortezomib promotes apoptosis. Leukemia Res. 28, 845-850. 10.1016/j.leukres.2003.12.010 [DOI] [PubMed] [Google Scholar]
- Laude A. J. and Prior I. A. (2008). Palmitoylation and localisation of RAS isoforms are modulated by the hypervariable linker domain. J. Cell Sci. 121, 421-427. 10.1242/jcs.020107 [DOI] [PubMed] [Google Scholar]
- Liu P., Xu B., Li J. and Lu H. (2009). BAG3 gene silencing sensitizes leukemic cells to Bortezomib-induced apoptosis. FEBS Lett. 583, 401-406. 10.1016/j.febslet.2008.12.032 [DOI] [PubMed] [Google Scholar]
- Maekawa M., Lee M., Wei K., Ridgway N. D. and Fairn G. D. (2016). Staurosporines decrease ORMDL proteins and enhance sphingomyelin synthesis resulting in depletion of plasmalemmal phosphatidylserine. Sci. Rep. 6, 35762 10.1038/srep35762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell K. N., Zhou Y. and Hancock J. F. (2018). Rac1 nanoscale organization on the plasma membrane is driven by lipid binding specificity encoded in the membrane anchor. Mol. Cell. Biol. 38, e00186-e00118 10.1128/MCB.00186-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki R., Morimatsu M., Uemura T., Waguri S., Miyoshi E., Taniguchi N., Matsuda M. and Taguchi T. (2010). Palmitoylated Ras proteins traffic through recycling endosomes to the plasma membrane during exocytosis. J. Cell Biol. 191, 23-29. 10.1083/jcb.200911143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri G., Bergamo P., Luini A., Ruvo M., Gogliettino M., Langella E., Saviano M., Hegde R. N., Sandomenico A. and Rossi M. (2011). Acylpeptide hydrolase inhibition as targeted strategy to induce proteasomal down-regulation. PLoS ONE 6, e25888 10.1371/journal.pone.0025888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo R., Gogliettino M., Cocca E., Iannitti R., Sandomenico A., Ruvo M., Balestrieri M., Rossi M. and Palmieri G. (2016). APEH inhibition affects osteosarcoma cell viability via downregulation of the proteasome. Int. J. Mol. Sci. 17, 1614 10.3390/ijms17101614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman S. J., Muncke C., Parton R. G. and Hancock J. F. (2005). H-ras, K-ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton. Proc. Natl. Acad. Sci. 102, 15500-15505. 10.1073/pnas.0504114102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior I. A. and Hancock J. F. (2001). Compartmentalization of Ras proteins. J. Cell Sci. 114, 1603-1608. [DOI] [PubMed] [Google Scholar]
- Prior I. A. and Hancock J. F. (2012). Ras trafficking, localization and compartmentalized signalling. Semin. Cell Dev. Biol. 23, 145-153. 10.1016/j.semcdb.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior I. A., Muncke C., Parton R. G. and Hancock J. F. (2003a). Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 160, 165-170. 10.1083/jcb.200209091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior I. A., Parton R. G. and Hancock J. F. (2003b). Observing cell surface signaling domains using electron microscopy. Sci. STKE 2003, pl9 10.1126/scisignal.1772003pl9 [DOI] [PubMed] [Google Scholar]
- Prior I. A., Lewis P. D. and Mattos C. (2012). A comprehensive survey of Ras mutations in cancer. Cancer Res. 72, 2457-2467. 10.1158/0008-5472.CAN-11-2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley B. (1977). Modelling spatial patterns (with discussion). J. R. Statist. Soc. B 39, 172-212. 10.1111/j.2517-6161.1977.tb01615.x [DOI] [Google Scholar]
- Rocks O., Peyker A., Kahms M., Verveer P. J., Koerner C., Lumbierres M., Kuhlmann J., Waldmann H., Wittinghofer A. and Bastiaens P. I. H. (2005). An acylation cycle regulates localization and activity of palmitoylated ras isoforms. Science 307, 1746-1752. 10.1126/science.1105654 [DOI] [PubMed] [Google Scholar]
- Rocks O., Gerauer M., Vartak N., Koch S., Huang Z.-P., Pechlivanis M., Kuhlmann J., Brunsveld L., Chandra A., Ellinger B. et al. (2010). The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell 141, 458-471. 10.1016/j.cell.2010.04.007 [DOI] [PubMed] [Google Scholar]
- Rodman J. S. and Wandinger-Ness A. (2000). Rab GTPases coordinate endocytosis. J. Cell Sci. 113, 183-192. [DOI] [PubMed] [Google Scholar]
- Roepstorff K., Grandal M. V., Henriksen L., Knudsen S. L. J., Lerdrup M., Grøvdal L., Willumsen B. M. and Van Deurs B. (2009). Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic 10, 1115-1127. 10.1111/j.1600-0854.2009.00943.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky E. K. (2006). Lately, it occurs to me what a long, strange trip it's been for the farnesyltransferase inhibitors. J. Clin. Oncol. 24, 2981-2984. 10.1200/JCO.2006.05.9808 [DOI] [PubMed] [Google Scholar]
- Salim A. A., Xiao X., Cho K.-J., Piggott A. M., Lacey E., Hancock J. F. and Capon R. J. (2014). Rare Streptomyces sp. polyketides as modulators of K-Ras localisation. Org. Biomol. Chem. 12, 4872-4878. 10.1039/C4OB00745J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandomenico A., Russo A., Palmieri G., Bergamo P., Gogliettino M., Falcigno L. and Ruvo M. (2012). Small peptide inhibitors of acetyl-peptide hydrolase having an uncommon mechanism of inhibition and a stable bent conformation. J. Med. Chem. 55, 2102-2111. 10.1021/jm2013375 [DOI] [PubMed] [Google Scholar]
- Schmick M., Vartak N., Papke B., Kovacevic M., Truxius D. C., Rossmannek L. and Bastiaens P. I. H. (2014). KRas localizes to the plasma membrane by spatial cycles of solubilization, trapping and vesicular transport. Cell 157, 459-471. 10.1016/j.cell.2014.02.051 [DOI] [PubMed] [Google Scholar]
- Schmick M., Kraemer A. and Bastiaens P. I. H. (2015). Ras moves to stay in place. Trends Cell Biol. 25, 190-197. 10.1016/j.tcb.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Sebti S. M. and Der C. J. (2003). Searching for the elusive targets of farnesyltransferase inhibitors. Nat. Rev. Cancer 3, 945 10.1038/nrc1234 [DOI] [PubMed] [Google Scholar]
- Shimizu K., Fujino T., Ando K., Hayakawa M., Yasuda H. and Kikugawa K. (2003). Overexpression of oxidized protein hydrolase protects COS-7 cells from oxidative stress-induced inhibition of cell growth and survival. Biochem. Biophys. Res. Commun. 304, 766-771. 10.1016/S0006-291X(03)00657-0 [DOI] [PubMed] [Google Scholar]
- Shimizu K., Kiuchi Y., Ando K., Hayakawa M. and Kikugawa K. (2004). Coordination of oxidized protein hydrolase and the proteasome in the clearance of cytotoxic denatured proteins. Biochem. Biophys. Res. Commun. 324, 140-146. 10.1016/j.bbrc.2004.08.231 [DOI] [PubMed] [Google Scholar]
- Tan L., Cho K.-J., Neupane P., Capon R. J. and Hancock J. F. (2018). An oxanthroquinone derivative that disrupts RAS plasma membrane localization inhibits cancer cell growth. J. Biol. Chem. 293, 13696-13706. 10.1074/jbc.RA118.003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai F. D., Lopes M. S., Zhou M., Court H., Ponce O., Fiordalisi J. J., Gierut J. J., Cox A. D., Haigis K. M. and Philips M. R. (2015). K-Ras4A splice variant is widely expressed in cancer and uses a hybrid membrane-targeting motif. Proc. Natl. Acad. Sci. USA 112, 779-784. 10.1073/pnas.1412811112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven D., Cho K.-J., Zhou Y., Ma X., Chen W., Naji A., Montufar-Solis D., Zuo Y., Kovar S. E. and Levental K. R. (2018). Sphingomyelin metabolism is a regulator of K-Ras function. Mol. Cell. Biol. 38, e00373-e00317. 10.1128/MCB.00373-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung B. H., Huang D.-C. and Sinicrope F. A. (2006). PS-341 (bortezomib) induces lysosomal cathepsin B release and a caspase-2-dependent mitochondrial permeabilization and apoptosis in human pancreatic cancer cells. J. Biol. Chem. 281, 11923-11932. 10.1074/jbc.M508533200 [DOI] [PubMed] [Google Scholar]
- Yeung T., Gilbert G. E., Shi J., Silvius J., Kapus A. and Grinstein S. (2008). Membrane phosphatidylserine regulates surface charge and protein localization. Science 319, 210-213. 10.1126/science.1152066 [DOI] [PubMed] [Google Scholar]
- Yu J., Tiwari S., Steiner P. and Zhang L. (2003). Differential apoptotic response to the proteasome inhibitor bortezomib (VELCADETM, PS-341) in bax-deficient and p21-deficient colon cancer cells. Cancer Biol. Ther. 2, 694-699. 10.4161/cbt.2.6.573 [DOI] [PubMed] [Google Scholar]
- Zeng Z., Rulten S. L., Breslin C., Zlatanou A., Coulthard V. and Caldecott K. W. (2017). Acylpeptide hydrolase is a component of the cellular response to DNA damage. DNA Repair 58, 52-61. 10.1016/j.dnarep.2017.08.008 [DOI] [PubMed] [Google Scholar]
- Zhang X., Ye J., Engholm-Keller K. and Højrup P. (2011). A proteome-scale study on in vivo protein Nα-acetylation using an optimized method. Proteomics 11, 81-93. 10.1002/pmic.201000453 [DOI] [PubMed] [Google Scholar]
- Zhou Y. and Hancock J. F. (2015). Ras nanoclusters: versatile lipid-based signaling platforms. Biochim. Biophys. Acta 1853, 841-849. 10.1016/j.bbamcr.2014.09.008 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Prakash P., Liang H., Cho K.-J., Gorfe A. A. and Hancock J. F. (2017). Lipid-sorting specificity encoded in K-Ras membrane anchor regulates signal output. Cell 168, 239-251.e16. 10.1016/j.cell.2016.11.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G., Papke B., Ismail S., Vartak N., Chandra A., Hoffmann M., Hahn S. A., Triola G., Wittinghofer A. and Bastiaens P. I. H. (2013). Small molecule inhibition of the KRAS–PDEδ interaction impairs oncogenic KRAS signalling. Nature 497, 638 10.1038/nature12205 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.