ABSTRACT
Motile cilia generate directed hydrodynamic flow that is important for the motility of cells and extracellular fluids. To optimize directed hydrodynamic flow, motile cilia are organized and oriented into a polarized array. Basal bodies (BBs) nucleate and position motile cilia at the cell cortex. Cytoplasmic BB-associated microtubules are conserved structures that extend from BBs. By using the ciliate, Tetrahymena thermophila, combined with EM-tomography and light microscopy, we show that BB-appendage microtubules assemble coincidently with new BB assembly and that they are attached to the cell cortex. These BB-appendage microtubules are specifically marked by post translational modifications of tubulin, including glycylation. Mutations that prevent glycylation shorten BB-appendage microtubules and disrupt BB positioning and cortical attachment. Consistent with the attachment of BB-appendage microtubules to the cell cortex to position BBs, mutations that disrupt the cellular cortical cytoskeleton disrupt the cortical attachment and positioning of BBs. In summary, BB-appendage microtubules promote the organization of ciliary arrays through attachment to the cell cortex.
KEY WORDS: Cilia, Basal bodies, Microtubule, Ciliary array, Multiciliated cells, Tubulin post-translational modifications
Highlighted Article: Motile cilia are positioned at the cell cortex by basal bodies (BB). Tubulin glycylation promotes elongation of BB-associated microtubules and structural attachment of BBs to the cortical cytoskeleton.
INTRODUCTION
Motile cilia produce directed fluid flow to enable fundamental biological activities, including cell movement through aqueous environments and mucociliary clearance in respiratory airways. To maximize fluid flow, cells employ hundreds of motile cilia that are arranged into polarized arrays. Each cilium beats in a coordinated and asymmetric pattern to generate fluid flow. Cilia are nucleated, positioned and oriented at the cell cortex by basal bodies (BBs). BBs comprise a cylinder of nine symmetrically arranged triplet microtubules. The distal ends of BBs attach to the cell cortex through fibers that symmetrically emanate from the ends of each microtubule triplet (Burke et al., 2014; Siller et al., 2017; Yang et al., 2018). Additional BB-associated structures project asymmetrically from BBs, linking them to other cytoskeletal elements within the cell cortex (Herawati et al., 2016; Kunimoto et al., 2012; Tateishi et al., 2017; Turk et al., 2015; Iftode et al., 1996). Such linkages ensure BB organization and orientation even when exposed to forces produced by motile cilia (Mahuzier et al., 2018; Galati et al., 2014; Werner et al., 2011). Thus, cilia position and polarity are directed by the linear organization and orientation of BBs.
Microtubules associated with BBs promote cilia organization. In mouse ciliated epithelial cells, BB-associated microtubules create a network of interconnected BBs that organize and maintain the correct spacing and orientation of cilia (Kunimoto et al., 2012; Tateishi et al., 2017; Herawati et al., 2016). BB-associated microtubules in multi-ciliated epithelia of Xenopus laevis skin ensure uniform spacing between BBs, which is important for effective hydrodynamic flow (Werner et al., 2011; Elgeti and Gompper, 2013). How BB-appendage microtubules connect to the rest of the cell is not yet known.
The tubulin subunits in BBs, cilia and BB-associated microtubules undergo post-translational modifications (PTMs), including acetylation, glutamylation and glycylation (Akella et al., 2010; Redeker et al., 1994; Vladar et al., 2012; Janke et al., 2005; Valenstein and Roll-Mecak, 2016; Xu et al., 2017). Microtubule PTMs contribute to microtubule plus-end dynamics, force production by motor proteins and mechanical properties of microtubules (Gadadhar et al., 2017a; Janke, 2014). Acetylation occurs through enzymatic activity of the α-tubulin acetyltransferase 1 (ATAT1, also known as αTAT1 and MEC17) at the lysine (K) 40 residue of α-tubulin located in the lumen of microtubules (Akella et al., 2010; Shida et al., 2010). Most other tubulin modifications occur at α- and β-tubulin C-terminal tails that are exposed on the microtubule outer surface. Polymeric tail modifications are catalyzed by the tubulin tyrosine ligase-like (TTLL) family of enzymes (Wloga et al., 2009; Janke et al., 2005; Rogowski et al., 2009). Microtubule glycylation is initiated by addition of a glycine to a glutamic acid side chain within the tubulin C-terminal tail sequence (Redeker et al., 1994). This monoglycylation is catalyzed by Ttll3 or Ttll8. Monoglycylation may be followed by addition of multiple glycine residues, a process that is catalyzed by the polyglycylase, Ttll10 (Rogowski et al., 2009; Wloga et al., 2009). Polyglycylases require monoglycylated microtubules as a substrate; i.e. when monoglycylation is absent, microtubules are not glycylated (Ikegami and Setou, 2009; Rogowski et al., 2009). Ttll3-dependent glycylation promotes cilia formation and elongation, presumably by affecting properties of axonemal microtubules (Bosch Grau et al., 2017; Wloga et al., 2009; Gadadhar et al., 2017b). Morpholino oligomers targeting zebrafish Ttll3 disorient cilia in pronephric ciliary arrays, suggesting that tubulin glycylation is important for either BBs or cilia (Pathak et al., 2011). However, how glycylation promotes cilia orientation is still unknown.
Like vertebrates and other protists, Tetrahymena cells have BB-associated microtubules or BB-appendages that are important to organize and orient cilia and BBs into polarized arrays. BB-appendage microtubules undergo PTMs; however, a role for these modifications in organizing and orienting cilia and BBs has not been identified (Callen et al., 1994; Akella et al., 2010; Tassin et al., 2015; Wloga et al., 2008). BBs are endowed with three BB-appendage structures: striated fibers (SFs), transverse microtubules (tMTs) and post-ciliary microtubules (pcMTs). SFs extend towards the cell anterior, and establish and maintain BB organization and orientation (Galati et al., 2014; Jerka-Dziadosz et al., 1995). tMTs and pcMTs are composed of microtubule bundles that nucleate from the BB base and extend transversely and posteriorly, respectively, towards the cortical cytoskeleton of the cell (Fig. 1). The consistent geometric orientation of the three BB-appendages is suggested to be ideal to secure BBs to the cell cortex while ensuring BB organization and orientation (Allen, 1967; Iftode et al., 1996; Pitelka, 1961). However, the development, molecular regulators and functions of BB-appendage microtubules in creating connections with the cell cortex have not yet been closely studied.
Fig. 1.
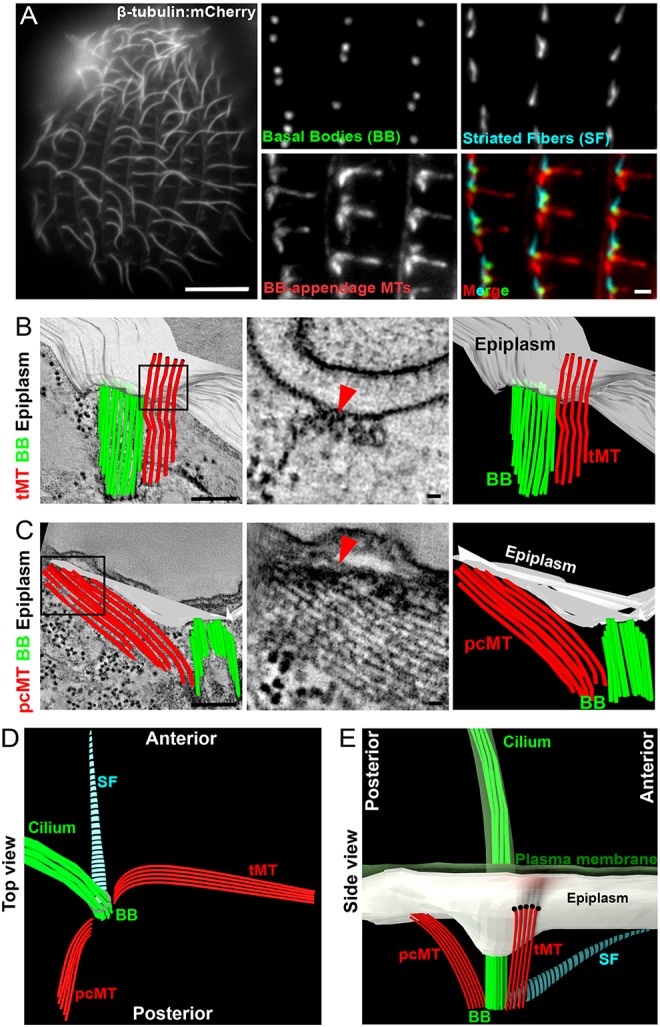
The BB-associated cortical cytoskeleton forms an organized pattern. (A) Left panel: Fluorescence image of the Tetrahymena microtubule and cilia array (β-tubulin:mCherry, grayscale). Scale bar: 10 µm. Right panels: BBs (Centrin, green), transverse (tMT) and post-ciliary (pcMT) microtubules (acetylated tubulin, red), and striated fibers (Bbc39:mCherry, cyan). Scale bar: 1 µm. (B) Left panel: 3D model of epiplasm (white), BB (green) and tMTs (red) projected on a tomographic slice. Boxed region highlights the tMT bundle running directly below the epiplasm. Scale bar: 200 nm. Middle panel: Tomographic slice from the boxed region shows tMT connections with the cortical epiplasm (red arrowhead). Scale bar: 20 nm. Right panel: 3D model of the BB unit derived from EM tomographic reconstruction. (C) Left panel: 3D model of epiplasm (white), BB (green) and pcMTs (red) projected on a tomographic slice. Boxed region highlights pcMTs ending in the epiplasm. Scale bar: 200 nm. Middle panel: projected tomographic slices from the boxed region showing pcMTs ending in the cortical epiplasm (red arrowhead). Scale bar: 20 nm. Right panel: 3D model of the BB unit derived from EM tomographic reconstruction. (D) Top view model of a cilium: BB and BB-appendages. (E) Longitudinal view model of a cilium, BB and BB-appendages relative to the plasma membrane and the epiplasm.
The cortical cytoskeleton is a network of cytoskeletal filaments that lies just below the plasma membrane. This cortical cytoskeleton affects cell morphology, surface tension and elasticity, while ensuring the positioning and orientation of BBs and their associated motile cilia (Discher et al., 1994; Zhang et al., 2017; Mahuzier et al., 2018; Herawati et al., 2016; Werner et al., 2011). During new BB assembly in ciliates, BBs are inserted through and attached to the cortical cytoskeleton (Aubusson-Fleury et al., 2013; Argetsinger, 1965; Allen, 1967; Pitelka, 1961). A main component of the ciliate cortical cytoskeleton, the epiplasm, is composed of intermediate filament-like proteins that form a dense fibrous network below the plasma membrane (Pitelka, 1961; Allen, 1967; Bouchard et al., 2001). Epiplasmin C (Epc1) is the most abundant epiplasm component in Tetrahymena, and epc1Δ mutants exhibit BB disorganization (Williams, 2004). Moreover, BB-appendage microtubules are physically linked to the epiplasm (Hufnagel, 1969; Allen, 1971, 1967; Iftode et al., 1996; Pitelka, 1961). These early studies postulate that BB-appendages are firmly attached to the epiplasm to maintain BB organization and orientation, while resisting mechanical forces from beating cilia that would otherwise cause BBs to disorganize and disorient.
Here, we investigated the development of BB-appendage microtubules following new BB assembly, and the molecular events that promote BB cortical attachment, positioning and orientation within ciliary arrays. To understand the molecular bases that govern the growth and attachment of BB-associated microtubules to the cortical cytoskeleton, we examined the effects of microtubule PTMs on BB organization. We identified microtubule glycylation to be required for normal elongation of BB-appendage microtubules, and normal cortical attachment, organization and orientation of BBs. Thus, microtubule glycylation has an important role in promoting BB and cilia positioning through associations with the cell cortex.
RESULTS
BB-associated microtubules contact the cortical cytoskeleton
Tetrahymena ciliary arrays are composed of hundreds of motile cilia (Fig. 1A). Cilia are nucleated and positioned by BBs, which have three appendage structures: SFs, tMTs and pcMTs. BBs and their associated appendage structures are organized into polarized rows, and the direction each appendage extends to is uniform (Wloga and Frankel, 2012; Allen, 1967). We observed similar orientation by using immunofluorescence (Fig. 1A and Fig. S1A-D). Gaps in the labeling of acetylated tMTs along the lengths of the tMTs are attributed to masking of epitopes rather than a discontinuity within the microtubule structure. Thin section electron microscopy of Paramecium and Tetrahymena identified linkages between BB-appendage microtubules and the cortical epiplasm (Iftode et al., 1996; Allen, 1967; Pitelka, 1961). By using the improved 3D spatial resolution of electron tomography, we show that BB-appendage microtubules extend toward the cell cortex and contact the cortical epiplasm. tMTs extend from the anterior side of the BB toward the adjacent ciliary row (Fig. 1A,B; Allen, 1967). Electron-dense linkages are detected at sites where the tMTs and the epiplasm are in close proximity (Fig. 1B, Fig. S1E and Movie 1). Similarly, pcMTs emerge from the posterior face of BBs and extend posteriorly to contact the cortical epiplasm when at two unique conformations (Fig. 1C, Fig. S1F and Movie 2). First, the pcMT tips are embedded in an electron-dense region of the epiplasm. Second, the pcMT ends connect with the cortical epiplasm through microtubule end-on linkages between the bundles of microtubules that run perpendicular to the cortical epiplasm. The two conformations of pcMT interactions with the cortical epiplasm might reflect distinct mechanisms of BB anchoring and unique roles in resisting mechanical forces from beating cilia. Thus, BBs and BB-appendage microtubules are organized within ciliary arrays, and are connected by distinct structural mechanisms to the cortical epiplasm (Fig. 1B-E and Fig. S1E,F).
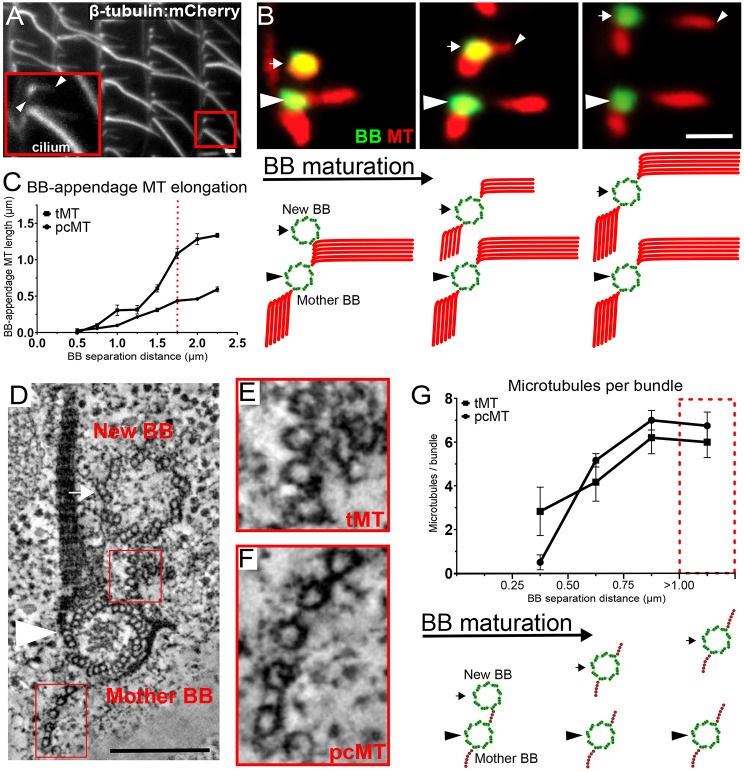
BB-appendage microtubules nucleate and elongate during maturation of new BBs
During Tetrahymena cellular growth, new BBs assemble into and expand the ciliary array (Nanney et al., 1975; Allen, 1969). New BBs form at the base of ciliated mother BBs, and mature as they migrate and separate in the anterior direction of the cell, away from the mother BB (Fig. 2). The separation distance between mother and daughter BBs serves as a proxy for BB maturity (Pearson et al., 2009a; Perlman, 1973; Nanney et al., 1975). Once a new BB has nucleated a cilium, it is considered mature. Because the maximum frequency of ciliated BBs (86%) was observed at a separation distance of 1.75 µm from the mother BB (Fig. S2A), BBs separated by <1.75 µm are, on average, still developing. To determine when maturing BBs acquire BB-appendage microtubules, the length of BB-appendage microtubules was measured relative to the BB separation distance (Fig. 2A-C). BBs and BB-appendage microtubules were labeled with mCherry (β-tubulin:mCherry). The tMTs and pcMTs of new BBs initiate elongation when new BBs can be resolved (by light microscopy) from mother BBs. As new BBs mature, BB-appendage microtubules elongate to a mature mean length of 1.4 µm and 0.5 µm for tMTs and pcMTs, respectively. Most BBs are ciliated at a separation distance of 1.75 µm (Fig. S2A), the same distance at which elongation of BB-appendage microtubules begins to plateau, suggesting that tMTs and pcMTs complete elongation coincidentally with ciliogenesis. BB-associated microtubules are formed of linear microtubule bundles (Fig. 2D-F). The pcMT and tMT bundles possess crosslinking densities between individual microtubules, thereby creating equal spacing between microtubules (Fig. 2E,F). Using EM tomography, we measured the total number of BB-appendage microtubules of new BBs relative to the distance they were separated from mother BBs (Fig. 2G and Fig. S2B). New BBs adjacent to their mother BBs do not possess BB-appendage microtubules. The number of microtubules in both tMT and pcMT bundles increases as new BBs mature, reaching a maximum of six microtubules. Thus, bundles of BB-appendage microtubules are of full length and number before ciliogenesis. We hypothesize that BB-appendage microtubules achieve cortical attachment prior to ciliogenesis and before BBs experience ciliary forces. This may preserve the organization and orientation of BBs.
Fig. 2.
BB-appendage microtubule assembly during new BB maturation. (A) BBs with associated cilia and BB-appendage microtubules (β-tubulin: mCherry, grayscale). Inset shows a mother and new BB with arrowheads denoting the tMTs and pcMTs of the new BB. Scale bar: 1 µm. (B) Top panels: tMTs and pcMTs elongation is coincident with BB maturation, as determined by the separation distance between mother (arrowheads) and new BBs (arrows). BBs (Centrin, green) and microtubules (acetylated-tubulin, red). Gap in tMTs is likely due to loss of antibody accessibility. Scale bar: 1 µm. Bottom panels: Model of BB-appendage elongation with BB maturation, corresponding to images above. Arrows indicate new BBs, arrowheads indicate mother BBs. (C) tMTs and pcMTs elongate during BB maturation (n=82 cells, 422 tMTs, 528 pcMTs). Dotted red line denotes BB separation distances when most BBs are ciliated (Fig. S2A). Quantification was performed on β-tubulin:mCherry cells (Fig. 2A). (D) EM tomographic slice of a mother BB and new BB pair. Scale bar: 200 nm. (E,F) Insets of the mother BB tMT (E) and pcMT (F). Inset width 100 nm. (G) Top panel: The number of microtubules (red circles) in tMT and pcMT bundles increases with BB maturation (n=5 tomograms, 17 new BBs and corresponding tMT and pcMT bundles). Bottom panel: Model showing the increasing number of BB-appendage microtubules with BB maturation.
Glycylation promotes BB organization and orientation
BBs and BB-appendage microtubules are dynamic structures that experience maturation, movement and ciliary forces. BBs and BB-appendage microtubules carry several types of conserved tubulin PTMs, which might be important in these processes. To determine the localization of tubulin PTMs at BBs and BB-appendage microtubules, we utilized immunofluorescence imaging of PTM-specific antibodies (Fig. 3). Glutamylated tubulin is enriched at BBs but is not detected at BB-appendage microtubules, consistent with our previous findings (Bayless et al., 2016). Glutamylation of tMTs and pcMTs has been described previously (Wloga et al., 2008). However, after exploring multiple immunofluorescence sample preparations, we found that glutamylated tubulin predominantly localized to BBs and detected only low levels of glutamylated tubulin at tMTs and pcMTs. In contrast, acetylated and glycylated tubulin are both present at tMTs and pcMTs (Fig. 3; Thazhath et al., 2002; Tassin et al., 2015; Iftode et al., 2000). Antibodies recognizing microtubule monoglycylation and polyglycylation mark both tMTs and pcMTs. These observations indicate that acetylation and glycylation tubulin PTMs localize to BB-appendage microtubules.
Fig. 3.

BB-appendage microtubules are enriched with acetylation and glycylation PTMs. Top panel: Image of Tetrahymena cell BBs (Centrin, green) and microtubules (acetylated-tubulin, red). Scale bar: 10 µm. Bottom panels: Images show localization of tubulin and tubulin PTMs (red) relative to the BBs (Centrin, green). White arrows denote the locations of tMTs and pcMTs. Scale bar: 1 µm.
To test whether microtubule acetylation promotes BB organization, we visualized BB organization in mutants that lack microtubule acetylation. Both mec17Δ (acetyltransferase knockout) and atu1-K40R (non-acetylatable α-tubulin mutant) mutants do not show detectable microtubule acetylation (Gaertig et al., 1995; Akella et al., 2010). However, both mutant cell lines exhibit normal BB organization (Fig. S2C,D).
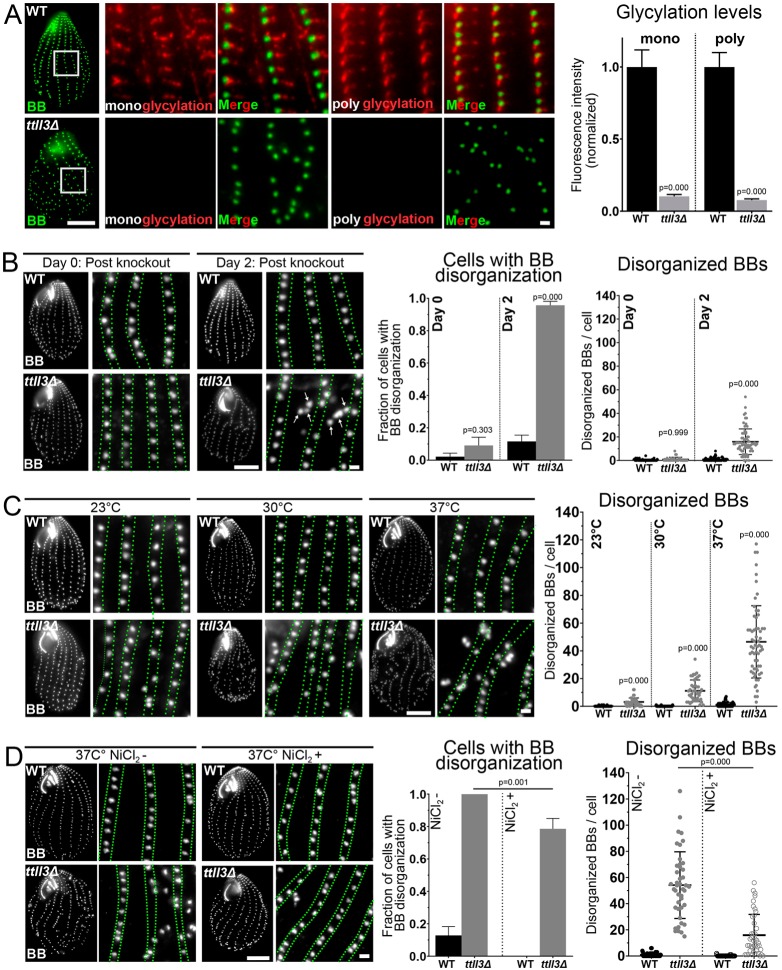
Glycylation modifies tubulin C-terminal tails and is present at BB-appendage microtubules (Fig. 3). Ttll3A, B, C, D, E and F include all Tetrahymena proteins with homology to the Ttll3 and Ttll8 monoglycylases. To test whether microtubule glycylation promotes BB organization, we examined the glycylase mutants ttll3aΔ, ttll3bΔ, ttll3cΔ, ttll3dΔ, ttll3eΔ and ttll3fΔ (hereafter referred to as ttll3Δ) (Wloga et al., 2009). After TTLL3 knockout, cells lose microtubule glycylation (Fig. 4A). Prior studies of these mutants reported reduced rates of cell multiplication and shorter cilia but not BB organization defects (Wloga et al., 2009). However, we show ttll3Δ cells to exhibit BB disorganization. BB disorganization is defined as cells with more than three BBs that deviate from the ciliary row axis by at least 1 µm. Immediately after TTLL3 knockout (day 0), BBs are organized correctly in ttll3Δ cells (Fig. 4B). This is likely because the progeny cells have remaining parental Ttll3 protein (Fig. 4B and Fig. S3A). The BB disorganization increases and peaks 2 days after TTLL3 knockout, when 94% of ttll3Δ cells possess disorganized BBs. The severity of BB disorganization in the ttll3Δ cells ranges from 3 to 54 disorganized BBs per cell (Fig. 4B and Fig. S3A). Moreover, cell morphology is commonly disrupted in cells with BB disorganization. In summary, Ttll3, likely through microtubule glycylation, is important for organizing BBs within ciliary arrays.
Fig. 4.
Microtubule glycylation promotes BB organization to resist forces from ciliary beating. (A) Left-most panels: WT and ttll3Δ BBs (Centrin, green). Scale bar: 10 µm. Images on right show loss of mono- and poly-glycylation in ttll3Δ cells two days after TTLL3 knockout (BB, Centrin, green; BB-appendage microtubules, glycylation, red). Scale bar: 1 µm. Right panel: ttll3Δ cells have reduced glycylation (WT monoglycylation: n=42 cells, ttll3Δ monoglycylation: n=33 cells, WT polyglycylation: n=34 cells, ttll3Δ polyglycyation: n=42 cells). (B) Left panels: WT and ttll3Δ cells cultured at 30°C 0 and 2 days post knockout (BB, Centrin, grayscale). Green lines denote boundaries of BB rows. Arrows denote disorganized BBs. Scale bars: 10 and 1 (inset) µm. Right panels: Increased fraction of ttll3Δ cells have BB disorganization on Day 2 (WT day 0: n=46 cells, ttll3Δ day 0: n=33 cells, WT day 2: n=69 cells, ttll3Δ day 2: n=70 cells). ttll3Δ cells at 30°C have more disorganized BBs per cell on Day 2 (WT day 0: n=46 cells, ttll3Δ day 0: n=33 cells, WT day 2: n=69 cells, ttll3Δ day 2: n=70 cells). (C) Left panels: Images of WT and ttll3Δ cells cultured at 23°C, 30°C and 37°C (BB, Centrin, grayscale). Green lines denote boundaries of BB rows. Scale bars: 10 and 1 (inset) µm. Right panels: ttll3Δ cells have increasing BB disorganization with increasing temperature (WT 23°C: n=35 cells, ttll3Δ 23°C: n=42 cells, WT 30°C: n=40 cells, ttll3Δ 30°C: n=43 cells, WT 37°C: n=54 cells, ttll3Δ 37°C: n=63 cells). (D) Left panels: WT and ttll3Δ cells cultured at 37°C in the presence or absence of NiCl2 (BB, Centrin, grayscale). Green lines denote boundaries of BB rows. Scale bars: 10 and 1 (inset) µm. Right panels: NiCl2 treatment of ttll3Δ cells partially reduces the fraction of cells with BB (middle graph) disorganization and the severity of BB disorganization (WT NiCl2-: n=40 cells, ttll3Δ NiCl2-: n=43 cells, WT NiCl2+: n=38 cells, ttll3Δ NiCl2+: n=42 cells).
Motile cilia are oriented along the anterior–posterior axis of the cell (Fig. 1). To determine whether BBs in ttll3Δ cells have orientation defects, we quantified the orientation of BBs in ttll3Δ cells that were either inside (organized) or outside (disorganized) of ciliary rows, by using the SF BB-appendage as a BB polarity marker (Fig. S3B). Circular statistics was used to assess BB orientation. An R-value of 1.00 indicates perfectly orientated BBs, whereas an R-value of 0.00 indicates random BB orientation. ttll3Δ BBs inside ciliary rows (organized) were 6% less oriented (R-value=0.93) than WT BBs (R-value=0.99). Additionally, disorganized ttll3Δ BBs were less oriented than organized ttll3Δ BBs (R-value=0.75). Disorganized BBs were not detected in WT cells. Thus, microtubule glycylation promotes the organization and orientation of BBs.
Glycylation stabilizes BB organization against forces from ciliary beating
BBs remain organized and oriented while anchoring motile cilia that produce mechanical forces. Ciliary beat frequency and ciliary forces transmitted to BBs can be manipulated in Tetrahymena by increasing the temperature or viscosity of the medium (Galati et al., 2014; Pearson et al., 2009b; Goto et al., 1982). Disorganization of BBs in ttll3Δ cells was found to recover at lower (23°C) temperatures (Fig. 4C and Fig. S3A). Because low temperatures reduce ciliary beating, this result suggested that the BB organization phenotypes in ttll3Δ cells are responsive to ciliary forces. After prolonged culture at 23°C, ttll3Δ cells were shifted to 30°C or 37°C for 24 h (Fig. 4C). 93% of ttll3Δ cells shifted to 30°C exhibited an intermediate level of BB disorganization. Finally, after 24 h at 37°C, 100% of ttll3Δ cells showed severe disorganization of BBs. The severe BB disorganization in ttll3Δ cells at 37°C was reduced when ciliary beating was decreased with NiCl2, an inhibitor of ciliary inner dynein arms (Fig. 4D; Larsen and Satir, 1991). Tetrahymena cells divide more rapidly at elevated temperatures and biogenesis and organization of new BBs is maintained through these rapid cell divisions (Frankel, 1962; Frankel and Nelsen, 2001; Hallberg and Hallberg, 1983). To further test whether the BB disorganization in ttll3Δ cells at elevated temperature is a result of increased ciliary forces instead of aberrant maturation due to increased cell division and BB assembly rates, we measured cell division rates of ttll3Δ cells treated with and without NiCl2 (Fig. S3C). No difference was observed, suggesting that rapid BB assembly and maturation is not responsible for BB disorganization in ttll3Δ cells. Surprisingly, BB disorganization was not elevated in ttll3Δ cells that were exposed to medium of increased viscosity, which also elevates mechanical forces on cilia (Fig. S3D). This suggests that glycylation protects the organized ciliary array from temperature-induced high frequency ciliary forces but has no role in resisting viscosity-induced elevated ciliary load. This is in contrast to strains carrying a mutated version of the SF BB-appendage protein DISorientation of basal bodies A (DisA; TTHERM_00941400), which are sensitive to increases in ciliary load, and suggests that different appendage structures have distinct roles at the BB by counteracting forces due to ciliary beating (Galati et al., 2014; Jerka-Dziadosz et al., 1995). In summary, tubulin glycylation is important to maintain the organization of BBs when confronted with rapid ciliary beating.
Our studies suggest that tubulin glycylation is important for maintaining the organization and orientation of BBs even in the face of ciliary forces. Tetrahymena cells are asymmetric, with dorsal–ventral and anterior–posterior axes, and we hypothesized that specific regions of the cell are more sensitive to ciliary forces. To test whether specific regions of the Tetrahymena cell are more sensitive to BB disorganization, we quantified the position of disorganized BBs. We found that BB disorganization is most frequent in medial and posterior regions along the dorsal axis of the cell (Fig. S4A). This suggests that BBs and BB-appendage microtubules at the dorsal and medial regions of Tetrahymena cells are subjected to greater physical forces from beating cilia.
The disorganization and disorientation of BBs observed in ttll3Δ cells suggests that tubulin glycylation promotes attachments between BBs and the cell cortex. To determine whether BBs in ttll3Δ cells are cortically detached, we visualized BBs relative to the cell cortex. Cortically detached BBs were defined as BBs that are internally separated from the cell cortex by >1 µm. At 30°C, 24% of ttll3Δ cells possessed BBs that were detached from the cell cortex compared to 5% of WT cells (Fig. 5A and Fig. S4B). Cortically detached BBs were observed as either individual BBs that were 1–3 µm away from the cell cortex or as clustered BBs located within the cell's interior (Fig. S4B). In summary, reduced microtubule glycylation results in detachment of BBs from the cell cortex.
Fig. 5.
Glycylation promotes BB cortical attachment and BB-appendage microtubule length. (A) Left panels: Images of longitudinal sections through BBs (Centrin, green) and epiplasm (Epc1, grayscale) in WT and ttll3Δ cells. Right panel, graph showing ttll3Δ cells have more cortically detached BBs at 30°C (WT: n=55, ttll3Δ: n=63). Scale bar: 1 µm. (B) Left panels: Images show BBs (Centrin, green) and BB-appendage microtubules (α-tubulin, red) in WT and ttll3Δ cells at 30°C. Scale bar: 1 μm. Right panel: Graph showing tMTs and pcMTs of mature BBs are shorter in ttll3Δ cells (WT: n=67 cells, 156 tMTs, 194 pcMTs; ttll3Δ: n=75 cells, 141 tMTs, 155 pcMTs).
Glycylation promotes BB-appendage length
The reductions in attachment, organization and orientation of BBs in ttll3Δ mutant cells suggest that glycylation promotes positioning of BBs by promoting their attachment to the cortical epiplasm. BB-appendage microtubules are near their fully elongated state by the time of ciliogenesis, when daughter BBs are separated from mother BBs by 1.75 µm (Fig. 2). We, therefore, hypothesized that elongation of BB-appendage microtubules ensures BB attachment to the cell cortex and next explored whether glycylation promotes the normal lengths of BB-appendage microtubules. To quantify the lengths of tMTs and pcMTs in WT and ttll3Δ cells, antibodies specific to BBs and α-tubulin were used to mark these structures, and the distances between the BB centroid to the ends of the tMTs and pcMTs were measured. BBs less than 1.75 µm apart were considered immature. tMTs and pcMTs associated with both developing and mature BBs are shorter in ttll3Δ cells than in WT controls (Fig. 5B and Fig. S4C). Therefore, microtubule glycylation accelerates the rate of new BB-appendage microtubule elongation and promotes increased length for these microtubules at maturity.
The shorter BB-appendage microtubules in ttll3Δ cells lead us to the hypothesis that BBs with shorter appendage microtubules are more likely to be disorganized. To determine if disorganized BBs in ttll3Δ cells have shorter BB-appendage microtubules than organized BBs, we measured the distances between the BB centroid and the ends of tMTs and pcMTs of organized and disorganized BBs. These measurements were analyzed independently of BB maturity, revealing that tMTs and pcMTs of disorganized BBs are shorter than those of organized BBs (Fig. S4D).
BB organization and attachment to the cortical epiplasm
BB-appendage microtubules contact the cell cortex by attaching to the cortical epiplasm that lies just below the plasma membrane (Fig. 1 and Fig. S1). Therefore, we hypothesized that the disruption of the cortical epiplasm would produce BB positioning defects that are analogous to those observed in ttll3Δ mutant cells. Indeed, BB organization and cortical attachment is disrupted in epc1Δ epiplasm mutant cells (Fig. 6; Williams, 2004). Consistent with our observations in ttll3Δ cells, Williams reported that, after EPC1 knockout, BB organization returned to normal within ∼20 generations. The BB-appendage microtubules of organized BBs in epc1Δ cells display normal orientation but, as expected, this orientation is lost in disorganized BBs (Fig. S5A). To test if Epc1 has a role in maintaining BB organization when ciliary forces are increased, we measured BB disorganization in epc1Δ cells cultured at elevated temperatures. At 23°C, epc1Δ cells did not exhibit detectable BB disorganization. However, upon shifting epc1Δ cells to 30°C and 37°C, BB disorganization increased (Fig. 6B). Thus, the epiplasm is part of the system that maintains BB organization in the face of ciliary forces.
Fig. 6.
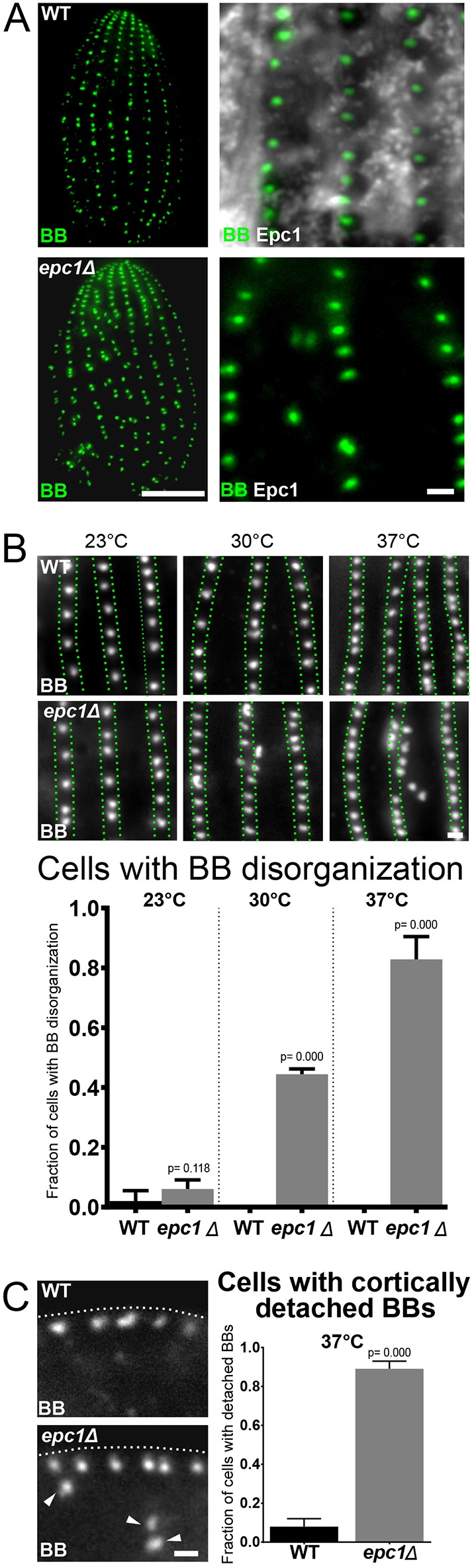
Cortical epiplasm is required for BB organization and cortical attachment. (A) Images showing the dorsal side (side without oral apparatus) of WT and epc1Δ cells labeled for BBs (Centrin, green). Scale bar: 10 µm. Magnified images from of WT and epc1Δ cells showing BBs (Centrin, green) and epiplasm (Epc1, grayscale). Scale bar: 1 µm. epc1Δ cells have disorganized BBs. (B) Top panels: Images of WT and epc1Δ cells grown at 23°C, 30°C and 37°C for 24 h, labeled for BBs (Centrin, grayscale). Scale bar: 1 µm. Green dotted lines denote boundaries of BB rows. Bottom panels: Elevated temperature increases BB disorganization in epc1Δ cells (WT 23°C: n=99 cells, epc1Δ 23°C: n=99 cells, WT 30°C: n=99 cells, epc1Δ 30°C: n=99 cells, WT 37°C: n=99 cells, epc1Δ 37°C: n=99 cells). (C) Left panel: Images showing a longitudinal section through BBs (Centrin, grayscale) at the cell cortex in WT and epc1Δ cells. Arrowheads denote BBs that are detached from the cell cortex. Dotted white line denotes the location of cell cortex as determined by the cellular fluorescence background. Scale bar: 1 µm. Right panel: Graph shows quantification of the proportion of cells with detached BBs. At 37°C, epc1Δ cells have more cortically detached BBs than WT cells (WT 37°C: n=63 cells, epc1Δ 37°C: n=64 cells).
The role of Epc1 in organizing BBs and, possibly, in resisting forces from cilia beating suggests that the epiplasm is important to anchor BBs to the cell cortex. To test this, we measured the cortical attachment of BBs in epc1Δ cells cultured at 37°C, when BBs are severely disorganized. BB attachment to the cell cortex was determined by BB localization relative to the cell cortex. 90% of epc1Δ cells exhibited cortically detached BBs (Fig. 6C). Together, this suggests that the epiplasm and glycylated BB-appendage microtubules are both important for organizing and cortically attaching BBs.
DISCUSSION
Cilia and BBs are attached to the cell cortex, and organized and oriented within polarized arrays to produce an effective hydrodynamic flow. BB-associated microtubules are vital to ensure this organization (Werner et al., 2011; Herawati et al., 2016; Kunimoto et al., 2012). We show that Tetrahymena BB-appendage microtubules nucleate and elongate from new BBs coincidently with BB maturation, so they are poised for cortical attachment by the time of ciliogenesis. Ttll3 glycylases, probably through microtubule glycylation, promote efficient elongation of BB-appendage microtubules, and maintain BB organization and orientation. Further, both BB-appendage microtubules and the cortical cytoskeleton they attach to promote BB attachment and organization. This study illuminates the importance of post-translationally modified BB-associated microtubules in connecting BBs to the cortical cytoskeleton, in order to resist forces from beating cilia.
BB-appendage microtubule length for cortical attachment of BBs
Ttll3 knockdown in zebrafish reduces polarized ciliary beating, suggesting that tubulin glycylation is important for either cilium beating and/or BB orientation (Pathak et al., 2011). In ciliates, BB-appendage microtubules are post-translationally modified; however, it was so far unknown whether and how PTMs promote BB organization and orientation (Fig. 3; Wloga et al., 2009; Callen et al., 1994; Tassin et al., 2015; Gaertig et al., 1995). Here, we provide the first evidence that glycylation of Tetrahymena microtubules promotes BB organization, orientation and cortical attachment.
Tubulin glycylation occurs at cilia, BBs and BB-appendage microtubules. BBs are glycylated, and this may be responsible for promoting BB attachment to the cell cortex. However, we favor a model where BB-appendage microtubules contribute to attachment of BBs to the cell cortex and to BB organization. Loss of microtubule glycylation reduces the length of BB-appendage microtubules and disorganizes BBs. Given that BB-appendage microtubules in ttll3Δ cells are shorter during BB maturation, microtubule glycylation is likely to affect elongation of these microtubules. BB-appendage microtubules of disorganized BBs are shorter than those of organized BBs in ttll3Δ cells (Fig. S4D). This is consistent with the role of tubulin glycylation in promoting the elongation of Tetrahymena cilia as well as in maintaining the lengths of mouse ependymal cells and retinal cell cilia (Bosch Grau et al., 2013, 2017; Wloga et al., 2009). Moreover, BB maturation and ciliogenesis are delayed in ttll3Δ cells (Fig. S4E). Thus, glycylation might have a general role in the development of BBs as they become competent to assemble cilia and appendage structures. We suggest that the length and development of BB-appendage microtubules is important for cortical contacts in order to anchor BBs and reduce BB mobility resulting from ciliary beating forces.
Upon cilia formation and beating, BBs experience mechanical forces from undulating cilia (Galati et al., 2014; Pearson et al., 2009b; Bayless et al., 2016). Consistent with this, when BB-appendage microtubules or the cortical cytoskeleton are disrupted, BB disorganization is increased when ciliary beating is elevated by increasing temperature. Reduced ciliary beating – even at high temperatures – reduces this BB disorganization. Because cilia defects occur in ttll3Δ cells, ciliary forces on BBs are likely to be reduced; and this, in turn, decreases the disorganization. If normal ciliary forces were imposed upon ttll3Δ BBs, we expect that the BB disorganization would increase. Surprisingly, ttll3Δ mutants are not sensitive to increased ciliary load when cells are subjected to increased viscosity. This contrasts the effects on the disA-1 SF mutant, which is sensitive to both elevated temperature and viscosity (Galati et al., 2014). Elevated viscosity has been reported to both alter ciliary and flagellar waveforms and to decrease ciliary beat frequency (Sleigh, 1966; Rikmenspoel, 1971; Minoura and Kamiya, 1995; Machemer, 1972). Ciliary dynein arm mutations alter the ciliary beat pattern and frequency when exposed to higher viscosity media, underscoring the importance of ciliary structure for cellular response to high viscosity conditions (Wilson et al., 2015). Because ttll3Δ mutants harbor shorter cilia, we suspect that ciliary beat patterns of these mutants are affected in ttll3Δ mutant cells at high viscosity to somehow reduce the forces transduced to BBs. Regardless, glycylation of BB-appendage microtubules promotes their elongation and attachment to the cell cortex in order to resist mechanical forces from ciliary beating.
Glycylation of BB-appendage microtubules for cortical attachment of BBs
As BB-appendage microtubule bundles elongate, the microtubule surface area juxtaposed to the epiplasm increases (Fig. 1D,E). We hypothesize that this increases the number of cortical contacts that anchor BBs and reduce BB movements that result from forces of ciliary beating. Tubulin glycylation modifies the surface of microtubules by creating a layer of flexible, uncharged residues (Wall et al., 2016; Gadadhar et al., 2017a). Thus, in addition to promoting growth of BB-appendage microtubules, glycylation may also control the accessibility of the charged residues on the C-terminal tail of tubulin. This may alter protein associations with these microtubules and promote BB organization by increasing the affinity of BB-appendage microtubules for the epiplasm. Thin-section EM studies and our electron tomography data show electron-dense connections between the epiplasm and tMTs (Fig. 1B and Fig. S1E; Allen, 1967; Iftode et al., 1996). This suggests that these microtubules do not need to contact the epiplasm directly but that they are physically linked through associated proteins that remain to be discovered. The ends of pcMTs are embedded within the epiplasm. This interaction could be direct or might rely on additional proteins. Given that both tMTs and pcMTs are grossly modified in similar ways, how their interactions with the cell cortex are differentially regulated is an interesting question. There may be differences in the length of glycine residue chains or in specific residues on the tubulin C-terminal tails that receive modifications.
Recovery of BB organization and orientation
Despite the loss of BB-appendage microtubule glycylation, the BB disorganization phenotype in ttll3Δ cells recovers several days after initiation of TTLL3 knockout (Fig. S3A). BB disorganization can almost completely recover when ttll3Δ cells are grown for several weeks at 23°C, where ciliary forces are reduced (Fig. 4C). Thus, Tetrahymena cells compensate for the loss of microtubule glycylation and recover from BB disorganization. However, the reorganized BBs in ttll3Δ cells remain sensitive to high temperature. We suggest that the temperature sensitivity results from the elevated ciliary forces that are associated with temperature-induced high-frequency cilia beating. Similar to the compensation observed in ttll3Δ cells, BB disorganization in epc1Δ cells recovers after extended growth at low temperature (Williams, 2004). Thus, Tetrahymena cells compensate for cortical morphology defects that arise from disruption of tubulin glycylation and the cortical cytoskeleton they attach to. We did not observe changes to the levels or localization of Epc1 in ttll3Δ cells. Moreover, the length of BB-associated microtubules, and the levels and localization of acetylation and glycylation remained unchanged in epc1Δ cells with BB disorganization. Tubulin glutamylation increases in ttll3Δ cells (Wloga et al., 2009); however, this increase is not due to glutamylation of BB-appendage microtubules (Fig. S5B). It is possible that the compensation is mediated by other cortical cytoskeletal proteins, such as epiplasmin or K-antigen proteins (Williams et al., 1995, 1990; Honts and Williams, 2003). The localization of these cortical proteins is consistent with the sites at which BB-appendage microtubules associate with the epiplasm, and their activities may be reinforced to promote BB organization. In summary, our study suggest that multiple pathways promote effective organization and orientation of BBs.
Glycylation of non-tubulin targets
Tubulin is not the only protein that is glycylated by members of the TTLL family of enzymes. Nucleosome assembly protein 1 (NAP-1) is glycylated by Ttll10 in addition to other nucleosome proteins that are glycylated by Ttll8 in Drosophila (Ikegami et al., 2008; Rogowski et al., 2009). However, there is no indication that glycylation of nucleosome components regulates BB organization. Moreover, Giardia TTLL3 polygylcylates C-terminal glutamate residues on 14-3-3 protein (epsilon-like) that regulates actin organization (Lalle et al., 2011, 2006; Krtkova et al., 2017). Ftt18, a Tetrahymena 14-3-3 protein, has been previously reported at BBs and pcMTs (Kilburn et al., 2007). However, it is not known whether Tetrahymena Ftt18 is glycylated. In fact, tubulin and the Hsp70 family protein Pgp1, are the major glycylated Tetrahymena proteins (Xie et al., 2007). Pgp1 is an essential ER-associated chaperone protein that responds to protein folding stress, including folding stress at elevated temperatures. Interestingly, Pgp1 glycylation is likely to occur within the ER, through Ttll enzymes with ER signal sequences (Xie et al., 2007). Importantly, the antibodies against glycylated tubulin that we used in our study do not recognize the ER. Taken together, although we cannot exclude contributions from other glycylated proteins, the observed phenotypes in ttll3Δ cells are consistent with tubulin as the primary glycylated substrate for BB positioning and attachment to the cell cortex.
BB-appendages and cortical attachments for cell morphology
BB-associated microtubules provide a structural framework to establish and maintain cell shape (Pitelka, 1961; Allen, 1967; Tilney et al., 1966; Tilney and Porter, 1965). Consistent with this, Tetrahymena ttll3Δ cells are rounder and more variable in shape than WT cells (Fig. 4, Figs S3 and S4). The shape of ttll3Δ cells becomes increasingly irregular with more-severe BB disorganization. We suggest that reduced cortical attachments of BBs and BB-appendage microtubules decreases their ability to effectively serve as a structural scaffold. Moreover, BB rows in ttll3Δ cells are commonly skewed relative to the geometry of the cell, thereby creating ciliary rows that spiral around the cell (Fig. 4). This is reminiscent of the twi1-1 (scr1-1) mutant that exhibits twisting of BB rows in a temperature-sensitive manner (Frankel, 2008). Additionally, we find that the medial and posterior regions along the dorsal surface of ttll3Δ cells have a higher number of disorganized BBs. We suggest that BBs within these regions of Tetrahymena cells experience greater ciliary forces. In summary, tubulin glycylation of BB-appendage microtubules is important for local BB organization and orientation as well as global cellular morphology. Glycylated BB-appendage microtubules, in combination with the cortical cytoskeleton, provide a structural framework to maintain cell shape.
MATERIALS AND METHODS
Tetrahymena strains
Tetrahymena thermophila cells (CU428, SB1969 and B2086) were obtained from the Tetrahymena Stock Center (tetrahymena.vet.cornell.edu/index). β-tubulin:mCherry is in the B2086 strain background and Bbc39:mCherry and Epc1:mCherry are in the SB1969 strain background. Acetylation (mec17Δ and K40R) and glycylation (ttll3Δ) mutant strains were obtained from the Tetrahymena Stock Center (Akella et al., 2010; Gaertig et al., 1995; Wloga et al., 2009). Epiplasm mutants (epc1Δ) were obtained from (Williams, 2004).
Tetrahymena cell culture
T. thermophila strains were cultured in 2% SPP medium (2% protease peptone, 0.1% yeast extract, 0.2% glucose and 0.003% ferric EDTA) at 30°C unless otherwise indicated. ttll3Δ, epc1Δ, and corresponding control cells were cultured in MEPP medium (2% protease peptone, 2 mM Na3 citrate 2H2O, 1 mM FeCl3, 30 μM CuSO4•5H2O, 1.7 μM CaCl2; Orias and Rasmussen, 1976). Cells collected for analysis were grown to mid-log phase (∼3×105 cells/ml). Cell counts were determined by using a Coulter Counter Z1 (Beckman Coulter). ttll3Δ and control (bld10miniΔ) cells were generated by mixing germline mutant heterokaryon cells of complementary mating types in Dryl's medium for 20 h, followed by 7 h of recovery in MEPP and subsequent treatment with paromomycin (200 µg/ml; Wloga et al., 2009). The WT control cells do not have detectable growth, morphology, BB synthesis or BB organization defects.
The forces from ciliary beating were manipulated by increasing temperature and medium viscosity (Goto et al., 1982; Galati et al., 2014). For temperature shift experiments, cells were transferred into fresh MEPP medium and incubated for 24 h at specified temperatures. Ciliary beating was inhibited using 100 µM NiCl2 (Bayless et al., 2016; Larsen and Satir, 1991). Medium viscosity was increased using 3% polyethylene oxide (PEO, MW 900,000, Acros Organics). PEO was dissolved at 37°C for 6 h and then incubated for 24 h at 25°C on a nutator. Cells were suspended in MEPP medium with 3% PEO and then cultured at 30°C for 24 h (Galati et al., 2014).
Light microscopy
Immunofluorescence imaging procedures were performed as previously described (Bayless et al., 2016; Thazhath et al., 2002; Williams et al., 1995). For BB visualization, i.e. staining for centrin, cells were washed in 10 mM Tris HCl pH 7.4, followed by PHEM buffer [60 mM 1,4-piperazinediethanesulfonic acid, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 10 mM EGTA, and 2 mM MgCl2, pH 6.9], and then fixed in a Triton X-100/paraformaldehyde solution (0.5% Triton X-100 and 3.2% paraformaldehyde in PHEM buffer) for 5 min. Cells were then washed three times in 0.1% bovine serum albumin (BSA) in PBS (BSA-PBS) before a 24 h incubation with primary antibody against centrin 1 of Tetrahymena thermophila (TtCen1; Stemm-Wolf et al., 2005) diluted in 1.0% BSA-PBS at 4°C. Cells were then washed three times in 0.1% BSA-PBS before a 2 h incubation with secondary antibody diluted in 1.0% BSA-PBS at 23°C. Cells were then washed three times in 0.1% BSA-PBS and pelleted. From the pellet, 2 µl of cells were added to a coverslip and mounted in 6.5 µl Citifluor mounting medium (Ted Pella). Samples were then sealed using clear nail polish.
For visualization of microtubules and tubulin PTMs, cells were washed in 10 mM Tris HCl pH 7.4, deciliated by using deciliation medium [10% Ficoll 400, 10 mM sodium acetate, 10 mM CaCl2, 10 mM EDTA pH 4.2 (adjusted with HCl)], and incubated for 60 s. The pH was rebalanced with 15 mM Tris-HCl (pH 7.9), followed by washes with 10 mM Tris-HCl pH 7.4, and PHEM pH 6.9. Cells were permeabilized with 0.5% Triton X-100 in PHEM for 50 s and then fixed in 2.0% paraformaldehyde. Cells were added to coverslips, dried at 42°C for 20 min, rehydrated in 0.1% BSA-PBS for 12 min before a 24 h incubation at 4°C with primary antibody diluted in 1.0% BSA-PBS. Cells were then washed three times in 0.1% BSA-PBS before incubation for 2 h at 23°C with secondary antibody diluted in 1.0% BSA-PBS. Cells were washed three times in 0.1% BSA-PBS and mounted with 6.5 µl Citifluor mounting medium (Ted Pella). Samples were sealed using clear nail polish. Immunofluorescence for epiplasm was performed as describe previously (Williams et al., 1995). Briefly, cells were incubated on ice for 5 min, fixed in 35% ethanol+0.5% Triton X-100 on ice for 20 min, washed three times in 0.1% BSA-PBS before incubation for 1 h at 23°C with primary antibody diluted in 1.0% BSA-PBS, and washed three times in 0.1% BSA-PBS before incubation for 1 h at 23°C with secondary antibody diluted in 1.0% BSA-PBS. Cells were then washed three times in 0.1% BSA-PBS before mounting with 6.5 µl Citifluor mounting medium (Ted Pella). Samples were sealed using clear nail polish.
The primary antibodies used in this study were against TtCen1 (1:1000; Stemm-Wolf et al., 2005) to mark BBs, monoglycylated tubulin (1:500, TAP952, EMD Millipore MABS277; Callen et al., 1994), polyglycylated tubulin (1:500, AXO49, EMD Millipore MABS276; Callen et al., 1994), glutamylated tubulin (1:1000, GT335, Adipogen AG-20B-0020-C100; Wolff et al., 1992), striated ciliary rootlets or striated fibers (1:1000, 5D8; Jerka-Dziadosz et al., 1995), α-tubulin (1:200, 12G10, DSHB AB_1157911; Thazhath et al., 2002), acetylated tubulin (1:200, 6-11 B-1, Abcam ab24610; LeDizet and Piperno, 1991) and Epc1 (1:500, 8G1; Williams et al., 1995). Secondary antibodies were polyclonal goat anti-rabbit IgG conjugated to Alexa Fluor 488 (Invitrogen A11034), Alexa Fluor 594 (Invitrogen A32740) or Alexa Fluor 647 (Invitrogen A21244), and goat anti-mouse IgG conjugated to Alexa Fluor 488 (Invitrogen A11029), Alexa Fluor 594 (Invitrogen A32742) or Alexa Fluor 647 (Invitrogen A21236); all of which were used at a dilution of 1:1000.
Fluorescence imaging was performed as previously described (Bayless et al., 2016). Briefly, either a Nikon wide-field TiE fluorescence microscope stand equipped with a 100× NA 1.4 Plan Apo objective and an Andor Xyla 4.2 CMOS camera or a Nikon swept-field confocal TiE fluorescence microscope stand equipped with a 100× NA 1.4 Plan Apo objective and an Andor iXon EMCCD camera were used. Images were acquired using NIS Elements software (Nikon). All fluorescence imaging was conducted at 25°C and exposure times were between 50 ms and 500 ms, depending on the experiment. For experiments requiring quantitative comparisons of fluorescence intensity, matched samples were imaged on the same day with identical acquisition settings; representative images were scaled equally.
Quantification of BB parameters
Elongation and length of BB-appendage microtubules
Images acquired for analysis were from samples obtained under matched experimental, preparation and image-acquisition conditions. Microtubule length and elongation of BB-appendages was measured as the distance from the approximated centroid of β-tubulin:mCherry BB signal to the tips of BB-appendage microtubules minus the half-width half-maximum of the BB signal. BB-appendage microtubule extension from BBs in WT and ttll3Δ cells was measured as the distance from the BB centroid (brightest centrin pixel after application of Gaussian blur) to the approximated tip of the microtubule bundle (after immunostaining with 12G10 anti-α-tubulin antibody). These measurements consider the distance of BB-appendage microtubule tips from the BB in xy dimensions but not the distance these microtubules extend in the z dimension. Measurements were performed using ImageJ (NIH image).
Total cell fluorescence
Full cell z-stacks were sum projected and were background subtracted using ImageJ. The corrected total cell fluorescence intensity was obtained by measuring the integrated intensity of the total outlined cell and background subtracted using the integrated intensity of a region outside of the cell.
BB disorganization
Disorganized BBs were identified as any BB with an approximated centroid deviating by >1.0 µm from the boundary of the BB row. The BB row boundary was defined by the edges of the nearest organized anterior and posterior BBs. BBs that were ≥1.0 µm outside the bounds of a row were considered disorganized. Only BBs from the medial and anterior portions of the cell were counted due to the difficulty of assigning BB rows in the posterior end of the cell where BBs are less dense and have less-defined positioning.
BB attachment to the cortex
BBs were considered cortically detached when they were >1.0 µm interior to the adjacent cortically attached BBs. BB positioning was also measured relative to the cell cortex using epiplasm (Epc1:mCherry) and DIC imaging.
Dorsal and ventral comparison
BBs were defined as ventral when they were within or adjacent to the two rows posterior to the oral apparatus (post oral rows) or the two row flanking the oral apparatus (two left and two right, total of six rows of BBs). The oral apparatus is the BB-rich feeding structure at the anterior end of the cell and defines the ventral side of the cell. BBs in the six rows opposite to the ventral or oral apparatus side were defined as dorsal. Only BBs in the medial region that were posterior to the oral apparatus were analyzed.
Electron tomography
Cells were prepared for electron tomography as previously described (Giddings et al., 2010; Meehl et al., 2009). Cells were gently spun into 15% dextran (molecular weight 9000–11,000; Sigma-Aldrich) with 5% BSA in 2% SPP. A small volume of concentrated cells was transferred to a sample holder and high-pressure frozen using a Wohlwend Compact 02 high pressure freezer (Technotrade International). After low-temperature freeze substitution in 0.25% glutaraldehyde and 0.1% uranyl acetate in acetone, cells were slowly infiltrated with Lowicryl HM20 resin. Serial 250–300 nm-thick sections were cut using a Leica UCT ultramicrotome. Sections were collected on Formvar-coated copper slot grids followed by staining with 2% aqueous uranyl acetate for 4 min, followed by Reynold's lead citrate for 3 min.
Dual-axis tilt series (−60 to +60°) of Tetrahymena cells were collected by using a Tecnai F30 intermediate voltage electron microscope (FEI). Images were acquired using the SerialEM acquisition program with a Gatan CCD camera at 1.2 nm/pixel. Serial section tomograms of Tetrahymena cortical structures were generated using the IMOD software package (Giddings et al., 2010; Kremer et al., 1996; Mastronarde, 1997). In total, eight tomograms were reconstructed. Three-dimensional (3D) models were created by using the IMOD software package (bio3d.colorado.edu/imod/). Diagrams of 3D structures in Fig. 1D were generated by using Blender Software (blender.org).
Statistical analyses
All experimental data sets represent a minimum of three biological replicates. The total number of cells and structures analyzed for each data set is provided in the figure legends. Cells were excluded from analyses if they were not at stage 1 (G1) of the Tetrahymena duplication cycle or if they were visibly damaged by the fixation and staining process (pre-established criteria). The morphological differences between control and mutant cells did not allow us to blind between experimental samples. Statistical tests were run using Prism8 (GraphPad Software). Categorical data sets were analyzed by using Fisher's exact test. Normally distributed continuous data sets were analyzed using unpaired, two-tailed Student's t-test. Non-normally distributed continuous data sets were analyzed using unpaired, two-tailed Mann–Whitney or Kruskal–Wallis tests (multiple comparisons). Unless otherwise noted, all P-values are numerically presented. Circular and directional statistical tests and plots were generated using ORIANA software (Kovach) to calculate R-values (mean-vector). Error bars in bar graphs and xy plots indicate the ±standard error of the mean (±s.e.m.). Lines and bars on all dot plots indicate the mean and ±standard deviation, respectively.
Supplementary Material
Acknowledgements
The authors thank Alexander Stemm-Wolf and Marisa Ruehle (University of Colorado School of Medicine) for critical reading of and feedback regarding this manuscript. We appreciate the discussions and expertise provided by the Pearson Lab. We also thank the Tetrahymena Stock Center (Cornell University) for strains and information to culture Tetrahymena. Specimen preparation and electron tomography was performed in the Boulder Electron Microscopy Services at the University of Colorado, Boulder.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.D.J., C.G.P.; Methodology: A.D.J., C.G.P.; Formal analysis: A.D.J., A.W.J.S., E.T.O.; Investigation: A.D.J., A.W.J.S., E.T.O., C.G.P.; Resources: J.B.M., M.G., M.W., J.E.H., J.G., C.G.P.; Data curation: A.D.J., C.G.P.; Writing - original draft: A.D.J., C.G.P.; Writing - review & editing: A.D.J., A.W.J.S., E.T.O., C.G.P.; Supervision: A.D.J., C.G.P.; Project administration: C.G.P.; Funding acquisition: C.G.P.
Funding
This work was supported by the National Institutes of Health (NIH-NIGMS RO1GM127571 to M.W., NIH-NICHD R21HD092809 to J.G., and NIH-NIGMS R01GM099820 to C.G.P.) and the American Cancer Society (ACS-RSG-16-157-01-CCG to C.G.P.). Deposited in PMC for release after 12 months.
Data availability
Image data are available upon request.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.233726.supplemental
References
- Akella J. S., Wloga D., Kim J., Starostina N. G., Lyons-Abbott S., Morrissette N. S., Dougan S. T., Kipreos E. T. and Gaertig J. (2010). MEC-17 is an alpha-tubulin acetyltransferase. Nature 467, 218-222. 10.1038/nature09324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D. (1967). Fine structure, reconstruction and possible functions of components of the cortex of Tetrahymena pyriformis. J. Protozool. 14, 553-565. 10.1111/j.1550-7408.1967.tb02042.x [DOI] [PubMed] [Google Scholar]
- Allen R. D. (1969). The morphogenesis of basal bodies and accessory structures of the cortex of the ciliated protozoan Tetrahymena pyriformis. J. Cell Biol. 40, 716-733. 10.1083/jcb.40.3.716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen R. D. (1971). Fine structure of membranous and microfibrillar systems in the cortex of Paramecium caudatum. J. Cell Biol. 49, 1-20. 10.1083/jcb.49.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argetsinger J. (1965). The isolation of ciliary basal bodies (kinetosomes) from Tetrahymena pyriformis. J. Cell Biol. 24, 154 10.1083/jcb.24.1.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubusson-Fleury A., Bricheux G., Damaj R., Lemullois M., Coffe G., Donnadieu F., Koll F., Viguès B. and Bouchard P. (2013). Epiplasmins and epiplasm in paramecium: the building of a submembraneous cytoskeleton. Protist 164, 451-469. 10.1016/j.protis.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Bayless B. A., Galati D. F., Junker A. D., Backer C. B., Gaertig J. and Pearson C. G. (2016). Asymmetrically localized proteins stabilize basal bodies against ciliary beating forces. J. Cell Biol. 215, 457-466. 10.1083/jcb.201604135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch Grau M., Gonzalez Curto G., Rocha C., Magiera M. M., Marques Sousa P., Giordano T., Spassky N. and Janke C. (2013). Tubulin glycylases and glutamylases have distinct functions in stabilization and motility of ependymal cilia. J. Cell Biol. 202, 441-451. 10.1083/jcb.201305041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch Grau M., Masson C., Gadadhar S., Rocha C., Tort O., Marques Sousa P., Vacher S., Bieche I. and Janke C. (2017). Alterations in the balance of tubulin glycylation and glutamylation in photoreceptors leads to retinal degeneration. J. Cell Sci. 130, 938-949. 10.1242/jcs.199091 [DOI] [PubMed] [Google Scholar]
- Bouchard P., Chomilier J., Ravet V., Mornon J. P. and Vigues B. (2001). Molecular characterization of the major membrane skeletal protein in the ciliate Tetrahymena pyriformis suggests n-plication of an early evolutionary intermediate filament protein subdomain. J. Cell Sci. 114, 101-110. [DOI] [PubMed] [Google Scholar]
- Burke M. C., Li F.-Q., Cyge B., Arashiro T., Brechbuhl H. M., Chen X., Siller S. S., Weiss M. A., O'Connell C. B., Love D. et al. (2014). Chibby promotes ciliary vesicle formation and basal body docking during airway cell differentiation. J. Cell Biol. 207, 123-137. 10.1083/jcb.201406140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callen A.-M., Adoutte A., Andrew J. M., Baroin-Tourancheau A., Bré M.-H., Ruiz P. C., Clérot J.-C., Delgado P., Fleury A., Jeanmaire-Wolf R. et al. (1994). Isolation and characterization of libraries of monoclonal antibodies directed against various forms of tubulin in Paramecium. Biol. Cell 81, 95-119. 10.1016/S0248-4900(94)80002-2 [DOI] [PubMed] [Google Scholar]
- Discher D. E., Mohandas N. and Evans E. A. (1994). Molecular maps of red cell deformation: hidden elasticity and in situ connectivity. Science 266, 1032-1035. 10.1126/science.7973655 [DOI] [PubMed] [Google Scholar]
- Elgeti J. and Gompper G. (2013). Emergence of metachronal waves in cilia arrays. Proc. Natl. Acad. Sci. USA 110, 4470-4475. 10.1073/pnas.1218869110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J. (1962). The effects of heat, cold and para-fluorophenylalanine on morphogensis in synchronized tetrahymena-pyriformis gl. Comptes rendus des travaux du laboratoire carlsberg 33, 1. [Google Scholar]
- Frankel J. (2008). What do genic mutations tell us about the structural patterning of a complex single-celled organism? Eukaryot. Cell 7, 1617-1639. 10.1128/EC.00161-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J. and Nelsen E. M. (2001). The effects of supraoptimal temperatures on population growth and cortical patterning in Tetrahymena pyriformis and Tetrahymena thermophila: a comparison. J. Eukaryot. Microbiol. 48, 135-146. 10.1111/j.1550-7408.2001.tb00296.x [DOI] [PubMed] [Google Scholar]
- Gadadhar S., Bodakuntla S., Natarajan K. and Janke C. (2017a). The tubulin code at a glance. J. Cell Sci. 130, 1347-1353. 10.1242/jcs.199471 [DOI] [PubMed] [Google Scholar]
- Gadadhar S., Dadi H., Bodakuntla S., Schnitzler A., Bieche I., Rusconi F. and Janke C. (2017b). Tubulin glycylation controls primary cilia length. J. Cell Biol. 216, 2701-2713. 10.1083/jcb.201612050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J., Cruz M. A., Bowen J., Gu L., Pennock D. G. and Gorovsky M. A. (1995). Acetylation of lysine 40 in alpha-tubulin is not essential in Tetrahymena thermophila. J. Cell Biol. 129, 1301-1310. 10.1083/jcb.129.5.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati D. F., Bonney S., Kronenberg Z., Clarissa C., Yandell M., Elde N. C., Jerka-Dziadosz M., Giddings T. H., Frankel J. and Pearson C. G. (2014). DisAp-dependent striated fiber elongation is required to organize ciliary arrays. J. Cell Biol. 207, 705-715. 10.1083/jcb.201409123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giddings T. H. Jr, Meehl J. B., Pearson C. G. and Winey M. (2010). Electron tomography and immuno-labeling of Tetrahymena thermophila basal bodies. Methods Cell Biol. 96, 117-141. 10.1016/S0091-679X(10)96006-8 [DOI] [PubMed] [Google Scholar]
- Goto M., Ohki K. and Nozawa Y. (1982). Evidence for a correlation between swimming velocity and membrane fluidity of Tetrahymena cells. Biochim. Biophys. Acta 693, 335-340. 10.1016/0005-2736(82)90440-0 [DOI] [PubMed] [Google Scholar]
- Hallberg R. L. and Hallberg E. M. (1983). Characterization of a cycloheximide-resistant Tetrahymena thermophila mutant which also displays altered growth properties. Mol. Cell. Biol. 3, 503-510. 10.1128/MCB.3.4.503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herawati E., Taniguchi D., Kanoh H., Tateishi K., Ishihara S. and Tsukita S. (2016). Multiciliated cell basal bodies align in stereotypical patterns coordinated by the apical cytoskeleton. J. Cell Biol. 214, 571-586. 10.1083/jcb.201601023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honts J. E. and Williams N. E. (2003). Novel cytoskeletal proteins in the cortex of Tetrahymena. J. Eukaryot. Microbiol. 50, 9-14. 10.1111/j.1550-7408.2003.tb00100.x [DOI] [PubMed] [Google Scholar]
- Hufnagel L. A. (1969). Cortical ultrastructure of Paramecium aurelia. Studies on isolated pellicles. J. Cell Biol. 40, 779-801. 10.1083/jcb.40.3.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftode F., Adoutte A. and Fleury A. (1996). The surface pattern of Paramecium tetraurelia in interphase: an electron microscopic study of basal body variability, connections with associated ribbons and their epiplasmic environment. Eur. J. Protistol. 32, 46-57. 10.1016/S0932-4739(96)80076-9 [DOI] [Google Scholar]
- Iftode F., Clérot J.-C., Levilliers N. and Bré M.-H. (2000). Tubulin polyglycylation: a morphogenetic marker in ciliates. Biol. Cell 92, 615-628. 10.1016/S0248-4900(00)01107-2 [DOI] [PubMed] [Google Scholar]
- Ikegami K. and Setou M. (2009). TTLL10 can perform tubulin glycylation when co-expressed with TTLL8. FEBS Lett. 583, 1957-1963. 10.1016/j.febslet.2009.05.003 [DOI] [PubMed] [Google Scholar]
- Ikegami K., Horigome D., Mukai M., Livnat I., Macgregor G. R. and Setou M. (2008). TTLL10 is a protein polyglycylase that can modify nucleosome assembly protein 1. FEBS Lett. 582, 1129-1134. 10.1016/j.febslet.2008.02.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C. (2014). The tubulin code: molecular components, readout mechanisms, and functions. J. Cell Biol. 206, 461-472. 10.1083/jcb.201406055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C., Rogowski K., Wloga D., Regnard C., Kajava A. V., Strub J. M., Temurak N., van Dijk J., Boucher D., van Dorsselaer A. et al. (2005). Tubulin polyglutamylase enzymes are members of the TTL domain protein family. Science 308, 1758-1762. 10.1126/science.1113010 [DOI] [PubMed] [Google Scholar]
- Jerka-Dziadosz M., Jenkins L. M., Nelsen E. M., Williams N. E., Jaeckel-Williams R. and Frankel J. (1995). Cellular polarity in ciliates: persistence of global polarity in a disorganized mutant of Tetrahymena thermophila that disrupts cytoskeletal organization. Dev. Biol. 169, 644-661. 10.1006/dbio.1995.1176 [DOI] [PubMed] [Google Scholar]
- Kilburn C. L., Pearson C. G., Romijn E. P., Meehl J. B., Giddings T. H. Jr, Culver B. P., Yates J. R. III and Winey M. (2007). New Tetrahymena basal body protein components identify basal body domain structure. J. Cell Biol. 178, 905-912. 10.1083/jcb.200703109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer J. R., Mastronarde D. N. and Mcintosh J. R. (1996). Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71-76. 10.1006/jsbi.1996.0013 [DOI] [PubMed] [Google Scholar]
- Krtkova J., Xu J., Lalle M., Steele-Ogus M., Alas G. C. M., Sept D. and Paredez A. R. (2017). 14-3-3 regulates actin filament formation in the deep-branching eukaryote Giardia lamblia. mSphere 2 10.1128/mSphere.00248-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto K., Yamazaki Y., Nishida T., Shinohara K., Ishikawa H., Hasegawa T., Okanoue T., Hamada H., Noda T., Tamura A. et al. (2012). Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell 148, 189-200. 10.1016/j.cell.2011.10.052 [DOI] [PubMed] [Google Scholar]
- Lalle M., Salzano A. M., Crescenzi M. and Pozio E. (2006). The Giardia duodenalis 14-3-3 protein is post-translationally modified by phosphorylation and polyglycylation of the C-terminal tail. J. Biol. Chem. 281, 5137-5148. 10.1074/jbc.M509673200 [DOI] [PubMed] [Google Scholar]
- Lalle M., Camerini S., Cecchetti S., Fantauzzi C. B., Crescenzi M. and Pozio E. (2011). Giardia duodenalis 14-3-3 protein is polyglycylated by a tubulin tyrosine ligase-like member and deglycylated by two metallocarboxypeptidases. J. Biol. Chem. 286, 4471-4484. 10.1074/jbc.M110.181511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J. and Satir P. (1991). Analysis of Ni(2+)-induced arrest of Paramecium axonemes. J. Cell Sci. 99, 33-40. [DOI] [PubMed] [Google Scholar]
- Ledizet M. and Piperno G. (1991). Detection of acetylated alpha-tubulin by specific antibodies. Methods Enzymol. 196, 264-274. 10.1016/0076-6879(91)96025-M [DOI] [PubMed] [Google Scholar]
- Machemer H. (1972). Ciliary activity and the origin of metachrony in paramecium: effects of increased viscosity. J. Exp. Biol. 57, 239-259. [DOI] [PubMed] [Google Scholar]
- Mahuzier A., Shihavuddin A., Fournier C., Lansade P., Faucourt M., Menezes N., Meunier A., Garfa-Traoré M., Carlier M.-F., Voituriez R. et al. (2018). Ependymal cilia beating induces an actin network to protect centrioles against shear stress. Nat. Commun. 9, 2279 10.1038/s41467-018-04676-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde D. N. (1997). Dual-axis tomography: an approach with alignment methods that preserve resolution. J. Struct. Biol. 120, 343-352. 10.1006/jsbi.1997.3919 [DOI] [PubMed] [Google Scholar]
- Meehl J. B., Giddings T. H. Jr and Winey M. (2009). High pressure freezing, electron microscopy, and immuno-electron microscopy of Tetrahymena thermophila basal bodies. Methods Mol. Biol. 586, 227-241. 10.1007/978-1-60761-376-3_12 [DOI] [PubMed] [Google Scholar]
- Minoura I. and Kamiya R. (1995). Strikingly different propulsive forces generated by different dynein-deficient mutants in viscous media. Cell Motil. Cytoskeleton 31, 130-139. 10.1002/cm.970310205 [DOI] [PubMed] [Google Scholar]
- Nanney D. L., Chow M. and Wozencraft B. (1975). Considerations of symmetry in the cortical integration of tetrahymena doublets. J. Exp. Zool. 193, 1-14. 10.1002/jez.1401930102 [DOI] [PubMed] [Google Scholar]
- Orias E. and Rasmussen L. (1976). Dual capacity for nutrient uptake in Tetrahymena: IV. Growth without food vacuoles and its implications. Exp. Cell Res. 102, 127-137. 10.1016/0014-4827(76)90307-4 [DOI] [PubMed] [Google Scholar]
- Pathak N., Austin C. A. and Drummond I. A. (2011). Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. J. Biol. Chem. 286, 11685-11695. 10.1074/jbc.M110.209817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C. G., Giddings T. H. Jr and Winey M. (2009a). Basal body components exhibit differential protein dynamics during nascent basal body assembly. Mol. Biol. Cell 20, 904-914. 10.1091/mbc.e08-08-0835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C. G., Osborn D. P. S., Giddings T. H. Jr, Beales P. L. and Winey M. (2009b). Basal body stability and ciliogenesis requires the conserved component Poc1. J. Cell Biol. 187, 905-920. 10.1083/jcb.200908019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman B. S. (1973). Basal body addition in ciliary rows of Tetrahymena pyriformis. J. Exp. Zool. 184, 365-368. 10.1002/jez.1401840310 [DOI] [PubMed] [Google Scholar]
- Pitelka D. R. (1961). Fine structure of the silverline and fibrillar systems of three tetrahymenid ciliates. J. Protozool. 8, 75-89. 10.1111/j.1550-7408.1961.tb01186.x [DOI] [Google Scholar]
- Redeker V., Levilliers N., Schmitter J. M., Le Caer J. P., Rossier J., Adoutte A. and Bre M. H. (1994). Polyglycylation of tubulin: a posttranslational modification in axonemal microtubules. Science 266, 1688-1691. 10.1126/science.7992051 [DOI] [PubMed] [Google Scholar]
- Rikmenspoel R. (1971). Contractile mechanisms in flagella. Biophys. J. 11, 446-463. 10.1016/S0006-3495(71)86227-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogowski K., Juge F., van Dijk J., Wloga D., Strub J.-M., Levilliers N., Thomas D., Bré M.-H., van Dorsselaer A., Gaertig J. et al. (2009). Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell 137, 1076-1087. 10.1016/j.cell.2009.05.020 [DOI] [PubMed] [Google Scholar]
- Shida T., Cueva J. G., Xu Z., Goodman M. B. and Nachury M. V. (2010). The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc. Natl. Acad. Sci. USA 107, 21517-21522. 10.1073/pnas.1013728107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siller S. S., Sharma H., Li S., Yang J., Zhang Y., Holtzman M. J., Winuthayanon W., Colognato H., Holdener B. C., Li F.-Q. et al. (2017). Conditional knockout mice for the distal appendage protein CEP164 reveal its essential roles in airway multiciliated cell differentiation. PLoS Genet. 13, e1007128 10.1371/journal.pgen.1007128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. A. (1966). The co-ordination and control of cilia. Symp. Soc. Exp. Biol. 20, 11-31. [PubMed] [Google Scholar]
- Stemm-Wolf A. J., Morgan G., Giddings T. H. Jr, White E. A., Marchione R., Mcdonald H. B. and Winey M. (2005). Basal body duplication and maintenance require one member of the Tetrahymena thermophila centrin gene family. Mol. Biol. Cell 16, 3606-3619. 10.1091/mbc.e04-10-0919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin A.-M., Lemullois M. and Aubusson-Fleury A. (2015). Paramecium tetraurelia basal body structure. Cilia 5, 6 10.1186/s13630-016-0026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K., Nishida T., Inoue K. and Tsukita S. (2017). Three-dimensional organization of layered apical cytoskeletal networks associated with mouse airway tissue development. Sci. Rep. 7, 43783 10.1038/srep43783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thazhath R., Liu C. and Gaertig J. (2002). Polyglycylation domain of beta-tubulin maintains axonemal architecture and affects cytokinesis in Tetrahymena. Nat. Cell Biol. 4, 256-259. 10.1038/ncb764 [DOI] [PubMed] [Google Scholar]
- Tilney L. G. and Porter K. R. (1965). Studies on microtubules in Heliozoa. I. The fine structure of Actinosphaerium nucleofilum (Barrett), with particular reference to the axial rod structure. Protoplasma 60, 317-344. 10.1007/BF01247886 [DOI] [PubMed] [Google Scholar]
- Tilney L. G., Hiramoto Y. and Marsland D. (1966). Studies on the microtubules in heliozoa: III. A pressure analysis of the role of these structures in the formation and maintenance of the Axopodia of Actinosphaerium nucleofilum (Barrett). J. Cell Biol. 29, 77-95. 10.1083/jcb.29.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E., Wills A. A., Kwon T., Sedzinski J., Wallingford J. B. and Stearns T. (2015). Zeta-tubulin is a member of a conserved tubulin module and is a component of the centriolar basal foot in multiciliated cells. Curr. Biol. 25, 2177-2183. 10.1016/j.cub.2015.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein M. L. and Roll-Mecak A. (2016). Graded control of microtubule severing by tubulin glutamylation. Cell 164, 911-921. 10.1016/j.cell.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladar E. K., Bayly R. D., Sangoram A. M., Scott M. P. and Axelrod J. D. (2012). Microtubules enable the planar cell polarity of airway cilia. Curr. Biol. 22, 2203-2212. 10.1016/j.cub.2012.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall K. P., Pagratis M., Armstrong G., Balsbaugh J. L., Verbeke E., Pearson C. G. and Hough L. E. (2016). Molecular determinants of tubulin's C-terminal tail conformational ensemble. ACS Chem. Biol. 11, 2981-2990. 10.1021/acschembio.6b00507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M. E., Hwang P., Huisman F., Taborek P., Yu C. C. and Mitchell B. J. (2011). Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol. 195, 19-26. 10.1083/jcb.201106110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams N. E. (2004). The epiplasm gene EPC1 influences cell shape and cortical pattern in Tetrahymena thermophila. J. Eukaryot. Microbiol. 51, 201-206. 10.1111/j.1550-7408.2004.tb00546.x [DOI] [PubMed] [Google Scholar]
- Williams N. E., Honts J. E. and Kaczanowska J. (1990). The formation of basal body domains in the membrane skeleton of Tetrahymena. Development 109, 935-942. [DOI] [PubMed] [Google Scholar]
- Williams N. E., Honts J. E., Dress V. M., Nelsen E. M. and Frankel J. (1995). Monoclonal antibodies reveal complex structure in the membrane skeleton of Tetrahymena. J. Eukaryot. Microbiol. 42, 422-427. 10.1111/j.1550-7408.1995.tb01606.x [DOI] [PubMed] [Google Scholar]
- Wilson K. S., Gonzalez O., Dutcher S. K. and Bayly P. V. (2015). Dynein-deficient flagella respond to increased viscosity with contrasting changes in power and recovery strokes. Cytoskeleton 72, 477-490. 10.1002/cm.21252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D. and Frankel J. (2012). From molecules to morphology: cellular organization of Tetrahymena thermophila. Methods Cell Biol. 109, 83-140. 10.1016/B978-0-12-385967-9.00005-0 [DOI] [PubMed] [Google Scholar]
- Wloga D., Rogowski K., Sharma N., van Dijk J., Janke C., Eddé B., Bré M.-H., Levilliers N., Redeker V., Duan J. et al. (2008). Glutamylation on alpha-tubulin is not essential but affects the assembly and functions of a subset of microtubules in Tetrahymena thermophila. Eukaryot. Cell 7, 1362-1372. 10.1128/EC.00084-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D., Webster D. M., Rogowski K., Bré M.-H., Levilliers N., Jerka-Dziadosz M., Janke C., Dougan S. T. and Gaertig J. (2009). TTLL3 Is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell 16, 867-876. 10.1016/j.devcel.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Wolff A., de Nechaud B., Chillet D., Mazarguil H., Desbruyeres E., Audebert S., Edde B., Gros F. and Denoulet P. (1992). Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur. J. Cell Biol. 59, 425-432. [PubMed] [Google Scholar]
- Xie R., Clark K. M. and Gorovsky M. A. (2007). Endoplasmic reticulum retention signal-dependent glycylation of the Hsp70/Grp170-related Pgp1p in Tetrahymena. Eukaryot. Cell 6, 388-397. 10.1128/EC.00366-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Schaedel L., Portran D., Aguilar A., Gaillard J., Marinkovich M. P., Théry M. and Nachury M. V. (2017). Microtubules acquire resistance from mechanical breakage through intralumenal acetylation. Science 356, 328-332. 10.1126/science.aai8764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. T., Chong W. M., Wang W.-J., Mazo G., Tanos B., Chen Z., Tran T. M. N., Chen Y.-D., Weng R. R., Huang C.-E. et al. (2018). Super-resolution architecture of mammalian centriole distal appendages reveals distinct blade and matrix functional components. Nat. Commun. 9, 2023 10.1038/s41467-018-04469-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Abiraman K., Li H., Pierce D. M., Tzingounis A. V. and Lykotrafitis G. (2017). Modeling of the axon membrane skeleton structure and implications for its mechanical properties. PLoS Comput. Biol. 13, e1005407 10.1371/journal.pcbi.1005407 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.