Abstract
Background:
Assays identifying circulating tumor cells (CTCs) may allow for noninvasive and sequential monitoring of lung cancer. We investigated whether serial CTC analysis could complement conventional imaging for detecting recurrences after treatment in patients with locally advanced non-small cell lung cancer (LA-NSCLC).
Methods:
Patients with LA-NSCLC (stage II-III) who definitively received concurrent chemoradiation were prospectively enrolled, with CTCs from peripheral blood samples identified using an adenoviral probe that detects elevated telomerase activity present in nearly all lung cancer cells. A "detectable" CTC level was defined as 1.3 GFP-positive cells/mL of collected blood. Samples were obtained before, during (weeks 2,4,6), and after treatment (post-radiation therapy (RT); months 1,3,6,12,18,24).
Results:
48 patients were enrolled. At a median follow-up of 10.9 months, 22 (46%) patients recurred at a median time of 7.6 months post-RT (range 1.3-32.0). Of the 20 of 22 patients for whom post-RT samples were obtained, 15 (75%) had a rise in CTC counts post-RT. In 10 of these 15 patients, CTCs were undetectable on initial post-RT draw but were then detected again prior to radiographic detection of recurrence, with a median lead time of 6.2 months and mean lead time of 6.1 months (range 0.1-12.0) between CTC rise and radiographic evidence of recurrence. One patient with an early recurrence (4.7 months) had persistently elevated detectable CTC levels during and after treatment.
Conclusions:
These results indicate that longitudinal CTC monitoring in patients with LA-NSCLC treated with chemoradiation is feasible, and that detectable CTC levels in many patients meaningfully precede radiologic evidence of disease recurrence.
Keywords: radiation oncology, CTC, LA-NSCLC, prospective trial
MicroAbstract
We investigated the potential usefulness of sequential circulating tumor cell (CTC) analysis for patients treated for locally advanced non-small cell lung cancer (LA-NSCLC). We found CTC rise gave median and mean lead-time notice of progression of disease of about 6 months ahead of radiographic evidence. This telomerase-based CTC assay may thus complement conventional imaging for post-treatment monitoring of patients with LA-NSCLC.
INTRODUCTION
Lung cancer remains the second most commonly diagnosed cancer in both men and women, with an estimated 228,150 new cases of lung cancer expected in 2019, and accounts for about 13% of all cancer diagnoses. It is responsible for more deaths than any other type of cancer in both men and women and is the leading cause of cancer death in men and women above 40 years of age.1 Non-small cell lung cancer (NSCLC) accounts for over 85% of these cases and the 5 year survival rate for locally advanced NSCLC (LA-NSCLC) ranges falls precipitously from 53% for stage IIB disease, to 36% for stage IIIA disease, to 26% for stage IIIB disease.2,3
New developments in radiation therapy (RT), imaging techniques, chemotherapy, surgery, and biological agents offer renewed hope for prolonging survival and improving outcomes. Optimizing monitoring of disease status to guide treatment throughout each patient’s clinical course and after treatment completion, including with novel biomarkers, could allow for improved efficacy of the combined modality therapies and improved outcomes for salvage therapies.4-6
Currently, the standard of care for patients having completed chemoradiation treatment for LA-NSCLC usually includes serial history and physical examinations, along with follow-up imaging, such as positron emission tomography (PET) or chest computed tomography (CT) scans. However, post-radiation changes and fibrosis often render detection of recurrent disease difficult to identify on imaging. In such situations where conventional imaging is unclear, circulating tumor cell (CTCs) assays could be illuminating. There is a relative paucity of information regarding CTC trends in patients with localized, non-metastatic lung cancer, including monitoring for treatment response or assessing for tumor recurrence following definitive chemoradiation. We therefore performed a prospective clinical trial with a primary endpoint to assess CTCs as a potential biomarker for patients with LA-NSCLC definitively treated with chemoradiation. A secondary goal was to assess whether CTC trends may precede conventional imaging in detecting recurrences.
MATERIALS AND METHODS
Patient Eligibility
All consecutive patients with LA-NSCLC (group stage II-III, as determined by 7th edition of the AJCC staging system) prospectively enrolled in this IRB-approved clinical trial were included in the analysis. Enrollment criteria included patients age 18 years or older who were planned to receive concurrent chemoradiation. Patients with a prior active malignancy in the last 5 years were ineligible. All patients involved were discussed in Thoracic Malignancy Multi-disciplinary Clinic and evaluated for surgery, but were deemed unresectable by consensus typically due to tumor location, extent, or excessive predicted peri-operative risks, generally due to pre-existing cardiopulmonary comorbidities and/or limited performance status.
Circulating Tumor Cell Assay
The CTC assay utilized in this study has been previously described for patients with NSCLC, glioma, melanoma, bladder and other types of solid tumors.7-10 It employs a replication-competent adenovirus that detects elevated telomerase expression manifested by almost all tumor cells (but which is not elevated in the majority of normal cells). The replication of the vector is regulated by the human telomerase reverse transcriptase (hTERT) promoter element, ultimately driving GFP expression that can be imaged and quantified by fluorescence microscopy. The pre-clinical validation process utilized testing of the probe in peripheral blood samples from healthy volunteers with and without spiking them with NSCLC cells.7 A logistic regression-based classifier was applied to these data to define the threshold for “CTC positivity” at 1.3 GFP-expressing cells per mL of collected blood. Key aspects of the validation process and assay involve depletion of erythrocytes, lymphocytes, granulocytes and mononuclear cells via Oncoquick gradient centrifugation8 gating via cell size to further exclude the smaller normal cells (such as monocytes),9 and utilizing a low titer of virus. These steps are essential to minimize false positives and are far superior to simple RBC lysis. (Supplemental Figure and data not shown).
Collection of Blood Samples from Patients
Peripheral blood samples (1 tube, typically between 7-10mL) were obtained from patients to assess for CTCs using the above-mentioned assay. Patients were assayed before chemoradiation (pre-RT), at 3 time points during concurrent chemoradiation (weeks 2, 4, and 6), and after chemoradiation completion (post-RT) (months 1, 3, 6, 12, 18, and 24). All care providers and laboratory personnel were blinded to quantitative and qualitative CTC analysis, and no treatment decisions were made based on the results of these assays.
Treatment and Follow-Up
Patients were treated to 6660 cGy in 37 fractions of external beam radiation therapy (180 cGy/fraction). In all cases, the radiation therapy was delivered to the primary tumor and to sites of nodal metastatic disease based on size criteria, PET avidity, or pathologic confirmation of nodal metastasis. In all cases, patients received concurrent chemotherapy that was a platinum based doublet, either cisplatin and etoposide or carboplatin and paclitaxel. Patients then underwent serial radiographic surveillance with either PET/CT or CT scans obtained just prior to each of their follow up appointments (every 3 months in years 1-2, every 4-6 months thereafter), or with additional imaging at the discretion of the patient’s physicians, all of whom are blinded to the results of the CTC analyses. The time to local failure was defined as at least a 20% increase in the diameter of the treated lesion or histologic confirmation of disease and was marked from the date of histologic confirmation or empiric treatment of disease, while local control was defined as absence of local failure. Nodal and distant control were defined as absence of disease in the lymph nodes or absence of extrathoracic metastases, respectively. Date of death was as recorded in the death certificate or as noted in the electronic medical records of each patient.
Statistical Analysis
Overall survival was defined as the duration from the start of radiation treatment to the date of death or last follow-up. Progression free survival was measured from the start of radiation treatment to the date of any disease event or last follow-up. Survival curves were calculated via Kaplan-Meier methods. Statistical analysis was performed using SAS/STAT software, version 9.4 of the SAS System (SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient Characteristics
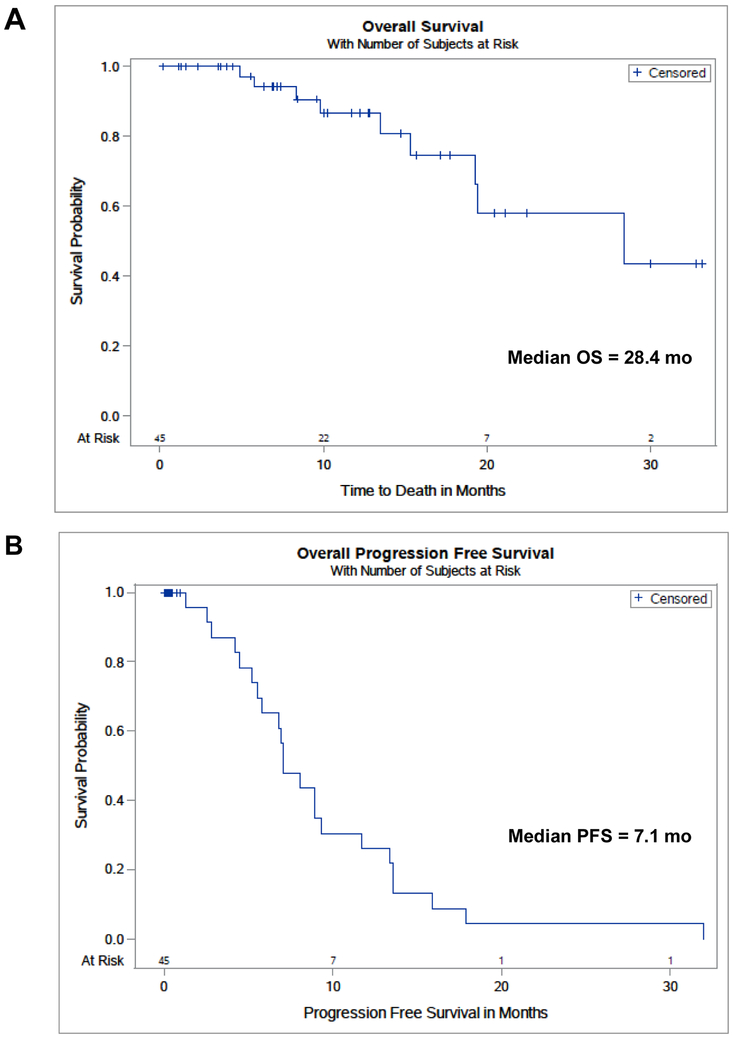
Forty-eight patients were sequentially enrolled. Patient composition included the following: male (54%), Caucasian (69%) or African American (21%), and former (77%) or current (21%) smokers. The median age of the patients was 66 years old (range, 31-84). Adenocarcinoma (46%) or squamous cell carcinoma (48%) comprised most of the histologies of the tumors. The primary tumor median size was 3.7 cm (range, 1.510.0). All patients were established to have histological confirmation of NSCLC and clinically have LA-NSCLC, defined as clinical group stage II or III. Patients predominantly were comprised of stage IIIA (54%) or IIIB (33%) disease, and they had cN2 (60%) or cN3 (23%) nodal disease (Table 1). The median overall survival for this patient population was 28.4 months (Fig. 1a), a duration comparable to the best arm survival rate seen in the RTOG-0617 clinical trial.11 Median progression free survival was 7.1 months, within the range of 6-12 months often described for LA-NSCLC patients (Fig. 1b).11-14 The number of patients at each stage and the corresponding blood draws are delineated in the Consort Diagram in the Supplemental Figure section.
Table 1:
Characteristics of the 48 patients that were sequentially enrolled
| Sex (%) | Male: 26 (54) |
| Female: 22 (46) | |
| Ethnicity (%) | White: 33 (69) |
| African American: 10 (21) | |
| Other: 5 (10) | |
| Age (yrs) | Median: 66 |
| Mean: 66 | |
| Range: 31-84 | |
| Smoking History (%) | Former: 37 (77) |
| Current: 10 (21) | |
| Never: 1 (2) | |
| Tumor Size (cm) | Median: 3.7 |
| Mean: 3.98 | |
| Range: 1.5-10 | |
| Tumor SUVmax | Median: 13 |
| Mean: 13.9 | |
| Range: 1.6-30 | |
| Histology (%) | Adeno: 22 (46) |
| Squamous: 23 (48) | |
| Other: 3 (6) | |
| Stage (%) | IIA: 1(2) |
| IIB: 5 (11) | |
| IIIA: 26 (54) | |
| IIIB: 16 (33) | |
| T-Stage (%) | T1: 13 (27) |
| T2: 14 (29) | |
| T3: 14 (29) | |
| T4: 7 (15) | |
| N-Stage (%) | N0: 6(13) |
| N1: 2 (4) | |
| N2: 29 (60) | |
| N3: 11 (23) |
Figure 1A and 1B: Overall and Progression Free Survival.
The median overall survival for this patient population was 28.4 months (Fig. 1A), while the median progression free survival was 7.1 months (Fig. 1B)
CTC analysis provides lead-time notice of clinical recurrence of disease
Thirty-five of the 48 patients (73%) had detectable levels of CTCs in their pretreatment samples, and all of these patients noted decreases in CTC counts after completion of treatment. At a median follow-up of 10.9 months, 22 of 48 patients (46%) had disease recurrence at a median time of 7.6 months post-RT (range 1.3-32.0). There was a near even distribution between adenocarcinoma and squamous histology in these patients and no obvious difference in outcomes between the two histologies was observed. Post-RT blood samples for CTC analysis were obtained in 20 of 22 recurrent patients (two patients were not able to return for CTC analysis). Of these 20 patients, 15 (75%) had a detectable rise in CTC counts post-RT. In 10 of these 15 patients (67%), CTC counts were undetectable on initial post-RT draw and rose to detectable levels prior to radiographic detection of recurrence, with a median lead-time of 6.2 months and mean lead-time of 6.1 months (range 0.1-12.0) between the CTC rise and radiographic evidence of recurrence. In these 10 patients, an average of 182 days (~6 months) elapsed between when CTC increases were detected and when disease recurrence was clinically noted. Specific details about the ten patients in whom CTC rises preceded disease recurrence are included in Table 2.
Table 2:
Details of the ten patients in whom CTC rises preceded radiographic evidence of disease recurrence.
| Date RT End |
Post-RT CTC Nadir |
Date CTC Rise |
CTC count at rise |
Site of Recurrence |
Date of Recurrence |
Days elapsed: rise to recur |
Vital status | Histology |
|---|---|---|---|---|---|---|---|---|
| 6/17/13 | 0.3 | 1/19/14 | 2.5 | Brain | 1/19/14 | 3 | Deceased | Squamous |
| 12/3/13 | 0 | 1/13/15 | 1.5 | Lung | 1/20/15 | 7 | Deceased | Squamous |
| 3/30/15 | 0.7 | 6/25/15 | 1.7 | Brain | 11/1/15 | 129 | Alive | Adeno |
| 9/16/15 | 0.1 | 12/2/15 | 1.5 | Lung | 5/18/16 | 168 | Alive | Adeno |
| 8/27/15 | 0.1 | 12/17/15 | 2 | Lung | 6/6/16 | 172 | Alive | Adeno |
| 6/22/15 | 0.7 | 1/12/16 | 2.3 | Lung | 8/1/16 | 202 | Alive | Adeno |
| 12/26/13 | 0.4 | 1/4/16 | 36.5 | Liver | 8/24/16 | 233 | Alive | Squamous |
| 12/29/14 | 0 | 6/24/15 | 1.4 | Brain | 2/15/16 | 236 | Deceased | Squamous |
| 1/2/15 | 0 | 6/24/15 | 5.9 | Bone | 4/29/16 | 310 | Alive | Adeno |
| 12/26/14 | 0 | 6/25/15 | 1.7 | Bone | 6/23/16 | 364 | Deceased | Squamous |
Of the five patients in whom the number of CTCs increased into the detectable range after radiographic detection of recurrence, one patient with an early recurrence (4.7 months) had persistently detectable CTC levels during and after treatment. The CTC counts in the four remaining patients decreased to a nadir, but then had radiographic evidence of disease progression or recurrence prior to the rise in CTCs. It should be noted, however, that several of these patients missed clinic appointments or had follow-up care conducted at outside facilities and so, were unable to undergo blood draw and CTC analysis at protocol-specified intervals. While these missed serial CTC assays were not classified as protocol violations, it is plausible that at least some of these would have also resulted in lead-time notice preceding radiographic evidence of disease progression or recurrence.
We also assessed whether CTC status prior to the start of treatment may have bearing on recurrence. As mentioned previously, 13 patients did not have detectable CTCs prior to treatment; of these 5 (or 38%) have been found to have radiographic evidence of recurrence. In contrast, 17 of the 35 (or 49%) patients with CTCs detectable prior to treatment have recurred. The difference did not appear to be statistically significant. Furthermore, as CTCs tend to be rare, additional pre-treatment blood draws would likely increase the probability of detection. Other potential reasons for the lack of CTC detectability are addressed in the discussion.
Illustrative Clinical Vignette
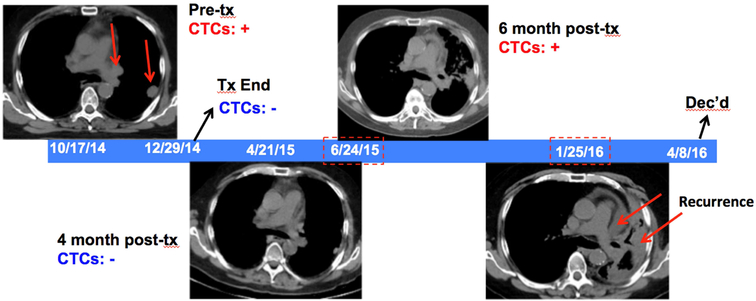
A 70-year-old male presented with stage IIIB squamous cell lung cancer (T3N2M0). On pretreatment PET/CT, the patient was noted to have a 1.5cm mass with SUV 13.9 in the left lung, with FDG avidity in the prevascular and left hilar nodes, and detectable CTCs pre-treatment. At the end of chemoradiation, CTCs were no longer detectable, and they continued to be undetectable during the first two post-treatment CTC evaluations, with a post-treatment CTC nadir of 0. At the patient’s 6-month posttreatment follow up visit though, CTCs were again detectable (1.4 CTCs/mL). However, the official interpretation of the CT scan obtained for this visit was: “Evolution of radiation changes and increase in prominence of mediastinal lymphoid tissue may be reactive.” Seven months later, the patient was unambiguously deemed to have recurrent disease with CT scans now demonstrating widespread progression in the left lung and lymphadenopathy. One month later, the patient developed brain metastases and passed away two months after that. In this patient’s case, 236 days passed between when a positive CTC count was first noted and when the patient was clinically deemed based on imaging to have recurred. A graphical representation of this case, with imaging scans, is shown in Fig. 2.
Figure 2: Sample Case.
Temporal graphic illustrating the disease course of a representative patient (clinical vignette), with serial CTC assays and CT imaging scans showing the status of the tumor. Periods when the CTC counts were elevated (“CTCs + “, in red) or not (“CTCs − “, in blue) are as indicated, along with when disease was detected radiographically (red arrows). CTCs became detectable again on 6/14/2015, preceding CT scans showing disease recurrence in 1/25/2016
DISCUSSION
This pilot trial is, to our knowledge, the largest prospective study assessing CTCs in patients with LA-NSCLC undergoing chemoradiation and the first compelling report indicating that CTC monitoring in such patients is feasible and clinically useful. CTC elevations into the detectable range in many patients meaningfully precede radiologic evidence of disease recurrence. The six-month lead-time notice of recurrence afforded by CTC analyses in this study (and in a population of patients with median progression-free survival of only about seven months) potentially may allow the initiation of retreatment or early salvage strategies at a time when tumor burden remains lower15.
The earliest possible detection of recurrences aided by CTC assays would allow the greatest number of patients to be eligible for effective salvage treatment. For example, an increasing body of prospective data indicate that reirradiation for local and regional non-small cell lung cancer recurrences allows for long-term survival, aided by recent advances in radiation therapy techniques and technology.16 Such treatment is, however, contingent on smaller treatment volumes to minimize the risk of complications. Prompt institution of effective salvage therapy may, therefore, lead to better outcomes and better preservation of quality of life.14 The development and introduction of immunotherapy and other novel effective treatments such as immune checkpoint inhibitors offer further opportunities for extending survival with early detection of recurrences. At the time of analysis, no subject in this trial had received immunotherapy. The demonstrated efficacy of durvalumab, a PD-L1 inhibitor, in the recently reported PACIFIC trial suggests the kind of additional salvage treatment options that may be rapidly implemented with early detection of recurrence afforded by CTC analyses.17
A number of factors may have accounted for the lack of detectable CTCs prior to treatment in some of the patients. The concentration of CTCs is likely to be very low with estimates of 1 CTC per every 1 billion nucleated cells and thus a degree of sampling error may be possible, especially when the volume of blood collected is low.18 In support of this possibility, wide variations in the rate of CTC release have been observed with real-time continuous bloodstream monitoring in preclinical studies.19 Due to discontinuous shedding of CTCs from the primary tumor, it may be ideal in future studies to include additional pretreatment draws for each patient in order to achieve maximal sensitivity.20 We speculate that there may also be alterations in physiology or biology (e.g. poorly perfused tumors, such that tumor cells have difficulty migrating into the systemic circulation), and such factors may also account for why rises in CTC counts were not observed in a subset (5 of 20 [25%]) of patients with clear evidence of disease recurrence.
It should also be noted that there were often logistical limitations in the time points utilized to collect follow-up CTC samples. The protocol was designed for serial CTC assays during a year after completion of treatment (at 1, 3, 6, 12 months). However, after completion of treatment at our quaternary care center, many patients elect to have most or all of the follow-up care performed closer to home due to expense, convenience, and other logistical factors. Consequently, for three of the four patients for whom a rise in CTC counts was detected after conventional imaging, CTC collection time points were missed due to such logistical reasons such as follow-up visits or diagnostic imaging scheduled outside the home institution during which sample collection for CTC analysis was not possible. In future studies, fewer time points may be missed if CTC collection could be performed in community settings. This would require methods of shipping and handling that allow for the stable survival of cells, such as through temperature-controlled shipping containers (different versions of which are commercially available right now). We are actively exploring such options.
Although these CTC assay results are promising, other circulating tumor materials are also being investigated for diagnostic or prognostic monitoring of cancer, such as cfDNA/ctDNA (cell free DNA/circulating tumor DNA) and exosomes.21-24 Future studies may indicate strategies through which CTC assays may complement assays of these other circulating tumor material. Furthermore, the standard of care for these patients now would be to receive one year of consolidation therapy with the PDL1 checkpoint inhibitor, durvalumab, which could impact the time to first rise in CTCs or the lead time in detecting recurrences, and further investigation in immunotherapy-treated patients is indicated. Ultimately, further analytical validation and testing are warranted for any of these assays to guide treatment and management decisions to reduce lung cancer-associated morbidity or mortality. Based on these compelling findings, an NRG Oncology NCI-funded cooperative group study “NRG-LU004: Phase I Trial of Accelerated or Conventionally Fractionated Radiotherapy Combined With MEDI4736 (durvalumab) in PD-L1 High Locally Advanced Non-Small Cell Lung Cancer (NSCLC) (ARCHON-1)” has just been activated that will incorporate CTC analyses in all patients which aims to provide multi-center validation of our findings and test the assay in immunotherapy-treated patients.
Clinical Practice Points
Analyses of circulating tumor material, including circulating tumor cells (CTCs), have attracted much recent interest. These assays are based on peripheral blood samples and are thus noninvasive and can be performed sequentially throughout a patient’s treatment course. The clinical usefulness of CTC analysis for locally advanced non-small cell lung cancer (LA-NSCLC), however, is not yet defined.
We investigated a CTC assay that detects the elevated telomerase activity in almost all cancer cells and which helps confer lack of senescence and immortality. In contrast, telomerase activity is not elevated in almost all normal cells so normal cells undergo senescence.
Consecutive patients treated with chemoradiation (standard fractionation and fields with concurrent platinum-based chemotherapy) for stage II-III LA-NSCLC were prospectively assayed before, during, and at months 1, 3, 6, 12, 18, and 24 after radiation therapy completion (post-RT). Patients underwent serial radiographic imaging with either PET/CT or CT scans before treatment and at each of their follow-up appointments.
22 of 48 (or 46%) patients recurred at a median time of 7.6 months post-RT. 15 of 20 (75%) patients for whom post-RT samples were obtained had a rise in CTC counts. In 10 of these 15 patients, CTCs were undetectable on initial post-RT draw but were then detected again prior to radiographic detection of recurrence, with a median lead time of 6.2 months and mean lead time of 6.1 months between CTC rise and radiographic evidence of progression.
CTC assay may enable implementation of salvage treatment at the earliest time of tumor recurrence or progression.
Supplementary Material
Supplemental Figure: Testing of TelomeScan virus with blood from healthy volunteers to establish ideal assay conditions associated with minimal “false positive” background
Consort diagram:
Systematic delineation of the patients sequentially enrolled in the study and the number active as the study progressed
ACKNOWLEDGMENTS
We acknowledge Yasunari Kashihara and Yasuo Urata of Oncolys Biopharma, Inc. for graciously supplying the adenoviral vector utilized in this research. We are grateful to Kaysee Baker and Susan Mazzoni of the University of Pennsylvania Department of Radiation Oncology and the other clinical research coordinators, for essential assistance with the clinical protocol on which the patients reported here were enrolled.
GRANT SUPPORT
This work was supported by the following grants from National Institutes of Health: grant R01 CA201071 from the National Cancer Institute and grant K08 NS076548-01 from the National Institute of Neurological Disorders and Stroke. J.F.D. was also supported by a Burroughs Welcome Career Award for Medical Scientists (1006792).
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The University of Pennsylvania has filed patent applications based on associated research and is ultimately the license owner. Drs. Hahn, Dorsey, and Kao are cofounders of Liquid Biotech USA through the University of Pennsylvania UPSTART Program.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Siegel RL, Miller KD & Jemal A Cancer statistics, 2019. CA. Cancer J. Clin. 69, 7–34 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Traynor AM & Schiller JH Systemic treatment of advanced non-small cell lung cancer. Drugs Today 40, 697 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Non-Small Cell Lung Cancer Survival Rates, by Stage. Available at: https://www.cancer.org/cancer/non-small-cell-lung-cancer/detection-diagnosis-staging/survival-rates.html. (Accessed: 16th January 2019)
- 4.Vachani A, Sequist LV & Spira A AJRCCM: 100-Year Anniversary. The Shifting Landscape for Lung Cancer: Past, Present, and Future. Am. J. Respir. Crit. Care Med. 195, 1150–1160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim C et al. Improving molecular testing and personalized medicine in non-small-cell lung cancer in Ontario. Curr. Oncol. 24, 103–110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong F.-M. (Spring), Hirsch FR & Machtay M Potential future consideration for imaging and blood-based biomarkers for precision medicine in lung cancer. Transl. Lung Cancer Res. 6, 713–715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorsey JF et al. Tracking Viable Circulating Tumor Cells (CTCs) in the Peripheral Blood of Non-Small Cell Lung Cancer Patients Undergoing Definitive Radiation Therapy: Pilot Study Results. Cancer 121, 139–149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clawson GA et al. Circulating tumor cells in melanoma patients. PloS One 7, e41052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabusaki M et al. Detection and preliminary evaluation of circulating tumor cells in the peripheral blood of patients with eight types of cancer using a telomerase-specific adenovirus. Oncol. Rep. 32, 1772–1778 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu MJ et al. Circulating Tumor Cells, DNA, and mRNA: Potential for Clinical Utility in Patients With Melanoma. The Oncologist 21, 84–94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley JD et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 16, 187–199 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal GM et al. Overall Response Rate, Progression-Free Survival, and Overall Survival With Targeted and Standard Therapies in Advanced Non-Small-Cell Lung Cancer: US Food and Drug Administration Trial-Level and Patient-Level Analyses. J. Clin. Oncol. 33, 1008–1014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provencio M, Isla D, Sánchez A & Cantos B Inoperable stage III non-small cell lung cancer: Current treatment and role of vinorelbine. J. Thorac. Dis. 3, 197–204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao H-H et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 12, 281–292 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Lee P et al. Metabolic tumor burden predicts for disease progression and death in lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 69, 328–333 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Vyfhuis MAL et al. Reirradiation for locoregionally recurrent non-small cell lung cancer. J. Thorac. Dis. 10, S2522–S2536 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonia SJ et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 377, 1919–1929 (2017). [DOI] [PubMed] [Google Scholar]
- 18.West H. (Jack) & Jin J. o. Circulating Tumor Cells. JAMA Oncol. 1, 394–394 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Juratli MA et al. Dynamic Fluctuation of Circulating Tumor Cells during Cancer Progression. Cancers 6, 128–142 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocellin S, Keilholz U, Rossi CR & Nitti D Circulating tumor cells: the ‘leukemic phase’ of solid cancers. Trends Mol. Med. 12, 130–139 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Calabuig-Fariñas S, Jantus-Lewintre E, Herreros-Pomares A & Camps C Circulating tumor cells versus circulating tumor DNA in lung cancer—which one will win? Transl. Lung Cancer Res. 5, 466–482 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold B, Cankovic M, Furtado LV, Meier F & Gocke CD Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the association for molecular pathology. J. Mol. Diagn. JMD 17, 209–224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandfeld-Paulsen B et al. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 10, 1595–1602 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilie M et al. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann. Transl. Med. 2, 107 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure: Testing of TelomeScan virus with blood from healthy volunteers to establish ideal assay conditions associated with minimal “false positive” background