Abstract
The blood retinal barrier (BRB) closely regulates the retinal microenvironment. Its compromise leads to the accumulation of retinal fluid containing potentially harmful plasma components. While eyes with non-exudative age-related macular degeneration (AMD) were previously felt to have an intact BRB, we propose that the BRB in non-exudative AMD eyes may be subclinically compromised, allowing entry of retina-toxic plasma proteins. We test this hypothesis by measuring retinal levels of abundant plasma proteins that should not cross the intact BRB. Two cohorts of frozen, post mortem neurosensory retinas were studied by Western analysis. One cohort from Alabama had 4 normal controls and 4 eyes with various forms of AMD. Another cohort from Minnesota had 5 intermediate AMD and 5 normals. Both cohorts were age/post mortem interval (PMI) matched. The non-exudative AMD retinas in the Alabama cohort had significantly higher levels of albumin and complement component 9 (C9) than normal controls. The positive control exudative AMD donor retina had higher levels of all but one serum protein. In both macular and peripheral neurosensory retina samples, intermediate AMD retinas in the Minnesota cohort had significantly higher levels of albumin, fibrinogen, IgG, and C9 than controls. Our results suggest that there may be moderate subclinical BRB leakage in non-exudative AMD. Potentially harmful plasma components including complement or iron could enter the neurosensory retina in AMD patients prior to advanced disease. Thus, therapies aiming to stabilize the BRB might have a role in the management of non-exudative AMD.
Keywords: age-related macular degeneration (AMD), blood retinal barrier (BRB), albumin, fibrinogen, IgG, complement factor 9 (C9), iron, retina
Age-related macular degeneration (AMD) is the leading cause of irreversible blindness among adults over the age of 65 in the United States (Bressler, 2004). It is characterized by progressive degeneration of the retina, retinal pigment epithelium (RPE), and choroid that leads to central vision loss. There are two distinct types of AMD. Non-exudative AMD is classified by the presence of drusen (early AMD) or geographic atrophy (late, non-exudative AMD). In contrast, exudative AMD is classified by the presence of neovascular vessels that invade and leak plasma into the retina. This can lead to accumulation of intraretinal and/or subretinal fluid and may result in sudden vision loss (Cunha-Vaz, 2017). Leakage of plasma may also allow for inappropriate accumulation of harmful agents in the photoreceptor layer, such as circulating complement components and iron that have been implicated in the pathogenesis of AMD (Hahn, 2003; Johnson et al., 2001; Kawa et al., 2014; Wysokinski et al., 2013; 2015; 2012; Zipfel et al., 2010).
Normally, the blood-retinal barrier (BRB) prevents influx of plasma and its damaging constituents into the retina. The outer BRB is formed by tight junctions between the RPE and the inner BRB is comprised of tight junctions between the retinal vascular endothelial cells. Both barriers contain highly selective transcellular active-transport systems that allow for close regulation of the specialized retinal microenvironment (Cunha-Vaz, 1979). Thus, the formation and maintenance of the BRB play a fundamental role in retinal health and vision, and its disruption has been linked to the development of many retinal diseases, including exudative AMD (Cunha-Vaz, 1976; Runkle and Antonetti, 2011).
Integrity of the BRB can be assessed using clinical imaging methods such as fluorescein angiography (FA). A direct correlation between fluorescein leakage and BRB breakdown has been demonstrated, as fluorescein has low permeability through the intact blood retinal barrier (Cunha-Vaz and Maurice, 1967; Engler et al., 1994; Moldow et al., 2001). Exudative AMD, involving disruption of the BRB, is diagnosed with clinical imaging techniques. Specifically, FA shows neovascular vessel leakage, and optical coherence tomography (OCT) shows subretinal and/or intraretinal fluid. In contrast, it has been widely accepted that the BRB remains intact in eyes with non-exudative AMD (Cunha-Vaz, 2009), which lack the positive findings on FA or OCT.
However, prior studies hint that non-exudative AMD eyes might have subclinical leakage. First, elevated iron levels were found in post mortem AMD retinas from donors with all stages of the disease (Hahn, 2003), suggesting aberrant iron transport and/or iron leakage from the plasma into the retina. Second, in a study evaluating quantity and location of the membrane attack complex (MAC) in aging and AMD eyes, a human eye with geographic atrophy showed moderate immunolabeling with an anti-MAC antibody in the photoreceptor outer segments (fig 4E in Mullins et al., 2014). Under normal circumstances, circulating complement proteins are too large to cross the BRB (Astafurov et al., 2014). While some complement proteins are normally expressed in the retina at low levels (Stasi et al., 2006), terminal complement proteins such as C9 are not locally expressed, and are essential components for MAC assembly (Anderson et al., 2010). Third, Hudson et al. showed that circadian regulation of claudin-5 facilitates BRB leakiness at night, and showed that BRB leakage can lead to retinal degeneration with features of AMD in mice (Hudson et al., 2018). Together, these findings suggest the possibility of more BRB leakage than previously appreciated in non-exudative AMD, which may contribute to AMD pathogenesis.
In the present study, we propose that the BRB in non-exudative AMD eyes might be moderately compromised in a manner that has been difficult to detect by clinical techniques such as FA or OCT. This would be the case if there is very slow, diffuse leakage, which FA is not well-suited to detect, or if the leakage is intermittent and mainly at night, when FA is not typically performed. Thus, the hypothesis that BRB leakiness occurs in non-exudative AMD was tested using semiquantitative Western analysis on post mortem AMD and normal eyes to measure neurosensory retina levels of abundant plasma proteins and immune factors: albumin, fibrinogen, IgG, and C9. These are large proteins found in the systemic circulation with very limited penetration through the BRB due to the intercellular tight junctions and lack of specific transport carrier systems for these molecules. Consequently, the leakage of albumin, fibrinogen, and IgG into the neurosensory retina (NSR, defined as all retinal cell types except RPE) has been used previously to demonstrate BRB dysfunction in diabetic retinopathy (Cheung et al., 2005; Murata et al., 1992; Vinores et al., 1989). In this study, detection of elevated levels of albumin, fibrinogen, IgG, C9, within the NSR would suggest BRB dysfunction and subsequent leakage from the systemic circulation.
Frozen, post mortem whole NSRs were obtained from the Alabama Eye Bank and the Minnesota Lions Eye Bank. All eyes were obtained with the written consent of the donor or the donor’s next of kin in accordance with the Declaration of Helsinki. The Alabama Eye Bank provided 4 normal eyes comprised of 2 females and 2 males: mean age, 83 ± 2.7 years. The 3 non-exudative AMD eye donors comprised 2 females and 1 male: mean age, 82 ± 4.0 years. The non-exudative AMD group comprised 1 eye with geographic atrophy (GA) and 2 eyes with intermediate AMD. An additional donor eye with exudative AMD was obtained as a positive control but was excluded from statistical analysis. All eyes had post mortem interval (PMI), <6 hours. Ascertainment of AMD in donor eyes were based on independent gross evaluations by an AMD histopathologist and an AMD pathologist/AMD clinician (Chowers et al., 2006). Donor cause of death did not include sepsis. Eyes were dissected, stored, and NSR protein was extracted for Western analysis as previously described (Chowers et al., 2006; C.-M. Li et al., 2005)
An additional cohort of age/PMI matched donor eyes was obtained from the Minnesota Lions Eye Bank. The 5 normal (MGS1) eye donors comprised 2 females and 3 males: mean age, 78 ± 0.9 years; mean PMI, 19 ± 1.7. The 5 intermediate AMD (MGS3) eye donors comprised 3 females and 2 males: mean age, 78 ± 0.9 years; mean PMI, 19 ± 0.9. Ascertainment of AMD in donor eyes was determined by a board certified Ophthalmologist using the criteria of the Minnesota Grading System (MGS) (Decanini et al., 2007; Olsen and Feng, 2004). Donors who died from sepsis were excluded from the study. Tissue handling, storage, and donor exclusion criteria were described previously (Karunadharma et al., 2010; Terluk et al., 2015). NSR protein lysates were extracted using Laemmli SDS lysis buffer supplemented with protease/phosphatase inhibition mixture and PMSF (Cell Signaling Technology, Danvers, MA, USA) according to standard methods (Y. Li et al., 2015). Protein concentration was measured using the Pierce BCA Protein Assay Kit (23225; Thermo Scientific, Rockford, IL, USA) according to the manufacturer’s protocol.
NSR lysates from both cohorts were studied by Western analysis as previously described (Y. Li et al., 2015). Primary antibodies used were as follows: rabbit anti-albumin (1:1,000; Cell Signaling Technology, Danvers, MA, USA), mouse anti-fibrinogen (1:1,000; Abcam, Cambridge, MA, USA), goat anti-IgG with HRP conjugate (1:10,000; Invitrogen, Carlsbad, CA, USA), mouse anti-hC9 (1:1000; R & D systems, Minneapolis, MN, USA). Secondary antibodies used were as follows: donkey anti-rabbit (1:5,000; ECL Rabbit IgG, HRP-linked whole antibody), and donkey anti-mouse (1:5,000; ECL Mouse IgG, HRP-linked whole antibody) (GE Healthcare, Chicago, IL, USA). Mouse anti-a-tubulin (1:10,000; Sigma-Aldrich, Inc., St Louis, MO, USA) served as an internal loading control. Immunoblots were probed for plasma proteins, stripped with Restore Western Blot Stripping Buffer (21059; Thermo Scientific, Rockford, IL, USA) for 15 minutes, re-probed for additional plasma proteins, and lastly probed for a-tubulin. Imaging was performed using the GE Amersham Imager 600 (GE Healthcare, Chalfont St. Giles, UK). FIJI software was used for band densitometry (Schindelin et al., 2012). Mean +/− SEM was calculated for each group. Student’s two-group, two-tailed unpaired t-test was used for statistical analysis of relative pixel density. The exudative AMD sample was excluded from statistical analysis because this disease process is known to involve neovascular blood vessel leakage. All statistical analyses were performed using GraphPad Prism 7.0 (San Diego, CA, USA).
Western analysis was performed on a cohort of normal and AMD eyes from the Alabama Eye Bank with antibodies to detect the serum proteins albumin and fibrinogen. In NSR protein extracts, levels of albumin were significantly higher in non-exudative AMD than in normals (Fig. 1A–B). There was a non-significant 2.7 fold increase in fibrinogen levels in non-exudative AMD as compared to normals (Fig. 1 C–D). Immunoblots were also performed with antibodies that detect the immune mediators IgG and C9. There was a non-significant 2.8 fold increase in IgG levels in non-exudative AMD as compared to normal (Fig. 1E–F). In addition, the levels of C9 were significantly higher in non-exudative AMD than in normal (Fig. 1G). All serum protein levels, except for albumin, were highest in the NSR of the exudative AMD donor. These findings were expected, because BRB leakage is known to occur in exudative AMD.
Fig. 1:
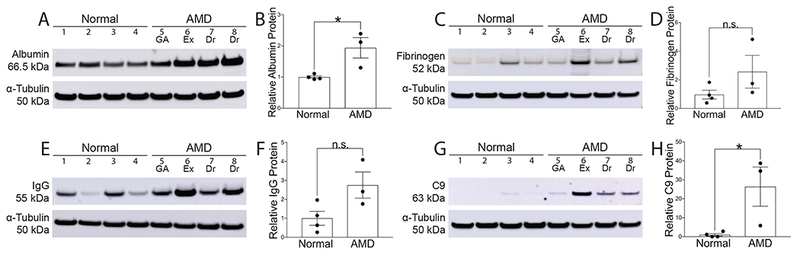
Western analysis of post mortem NSR protein from the Alabama Eye Bank with corresponding pixel density graphs. Immunoblots were done with the following antibodies: albumin (A), fibrinogen (B), IgG (C), C9 (D). Loading control α-tubulin (50kDa) bands are shown below each set of lanes. Graphs show band densitometry normalized to loading control calculated using Image J software. Numbers represent mean values (±SEM). AMD human NSR (n=3) and normal human NSR (n=4). Exudative AMD (n=1) served as a positive control and was excluded from statistical analysis. GA, geographic atrophy; Ex, exudative AMD; Dr, drusen only. Statistical analysis was performed using student’s two-tailed unpaired t-test. * p<0.05, ** p<0.01
To further investigate validity of the initial findings, Western analysis was performed on an independent cohort of eyes classified as MGS3 (intermediate AMD) and MGS1 (normal) from a different eye bank. In this group, MGS3 and MGS1 eyes were matched for age and PMI, and samples were obtained from both the macula and nasal periphery to see if BRB compromise was limited to the macula. In NSR protein extracts from both macula and nasal periphery, the levels of albumin, fibrinogen, IgG, and C9 were significantly higher in the MGS3 group than in the MGS1 group (Fig. 2).
Fig. 2:
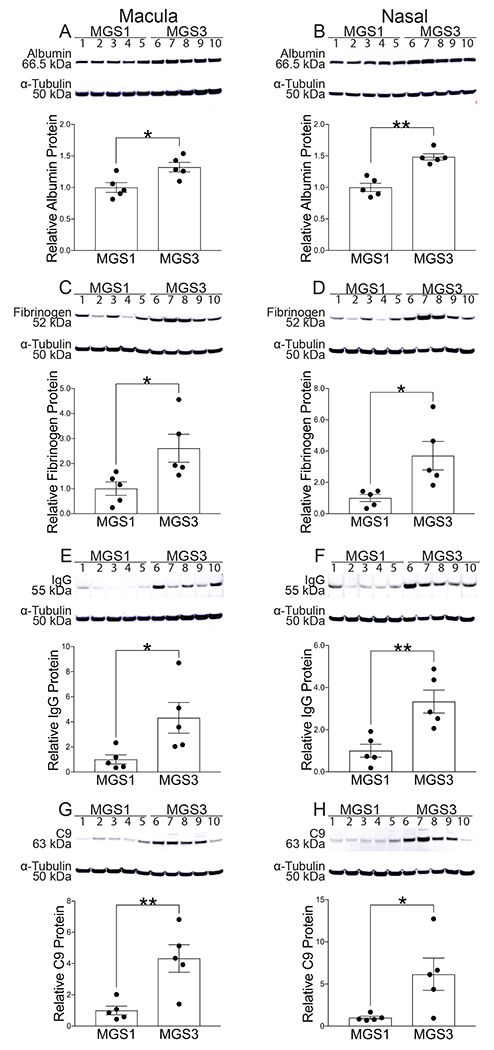
Western analysis of post mortem NSR protein from macula with corresponding pixel density graphs (A, C, E, G) and from nasal periphery with corresponding pixel density graphs (B, D, F, H) obtained from the Minnesota Lions Eye Bank. Immunoblots were done with the following antibodies: albumin (A,B), fibrinogen (C,D), IgG (E,F), C9(G,H). Loading control α-tubulin (50kDa) bands are shown below each set of lanes. Graphs show band densitometry normalized to loading control calculated using Image J software. Numbers represent mean values (±SEM). MGS1 (n=5) indicates normal, MGS3 (n=5) indicates intermediate AMD. Statistical analysis was performed using student’s two-tailed unpaired t-test. p<0.05, ** p<0.01.
Overall, we found increased levels of serum proteins including immune mediators within the NSR of eyes with intermediate non-exudative AMD as compared to normals. These findings were observed across two distinct cohorts that were both age and PMI matched. Together, these results suggest the possibility of BRB leakage not previously appreciated in non-exudative AMD. Such consistent results across two cohorts strengthen our findings. While this is a novel concept in AMD, it has been observed in other age-related neurodegenerative disorders. Early blood-brain barrier (BBB) dysfunction with subsequent leakage of plasma proteins into the CNS has been shown in mouse models of Alzheimer’s disease (AD) and in post mortem human brains with early AD. In addition, BBB and blood spinal cord barrier leakiness have been shown to contribute to amyotrophic lateral sclerosis, Parkinson’s disease, and Huntington’s disease (Nelson et al., 2016).
Interestingly, increased levels of serum proteins in the NSR of intermediate AMD eyes as compared to normal controls were found in tissue obtained from both the macula and periphery. While leakage and accumulation of harmful serum components is known to contribute to degeneration of the macula, damage to the peripheral retina has been previously underappreciated in AMD. However, with the advent of peripheral retinal imaging systems, associations between AMD OCT macular findings and peripheral lesions have been documented (Ung et al., 2019), and peripheral retinal changes were found to be more prevalent in eyes with AMD than in control eyes (Domalpally et al., 2017). Additionally, equivalent mitochondrial DNA damage has been found in macular and peripheral RPE from AMD donor eyes. This suggests damage in the peripheral retina along with the macula, as there is evidence that mitochondrial dysfunction may play a key role in the pathological mechanism of AMD (Terluk et al., 2015).
Prior studies have shown that intraretinal fluid (IRF) or subretinal fluid (SRF) are found in about 20% of intermediate AMD eyes in the absence of choroidal neovascularization (Lek et al., 2018; Sleiman et al., 2017). This fluid accumulation could result from leakage through the RPE or vascular endothelial cells, which would support our hypothesis that there is BRB dysfunction in non-exudative AMD. However, our data also suggest that subclinical leakage likely occurs in the majority of intermediate AMD eyes, and thus in most instances is not detectable by FA or OCT. This could be due to slow plasma leakage, with reabsorption of aqueous plasma content, but not plasma proteins, prior to clinically observable buildup.
It is important to note the possibility that our findings might simply reflect increased levels of protein within the plasma of patients with AMD, as the samples contained blood within the retinal vasculature. However, this explanation is unlikely, as a previous study found no significant difference in the concentration of plasma proteins such as total IgG and albumin in protein extracts from Bruch’s membrane and choroid in MGS3 donor eyes as compared to MGS1(Loyet et al., 2012). In addition, The Blue Mountains Eye Study showed that while the plasma fibrinogen level is significantly associated with late AMD, there is no association between the plasma fibrinogen level and earlier stages of AMD (Smith et al., 1998).
Another alternative explanation for our findings might be that there is de novo synthesis of the measured proteins within the diseased retina. However, this is unlikely to account for our results, as a database of AMD transcriptomics from peripheral NSR (Brooks et al., 2019; Ratnapriya et al., 2019) and macula (Kwicklis, M, and Swaroop, A, unpublished) showed no difference in albumin mRNA levels in the NSR of intermediate AMD (MGS3) eyes as compared to age-matched normals (MGS1). Additionally, mRNA transcripts for fibrinogen, IgG, and C9 were undetectable, suggesting that synthesis of these proteins does not occur within the NSR of AMD or normal eyes.
Further investigation of the role of BRB dysfunction in non-exudative AMD is needed. The present study does not show localization of serum proteins within the neural retina, which would be worthwhile in a future analysis. Preliminary immunolabeling suggests albumin and IgG can localize to the photoreceptor outer segments, while the membrane attack complex (MAC), a component of the terminal complement cascade, can localize to the outer plexiform layer (data not shown). In addition, clinical studies aiming to detect slow or intermittent BRB leakage in patients with intermediate AMD are warranted. These could include serial OCT and FA, including nighttime imaging.
In conclusion, we show increased abundance of serum proteins including immune system components in the NSR of eyes with intermediate non-exudative AMD. This suggests that BRB dysfunction may play a role in non-exudative AMD and could inform new therapeutic directions. Current treatments for exudative AMD target abnormal retinal vascular permeability and reverse the effects of BRB breakdown, such as accumulation of intraretinal and subretinal fluid. However, other than AREDS2 vitamins, which reduce the risk of progression from early to advanced AMD by 25% (Age-Related Eye Disease Study 2 Research Group, 2013) there are currently no widely accepted treatments for non-exudative AMD, largely due to limited understanding of its pathophysiology (Arroyo, 2006). If leakage and subsequent accumulation of harmful agents in the photoreceptor layer might contribute to disease progression of non-exudative AMD, therapies that aim to tighten the BRB could have a therapeutic role. For example, the VEGF pathway and the β-catenin-dependent signaling of the Wnt pathway have been shown to play central roles in BBB and BRB development and maintenance. Thus, pharmacologic inhibition of VEGF or enhancement of Wnt signaling could possibly be exploited to limit BRB leakage. Wnt agonists with therapeutic potential have been developed (Janda et al., 2017; Wang et al., 2012). Additional molecules that have been shown to promote microvascular stabilization include activated protein C (APC), CypaA inhibitors, and MMP-9 inhibitors, and could be further studied as possible pharmacological interventions for non-exudative AMD (Nelson et al., 2016). Further, the finding of elevated C9 within AMD NSR retinas suggests that anti-complement therapeutics may need to achieve therapeutic levels within the NSR.
Highlights:
Non-exudative AMD retinas have elevated levels of plasma proteins compared to normals
Serum immune system proteins IgG and complement C9 are more abundant in AMD retinas
Subclinical BRB leakage in non-exudative AMD would explain these findings
Acknowledgements:
We are grateful to Christine Curcio (UAB) for sharing post mortem eyes and to Madeline Kwicklis, Nivedita Singh and Anand Swaroop (NIH/NEI) for sharing unpublished macula transcriptome information
Funding: This work was funded in the lab of JLD by: NIH/NEI EY015240, Research to Prevent Blindness Medical Student Research Award and unrestricted funds, the Jeffrey W. Berger, MD, PhD Foundation, the F.M. Kirby Foundation, a gift in memory of Lee F. Mauger, MD, and the Paul andEvanina BellMackall Foundation Trust. Funding for DAF: NIH/NEIEY026012, Helen Lindsay Foundation, Larson Endowed Vision Research Chair, and an Anonymous Donor for AMD Research.
Non Standard Abbreviations:
- BRB
blood-retinal barrier
- AMD
age-related macular degeneration
- PMI
post mortem interval
- C9
complement component 9
- MAC
membrane attack complex
- RPE
retinal pigment epithelium
- FA
fluorescein angiography
- OCT
optical coherence tomography
- BBB
blood-brain barrier
- NSR
neurosensory retina (includes all retinal cell types except RPE)
- GA
geographic atrophy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Age-Related Eye Disease Study 2 Research Group, 2013. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 309, 2005–2015. doi: 10.1001/jama.2013.4997 [DOI] [PubMed] [Google Scholar]
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV, 2010. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 29, 95–112. doi : 10.1016/j.preteyeres.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo JG, 2006. A 76-year-old man with macular degeneration. JAMA 295, 2394–2406. doi: 10.1001/jama.295.20.2394 [DOI] [PubMed] [Google Scholar]
- Astafurov K, Dong CQ, Panagis L, Kamthan G, Ren L, Rozenboym A, Perera TD, Coplan JD, Danias J, 2014. Complement expression in the retina is not influenced by short-term pressure elevation. Mol. Vis. 20, 140–152. [PMC free article] [PubMed] [Google Scholar]
- Bressler NM, 2004. Age-related macular degeneration is the leading cause of blindness… JAMA 291, 1900–1901. [DOI] [PubMed] [Google Scholar]
- Brooks BP, Song MJ, Swaroop A, 2019. Unpublished data via NEI commons. doi:Cited 2019 March 29 [Google Scholar]
- Cheung AKH, Fung MKL, Lo ACY, Lam TTL, So K-F, Chung SSM, Chung SK, 2005. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes 54, 3119–3125. [DOI] [PubMed] [Google Scholar]
- Chowers I, Wong R, Dentchev T, Farkas RH, Iacovelli J, Gunatilaka TL, Medeiros NE, Presley JB, Campochiaro PA, Curcio CA, Dunaief JL, Zack DJ, 2006. The Iron Carrier Transferrin Is Upregulated in Retinas from Patients with Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 47, 2135–6. doi: 10.1167/iovs.05-1135 [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, 2017. The Blood-Retinal Barrier in the Management of Retinal Disease: EURETINA Award Lecture. Ophthalmologica 237, 1–10. doi: 10.1159/000455809 [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, 1979. The blood-ocular barriers. Surv Ophthalmol 23, 279–296. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, President, Association for Innovation and Biomedical Research on Light and Image, 2009. The Blood-Retinal Barrier in Retinal Disease. European Ophthalmic Review 03, 105–4. doi: 10.17925/EOR.2009.03.02.105 [DOI] [Google Scholar]
- Cunha-Vaz JG, 1976. The blood-retinal barriers. Doc Ophthalmol 41, 287–327. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz JG, Maurice DM, 1967. The active transport of fluorescein by the retinal vessels and the retina. J. Physiol. (Lond.) 191, 467–486. doi: 10.1111/(ISSN)1469-7793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decanini A, Nordgaard CL, Feng X, Ferrington DA, Olsen TW, 2007. Changes in select redox proteins of the retinal pigment epithelium in age-related macular degeneration. Am. J. Ophthalmol. 143, 607–615. doi: 10.1016/j.ajo.2006.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler CB, Sander B, Larsen M, Dalgaard P, Lund-Andersen H, 1994. Fluorescein transport across the human blood-retina barrier in the direction vitreous to blood. Quantitative assessment in vivo. Acta Ophthalmol 72, 655–662. [DOI] [PubMed] [Google Scholar]
- Hahn P, 2003. Maculas Affected by Age-Related Macular Degeneration Contain Increased Chelatable Iron in the Retinal Pigment Epithelium and Bruch’s Membrane. Archives of Ophthalmology 121, 1099–1105. doi: 10.1001/archopht.121.8.1099 [DOI] [PubMed] [Google Scholar]
- Hudson N, Celkova L, Fahey E, Ozaki E, Doyle S, Campbell M, 2018. Aberrant BMAL1 dependent claudin-5 cycling induces geographic atrophy. XVIII INTERNATIONAL SYMPOSIUM ON RETINAL DEGENERATION RD2018 Sept. 03 - 08, 2018 The Great Southern Hotel and Conference Center Killarney, Ireland [Google Scholar]
- Janda CY, Dang LT, You C, Chang J, de Lau W, Zhong ZA, Yan KS, Marecic O, Siepe D, Li X, Moody JD, Williams BO, Clevers H, Piehler J, Baker D, Kuo CJ, Garcia KC, 2017. Surrogate Wnt agonists that phenocopy canonical Wnt and β-catenin signalling. Nature 545, 234–237. doi: 10.1038/nature22306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LV, Leitner WP, Staples MK, Anderson DH, 2001. Complement activation and inflammatory processes in Drusen formation and age related macular degeneration. Experimental Eye Research 73, 887–896. doi: 10.1006/exer.2001.1094 [DOI] [PubMed] [Google Scholar]
- Karunadharma PP, Nordgaard CL, Olsen TW, Ferrington DA, 2010. Mitochondrial DNA damage as a potential mechanism for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 51, 5470–5479. doi: 10.1167/iovs.10-5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa MP, Machalinska A, Roginska D, Machalinski B, 2014. Complement system in pathogenesis of AMD: dual player in degeneration and protection of retinal tissue. J Immunol Res 2014, 483960–12. doi: 10.1155/2014/483960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lek JJ, Caruso E, Baglin EK, Sharangan P, Hodgson LAB, Harper CA, Rosenfeld PJ, Luu CD, Guymer RH, 2018. Interpretation of Subretinal Fluid Using OCT in Intermediate Age-Related Macular Degeneration. Ophthalmology Retina 2, 792–802. doi: 10.1016/j.oret.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Li C-M, Presley JB, Zhang X, Dashti N, Chung BH, Medeiros NE, Guidry C, Curcio CA, 2005. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J. Lipid Res. 46, 628–640. doi: 10.1194/jlr.M400428-JLR200 [DOI] [PubMed] [Google Scholar]
- Li Y, Song D, Song Y, Zhao L, Wolkow N, Tobias JW, Song W, Dunaief JL, 2015. Iron-induced Local Complement Component 3 (C3) Up-regulation via Non-canonical Transforming Growth Factor (TGF)β Signaling in the Retinal Pigment Epithelium. J. Biol. Chem. 290, 11918–11934. doi: 10.1074/jbc.M115.645903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyet KM, Deforge LE, Katschke KJ, Diehl L, Graham RR, Pao L, Sturgeon L, Lewin-Koh S-C, Hollyfield JG, van Lookeren Campagne M, 2012. Activation of the alternative complement pathway in vitreous is controlled by genetics in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 53, 6628–6637. doi: 10.1167/iovs.12-9587 [DOI] [PubMed] [Google Scholar]
- Domalpally A, Clemons TE, Danis RP, Sadda SR, Cukras CA, Toth CA, Friberg TR, Chew EY, 2017. Peripheral Retinal Changes Associated with Age-Related Macular Degeneration in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 12 by the Age-Related Eye Disease Study 2 Optos PEripheral RetinA (OPERA) Study Research Group*. Ophthalmology 124, 479–487. doi: 10.1016/j.ophtha.2016.12.004 [DOI] [PubMed] [Google Scholar]
- Moldow B, Larsen M, Sander B, Lund-Andersen H, 2001. Passive permeability and outward active transport of fluorescein across the blood-retinal barrier in early ARM. Br J Ophthalmol 85, 592–597. doi: 10.1136/bjo.85.5.592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Schoo DP, Sohn EH, Flamme-Wiese MJ, Workamelahu G, Johnston RM, Wang K, Tucker BA, Stone EM, 2014. The membrane attack complex in aging human choriocapillaris: relationship to macular degeneration and choroidal thinning. Am. J. Pathol. 184, 3142–3153. doi: 10.1016/j.ajpath.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Ishibashi T, Inomata H, 1992. Immunohistochemical detection of extravasated fibrinogen (fibrin) in human diabetic retina. Graefes Arch. Clin. Exp. Ophthalmol. 230, 428–431. [DOI] [PubMed] [Google Scholar]
- Nelson AR, Sweeney MD, Sagare AP, Zlokovic BV, 2016. Neurovascular dysfunction and neurodegeneration in dementia and Alzheimer’s disease. Biochim. Biophys. Acta 1862, 887–900. doi: 10.1016/j.bbadis.2015.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen TW, Feng X, 2004. The Minnesota Grading System of eye bank eyes for age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 45, 4484–4490. doi: 10.1167/iovs.04-0342 [DOI] [PubMed] [Google Scholar]
- Ratnapriya R, Sosina OA, Starostik MR, Kwicklis M, Kapphahn RJ, Fritsche LG, Walton A, Arvanitis M, Gieser L, Pietraszkiewicz A, Montezuma SR, Chew EY, Battle A, Abecasis GR, Ferrington DA, Chatterjee N, Swaroop A, 2019. Retinal transcriptome and eQTL analyses identify genes associated with age-related macular degeneration. Nat. Genet. 15, 151. doi: 10.1038/s41588-019-0351-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkle EA, Antonetti DA, 2011. The blood-retinal barrier: structure and functional significance. Methods Mol. Biol. 686, 133–148. doi: 10.1007/978-1-60761-938-3_5 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A, 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiman K, Veerappan M, Winter KP, McCall MN, Yiu G, Farsiu S, Chew EY, Toth CA, Wong W, Huang T, Hubbard GB, Srivastava S, McCall M, Winter K, Sarin N, Hall K, McCollum P, Curtis L, Schuman S, Chiu SJ, Tai V, Clemons T, Chew E, 2017. Optical Coherence Tomography Predictors of Risk for Progression to Non-Neovascular Atrophic Age-Related Macular Degeneration. Ophthalmology 124, 1764–1777. doi: 10.1016/j.ophtha.2017.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W, Mitchell P, Leeder SR, Wang JJ, 1998. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Archives of Ophthalmology 116, 583–587. [DOI] [PubMed] [Google Scholar]
- Stasi K, Nagel D, Yang X, Wang R-F, Ren L, Podos SM, Mittag T, Danias J, 2006. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest. Ophthalmol. Vis. Sci. 47, 1024–1029. doi: 10.1167/iovs.05-0830 [DOI] [PubMed] [Google Scholar]
- Terluk MR, Kapphahn RJ, Soukup LM, Gong H, Gallardo C, Montezuma SR, Ferrington DA, 2015. Investigating mitochondria as a target for treating age-related macular degeneration. J. Neurosci. 35, 7304–7311. doi: 10.1523/JNEUROSCI.0190-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung C, Lains I, Woods RL, Park DH, Mukai R, Silverman R, Oellers P, Kim IK, Vavvas D, Miller JW, Miller JB, Husain D, 2019. Associations between macular OCT findings and peripheral changes in AMD. ARVO Annual Meeting 2019. [Google Scholar]
- Vinores SA, Gadegbeku C, Campochiaro PA, Green WR, 1989. Immunohistochemical localization of blood-retinal barrier breakdown in human diabetics. Am. J. Pathol. 134, 231–235. [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rattner A, Zhou Y, Williams J, Smallwood PM, Nathans J, 2012. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell 151, 1332–1344. doi: 10.1016/j.cell.2012.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysokinski D, Danisz K, Blasiak J, Dorecka M, Romaniuk D, Szaflik J, Szaflik JP, 2013. An association of transferrin gene polymorphism and serum transferrin levels with age-related macular degeneration. Experimental Eye Research 106, 14–23. doi: 10.1016/j.exer.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Wysokinski D, Danisz K, Pawlowska E, Dorecka M, Romaniuk D, Robaszkiewicz J, Szaflik M, Szaflik J, Blasiak J, Szaflik JP, 2015. Transferrin receptor levels and polymorphism of its gene in age-related macular degeneration. Acta Biochimica Polonica 62, 177–184. doi: 10.18388/abp.2014_843 [DOI] [PubMed] [Google Scholar]
- Wysokinski D, Zaras M, Dorecka M, Waszczyk M, Szaflik J, Blasiak J, Szaflik JP, 2012. An association between environmental factors and the IVS4+44C>A polymorphism of the DMT1 gene in age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 250, 1057–1065. doi: 10.1007/s00417-012-1966-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, Lauer N, Skerka C, 2010. The role of complement in AMD. Adv. Exp. Med. Biol. 703, 9–24. doi: 10.1007/978-1-4419-5635-4_2 [DOI] [PubMed] [Google Scholar]


