SUMMARY
Staphyloxanthin, a carotenoid in S. aureus, is a powerful antioxidant against oxidative stresses. The crtOPQMN operon driving pigment synthesis is under the control of σB. CspA, a cold-shock protein, is known to control σB activity. To ascertain genes that regulate cspA, we screened a transposon library that exhibited reduced cspA expression and pigmentation. We found that the adaptor protein YjbH activates cspA expression. Spx, the redox-sensitive transcriptional regulator and a proteolytic target for YjbH and ClpXP, complexes with αCTD of RNAP prior to binding the cspA promoter to repress cspA activity. Increased cspA expression in trans in the inactive spx C10A mutant of JE2 did not enhance pigment production while it did in JE2, suggesting that cspA is downstream to Spx in pigmentation control. As the staphyloxanthin pigment is critical to S. aureus survival in human hosts, we demonstrated that the cspA and yjbH mutants survived less well than the parent in whole blood killing assay. Collectively, our studies suggest a pathway wherein YjbH and ClpXP proteolytically cleave Spx, a repressor of cspA transcription, to affect σB-dependent carotenoid expression, thus providing a critical link between intracellular redox sensing by Spx and carotenoid production to improve S. aureus survival during infections.
Keywords: Carotenoid, oxidative stress, Spx, YjbH, CspA, S. aureus
GRAPHICAL ABSTRACT:
This study investigates the pathway whereby YjbH and ClpXP proteolytically cleave Spx, a repressor of cspA transcription, to affect σB-dependent carotenoid expression, thus providing a critical link between intracellular redox sensing by Spx and carotenoid production to improve S. aureus survival during infections.
ABBREVIATED SUMMARY:
The carotenoid pigment (also called staphyloxanthin) in S. aureus is a powerful antioxidant that protects the bacteria from oxidative stresses encountered during growth and infection. In this paper, we conducted experiments to ascertain genetic factors that govern the expression of this pigment in S. aureus.
INTRODUCTION
The golden pigmentation of the human pathogen Staphylococcus aureus results from accumulation of staphyloxanthin, a membrane-localized triterpenoid carotenoid (Katzif et al., 2003; Duval et al., 2010). This compound is thought to act as a free-radical scavenger and singlet oxygen quencher, enabling S. aureus to survive oxidative stresses stemming from ROS (Pelz et al., 2005). The crtOPQMN operon encodes the proteins responsible for the biosynthesis of staphyloxanthin (Pelz et al., 2005), and is regulated by a series of regulators (Lan et al., 2010; Fey et al., 2012) within the alternative sigma factor σB system (Kullik et al., 1998). In unstressed conditions, σB (encoded by sigB) is bound in an inactive state by its anti-sigma factor RsbW, and is only freed to bind RNA polymerase to initiate transcription at σB-dependent promoters when its upstream regulator RsbU dephosphorylates RsbV to disrupt the σB-RsbW complex (Benson and Haldenwang, 1993; Senn et al., 2005).
Other regulators of pigmentation in S. aureus include cspA [also called msaB (Elbarasi, 2014)] and cspB (Katzif et al., 2005; Duval et al., 2010) wherein σB activity appears to be positively dependent on cspA (Katzif et al., 2005). Csps are small (~7 kD) proteins found in bacteria (Schindler et al., 1999) and archaea (Giaquinto et al., 2007) that have a compact β-barrel structure for binding single-stranded DNA (Zeeb, 2003) and RNA (Bae et al., 2000), presumably to stabilize mRNA and promoters and to enhance translation (Phadtare and Severinov, 2010).
All Csps in S. aureus share considerable identity to those in E. coli (~60%) and B. subtilis (~76%), as well as amongst themselves (~80–90%). However, each Csp appears to impact different pathways in S. aureus (Duval et al., 2010; Lioliou et al., 2012), with cspA primarily affecting biofilm development (Elbarasi, 2014), protease production (Sahukhal and Elasri, 2014) and cold shock response (Katzif et al., 2005; Anderson et al., 2006). Regulation of these Csp homologs appears equally as diverse, with some csp promoters controlled by transcription factors (Constantinidou et al., 2006; Uppal et al., 2014), while other csp genes are modulated by regulatory RNAs at their 5’ untranslated regions (Jiang et al., 1996) that are then targeted post-transcriptionally for degradation by RNases (Lioliou et al., 2012) and toxin-antitoxin modules (Younghoon Kim et al., 2010; Samin Kim et al., 2016). Post-translational regulation may also occur, as with the intracellular protease Lon which can affect CspD levels in E. coli (Langklotz and Narberhaus, 2011).
In this study, we sought to explore the role of csp homologs and other genes in controlling σB and the ensuing carotenoid pigmentation (i.e. staphyloxanthin) in S. aureus. We have identified that YjbH, an adaptor of the ClpXP proteolytic system (Engman et al., 2012), acts indirectly as an activator of cspA. In addition, Spx, a redox-sensing transcription factor targeted by ClpXP and YjbH for degradation, was found to repress cspA levels by directly interacting with the cspA promoter via the α-subunit of RNA polymerase (Michiko M Nakano et al., 2010). Activation of CspA via Spx degradation by the YjbH-ClpXP proteasome complex would lead to increased pigmentation via enhanced σB activity. Together, these results reveal a novel pathway wherein staphyloxanthin, a S. aureus pigment that enhances bacterial survival upon oxidative stress, is controlled by a redox-sensitive regulator in S. aureus.
RESULTS
Carotenoid expression in csp mutants.
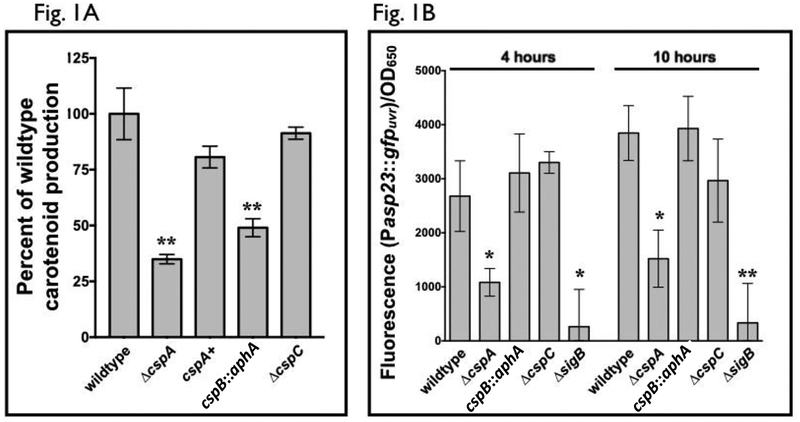
In prior studies, it has been reported that cspA (Katzif et al., 2005) and cspB mutants (Duval et al., 2010) harbor lower levels of carotenoid pigment. Given the role staphyloxanthin plays in S. aureus resistance to oxidative stress, we wanted to further evaluate this phenomenon since individual mutants have not been compared to each other on carotenoid levels in identical genetic background nor has the impact of cspC been examined. Accordingly, we created cspA and cspC mutants of S. aureus strain SH1000 using allelic replacement (Arnaud et al., 2004). Due to the reported slower growth (Duval et al., 2010) for the cspB mutant, a SH1000 strain disrupted for cspB was created via transduction of the kanamycin resistance cassette from strain BD1 to improve the frequency of homologous recombination. We observed that both cspA and cspB mutants revealed lower pigmentation than the wildtype SH1000, with the ΔcspA strain having the lower of the two. In contrast, a ΔcspC strain of SH1000 retained pigmentation comparable to the wildtype SH1000 (Fig. 1A), indicating cspC is not a major determinant of staphyloxanthin pigmentation in S. aureus.
Figure 1: The impact of S. aureus csp homologs on carotenoid production and σB activity.
(A) Carotenoid was extracted from each of three biological replicates of S. aureus SH1000 wildtype and csp mutant strains that were grown overnight on tryptic soy agar. These levels were normalized to cell density and compared to one another as a percentage of wildtype. The experiments were performed thrice at different days, with consistent data within each experiment, but showing variation between experiment. Accordingly, percentages from one representative experiment were displayed. The cspA+ strain denotes the cspA complement mutant strain ALC8212, and cspB::aphA indicates the cspB mutant strain ALC8581.
(B) GFP fluorescence generated from σB-dependent asp23 promoter was measured for S. aureus SH1000 wildtype, ΔsigB and csp mutant strains transformed with the plasmid pALC2201 carrying the asp promoter driving gfp, at 4 and 10 hours of growth in TSB. Cell densities were simultaneously measured at OD650, and fluorescence was normalized to this value. The experiments were performed using three biological replicates and repeated three times. A representative experiment is displayed.
The asterisks in Fig. 1A and 1B indicate statistical significance between wildtype and mutant strains, determined using Student t-test (*, P < 0.05; **, P < 0.005).
σB activity is reduced in a strain lacking cspA but not cspB.
Given that the ΔsigB, ΔcspA and ΔcspB strains of S. aureus all exhibited reduced carotenoid levels, the contribution of each csp gene product to σB activity was examined. In contrast to other protein-based regulators, σB activity cannot be determined by Western blot because it is the free form of σB, not the σB-RsbW complex, that determines SigB activity. For this reason, transcription from the σB-dependent asp23 promoter (Gertz et al., 1999) is a more reliable measure of overall σB activity. Accordingly, S. aureus reporter strains with the plasmid containing a transcriptional fusion of the asp23 promoter to gfpuvr (pALC2201) were constructed (Palma et al., 2006). Overnight cultures of these strains with pALC2201 (Cm resistant) were back-diluted to an OD650=0.1 in fresh TSB with chloramphenicol followed by serial GFP fluorescence and OD650 measurements. While the asp23-dependent GFP levels were unaltered in ΔcspB and ΔcspC mutants compared to the wildtype strain SH1000 (Fig. 1B), the GFP level in the ΔcspA strain of SH1000 was significantly lower, but not completely absent. This result indicated that among the three csp genes in S. aureus, only cspA was involved in carotenoid production that is linked to the σB-dependent asp23 promoter. We thus elected to concentrate our ensuing analysis on factors affecting sigB-dependent pigmentation attributable to cspA.
yjbH is an activator of cspA transcription.
The transcription of cspA is initiated from two promoters, one 514 bp upstream of the cspA translation start site that co-transcribes msaA and cspA (PmsaA) (Sahukhal and Elasri, 2014), and the other from a σA-dependent promoter 112 bp upstream of the cspA initiation codon (PcspA) (Uppalapati et al., 2017). While PcspA is better characterized (Katzif et al., 2003; Lioliou et al., 2012; Uppalapati et al., 2017) than PmsaA, there were conflicting data (Sahukhal and Elasri, 2014) regarding the relative activities of these two promoters. To address this, we created transcriptional fusions of each of these promoters to gfpuvr and compared their fluorescence in wildtype SH1000. We observed that the promoter upstream of cspA (PcspA) was consistently much stronger than that of msaA (PmsaA) (Fig. S1). Due to this significant difference, we limited further investigations to the transcriptional regulation of PcspA.
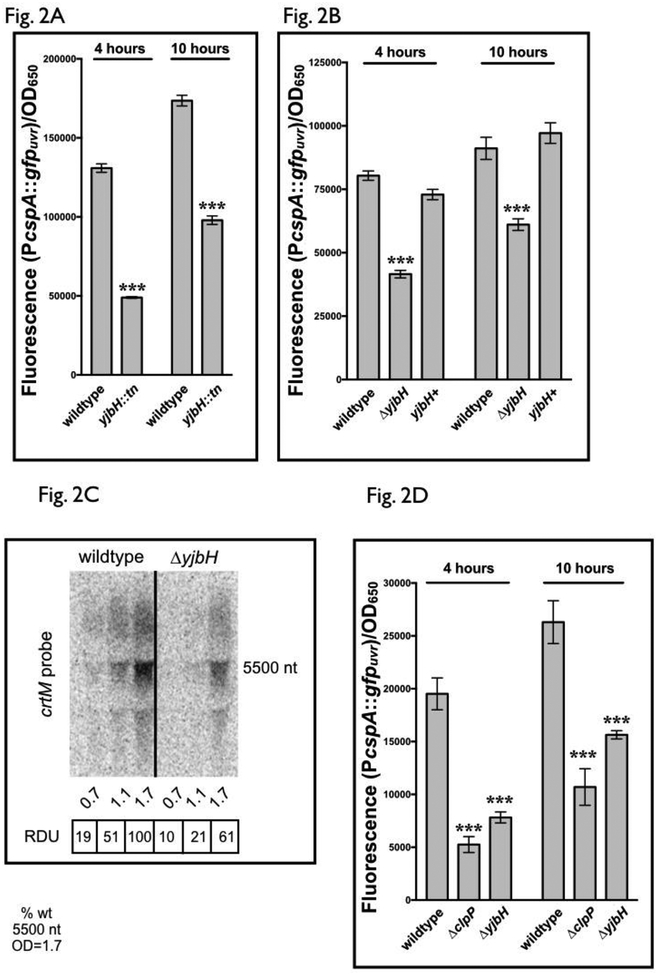
To expand our understanding on how pigmentation is controlled through sigB and cspA, we screened the Nebraska JE2 transposon library for mutants with pigmentation defects (Fey et al., 2012). These mutant strains were transformed with pALC8135 (PcspA::gfpuvr) and fluorescence compared to wild-type JE2 containing the identical plasmid. Using this technique to screen the entire mutant library, a mutant strain with diminished cspA promoter activity was found to have a transposition insertion into yjbH (NE896) (Fig. 2A). To validate this, a SH1000 strain with a clean deletion of yjbH was created using allelic exchange (Arnaud et al., 2004), as well as its subsequent chromosomal replacement. These strains, together wild type SH1000, were transformed with pALC8135. Similar to our results in the JE2 transposon mutant, deletion of yjbH in SH1000 resulted in decreased cspA transcription (Fig. 2B) compared to wild type and chromosomal replacement strains, validating the results of our screen and suggesting that yjbH plays a role in activating cspA transcription.
Figure 2: yjbH regulates cspA promoter activity in S. aureus.
(A) cspA promoter activity in S. aureus JE2 wildtype and a strain disrupted for yjbH by the mariner transposon. GFP fluorescence generated from the cspA promoter was measured at 4 and 10 hours for S. aureus JE2 wildtype and NE896 (yjbH transposon mutant), carrying the plasmid pALC8135 with cspA promoter driving gfp. Cell densities were simultaneously measured at OD650 and fluorescence normalized to OD650. The value for each strain represents the mean of two biological replicates that were read in triplicate, and the experiments were repeated three times. A representative experiment is displayed.
(B) cspA promoter activity in S. aureus SH1000 wildtype, de-novo constructed ΔyjbH strains and yjbH mutant with chromosomal replacement. Fluorescence of S. aureus SH1000 wildtype, ΔyjbH and yjbH+, its chromosomally complemented strain, carrying the plasmid pALC8135 was measured at 4 and 10 hours of growth in TSB. Cell densities were simultaneously measured at OD650 and fluorescence normalized by it. The value for each strain represents the mean read in triplicate and the experiments were repeated three times. A representative experiment is displayed. The yjbH+ strain indicates the yjbH complement strain ALC8121.
(C) A representative Northern blot of the crtOPQMN transcript in SH1000 wildtype and ΔyjbH strains. RNA obtained from SH1000 wildtype and ΔyjbH cells at various optical densities (15 μg of cellular RNA per lane) was resolved on a denaturing agarose gel, blotted to Hybond XL membrane and hybridized with a 300-bp 32P-radiolabeled crtM DNA probe. The relative densitometric units (RDU) of each ~5.5k nt band were calculated relative to wildtype at OD650=1.7. The experiment was repeated at least two times with consistent results.
(D) cspA promoter activity in S. aureus SH1000 wildtype and strains deleted for ΔclpP and ΔyjbH. Fluorescence of S. aureus SH1000 wildtype, ΔclpP and ΔyjbH carrying the plasmid pALC8135 was measured at 4 and 10 hours of growth in TSB. Cell densities were simultaneously measured at OD650 and fluorescence normalized by it. The value for each strain represents the mean of two biological replicates that were read in triplicate, and the experiments were repeated three times. A representative experiment is displayed.
The asterisks in Fig. 2A, 2B and 2D indicate statistical significance between wildtype and mutant strains, determined using Student t-test (***, P <0.0005).
Subsequent transcriptional analyses of cspA in yjbH mutants by Northern blots were unfortunately complicated by apparent cross-hybridization among csp homologs. For example, a cspA probe still hybridized with a transcript in a ΔcspA mutant, and similar result was seen for a cspC probe to RNA from the ΔcspC strain of SH1000 (Fig. S2A). This is likely due to the significant homology shared among the csp paralogs in S. aureus [e.g. cspB and cspC share 68% and 78% nucleotide identity to cspA, respectively (Fig. S2B)]. To verify that the decreased pigmentation in a ΔyjbH mutant occurred through changes in crt mRNA levels, analysis of the crt transcript was examined by Northern blotting. Overall, the crt transcript encoding crtOPQMN was lower in a ΔyjbH strain compared to the wildtype SH1000 (Fig. 2C), indicating that deletion of yjbH leads to reduced carotenoid production, likely due to decreased crt transcription by way of diminished cspA expression.
In S. aureus and B. subtilis, YjbH functions as an adaptor for the ClpXP proteolytic complex by recognizing and ushering specific proteins such as redox-sensitive Spx (Kommineni et al., 2011; Engman et al., 2012) to the proteolytic pocket of ClpP for processing. To determine this possibility for regulation of cspA by YjbH, we examined whether a S. aureus clpP mutant displayed a similar decrease in cspA transcription. Indeed, a SH1000 ΔclpP strain containing pALC8135 displayed decreased fluorescence comparable to a SH1000 ΔyjbH strain (Fig. 2D), suggesting that yjbH-mediated regulation of cspA may occur through ClpP-dependent proteolytic activity.
Given the linkage of cspA to proteolytic components of S. aureus, we examined whether direct proteolytic control of CspA exists in S. aureus. In E. coli, CspD is degraded by the Lon protease (Langklotz and Narberhaus, 2011); however, S. aureus lacks a Lon ortholog and, in its place, ClpP controls S. aureus various protein levels via ClpP-mediated degradation (Donegan et al., 2010; Cohn et al., 2011). Thus, a SH1000 strain containing a plasmid (pALC7342) with xylose-inducible expression of CspA (Forsyth et al., 2002) was used to follow CspA protein levels over time. Aliquots of cells were taken pre- (t=0’) and post-translational stalling (t=60’) by adding 50 μg/ml erythromycin at various growth phases, as well as during both cold (16oC) and heat (44oC) shock. CspA levels were then examined by Western blots with a rabbit anti-CspA antibody (a kind gift of Sam Katzif, Midwestern University). Under no condition was CspA observed to be degraded (data not shown), suggesting that CspA is not post-translationally regulated.
cspA transcription is subject to negative autoregulation.
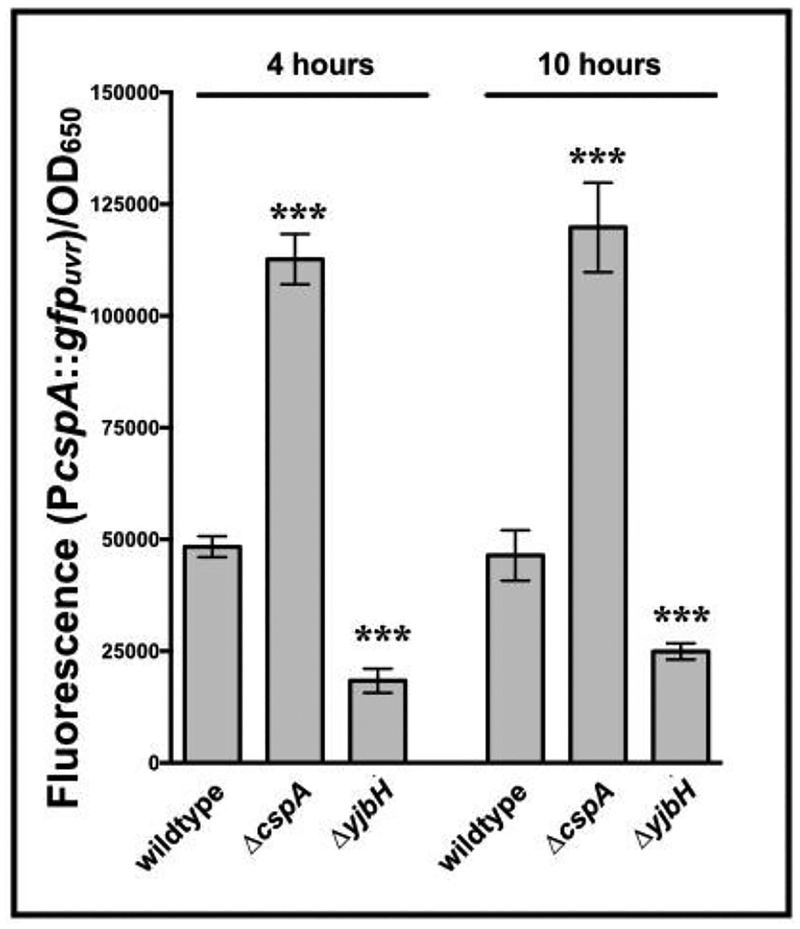
As the 5’ UTR of cspA in both E. coli and S. aureus is controlled at both the transcriptional and post-transcriptional levels (Jiang et al., 1996; Uppalapati et al., 2017), we were interested in whether a S. aureus strain lacking cspA showed changes in cspA promoter activity. Fluorescence levels for a ΔcspA strain of SH1000 carrying pALC8135 were compared to wildtype with pALC8135. Cells lacking cspA showed a strong increase in PcspA-dependent fluorescence compared to wildtype (Fig. 3), suggesting a negative feedback loop for the cspA locus.
Figure 3: cspA represses the activity of its own promoter and is regulated by the hbo-yjbH locus.
cspA promoter activity in S. aureus SH1000 strains deleted for cspA or yjbH. Fluorescence of S. aureus SH1000 wildtype, ΔcspA and ΔyjbH strains carrying the plasmid pALC8135 at 4 and 10 hours of growth in TSB. Cell densities were simultaneously measured at OD650 and fluorescence normalized by it. The value for each strain represents the mean of two biological replicates that were read in triplicate, and the experiments were repeated three times. A representative experiment is displayed. The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (***, P <0.0005).
Impact of cspA on transcription of the yjbH locus.
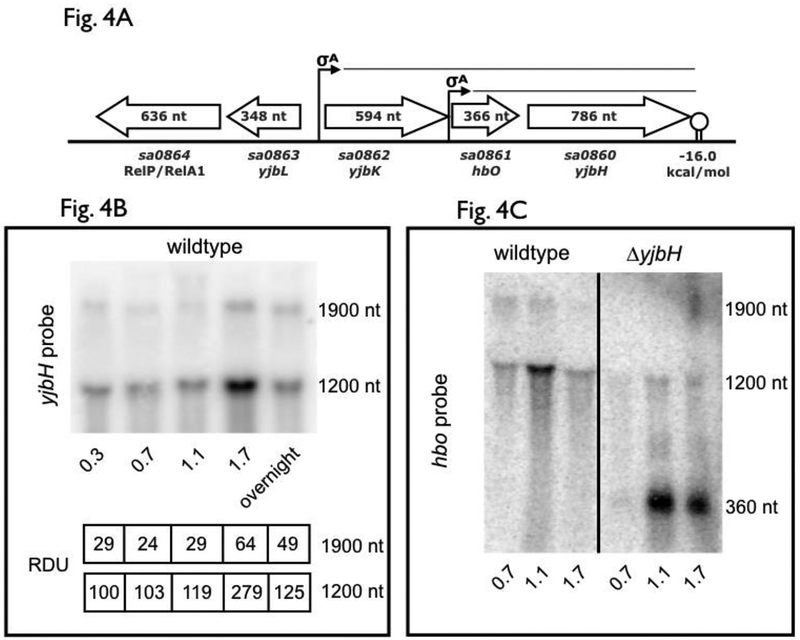
To ascertain if cspA can impact yjbH, we first examined the transcription of the yjbH locus (Fig. 4A). Northern blot analysis revealed two distinct transcripts of ~1.2 kb and 1.9 kb that hybridized with an yjbH probe (Fig. 4B), with the smaller of the two showing greater intensity and peaking at stationary phase (OD650=1.7). The proximity of the hbo gene, also called yjbI (Austin et al. 2019) and located just 44 bp upstream of yjbH, coupled with a strong rho-independent terminator (ΔG=−16 kcal/mol) (de Hoon et al., 2005) downstream of yjbH (Fig. 4A), implicates the 1.2 kb transcript to encompass hbo-yjbH. The larger 1.9 kb yjbH-containing transcript likely originates from a σA promoter upstream of sa0862, an uncharacterized gene immediately upstream of hbo. The exact function of the hbo (yjbI), coding for a putative truncated hemoglobin homolog with high oxygen affinity (Pathania, 2002), and its relationship to YjbH is unclear.
Figure 4: yjbH operon structure and transcriptional analysis.
(A) Operon and promoters of genetic elements neighboring yjbH. The location for the σA-dependent promoters directing hbo (also called yjbI) and yjbH transcription as well as that for sa0862-hbo-yjbH and the coding sequences for yjbH and its surrounding genes are shown in relation to one another. Predicted yjbH transcripts from transcriptional analyses and in vitro terminator data are also shown.
(B) yjbH transcription in SH1000 during logarithmic and stationary growth phases. RNA taken from SH1000 wildtype cells (15 μg per lane) at time points was resolved on a denaturing agarose gel, blotted to Hybond XL membrane and hybridized with a 300-bp 32P-radiolabeled yjbH DNA fragment. The relative densitometric units (RDU) of the 1.9k and 1.2k nt bands were calculated relative to 1.2k nt levels in the wildtype at OD650=0.7 (set at 100). This is a representative blot of an experiment that had been repeated at least two times, with consistent results across different experiments
(C) hbo transcription in SH1000 wildtype and ΔyjbH strains. RNA obtained from SH1000 wildtype and ΔyjbH cells at various optical densities (15 μg per lane) was resolved on a denaturing agarose gel, blotted to Hybond XL membrane and hybridized with a 200-bp 32P-radiolabeled hbo DNA fragment.
The linkage of hbo to yjbH was verified by probing for hbo transcript in wildtype and ΔyjbH strains (see Fig. 4A). The 1.2 kb and 1.9 kb bands in the SH1000 wildtype strain were replaced by ~360 and a ~1.0 kb bands in the isogenic ΔyjbH strain (Fig. 4C), indicating that hbo was part of both abbreviated transcripts that encompass yjbH. Therefore, the promoters of both hbo and sa0862 are active, yielding co-transcription with yjbH, with the proximal (Phbo) promoter stronger than the distal promoter (Psa0862).
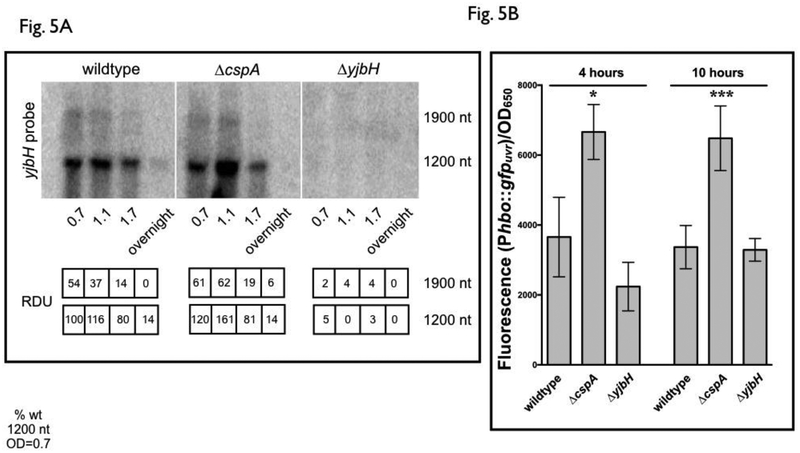
With the above information available, the effect of cspA on yjbH transcription was then evaluated by Northern blots of RNA from SH1000 wildtype, isogenic ΔcspA and ΔyjbH strains with a radiolabeled yjbH probe. As shown in Fig. 5A, the 1.2 kb hbo-yjbH transcript at an OD650nm of 1.1 was increased in a ΔcspA mutant compared to the wildtype, while the effect was not as prominent as the lesser transcribed 1.9 kb sa0862-hbo-yjbH transcript (Fig. 5A). These results indicate that yjbH is repressed by cspA and that most of the effects of cspA on yjbH transcription is through the 1.2 kb hbo-yjbH transcript. We then confirmed this finding of cspA-mediated repression of yjbH with the Phbo promoter that controls hbo-yjbH transcription (Fig. 5B) as this promoter appeared to be the stronger of the two and also mostly affected by cspA (Fig. 5A). We constructed the plasmid (pALC8134) containing the 90 bp region upstream of hbo containing the putative hbo promoter (Pathania, 2002), and transcriptionally fused it to gfpuvr. The recombinant plasmid was transformed into SH1000 wildtype, ΔcspA and ΔyjbH strains. Similar to the results of the Northern blot of yjbH in a ΔcspA strain, deletion of cspA resulted in increased Phbo activity as detected by fluorescence (Fig. 5B). As we have shown (Fig. 3) that a regulatory loop exists for the regulation of the cspA promoter in a cspA mutant, we also assessed whether a similar type of regulation existed for yjbH. However, no variation of fluorescence was observed between wildtype and the ΔyjbH mutant harboring pALC8134, indicating a lack of feedback of YjbH on its own promoter (Fig. 5B).
Figure 5: Transcriptional activity of the hbo-yjbH promoter is affected by cspA.
(A) yjbH transcription in SH1000 wildtype, ΔcspA and ΔyjbH strains. RNA obtained from SH1000 wildtype, ΔcspA and ΔyjbH cells at various optical densities (15 μg per lane) was resolved on a denaturing agarose gel, blotted to Hybond XL membrane and hybridized with a 200-bp 32P-radiolabeled yjbH DNA fragment. The relative densitometric units (RDU) of the 1.9k and 1.2k nt bands were calculated relative to 1.2k nt levels in the wildtype at OD650=0.7 (set at 100). The hybridization analysis was repeated twice with similar results. A representative blot was shown.
(B) hbo-yjbH promoter activity in S. aureus SH1000 strains deleted for cspA or yjbH. Cell density-normalized GFP fluorescence of S. aureus SH1000 wildtype, ΔcspA and ΔyjbH strains carrying the hbo-yjbH promoter reporter plasmid (pALC8134) at 4 and 10 hours of growth in TSB was shown. Cell densities were simultaneously measured at OD650 and fluorescence normalized by it. The value for each strain represents the mean of two biological replicates that were read in triplicate, and the experiments were repeated three times. A representative experiment is displayed. The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (*, P < 0.05; ***, P <0.0005).
Expression of cspA can complement the loss of yjbH.
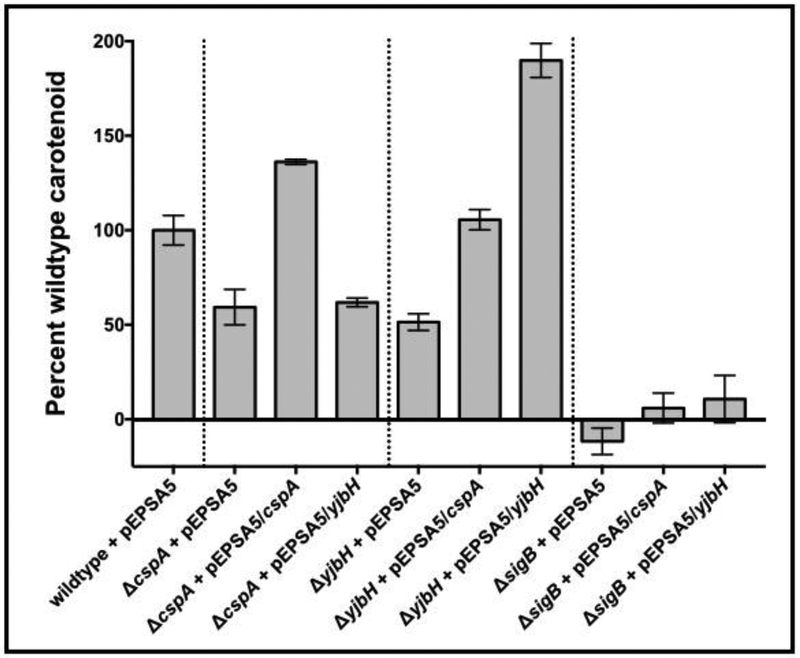
As yjbH and cspA are necessary for full pigmentation in S. aureus (Engman et al., 2012; Fey et al., 2012) (Fig. 1A), we examined whether these two genes control carotenoid production along a similar pathway. Accordingly, we created ΔyjbH and ΔcspA strains with a multi-copy plasmid (pEPSA5) capable of inducible cspA (pALC7342) or yjbH (pALC7091) expression. The empty vector was used as a control. Carotenoid was then extracted from cells grown overnight at 37oC with 0.5% xylose induction and normalized to cell density. Notably, pigmentation was not restored in ΔsigB strains expressing either yjbH or cspA (Fig. 6), indicating that sigB is epistatic to both yjbH and cspA. However, pigment production was diminished in the ΔyjbH strain but was restored upon expressing cspA exogenously under a xylose-inducible promoter, while a ΔcspA strain carrying pEPSA5::yjbH was not able to restore pigmentation (Fig. 6). As expected, the ΔyjbH mutant strain expressing yjbH from the pEPSA5 plasmid, displayed high pigmentation. These data imply that cspA and yjbH share a common pathway in the synthesis of carotenoid, with cspA epistatic to yjbH.
Figure 6: Expression of cspA in trans cross-complements carotenoid production in a SH1000 ΔyjbH strain.
Carotenoid was extracted from two biological replicates each of S. aureus SH1000 wildtype, ΔcspA, ΔyjbH and ΔsigB mutant strains with either pEPSA5, pALC7091 (pEPSA5::yjbH) or pALC7342 (pEPSA5::cspA) that were grown overnight on solid Tryptic Soy agar. The carotenoid levels were normalized to cell density and compared as a percentage of wildtype set at 100%. The experiments were repeated three times, with representative percentages from one experiment displayed. The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (**, P < 0.005; ***, P <0.0005).
yjbH regulates the spx promoter through Spx.
Spx is a redox-sensitive transcription factor that responds to redox and oxidative stresses (Zuber, 2004), and adjusts its transcriptome accordingly via changes in its interaction presumably with the α subunit of RNA polymerase (Nakano et al., 2010). However, we have also encountered a scenario where Spx of S. aureus can bind directly to the target promoter (unpublished data). Importantly, intracellular Spx levels are controlled by YjbH, an adaptor protein that directs Spx for ClpXP-mediated proteolysis (Engman et al., 2012). Given this involvement of YjbH in Spx and also in carotenoid regulation as delineated above, we surmise that cspA expression may be linked to Spx.
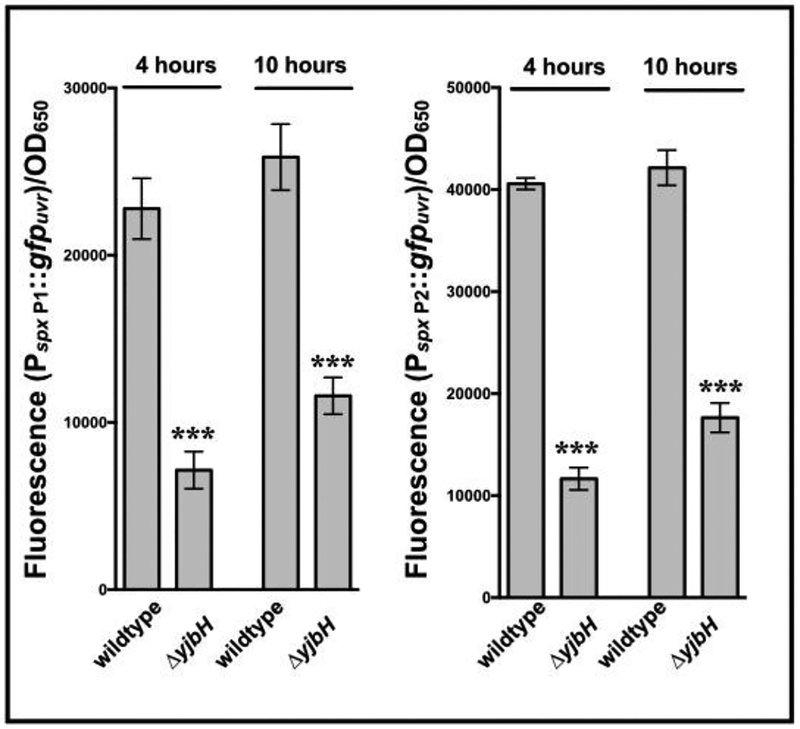
Transcriptional analyses have revealed spx to be transcribed from both a proximal (P1) and distal (P2) promoters (Jousselin et al., 2013). Upon introducing plasmids containing spx P1 or P2 promoter fused to gfpuvr into isogenic yjbH strains, it was observed that there was less fluorescence from both spx P1 and P2 promoters in the yjbH mutant compared to wildtype (Fig. 7). This finding concurs with a previous report (Jousselin et al., 2013), showing that spx transcription from its promoter is likely subject to increased auto-repression from elevated Spx levels, which accumulate in the absence of YjbH-mediated degradation by ClpXP.
Figure 7: Activation of spx promoter in a ΔyjbH strain vs. the parent.
Promoter activity from both the proximal (P1) and distal (P2) spx promoters were measured in S. aureus SH1000 strains deleted for yjbH. Cell density-normalized GFP fluorescence of S. aureus SH1000 wildtype and ΔyjbH strains carrying the P1 (pALC7803) and P2 (pALC7804) transcriptional fusion plasmids were taken at 4 and 10 hours of growth in TSB. Cell densities were simultaneously measured at OD650 and fluorescence normalized by it. The value for each strain represents the mean of three technical triplicates of two biological replicates, and the experiments were repeated three times. A representative experiment is displayed. The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (***, P <0.0005).
The effect of Spx on cspA transcription.
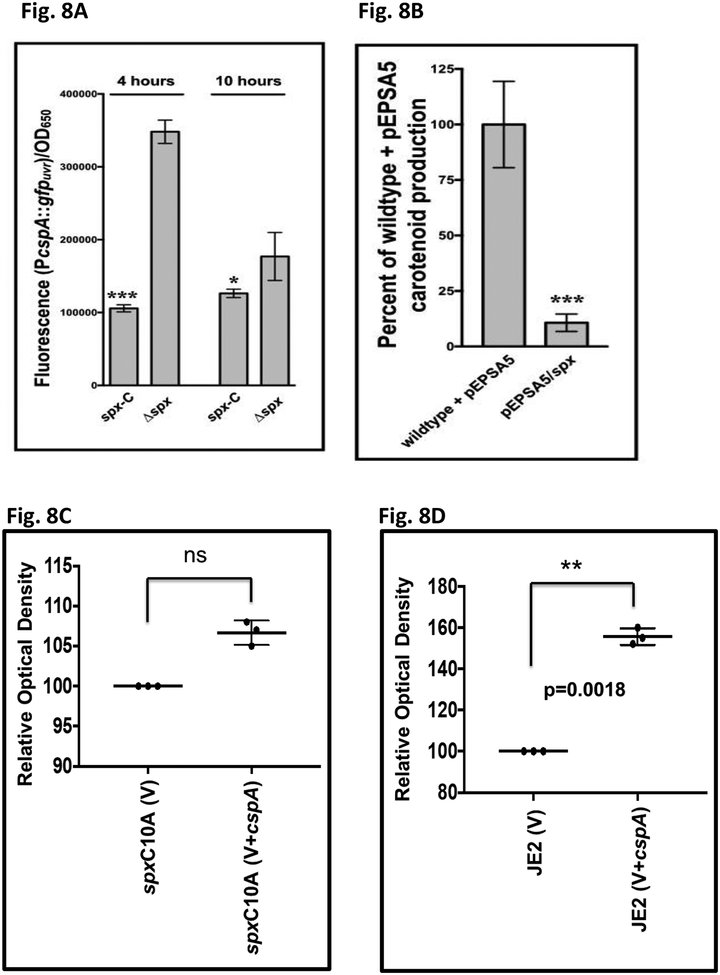
As Spx and CspA are involved in redox stress and cold shock response, respectively, it is reasonable to surmise that Spx may somehow interact with cspA, given that Spx has been shown to be preferentially enriched on all three csp promoters in B. subtilis (Rochat et al., 2012). Accordingly, a Δspx mutant of S. aureus strain 8325–4 and the chromosomal replacement strain spx-c (by inserting a native copy of spx into Δspx strain), both containing the cspA promoter::gfp construct (pALC8135), were grown and assessed for absorbance and GFP fluorescence. A potential issue for the Δspx mutant in the 8325–4 background is that it may harbor suppressor mutations and possibly a mild fitness defect (Pamp et al., 2006). Therefore, the Δspx mutant of 8325–4 was complemented with a native copy of spx chromosomally for comparison, expecting whatever mutations in the Δspx mutant would be carried over to the chromosomal replacement strain. Our results (Fig. 8A) showed that cspA promoter activity was higher in the 8325–4 Δspx mutant than the chromosomal replacement strain at both time points, indicating that spx acts as a repressor for cspA. Consistent with this observation is that over-expression of spx from pEPSA5 (pALC7113) in wild type strain SH1000, predicted to have lower cspA expression due to the native and exogenous copy of spx (Fig. 8A), had a lower level of carotenoid pigment vs. the vector control (Fig. 8B), consistent with our hypothesis that spx is a repressor of cspA, a positive regulator of σB and carotenoid expression.
Fig. 8: Repression of the cspA promoter in a spx strain and effect of spx on pigment production.
(A) S. aureus 8325–4 Δspx and its restored complement spx-C transformed with pALC8135 (PcspA::gfp) were examined for fluorescence during growth in TSB at 4 and 10 hours. Cell densities at OD650 were measured simultaneously, and fluorescence normalized by it. The value for each strain represents the mean of three technical triplicates of two biological replicates, and the experiments were repeated three times. A representative experiment is displayed.
The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (*, P < 0.05; **, P < 0.005).
(B) S. aureus SH1000 wildtype with either empty vector (pEPSA5) or vector over-expressing spx (pEPSA5::spx, pALC7133) were grown at 37oC overnight on solid agar with 0.2% xylose. Cells were scraped and resuspended in PBS, whereupon the cell density was measured, and carotenoid pigment extracted. Carotenoid levels were then normalized to cell density and compared to one another as a percentage of wildtype set at 100%. The experiments were repeated three times, with representative percentages from one experiment displayed. The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (***, P <0.0005).
(C) Relative carotenoid pigment expression in spx C10A mutant carrying either pEPSA5 (V) or pEPSA5::cspA (V+cspA). Pigment from cells was extracted in triplicate as described in Fig. 8B. The spx C10A mutant with vector (V) was set at 100%. There was no significant difference between vector alone and vector expressing cspA.
(D) Relative carotenoid pigment expression in wild type JE2 cells carrying either pEPSA5 (V) or pEPSA5::cspA (V+cspA). Pigment from cells was extracted in triplicate as described above. The JE2 cells with vector (V) was set at 100%. Student t test was used to assess significance, with P value shown.
The cspA promoter is directly controlled by Spx.
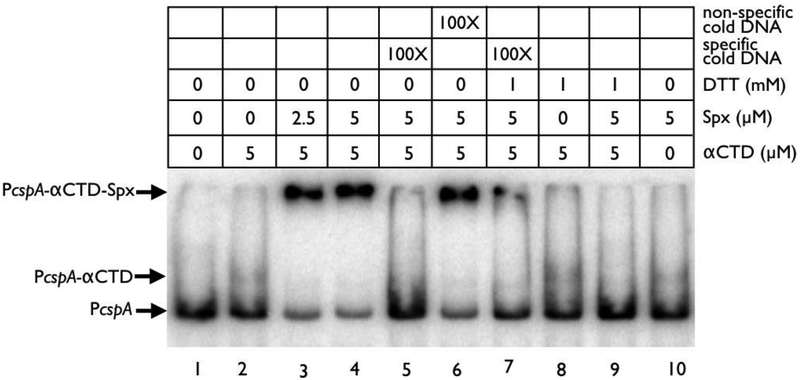
We next examined the mechanistic control of Spx on the cspA promoter. While Spx has been shown to bind DNA when complexed with the α subunit of RNA polymerase, RpoA (Shunji Nakano et al., 2004; Michiko M Nakano et al., 2010), it does not eliminate the possibility that Spx can interact solely with the target promoter. With regard to the binding of RpoA, studies have shown that the C-terminal domain of RpoA, called the αCTD, recognizes UP elements in promoter DNA and interacts with transcription regulators like Spx in an association that can either enhance or occlude nearby DNA elements (Shunji Nakano et al., 2004; Chan et al., 2012). To evaluate this possibility for the cspA promoter, combinations of purified αCTD and Spx were evaluated for their ability to bind a 300 bp cspA promoter fragment in EMSA studies.
On its own, αCTD and Spx shifted radiolabeled cspA promoter DNA poorly (Fig. 9, lanes 2 and 10). However, when both proteins were pre-incubated together, more intense shifting of the cspA promoter fragment was observed (Fig. 9, lanes 3 and 4). The binding of αCTD and Spx together was also specific for the cspA promoter, as challenge with 100-fold excess of unlabeled cspA promoter was able to abolish this shift (Fig. 9, lane 5), while challenge with cold non-specific DNA did not (Fig. 9, lane 6).
Figure 9: Spx and αCTD interact with the cspA promoter.
EMSA analysis of αCTD and Spx binding to the cspA promoter probe in reactions containing Spx or mixtures of Spx with αCTD under various conditions. Bands corresponding to the cspA promoter/αCTD and cspA promoter/Spx/αCTD complexes are marked with arrows. The 32P cspA promoter probe was generated by labeling a PCR product of the cspA promoter (−300 to +1) with γ−32P dATP using T4 polynucleotide kinase. An internal fragment of sarX was used as the non-specific cold challenge DNA, while unlabeled cspA promoter (−300 to +1) was used as the specific cold challenge DNA.
Given Spx’s role as a redox-sensitive transcription factor in both S. aureus (Pamp et al., 2006) and B. subtilis (Michiko M Nakano et al., 2010), we were curious whether Spx in S. aureus would show redox-dependent binding to αCTD and the cspA promoter. Indeed, the presence of 1 mM DTT which reduces Spx to an inactive state abolished a shift of the radiolabeled cspA promoter with αCTD and Spx (Fig. 9, Lane 9), suggesting that the oxidized form of Spx is important for this interaction.
Effect on Spx on pigment production.
Recognizing that Spx interacts with αCTD to bind the cspA promoter, we were curious as to the biologic effect of this interaction on pigmentation, an important virulence factor of S. aureus. We have shown earlier that increased expression of spx repressed carotenoid pigment production (Fig. 8B). To confirm this finding, we examined pigment production in the spx C10A mutant in the JE2 background. This mutant (gift of Vanai Thomas, Univ. of Nebraska Medical School), lacking the CXXC motif to fabricate the active (oxidized) form of Spx (Shunji Nakano et al., 2004), has no growth defect and could elaborate strong carotenoid pigment (Fig 8C), consistent with high cspA expression (Fig. 8A) in association with elevated σB-dependent asp23 activity (Fig. 10A). Over-expression of cspA from an exogenous promoter in the spx C10A mutant with plasmid pALC7342 (V+cspA) led to a modest but insignificant increase in carotenoid production vs. the vector control (V) (Fig. 8C), consistent with already elevated cspA expression in this mutant. In contrast to the spx C10A mutant, enhanced expression of cspA with pALC7342 in the wild type JE2 resulted in a significantly higher carotenoid level than its counterpart carrying the pEPSA5 vector alone (Fig. 8D). Together, these data support a model whereby Spx represses cspA expression to reduce carotenoid expression.
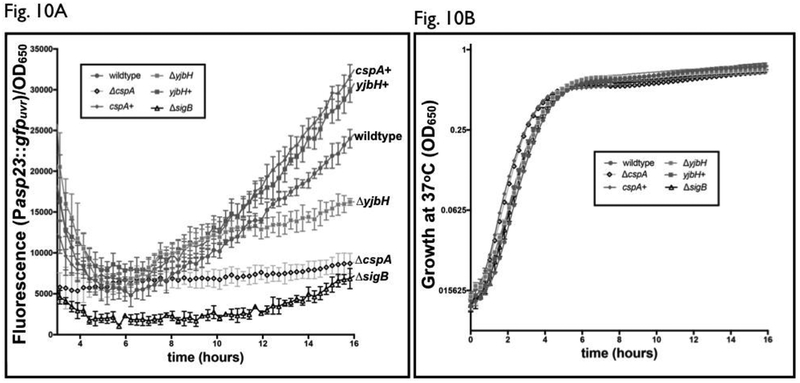
Figure 10: Strains lacking ΔcspA or ΔyjbH have differing profiles of the σB-dependent asp23 promoter activity.
(A) Fluorescence and cell density (OD650) were simultaneously measured in S. aureus wildtype (closed circle), ΔcspA (open diamond), ΔyjbH (lighter closed square) and ΔsigB mutant (open triangle) strains and their chromosomally complemented equivalents (cspA+, closed diamond, yjbH+, darker closed square) carrying the plasmid pALC2201 (asp23 promoter driving gfp) during 16 hours of growth in TSB. GFP fluorescence was then normalized to OD650, with each value representing the mean of two biological replicates that were read in triplicate.
(B) Growth curve of SH1000 wildtype and mutant strains. Cell densities of S. aureus wildtype (closed circle), ΔcspA (open diamond), ΔyjbH (lighter square) and ΔsigB mutant (open triangle) strains and their chromosomally complemented equivalents (cspA+, closed diamond, yjbH+, darker square) carrying the plasmid pALC2201 were measured at OD650, with each value representing the mean of two biological replicates that were read in triplicate.
The level of asp23 transcription is temporally different in cspA and yjbH mutants.
Given that cspA regulates pigment through sigB (Katzif et al., 2005), and yjbH is necessary for cspA transcription via reduced Spx expression, we measured the impact of each on σB activity over time by following the fluorescence in strains with an asp23 promoter fusion with GFP. As a control, a ΔsigB strain was provided as a baseline for the impacts of cspA and yjbH on σB activity (Katzif et al., 2005). As displayed in Fig. 10A, σB-dependent asp23 promoter activity in a ΔcspA mutant of SH1000 was lower compared to the wild-type, but still higher than that found in a ΔsigB strain despite a lack of differences in growth among strains (Fig. 10B), thus mirroring the significant, but not total, decrease in pigmentation in a ΔcspA mutant (Fig. 1A). In contrast, the asp23 activity of the ΔyjbH strain of SH1000 was equivalent to that seen in wild type up through the end of post-exponential phase (Fig. 10A). However, following entry into late or post stationary phase, asp23 promoter activity diverged, with levels increasing in a wildtype strain, while the level of asp23 promoter activity of the ΔyjbH strain had a modest increase but significantly lower than that of the wild type, consistent with a lower σB activity in the mutant. We also observed that chromosomal replacement in the respective cspA and yjbH mutants (csp+ and yjbH+) yielded slightly higher asp23 promoter activity than the wild type. The reason for this moderate increase is not clear at this point.
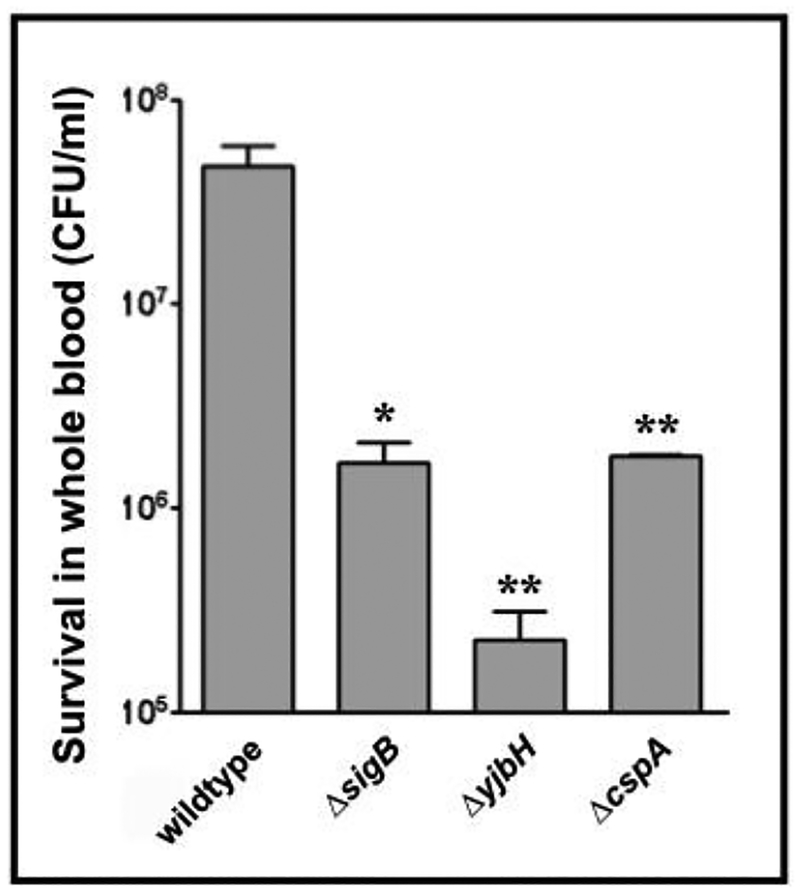
Both cspA and yjbH are necessary for survival in whole blood.
cspA has been previously shown to be important for survival of S. aureus phagocytosed by neutrophils (Samanta et al., 2016), purportedly due to alteration of capsular activity via binding of CspA to the cap promoter. We were interested as to whether the increased susceptibility of a S. aureus cspA mutant to neutrophils extended to strains lacking ΔyjbH, given the genetic linkage between yjbH and cspA delineated above. Furthermore, as sigB has also been shown to be involved in capsule regulation via the arlRS and yabJ-spoVG operons (Meier et al., 2007), a S. aureus ΔsigB strain was included as a control in this experiment. Accordingly, we exposed S. aureus wild type, ΔcspA, ΔyjbH and ΔsigB strains to human phagocytic cells (mostly PMNs) in a whole blood killing assay. Both ΔsigB and ΔcspA strains were much less able to survive compared to the wildtype (Fig. 11). Importantly, a SH1000 ΔyjbH strain survived even less well than the sigB and cspA mutants, suggesting a greater involvement of yjbH in resistance to oxidative stress and oxidative killing by PMN, possibly through post-translational control of a cspA-independent but yjbH-dependent pathway in S. aureus.
Figure 11: Human whole blood killing assay of SH1000 and mutant strains.
Blood from human volunteers was mixed with wildtype or mutant S. aureus strains (1 × 105 CFU/ml). Shown are surviving CFU at 3h. The experiment was performed three times and a representative experiment is displayed. The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (*, P < 0.05; **, P < 0.005; ***, P <0.0005).
DISCUSSION
Staphyloxanthin, the carotenoid pigment accounting for the golden yellow color in S. aureus, is a major virulence factor of S. aureus (Song et al., 2009). During infections, staphyloxanthin, as a powerful antioxidant, has been shown to impair neutrophil killing by evading death due to reactive oxygen species produced by the neutrophils (Liu et al., 2005). In previous studies, the cold shock protein CspA has been found to regulate sigB-dependent carotenoid production in S. aureus (Katzif et al., 2005). However, the pathways that control CspA expression have not been well defined. In addition, there are two additional Csp paralogs (e.g. CspB and CspC) the function of which is poorly defined. Our study here has clarified the roles of these Csp paralogs in carotenoid pigment production in S. aureus. In previous reports (Katzif et al., 2005; Duval et al., 2010), attempts at determining the csp-dependent phenotypes were ill-defined as strains were created with polar disruptions of csp paralogs in divergent backgrounds or large deletion of chromosomal region surrounding csp genes (Sahukhal and Elasri, 2014). In this study, only the impact of clean deletions and/or non-polar insertions in csp genes in a single genetic background (i.e. SH1000) on carotenoid production were examined and compared to a ΔsigB strain. We established that cspA plays a larger role in carotenoid production than cspB and that cspC has no role in S. aureus pigmentation (Fig. 1A). In contrast to CspA, the part played by CspB in pigmentation is independent of σB. The significance of this is yet unclear, but a screen of carotenoid-deficient mutants in the JE2 transposon library (Fey et al., 2012) with unaltered asp23 activity also revealed σB-independent expression of staphyloxanthin in diverse strains (data not shown). This is in agreement with a previous report, showing that both σB-dependent and -independent pathways are capable of modulating carotenoid production in S. aureus (Lan et al., 2010).
A recent study (Sahukhal and Elasri, 2014) suggested that the vast majority of cspA mRNA in S. aureus existed as a single-gene transcript generated by post-transcriptional processing of a larger msaA-cspA transcript (Sahukhal and Elasri, 2014). However, the results from our studies (Fig. 2B) and other labs (Uppalapati et al., 2017) support the notion that the cspA promoter is considerably more active than the msaA promoter (Fig. S1). One possibility for this difference could be the use of luminescent construct in other studies (Sahukhal and Elasri, 2014) vs. the fluorescent reporter in our studies, as luminescence in S. aureus is affected by intracellular redox state and growth phase (e.g. less activity during stationary phase) (Galluzzi and Karp, 2007).
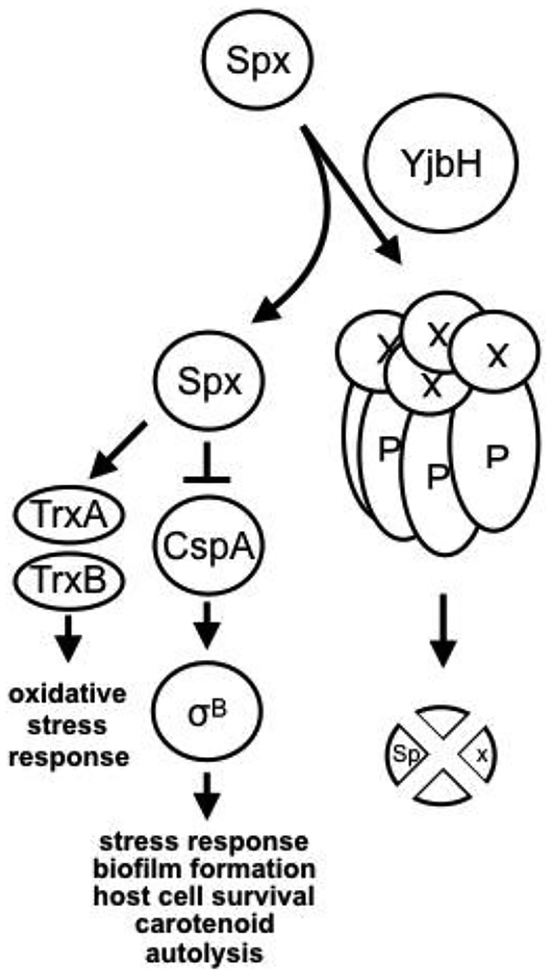
In elucidating the mechanism that controls carotenoid production in S. aureus, we have discovered a novel pathway wherein the stress-response pathway of CspA-σB is linked to the redox sensitive transcriptional regulator Spx (Fig. 12). The discovery of Spx in the repression of cspA (Fig. 8) has prompted us to examine factors that mediate post-transcriptional control of Spx. In conducting a de novo screen of the Nebraska JE2 transposon library for mutants that exhibited reduced pigmentation, we discovered one of the mutants to be an yjbH mutant. YjbH is an adaptor protein that ushers specific protein(s) to the ClpXP proteolytic core for degradation (Fig. 12). Our observation that the ΔyjbH and the ΔclpP mutants displayed reduced cspA promoter activity vs. the parent SH1000 (Fig. 2D) is consistent with increased Spx level due to reduced Spx degradation in the mutants which, in turn, represses cspA more than the wildtype.
Figure 12: Proposed model for sigB-mediated carotenoid regulation in S. aureus.
The membrane pigmentation, staphyloxanthin, is a triterpenoid carotenoid. Staphyloxanthin encoded by the crtOPQMN operon is transcribed from a σB-dependent promoter. CspA acts as an activator of σB and its transcription is directly repressed by the redox-responsive transcriptional regulator Spx. Besides transcriptional control of cspA, there is also post-translational control because Spx is subjected to proteolytic degradation by the ClpXP protease as assisted by the adaptor YjbH. Spx also represses its own transcription. As part of the feedback, CspA can repress yjbH transcription.
Our model (Fig. 12) also predicts reduced carotenoid production in the ΔyjbH mutant (Fig. 6). As both cspA and yjbH positively regulate pigment production, we also verified that cspA is epistatic to yjbH as supported by findings that complementation of cspA in a ΔyjbH mutant could restore carotenoid production (while the reverse was not possible) (Fig. 6). YjbH regulates Spx post-translationally by recognizing a C-terminal signal on Spx and guiding it to the chaperone ClpX, which channels it for degradation by ClpP (Chan et al., 2012). Together, these data suggest that yjbH lies upstream of spx and cspA in a pathway controlling sigB-dependent carotenoid production.
Interestingly, a recent paper by Austin et al also reported the contribution of YjbH to pigmentation and host colonization in S. aureus (Austin et. Al. 2019). While some of our findings are complementary (e.g. YjbH controls σB and pigmentation by altering Spx level), other findings in this paper are additive (e.g. cspA is the intermediary between Spx and σB. We have also provided a more comprehensive model to explain the genetic pathway that controls pigmentation. It is surprising that Austin et al described increased colonization of the yjbH mutant in kidneys and spleens vs. the wild type in a murine eye infection model, in contrast to our human whole blood killing assay wherein the yjbH mutant was more susceptible than the wild type. As mouse survival with these mutants was not measured in the Austin paper, it is unclear if these mutants would impact survival in this particular murine model. It should be mentioned that infection of an eye where it is immunologically inert is an unusual model for disseminated infection for S. aureus. Based on our whole blood killing assay, we would predict decreased survival of the yjbH mutant vs. the parental strain in the murine dissemination model of infection due to reduced pigment production.
Spx contains an internal disulfide redox sensing motif 10-CXXC-14 that affects its interaction with RNA polymerase (Shunji Nakano et al., 2004). The function of Spx as an activator or repressor appears to requires a small G/C rich element upstream of RpoA αCTD DNA binding site (Rochat et al., 2012). As our EMSA data show that Spx interacts with the cspA promoter through the αCTD of RpoA, we conclude that Spx is a direct repressor of the cspA promoter. Such regulation is consistent with ChIP-chip data from B. subtilis, showing that Spx is enriched at promoters of all three csp homologs (Rochat et al., 2012). Notably, failure of a previous two-dimensional fluorescence difference gel electrophoresis (2D-DIGE) to detect CspA (~7 kD) in the proteome of Spx (Pamp et al., 2006) was likely due to its inability to detect proteins below 20 kD. In addition, we also showed that the repressive effect of Spx on carotenoid production can be bypassed by expressing cspA from xylose-inducible promoter whereas this induction effect is muted in a spx C10A mutant where cspA expression is already high.
It is still possible that Spx governs cspA expression through other mechanisms of regulation. Maturation of the cspA transcript has been shown to be modulated by the binding of msaR, an antisense small RNA to the 5’ UTR of cspA. Generation of this small RNA and the subsequent maturation of the cspA transcript is controlled by ribonucleases (Galluzzi and Karp, 2007; Lioliou et al., 2012) which themselves could be targets for Spx-mediated regulation.
We have demonstrated auto-repression of cspA, based on up-regulation of the cspA promoter in a cspA mutant. Predicated on the up-regulation of yjbH in a cspA mutant (Fig. 5A), we propose that the pathway for auto-repression of cspA may occur via YjbH and Spx which, in turn, binds αCTD of RNAP and then onto the cspA promoter to repress cspA transcription (Fig. 3). According to this scheme, transcription of hbo-yjbH is up-regulated in a cspA mutant (Fig. 5A and 5B), increasing YjbH levels which contribute to increased Spx proteolysis. Depletion of Spx would lead to de-repression of the cspA promoter (Fig. 8A), resulting in up-regulation of the cspA promoter in a cspA mutant (Fig. 3). Alternatively, we recognize that Csp homologs can alter gene expression through their ability to bind mRNA and DNA promoter and terminator elements (Phadtare and Severinov, 2010), thus CspA may bind the cspA promoter directly for the repressive effect.
The significance of the transcription of hbo and yjbH (or yjbIH) as an operon and its relevance to the larger cspA/sigB regulatory network is still unclear. The putative truncated hemoglobin protein produced from the hbo gene was recently shown to have a high oxygen affinity and provide an advantage for growth under low oxygen conditions when expressed exogenously in E. coli (Pathania, 2002). Any relationship linking these characteristics of Hbo to oxidative stress or carotenoid production is not clear at this point. Interestingly, we have also found via cspA promoter-GFP fusion studies with pALC8135 that the cspA promoter activity in strain SH1000 was significantly elevated upon exposure to 10 mM of hydrogen peroxide, suggesting that the cspA gene is also responsive to oxidative stress (Fig. S4). Given the role of Spx, CspA and carotenoid in response and resistance to oxidative stress, the linkage seems plausible.
Carotenoid pigment is a powerful antioxidant that has been found to impair neutrophil killing mediated by the superoxide anion that is generated as a result of the oxidative burst. As we have uncovered the role of yjbH-spx-cspA-sigB axis (Fig. 12) in the control of pigment production, we wanted to verify the survival of the yjbH and cspA mutant in whole blood killing assay where PMNs are the predominant phagocytic cells capable of generating superoxide anion and hydrogen peroxide inside the phagolysosomes. We observed that both the yjbH and cspA mutants survived less well than the parent SH1000. Remarkably, the yjbH mutant has the lower survival rate than sigB and cspA mutants, suggesting that YjbH may harbor cspA-independent pathway for resistance to oxidative killing by PMNs. Accordingly, a transcriptomic analysis of cspA and yjbH mutant may provide clues for the differential survival rates between these two mutants.
EXPERIMENTAL PROCEDURES:
Bacterial strains and culture conditions.
Table 1 contains a list of bacterial strains used in these studies. E. coli strains were grown in Lysogeny Broth (LB), and S. aureus grown in Trypticase Soy Broth (TSB), both shaking at 250 rpm at 37°C. Cell density was measured via optical densities (OD) at 650 nm using either an 18-mm borosilicate glass tube in a Spectronic 20D+ spectrophotometer or an Infinite M1000 Pro reader (Tecan).
TABLE 1:
| Strain | Genotype | Source or reference |
|---|---|---|
|
S. aureus |
||
| BD1 | COL with aphA inserted upstream of cspB | (Duval et al., 2010) |
| JE2 | USA300 LAC derivative cured of its plasmids | (Fey et al., 2012) |
| NE896 | JE2 yjbH::tn | (Fey et al., 2012) |
| SH1000 | 8325–4 strain with repaired rsbU gene | (Horsburgh et al., 2002) |
| SK12 | SH1000 with Tn551 insertion upstream of cspA | (Katzif et al., 2003) |
| Δspx | AR738, 8325–4 Δspx | (Pamp et al., 2006) |
| spx-c | 8325–4 spx mutant with a native chromosomal copy of spx | (Pamp et al., 2006) |
| ALC3085 | SH1000 sigB::erm | (Schmidt et al., 2004) |
| ALC5105 | SH1000 ΔclpP | (Donegan et al., 2010) |
| ALC7137 | SH1000 ΔyjbH | (Donegan et al., 2014) |
| ALC8112 | SH1000 ΔcspA | This work |
| ALC8121 | SH1000 yjbH mutant with a native chromosomal copy of yjbH | This work |
| ALC8212 | SH1000 cspA mutant with a native chromosomal copy of cspA | This work |
| ALC8249 | SH1000 ΔcspC | This work |
| ALC8581 | SH1000 cspB::aphA | This work |
| ALC8702 | MRSA JE2 | (Fey et al., 2012) |
| ALC8704 | JE2 with spx C10A mutation | Vanai Thomas, Univ. of Nebraska Medical School |
| ALC8706 | JE2 with spx C10A mutation chromosomally repaired | Vanai Thomas, Univ. of Nebraska Medical School |
| ALC8971 | ALC8702 with pALC8135 (pALC1484 with cspA promoter driving gfpuvr) | This work |
| ALC8972 | ALC8704 with pALC8135 | This work |
| ALC8973 | ALC8706 with pALC8135 | This work |
| ALC8974 | ALC8702 with pEPSA5 containing a xylose-inducible promoter | This work |
| ALC8975 | ALC8702 with pALC7342 (pEPSA5::cspA) | This work |
| ALC8976 | ALC8704 with pEPSA5 | This work |
| ALC8977 | ALC8704 with pALC7342 | This work |
| E. coli | ||
| XL-1 Blue | General cloning strain | Agilent |
| BL21 (DE3) pLysS | General expression strain | (Studier et al., 1990) |
| ALC7884 | DC10B with pCR2.1-AmpS::hsdMS-2CC8 | (Jones et al., 2015) |
| ALC7885 | DC10B with pACYC184::hsdMS-2CC8 | (Jones et al., 2015) |
| Plasmid | Characteristics | Source or reference |
| pMAD | E. coli/S. aureus shuttle plasmid with the ori pE194ts; bgaB Ampr Ermr | (Arnaud et al., 2004) |
| pET28a | E. coli expression vector | EMD Millipore |
| pEPSA5 | Xylose-inducible shuttle vector; Ampr Cmr | (Forsyth et al., 2002) |
| pSK236 | S. aureus/E. coli shuttle vector with pUC19 cloned into the HindIII site of pC194 | (Gaskill and Khan, 1988) |
| pALC1484 | pSK236 with a promoterless gfpuvr, a cycle-3 gfp allele. | (Kahl et al., 2000) |
| pALC2201 | pALC1484 with asp23 promoter fragment | (Palma et al., 2006) |
| pALC7091 | pEPSA5 with yjbH in EcoRI and BamHI sites and sarA ribosome binding site | This work |
| pALC7113 | pEPSA5 with spx in EcoRI and BamHI sites and sarA ribosome binding site | This work |
| pALC7342 | pEPSA5 with cspA in EcoRI and BamHI sites and sarA ribosome binding site | This work |
| pALC7803 | pALC1484 with spx P1 promoter in EcoRI and XbaI sites preceding the gfpuvr gene | (Donegan et al., 2014) |
| pALC7804 | pALC1484 with spx P2 promoter in EcoRI and XbaI sites preceding the gfpuvr gene | (Donegan et al., 2014) |
| pALC8134 | pALC1484 with hbo-yjbH promoter in EcoRI and XbaI sites preceding the gfpuvr gene | This work |
| pALC8135 | pALC1484 with cspA promoter in EcoRI and XbaI sites preceding the gfpuvr gene | This work |
| pALC8640 | pALC1484 with msaA promoter in EcoRI and XbaI sites preceding the gfpuvr gene | This work |
| pALC8681 | pET28a with spx and N-terminal hexahistidine fusion. | This work |
| pALC8682 | pET28a with αCTD of S. aureus and N-terminal hexahistidine fusion. | This work |
DNA manipulations.
E. coli plasmid purification was performed per the manufacturer’s instructions (Omega), while plasmid isolation from S. aureus was performed as described previously (Schenk and Laddaga, 1992). Transformation of S. aureus was achieved via electroporation with a MicroPulser (Biorad), using plasmid DNA directly isolated from either E. coli ALC7884 or ALC7885 (Jones et al., 2015), for p15A and pUC-based vectors, respectively. Oligonucleotides were synthetized by IDT Inc.
Protein expression and purification.
Expression of Spx or αCTD (residues 245 to 314 of the RNA polymerase α subunit RpoA) was performed by PCR amplification of each gene with 5’ NdeI and 3’ EcoRI sites, digesting with the same enzymes, and subcloning into the equivalently digested pET28a. E. coli BL21(DE3)pLysS strains harboring each resulting plasmid (pALC8681 or pALC8682) were induced with 1 mM IPTG at 37oC for 4 hours (Studier et al., 1990). Harvested cells were lysed ultrasonically and purified with HisPur Cobalt Resin (ThermoFisher). His6-αCTD was digested with thrombin to remove the hexahistidine tag using a Thrombin Cleavage/Capture Kit (Millipore). Protein was then aliquoted, stored in 10 mM Tris-Cl, 1 mM EDTA, 100 mM NaCl, 10% glycerol, pH 8.0 at −80oC, verified for purity with AcquaStain (Bulldog Bio) and authenticated by mass spectrometry at the University of Vermont Spectrometry Facility.
in trans expression in S. aureus.
Inducible systems in pEPSA5 for cspA, yjbH and spx expression were created by PCR amplifying each gene with a sarA ribosome binding site and 5’ EcoRI and 3’ BamHI sites, digesting the PCR product with these enzymes, and ligating into similarly digested pEPSA5 (Forsyth et al., 2002).
Cell lysate preparation and Western blot analyses.
Protein lysates were prepared as described before (Donegan et al., 2010) except that translation was stalled with 50 μg/ml erythromycin upon harvest.
Transcriptional fusions to gfpuvr.
Constructs containing the promoter regions of cspA, msaA (the gene upstream of cspA) and hbo-yjbH (also called yjbIH) were created to control gfpuvr by PCR amplification of each region with flanking 5’ EcoRI and 3’ XbaI restriction sites. These regions corresponded to: Phbo, 90bp upstream of the hbo start codon; PmsaA, 500bp upstream of the msa start codon; PcspA, 500bp upstream of the cspA start codon. The correspondingly digested PCR fragments were then ligated into the EcoRI and XbaI sites upstream of promoterless gfpuvr in pALC1484.
Construction of deletion and mutant strains.
Strains deleted for cspA (sa1234), cspC (sa0747) or yjbH (sa0860) were constructed using the temperature-sensitive allelic replacement plasmid pMAD as described (Arnaud et al., 2004). In brief, chromosomal regions 1000 bp up- and downstream of each gene of interest were amplified by PCR with primers proximal to the deletion site, joined by gene sewing, ligated into pMAD, transformed into ALC7885 (a derivative of E. coli DC10B) and then electroporated into S. aureus strain SH1000. The mutant was then generated by a temperature shift strategy to yield the mutant (Arnaud et al., 2004). The pMAD constructs used to replace deletion of either cspA or yjbH were created by amplifying the appropriate gene and 1000 bp flanking regions from the wild type SH1000 chromosome, and then utilizing allelic replacement as described above. As the genes replaced above are either transcribed monocistronically (e.g. cspC or cspA where the major transcript is monocistronic) or the last gene in a two-gene transcript (e.g. yjbH), it is unlikely that the chromosomal gene replacement would have significant polar effect downstream. Successful allelic exchanges were verified by colony PCR and chromosomal sequencing. Transduction of the kanamycin-resistance cassette aphA immediately upstream of cspB from S. aureus strain COL to SH1000 was performed using phage 80α (Schwesinger and Novick, 1975).
A chromosomal C10A mutation of spx in MRSA strain JE2 was a gift from Vanai Thomas at the University of Nebraska. The spx C10A mutant cannot be oxidized due to the disruption of the 10-CXXC-14 motif.
Isolation of RNA and Northern blot hybridization.
RNA isolation and purification and subsequent Northern blot analysis were performed as previously reported (Cheung et al., 1994).
Electrophoretic mobility shift assays (EMSA).
To decipher interactions between proteins and the cspA promoter, a PCR product consisting of −300 to +1 of the cspA promoter was first end-labeled with γ−32P-ATP (Perkin Elmer) using T4 polynucleotide kinase (NEB) and purified by MicroSpin G-50 columns (GE). His6-Spx and/or αCTD were pre-incubated for 10 min at 25oC in 18 μL of binding buffer (20 mM Tris-HCl pH 7.8, 50 mM NaCl, 5 mM MgCl2, 10% glycerol) containing 0.5 μg of calf thymus DNA. 2 μL of radiolabeled DNA (20,000 cpm) was then added and incubated at 25oC for 15’. Non-radioactive competitor DNA was either the cspA promoter (specific) or a 285 bp sarX fragment (non-specific). Samples were then resolved on 6% polyacrylamide gels in TGE buffer (50 mM Tris, 380 mM glycine, 2 mM EDTA) at 150 V and visualized with a Typhoon Phosphorimager (GE).
Carotenoid assay.
Strains were grown overnight at 37oC on Tryptic Soy agar (TSA) with antibiotics and, as needed, 0.2% xylose. Cells were scraped into 500 μL of phosphate buffered saline (PBS), vortexed 5’ for homogenization and measured for OD650 in a microplate reader to determine cell density. The culture was pelleted and resuspended in 1 ml methanol and pigment extracted by vortexing for 5’ and incubation at 55oC for 10’. After brief centrifugation, the supernatant was measured at OD450 in a microplate reader and normalized for cell density at OD650.
Fluorescence analysis.
For static assays, GFP fluorescence was measured at 485 nm and 516 nm for excitation and emission, respectively, and cell density recorded by measuring absorbance at 650 or 600 nm. For kinetic assays, 150 μL of cells were grown at 37oC with shaking (250 rpm) in a sterile 96 well plate with a transparent bottom (Costar plate 3632). Auto-fluorescence from sterile media was subtracted from each time-point and divided by OD650 to normalize for cell density.
Whole blood killing assay.
A whole blood killing assay with S. aureus strains was performed as previously described (Kyme et al., 2012).
Software analysis.
Scanned images from non-saturated exposed film were analyzed densitometrically using ImageJ 1.51g (NIH). Statistical significance was determined with the unpaired two tailed t-test using Prism 7.0a (Graphpad) where needed.
STUDY APPROVAL
Peripheral blood was obtained from healthy adult donors by the General Clinical Research Center at Cedars-Sinai Medical Center. The study was approved by the Cedars-Sinai Medical Center Institutional Review Board and Office of Research Compliance.
Supplementary Material
Figure S1: Relative activities of msaA and cspA promoters in S. aureus. S. aureus SH1000 wildtype transformed with either pALC8135 (PcspA::gfp) or pALC8640 (PmsaA::gfp) was examined for fluorescence during growth in TSB at 4 and 10 hours. Cell densities at OD650 were measured simultaneously, and fluorescence normalized by it. The value for each strain represents the mean of three technical triplicates of two biological replicates, and the experiments were repeated three times. A representative experiment is displayed. The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (***, P <0.0005).
Figure S2: Cross-hybridization between high homology csp genes in S. aureus. (A) Cross-hybridization of cspA and cspC transcripts in S. aureus. RNA was obtained from SH1000 wildtype, ΔcspA and ΔcspC strains at various optical densities (15 μg per lane) and was on a denaturing agarose gel, blotted to Hybond XL membrane and hybridized with either a 200-bp 32P-radiolabeled cspA or cspC DNA fragment. (B) Significant nucleotide identity between S. aureus csp homologs. CLUSTAL nucleotide alignment of S. aureus csp homologs from the S. aureus reference genome N315 is shown.
Figure S3: Production of αCTD and Spx. E. coli cells expressing the Spx and αCTD were grown in LB, induced, lysed and purified as described in Materials and Methods. 5 μg of each purified protein was then resolved by SDS-polyacrylamide gel electrophoresis and visualized by staining. Lanes: 1, Spx with N-terminal His6 tag; 2, molecular weight marker; 3, αCTD with N-terminal His6 tag; 4, αCTD following thrombin cleavage and cleanup.
Figure S4: Analysis of the cspA promoter fused to GFP reporter system in the wild type SH1000 under oxidative and redox stresses. Assays were performed after adding 5 mM diamide and 10 mM hydrogen peroxide at 1.2 O.D. at 600 nm followed by serial fluorescence measurements normalized to OD600nm. The amount of each stressor was chosen to promote stress but not affecting growth. NS, not significant, * P values <0.05, ** P values <0.005 and *** P values <0.0005. There was modest increase in activity at 20 min after diamide exposure, but the value did not reach statistical significance.
ACKNOWLEDGEMENTS:
This work was supported by NIH grant R21AI119570 to ALC. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The authors want to thank Vanai Thomas for allowing us to use the spx C10A mutant and Stephen Costa for helping with graphics.
Footnotes
Conflict of Interest Statement: There is no conflict of interest in this manuscript among authors.
REFERENCES:
- Anderson KL, Roberts C, Disz T, Vonstein V, Hwang K, Overbeek R, et al. (2006) Characterization of the Staphylococcus aureus Heat Shock, Cold Shock, Stringent, and SOS Responses and Their Effects on Log-Phase mRNA Turnover. Journal of Bacteriology 188: 6739–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud M, Chastanet A, and Debarbouille M (2004) New Vector for Efficient Allelic Replacement in Naturally Nontransformable, Low-GC-Content, Gram-Positive Bacteria. Appl Environ Microbiol 70: 6887–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CM, Garabaglu S, Krute CN, Ridder MJ, Seawell NA, Markiewicz MA, Boyd JM and Bose JL Contribution of and Bose JL (2019) Contribution of jbH to virulence factor expression and host colonization in Staphylococcus aureus. Infect. Immun In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae W, Xia B, Inouye M, and Severinov K (2000) Escherichia coli CspA-family RNA chaperones are transcription antiterminators. Proceedings of the National Academy of Sciences 97: 7784–7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson AK, and Haldenwang WG (1993) Bacillus subtilis sigma B is regulated by a binding protein (RsbW) that blocks its association with core RNA polymerase. Proceedings of the National Academy of Sciences 90: 2330–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CM, Garg S, Lin AA, and Zuber P (2012) Geobacillus thermodenitrificans YjbH recognizes the C-terminal end of Bacillus subtilis Spx to accelerate Spx proteolysis by ClpXP. Microbiology 158: 1268–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AL, Eberhardt KJ, and Fischetti VA (1994) A Method to Isolate RNA from Gram-Positive Bacteria and Mycobacteria. Anal Biochem 222: 511–514. [DOI] [PubMed] [Google Scholar]
- Cohn MT, Kjelgaard P, Frees D, Penades JR, and Ingmer H (2011) Clp-dependent proteolysis of the LexA N-terminal domain in Staphylococcus aureus. Microbiology 157: 677–684. [DOI] [PubMed] [Google Scholar]
- Constantinidou C, Hobman JL, Griffiths L, Patel MD, Penn CW, Cole JA, and Overton TW (2006) A Reassessment of the FNR Regulon and Transcriptomic Analysis of the Effects of Nitrate, Nitrite, NarXL, and NarQP as Escherichia coliK12 Adapts from Aerobic to Anaerobic Growth. Journal of Biological Chemistry 281: 4802–4815. [DOI] [PubMed] [Google Scholar]
- de Hoon M, Makita Y, Nakai K, and Miyano S (2005) Prediction of transcriptional terminators in Bacillus subtilis and related species. PLoS Comput Biol 1: e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan NP, Marvin JS, and Cheung AL (2014) Role of Adaptor TrfA and ClpPC in Controlling Levels of SsrA-Tagged Proteins and Antitoxins in Staphylococcus aureus. Journal of Bacteriology 196: 4140–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donegan NP, Thompson ET, Fu Z, and Cheung AL (2010) Proteolytic Regulation of Toxin-Antitoxin Systems by ClpPC in Staphylococcus aureus. Journal of Bacteriology 192: 1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval BD, Mathew A, Satola SW, and Shafer WM (2010) Altered Growth, Pigmentation, and Antimicrobial Susceptibility Properties of Staphylococcus aureus Due to Loss of the Major Cold Shock Gene cspB. Antimicrob Agents Chemother 54: 2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbarasi A (2014) Identification and Characterization of msaB Gene Involved in Biofilm Formation and Virulence in Staphylococcus aureus. [Google Scholar]
- Engman J, Rogstam A, Frees D, Ingmer H, and Wachenfeldt, von C (2012) The YjbH Adaptor Protein Enhances Proteolysis of the Transcriptional Regulator Spx in Staphylococcus aureus. Journal of Bacteriology 194: 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, and Bayles KW (2012) A Genetic Resource for Rapid and Comprehensive Phenotype Screening of Nonessential Staphylococcus aureus Genes. MBio 4: e00537–12–e00537–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth RA, Haselbeck RJ, Ohlsen KL, Yamamoto RT, Xu H, Trawick JD, et al. (2002) A genome-wide strategy for the identification of essential genes in Staphylococcus aureus. Mol Microbiol 43: 1387–1400. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, and Karp M (2007) Intracellular redox equilibrium and growth phase affect the performance of luciferase-based biosensors. Journal of Biotechnology 127: 188–198. [DOI] [PubMed] [Google Scholar]
- Gaskill ME, and Khan SA (1988) Regulation of the enterotoxin B gene in Staphylococcus aureus. J Biochem 263: 6276–6280. [PubMed] [Google Scholar]
- Gertz S, Schmid R, Ohlsen K, Hacker J, and Hecker M (1999) Regulation of sigmaB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol Gen Genet 261: 558–566. [DOI] [PubMed] [Google Scholar]
- Giaquinto L, Curmi PMG, Siddiqui KS, Poljak A, DeLong E, DasSarma S, and Cavicchioli R (2007) Structure and Function of Cold Shock Proteins in Archaea. Journal of Bacteriology 189: 5738–5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, and Foster SJ (2002) sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325–4. Journal of Bacteriology 184: 5457–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Fang L, and Inouye M (1996) The role of the 5’-end untranslated region of the mRNA for CspA, the major cold-shock protein of Escherichia coli, in cold-shock adaptation. Journal of Bacteriology 178: 4919–4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MJ, Donegan NP, Mikheyeva IV, and Cheung AL (2015) Improving Transformation of Staphylococcus aureus Belonging to the CC1, CC5 and CC8 Clonal Complexes. PLoS ONE 10: e0119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousselin A, Kelley WL, Barras C, Lew DP, and Renzoni A (2013) The Staphylococcus aureus Thiol/Oxidative Stress Global Regulator Spx Controls trfA, a Gene Implicated in Cell Wall Antibiotic Resistance. Antimicrob Agents Chemother 57: 3283–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl BC, Goulian M, van Wamel W, Herrmann M, Simon SM, Kaplan G, et al. (2000) Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infect Immun 68: 5385–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzif S, Danavall D, Bowers S, Balthazar JT, and Shafer WM (2003) The Major Cold Shock Gene, cspA, Is Involved in the Susceptibility of Staphylococcus aureus to an Antimicrobial Peptide of Human Cathepsin G. Infect Immun 71: 4304–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzif S, Lee EH, Law AB, Tzeng YL, and Shafer WM (2005) CspA Regulates Pigment Production in Staphylococcus aureus through a SigB-Dependent Mechanism. Journal of Bacteriology 187: 8181–8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Corvaglia A-R, Léo S, Cheung AL, and Francois P (2016) Characterization of RNA Helicase CshA and Its Role in Protecting mRNAs and Small RNAs of Staphylococcus aureus Strain Newman. Infect Immun 84: 833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Wang X, Zhang X-S, Grigoriu S, Page R, Peti W, and Wood TK (2010) Escherichia coli toxin/antitoxin pair MqsR/MqsA regulate toxin CspD. Environmental Microbiology 12: 1105–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommineni S, Garg SK, Chan CM, and Zuber P (2011) YjbH-Enhanced Proteolysis of Spx by ClpXP in Bacillus subtilis Is Inhibited by the Small Protein YirB (YuzO). Journal of Bacteriology 193: 2133–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullik I, Giachino P, and Fuchs T (1998) Deletion of the alternative sigma factor sigmaB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. Journal of Bacteriology 180: 4814–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyme P, Thoennissen NH, Tseng CW, Thoennissen GB, Wolf AJ, Shimada K, et al. (2012) C/EBPε mediates nicotinamide-enhanced clearance of Staphylococcus aureus in mice. J Clin Invest 122: 3316–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L, Cheng A, Dunman PM, Missiakas D, and He C (2010) Golden Pigment Production and Virulence Gene Expression Are Affected by Metabolisms in Staphylococcus aureus. Journal of Bacteriology 192: 3068–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langklotz S, and Narberhaus F (2011) The Escherichia coli replication inhibitor CspD is subject to growth-regulated degradation by the Lon protease. Mol Microbiol 80: 1313–1325. [DOI] [PubMed] [Google Scholar]
- Lioliou E, Sharma CM, Caldelari I, Helfer A-C, Fechter P, Vandenesch F, et al. (2012) Global Regulatory Functions of the Staphylococcus aureus Endoribonuclease III in Gene Expression. PLoS Genet 8: e1002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, et al. (2005) Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. Journal of Experimental Medicine 202: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S, Goerke C, Wolz C, Seidl K, Homerova D, Schulthess B, et al. (2007) sigmaB and the sigmaB-dependent arlRS and yabJ-spoVG loci affect capsule formation in Staphylococcus aureus. Infect Immun 75: 4562–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Lin A, Zuber CS, Newberry KJ, Brennan RG, and Zuber P (2010) Promoter Recognition by a Complex of Spx and the C-Terminal Domain of the RNA Polymerase α Subunit. PLoS ONE 5: e8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano S, Erwin KN, Ralle M, and Zuber P (2004) Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol Microbiol 55: 498–510. [DOI] [PubMed] [Google Scholar]
- Palma M, Bayer A, Kupferwasser LI, Joska T, Yeaman MR, and Cheung AL (2006) Salicylic Acid Activates Sigma Factor B by rsbU-Dependent and -Independent Mechanisms. Journal of Bacteriology 188: 5896–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamp SJ, Frees D, Engelmann S, Hecker M, and Ingmer H (2006) Spx Is a Global Effector Impacting Stress Tolerance and Biofilm Formation in Staphylococcus aureus. Journal of Bacteriology 188: 4861–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania R (2002) Molecular, biochemical and genetic studies on truncated hemoglobins. [Google Scholar]
- Pelz A, Wieland K-P, Putzbach K, Hentschel P, Albert K, and Gotz F (2005) Structure and Biosynthesis of Staphyloxanthin from Staphylococcus aureus. Journal of Biological Chemistry 280: 32493–32498. [DOI] [PubMed] [Google Scholar]
- Phadtare S, and Severinov K (2010) RNA remodeling and gene regulation by cold shock proteins. RNA Biol 7: 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat T, Nicolas P, Delumeau O, Rabatinová A, Korelusová J, Leduc A, et al. (2012) Genome-wide identification of genes directly regulated by the pleiotropic transcription factor Spx in Bacillus subtilis. Nucl Acids Res 40: 9571–9583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahukhal GS, and Elasri MO (2014) Identification and characterization of an operon, msaABCR, that controls virulence and biofilm development in Staphylococcus aureus. BMC Microbiol 14: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta D, Elasri MO, and Batte JL (2016) MsaB activates capsule production at the transcription level in Staphylococcus aureus. Microbiology 162: 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, and Laddaga RA (1992) Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett 73: 133–138. [DOI] [PubMed] [Google Scholar]
- Schindler T, Graumann PL, Perl D, Ma S, Schmid FX, and Marahiel MA (1999) The family of cold shock proteins of Bacillus subtilis. Stability and dynamics in vitro and in vivo. Journal of Biological Chemistry 274: 3407–3413. [DOI] [PubMed] [Google Scholar]
- Schmidt KA, Donegan NP, Kwan WA Jr, and Cheung AL (2004) Influences of σ Band agron expression of staphylococcal enterotoxin B (seb) in Staphylococcus aureus. Can J Microbiol 50: 351–360. [DOI] [PubMed] [Google Scholar]
- Schwesinger MD, and Novick RP (1975) Prophage-dependent plasmid integration in Staphylococcus aureus. Journal of Bacteriology 123: 724–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn MM, Giachino P, Homerova D, Steinhuber A, Strassner J, Kormanec J, et al. (2005) Molecular Analysis and Organization of the B Operon in Staphylococcus aureus. Journal of Bacteriology 187: 8006–8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Liu C-I, Lin F-Y, No JH, Hensler M, Liu Y-L, et al. (2009) Inhibition of staphyloxanthin virulence factor biosynthesis in Staphylococcus aureus: in vitro, in vivo, and crystallographic results. J Med Chem 52: 3869–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier FW, Rosenberg AH, Dunn JJ, and Dubendorff JW (1990) Use of T7 RNA polymerase to direct expression of cloned genes. Meth Enzymol 185: 60–89. [DOI] [PubMed] [Google Scholar]
- Uppal S, Shetty DM, and Jawali N (2014) Cyclic AMP Receptor Protein Regulates cspD, a Bacterial Toxin Gene, in Escherichia coli. Journal of Bacteriology 196: 1569–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppalapati CK, Gutierrez KD, Buss-Valley G, and Katzif S (2017) Growth-dependent activity of the cold shock cspA promoter + 5′ UTR and production of the protein CspA in Staphylococcus aureus Newman. BMC Res Notes 10: 626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb M (2003) Single-stranded DNA binding of the cold-shock protein CspB from Bacillus subtilis: NMR mapping and mutational characterization. Protein Sci 12: 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P (2004) Spx-RNA Polymerase Interaction and Global Transcriptional Control during Oxidative Stress. Journal of Bacteriology 186: 1911–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Relative activities of msaA and cspA promoters in S. aureus. S. aureus SH1000 wildtype transformed with either pALC8135 (PcspA::gfp) or pALC8640 (PmsaA::gfp) was examined for fluorescence during growth in TSB at 4 and 10 hours. Cell densities at OD650 were measured simultaneously, and fluorescence normalized by it. The value for each strain represents the mean of three technical triplicates of two biological replicates, and the experiments were repeated three times. A representative experiment is displayed. The asterisks indicate statistical significance between wildtype and mutant strains, determined using Student t-test (***, P <0.0005).
Figure S2: Cross-hybridization between high homology csp genes in S. aureus. (A) Cross-hybridization of cspA and cspC transcripts in S. aureus. RNA was obtained from SH1000 wildtype, ΔcspA and ΔcspC strains at various optical densities (15 μg per lane) and was on a denaturing agarose gel, blotted to Hybond XL membrane and hybridized with either a 200-bp 32P-radiolabeled cspA or cspC DNA fragment. (B) Significant nucleotide identity between S. aureus csp homologs. CLUSTAL nucleotide alignment of S. aureus csp homologs from the S. aureus reference genome N315 is shown.
Figure S3: Production of αCTD and Spx. E. coli cells expressing the Spx and αCTD were grown in LB, induced, lysed and purified as described in Materials and Methods. 5 μg of each purified protein was then resolved by SDS-polyacrylamide gel electrophoresis and visualized by staining. Lanes: 1, Spx with N-terminal His6 tag; 2, molecular weight marker; 3, αCTD with N-terminal His6 tag; 4, αCTD following thrombin cleavage and cleanup.
Figure S4: Analysis of the cspA promoter fused to GFP reporter system in the wild type SH1000 under oxidative and redox stresses. Assays were performed after adding 5 mM diamide and 10 mM hydrogen peroxide at 1.2 O.D. at 600 nm followed by serial fluorescence measurements normalized to OD600nm. The amount of each stressor was chosen to promote stress but not affecting growth. NS, not significant, * P values <0.05, ** P values <0.005 and *** P values <0.0005. There was modest increase in activity at 20 min after diamide exposure, but the value did not reach statistical significance.