Abstract
Background:
Conditional recurrence-free survival (RFS) probability, i.e., recurrence probability after a given interval without recurrence, has not been reported after resection of colorectal liver metastases (CLM). We aimed to estimate conditional RFS and identify factors affecting conditional RFS.
Study design:
Patients undergoing initial resection of CLM during 2000-2016 with mutation data were identified. RFS and risk factors for recurrence were evaluated at the time of resection for all patients and at 1 year and 2 years after resection for patients who remained recurrence-free.
Results:
Of 2118 patients, 485 met the inclusion criteria, of whom 225 were recurrence-free at 1 year and 109 were recurrence-free at 2 years. The 5-year RFS rates were 17.3%, 36.8%, and 70.7% for all patients and the 1-year and 2-year recurrence-free groups, respectively, when assessed from the time of initial CLM resection. RAS/TP53 co-mutation was the only factor independently associated with increased risk of recurrence for all groups (all patients, hazard ratio [HR] 1.47, 95% confidence interval [CI] 1.19–1.82, P<0.001; 1-year recurrence-free, HR 1.69, 95% CI 1.17–2.43, P=0.005; 2-year recurrence-free, HR 2.41, 95% CI 1.12–5.17, P=0.024). T category, extrahepatic disease, multiple CLM, largest CLM diameter, and surgical margin status were risk factors for recurrence in all patients and/or the 1-year recurrence-free group but not the 2-year recurrence-free group. Median RFS was lower for patients with RAS/TP53 co-mutation than for those with RAS/TP53 wild-type in the 1-year (1.5 vs. 2.8 years; P=0.006) and 2-year recurrence-free groups (3.0 vs. 5.9 years; P=0.024).
Conclusion:
Conditional RFS is useful for updating prognosis after a given time interval without recurrence after CLM resection. Importantly, RAS/TP53 co-mutation has a persistent deleterious association with recurrence.
Precis
Conditional recurrence-free survival probability, ie recurrence probability after a given interval without recurrence is useful for updating prognosis in patients undergoing resection of colorectal liver metastases. RAS/TP53 co-mutation has a persistent deleterious association with recurrence for patients free from recurrence at 1 year and 2 years.
INTRODUCTION
Surgical resection is the current standard of care for colorectal liver metastases (CLM), producing 5-year overall survival (OS) rates that range from 40% to 58% (1-3). However, most patients undergoing liver resection for CLM experience recurrence; 5-year recurrence-free survival (RFS) rates after CLM resection range from 20% to 35% (1-4). Various clinicopathologic factors have been shown to be association with risk of recurrence, including factors related to the primary colorectal cancer and factors reflecting the extent of liver involvement (5-8). In addition, molecular biomarkers have emerged as important prognostic factors to guide systemic therapy and patient selection for surgery. Previous studies showed that somatic mutations of BRAF, RAS, TP53, APC, and PIK3CA were associated with prognosis after CLM resection (9-13). Specifically, our group has reported that RAS and TP53 co-mutation was associated with worse prognosis than RAS mutation alone (14).
Traditionally, to estimate a patients’ risk of recurrence and death after CLM resection, clinicopathologic factors and molecular biomarkers are assessed once at the time of liver resection. However, the risks of recurrence and death after CLM resection are not constant over time (15, 16). The risk of death is reported to be highest within the first year after CLM resection and to decline over time (16, 17). Because patients living longer want to understand their prognosis better (18), conditional survival, defined as the survival probability after a given length of survival, has been advocated as an alternative meaningful measure of survival probability (16, 19-22). Conditional survival estimates can provide useful prognostic information. However, such estimates do not provide information about the risk of recurrence. Two previous reports focused on conditional OS after resection of CLM (16, 17). However, to our knowledge, no study has focused on conditional RFS (i.e., the recurrence probability after a given interval without recurrence) or changes over time in risk factors for recurrence after CLM resection.
To address these gaps, we assessed changes over time in recurrence probability and risk factors for recurrence in patients who had not experienced recurrence at predefined times after CLM resection.
METHODS
Study population
A prospectively compiled database was searched to identify patients who underwent liver resection for CLM at The University of Texas MD Anderson Cancer Center from 2000 to 2016. Only patients undergoing a first-time liver resection for CLM with curative intent were included for analysis. To permit assessment of the impact of tumor mutation profile, patients without genetic sequencing were excluded. The study was approved by the institutional review board at MD Anderson Cancer Center.
Institutional approach to surgical management of CLM
At our institution, the vast majority of patients with CLM receive preoperative chemotherapy. During preoperative chemotherapy, restaging is performed. CLM are deemed resectable when a hepatectomy can achieve a negative margin while preserving more than 20% to 30% of the total estimated liver volume, sparing 2 contiguous hepatic segments, and maintaining vascular inflow, vascular outflow, and biliary drainage (23). In the case of disease progression or suboptimal tumor response after first-line chemotherapy, second-line chemotherapy is considered (24). For patients with synchronous CLM and an intact primary tumor, decisions regarding the treatment sequence (primary tumor first, combined, or liver first) are discussed at a multidisciplinary conference, where decisions are primarily based on the extent of the primary tumor and CLM (11). For patients with an anticipated insufficient future liver remnant, preoperative portal vein embolization and staged hepatectomy are proposed. Postoperative chemotherapy is typically administered to complete a total of 12 cycles, including those given preoperatively (25). The interval between the last dose of chemotherapy and liver resection is 3–6 weeks. Patients are routinely followed after resection with history, physical examination, laboratory evaluation, and axial imaging every 3 to 4 months for the first 2 years and every 4 to 6 months for the subsequent 3 years.
Somatic gene mutation profiling
Tumor DNA was isolated from 5-mm-thick unstained sections from tumor tissue blocks or slides from primary colorectal cancer or CLM specimens. Macrodissection was performed in cases of low tumor cellularity. Next-generation sequencing was performed with a multigene panel (eAppendix 1) using the Ion Torrent Personal Genome Machine (Life Technologies, CA) in a Clinical Laboratory Improvement Amendment-certified molecular diagnostic laboratory (26). RAS mutation analysis included analysis of KRAS exons 2–4 (codons 5–66 and 114–150), NRAS exons 2–4 (codons 3–31, 43–69, and 112–150), and HRAS exons 2 to 3 (codons 5–35 and 42–82).
Estimation of changing probability of recurrence and conditional RFS and analysis of changes over time in risk factors for recurrence
Changing probability of recurrence was estimated using the Kernel-smoothed hazard estimate method (27, 28). We defined the conditional RFS rate as the recurrence probability calculated in patients who had not experienced recurrence by a predefined time after CLM resection, based on the concept of “conditional survival” estimate analysis (20). RFS and risk factors for recurrence after CLM resection were evaluated at the time of liver resection for all patients. For patients who remained recurrence-free 1 year after CLM resection (the 1-year recurrence-free group), risk factors for subsequent recurrence were analyzed. The same sub-analysis was performed for patients who remained recurrence-free 2 years after CLM resection (the 2-year recurrence-free group).
Definitions
Synchronous metastases were defined as metastases diagnosed within 12 months of primary tumor diagnosis. A positive surgical margin was defined as the presence of tumor cells within 1 mm of the transection line. The number and diameter of liver metastases were determined from examination of surgical pathology specimens. Pathologic response was defined as less than 50% residual cancer cells remaining (29). T category was classified according to the staging system in the AJCC Cancer Stating Manual, seventh edition (30). Preoperative chemotherapy regimens were recorded and further categorized according to whether they included an anti-VEGF or anti-EGFR agent.
Statistical analysis
Categorical variables were expressed in numbers and percentages and were compared among groups using Fisher’s exact test or the chi-square test, as appropriate. Continuous variables were expressed as median values with the interquartile range (IQR) and were compared using Wilcoxon’s rank-sum test. OS and RFS curves were constructed using the Kaplan-Meier method and compared using the log-rank test. Deaths without recurrence were censored for the RFS analysis. A Cox proportional hazards model analysis initially included the following factors: age (> 65 vs. ≤ 65 yr), sex, primary tumor location, T category, primary lymph node metastasis, prehepatectomy carcinoembryonic antigen level (> 5.0 vs. ≤ 5.0 ng/mL), timing of metastasis (synchronous vs. metachronous), prehepatectomy chemotherapy, extrahepatic disease, number of CLM (multiple vs. single), largest CLM diameter (> 5 cm vs. ≤ 5 cm), surgical margin status (R1 vs. R0), pathologic response, BRAF mutation, RAS mutation, TP53 mutation, APC mutation, and PIK3CA mutation. A backward elimination with a threshold P value of 0.05 was used to select variables for the final models. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for each factor. P ≤ 0.05 was considered statistically significant. Statistical analysis was conducted with SAS (SAS Institute, Cary, NC).
RESULTS
Study population
Of 2118 patients who underwent CLM resection during the study period, 485 patients were included in the analysis (eFigure 1). Demographic and clinicopathologic characteristics are summarized in Table 1. The median duration of follow-up was 3.1 years (IQR, 2.1–4.7 years). Of the 485 patients, 256 patients experienced recurrence and 4 patients were censored within 1 year of CLM resection, leaving 225 patients in the 1-year recurrence-free group. Of these 225 patients, 106 experienced recurrence and 10 patients were censored between 1 and 2 years after CLM resection, leaving 109 patients in the 2-year recurrence-free group. The median duration of follow-up for patients in the 2-year recurrence-free group was 3.6 years (range, 2.0–11.6 years).
Table 1.
Demographic and Clinicopathologic Characteristics of 485 Patients Who Underwent Resection of Colorectal Liver Metastases
| Characteristic | Value |
|---|---|
| Patient factor | |
| Age, y, median (IQR) | 55 (46–62) |
| Sex, n (%) | |
| Male | 274 (56.5) |
| Female | 211(43.5) |
| ASA score ≥ 3, n (%) | 418 (86.2) |
| Primary lesion factor, n (%) | |
| Location, n (%) | |
| Colon | 333 (68.7) |
| Rectum | 152 (31.3) |
| T category ≥ 3, n (%)* | 420 (86.6) |
| Lymph node metastasis, n (%)* | 333 (68.7) |
| Liver metastasis clinical factor | |
| Prehepatectomy CEA level, ng/mL, median (IQR) | 4.0 (2.2–12.4) |
| Synchronous metastasis, n (%) | 372 (76.7) |
| Extrahepatic metastasis, n (%) | 80 (16.5) |
| Prehepatectomy chemotherapy, n (%) | 439 (90.5) |
| > 6 cycles | 139 (28.7) |
| Oxaliplatin-containing regimen | 362 (74.6) |
| Irinotecan-containing regimen | 103 (21.2) |
| Anti-VEGF agent-containing regimen | 345 (71.1) |
| Anti-EGFR agent-containing regimen | 38 (7.8) |
| Liver metastasis histopathologic factor | |
| Tumor number, median (IQR) | 2 (1–4) |
| Maximum tumor size, cm, median (IQR) | 2.5 (1.5–4.0) |
| R1 surgical margin, n (%) | 98 (20.2) |
| Pathologic response, n (%)* | 236 (48.7) |
| Somatic mutation, n (%) | |
| BRAF | 11 (2.3) |
| RAS† | 246 (50.7) |
| TP53† | 336 (69.3) |
| APC | 253 (52.2) |
| PIK3CA | 79 (16.3) |
Data missing on T category for 6 patients, lymph node metastasis for 15 patients, and pathologic response for 19 patients.
RAS/TP53 co-mutation, 155 (32.0%)
IQR, interquartile range; ASA, American Society of Anesthesiologists; CEA, carcinoembryonic antigen; VEGF, vascular endothelial growth factor; EGFR, epidermal growth factor receptor
Changing probability of recurrence
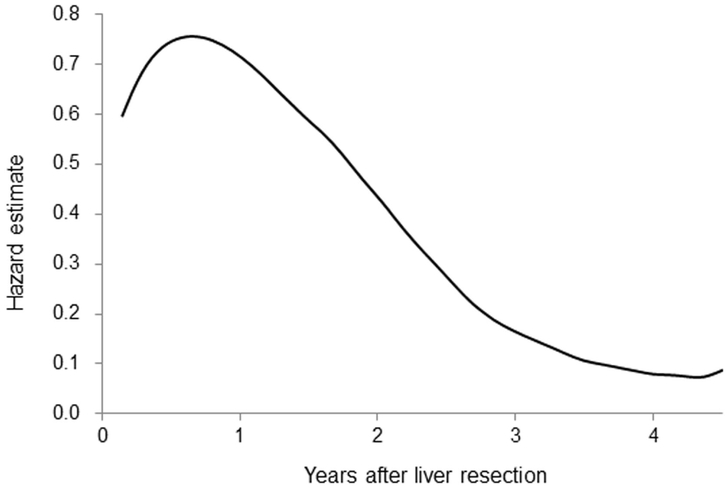
In the overall study group, the probability of recurrence peaked at approximately 1 year and diminished thereafter (Figure 1). The fact that the risk of recurrence changed over time implied that conditional RFS and the risk factors for recurrence might change over time too. Therefore, we examined changes over time in conditional RFS and risk factors for recurrence.
Figure 1.
Risk of recurrence over time after resection of colorectal liver metastases. The risk of recurrence peaked at approximately 1 year, diminished from 1 year to 3 years, and remained fairly steady after 4 years.
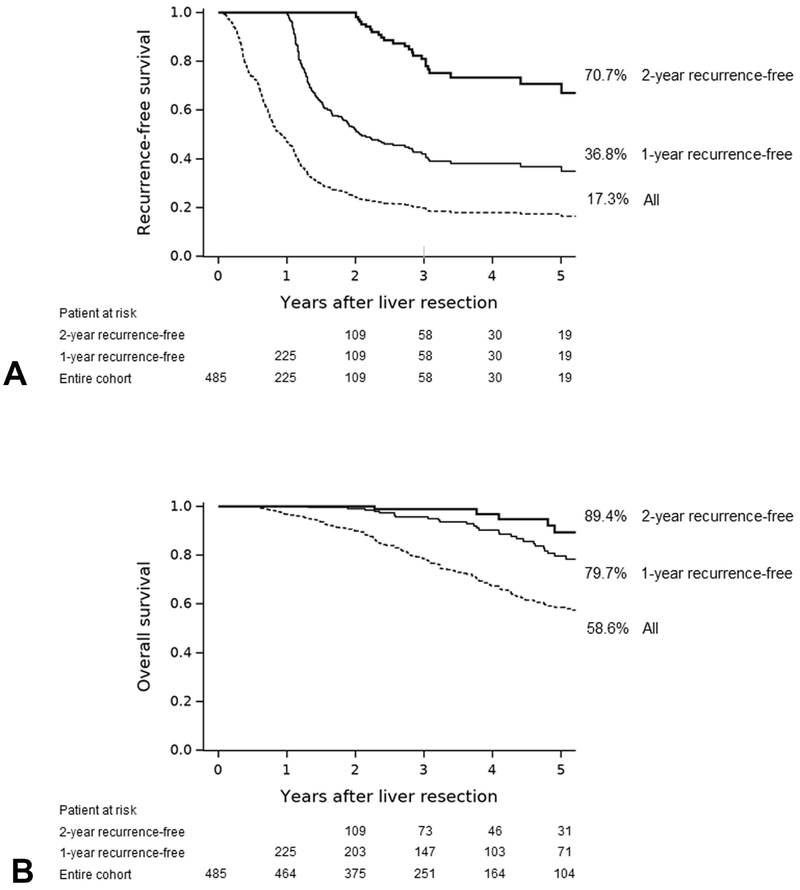
RFS and OS estimates for the entire group and the 1-year and 2-year recurrence-free groups
RFS curves for the entire cohort and the 1-year and 2-year recurrence-free groups are shown in Figure 2A. The 5-year RFS rates were 17.3%, 36.8%, and 70.7% in the entire cohort, the 1-year recurrence-free group, and the 2-year recurrence-free group, respectively. The 5-year OS rates were 58.6%, 79.7%, and 89.4% in the entire cohort, the 1-year recurrence-free group, and the 2-year recurrence-free group, respectively (Figure 2B).
Figure 2.
(A) Recurrence-free and (B) overall survival in the entire cohort, 1-year recurrence-free group, and 2-year recurrence-free group.
Changes in risk factors for recurrence after given time intervals free from recurrence
Risk factors for recurrence after given time intervals free from recurrence are summarized in Table 2. A multivariable Cox proportional hazards model identified RAS/TP53 co-mutation as the only factor significantly associated with RFS for every group (Table 2). In addition, T category ≥ 3, extrahepatic disease, Multiple CLM, largest CLM diameter > 5 cm, and surgical margin status were associated with recurrence in all patients at the time of liver resection, and largest CLM diameter > 5 cm were associated with recurrence in the 1-year recurrence-free group.
Table 2.
Multivariable Cox Proportional Hazards Model Analysis for Recurrence-Free Survival for All Patients and for the Patient Subgroup Observed to Be Recurrence-Free at 1 Year and at 2 Years After Colorectal Liver Metastasis Resection
| Variable* | At the time of liver resection (N = 479)† |
1-year recurrence-free (N = 224)‡ |
2-year recurrence-free (N = 109)§ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Multivariable HR |
95% CI | p Value | Multivariable HR |
95% CI | p Value | Multivariable HR¶ |
95% CI | p Value | |
| T category ≥ 3 | 1.38 | 1.00–1.91 | 0.049 | 1.30 | 0.79–2.16 | 0.30 | – | – | – |
| Extrahepatic disease | 1.46 | 1.12–1.89 | 0.004 | 1.54 | 0.95–2.51 | 0.08 | – | – | – |
| Multiple CLM (vs single) | 1.27 | 1.02–1.57 | 0.029 | 0.96 | 0.68–1.35 | 0.81 | – | – | – |
| Largest CLM diameter > 5 cm | 1.78 | 1.34–2.37 | < 0.001 | 2.79 | 1.63–4.79 | < 0.001 | 2.41 | 0.37–22.19 | 0.40 |
| Surgical margin status, R1 | 1.35 | 1.05–1.73 | 0.021 | 1.00 | 0.60–1.66 | 0.99 | – | – | – |
| RAS/TP53 co-mutation | 1.47 | 1.19–1.82 | < 0.001 | 1.69 | 1.17–2.43 | 0.005 | 2.41 | 1.12–5.17 | 0.024 |
CLM, colorectal liver metastasis; HR, hazard ratio
Based on analysis of data from 468 patients with complete data on T category and lymph node metastasis, 7 variables were selected for the final model as follows: T category (≥ 3 vs. < 3), extrahepatic disease, multiple CLM (vs. single), largest CLM diameter (> 5 cm vs. ≤ 5 cm), surgical margin status (R1 vs. R0), RAS mutation, and TP53 mutation.
Based on analysis of data from 479 patients at the time of liver resection with complete data on T category. Multivariable hazard ratio was calculated with these variables after grouping RAS mutation and TP53 mutation as RAS/TP53 co-mutation.
Based on analysis of data from 224 patients recurrence free at 1 year with complete data on T category. Multivariable hazard ratio was calculated with T category, extrahepatic disease, multiple CLM, largest CLM diameter, surgical margin status, and RAS/TP53 co-mutation.
Based on analysis of data from 109 patients recurrence free at 2 years. Multivariable hazard ratio was calculated with largest CLM diameter and RAS/TP53 co-mutation.
T category, extrahepatic disease, multiple CLM, and surgical margin status were not included in the model because these factors were not associated with risks of recurrence in patients who were recurrence-free at 1 year.
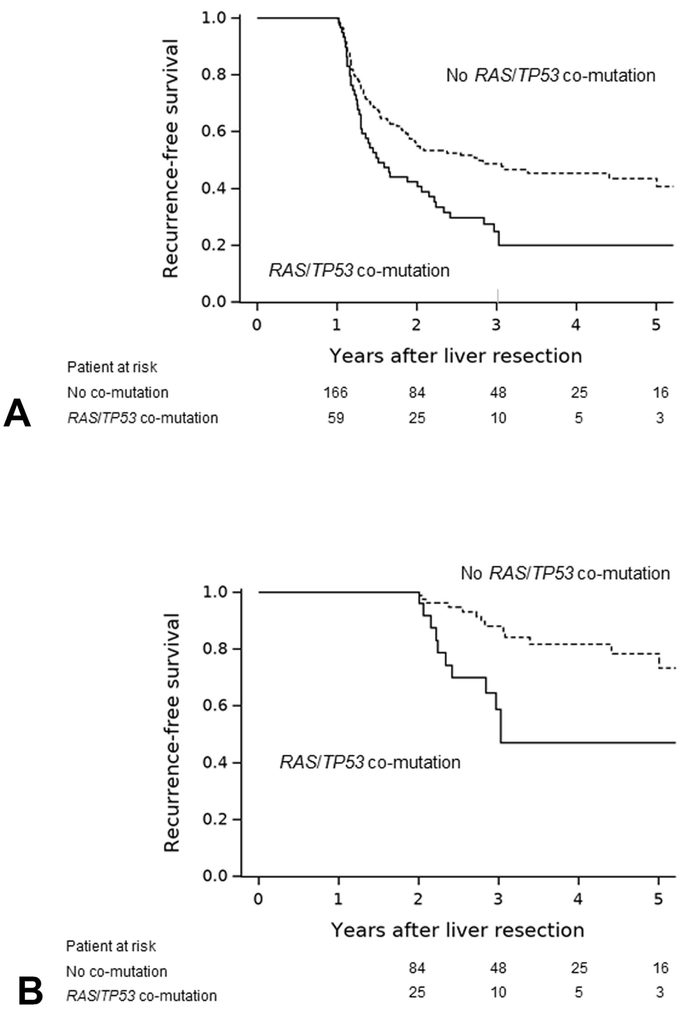
Conditional RFS by RAS/TP53 co-mutation
RFS was significantly shorter in patients with RAS/TP53 co-mutation than in those without RAS/TP53 co-mutation (Figure 3) in both the 1-year recurrence-free group (median RFS, 1.5 years vs. 2.8 years; P = 0.006) and the 2-year recurrence-free group (median RFS, 3.0 years vs. 5.9 years; P = 0.024).
Figure 3.
Conditional recurrence-free survival (RFS) by RAS/TP53 co-mutation status. (A) In the 1-year recurrence-free group (n=225), median RFS was 1.5 years in patients with RAS/TP53 co-mutation (n=59) vs 2.8 years in patients without RAS/TP53 co-mutation (n=166) (P = 0.006). (B) In the 2-year recurrence-free group (n=109), median RFS was 3.0 years in patients with RAS/TP53 co-mutation (n=25) vs 5.9 years in patients without RAS/TP53 co-mutation (n=84) (P = 0.024).
DISCUSSION
The findings of this study show that the rates of RFS for patients who survive without recurrence progressively improve with the duration of time without recurrence after CLM resection. Furthermore, risk factors for recurrence may have changed with the recurrence-free interval. Importantly, RAS/TP53 co-mutation had a persistent deleterious association with recurrence over time; RAS/TP53 co-mutation was a risk factor not only for patients at the time of CLM resection but also for patients who remained recurrence-free at 1 year or 2 years after resection. Conditional RFS estimates and information about changes in risk factors with increasing recurrence-free interval should be taken into account in individualizing postoperative therapy and surveillance intensity and can be used to update patients’ prognosis. The current study assessed conditional RFS (i.e., RFS probability after a given time interval without recurrence) and demonstrated that the risk of recurrence was lower in patients who did not experience recurrence within 1 or 2 years after CLM resection than in all patients at the time of resection. At 5 years, 17.3% of all patients were estimated to be recurrence-free. However, the proportion of patients recurrence-free at 5 years was 36.8% among patients who had not experienced a recurrence within 1 year of CLM resection. The recurrence-free rate at 5 years was even higher, 70.7%, for patients who remained free of recurrence 2 years after CLM resection.
OS rates were also higher in patients remaining free of recurrence at 1 or 2 years than in the entire group of patients from the time of CLM resection. Conditional OS estimates update the survival probability for a patient but do not stratify the changing survival probabilities based on whether or not a patient has experienced recurrence (16, 17). Conditional OS is clearly important. However, for surgeons and/or medical oncologists caring for patients after CLM resection, the most important considerations after CLM resection revolve around preventing and accurately detecting disease recurrence. Thus, we believe that the concept of conditional RFS provides a more useful tool for clinical decision-making such as selecting between repeat resection or chemotherapy, determining surveillance frequency and intensity, and scheduling clinical follow-up visits.
We previously showed that RAS/TP53 co-mutation is a strong independent predictor of worse survival after CLM resection (14). In line with those findings, we found in the current study that RAS/TP53 co-mutation exerted a persistent deleterious effect on the risk of recurrence. Patients with this co-mutation remained at increased risk for recurrence even if they had been recurrence-free for 2 years after CLM resection and may need to be followed more stringently.
Similar to previous studies (5, 31), our current study showed that traditional clinicopathologic factors, including T category, extrahepatic disease, largest CLM diameter, surgical margin status were association with risk of recurrence and were clinically relevant for counseling patients in the immediate postoperative follow-up period. However, these traditional clinicopathologic factors were no longer significantly associated with risk of recurrence once a patient had survived 2 years without recurrence.
This study has several limitations. First, only one-fifth of all patients who underwent CLM resection at our institution during the study period were analyzed as multigene panel testing was not performed in patients undergoing CLM resection before 2012. Mutations were determined through analysis of primary tumor tissue in some patients and CLM tissue in others. However, many studies suggest a high rate of concordance (> 90%) for RAS, TP53, APC, and PIK3CA mutations between primary tumors and metastases (32-35). Another limitation is that we estimated conditional RFS and risk factors for recurrence after given time intervals free from recurrence only for patients remaining free of recurrence at 1 and 2 years after resection of CLM. From a clinical standpoint, it may be useful to know factors associated with recurrence for patients who have survived 3 or more years without recurrence. However, because 75% of the patients in our series had recurrence within 2 years, this analysis was not performed. Additionally, the number of events for patients who were recurrence free at 2 years also decreases, thus limiting the number of variables that can be included in a proportional hazard model.
CONCLUSIONS
In conclusion, conditional RFS estimates and knowledge of the risk factors for recurrence at specific recurrence-free intervals after initial liver resection for CLM allow clinicians to update a patient’s prognosis after a given time interval without recurrence and may allow oncologists to offer precision clinical care by altering surveillance intensity and follow-up periods in accordance with prognostic risk changes. In particular, patients with RAS/TP53 co-mutation may need to have more intense surveillance strategy as RAS/TP53 co-mutation exhibits a persistent deleterious association with recurrence for patients who have not yet experienced recurrence.
Supplementary Material
eFigure 1. Patient selection. *In patients who underwent successful planned 2-stage hepatectomy, only the second-stage hepatectomy was included. The first-stage hepatectomy was excluded so that we would not include patients twice in our analysis. CLM, colorectal liver metastases
Acknowledgement
The authors thank Dr Mario De Bellis for reviewing the data used in the study, Ms Ruth Haynes for administrative support in the preparation of this manuscript, and Ms Stephanie Deming, an employee of the Department of Scientific Publications at MD Anderson Cancer Center, for copyediting the manuscript.
Support: This study was supported by the National Cancer Institute under award number P30 CA016672, which supports the MD Anderson Cancer Center Clinical Trials Support Resource.
ABBREVIATIONS
- CLM
colorectal liver metastasis
- OS
overall survival
- RFS
recurrence-free survival
- IQR
interquartile range
- HR
hazard ratio
- CI
confidence interval
Footnotes
Presented at the Western Surgical Association 126th Scientific Session, San Jose del Cabo, Mexico, November 2018.
Disclosure Information: Nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdalla EK, Vauthey J-N, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 Fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol 2011;18:1096–1103. [DOI] [PubMed] [Google Scholar]
- 5.Vigano L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol 2014;21:1276–1286. [DOI] [PubMed] [Google Scholar]
- 6.Nuzzo G, Giuliante F, Ardito F, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery 2008;143:384–393. [DOI] [PubMed] [Google Scholar]
- 7.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 2009;250:440–448. [DOI] [PubMed] [Google Scholar]
- 8.Hallet J, Sa Cunha A, Adam R, et al. Factors influencing recurrence following initial hepatectomy for colorectal liver metastases. Br J Surg 2016;103:1366–1376. [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Rocken C, Lofton-Day C, et al. Molecular analysis of APC promoter methylation and protein expression in colorectal cancer metastasis. Carcinogenesis 2005;26:37–43. [DOI] [PubMed] [Google Scholar]
- 10.Mollevi DG, Serrano T, Ginesta MM, et al. Mutations in TP53 are a prognostic factor in colorectal hepatic metastases undergoing surgical resection. Carcinogenesis 2007;28:1241–1246. [DOI] [PubMed] [Google Scholar]
- 11.Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013;258:619–626; discussion 626-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karagkounis G, Torbenson MS, Daniel HD, et al. Incidence and prognostic impact of KRAS and BRAF mutation in patients undergoing liver surgery for colorectal metastases. Cancer 2013;119:4137–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loes IM, Immervoll H, Sorbye H, et al. Impact of KRAS, BRAF, PIK3CA, TP53 status and intraindividual mutation heterogeneity on outcome after liver resection for colorectal cancer metastases. Int J Cancer 2016;139:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun YS, Passot G, Yamashita S, et al. Deleterious Effect of RAS and Evolutionary High-risk TP53 Double Mutation in Colorectal Liver Metastases. Ann Surg 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg 1995;19:59–71. [DOI] [PubMed] [Google Scholar]
- 16.Nathan H, de Jong MC, Pulitano C, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg 2010;210:755–764, 764–756. [DOI] [PubMed] [Google Scholar]
- 17.Margonis GA, Buettner S, Andreatos N, et al. Prognostic factors change over time after hepatectomy for colorectal liver metastases: a multi-institutional, international analysis of 1099 patients. Ann Surg 2018. January 18. doi: 10.1097/SLA.0000000000002664. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193. [DOI] [PubMed] [Google Scholar]
- 19.Henson DE, Ries LA, Carriaga MT. Conditional survival of 56,268 patients with breast cancer. Cancer 1995;76:237–242. [DOI] [PubMed] [Google Scholar]
- 20.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum 1998;41:1097–1106. [DOI] [PubMed] [Google Scholar]
- 21.Fuller CD, Wang SJ, Thomas CR Jr., et al. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973-1998. Cancer 2007;109:1331–1343. [DOI] [PubMed] [Google Scholar]
- 22.Choi M, Fuller CD, Thomas CR Jr., et al. Conditional survival in ovarian cancer: results from the SEER dataset 1988-2001. Gynecol Oncol 2008;109:203–209. [DOI] [PubMed] [Google Scholar]
- 23.Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg 2009;250:540–548. [DOI] [PubMed] [Google Scholar]
- 24.Chan AO, Jim MH, Lam KF, et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA 2007;298:1412–1419. [DOI] [PubMed] [Google Scholar]
- 25.Brouquet A, Abdalla EK, Kopetz S, et al. High survival rate after two-stage resection of advanced colorectal liver metastases: response-based selection and complete resection define outcome. J Clin Oncol 2011;29:1083–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh RR, Patel KP, Routbort MJ, et al. Clinical validation of a next-generation sequencing screen for mutational hotspots in 46 cancer-related genes. J Mol Diagn 2013;15:607–622. [DOI] [PubMed] [Google Scholar]
- 27.Gasser TaM HG Smoothing Techniques for Curve Estimation. Springer (Berlin) 1979:23–68. [Google Scholar]
- 28.Hess KR, Levin VA. Getting more out of survival data by using the hazard function. Clin Cancer Res 2014;20:1404–1409. [DOI] [PubMed] [Google Scholar]
- 29.Blazer DG 3rd, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol 2008;26:5344–5351. [DOI] [PubMed] [Google Scholar]
- 30.Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017;67:93–99. [DOI] [PubMed] [Google Scholar]
- 31.Bhogal RH, Hodson J, Bramhall SR et al. Predictors of Early Recurrence after Resection of Colorectal Liver Metastases. In World J Surg Oncol, Edition London: BioMed Central 2015; 135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemeny NE, Chou JF, Capanu M, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 2014;120:3965–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knijn N, Mekenkamp LJ, Klomp M, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer 2011;104:1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baas JM, Krens LL, Guchelaar HJ, et al. Concordance of predictive markers for EGFR inhibitors in primary tumors and metastases in colorectal cancer: a review. Oncologist 2011;16:1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vakiani E, Janakiraman M, Shen R, et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol 2012;30:2956–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Patient selection. *In patients who underwent successful planned 2-stage hepatectomy, only the second-stage hepatectomy was included. The first-stage hepatectomy was excluded so that we would not include patients twice in our analysis. CLM, colorectal liver metastases